ABSTRACT
Mobile colistin resistance (mcr) genes represent an emerging challenge. Here we describe a novel mcr gene, mcr-10, on an IncFIA plasmid of an Enterobacter roggenkampii clinical strain. mcr-10 has the highest nucleotide identity (79.69%) with mcr-9 and encodes MCR-10 with 82.93% amino acids identical to MCR-9. mcr-10 confers 4-fold increase in colistin MIC (from 1 to 4 mg/L) when cloned into a colistin-susceptible E. roggenkampii strain. By screening GenBank, mcr-10 was found in various Enterobacteriaceae species of countries in four continents, suggesting that this gene has widely spread. MCR-10 shows 79.04% to 83.67% amino acid identity and highly conserved predicted protein structures with chromosomally encoded MCR-like phosphoethanolamine transferases (designated MCR-B here) of various Buttiauxella species. MCR-10, MCR-9 and MCR-B proteins may, therefore, originate from a common ancestor. mcr-10 was adjacent to a site-specific recombinase-encoding gene and was bracketed by IS903 and may be mobilized by site-specific recombination or composite transposon. Our results indicate that mcr-10 is a novel plasmid-borne colistin resistance gene and warrants immediate monitoring and further studies.
KEYWORDS: Colistin resistance, mcr, mcr-10, plasmid, Enterobacter roggenkampii
Introduction
Colistin is a last resort antimicrobial agent against carbapenem-resistant Gram-negative bacteria including Enterobacteriaceae but strains with acquired colistin resistance have also emerged worldwide [1]. Colistin resistance in the Enterobacteriaceae can be due to chromosomal mechanisms and plasmid-borne mobile colistin resistance genes (mcr). Since the report of the first mcr gene, mcr-1, in 2016 [2], a few other mcr genes including mcr-2 [3], mcr-3 [4], mcr-4 [5], mcr-5 [6], mcr-6 [7], mcr-7 [8], mcr-8 [9] and mcr-9 [10] have been described in Enterobacteriaceae and mcr-1 and mcr-4 have also been reported in Acinetobacter spp.[11,12] or Pseudomonas spp. (mcr-1 only) [12]. All of the MCR proteins are phosphoethanolamine (PEA) transferases [13]. These PEA transferases catalyse the attachment of PEA to lipopolysaccharides (LPS)-lipid A, lead to a reduction of the negative charge of LPS upon structural alteration of lipid A and therefore result in resistance to colistin [13]. Of note, the discovery of mcr-9 in colistin-susceptible strains suggests that strains carrying mcr genes may not exhibit colistin resistance phenotype due to low-level gene expression [10]. This promotes us to investigate the presence of mcr-like genes in a colistin-susceptible Enterobacter clinical strain, for which the minimum inhibitory concentration (MIC) of colistin was 2 mg/L, close to the 4 mg/L resistance breakpoint defined by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (http://www.eucast.org/). We found a new mcr variant, designated mcr-10, in the strain and report the findings here.
Methods
The strain, in vitro susceptibility testing
Strain 090065 (also called WCHER090065) was a clinical isolate recovered in 2016 at West China Hospital. This study was approved by the Ethical Committee of West China Hospital with waiving the inform consent. MICs of aztreonam, cefepime, ceftazidime, colistin, imipenem, meropenem, piperacillin-tazobactam, and tigecycline were determined using the broth microdilution method of the Clinical and Laboratory Standards Institute (CLSI) [14]. For colistin and tigecycline, the breakpoints defined by EUCAST (http://www.eucast.org/) were applied.
Genome sequencing and analysis
Strain 090065 was subjected to whole-genome sequencing using the HiSeq X10 (Illumina; San Diego, CA, USA) according to the manufacturer’s instructions. Genomic DNA was prepared using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). Generated reads were de novo assembled into contigs using SPAdes v3.13.0 [15] applying the careful and auto-cut-off modes. To determine the location of mcr-10, strain 090065 was also subjected to long-read whole-genome sequencing using a MinION Sequencer (Nanopore; Oxford, UK). The de novo hybrid assembly of both short (Illumina) and long reads was performed using Unicycler v0.4.3 [16] under conservative mode for increased accuracy. Pilon v1.22 [17] was used to correct complete circular contigs with Illumina reads for several rounds until no change was detected.
Prokka v1.13 [18] was used to annotate the genome sequence. Antimicrobial resistance genes were identified from genome sequences using the ABRicate program (https://github.com/tseemann/abricate) to query the ResFinder database (https://cge.cbs.dtu.dk/services/ResFinder/). Plasmid replicons were identified using PlasmidFinder 2.0 (https://cge.cbs.dtu.dk/services/PlasmidFinder/).
Nucleotide sequence accession numbers
The complete sequence of the chromosome and plasmids of strain 090065 has been deposited into GenBank under the accession no. CP045064-CP045066. The sequence of mcr-10 has been deposited into GenBank under the accession no. MN179494.
Precise species identification
For precise species identification, the pair-wise average nucleotide identity (ANI) between strain 090065 and type strains of Enterobacter species was determined using JSpeciesWS based on BLAST [19]. A ≥ 95–96% ANI cut-off was used to define a bacterial species [20].
Cloning
The −10, −35 boxes of the promoter of mcr-10 were predicted using the online tool BPROM (http://www.softberry.com/berry.phtml?topic=bprom&group=programs&subgroup=gfindb). The complete coding sequence of mcr-10 and its promoter region were amplified with primers 090065_up_SacI (5′-AAAAAAGAGCTCTCCGCTTTGTATCCCAATAC; restriction site is underlined) and 090065_down_EcoRI (5′-AAAAAAGAATTCTTTTATAATTTCCGGCAGCA) using PrimeSTAR Max DNA Polymerase (Takara, Dalian, China). PCR amplicons and the vector pBC SK (Stratagene, La Jolla, CA, USA) were digested using SacI and EcoRI (New England Biolabs, Ipswich, MA, USA) and were ligated to the pBC SK vector using T4 ligase (New England Biolabs) to construct pBC SK-mcr10. pBC SK-mcr10 was transformed into a colistin-susceptible E. roggenkampii clinical strain, named 120033, by chemical transformation. Potential transformants containing mcr-10 were selected on Luria–Bertani agar plates (Sigma; St. Louis, MO, USA) containing 30 mg/L chloramphenicol. Colonies on plates were screened for mcr-10 by PCR using primers 090065_up_SacI/090065_down_EcoRI and the presence of mcr-10 was confirmed by Sanger sequencing amplicons. MICs of colistin were determined for transformants containing pBC SK-mcr10 using the CLSI broth microdilution method.
Induction tests
To examine whether the expression of mcr-10 is inducible as reported for mcr-9 [10], strain 090065 was subjected to induction with IPTG (isopropyl-β-d-thiogalactopyranoside; BBI, Shanghai, China) or lactose (Meilun, Dalian, China) as described previously [21]. After induction, MIC of colistin for strain 090065 was determined in the absence or presence of 1 or 3 mmol/L lactose or 0.4 or 1 mmol/L IPTG.
Conjugation and electroporation experiments
Conjugation experiments were carried out in broth and on filters with the azide-resistant E. roggenkampii strain 120033 AizR (an azide-resistant variant of 120033) as the recipient at both 25°C and 37°C as described previously [22]. Potential transconjugants were selected on LB agar plates containing 2 mg/L colistin and 150 mg/L azide. Electroporation was performed with Escherichia coli DH5α as described previously [23]. Transformants were selected on Luria–Bertani agar plates containing 2 mg/L colistin. The presence of mcr-10 in the transformant was confirmed by PCR with primers 090065_up_SacI/090065_down_EcoRI and subsequent Sanger sequencing. MICs of aztreonam, ceftazidime, colistin, and meropenem were determined as described above.
Screening the presence of mcr-10 in GenBank
We screened the presence of mcr-10 in sequences including complete or draft genome sequences deposited in GenBank by BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed by 30 August 2019). Matches with >90% identity and >90% coverage were retrieved from GenBank.
Structure comparisons of MCR-10, MCR-9 and MCR-Bs
The amino acid sequences of MCRs were retrieved from Bacterial Antimicrobial Resistance Reference Gene Database (BioProject no. PRJNA313047). MCR-B proteins from genus Buttiauxella were retrieved from their whole-genome assemblies. Along with the two alleles of MCR-10 found in this study (see below for details), the amino acid sequences of all genes (n = 75) were aligned using Prank v1.70427 [24] with 50 iterations first, followed by aligning corresponding nucleotide sequences using aligned amino acid sequences as the guide in the same program. The aligned nucleotide and amino acid sequences were fed into RAxML v8.2.12 [25] with a 1000-bootstrap test under GTRGAMMA and PROTGTRGAMMA model, respectively, for inferring maximum-likelihood phylogenies.
Three-dimensional (3D) structural models of MCR-10, other reported MCR proteins (MCR-1 to -9) and MCR-B of various Buttiauxella species based on lipid A PEA transferase [26] were constructed using Phyre2 [27] and were visualized using UCSF Chimera [28]. Secondary structures of MCR-9, MCR-10 and MCR-Bs were then predicted using ESPript 3 [29].
Results
A novel mcr, mcr-10, was identified in strain 090065 of E. roggenkampii
Strain 090065 (also called WCHER090065) was recovered from an ascites sample of a patient in 2016 at West China Hospital. Strain 090065 (also called WCHER090065) was resistant to aztreonam (MIC, 256 mg/L), ceftazidime (MIC, 64 mg/L), imipenem (MIC, 32 mg/L), meropenem (MIC, 16 mg/L), intermediate to piperacillin-tazobactam (MIC, 32/4 mg/L) but susceptible to cefepime (MIC, 2 mg/L), colistin (MIC, 2 mg/L), and tigecycline (MIC, 2 mg/L). Short-read whole-genome sequencing of strain 090065 generated 1.77 clean gigabases (367.6× coverage), which were de novo assembled into 137 contigs (N50, 134,938 bp). Strain 090065 belongs to E. roggenkampii, a recently described Enterobacter species [30] as it has 98.51% ANI value with the type strain of E. roggenkampii (strain DSM16690T, GenBank accession no. CP017184).
Strain 090065 has two known antimicrobial resistance genes, blaMIR-5 (a chromosomal ampC gene intrinsic to Enterobacter mediating resistance to aztreonam, 1st to 3rd cephalosporins, and penicillins) and fosA (mediating resistance to fosfomycin). Known mcr genes including mcr-1 to -9 were not identified in the draft genome sequence of strain 090065. However, a PEA transferase-encoding gene, which has 79.69% identity and 99% coverage with mcr-9.1 (GenBank accession no. NG_064792) [10], was identified. The PEA transferase encoded by the gene has 82.93% amino acid identity with MCR-9, suggesting that the transferase is a novel MCR-like protein.
To determine whether this novel mcr-like gene mediates colistin resistance or not, the gene was cloned on pBC SK (Stratagene, La Jolla, CA, USA) to construct pBC SK-mcr10, which was transferred into a colistin-susceptible E. roggenkampii clinical strain, named 120033. MIC of colistin against strain 120033 containing pBC SK-mcr10 and strain 120033 containing pBC SK alone was 4 and 1 mg/L, respectively. The four-fold increase in colistin MIC in the presence of mcr-10 suggests that this gene indeed mediates colistin resistance.
Although the precise cut-off to define MCR groups has been established, the amino acid identity of 88% to 96% has been commonly used the de facto cut-off [31]. After consulting the NCBI as suggested recently [31], the novel MCR identified in the present study is named MCR-10. MCR-10 has 29.31%, 27.09%, 61.60%, 42.49%, 28.94%, 26.53%, 58.26%, 35.81%, and 82.93% amino acid identity with MCR-1, MCR-2, MCR-3, MCR-4, MCR-5, MCR-6, MCR-7, MCR-8, and MCR-9, respectively. Induction with lactose and IPTG did not increase the MICs of colistin against 090065 as MIC of colistin remained 2 mg/L in the presence of 1 or 3 mmol/L lactose or 0.4 or 1 mmol/L IPTG. This suggests that the expression of mcr-10 may not be inducible.
mcr-10 was carried by an IncFIA plasmid in strain 090065
To determine the location of mcr-10, strain 090065 was also subjected to long-read whole-genome sequencing using MinION. The hybrid assemblies of Illumina and MinION reads revealed that strain 090065 had a 4.86-Mb circular chromosome and two plasmids, p1_090065 (10,944-bp, replicon type undetermined) and pMCR10_090065 (71,775-bp, containing an IncFIA(HI1) replicon).
Despite repeated attempts of conjugation experiments, transconjugants containing pMCR10_090065 were not obtained, suggesting that pMCR10_090065 was not self-transmissible. Examining the complete sequence of pMCR10_090065 revealed that there was no conjugation module on this plasmid. E. coli transformant carrying mcr-10 was obtained by electroporation, confirming that mcr-10 is plasmid-borne. mcr-10-carrying transformant was susceptible to colistin (MIC, 2 mg/L), meropenem (MIC, <1 mg/L), aztreonam (MIC, <1 mg/L), and ceftazidime (MIC, 4 mg/L). This suggests that the resistance to these agents seen in strain 090065 was not co-transferred with mcr-10.
mcr-10 is found in a few genera of the family Enterobacteriaceae and has a global distribution
In GenBank, a total of 34 matches that have >90% identity and >90% coverage with mcr-10 were identified including 30 draft genome sequences (Table 1) and four complete plasmid sequences (Table 2). In addition, an incomplete mcr-10 was found in the draft genome sequence of an E. coli strain (accession no. LLYM01000000), which was truncated by insertion sequence IS3. The mcr-10-containing strains belonged to 13 species of 6 genera (Citrobacter, Enterobacter, Escherichia, Klebsiella, Kluyvera, and Raoultella) of the family Enterobacteriaceae but most (24/35) were of the genus Enterobacter. The strains were found in 11 countries (Australia, Canada, China, France, Germany, Japan, the Netherlands, Spain, Thailand, USA and Vietnam) of four continents. Most (n = 30) of the 35 strains were from human, mcr-10 was also present in strains from animal (dog, n = 1) and environment (water, n = 4). As only contigs of the 31 draft genome sequences are available, we were unable to reliably determine the location (chromosome or plasmid) of mcr-10 for these strains. Nonetheless, all of the four mcr-10-carrying plasmids contained one or two IncF replicons (Table 2).
Table 1. Strains harbouring mcr-10 in GenBank.
| Host Species | Strain | Accession no. | Country | Year | Host | Source |
|---|---|---|---|---|---|---|
| Citrobacter freundii | B38a | GCF_001702455 | China | 1998 | Human | Leg ulcer |
| Enterobacter asburiae | KA2 | GCF_003023805 | Spain | 2014 | Human | Rectal colonization |
| Enterobacter cloacae | SB610 | GCA_900978275 | Netherlands | 2000 | Environment | Water |
| Enterobacter cloacae | PIMB10EC27a | GCF_002982195 | Vietnam | 2010 | Human | Urine |
| Enterobacter kobei | C7 | GCF_001276465 | Australia | 2003 | Human | Lungs |
| Enterobacter kobei | 24.1-R2 | GCF_002001845 | Australia | 2012 | Human | Feces |
| Enterobacter kobei | 4300STDY7045912 | GCA_900496815 | Thailand | 2016 | Human | NA |
| Enterobacter kobei | GEO_33_Down_A | GCF_004024245 | USA | 2017 | Environment | Water |
| Enterobacter kobei | GEO_23_Down_A | GCF_004024335 | USA | 2017 | Environment | Water |
| Enterobacter kobei | MGH132 | GCF_002151855 | USA | 2015 | Human | NA |
| Enterobacter kobei | UCI 39 | GCF_000534155 | USA | NA | Human | Urine |
| Enterobacter kobei | GN02570 | GCF_001022655 | USA | 2007 | Human | Bodily fluid |
| Enterobacter kobei | 1001_ECLO | GCF_001052605 | USA | NA | Human | NA |
| Enterobacter kobei | 1000_ECLO | GCF_001052055 | USA | NA | Human | NA |
| Enterobacter kobei | SMART_313 | GCF_001472135 | Vietnam | 2010 | Human | NA |
| Enterobacter roggenkampii | 49530189 | GCF_002208285 | Australia | 2017 | Human | Blood |
| Enterobacter roggenkampii | ECC1097 | GCF_002785795 | China | 2010 | Human | Urine |
| Enterobacter roggenkampii | GER_MD16_1505_Eko_090 | GCF_003331015 | Germany | 2015 | Dog | Feces |
| Enterobacter roggenkampii | ntmc-TH | GCF_003427235 | NA | 2017 | Human | Blood |
| Enterobacter roggenkampii | MGH 25 | GCF_000492995 | NA | NA | Human | Urine |
| Enterobacter roggenkampii | GN05753 | GCF_001518455 | USA | 2013 | Human | NA |
| Enterobacter roggenkampii | GN02204 | GCF_001023195 | USA | 2003 | Human | Body fluid |
| Enterobacter roggenkampii | GN02825 | GCF_001022915 | USA | 2009 | Human | Body fluid |
| Enterobacter sichuanensis | ECC1752 | GCF_002785945 | China | NA | Human | NA |
| Enterobacter sp. | 18A13a,b | AP019634 | Japan | 2018 | Environment | River water |
| Escherichia coli | AZ74c | GCF_001484485 | China | 2014 | Human | Gut |
| Escherichia coli | CRE54 | GCF_003401245 | USA | 2016 | Human | Blood |
| Klebsiella pneumoniae | 1323_ECLO | GCF_001053395 | USA | NA | Human | NA |
| Klebsiella pneumoniae | BIDMC 67 | GCF_000692255 | USA | 2013 | Human | Abscess |
| Klebsiella quasipneumoniae | 149G8 | GCF_003289115 | France | 2017 | Human | Urine |
| Klebsiella quasipneumoniae | CRE71 | GCF_003401175 | USA | 2016 | Human | Blood |
| Kluyvera sp. | TUM14004 | GCF_004310925 | Japan | 2013 | Human | Blood |
| Raoultella electrica | TUM14061 | GCF_004312065 | Japan | 2013 | Human | Blood |
| Raoultella ornithinolytica | FDAARGOS_431a | GCF_002635365 | Canada | 2015 | Human | Rectal swab |
Note: NA, not available.
aIn the four strains, mcr-10 is located on a plasmid (Table 2).
bmcr-10 in strain 18A13 encodes an MCR-10 variant with 15 amino acids different from MCR-10 encoded by mcr-10 genes in all other strains.
cmcr-10 in strain AZ74 is truncated.
Table 2. mcr-10-carrying plasmids.
| Plasmid | Accession no. | Species, strain | Source | Year | Country | Plasmid replicon |
|---|---|---|---|---|---|---|
| pMCR10_090065 | Enterobacter roggenkampii 090065 | Human ascites | 2016 | China | FIA | |
| pOZ172 | CP016763 | Citrobacter freundii B38 | Human leg ulcer | 1998 | China | FIB, FII |
| pEC27-2 | CP020091 | Enterobacter cloacae PIMB10EC27 | Human urine | 2010 | Vietnam | FII |
| unnamed1 | CP023893 | Raoultella ornithinolytica FDAARGOS_431 | Human rectal swab | 2015 | Canada | FIB, FII |
| pECC18A13-1a | AP019635 | Enterobacter spp. 18A13b | River water | 2018 | Japan | FIA |
aThe MCR-10 variant encoded by pECC18A13-1 has 15 amino acid substitutions compared with MCR-10 encoded by the other plasmids.
bThe strain is likely of a new Enterobacter species, which is most closely related to E. roggenkampii with a 94.51% ANI value.
The mcr-10 genes of 33 strains encode MCR-10 that has an identical amino acid sequence with that in strain 090065. The remaining sequence of the truncated mcr-10 (accession no. LLYM01000000) is also identical to that of strain 090065. However, another MCR-10 variant with 15 amino acid substitutions (97.2% [524/539] amino acid identity) is found in plasmid pECC18A13-1 of Enterobacter sp. 18A13 (all call as DSM16690; GenBank accession no. AP019635). The alignment of the two MCR-10 variants is shown in Figure S1 in the Supplementary file. This suggests that mcr-10 has been diverged.
mcr-10 may originate from a yet-to-identified species closely related to known Buttiauxella species
Like MCR-9 [10,32], MCR-10 shows significant amino acid identity with chromosomally encoded MCR-like PEA transferases of various Buttiauxella species, designated MCR-B here, from 79.04% (426/539 identical aa) with that of Buttiauxella agrestis (NCBI Reference Sequence no. WP_034495833.1) to 83.67% (451/539 identical aa) with those of Buttiauxella gaviniae (NCBI Reference Sequence no. WP_064511805.1) and Buttiauxella brennerae (NCBI Reference Sequence no. WP_064558897.1).
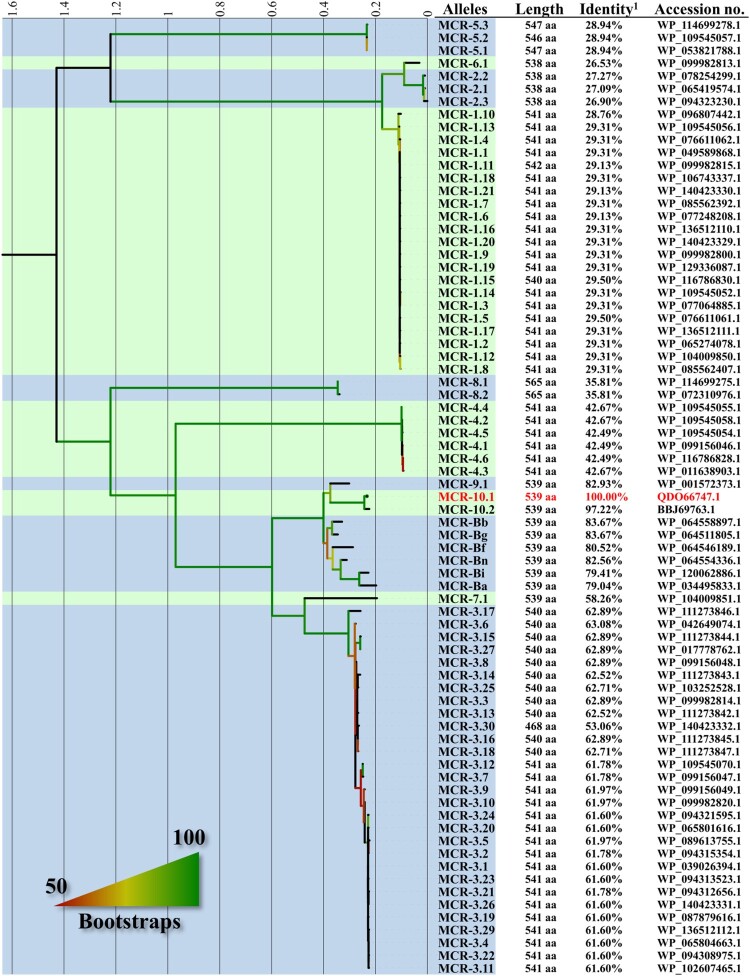
Comparison with known MCRs (MCR-1 to MCR-9) and MCR-B of various Buttiauxella species showed MCR-10 form a cluster with MCR-9 and MCR-B, which is well separated from other MCRs (Figure 1; a phylogenetic tree of mcr genes is shown as Figure S2 in the Supplementary file). Nonetheless, there are a large number of amino acid variations (≥89 aa) between MCR-10 and MCR-B proteins and the variations are diffusely distributed in the amino acid sequence of the MCR-B proteins (Figure S3 in the Supplementary file).
Figure 1.
Comparison of MCR-10 with other known MCRs and MCR-like proteins (MCR-B) in Buttiauxella species. This maximum-likelihood tree based on amino acid sequences was inferred using RAxML v8.2.12 [25] with a 1000-bootstrap test under the PROTGTRGAMMA model. The tree is middle-point rooted and the blue and green strips separate different MCR families with MCR-10 being highlighted in red. Bootstrap results are indicated by colour gradient on the branches, starting from 50% shown as red and up to 100% shown as green. MCR-like proteins in Buttiauxella species are named here according to the species. MCR-Ba, MCR-Bb, MCR-Bf, MCR-Bg, MCR-Bi, and MCR-Bn are MCR-like proteins from Buttiauxella agrestis strain ATCC 33320T (accession no. JMPI00000000), Buttiauxella brennerae ATCC 51605T (accession no. LXER00000000), Buttiauxella ferragutiae ATCC 51602T (accession no. LXEQ00000000), Buttiauxella gaviniae ATCC 51604T (accession no. LXEP00000000), Buttiauxella izardii CCUG35510T (accession no. QZWH01000000), and Buttiauxella noackiae ATCC 51607T (accession no. LXEO00000000), respectively.
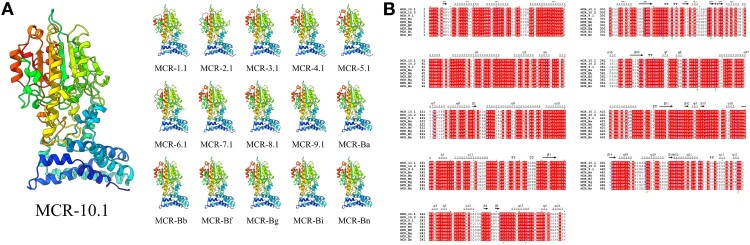
MCR-10, MCR-9 and MCR-Bs are highly similar at the structural level
Three-dimensional (3D) models showed that both the membrane-anchored domain and the soluble catalytic domain of MCR-1 to -10 and MCR-Bs had high levels of conservation (Figure 2(A)). The N-terminal membrane-anchored domain and the C-terminal soluble catalytic domain of these MCR proteins were conserved in both amino acids and structural elements (Figure 2(B)).
Figure 2.
Structures of MCR-10, other reported MCR proteins (MCR-1 to -9) and MCR-B of various Buttiauxella species. (A) Structural models were constructed using Phyre2 [27] and show the transmembrane-anchored and soluble periplasmic domains of the phosphoethanolamine transferase. (B) Secondary structures of MCR-10, MCR-9 and MCR-B. The alignment of amino acid sequences and the prediction of secondary structures were performed using ESPript 3 [29]. Secondary structure elements, helixes, sheets, and 310-helixes (representing by η), are indicated. β-strands are rendered as arrows, and strict β-turns are shown as TT letters.
The mobilization of mcr-10 may be due to site-specific recombination
On pMCR10_090065 and three other plasmids carrying mcr-10, mcr-10 was located at the immediate downstream of a XerC-type tyrosine recombinase-encoding gene, designated xerC here (Figure 3). It has been known that XerC-type tyrosine recombinases are able to mediate mobilization of adjacent genetic components including antimicrobial resistance genes via site-specific recombination [33,34]. In Enterobacter spp., an XerC-type tyrosine recombinase has been identified to mediate the mobilization of blaIMI, a carbapenemase gene [35]. Therefore, the mobilization of mcr-10 may also be mediated by the XerC-type tyrosine recombinase. As the downstream sequence of mcr-10 was truncated by insertion sequence ISEc36, it is therefore impossible to identify the recombination sites recognized by the XerC-type tyrosine recombinase. Of note, two copies of IS903 were located at upstream and downstream of xerC-mcr-10 on pMCR10_090065, respectively, and could form a composite transposon. On insertion, IS903 generates 9-bp direct target repeats but the 9-bp sequences abutting the two IS903 were different, suggesting that the region bracketed by IS903 was not due to direct insertion. Nonetheless, the IS903-formed composite transposon has the potential to mediate mobilization of the intervening genetic components. In contrast, IS903 has also been found upstream of mcr-9 but other insertion sequences such as IS26 are present downstream instead [32].
Figure 3.
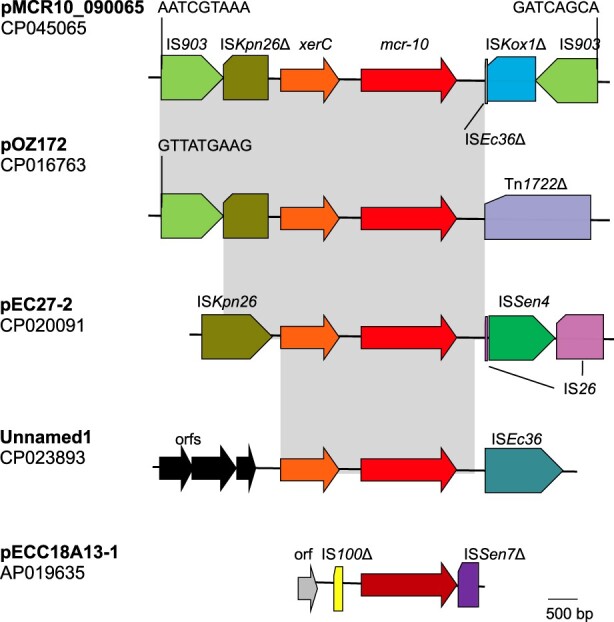
Genetic contexts of mcr-10. Gene xerC (shown in orange) encodes a XerC-type tyrosine recombinase, which may mediate mobilization of adjacent genetic components via site-specific recombination. Δ represents truncated insertion sequences or transposons. Identical regions are highlighted by grey rectangles. On pMCR10_090065, two copies of IS903 are located at upstream and downstream of xerC-mcr-10 (mcr-10 is shown in red) and the 9-bp abutting sequences are indicated. On pOZ171, there is an IS903 at upstream with the 9-bp abutting sequence being shown. However, there is no IS903 at downstream of xerC-mcr-10 but instead, transposon Tn1722 is presented. On pEC27-2, there is no IS903. A complete ISKpn26 is present at upstream of xerC-mcr-10 and an IS26, which is interrupted by the insertion of ISSen4, is at downstream. On an unnamed plasmid (accession no. CP023893), several open reading frames (orfs) without known function are present at upstream of xerC-mcr-10, while ISEc36 is present at downstream. On pECC18A13-1, the mcr-10 encodes an MCR-10 variant with 15 amino acids different from MCR-10 encoded by the other plasmids and is shown in dark red. No xerC is present, while truncated IS100 and truncated ISSen7 are located at upstream and downstream of the mcr-10, respectively.
Discussion
Enterobacteriaceae is the most common pathogen causing human infections [36]. Multi-drug resistant organisms such as carbapenem-resistant Enterobacteriaceae (CRE) have become a major global challenge [37]. Colistin is a last resort antimicrobial agent and one of the only options to treat serious infections caused by CRE. However, Enterobacteriaceae strains with acquired colistin resistance have emerged worldwide, which are significantly jeopardizing the efficacy of colistin [38]. Plasmid-borne mcr colistin resistance genes can be transmitted across Enterobacteriaceae species and may have spread to multiple continents, representing a particular threat to public health and clinical management in the whole world [39]. Monitoring the spread antimicrobial resistance is a core component of strategies for combating antimicrobial resistance [40]. Identification of new mcr genes can be used to improve the monitoring of plasmid-borne colistin resistance and may, therefore, help to develop effective control measures.
In this study, we identified a novel mcr gene in strain 090065 of Enterobacter roggenkampii. mcr-10 has the highest nucleotide identity (79.69%) with mcr-9 and encodes MCR-10 with 82.93% amino acids identical to MCR-9. mcr-10 confers 4-fold increase in colistin MIC (from 1 to 4 mg/L) when cloned into a colistin-susceptible E. roggenkampii strain. This suggests that mcr-10 was indeed a colistin resistance gene. Of note, strain 090065 carrying mcr-10 was susceptible to colistin (MIC, 2 mg/L), while the originally colistin-susceptible E. roggenkampii strain became resistant to colistin (MIC, 4 mg/L) after receiving mcr-10 by cloning. The discrepancy in colistin susceptibility is likely due to the expression as mcr-10 was carried by a large naturally occurring plasmid in strain 090065 but was cloned onto the small-size vector pBC SK in strain 120033. Nonetheless, such discrepancy warrants further studies. We revealed that mcr-10 was adjacent to site-specific recombinase-encoding gene and was bracketed by IS903 on an IncFIA plasmid in strain 090065, indicating that mcr-10 has the potential to be mobile. We also found that mcr-10 has been present in a few genera of the family Enterobacteriaceae with a global distribution. Of note, the earliest match of mcr-10 in GenBank was plasmid pOZ172 of a Citrobacter freundii clinical strain, which was recovered in 1998 in Guangzhou, southern China, suggesting that mcr-10 has been mobilized by plasmids within Enterobacteriaceae for decades. We showed that MCR-10, MCR-9 and MCR-B proteins originated from a common ancestor. The large number and diffuse distribution of amino acid variations between MCR-10 and MCR-B proteins suggest that MCR-10 is not directly derived from these Buttiauxella species but may have originated from a yet-to-identified species that are closely related to known Buttiauxella species.
In conclusion, we identified and characterized a novel mcr gene and we also found that the gene has been spread globally under the radar. This suggests that the novel mcr gene is of significance for health. Our findings are essential for developing effective countermeasures including surveillance.
Supplementary Material
Acknowledgements
We are grateful for Mrs. Xiaoxia Zhang to perform some experiments for this study.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China [project no. 81772233, 81661130159 and 81861138055], West China Hospital of Sichuan University [1.3.5 project for disciplines of excellence, project no. ZYYC08006], the Newton Advanced Fellowship, Royal Society, UK [NA150363]. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Li J, Nation RL, Turnidge JD, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6:589–601. doi: 10.1016/S1473-3099(06)70580-1 [DOI] [PubMed] [Google Scholar]
- 2.Liu Y-Y, Wang Y, Walsh TR, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7 [DOI] [PubMed] [Google Scholar]
- 3.Xavier BB, Lammens C, Ruhal R, et al. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium. Euro Surveill. 2016 Jun;21:30280. doi: 10.2807/1560-7917.ES.2016.21.27.30280 [DOI] [PubMed] [Google Scholar]
- 4.Yin W, Li H, Shen Y, et al. Novel plasmid-mediated colistin resistance gene mcr-3 in Escherichia coli. mBio. 2017;8:e00543-17. doi: 10.1128/mBio.00543-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carattoli A, Villa L, Feudi C, et al. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Euro Surveill. 2017;22:30589. doi: 10.2807/1560-7917.ES.2017.22.31.30589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borowiak M, Fischer J, Hammerl JA, et al. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in D-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J Antimicrob Chemother. 2017;72:3317–3324. doi: 10.1093/jac/dkx327 [DOI] [PubMed] [Google Scholar]
- 7.AbuOun M, Stubberfield EJ, Duggett NA, et al. mcr-1 and mcr-2 variant genes identified in Moraxella species isolated from pigs in Great Britain from 2014 to 2015. J Antimicrob Chemother. 2017;72:2745–2749. doi: 10.1093/jac/dkx286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang YQ, Li YX, Lei CW, et al. Novel plasmid-mediated colistin resistance gene mcr-7.1 in Klebsiella pneumoniae. J Antimicrob Chemother. 2018;73:1791–1795. doi: 10.1093/jac/dky111 [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Wang Y, Zhou Y, et al. Emergence of a novel mobile colistin resistance gene, mcr-8, in NDM-producing Klebsiella pneumoniae. Emerg Microbes Infect. 2018;7;122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll LM, Gaballa A, Guldimann C, et al. Identification of novel mobilized colistin resistance gene mcr-9 in a multidrug-resistant, colistin-susceptible Salmonella enterica serotype Typhimurium isolate. mBio. 2019;10:00853–19. doi: 10.1128/mBio.00853-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martins-Sorenson N, Snesrud E, Xavier DE, et al. A novel plasmid-encoded mcr-4.3 gene in a colistin-resistant Acinetobacter baumannii clinical strain. J Antimicrob Chemother. 2019;75:60–64. doi: 10.1093/jac/dkz413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caselli E, D'Accolti M, Soffritti I, et al. Spread of mcr-1-driven colistin resistance on hospital surfaces, Italy. Emerg Infect Dis. 2018;24:1752–1753. doi: 10.3201/eid2409.171386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun J, Zhang H, Liu YH, et al. Towards understanding MCR-like colistin resistance. Trends Microbiol. 2018;26:794–808. doi: 10.1016/j.tim.2018.02.006 [DOI] [PubMed] [Google Scholar]
- 14.CLSI . Performance standards for antimicrobial susceptibility testing; twenty-seventh informational supplement. M100-S27. Wayne (PA: ): Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- 15.Bankevich A, Nurk S, Antipov D, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wick RR, Judd LM, Gorrie CL, et al. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13:e1005595. doi: 10.1371/journal.pcbi.1005595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker BJ, Abeel T, Shea T, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 2014;9:e112963. doi: 10.1371/journal.pone.0112963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 19.Richter M, Rossello-Mora R, Glockner F O, et al. JSpeciesWS: a web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics. 2016;32:929–931. doi: 10.1093/bioinformatics/btv681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richter M, Rossello-Mora R.. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci U S A. 2009;106:19126–19131. doi: 10.1073/pnas.0906412106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dvorak P, Chrast L, Nikel PI, et al. Exacerbation of substrate toxicity by IPTG in Escherichia coli BL21(DE3) carrying a synthetic metabolic pathway. Microb Cell Fact. 2015;14:201. doi: 10.1186/s12934-015-0393-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coque TM, Oliver A, Perez-Diaz JC, et al. Genes encoding TEM-4, SHV-2, and CTX-M-10 extended-spectrum β-lactamases are carried by multiple Klebsiella pneumoniae clones in a single hospital (Madrid, 1989 to 2000). Antimicrob Agents Chemother. 2002;46:500–510. doi: 10.1128/AAC.46.2.500-510.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bio-Rad Laboratories I . Gene Pulser Xcell electroporation system instruction manual. Alfred Nobel Drive, CA. 2000.
- 24.Loytynoja A. Phylogeny-aware alignment with PRANK. Methods Mol Biol. 2014;1079:155–170. doi: 10.1007/978-1-62703-646-7_10 [DOI] [PubMed] [Google Scholar]
- 25.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anandan A, Evans GL, Condic-Jurkic K, et al. Structure of a lipid A phosphoethanolamine transferase suggests how conformational changes govern substrate binding. Proc Natl Acad Sci USA. 2017;114:2218–2223. doi: 10.1073/pnas.1612927114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelley LA, Mezulis S, Yates CM, et al. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pettersen EF, Goddard TD, Huang CC, et al. UCSF Chimera – a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084 [DOI] [PubMed] [Google Scholar]
- 29.Robert X, Gouet P.. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014;42:W320–W324. doi: 10.1093/nar/gku316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutton GG, Brinkac LM, Clarke TH, et al. Enterobacter hormaechei subsp. hoffmannii subsp. nov., Enterobacter hormaechei subsp. xiangfangensis comb. nov., Enterobacter roggenkampii sp. nov., and Enterobacter muelleri is a later heterotypic synonym of Enterobacter asburiae based on computational analysis of sequenced Enterobacter genomes. F1000Res. 2018;7:521. doi: 10.12688/f1000research.14566.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Partridge SR, Di Pilato V, Doi Y, et al. Proposal for assignment of allele numbers for mobile colistin resistance (mcr) genes. J Antimicrob Chemother. 2018;73:2625–2630. doi: 10.1093/jac/dky262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kieffer N, Royer G, Decousser JW, et al . mcr-9, an inducible gene encoding an acquired phosphoethanolamine transferase in Escherichia coli, and its origin. Antimicrob Agents Chemother. 2019;63:e00965-19. doi: 10.1128/AAC.00965-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castillo F, Benmohamed A, Szatmari G.. Xer site specific recombination: double and single recombinase systems. Front Microbiol. 2017;8:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Midonet C, Barre FX.. Xer site-specific recombination: promoting vertical and horizontal transmission of genetic information. Microbiol Spectr. 2014;2. DOI: 10.1128/microbiolspec.MDNA3-0056-2014. [DOI] [PubMed] [Google Scholar]
- 35.Antonelli A, D'Andrea MM, Di Pilato V, et al. Characterization of a novel putative Xer-dependent integrative mobile element carrying the blaNMC-A carbapenemase gene, inserted into the chromosome of members of the Enterobacter cloacae complex. Antimicrob Agents Chemother. 2015;59:6620–6624. doi: 10.1128/AAC.01452-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adeolu M, Alnajar S, Naushad S, et al. Genome-based phylogeny and taxonomy of the ‘enterobacteriales': proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int J Syst Evol Microbiol. 2016;66:5575–5599. doi: 10.1099/ijsem.0.001485 [DOI] [PubMed] [Google Scholar]
- 37.Nordmann P, Naas T, Poirel L.. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17:1791–1798. doi: 10.3201/eid1710.110655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tzouvelekis LS, Markogiannakis A, Psichogiou M, et al. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25:682–707. doi: 10.1128/CMR.05035-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poirel L, Jayol A, Nordmann P.. Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev. 2017;30:557–596. doi: 10.1128/CMR.00064-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.CDC . Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE). Atlanta (GA: ): CDC; 2015 Nov. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.