 Bioactive compounds isolated from plants are considered to be attractive candidates for cancer therapy.
Bioactive compounds isolated from plants are considered to be attractive candidates for cancer therapy.
Abstract
Bioactive compounds isolated from plants are considered to be attractive candidates for cancer therapy. In this study, we examined the effect of kaempferol, its derivatives, the polyphenol fraction (PF) and an extract (EX) isolated from the aerial parts of Lens culinaris Medik. on DNA damage induced by etoposide in human cells. We also studied the effect of these compounds and their combinations on cell viability. The studies were conducted on HL-60 cells and human peripheral blood mononuclear cells (PBMCs). We used the comet assay in the alkaline version to evaluate DNA damage. To examine cell viability we applied the trypan blue exclusion assay. We demonstrated that kaempferol glycoside derivatives isolated from the aerial parts of Lens culinaris Medik. reduce DNA damage induced by etoposide in PBMCs, but do not have an impact on DNA damage in HL-60 cells. We also showed that kaempferol induces DNA damage in HL-60 cells and leads to an increase of DNA damage provoked by etoposide. Our data suggest that kaempferol derivatives can be further explored as a potential agent protecting normal cells against DNA damage induced by etoposide. Moreover, kaempferol's ability to induce DNA damage in cancer cells and to increase DNA damage caused by etoposide may be useful in designing and improving anticancer therapies.
1. Introduction
Despite many years of research and high financial outlay, cancer remains one of the most significant public health problems. Chemotherapy applied during cancer treatment is toxic and accompanied by many side effects; hence the need to find antineoplastic drugs with reduced toxicity toward normal cells. On the other hand, compounds that would protect normal cells against the toxic effects of antineoplastic drugs are sought. Fruits and vegetables, which constitute important elements of human diet, are a rich source of compounds, such as polyphenols, flavonoids and carotenoids. A proper intake of dietary phytochemicals may contribute to reduced cancer risk.1 For this reason the interest in phytochemicals as elements of cancer prevention and adjuvant treatment is gradually increasing.
Lentil (Lens culinaris) is one of the oldest cultivated legumes. It belongs to the Neolithic crop package from the Near East, the so called “founder crops” with wheat, emmer wheat, barley, pea, chickpea, bitter vetch, and flax.2 Numerous reports showed that lentil constituents have a wide variety of beneficial effects on human health. Its anti-diabetic, antiobesity, cardioprotective and anti-microbial properties have been well established.3 Moreover, it has been presented in many studies that lentils have exceptionally high antioxidant capacity and ROS scavenging potential as compared to various fruits and vegetables. What is important, lentil consumption reduces the risk of incidence of a number of cancers.4–6 It was shown that compounds isolated from lentil seeds have chemo-preventive potential, acting through different mechanisms inside the cell.7,8 For example, it has been recently shown that complementarily administered sodium selenite, proteolytic enzymes and Lens culinaris lectin significantly reduced the defined side-effects (e.g. arthralgia, mucosal dryness) of adjuvant hormone therapy in patients with breast cancer.9
Kaempferol [3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one] (Fig. 1A) belongs to the group of flavonols and is found, among other things, in lentil, broccoli, tea, tomato, leek, cabbage and beans. Furthermore, many studies revealed that kaempferol and kaempferol glycosides offer a wide range of health benefits, including antioxidant, cardioprotective, anticancer, anti-inflammatory or neuroprotective benefits.10 Kaempferol exhibits many anticancer properties – it affects proliferation, apoptosis, autophagy, neoangiogenesis and metastasis.11,12 Moreover, kaempferol may cause epigenetic modifications of histones and DNA.13,14 A number of epidemiological studies indicate that a high intake of kaempferol may reduce the risk of cancer.15–17 Many studies also showed that kaempferol may display anticancer activity, but little is known about the effect of kaempferol on the activity of chemotherapeutic drugs toward both cancer and normal cells.
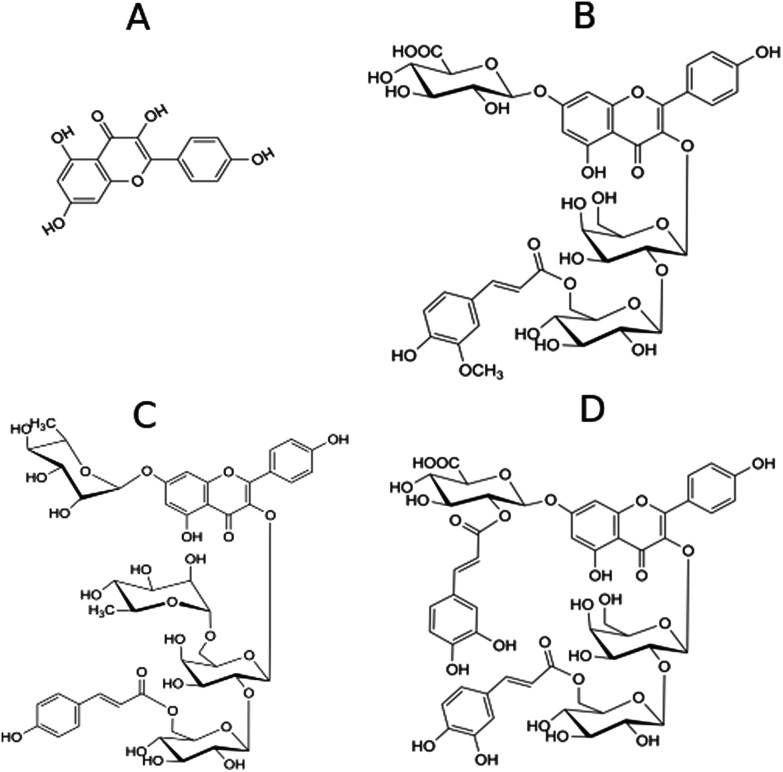
Fig. 1. Chemical structures of kaempferol (A) and kaempferol derivatives P7 (B), P8 (C) and P9 (D) isolated from Lens culinaris Medik.
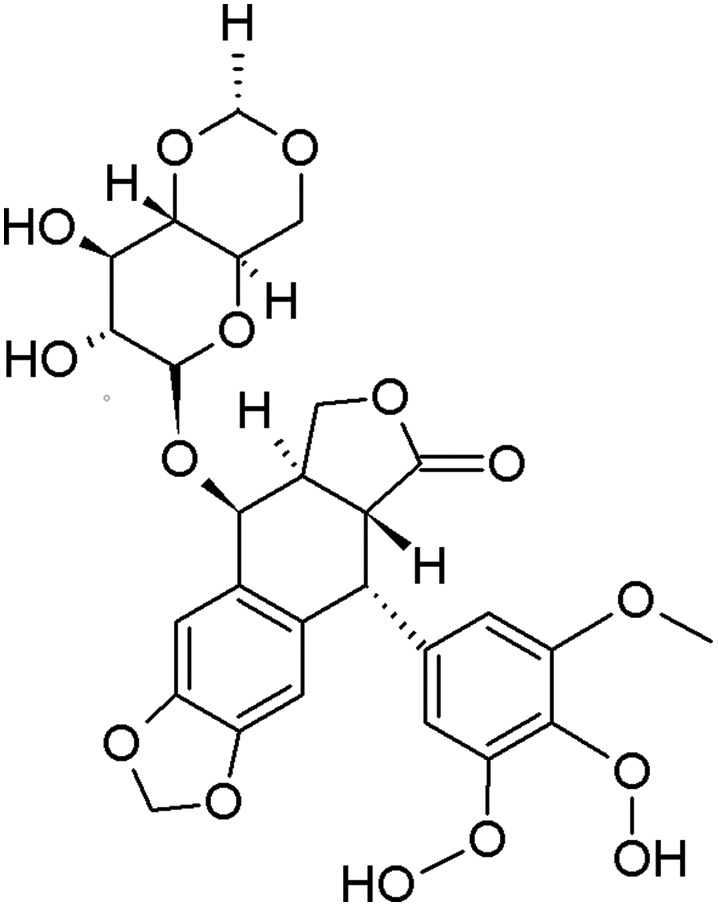
Etoposide is a chemotherapeutic agent used in the treatment of many types of cancer, including leukemia, approved by the FDA in 198318 (Fig. 2). Etoposide belongs to the class of DNA topoisomerase II inhibitors. Its cytotoxic effect is based on the stabilization of a covalent complex between DNA topoisomerase II and DNA, which increases the level of DNA strand breaks.19 DNA damage accumulation may initiate death cell pathways, such as apoptosis. Etoposide is usually administered intravenously in the form of infusion lasting for about 2 hours or orally (capsules). This drug has side effects, including bone marrow suppression, inflammation of mucous membranes and secondary leukemia.
Fig. 2. Chemical structure of etoposide.
The aim of this study was to evaluate the effect of kaempferol, its derivatives, the polyphenol fraction and an extract isolated from the aerial parts of Lens culinaris Medik. on DNA damage induced by etoposide in human peripheral blood mononuclear cells (PBMCs) and the HL-60 (human promyelocytic leukemia) cell line. The authors also evaluated the effect of the tested plant compounds on the viability of PBMCs and HL-60 cells.
2. Materials and methods
2.1. Reagents
3,4′,5,7-Tetrahydroxyflavone (Kaempferol) (K0133) and 4′-demethylepipodophyllotoxin 9-(4,6-O-ethylidene-β-d-glucopyranoside) (Etoposide) (E1383) were purchased from Sigma-Aldrich (St Louis, MO, USA). Kaempferol was dissolved in DMSO and stored at –20 °C. Etoposide was dissolved in methanol. Trypan blue dye (145-0013) was purchased from Bio-Rad Laboratories (Hercules, CA, USA). Low-melting-point (LMP) and normal-melting-point (NMP) agarose, DAPI (4′,6-diamidino-2-phenylindole), dimethyl sulfoxide (DMSO) and hydrogen peroxide (H2O2) were obtained from Sigma-Aldrich (St Louis, MO, USA).
2.2. Preparation of the lentil extract and phenolic fraction
The extract (EX) from the aerial parts of Lens culinaris Medik. was isolated according to the procedure described by Żuchowski et al. (2014).20 Briefly, lentil aerial parts were freeze-dried, powdered, and defatted with chloroform, using a Soxhlet extractor. A portion of the defatted plant material (250 g) was subjected to triple extraction with boiling 80% aqueous methanol (v/v; 2.5 L; 1 h) under reflux. The collected extract was filtered through a filter funnel, concentrated using a rotary evaporator (Heidolph, Schwabach, Germany), and freeze-dried. A portion of EX (7.03 g) was dissolved in 1% methanol solution, containing 0.1% formic acid, and loaded onto a C18 column (44 × 120 mm; Cosmosil 140C18-Prep, 140 μm). The column was washed with the same solvent to remove highly polar constituents of the extract, and phenolic compounds were subsequently eluted with 60% methanol. The obtained eluate was rotary evaporated, dissolved in water and freeze-dried, to yield 976 mg of the PF.
2.3. Kaempferol glycosides from the aerial parts of lentil
Kaempferol glycosides: kaempferol-3-O-[(6-O-E-feruloyl)-β-d-glucopyranosyl-(1→2)]-β-d-galactopyranoside-7-O-β-d-glucuropyranoside (P7), kaempferol-3-O-{[(6-O-E-p-coumaroyl)-β-d-glucopyranosyl-(1→2)]-α-l-rhamnopyranosyl(1→6)}-β-d-galactopyranoside-7-O-α-l-rhamnopyranoside (P8), and kaempferol-3-O-[(6-O-E-caffeoyl)-β-d-glucopyranosyl-(1→2)]-β-d-galactopyranoside-7-O-(2-O-E-caffeoyl’)-β-d-glucuropyranoside (P9) (Fig. 1) were isolated according to the procedure described by Żuchowski et al. (2014).20 Different glycosides of quercetin and kaempferol (mainly compounds acylated with hydroxycinnamic acids) are dominant phenolics of EX and PF. A detailed description of these compounds can be found in the work of Żuchowski et al. (2014).20 All isolated compounds and extracts were dissolved in 50% DMSO and stored at –20 °C.
2.4. Cell preparation
Peripheral blood mononuclear cells (PBMCs) were isolated from a leucocyte-buffy coat collected from the blood of healthy non-smoking donors from the Blood Bank in Lodz, Poland. A portion of the leucocyte-buffy coat was diluted in 1% phosphate buffer saline (PBS). Then, it was centrifuged in a density gradient of Lymphosep (Cytogen, Zgierz, Poland) at 200g for 20 min at room temperature. Next, the cells were collected and washed three times in 1% PBS. The supernatant was poured off and the pellet of the cells was resuspended in RPMI 1640 medium (Lonza, Basel, Switzerland). The study protocol was approved by the Committee for Research on Human Subjects of the University of Lodz (11/KBBN-UŁ/III/2018).
The HL-60 (human promyelocytic leukemia) cell line was obtained from the American Type Culture Collection (ATCC) and cultured in Iscove's Modified Dulbecco's Medium (IMDM) with 15% inactivated fetal bovine serum (FBS) from Biowest (Cytogen, Zgierz, Poland), 2 mM l-glutamine and 25 mM HEPES (Lonza, Basel, Switzerland) and penicillin/streptomycin solution (100 U ml–1 and 100 μg ml–1, respectively) (Cytogen, Zgierz, Poland). The HL-60 cells were cultured in flasks at 37 °C in 5% CO2 and sub-cultured every 3–4 days to maintain exponential growth.
2.5. Cell treatment
During the experiments, the cells were seeded in the culture medium and then incubated for 2 h at 37 °C with different concentrations (10–50 μg ml–1) of: kaempferol (K), kaempferol-3-O-[(6-O-E-feruloyl)-β-d-glucopyranosyl-(1→2)]-β-d-galactopyranoside-7-O-β-d-glucuropyranoside (P7), kaempferol-3-O-{[(6-O-E-p-coumaroyl)-β-d-glucopyranosyl-(1→2)]-α-l-rhamnopyranosyl(1→6)}-β-d-galactopyranoside-7-O-α-l-rhamnopyranoside (P8), kaempferol-3-O-[(6-O-E-caffeoyl)-β-d-glucopyranosyl-(1→2)]-β-d-galactopyranoside-7-O-(2-O-E-caffeoyl’)-β-d-glucuropyranoside (P9), the polyphenol fraction from the aerial parts of Lens culinaris Medik. (PF) and an extract from the aerial parts of Lens culinaris Medik. (EX).
The cells were treated with different concentrations of etoposide (E) (1 μM for HL-60 cells and 50 μM for PBMCs) and incubated for 2 h at 37 °C with the aim of inducing DNA damage.
To investigate the effect of lentil compounds on the geno- and cytotoxicity of etoposide, the cells were incubated simultaneously with these compounds and etoposide. The final concentration of DMSO and methanol in the samples did not exceed 0.5% and did not induce DNA damage or affect cell viability.
2.6. Cell viability
Cell viability was determined by the trypan blue exclusion assay. The cells were seeded at a density of 5 × 105 cell per ml in a complete medium and then treated as described above. After incubation with the tested compounds, 10 μl of the cell suspension was mixed in a 1 : 1 ratio with 0.4% trypan blue solution (Bio-Rad Laboratories, Hercules, CA, USA) and applied to a Bürker hemocytometer. Afterwards, 100 cells were counted under a light microscope. The experiment was repeated 3 times.
2.7. DNA damage – comet assay
DNA damage was estimated using the alkaline comet assay according to the procedure defined by Singh et al.21 described in detail by Tokarz et al.22 The HL-60 cells were separated to the density of 5 × 104 cell per ml and PBMCs were separated to 1 × 105 in the culture medium and treated as described above. Then, the cells were centrifuged and embedded in 0.75% low melting point agarose. One hundred comets were randomly selected from each sample and the percentage of DNA in the tail was measured. The comets were observed at 200× magnification in an Eclipse fluorescence microscope (Nikon, Japan) attached to a COHU 4910 video camera (Cohu, Inc., San Diego, CA, USA) equipped with a UV-1 A filter block and connected to the Lucia-Comet v. 6.0 image analysis system (Laboratory Imaging, Prague, Czech Republic). The positive control included cells incubated with 20 μM hydrogen peroxide (H2O2) for 15 min on ice.
2.8. Statistical analysis
Data are presented as mean values ± SEM. Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA). Statistical differences were determined by one-way ANOVA followed by post hoc Tukey's test. The differences were considered to be statistically significant when the p value was less than 0.05.
3. Results
3.1. Etoposide, kaempferol, kaempferol glycosides, polyphenol fraction and extract from Lens culinaris Medik. leaves do not affect the viability of HL-60 cells and PBMCs
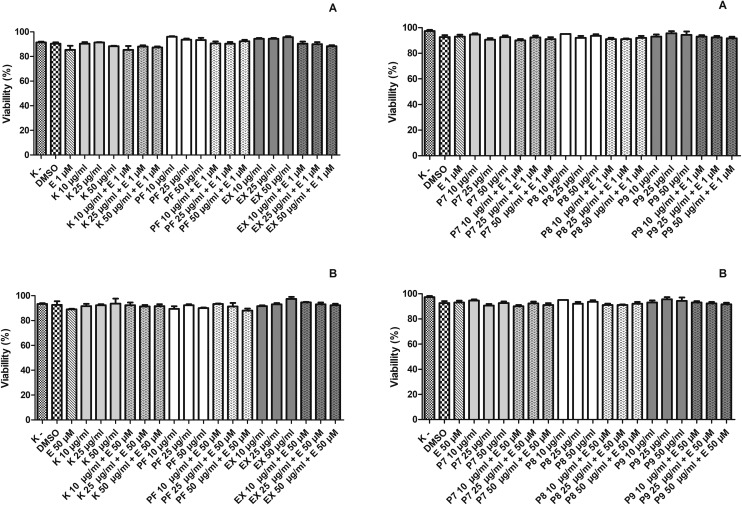
Firstly, we checked the cytotoxicity of the tested compounds using the trypan blue exclusion assay. The results indicated that kaempferol (K), kaempferol glycosides (P7–P9), polyphenol fraction (PF) and extract (EX) from the aerial parts of Lens culinaris Medik. were not toxic for HL-60 cells and PBMCs after 2 hours of incubation at 37 °C (Fig. 3).
Fig. 3. Effect of kaempferol (K), the polyphenol fraction from Lens culinaris Medik. (PF), an extract from Lens culinaris Medik. (EX) and kaempferol derivatives P7, P8 and P9 in the concentration of 10–50 μg ml–1 and etoposide (E) at 1 μM or 50 μM on the viability of HL-60 cells (A) and PBMCs (B) measured by the trypan blue exclusion method. Cells were incubated with the tested compounds for 2 h at 37 °C. The figure shows mean results from three independent experiments. Error bars denote SEM.
3.2. Kaempferol induces DNA damage in HL-60 cells
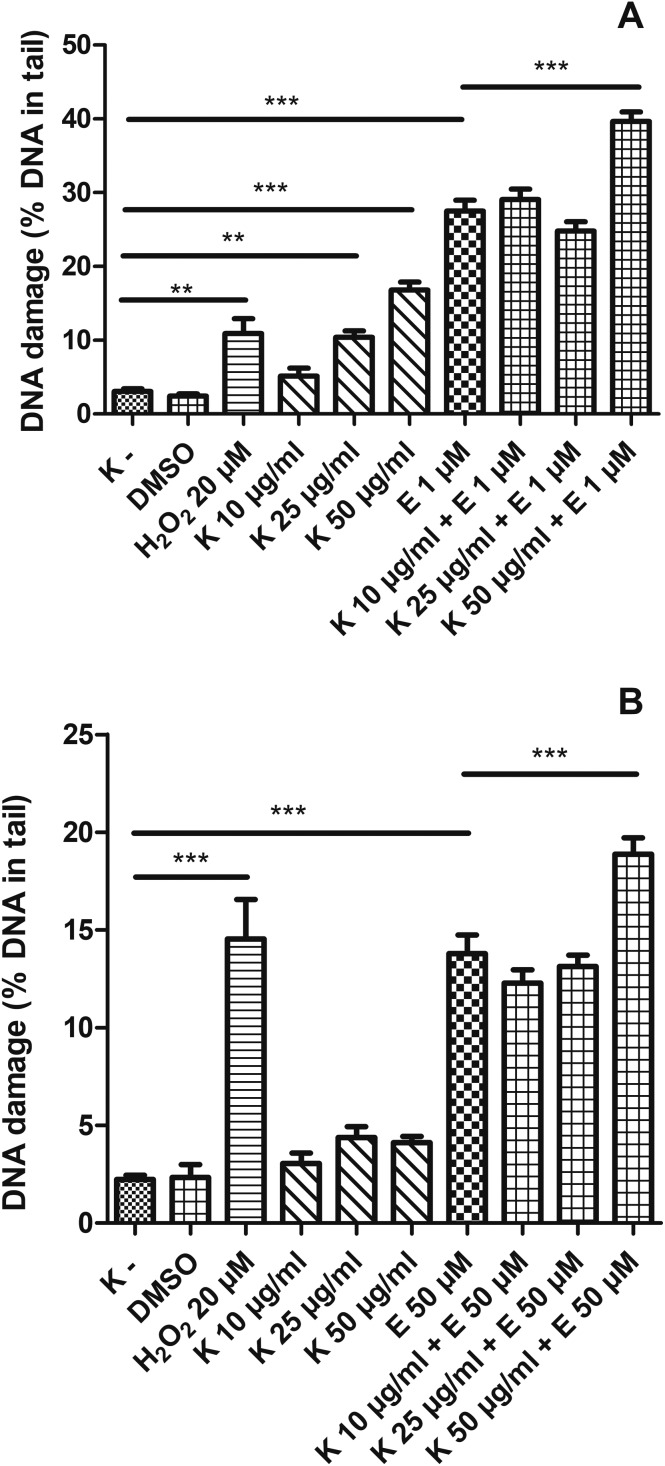
We found that kaempferol (K) in the concentrations of 25 μg ml–1 and 50 μg ml–1 induced statistically significant DNA damage in HL-60 cells (p < 0.01 and p < 0.001, respectively) (Fig. 4A). Additionally, the simultaneous incubation of HL-60 cells with etoposide and 50 μg ml–1 kaempferol led to an increased level of DNA damage (39.6%) compared to incubation only with etoposide (27.5%) (p < 0.001). We observed that kaempferol did not induce DNA damage in PBMCs (Fig. 4B). However, in PBMCs, kaempferol at 50 μg ml–1 increased DNA damage induced by etoposide (from 13.8% to 18.8%) (p < 0.001).
Fig. 4. DNA damage, measured as the comet tail DNA (%) of HL-60 cells (A) and PBMCs (B) incubated for 2 h at 37 °C with kaempferol (K) (10–50 μM) and etoposide (E) at 1 μM or 50 μM, analyzed by the alkaline comet assay. The figure shows mean results ± SEM, n = 100; **p < 0.01, ***p < 0.001.
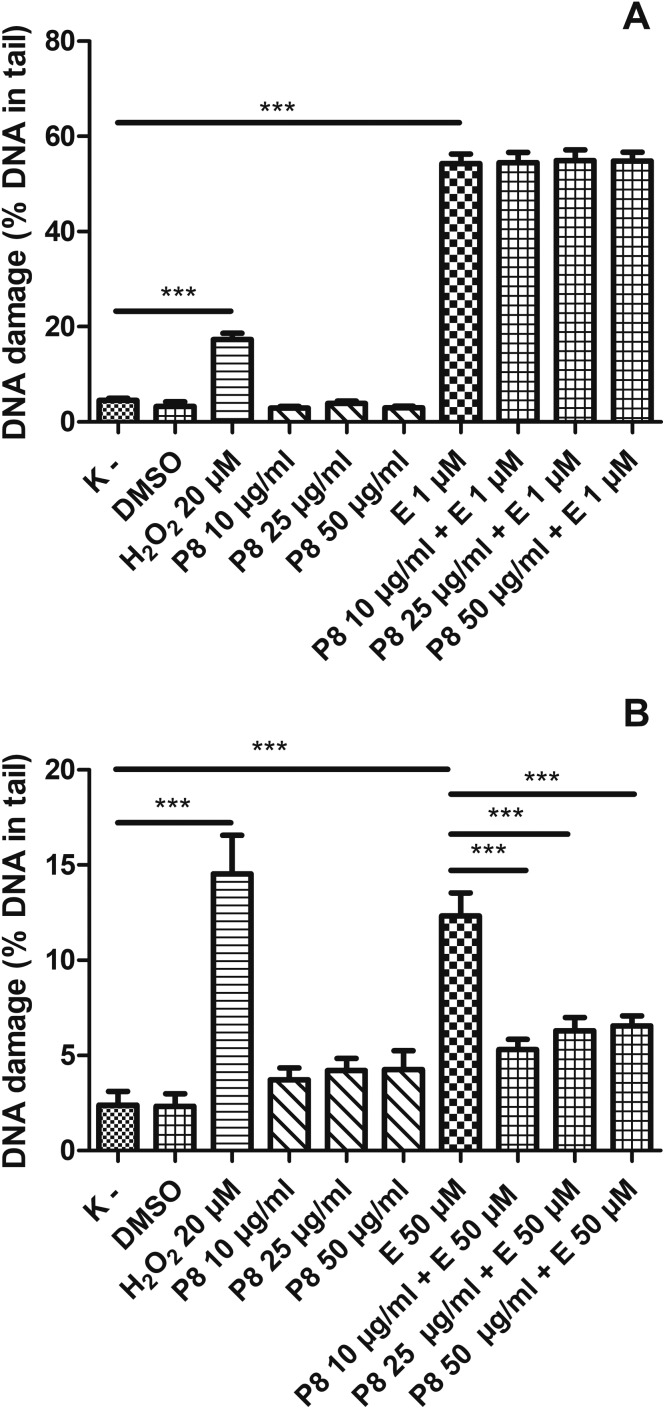
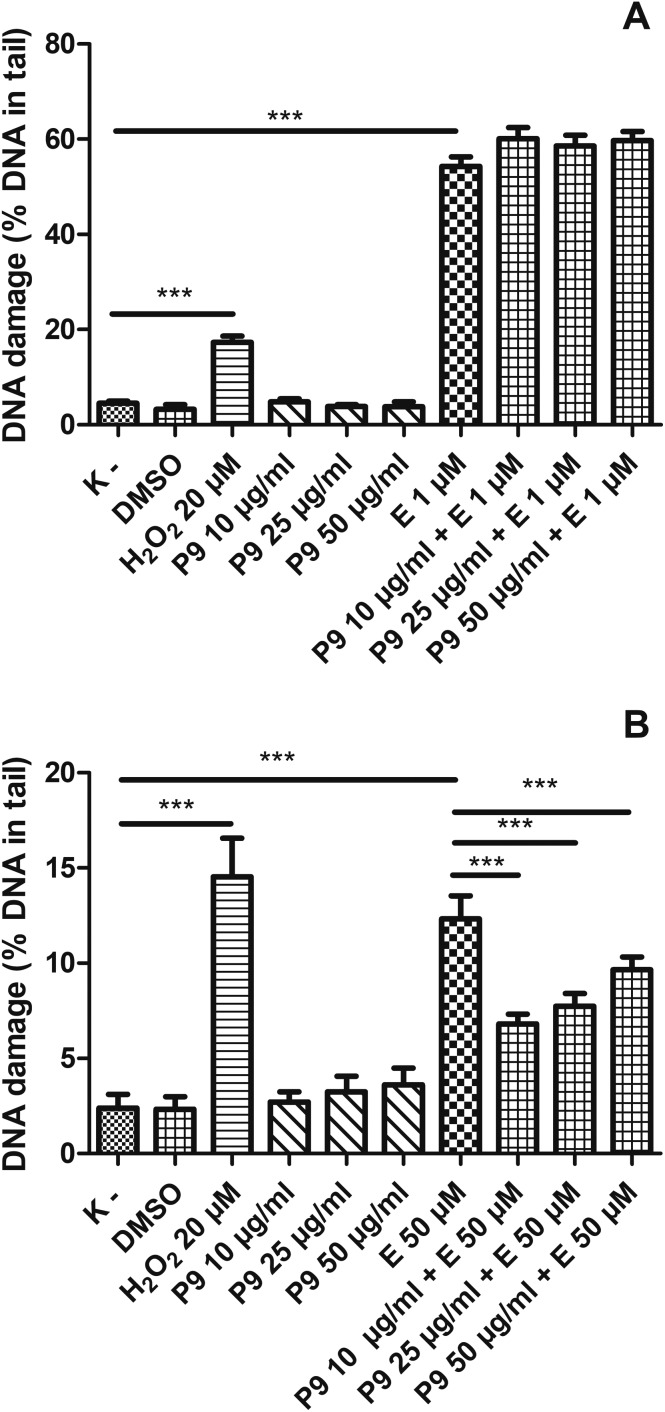
3.3. Kaempferol glycosides from the aerial parts of Lens Culinaris Medik. decrease DNA damage induced by etoposide in PBMCs and do not affect the level of DNA damage in HL-60 cells
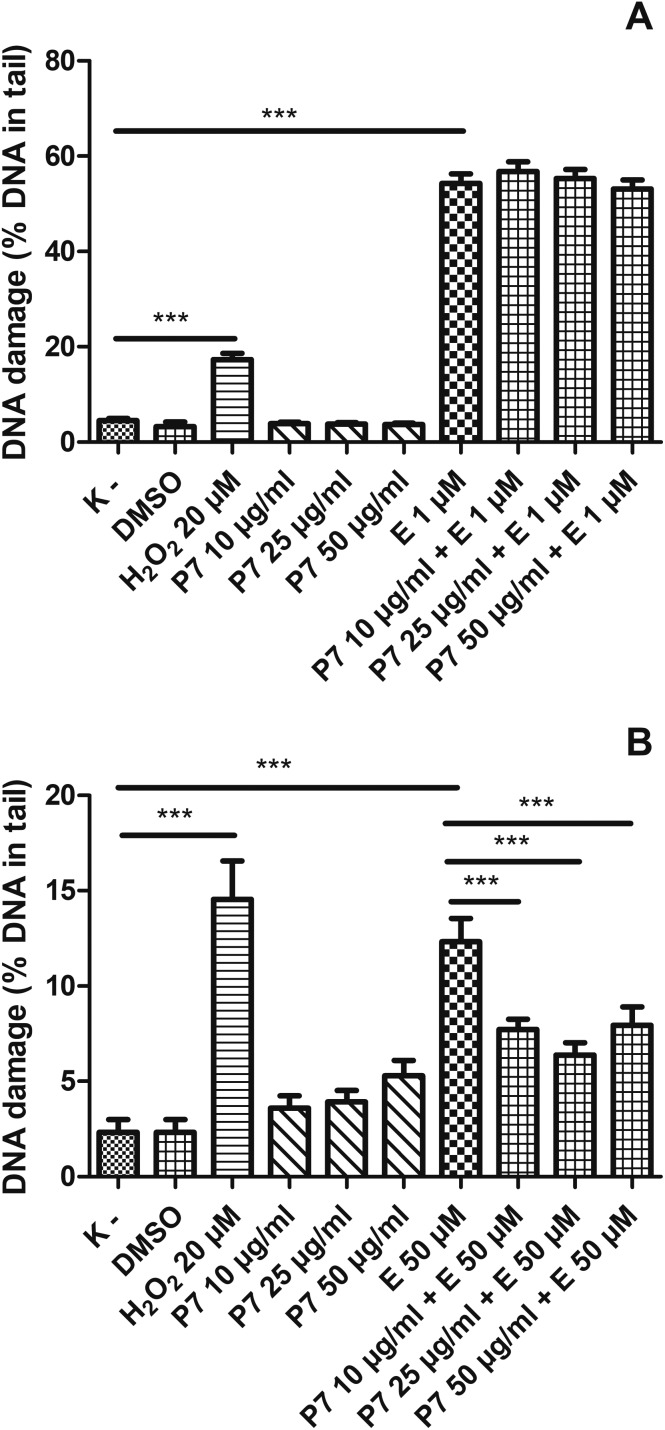
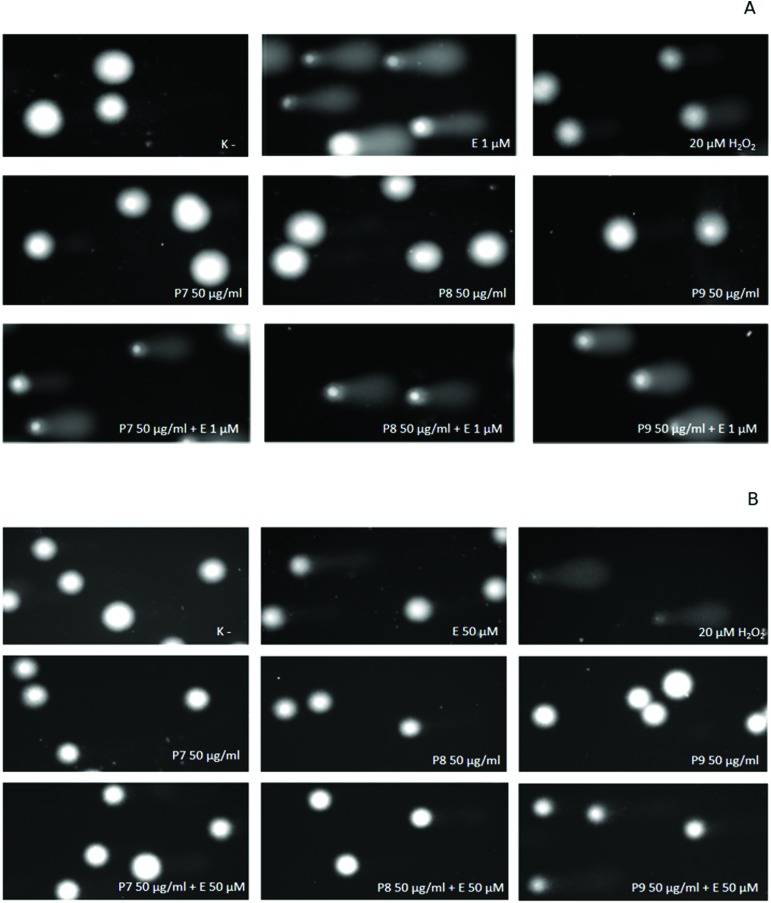
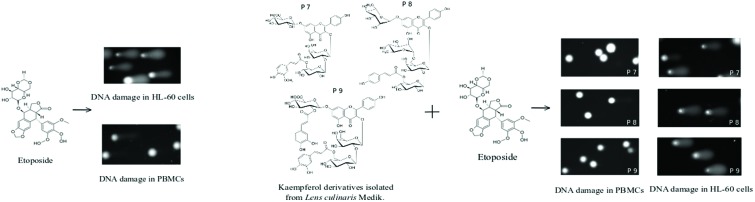
We showed that kaempferol glycosides did not cause DNA damage in both HL-60 cells and PBMCs (Fig. 5–7). Furthermore, all three of the tested kaempferol glycosides reduced the level of DNA damage induced by etoposide at 50 μM in PBMCs (Fig. 5B, 6B and 7B). For example, in the cells treated with etoposide at 50 μM and the P7 derivative at three different concentrations (10, 25 and 50 μg ml–1) the amount of DNA damage in the comet tail decreased from 12.3% to 7.7%, 6.4% and 7.9%, respectively (p < 0.001) (Fig. 5B). However, we did not observe any changes in the level of DNA damage caused by etoposide in the HL-60 cells incubated with kaempferol glycosides (Fig. 5A, 6A and 7A). Fig. 8 shows representative pictures of the comets obtained after the incubation of HL-60 cells (A) and PBMCs (B) with P7, P8 and P9 kaempferol derivatives at 50 μM and etoposide (E) at 1 μM or 50 μM.
Fig. 5. DNA damage, measured as the comet tail DNA (%) of HL-60 cells (A) and PBMCs (B) incubated for 2 h at 37 °C with the P7 derivative (10–50 μM) and etoposide (E) at 1 μM or 50 μM, analyzed by the alkaline comet assay. The figure shows mean results ± SEM, n = 100; **p < 0.01, ***p < 0.001.
Fig. 6. DNA damage, measured as the comet tail DNA of HL-60 cells (A) and PBMCs (B) incubated for 2 h at 37 °C with the P8 derivative (10–50 μM) and etoposide (E) at 1 μM or 50 μM, analyzed by the alkaline comet assay. The figure shows mean results ± SEM, n = 100; **p < 0.01, ***p < 0.001.
Fig. 7. DNA damage, measured as the comet tail DNA (%) of HL-60 cells (A) and PBMCs (B) incubated for 2 h at 37 °C with the P9 derivative (10–50 μM) and etoposide (E) at 1 μM or 50 μM, analyzed by the alkaline comet assay. The figure shows mean results ± SEM, n = 100; **p < 0.01, ***p < 0.001.
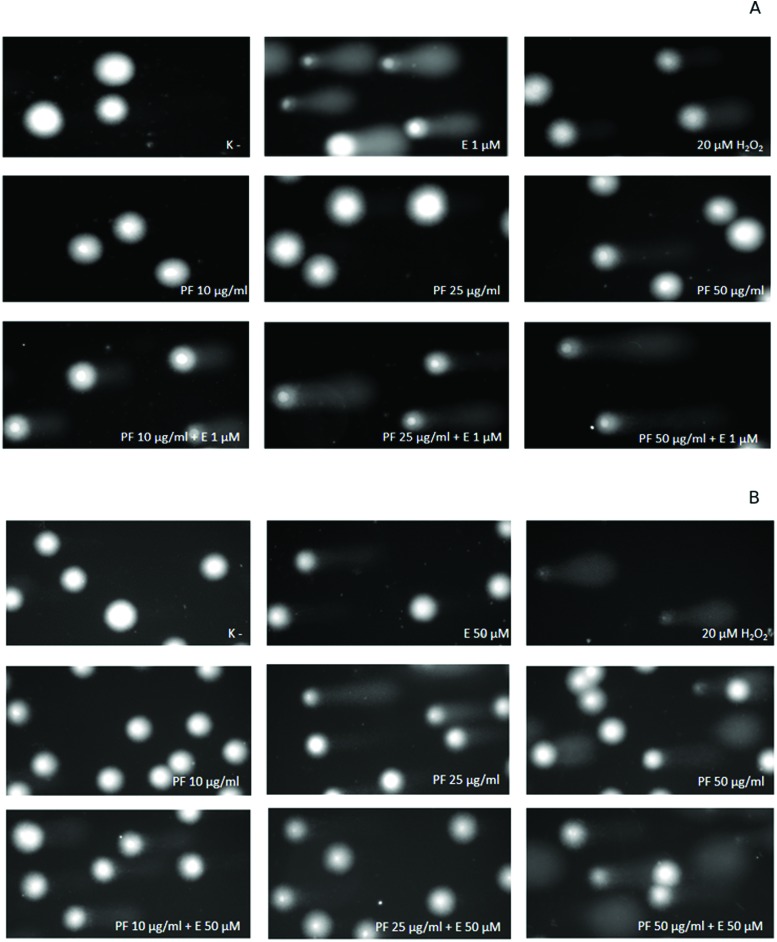
Fig. 8. Representative photos of comets, obtained in the alkaline version of the comet assay after the incubation of HL-60 cells (A) and PBMCs (B) with P7, P8 and P9 kaempferol derivatives at 50 μM and etoposide (E) at 1 μM or 50 μM. The figure also contains pictures of comets from the negative control (K–) and positive control (cells incubated with H2O2 at 20 μM for 15 min on ice).
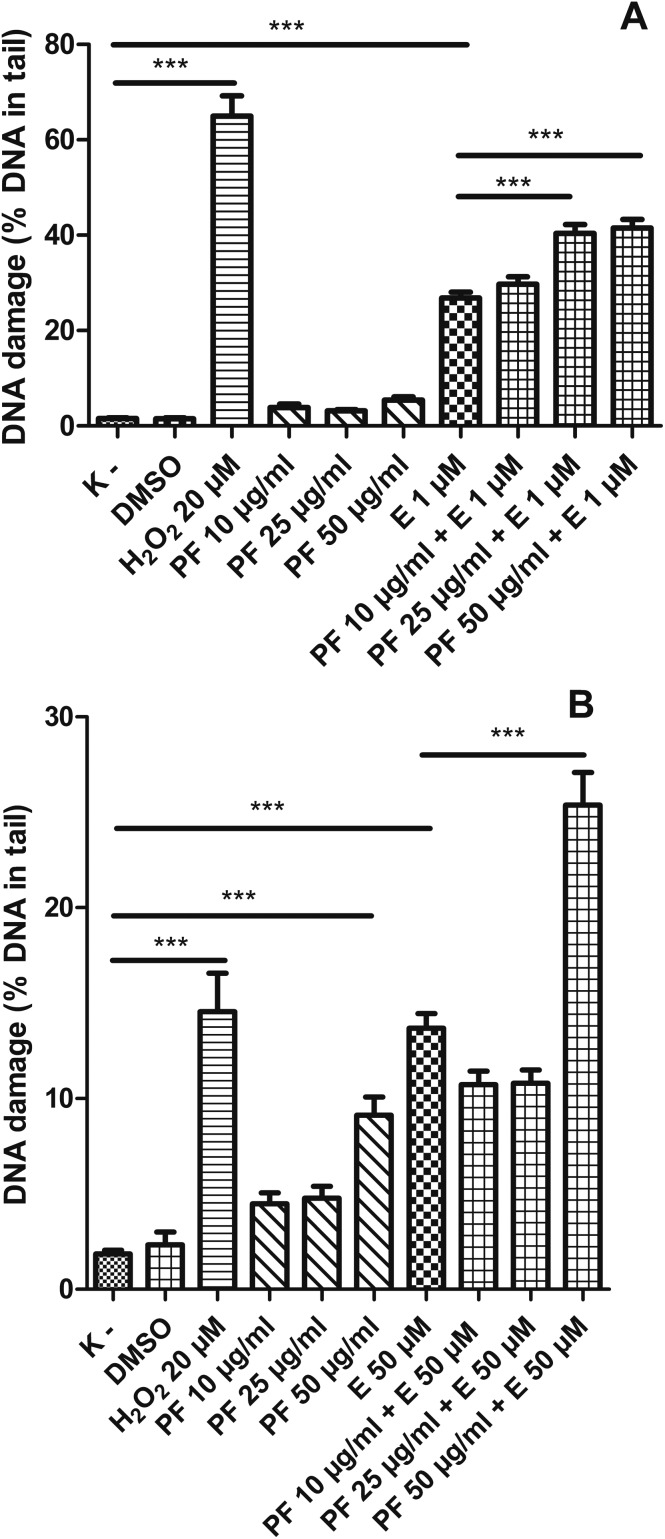
3.4. Polyphenol fraction from the aerial parts of Lens culinaris Medik. increases the level of DNA damage induced by etoposide in HL-60 cells and PBMCs
We noticed that the polyphenol fraction (PF) from the aerial parts of Lens culinaris Medik. at the concentration of 50 μg ml–1 induced DNA damage in PBMCs (Fig. 9B) at a statistically significant level (p < 0.001). Furthermore, the PF at this concentration increased DNA damage caused by etoposide. Etoposide induced 13.7% of DNA damage in PBMCs (p < 0.001), while a combination of etoposide and PF at 50 μg ml–1 caused 25.4% of DNA damage (p < 0.001). We did not observe any changes in the level of DNA damage at lower concentrations (10 and 25 μg ml–1) of the PF in PBMCs. The PF at the tested concentrations (10–50 μg ml–1) did not induce DNA damage in HL-60 cells (Fig. 9A). However, the PF at 25 μg ml–1 and 50 μg ml–1 caused an increase in DNA damage induced by etoposide from 26.8% to 40.4% and 41.5%, respectively (p < 0.001) (Fig. 9A). Fig. 10 shows representative pictures of the comets obtained after the incubation of HL-60 cells (A) and PBMCs (B) with the PF at 10, 25 and 50 μg ml–1 and etoposide (E) at 1 μM or 50 μM.
Fig. 9. DNA damage, measured as the comet tail DNA (%) of HL-60 cells (A) and PBMCs (B) incubated for 2 h at 37 °C with the polyphenol fraction (PF) from Lens culinaris Medik. (10–50 μg ml–1) and etoposide (E) at 1 μM or 50 μM, analyzed by the alkaline comet assay. The figure shows mean results ± SEM, n = 100; *p < 0.05, **p < 0.01, ***p < 0.001.
Fig. 10. Representative photos of comets, obtained in the alkaline version of the comet assay after the incubation of HL-60 cells (A) and PBMCs (B) with the polyphenol fraction (PF) at 10, 25 and 50 μg ml–1 and etoposide (E) at 1 μM or 50 μM. The figure also contains pictures of comets from the negative control (K–) and positive control (cells incubated with H2O2 at 20 μM for 15 min on ice).
3.5. Extract from the aerial parts of Lens culinaris Medik. increases the level of DNA damage induced by etoposide in HL-60 cells but does not affect DNA damage in PBMCs
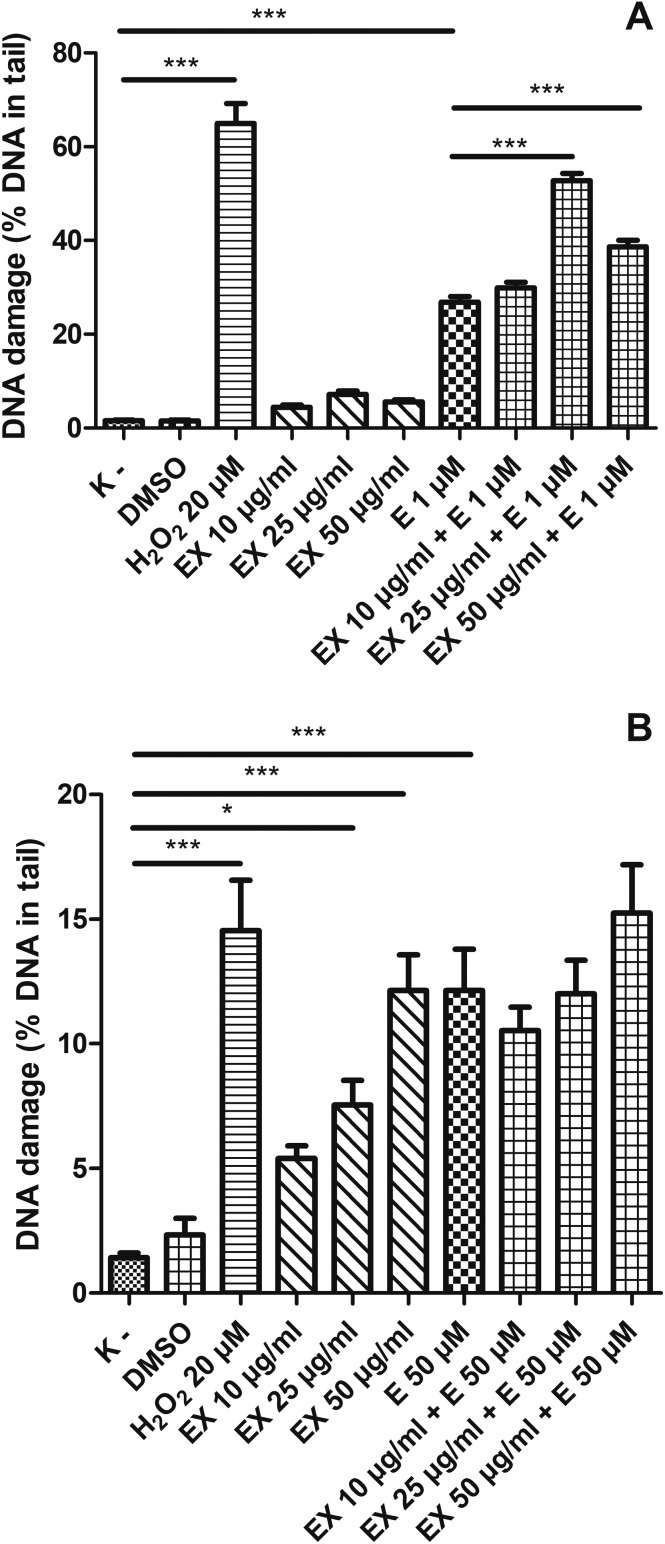
Finally, we examined the effect of an extract isolated from the aerial parts of Lens culinaris Medik. (EX) on etoposide treatment (Fig. 11). While the control PBMCs had about 1% of DNA lesions, the cells after incubation with EX at 25 μg ml–1 had 7.5% of DNA damage (p < 0.05) and after incubation with EX at 50 μg ml–1 showed 12.1% of DNA damage (p < 0.001) (Fig. 11B). Interestingly, the level of DNA damage caused by etoposide and EX at 50 μg ml–1 was at a similar level (around 12%) (p < 0.001). However, we did not observe any changes in the level of DNA damage in PBMCs caused after incubation with both etoposide and EX (Fig. 11B). EX did not induce DNA damage in HL-60 cells (Fig. 11A). We noticed an increase in the DNA damage level in HL-60 cells treated with etoposide and EX. Etoposide at 1 μM induced DNA damage in HL-60 cells at the level of 26.3% (p < 0.001). The simultaneous incubation of HL-60 cells with etoposide and EX at 25 μg ml–1 led to 52.7% of DNA damage (p < 0.001). The incubation of HL-60 cells with EX at 50 μg ml–1 and etoposide at 1 μM induced about 38.7% of DNA damage (p < 0.001) (Fig. 11A).
Fig. 11. DNA damage, measured as the comet tail DNA (%) of HL-60 cells (A) and PBMCs (B) incubated for 2 h at 37 °C with an extract (EX) from Lens culinaris Medik. (10–50 μM) and etoposide (E) at 1 μM or 50 μM, analyzed by the alkaline comet assay. The figure shows mean results ± SEM, n = 100; *p < 0.05, **p < 0.01, ***p < 0.001.
4. Discussion
Intracellular oxidative stress is directly associated with cancer.23 Kaempferol protects DNA, proteins and lipids against damage induced by oxidative stress. Therefore, the antioxidant properties of kaempferol can be used in cancer treatment and prevention. It was shown that 20 μM kaempferol protected erythrocytes against AAPH-induced oxidative damage.24 Additionally, it was noted that kaempferol increased the activity of antioxidative enzymes, such as GPx.25 Other studies showed that kaempferol at 50 μM for 24 h reduced the viability of human gastric cancer SNU-216 cells to 26.87 ± 3.18%, and kaempferol at 50 μM for 48 h reduced the viability of SNU-216 cells to 9.63 ± 4.28%.26 In the same study it was observed that kaempferol at 50 μM had no significant effect on the viability of human normal gastric GES-1 cells.26 In this study we evaluated whether polyphenols isolated from Lens culinaris Medik. could affect DNA damage caused by etoposide in HL-60 cells and PBMCs. These are the first studies showing the impact of kaempferol and its glycosides on etoposide activity in both neoplastic and normal cells. We showed that the tested compounds – kaempferol, its derivatives (P7, P8 and P9), the polyphenol fraction (PF) and an extract (EX) isolated from the aerial parts of Lens culinaris Medik., used at concentrations of 10, 25 and 50 μg ml–1, did not affect the viability of HL-60 cells and PBMCs (Fig. 3).
In this work we also demonstrated that kaempferol did not induce DNA damage in PBMCs (Fig. 4B). Our results are consistent with other studies conducted on normal lung and liver cells.27 The same studies showed that kaempferol isolated from S. anacardium protected these cells against DNA damage induced by H2O2.27 Kumar et al. also demonstrated that kaempferol-based treatment increased the expression of antioxidant genes encoding catalase and superoxide dismutase-2. Curcumin, i.e. another flavonoid, did not induce DNA damage in normal CD34+ cells and granulocytes.28 On the other hand, curcumin enhanced the cytogenotoxic effect of etoposide in HL-60 cells through the intensification of free radical production.28 We observed that kaempferol induced DNA damage only in cancer cells (Fig. 4A). This is consistent with the results obtained by Zhu.29 Kaempferol induced DNA damage in MDA-MB-231 breast cancer cells and increased the expression of the DNA damage-associated protein ATM.
Interestingly, kaempferol glycosides – P7, P8 and P9 derivatives isolated from the aerial parts of Lens culinaris Medik. – did not induce DNA damage in both HL-60 cells and PBMCs (Fig. 5–7) (Fig. 8). Moreover, kaempferol glycosides reduced DNA damage induced by etoposide in PBMCs (Fig. 5B, 6B and 7B) (Fig. 8B). The kaempferol derivatives tested by us contained additionally ferulic acid (P7), coumaric acid (P8) and caffeic acid (P9). These substances have antioxidant and protective properties. The protective effect of caffeic, ferulic and coumaric acids on DNA damage is due to the antioxidant activity of these compounds. Ferulic acid decreased the level of DNA damage induced by gamma irradiation in PBMCs. Its action is based on the ability of scavenging reactive oxygen species (ROS) and inducing DNA repair mechanisms.30 It was shown that caffeic acid decreased oxidative stress and protected liver cells against DNA damage induced by ethanol.31 Caffeic acid at 10 μM decreased DNA double-strand breaks (DSBs) induced by H2O2 in L-02 human liver cells. Caffeic acid also decreased the level of ROS in L-02 cells.32 The protective effect of caffeic acid was also tested on human lymphocytes exposed to UVB radiation. Pretreatment with caffeic acid before UVB-irradiation significantly decreased the level of DNA damage.33 Moreover, caffeic acid protected lymphocytes against DNA damage induced by mycotoxin Ochratoxin A.34 Caffeic acid treatment also protected human T-lymphoma Jurkat cells against H2O2-induced DNA damage.35 On the other hand, caffeic acid increased the level of DNA damage induced by radiation (6 Gy) in breast cancer cell lines T47D and MDA-MB-231.36 An extract from L. sibiricus, which has a high content of caffeic, ferulic and coumaric acids, protected CHO cells against DNA damage induced by H2O2.37 This protective effect was associated with the stimulation of DNA repair mechanisms and increased the expression of antioxidant genes (SOD2, CAT and GPx). Experiments carried out on pBR322 plasmid DNA showed that ferulic and caffeic acids protected DNA against mutagenic and toxic effects of UV and H2O2.38 Ferulic acid also decreased the level of oxidative DNA damage induced by methylglyoxal in plasmid DNA.39 The protective action of kaempferol glycosides, which we observed in PBMCs, may be related to the presence of the above-mentioned phenolic acids. Interestingly, we noticed that this protective effect did not occur in the case of cancer cells.
Furthermore other representatives of hydroxycinnamic acids have a protective effect on normal cells. Chlorogenic acid protected vascular smooth muscle cells from DNA damage induced by isoproterenol through the suppression of ROS generation.40 Additionally, chlorogenic acid protected DNA from damage induced by UV radiation in mouse fibroblast cells (CCRF).41 The main components of the extract isolated from Amaranthus viridis are chlorogenic acid, gulonic acid, and kaempferol, which protected human PBMCs from H2O2-induced DNA damage.42 Moreover, phenolic extracts isolated from different kinds of honey which contain seven phenolic acids including hydroxycinnamic acid protected mice lymphocytes from oxidative DNA damage induced by H2O2.43 It was suggested that these phenolic compounds can pass through the cell membrane and then scavenge hydrogen peroxide. Additionally, cinnamic acid at concentrations of 0.74–148 μg ml–1 reduced the level of DNA damage induced by hydrogen peroxide in human lymphocytes.44
Interestingly, other flavonol glycosides can protect cells from genotoxicity. Kaempferol 3-O-β-isorhamninoside and rhamnocitrin 3-O-β-isorhamninoside isolated from Rhamnus alaternus leaves decrease the amount of DNA damage induced by H2O2 in human lymphoblastoid TK6 cells and in the p53 deficient cell line NH32.45 Moreover, both tested compounds have antioxidant activity. These compounds chelate trace metals such as iron and copper which play a role in oxygen metabolism. The derivative P8 we tested contains a rhamnopyranoside moiety. It was shown that another flavonol glycoside containing this moiety, quercetin-3-O-α-l-rhamnopyranoside, also exhibits a protective activity against DNA damage induced by H2O2 in human umbilical vein endothelial cells (HUVECs).46 Flavonol glycosides can protect normal cells from damage in the genetic material but there is a need to further examine the mechanism of their action and the possibility of their use in the prevention of normal cells during chemotherapy.
5. Conclusion
In this paper we determined the effect of plant compounds isolated from the aerial parts of Lens culinaris Medik. on DNA damage induced by etoposide in HL-60 cells and PBMCs. We showed that kaempferol derivatives from lentils may be useful in protecting normal cells against DNA damage caused by etoposide. Furthermore, we demonstrated that kaempferol and an extract from the aerial parts of Lens culinaris Medik. increase the level of DNA damage induced by etoposide in HL-60 cells. Our results suggest that active compounds isolated from lentils may be successfully applied in anticancer therapy as substances protecting normal cells against the toxic effects of chemotherapeutics, e.g. etoposide. On the other hand, these compounds may enhance the chemotherapeutic effect of etoposide in cancer cells.
Abbreviations
- DAPI
4′,6-Diamidino-2-phenylindole
- DMSO
Dimethyl sulfoxide
- HL-60
Human promyelocytic leukemia cell line
- LMP
Low-melting-point agarose
- NMP
Normal-melting-point agarose
- PBMCs
Peripheral blood mononuclear cells
Conflicts of interest
The authors declare that there is no conflict of interest.
Acknowledgments
This work was supported financially by the University of Lodz, Poland, the Faculty of Biology and Environmental Protection.
References
- Rodriguez-Casado A. Crit. Rev. Food Sci. Nutr. 2016;56:1097–1107. doi: 10.1080/10408398.2012.755149. [DOI] [PubMed] [Google Scholar]
- Abbo S., Lev-Yadun S., Heun M., Gopher A. J. Exp. Bot. 2013;64:815–822. doi: 10.1093/jxb/ers373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan K., Xu B. J. Mol. Sci. 2017;18:2390. [Google Scholar]
- Adebamowo C. A., Cho E., Sampson L., Katan M. B., Spiegelman D., Willett W. C., Holmes M. D. Int. J. Cancer. 2005;114:628–633. doi: 10.1002/ijc.20741. [DOI] [PubMed] [Google Scholar]
- Caccialupi P., Ceci L. R., Siciliano R. A., Pignone D., Clemente A., Sonnante G. Food Chem. 2010;120:1058–1066. [Google Scholar]
- Perabo F. G., Von Löw E. C., Ellinger J., von Rücker A., Müller S. C. Prostate Cancer Prostatic Dis. 2008;11:6–12. doi: 10.1038/sj.pcan.4501000. [DOI] [PubMed] [Google Scholar]
- Faris M. A., Takruri H. R., Shomaf M. S., Bustanji Y. K. Nutr. Res. 2009;29:355–362. doi: 10.1016/j.nutres.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Spanou C., Stagos D., Tousias L., Angelis A., Aligiannis N., Skaltsounis A. L., Kouretas D. Anticancer Res. 2007;27:3403–3410. [PubMed] [Google Scholar]
- Beuth J., Schneider B., Van Leendert R., Uhlenbruck G. In Vivo. 2016;30:73–75. [PubMed] [Google Scholar]
- Calderón-Montaño J. M., Burgos-Morón E., Pérez-Guerrero C., López-Lázaro M. Mini-Rev. Med. Chem. 2011;11:298–344. doi: 10.2174/138955711795305335. [DOI] [PubMed] [Google Scholar]
- Avtanski D., Poretsky L. Mol. Med. 2018;24:29. doi: 10.1186/s10020-018-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imran M., Rauf A., Shah Z. A., Saeed F., Imran A., Arshad M. U., Ahmad B., Bawazeer S., Atif M., Peters D. G., Mubarak M. S. Phytother. Res. 2019;33:263–275. doi: 10.1002/ptr.6227. [DOI] [PubMed] [Google Scholar]
- Berger A., Venturelli S., Kallnischkies M., Böcker A., Busch C., Weiland T., Noor S., Leischner C., Weiss T. S., Lauer U. M., Bischoff S. C., Bitzer M. J. Nutr. Biochem. 2013;24:977–985. doi: 10.1016/j.jnutbio.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Qiu W., Lin J., Zhu Y., Zhang J., Zeng L., Su M., Tian Y. Cell. Physiol. Biochem. 2017;41:1325–1335. doi: 10.1159/000464435. [DOI] [PubMed] [Google Scholar]
- Bobe G., Sansbury L. B., Albert P. S., Cross A. J., Kahle L., Ashby J., Slattery M. L., Caan B., Paskett E., Iber F., Kikendall J. W., Lance P., Daston C., Marshall J. R., Schatzkin A., Lanza E. Cancer Epidemiol. Biomarkers Prev. 2008;17:1344–1353. doi: 10.1158/1055-9965.EPI-07-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Closas R., Gonzalez C. A., Agudo A., Riboli E. Cancer Causes Control. 1999;10:71–75. doi: 10.1023/a:1008867108960. [DOI] [PubMed] [Google Scholar]
- Gates M. A., Tworoger S. S., Hecht J. L., De Vivo I., Rosner B., Hankinson S. E. Int. J. Cancer. 2007;121:2225–2232. doi: 10.1002/ijc.22790. [DOI] [PubMed] [Google Scholar]
- Baldwin E. L., Osheroff E. Curr. Med. Chem. Anticancer Agents. 2005;5:363–372. doi: 10.2174/1568011054222364. [DOI] [PubMed] [Google Scholar]
- Montecucco A., Biamonti G. Cancer Lett. 2007;252:9–18. doi: 10.1016/j.canlet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Żuchowski J., Pecio Ł., Stochmal A. Molecules. 2014;19:18152–18178. doi: 10.3390/molecules191118152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. P., McCoy T., Tice R. R., Schneider E. L. Exp. Cell Res. 1988;175:184–192. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Tokarz P., Piastowska-Ciesielska A. W., Kaarniranta K., Blasiak J. Int. J. Mol. Sci. 2016;17:898. doi: 10.3390/ijms17060898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. K., Patel A. K., Shah N., Chaudhary A. K., Jha U. K., Yadav U. C., Gupta P. K., Pakuwal U. Asian Pac. J. Cancer Prev. 2014;15:4405–4409. doi: 10.7314/apjcp.2014.15.11.4405. [DOI] [PubMed] [Google Scholar]
- Wu P., Meng X., Zheng H., Zeng Q., Chen T., Wang W., Zhang X., Su J. Molecules. 2018;23:2592. doi: 10.3390/molecules23102592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W., Chen L., Ma X., Jiao R., Li X., Wang Y. Eur. J. Med. Chem. 2016;114:24–32. doi: 10.1016/j.ejmech.2016.02.045. [DOI] [PubMed] [Google Scholar]
- Zhang F., Ma C. Braz. J. Med. Biol. Res. 2019;52:e7843. doi: 10.1590/1414-431X20187843. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kumar A. D., Bevara G. B., Kaja L. K., Badana A. K., Malla R. R. BMC Complementary Altern. Med. 2016;16:376. doi: 10.1186/s12906-016-1354-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papież M. A., Krzyściak W., Szade K., Bukowska-Straková K., Kozakowska M., Hajduk K., Bystrowska B., Dulak J., Jozkowicz A. Drug Des., Dev. Ther. 2016;10:557–570. doi: 10.2147/DDDT.S92687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Xue L. Oncol. Res. 2019;27:629–634. doi: 10.3727/096504018X15228018559434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das U., Manna K., Khan A., Sinha M., Biswas S., Sengupta A., Chakraborty A., Dey S. Free Radic. Res. 2017;51:47–63. doi: 10.1080/10715762.2016.1267345. [DOI] [PubMed] [Google Scholar]
- Bispo V. S., Dantas L. S., Filho C. A. B., Pinto I. F. D., Silva R. P. D., Otsuka F. A. M., Santos R. B., Santos A. C., Trindade D. J., Matos H. R. An. Acad. Bras. Cienc. 2017;89:1095–1109. doi: 10.1590/0001-3765201720160581. [DOI] [PubMed] [Google Scholar]
- Li Y., Chen L. J., Jiang F., Yang Y., Wang X. X., Zhang Z., Li Z., Li L. Braz. J. Med. Biol. Res. 2015;48:502–508. doi: 10.1590/1414-431X20143729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad N. R., Jeyanthimala K., Ramachandran S. J. Photochem. Photobiol., B. 2009;95:196–203. doi: 10.1016/j.jphotobiol.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Cariddi L. N., Sabini M. C., Escobar F. M., Montironi I., Mañas F., Iglesias D., Comini L. R., Sabini L. I., Dalcero A. M. Environ. Toxicol. Pharmacol. 2015;39:1008–1018. doi: 10.1016/j.etap.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Kitsati N., Fokas D., Ouzouni M. D., Mantzaris M. D., Barbouti A., Galaris D. J. Agric. Food Chem. 2012;60:7873–7879. doi: 10.1021/jf301237y. [DOI] [PubMed] [Google Scholar]
- Khoram N. M., Bigdeli B., Nikoofar A., Goliaei B. J. Breast Cancer. 2016;19:18–25. doi: 10.4048/jbc.2016.19.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitarek P., Skała E., Wysokińska H., Wielanek M., Szemraj J., Toma M., Sliwiński T. Oxid. Med. Cell Longevity. 2016:5738193. doi: 10.1155/2016/5738193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevgi K., Tepe B., Sarikurkcu C. Food Chem. Toxicol. 2015;77:12–21. doi: 10.1016/j.fct.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Sompong W., Cheng H., Adisakwattana S. J. Physiol. Biochem. 2017;73:121–131. doi: 10.1007/s13105-016-0531-3. [DOI] [PubMed] [Google Scholar]
- Wang J., Li J., Liu J., Xu M., Tong X., Wang J., Mol. Med. Rep., 2016, 14 , 4063 –4068 10.3892/mmr.2016.5743 , . Epub 2016 Sep 15. PubMed PMID: 27634104; PubMed Central PMCID: PMC5101879 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y. H., Bahuguna A., Kim H. H., Kim D. I., Kim H. J., Yu J. M., Jung H. G., Jang J. Y., Kwak J. H., Park G. H., Kwon O. J., Cho Y. J., An J. Y., Jo C., Kang S. C., An B. J. J. Photochem. Photobiol., B. 2017;174:323–332. doi: 10.1016/j.jphotobiol.2017.08.015. [DOI] [PubMed] [Google Scholar]
- Kumari S., Elancheran R., Devi R. Indian J. Pharmacol. 2018;50:130–138. doi: 10.4103/ijp.IJP_77_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N., Wang Y., Cao W. Plant Foods Hum. Nutr. 2017;72:388–395. doi: 10.1007/s11130-017-0634-1. [DOI] [PubMed] [Google Scholar]
- Taner G., Özkan Vardar D., Aydin S., Aytaç Z., Başaran A., Başaran N. Drug Chem. Toxicol. 2017;40:183–190. doi: 10.1080/01480545.2016.1190740. [DOI] [PubMed] [Google Scholar]
- Bhouri W., Boubaker J., Kilani S., Ghedira K., Chekir-Ghedira L. S. Afr. J. Bot. 2012;80:57–62. [Google Scholar]
- Han H., Xu B., Amin A., Li H., Yu X., Gong M., Zhang L., Int. J. Mol. Med., 2019, 43 , 461 –474 10.3892/ijmm.2018.3976 , . Epub 2018 Nov 5. PubMed PMID: 30431061 . [DOI] [PubMed] [Google Scholar]