Abstract
Background
Primary central nervous system lymphoma (PCNSL) is rare and there is limited genomic and immunological information available. Incidental clinical and radiographic responses have been reported in PCNSL patients treated with immune checkpoint inhibitors.
Materials and Methods
To genetically characterize and ascertain if the majority of PCNSL patients may potentially benefit from immune checkpoint inhibitors, we profiled 48 subjects with PCNSL from 2013 to 2018 with (1) next-generation sequencing to detect mutations, gene amplifications, and microsatellite instability (MSI); (2) RNA sequencing to detect gene fusions; and (3) immunohistochemistry to ascertain PD-1 and PD-L1 expression. Tumor mutational burden (TMB) was calculated using somatic nonsynonymous missense mutations.
Results
High PD-L1 expression (>5% staining) was seen in 18 patients (37.5%), and intermediate expression (1–5% staining) was noted in 14 patients (29.2%). Sixteen patients (33.3%) lacked PD-L1 expression. PD-1 expression (>1 cell/high-power field) was seen in 12/14 tumors (85.7%), uncorrelated with PD-L1 expression. TMB of greater than or equal to 5 mutations per megabase (mt/Mb) occurred in 41/42 tumors, with 19% (n = 8) exhibiting high TMB (≥17 mt/Mb), 71.4% (n = 30) exhibiting intermediate TMB (7–16 mt/Mb), and 9.5% (n = 4) exhibiting low TMB (≤6 mt/Mb). No samples had MSI. Twenty-six genes showed mutations, most frequently in MYD88 (34/42, 81%), CD79B (23/42, 55%), and PIM1 (23/42, 55%). Among 7 cases tested with RNA sequencing, an ETV6-IGH fusion was found. Overall, 18/48 samples expressed high PD-L1 and 38/42 samples expressed intermediate to high TMB.
Conclusions
Based on TMB biomarker expression, over 90% of PCNSL patients may benefit from the use of immune checkpoint inhibitors.
Keywords: CNS, lymphoma, PD-L1, tumor mutational burden
Key Points.
Primary CNS lymphoma frequently expresses high PD-L1 and tumor mutational burden.
Further study of checkpoint inhibitor therapy in primary CNS lymphoma is warranted.
Importance of the Study.
As knowledge of the role of targeted therapies and immunotherapies in primary CNS malignancies continues to mature, the necessity of identifying biomarkers that can reliably predict response to treatment is of paramount importance. Our study represents the largest cohort of primary CNS lymphoma tumors that have been analyzed to date for immune checkpoint inhibition-related biomarkers, including PD-1/PD-L1 IHC, tumor mutational burden, and microsatellite instability as well as next-generation sequencing to identify key pathways for potential therapeutic targeting. We find that a significant proportion of PCNSLs appears to harbor features signifying potential responsiveness to immune checkpoint inhibition and targeted molecular therapy.
Primary central nervous system lymphoma (PCNSL) is an aggressive extranodal form of non-Hodgkin’s (eg, diffuse large B cell) lymphoma that is restricted to the brain, eyes, spinal cord, and surrounding cerebrospinal fluid. The incidence of PCNSL in immunocompetent patients is relatively rare, constituting 4% of all intracranial tumors and from 4% to 6% of all extranodal lymphomas.1 In recent years, the incidence in the elderly has been increasing.2 Although there have been significant advances in treatment with the use of high-dose methotrexate and rituximab, which has improved survival tremendously, overall survival relative to other forms of non-Hodgkin’s lymphoma remains poor, and disease recurrence is very common. Up to 50% of patients with PCNSL will relapse, and 10–15% demonstrate primary refractory disease, indicating a significant unmet therapeutic need.3
Immunotherapy in cancer, under development for many decades, reached a seminal moment in 2011, with the FDA approval of ipilimumab, which targeted CTLA-4 (cytotoxic T-lymphocyte-associated protein 4), shown to be therapeutically effective in metastatic melanoma.4 The PD-1–PD-L1 axis inhibits T-cell proliferation and antitumor immune effector activity in the latter stages of the immune response in the tumor microenvironment.5–7 The early successes of immune checkpoint inhibition targeting the PD-1–PD-L1 (programmed death 1 and its ligand) axes in metastatic melanoma and non-small cell lung cancer8–12 firmly established the importance of tumor-mediated mechanisms for evading immune response and triggered enthusiasm for the use of immunotherapies against a multitude of other solid tumors including lymphoid malignancies.13 Anti-PD-1 has demonstrated therapeutic effects in preclinical models of lymphoma14 and in a small cohort of patients with PCNSL.15
Currently, it is unclear whether immune checkpoint inhibition would be universally beneficial in treating patients with PCNSL or if a companion biomarker needs to be considered in the context of large-scale clinical trials. Thus far, there has been no prior systematic examination of predictive biomarkers for response to checkpoint inhibition in PCNSL. Tumor and tumor-infiltrating lymphocyte (TIL) expression of PD-L1 is recognized as one of the predictive biomarkers for immune checkpoint inhibitor responses in various solid tumors16–19 and for Hodgkin’s lymphoma.20 Concomitant immunogenicity, as characterized by tumor mutational burden (TMB) and hypermutability secondary to defective DNA mismatch repair, is also considered an enrichment biomarker for response to immune checkpoint inhibition.21–24 In view of ongoing studies of the use of immunomodulatory therapeutics for PCNSL, we analyzed PCNSL patients using immunohistochemistry (IHC) and next-generation sequencing (NGS) to ascertain the incidence of response biomarkers to immune checkpoint inhibitors and performed genetic characterization to ascertain the potential therapeutic opportunities for targeted therapy.
Materials and Methods
Ethics Statement
Human subjects were de-identified prior to analysis, and this research is exempt under the Code of Federal Regulations 45 CFR 46.101(b)(4) from 45 CFR part 46 requirements. We analyzed reported results for 48 patients from a database of PCNSL patients who underwent tumor profiling with Caris Life Sciences (Irving, TX), a CLIA-certified laboratory, from 2013 to 2018. Specimens were obtained from multiple research centers within the United States and had limited clinical annotation.
Next-Generation Sequencing
NGS was performed on genomic DNA isolated from formalin-fixed paraffin-embedded tissue using the Illumina NextSEQ platform, a 592-gene panel (n = 36) or 45-gene panel (n = 6) used to identify mutations and gene amplification as described (http://www.carislifesciences.com) in which there was sufficient tissue to identify potential therapeutic targets. Because synonymous and exonic mutations do not guide the selection of therapeutics at this time, these were not reported. Variants were detected with greater than 99% confidence based upon allele frequency and amplicon coverage, with an average sequencing depth of coverage greater than 500× and analytic sensitivity of 5% variant frequency. Variants were classified according to the American College of Medical Genetics and Genomics guidelines.25 Microsatellite instability (MSI) was tested by NGS. TMB was calculated using somatic nonsynonymous missense mutations in accordance with the TMB harmonization project (http://www.focr.org/tmb), adding nonsynonymous, nonsense, in-frame indel, and frameshift variants after filtering out presumed germline variants determined from the Genome Aggregation Database (release 2.1), the Single Nucleotide Polymorphism Database human build 151, and the Caris in-house benign database. Per tumor sample, a total of 1.4 Mb was sequenced.26 Low TMB was defined as less than or equal to 6 mutations per megabase (mt/Mb), intermediate TMB was defined as inclusive of 7 and 16 mt/Mb, and high TMB was defined as greater than or equal to 17 mt/Mb based on cut points established in other malignancies.27,28
Immunohistochemistry
PD-L1 IHC (SP142, rabbit) and PD-1 IHC (MRQ-22, mouse) expression was evaluated in tumor cells and in tumor-infiltrating immune cells as previously described.29 The respective negative controls were the Dako FLEX rabbit immunoglobulin fraction of serum from non-immunized rabbits, solid phase absorbed, code IS600, and the Ventana antibody (monoclonal, catalog number 760-2014). Low staining intensity was defined as 0%, intermediate staining intensity was defined as 1–4% (inclusive), and high PD-L1 staining intensity was defined as greater than or equal to 5% based on the cut point for clinical responses to immune checkpoint inhibitors.17,30
Statistical Analysis
Kruskal–Wallis, Pearson correlation coefficient, and linear regression tests for statistical significance were performed using GraphPad Prism version 9.2.1 for Windows, GraphPad Software, San Diego, CA, www.graphpad.com.
Results
Characteristics of the Analyzed Patient Cohort
Our study analyzed 48 patients diagnosed with PCNSL, ranging from 39 to 84 years old (mean age 66.9 years) with an even sex distribution (Table 1). Of the 31 tumors whose sampling sites were recorded, most were from the frontal lobe (n = 11), followed by parietal lobe (n = 5), temporal lobe (n = 3), basal ganglia (n = 3), occipital lobe (n = 2), ventricle (n = 2), thalamus (n = 2), cerebellum (n = 1), corpus callosum (n = 1), and hypothalamus (n = 1).
Table 1.
Characteristics of Patients With Primary Central Nervous System Lymphoma and Their Tumor Samples
| Average Age | 66.9 years | ||
|---|---|---|---|
| Age Range | 39–84 years | ||
| Specimen Site | N | Female | Male |
| Frontal lobe | 11 | 5 | 6 |
| Parietal lobe | 5 | 1 | 4 |
| Temporal lobe | 3 | 0 | 3 |
| Ventricle | 2 | 2 | 0 |
| Occipital lobe | 2 | 2 | 0 |
| Thalamus | 2 | 1 | 1 |
| Basal ganglia | 3 | 2 | 1 |
| Cerebellum | 1 | 1 | 0 |
| Corpus callosum | 1 | 0 | 1 |
| Hypothalamus | 1 | 1 | 0 |
| Brain, NOS | 17 | 9 | 8 |
| Total | 48 | 24 | 24 |
Mutations Identified by NGS and RNA Sequencing
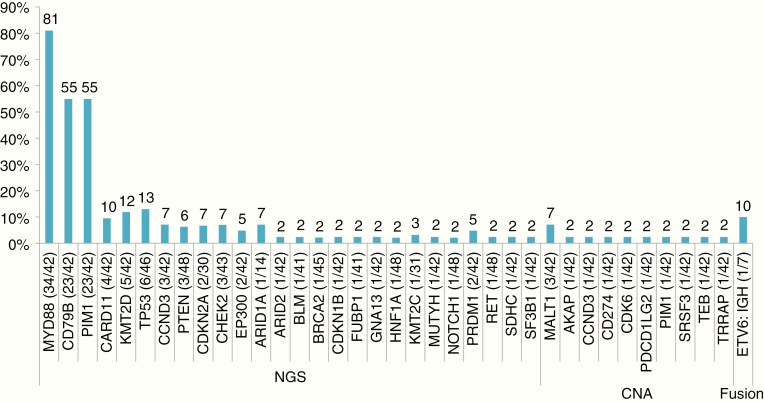
Mutations were found in 26 genes, the most frequent of which were in MYD88 (81%, 34/42), CD79B (55%, 23/42), and PIM1 (55%, 23/42). Other mutations were found in CARD11 (9.5%, 4/42), KMT2D (11.9%, 5/42), TP53 (13%, 6/46), CCND3 (7.1%, 3/42), PTEN (6.3%, 3/48), and CDKN2A (6.7%, 2/30) (Figure 1). Genetic co-amplification of PD-L1 and PD-L2 was seen in one sample. Of the 10 samples tested with RNA sequencing, one ETV6-IGH fusion was found. Most mutations in MYD88 and CD79B led to changes in the L265P and Y196 residues, respectively. Other genes, eg, PIM1 CARD11, KMT2D, and TP53, were found to have a variety of different mutations (Table 2). There was an association of changes in MYD88 and CD79B with frontal lobe tumors (Supplementary Figure S1); however, this was not statistically significant and is likely related to the minimal neurological risks of sampling a multifocal disease in this location.
Fig. 1.
Bar chart of the total number of primary central nervous system lymphomas (PCNSLs) detected with an alteration above the total N tested, shown in parentheses. Molecular alterations were detected by next-generation sequencing (NGS) in a total of 42 sequenced PCNSLs. NGS: mutations in DNA; CNA: copy number amplifications in DNA; Fusion: genetic fusion detected in RNA.
Table 2.
Protein Changes Seen for the Top 6 Most Frequently Mutated Genes in Patients With Primary Nervous System Lymphoma
| Gene | Protein Change | N | Total |
|---|---|---|---|
| MYD88 | L265P | 33 | 34 |
| V217F | 1 | ||
| CD79B | Y196 | 21 | 23 |
| L199P | 2 | ||
| CARD11 | E626K | 2 | 4 |
| C49Y | 1 | ||
| D230N | 1 | ||
| KMT2D | C189X | 1 | 4 |
| Q1557fs | 1 | ||
| R2687X | 1 | ||
| S3443fs | 1 | ||
| TP53 | R196X | 1 | 6 |
| R209fs | 1 | ||
| R333fs | 1 | ||
| R337C I255N | 1 | ||
| V218_P222del | 1 | ||
| PIM1 | E135K | 9 | 49 |
| G28D | 6 | ||
| M1I | 6 | ||
| P33S | 5 | ||
| G99D | 4 | ||
| E30K | 3 | ||
| K24N | 3 | ||
| L184F | 3 | ||
| E79D | 2 | ||
| P125S | 2 | ||
| S146R | 2 | ||
| S97N | 3 | ||
| K71N | 1 |
Tumor Mutational Burden and Microsatellite Instability
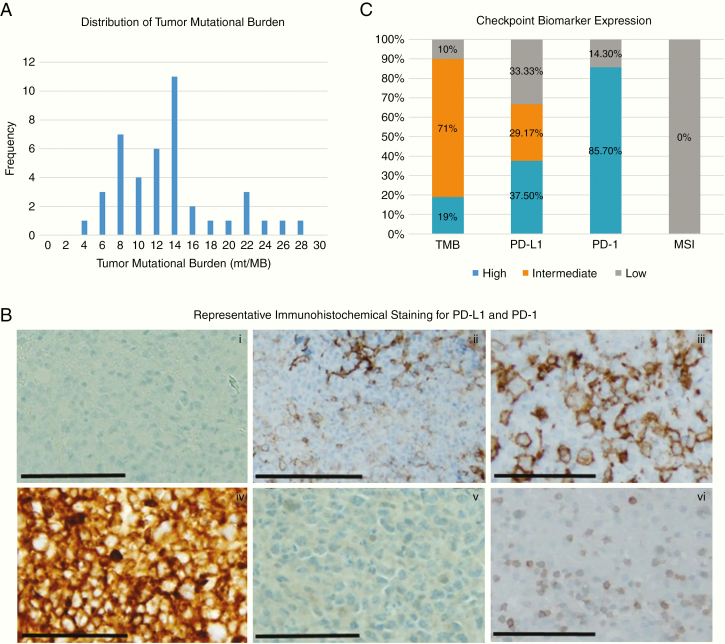
TMB was quantified via the 592-gene panel, and of the 42 samples from which we could obtain these data, 8 (19%) samples exhibited high TMB (≥17 mt/Mb), 30 (71.4%) samples exhibited intermediate TMB (7–16 mt/Mb), and 4 (9.5%) samples exhibited low TMB (≤6 mt/Mb) (Figure 2A). No samples exhibited high levels of MSI (Figure 2C).
Fig. 2.
(A) Bar chart depicting numbers of patients (y-axis) expressing specific tumor mutational burden values (x-axis). (B) Representative immunohistochemical micrographs demonstrating low (ii), intermediate (iii), and high (iv) PD-L1 staining and high (vi) PD-1 staining in PCNSL cases with negative controls for PD-L1 (i) and PD-1 (v), respectively. Bar = 1 mm at 20× magnification. (C) Bar chart demonstrating the frequency of expression of immune checkpoint biomarkers in PCNSLs.
Expression of PD-L1 and PD-1
High PD-L1 expression (>5% staining) was seen in 18 cases (37.5%), intermediate expression (1–5% staining) was noted in 14 cases (29.2%), and 16 cases (33.3%) showed no PD-L1 expression (Figure 2B and C). PD-1 expression in TILs (>1 cell/high-power field) was seen in 12/14 tumors (85.7%), but there was no association with concomitant PD-L1 expression (Figure 2C).
Immune Checkpoint Biomarker Association
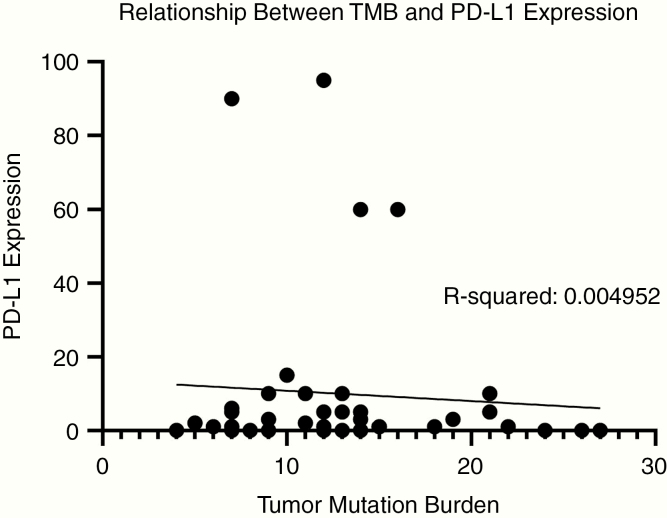
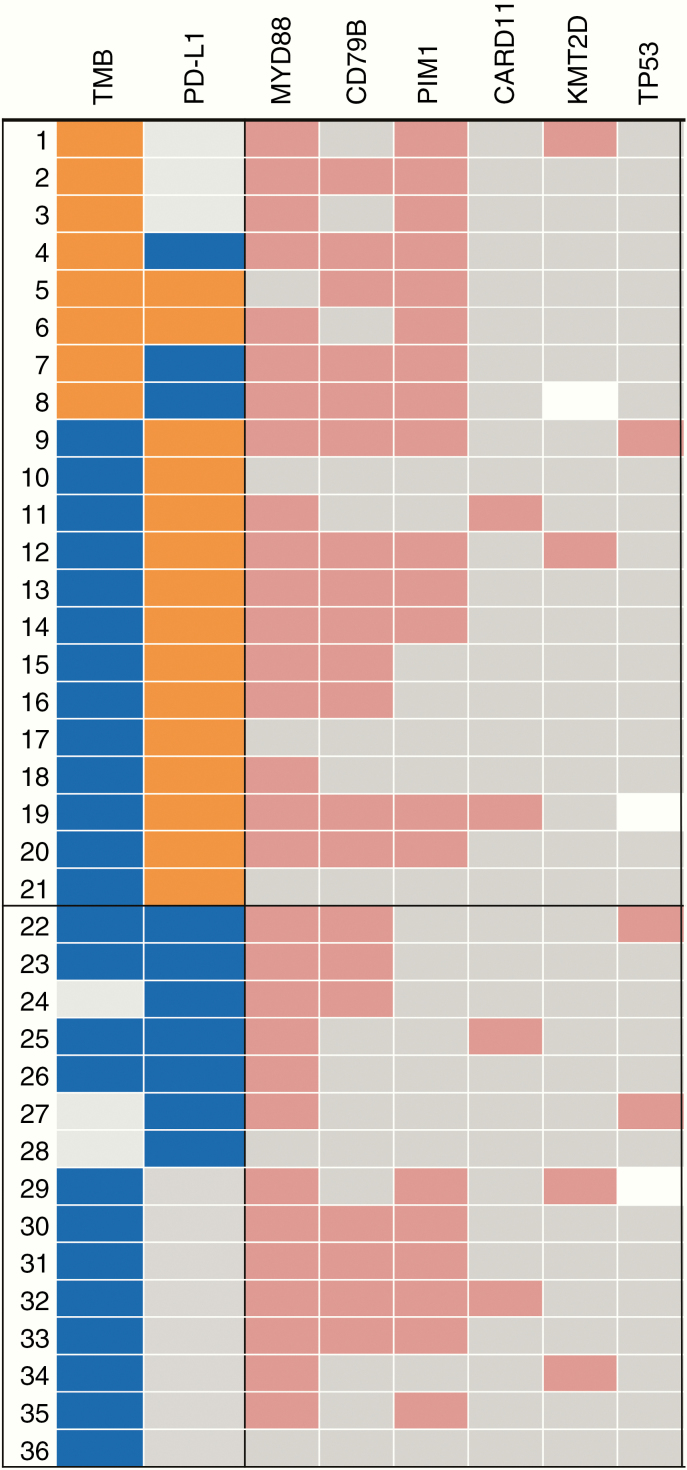
Overall, 54.8% of tumors expressed either high PD-L1 expression or high TMB. PD-L1 and TMB did not co-associate (Figure 3). Additionally, location was not significantly associated with either high TMB (P = .576) or PD-L1 expression (P = .0542) (Supplementary Figure S2). TMB was not significantly associated with MSI. The most common mutations such as MYD88, CD79B, and PIM1 were usually detected in cases that had high or intermediate TMB but not necessarily high PD-L1 expression (Figure 4). Based on the expression of either high PD-L1 expression or intermediate to high TMB, 37.5–90% of PCNSL patients may respond to immune checkpoint inhibitors. In addition, 85.7% of patients had positive PD-1 expression and 42.8% of patients with positive PD-1 expression also had high PD-L1 levels.
Fig. 3.
Scatter plot demonstrating the level of tumor mutational burden (TMB) as determined by NGS (x-axis), relative to the level of PD-L1 expression (y-axis) in patient samples.
Fig. 4.
Oncoprint demonstrating the association between biomarkers of immune checkpoint response and specific gene mutations. TMB high: ≥17 mt/Mb; intermediate: 16 mt/Mb ≥ TMB ≥7 mt/Mb; TMB low: ≤6 mt/Mb. PD-L1 high: staining intensity ≥5%; PD-L1 intermediate: 4% ≥ PDL1 ≥1% PD-L1; low: staining intensity = 0 (negative). High expression of TMB or PD-L1 is shown in orange and intermediate in blue. Gene mutations, amplifications, or gene fusions are shown in red; no alterations/wild type are in gray; blank indicates missing data.
Discussion
The purpose of this study was to determine the frequency of expression of immune checkpoint biomarkers and their association with known genetic alterations in PCNSL. To characterize the immunogenicity of PCNSL, we examined TMB and found nearly 90% of our tested cohort to have intermediate to high TMB. Based on their expression of either high PD-L1 or intermediate to high TMB, it appears that the majority of PCNSL patients may respond to immune checkpoint inhibitors. Higher rates of nonsynonymous TMB have been associated with favorable overall response rate, clinical benefit, and progression-free survival in patients who received anti-PD-1/PD-L1 monotherapy.7,22 The cut point at which TMB correlates with therapeutic responses may be lineage specific and may be as low as 6 mt/Mb.31 Based on our results and those of other researchers, it is likely that PCNSL patients with a high TMB—with or without concomitant high PD-L1 expression—may benefit from checkpoint inhibition. Interestingly, systemic lymphomas have a higher TMB32 than PCNSL, and it would be interesting to identify in matched secondary CNS lymphomas if TMB is lower than in the primary. As such, PCNSL may have less response to immune checkpoint inhibitors than other types of lymphoma, but further clinical study will be necessary to refine and validate TMB cutoffs in the PCNSL patient population.
Though MSI is known to be an important mechanism of neoantigen generation in a number of solid tumors (eg, colorectal, endometrial, and gastric) and HIV-related lymphomas, we did not find any evidence of MSI in our samples.33 MSI is characteristic of a tumor phenotype caused by defective DNA mismatch repair (leading to the accumulation of somatic mutations at repetitive microsatellite sequences contained in various target genes implicated in human cancers).34 Our findings are consistent with previous reports,28,35,36 which suggest that this mechanism is probably not a significant driver of mutational burden or PD-1–PD-L1 axis blockade efficacy in PCNSL.
The most common genes found to be altered in PCNSL were MYD88, CD79B, and PIM1 consistent with prior analyses.37,38 These genes are oncogenic drivers of the NF-kB pathway,39,40 which has been shown to be associated with PCNSL.41 There is preclinical data that HDAC inhibitors (eg, panobinostat) work synergistically with ibrutinib in lymphoma cases harboring MYD88 mutations, and patients with CD79B mutations tended to respond to ibrutinib.42,43 An ongoing clinical trial of ibrutinib and nivolumab in refractory PCNSL patients at MD Anderson Cancer Center open for accrual (NCT03770416). Notably, point mutations in the kinase PIM1, via altered interactions with upstream regulators as well as downstream signaling, in particular, have recently been shown to reduce the sensitivity of the activated B-cell subtype of diffuse large B-cell lymphoma to ibrutinib.44 A potential role for PIM1 as a driver of disease recurrence has also been described.33 CARD11, found to be mutated in 11% of our samples, is an important scaffold protein involved in signaling that controls antigen-induced B and T lymphocyte activation during the adaptive immune response, and gain-of-function mutations here lead to constitutive activation of NF-kB, JNK, and mTOR.45KMT2D encodes a DNA methyltransferase important for global H3K4 methylation in germinal-center B cells, and its inactivation early in B-cell development results in increased proliferation of germinal-center B cells.46 According to the literature, B-cell receptor/NF-kB signaling pathways are altered in more than 90% of PCNSLs, highlighting this receptor’s value for targeted therapy.39 Specific NF-kB inhibitors have been developed but are not yet available for use in clinical trials.
Despite promising findings, our study has notable limitations. We do not have the survival statistics of the patients whose samples were analyzed, which limits the predictive significance of our findings. A clinical trial evaluating the prognostic significance of PD-L1 is underway (NCT04158128) and transcript variants of high PD-1 expression were shown to confer a negative prognostic outcome.47 Second, it should be noted that patients who lack high TMB or PD-L1 expression can still have a clinical benefit from checkpoint inhibition,8,13,48 and we did not assess for other markers of potential response such as neoantigen burden or T-cell receptor clonality.49–51 There are likely other biomarkers at play that have not yet even been considered and/or devised. Third, with an arguably greater therapeutic need for relapsed or recurrent PCNSL, our findings do not distinguish between newly diagnosed and recurrent or relapsed disease. This concern may be mitigated by the prior work of McGranahan et al.,52 which supports the idea that clonal neoantigens—that is, those that occur early on in tumorigenesis rather than later subclonal neoantigens—are more important in responsiveness to immune checkpoint inhibition. Finally, as it has been previously shown that the relationship among TMB, MSI, and PD-L1 varies significantly by cancer type, biomarkers such as PD-L1 and TMB, although investigated in other solid tumors, have yet to be thoroughly validated in the PCNSL patient population. Our data would support the use of either anti-PD-1 or anti-PD-L1 therapy in a clinical trial in “all comers” with PCNSL with a retrospective determination of which biomarker correlated with potential clinical response. Clinical trials of patients with PCNSL being treated with anti-PD-1 are currently under way (NCT04052659; NCT03255018).
Funding
This research was supported by the National Institutes of Health [CA1208113 and P30 CA016672].
Disclosure of Potential Conflicts of Interest
D.S., Z.G., J.X., and M.K. are employees and A.B.H. serves on the advisory board of Caris Life Sciences.
Authorship Statement
The original idea was developed by A.S., S.P., D.S., S.M., A.B., M.P., S.K., M.K., S.M., and A.B.H. Sample collection and preparation was done by Z.G. and J.X. Benchwork was completed by Z.G. and J.X. Data analysis was performed by A.O., D.S., J.X., M.K., and A.B.H. Tables and figures were created by A.O., Z.G., J.X., and A.B.H. The manuscript was drafted by A.O. and A.B.H. All authors read and approved the final manuscript.
Supplementary Material
Acknowledgments
The authors acknowledge David M. Wildrick, PhD, and Audria Patrick for their editorial and administrative support.
References
- 1. Grommes C, DeAngelis LM. Primary CNS lymphoma. J Clin Oncol. 2017;35(21):2410–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011;105(9):1414–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Langner-Lemercier S, Houillier C, Soussain C, et al. Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network. Neuro Oncol. 2016;18(9):1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iwai Y, Hamanishi J, Chamoto K, Honjo T. Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci. 2017;24(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reck M, Rodríguez-Abreu D, Robinson AG, et al. ; KEYNOTE-024 Investigators Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. [DOI] [PubMed] [Google Scholar]
- 11. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. [DOI] [PubMed] [Google Scholar]
- 12. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. [DOI] [PubMed] [Google Scholar]
- 13. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qiu Y, Li Z, Pouzoulet F, et al. Immune checkpoint inhibition by anti-PDCD1 (anti-PD1) monoclonal antibody has significant therapeutic activity against central nervous system lymphoma in an immunocompetent preclinical model. Br J Haematol. 2018;183(4):674–678. [DOI] [PubMed] [Google Scholar]
- 15. Nayak L, Iwamoto FM, LaCasce A, et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129(23):3071–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Daud AI, Wolchok JD, Robert C, et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol. 2016;34(34):4102–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McDermott DF, Sosman JA, Sznol M, et al. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol. 2016;34(8):833–842. [DOI] [PubMed] [Google Scholar]
- 19. Peters S, Gettinger S, Johnson ML, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH). J Clin Oncol. 2017;35(24):2781–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roemer MGM, Redd RA, Cader FZ, et al. Major histocompatibility complex class II and programmed death ligand 1 expression predict outcome after programmed death 1 blockade in classic Hodgkin lymphoma. J Clin Oncol. 2018;36(10):942–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Campesato LF, Barroso-Sousa R, Jimenez L, et al. Comprehensive cancer-gene panels can be used to estimate mutational load and predict clinical benefit to PD-1 blockade in clinical practice. Oncotarget. 2015;6(33):34221–34227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schrock AB, Ouyang C, Sandhu J, et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol. 2019;30(7):1096–1103. [DOI] [PubMed] [Google Scholar]
- 24. Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Richards S, Aziz N, Bale S, et al. ; ACMG Laboratory Quality Assurance Committee Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stadler ZK, Battaglin F, Middha S, et al. Reliable detection of mismatch repair deficiency in colorectal cancers using mutational load in next-generation sequencing panels. J Clin Oncol. 2016;34(18):2141–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vanderwalde A, Spetzler D, Xiao N, Gatalica Z, Marshall J. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med. 2018;7(3):746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garber ST, Hashimoto Y, Weathers SP, et al. Immune checkpoint blockade as a potential therapeutic target: surveying CNS malignancies. Neuro Oncol. 2016;18(10):1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391(10122): 748–757. [DOI] [PubMed] [Google Scholar]
- 31. Goodman AM, Kato S, Bazhenova L, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16(11):2598–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liang WS, Vergilio JA, Salhia B, et al. Comprehensive genomic profiling of Hodgkin lymphoma reveals recurrently mutated genes and increased mutation burden. Oncologist. 2019;24(2):219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cuceu C, Hempel WM, Sabatier L, Bosq J, Carde P, M’Kacher R. Chromosomal instability in Hodgkin lymphoma: an in-depth review and perspectives. Cancers (Basel). 2018;10(4):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Duval A, Raphael M, Brennetot C, et al. The mutator pathway is a feature of immunodeficiency-related lymphomas. Proc Natl Acad Sci U S A. 2004;101(14):5002–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu-Monette ZY, Zhou J, Young KH. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018;131(1):68–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fukumura K, Kawazu M, Kojima S, et al. Genomic characterization of primary central nervous system lymphoma. Acta Neuropathol. 2016;131(6):865–875. [DOI] [PubMed] [Google Scholar]
- 38. Zorofchian S, El-Achi H, Yan Y, Esquenazi Y, Ballester LY. Characterization of genomic alterations in primary central nervous system lymphomas. J Neurooncol. 2018;140(3):509–517. [DOI] [PubMed] [Google Scholar]
- 39. Braggio E, Van Wier S, Ojha J, et al. Genome-wide analysis uncovers novel recurrent alterations in primary central nervous system lymphomas. Clin Cancer Res. 2015;21(17):3986–3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suehara Y, Sakata-Yanagimoto M, Hattori K, et al. Mutations found in cell-free DNAs of patients with malignant lymphoma at remission can derive from clonal hematopoiesis. Cancer Sci. 2019;110(10):3375–3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vater I, Montesinos-Rongen M, Schlesner M, et al. The mutational pattern of primary lymphoma of the central nervous system determined by whole-exome sequencing. Leukemia. 2015;29(3):677–685. [DOI] [PubMed] [Google Scholar]
- 42. Mondello P, Brea EJ, De Stanchina E, et al. Panobinostat acts synergistically with ibrutinib in diffuse large B cell lymphoma cells with MyD88 L265P mutations. JCI Insight. 2018;3(22):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21(8):922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kuo HP, Ezell SA, Hsieh S, et al. The role of PIM1 in the ibrutinib-resistant ABC subtype of diffuse large B-cell lymphoma. Am J Cancer Res. 2016;6(11):2489–2501. [PMC free article] [PubMed] [Google Scholar]
- 45. Bedsaul JR, Carter NM, Deibel KE, et al. Mechanisms of regulated and dysregulated CARD11 signaling in adaptive immunity and disease. Front Immunol. 2018;9:2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang J, Dominguez-Sola D, Hussein S, et al. Disruption of KMT2D perturbs germinal center B cell development and promotes lymphomagenesis. Nat Med. 2015;21(10):1190–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Takashima Y, Kawaguchi A, Sato R, et al. Differential expression of individual transcript variants of PD-1 and PD-L2 genes on Th-1/Th-2 status is guaranteed for prognosis prediction in PCNSL. Sci Rep. 2019;9(1):10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17(12):e542–e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pollari M, Brück O, Pellinen T, et al. PD-L1+ tumor-associated macrophages and PD-1+ tumor-infiltrating lymphocytes predict survival in primary testicular lymphoma. Haematologica. 2018;103(11):1908–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.