Abstract
Tooth enamel is a hard yet resilient biomaterial that derives its unique mechanical properties from decussating bundles of apatite crystals. To understand enamel crystal nucleation and growth at a nanoscale level and to minimize preparation artifacts, the developing mouse enamel matrix was imaged in situ using graphene liquid cells and atomic resolution scanning transmission electron and cryo-fracture electron microscopy. We report that 1–2nm diameter mineral precipitates aggregated to form larger 5nm particle assemblies within ameloblast secretory vesicles or annular organic matrix subunits. Further evidence for the fusion of 1–2nm mineral precipitates into 5nm mineral aggregates via particle attachment was provided by matrix-mediated calcium phosphate crystal growth studies. As a next step, aggregated particles organized into rows of 3–10 subunits and developed lattice suprastructures with 0.34nm gridline spacings corresponding to the (002) planes of apatite crystals. Mineral lattice suprastructures superseded closely matched organic matrix patterns, suggestive of a combination of organic/inorganic templates guiding apatite crystal growth. Upon assembly of 2–5nm subunits into crystal ribbons, lattice fringes suggesting the presence of a larger ordered crystallites were observed surrounding elongating crystal ribbons, presumably guiding the c-axis growth of composite apatite crystals. Cryofracture micrographs revealed reticular networks of organic matrix on the surface of elongating enamel crystal ribbons, suggesting that protein coats facilitate c-axis apatite crystal growth. Together, these data demonstrate (i) the involvement of particle attachment in enamel crystal nucleation, (ii) a combination of matrix and lattice-guided crystal growth, and (iii) fusion of individual crystals via a mechanism similar to Ostwald ripening.
Keywords: enamel, apatite, crystal growth, graphene liquid cell, atomic scale microscopy
The successful evolution of vertebrate organisms is tightly linked to the prominence of bones and teeth in their body plan.1 The unique biological, physical and mechanical properties of bones and teeth are the result of the involvement of organic protein matrices in the nucleation and growth of their mineral phase.2 These organic protein matrices form a lightweight organic framework that controls the growth and shape of calcium-ion based assemblies to produce an inorganic-organic bio-composite with well-defined mechanical properties.3 In bones and mesenchyme-derived dental tissues the major organic framework protein that shapes calcium phosphate crystal growth is collagen, while in tooth enamel the predominant matrix protein involved in the control of calcium phosphate crystal growth is amelogenin.
During tooth enamel development, enamel apatite crystals nucleate and grow from nanometer-sized mineral precipitates within a soft, aqueous matrix rich in proteins such as amelogenin to become mature enamel crystal measuring several hundreds of micrometers in length. In previous studies we established the essential role of the enamel matrix protein amelogenin for orderly matrix mediated enamel formation.4 visualized the functional role of enamel matrix subunit compartments during enamel crystal growth,5,6 and demonstrated the role of posttranslational amelogenin processing and resulting changes in matrix assembly.7,8 Recent studies using cryo-electron microscopy demonstrated the importance of amelogenin assemblies in the stabilization of mineral prenucleation clusters and their involvement in the formation of linear chains and needle-shaped mineral particles.9 Other studies have provided evidence for the hierarchical organization of the amelogenin matrix, beginning with 4–6nm subunits within nanospheres of 15–25nm radii and resulting in self-assembly of microribbons of > 100nm length.10,11
Enamel crystal nucleation is a process responsible for the orderly seeding and spacing of enamel crystals and of quintessential importance for its biological properties. In general, all biominerals rely on nanoparticle-protein complexes as building blocks that assemble using protein superstructures as growth templates.12 These protein superstructures consist of arrays of protein cages that provide a size- and space-constrained template for inorganic material mineralization, yielding nanoparticles with a high monodispersity and nano-stability.12 Nanoparticles then organize into complex inorganic nanostructures either through the assembly of protein particles mineralized with an inorganic core or as the result of continued mineralization on protein superstructures as a template.12 While the classical theory of crystal nucleation is based on monomer-by-monomer addition scenarios, more recent theories of crystal nucleation envision that crystallization occurs through the addition of individual particles ranging from multi-ion complexes to fully formed nanocrystals.13,14
Organic matrix mediated apatite crystal growth is essential for the life of many biological organisms, especially vertebrates and is also important for the design and function of biomaterials.1,15 However, apatites also grow in the absence of proteins and transition through stages similar to those that are known in biological minerals.2–4 In general, apatite crystals nucleate as amorphous calcium phosphate precursors (ACP), which transform into crystalline apatite through a number of intermediate stages.16,17 At the onset of calcium phosphate mineralization, ACP precursors form prenucleation clusters which result in the nucleation of ACP.18 Studies have demonstrated that nascent calcium phosphate precipitates as dual amorphous phases which transition between individual phases through dehydration.16,17 Further transformation into crystalline apatite occurs either through internal lattice rearrangements or reprecipitation of constantly dissipating units in contact with an aqueous solution.17 These multistep mineral growth mechanisms in conjunction with the continuous rearrangement of internal lattices during calcium phosphate crystal growth explain the structural adaptability of apatites in biological organisms and may also provide a mechanism for the crystal growth stages documented in the present study.
The purpose of the present study is to gain insights into the earliest stages of enamel crystal nucleation and to reveal mechanisms involved in enamel crystal growth on a nanoscale level. The present study takes advantage of recent advances in nanoscale and sub-nanoscale microscopy and liquid cell technology, eliminating artifacts due to fixation, staining, dehydration, and embedding. To visualize initial enamel crystal nucleation and elongation events, murine enamel matrix was immersed in a graphene coated liquid cell and visualized by aberration-corrected liquid-cell scanning transmission electron microscopy (STEM) in an unfixed and unstained native state. Enamel apatite nucleation simulation studies were performed in a metastable calcium phosphate solution combined with amelogenin enamel matrix protein. These samples were encapsulated between graphene sandwiches and visualized in our graphene liquid cell at atomic resolution. The interface between elongated enamel crystals and the organic matrix was imaged using a combination of cryo-fracture and cryo-scanning electron microscopy. Together, these minimally invasive ultrahigh resolution studies allowed for nanoscale and sub-nanoscale images of the earliest stages of enamel crystal nucleation and growth.
Results and Discussion
The present study is an ultra-high-resolution analysis of the earliest events in enamel crystal growth, including enamel protein matrix condensation, enamel crystal nucleation, and subsequent enamel crystal assembly, using fixation-free in situ nanoscale imaging. Our study overcomes several technical challenges posed by the aqueous, protein-rich enamel matrix,19 requiring invasive steps of sample preparation and dehydration necessary for electron imaging in high vacuum.8 To avoid the inherent artifacts associated with classical techniques and to study mineral/matrix relationships in the developing enamel matrix at a nanoscale level, ultrathin enamel matrix preparations have been encapsulated between few-layer graphene sheets, allowing for liquid-state atomic resolution matrix imaging using atomic resolution electron microscopy. There was no evidence of dissolution or phase transformation in our micrographs, suggesting that these images captured unperturbed events during the earliest stages of mouse amelogenesis. In addition to STEM imaging of developing enamel matrix preparations, calcium phosphate crystal growth studies using the enamel protein amelogenin as a templating matrix were performed in between adjacent graphene layers and directly observed in situ to overcome the limitations of dehydration, fixation, and contrasting agents. Only at later stages of enamel development (e.g. in 3 days postnatal mouse molars) sample thickness prohibited successful STEM imaging. At this stage the organic matrix in relationship to extended crystals and prisms was visualized using flash-frozen tooth organs which were subjected to cryofracture under high pressure and then observed under a high resolution cryo-scanning electron microscope. Together, the combination of in situ liquid cell STEM imaging, liquid cell crystal growth studies, and cryofracture imaging allowed us to generate a time-lapse reconstruction of the nanoscale and sub-nanoscale events involved in enamel crystal nucleation and growth with the least amount of sample preparation artifacts possible.
Earliest mineral precipitates formed and aligned within 20nm diameter organic matrix condensations
STEM imaging of the graphene liquid chamber containing native unmodified 1 day old mouse molar enamel matrix revealed annular matrix assemblies of approximately 20 nm in diameter with a 5 nm opening in the center of each assembly (Fig. 1A,B). Protein matrix assemblies contained one nanometer diameter electron dense particles aggregated into larger (5nm) clusters of amorphous material (Fig. 1A,B). Particles were identified as calcium phosphate via energy dispersive X-ray analysis (Figure 8, supporting information), and crystalline particles were confirmed as apatite based on lattice dimensions of 0.34 nm matching the d-spacing of the apatite 002 plane previously reported.20,21 There was little difference between the ultrastructure of the 20nm subunit compartment within the native enamel matrix (Fig. 1A,B), the subunit compartment within the sonicated mouse molar enamel layer (Fig. 1C–H) and the matrix isolated mechanically from the adjacent ameloblast cell layer (Fig. 1I–N). However, mineral density in the enamel matrix obtained through sonication was substantially higher when compared to the mineral deposits in the ameloblast secretory vesicle matrix, featuring multiple areas of dense mineral aggregation randomly dispersed across the protein matrix (Fig. 1C–H). The high mineral density in the sonicated enamel matrix was likely due to the ability of the enamel proteins to adsorb mineral and not necessarily a reflection of the in situ composition of the enamel matrix. In contrast, mineral accumulation appeared to only occur in one of the six corners of the 20nm subunit compartments of the ameloblast secretory matrix, resulting in a regular 5nm spacing between initial mineral assemblies within the ameloblast secretory matrix (Figs. 3G–L).
Figure 1. Enamel matrix subunit compartments from native enamel matrix, sonicated enamel matrix and in ameloblast secretory vesicles imaged using aberration corrected STEM imaging.

(A,B) Crystal nucleation in organic subunit compartments within the native enamel matrix of 1 days postnatal developing mouse molars. Note the individual 1–3nm diameter nucleation sites (arrows, A,B) randomly dispersed with a circular 20nm diameter organic matrix assembly (matrix)(A,B). (C-F) Network of 20nm subunit compartments generated from the sonicated 3dpn mouse molar enamel layer and its adjacent enamel matrix (matrix). Note the dense mineral assemblies (arrows) associated with protein subunits. The delineated square outlines the area selected for the individual subunit magnification in Figs. (G,H). (G,H) Nanoscale resolution image of the 20nm diameter organic matrix subunits from the sonicated enamel matrix. (I-N) Ultra high-resolution micrographs of the enamel matrix immediately adjacent to the secretory ameloblast layer. Note the presence of isolated mineral condensations within each protein subunit compartment (arrows). The rectangle demarks the area used for the high magnification micrographs in Figs. M,N. Nanoscale resolution image of the 20nm diameter organic matrix subunits from the ameloblast secretory vesicles (M,N). The site of mineral condensation (min) was restricted to one corner of the organic matrix (matrix) subunit compartment (N).
Figure 3. Enamel crystal growth studies within the STEM ARM liquid cell in vitro.
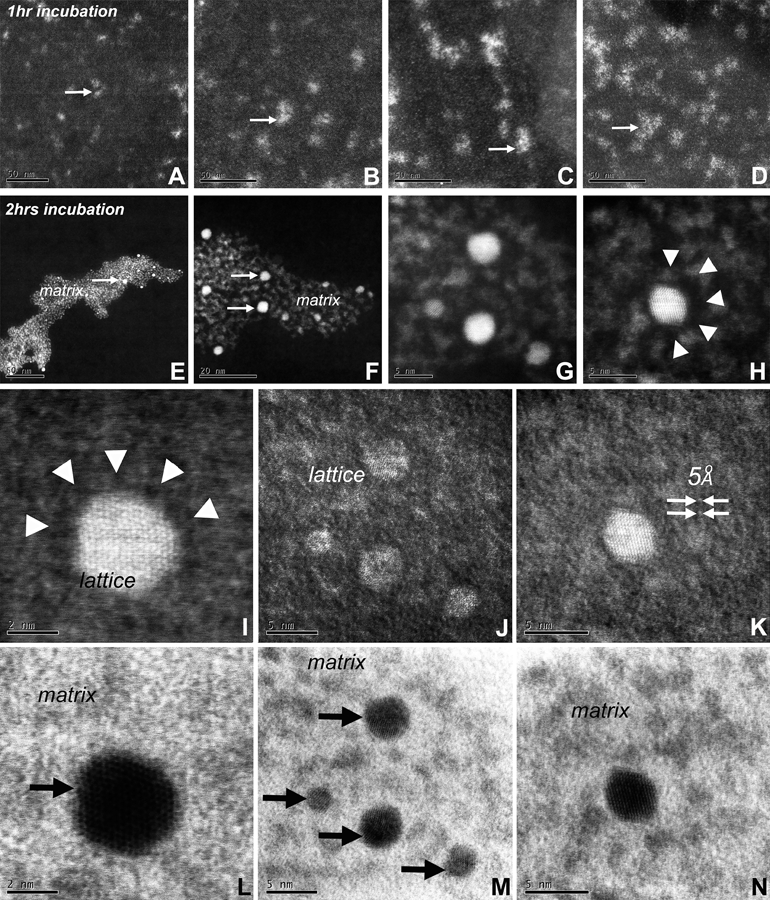
(A-D) Mineral deposition within the 20nm diameter organic matrix subunits after 1 hour incubation. Mineral precipitates are indicated with an arrow (A-D). (E-N) Mineral deposition and crystal nucleation within the 20nm diameter organic matrix subunits after 2 hours incubation. A-H were taken in high angle annular dark field mode, I-L in low angle annular dark field mode, and M-P in annular bright field mode. The position of the protein matrix (matrix), of the nucleating apatite crystals (HA) is indicated, and of the lattice spacings of newly formed crystals (lattice) is marked. Arrowheads (H, J) indicate the position of the protein- and mineral- free zone surrounding newly formed crystallites.
Our liquid cell/STEM approach identified individual annular matrix assemblies of approximately 20 nm in diameter with a 5 nm hollow opening in the center as individual subunits of the pre-crystallization enamel matrix of one day postnatal mouse molar matrices. Previous electron microscopic images of fixed enamel protein matrix had also demonstrated approximately 20nm diameter hollow and annular subunits, but these were part of an extensive network of similar structures that together formed the non-mineralized enamel protein matrix.4–6, 8 In the present study, immersion of unfixed pre-mineralization enamel matrix into the liquid cell and the atomic scale spatial resolution of our STEM resulted in the capture of these high-resolution micrographs of individual annular matrix subunits. The unique annular shape of these subunits may provide a structural template for mineral particle assembly to form higher ordered structures.
Our STEM images revealed that these initial protein assemblies were laden with 2nm diameter electron-dense particles. These particles are likely amorphous calcium phosphates, as no lattice fringes are visible in the images, and EDX analysis indicated the presence of calcium and phosphorous.5 The transition between amorphous precursor particles to rows of crystalline apatite nucleation clusters delineated by distinct crystal fringes lends support to the particle-attachment theory of crystal growth.13,14 According to this theory, crystals do not form through monomer-by-monomer addition but rather by the assembly and attachment of individual particles ranging from multi-ion complexes to fully formed nanocrystals.13 In support of the particle-attachment theory of crystal growth, our electron micrographs indicate that individual particles were nucleated apart from each other and crystals only grow by particle-mediated attachment of individual mineral particles until they eventually fused to become larger crystals.
Lattice-guided alignment of mineral precipitates
As a first step toward enamel crystal elongation, clusters of mineral particles formed linear rows of 1nm particles approximately 5nm apart from each other (Fig. 2A). Individual particle clusters were connected through matrix and elongated lattice structures in c-axis direction with lattice fringes perpendicular to the orientation of the linear rows (Fig. 2B–D). Following initial assembly into needle-shaped linear rows of mineralization crystals, a subsequent set of enamel crystal precursors consisted of linear rows of 2nm wide apatite crystals interspersed with non-crystalline mineral assemblies (Fig. 2E–J). There was a seamless transition between individual mineralization foci within the enamel matrix dispersed at a 0.3–0.4nm distance from each other (Fig. 2A–F) and the 0.3–0.4nm distance lattice intervals (Fig. 2A–F). At both stages, enamel crystals formed within a preformed template consisting of organic matrix and an apparent crystalline lattice. Even after individual 10nm crystals had assembled into an elongated compound enamel crystal (Fig. 2K), individual precursor crystals were distinguished by their orientations (Fig. 2K, A–F). High resolution micrographs revealed that early elongated enamel crystals were composite in nature (Fig. 2K–M) and consisted of individual subunits with major lattice fringes in between individual subunits forming a crisscross pattern with diametrically opposed 90-degree angles (Fig. 2L). The composite crystal was surrounded by a shaft with distinct lattice fringes parallel to the elongated crystal needle (Fig. 2L,M). The tip of the elongated crystal needle was surrounded by protein matrix and sub-nanoscale mineralization foci (Fig. 2M).
Figure 2. Particle-attachment mediated crystal growth and lattice-guided elongation in the native enamel matrix of 1 day postnatal developing mouse molars via atomic resolution STEM.

(A-D) Linear rows of longitudinally aligned individual nucleation sites (arrows) surrounded by organic matrix (matrix). Note the regular pattern of 0.3–0.4nm distance parallel lattice fringes perpendicular to the direction of individual nucleation sites indicative of the presence of crystallized matter. (A) Particle attachment stage, demonstrating three nucleation sites (arrows) surrounded by organic matrix (matrix), with individual particles aggregated in the proximity of each nucleation site. (B) Three rows of 5–7 linear arranged nucleation sites surrounded by organic matrix and connected through lattice fringes, each measuring approximately 20nm in length. (C,D) Lattice guided bridging of mineralization sites (arrows). Individual nucleation sites were 4–8nm apart from each other, and lattice fringes extended beyond nucleation sites. (E-J) Relationship between widened crystalline lattice structures (lattice) and matrix interfaces (matrix) during continued enamel apatite crystal growth. (E-G) High magnification STEM micrographs of three adjacent 3nm diameter crystals surrounded by organic matrix (matrix). Parallel lattice fringes at a 0.3–0.4nm distance from each other were indicative of the presence of newly formed apatite crystals within the enamel matrix. (H-J) High magnification focus stack of an initial apatite crystal nucleation site within the organic matrix (matrix), revealing parallel 0.3–0.4nm distance lattice fringes and grid patterns. (K-M) High resolution ultrastructure of an elongated composite enamel crystal. Note the composite structure of this initial enamel crystal needle consisting of individual crystalline subunits (a-f). (L) Criss-cross lattice structure in adjacent crystal subunits. Note the bright lattice fringes parallel to the shaft (shaft) of the crystal needle (double arrows, H and I). (M) Lattice organization of the crystalline subunit at the enamel crystal needle tip. Note the distinct lattice fringes parallel to the elongated crystal needle (double arrows). Protein matrix patterns (matrix) were detected in proximity to the tip of the elongated crystal.
Upon crystallization, initial mineral/protein assemblies formed an apparent lattice structure that closely matched the arrangement of electron-dense precipitates within the matrix as well as the 0.34nm lattice periodicities demonstrated in earlier studies.20,21 Previously, we have only observed the presence of electron-dense precipitates within the protein matrix in our organ culture studies.4,5,6,8 Here we attribute our ability to resolve mineralization foci within the native matrix to the high resolution of our STEM and to the darkfield imaging mode employed. In our samples, electron-dense particles were frequently aligned to form rows of individual particles, and a lattice pattern extended beyond the boundaries of individual mineralization particles. These data indicate that latent mineral nucleation events precede the precipitation of the first apatite crystals deposited in the enamel matrix close to ameloblast membrane or dentin boundaries and that the combined protein/mineral precipitation sites provide the basis for the crystal lattices formed shortly thereafter.
The overlapping nanothin protein and mineral lattices resembled the superlattices used in the semiconductor industry, where thin layers of different materials are sandwiched together to form semiconducting materials.22 Superlattice materials are lattice materials comprising two or more structural levels, which exhibit distinct features compared with single crystals.23 Specifically, superlattice materials are characterized by distinct lattice orientations across different levels as well as different materials components and symmetries of the superlattice unit cells. 23 Here our data demonstrate that initial protein-based templates were superimposed by a mineral lattice distinct from the underlying protein matrix in terms of spacing and orientation. Moroever, the lattice planes of the initial needle-shaped mineral assemblies were perpendicular to the overall orientation of the aligned mineral particle precipitates. The eventual fate of the templating protein lattice warrants further investigation, especially as to how enzymes might function to remove the proteins from these densely woven lattices or whether the protein lattice becomes entirely engulfed by the continuously growing mineral phase. Our data suggest that the orderly protein matrix serves as a transient template rather than an integral structural component because after initial protein-mediated crystal nucleation and alignment, mineral lattices extended beyond the boundaries of the protein template and formed a sheath surrounding assembled mineral particles in c-axis direction. These data suggest that following initial protein mediated assembly, the orderly aligned mineral lattices play a role in directional enamel crystal growth at a later stage of enamel crystal elongation.
Matrix reorganization in the immediate proximity of apatite crystal nucleation sites in vitro
To determine whether a combination of calcium phosphate together with enamel protein matrix suffice to promote the conversion of nanoscale mineral precipitates to hydroxyapatite crystals, a mixture of a calcium phosphate crystal growth solution in conjunction with the major amelogenin recombinant tooth enamel protein was established in a STEM liquid cell, and the mineral/protein solution was observed after one and two hours incubation (Fig. 3). STEM microscopy of the graphene liquid cell containing apatite mineralization conditions revealed initial mineral precipitates within an orderly organic matrix (Fig. 3A–D) featuring 20nm subunits after 1-hour incubation similar to those detected in the early enamel matrix (Fig. 3A,B compare to Fig. 1A,B). After 2 hours incubation, the mineral phase contained numerous 5nm diameter apatite crystals with high crystallinity as evidenced by a distinct lattice and hexagonal contours (Fig. 3E–N) in addition to the mineral precipitation foci that formed after the first hour of incubation (Fig. 3A–D). Apatite crystals were surrounded by an organic matrix featuring both greater 20nm assemblies and smaller 2–4 nm subunits (Figs. 3I–N) and crystals were interconnected through a surrounding larger crystal as evidenced by a discrete lattice pattern (Fig. 3J). Individual apatite crystals were surrounded by a 1nm diameter zone deplete of electron dense structures indicative of ions and/or enamel proteins (Figs. 3H,I).
Amorphous precursor particles such as amorphous calcium phosphate and intermediate phases such as octacalcium phosphate5,6 are common precursor stages of biominerals as they allow for the efficient transport of mineral constituents with low solubility to the crystallization site.13,24,25 This conversion from condensed assemblies of electron-dense amorphous particles into mineral crystals characterized by distinct crystal lattices was confirmed in our in situ mineralization studies in which a calcium phosphate enriched amelogenin matrix featured electron dense particles after one hour of incubation which were replaced by mineral crystals after another hour of incubation. Apparently, during that process, the periphery of larger particles was devoid of both mineral and protein, suggesting that the growth of larger, assembled crystals involved the attraction of both proteins and crystals from the immediate periphery by some unknown mechanism. Due to the lack of amelogenin protein cleavage, these in situ mineralization studies only mimic the early stages of calcium phosphate crystal growth, and further growth studies would require the presence of enamel proteases such as MMP20.
Developing enamel crystals were coated with a 50–100nm mesh protein network featuring 20nm subunits
In vivo studies of unfixed, unstained, and fresh enamel matrix using STEM imaging were limited to the study of the very initial enamel matrix because of the mineral density at successive stages. To study enamel matrix organization in the periphery of elongated enamel crystals at 3 days postnatal mouse molar development, freeze-fracture and cryo-scanning electron microscopy technology were applied (Fig. 4A). Scanning electron micrographs of the freeze fractured enamel crystal surface revealed 50–100nm annular matrix subunits containing smaller 20nm spherical matrix subunits positioned on the crystal surface (Fig. 4B–I).
Figure 4. Cryo-EM analysis of freeze-fractured 3 days postnatal developing tooth enamel and enamel organs.

(A) Overview micrograph illustrating the freeze-fracture topography at the interface between ameloblasts (amel), (dentin) (de) and predentin (pd). There was a thin layer of protein matrix (ma) between the ameloblast cell layer (amel) and the dentin layer (de). (B-K) are freeze-fracture cryo electron micrographs from the early enamel layer positioned between ameloblasts (amel) and dentin (de). (B) Freeze fracture topography of the organic matrix (prot) immediately associated with the inorganic crystal surface (cryst). (C,D) Identification of 50–100nm annular protein matrix assembly rings (arrowheads) on crystal surfaces. (D,E) 20nm spherical matrix subunit position on the crystal surface (double arrows). (F-I) Protein matrix at the ameloblast face of developing enamel crystals (arrows, G). The arrowheads in (H, I) point to annular subunit compartments measuring approximately 50–100nm in diameter. (J,K) image processing technologies were applied to either enhance the contrast (J) or to emboss the 3D surface relief (K) of the micrograph in (H) to further define the structural basis of enamel protein assemblies on crystal surfaces.
Our cryo-fracture scanning electron micrographs revealed 50–100nm diameter annular matrix assembly rings with 20nm subunits on the surface of elongating crystals. In previous studies we have identified the protein phase tightly associated with the enamel crystal surfaces as C-terminal fragments, while N-terminal fragments appeared to float freely in between maturing enamel crystals.8 Others have proposed that cleavage of the C-terminal fragment from the polyproline-rich domain will render the amelogenin carboxy-terminus hydrophobic,26 which would explain the self-assembled reticular networks on the growing enamel crystal surfaces as proline-rich self-assembled protein aggregates. Such protein-dense self-assembled reticular networks in between individual enamel crystals would provide spacing during crystal growth and and bulk calcium phosphate precipitation. Prevention of enamel crystal fusion in a- and b- axis direction by insulating individual enamel crystals from each other would facilitate the growth of needle-like crystal morphologies in c-axis direction and thus be essential for the maintenance of the unique biomechanical properties of enamel as a resilient yet flexible biomaterial.
Elongated enamel crystals were demarked by serrated or irregular outlines
Based on the aggregated composition of early enamel crystal assemblies we asked whether there was evidence for a composite nature of individual enamel crystals at later stages of enamel crystal formation. At 3 days postnatal mouse molar enamel development, both our STEM images (Fig. 5A–C) and our cryo-fracture micrographs (Fig. 5D,E) demonstrated serrated outlines (Fig. 5B,D) or individual electron dense subunits (Fig. 5C) along the c-axial borders of the elongated enamel crystal.
Figure 5. Nanoscale structure of unfixed 3 days postnatal first mouse molar enamel crystals as observed via atomic resolution STEM (A-C) and freeze-fracture scanning microscopy (D,E).
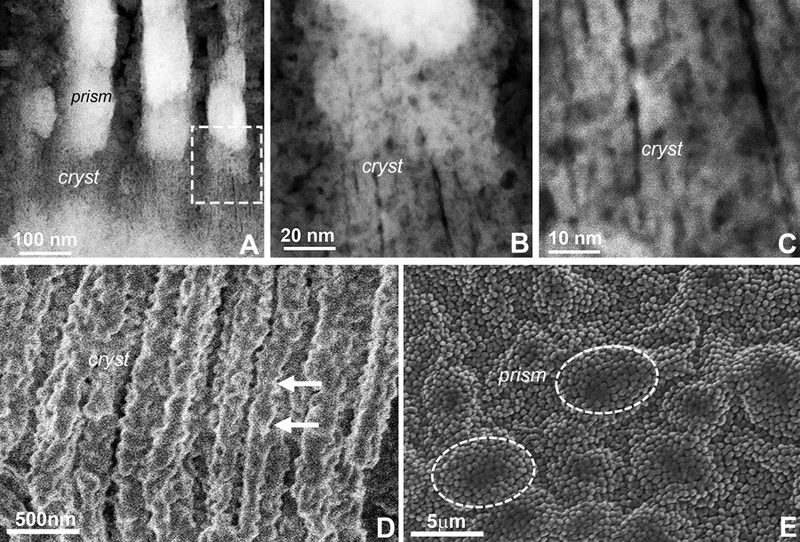
(A) Overview micrograph demonstrating individual crystals (cryst) organized into enamel prisms (prism). The insert indicates the area chosen for higher magnification micrographs in (B) and (C). (A-C) individual enamel crystals. Note the irregularities along the crystal c-axis and the close proximity between subunits from adjacent crystals (cryst). (D) Cryofracture micrographs illustrating enamel crystal morphology (cryst) in vertical direction. Note the serrated lateral surface of the elongated enamel crystals (arrowheads). (E) SEM visualization of the cryo-etched enamel prisms (prism). Prisms containing individual crystals are highlighted.
Our STEM and cryofracture micrographs provided evidence for the composite nature of initial and elongating enamel crystals, both in 1 day and 3 day postnatal developing mouse molar enamel samples. These micrographs revealed individual crystalline mineral particles aligned into needle shaped electron-dense enamel crystals. The composite nature of elongated enamel crystals was revealed as individual subunits on STEM images and through the serrated outlines enamel crystals on cryofracture micrographs. Together, serrated outlines in conjunction with the presence of crystal subunitsindicates that crystal assemblies were inhomogeneous and consisted of fused individual crystals and not a singular large crystal. The fusion of individual apatite crystals into mature enamel crystals through a process called Ostwald ripening has been discussed earlier.5,6,8,27–30 Here we show that the individual crystalline subunits were distinguished from each other by opposing lattice orientations, indicative of their independent origins within our sample. Moreover, elongated sub-assembled crystals were further surrounded by another crystalline structure, such that in addition to the protein phase, larger surrounding crystalline structures acted as contributors to directional enamel crystal growth.
Conclusion
Our data provide evidence for a four-pronged process that leads to enamel growth and maturation, involving (A) a stage of initial calcium phosphate particle precipitation and attachment within the organized enamel protein matrix, (B) the formation of initial crystal needles through lattice- and matrix-guided bridging of individual nucleation sites, (C) the lattice-guided alignment and extension of individual apatite crystals into elongated enamel crystals, and (D) the fusion and guided growth of individual enamel crystals by surrounding lattices (Fig. 6). This multi-step process explains the regular seeding of enamel crystals within a protein-rich enamel matrix8 and the step-wise elongation of individual crystals by continuous fusion and growth of individual calcium phosphate crystals. Packaging and coating of individual enamel crystals with reticular enamel protein networks (Figs. 4,6) then facilitates further c-axis direction crystal growth by spacing individual crystals, preventing crystal fusion, and limiting a- and b-axis crystal growth in favor of c-axis elongation.
Figure 6. (Sketch) Five successive stages of enamel crystal precipitation and elongation according to our STEM and cryo-fracture data.

(A) Stage of initial calcium phosphate particle precipitation and attachment within the organized enamel protein matrix, (B) Formation of initial crystal needles through lattice- and matrix-guided bridging of individual nucleation sites, (C) Lattice-guided alignment and extension of individual apatite crystals into elongated enamel crystals, (D) Recently fused early enamel crystal with surrounding lattices guiding further crystal elongation and growth, (E) Decussating arrangement of individual enamel crystals into enamel prisms (rods). The oblate ellipsoids on the surface of individual enamel crystals illustrate the shape of the protein coat based on our cryo-fracture micrographs.
Experimental Section
Animal studies and sample preparation
For the preparation of enamel matrix from postnatal mouse mandibular molars, mice were sacrificed according to UIC animal care guidelines. Five types of specimen were prepared for further analysis (i) unfixed 100nm ultrathin matrix from 1 day postnatal mouse molars, (ii) unfixed preparations of the innermost layer of the enamel organ from 3 days postnatal mouse molars for the analysis of ameloblast secretory vesicles, (iii) enamel matrix from 3 days postnatal mouse molars for further processing by 30 seconds sonication, (iv) the enamel layer of 3 days postnatal mouse molars for STEM enamel crystal studies was fixed in 2% glutaraldehyde for 2 hours, and (v) 3 days mouse molar tooth organs for cryo-fracture studies. The unfixed enamel matrix was prepared using a scalpel under a dissection microscope from the center of the mesial slopes of the central cusp of mandibular molars from 1 day postnatal mice by gently lifting the earliest enamel matrix of 1 day postnatal mouse molars from the underlying dentin (Fig. 7, Supporting Information).
STEM ARM electron microscopy of newly deposited enamel matrix
Unfixed initial enamel matrix of 1 day old postnatal mouse molars was immersed into filtered water without fixation. Encapsulation between two sheets of graphene supported on a 3mm TEM grid was carried out in several steps. Free floating graphene was prepared from commercial graphene grown on copper substrate. The graphene was freed from the copper by etching with ammonium persulfate solution (Transene Inc.) and transferred to pure water. A bottom layer of graphene was placed on a 3mm holey carbon TEM grid (EMS, Inc.) by bringing the clean carbon surface into contact with the floating graphene. After drying the sample solution was placed on the graphene-coated grid. A second layer encapsulating the sample was then added by bringing the sample into contact with another piece of floating graphene to generate a graphene liquid cell (GLC)(Fig. 7, Supporting Information). GLC preparation has been described in greater detail elsewhere.31 Preparation time for each GLC was approximately 1 hr, after which the GLC including sample was immediately transferred to the microscope for imaging. Imaging for each specimen was carried out over a single day. STEM studies were carried out using our aberration-corrected JEOL ARM-200CF. An accelerating voltage of 80 kV was used for imaging and EDS to reduce beam damage. The presence of water in the sample was confirmed by increasing the dose rate in certain areas, and confirming the formation of bubbles in those areas due to radiolysis of the water. Elemental analysis and mapping was conducted using energy dispersive X-ray analysis (EDS), with an Oxford X-max 100TLE windowless SDD X-ray detector. Lattice spacing measurements of newly formed apatite crystals were performed on enlarged micrographs at a 1nm square resolution. Enamel nucleation and crystal growth micrographs were sorted according to progressing mineral/crystal length and density.
In vitro biomineralization studies
Apatite biomineralization experiments were performed as previously described.7,32 Briefly, peptides and proteins were dissolved in distilled, deionized water (DDW) at a concentration of 4mg/ml and then adjusted to pH7.5–8.0 with 20mM NH4OH at 400C. Our reaction mixture contained 1mg/ml M179 amelogenin, 5 mM CaCl2 and 3 mM (NH4)2HPO4 which were mixed together and incubated for one hour or two hours before being diluted and encapsulated in between graphene layers. In order to prepare a graphene liquid cell grid, free-standing graphene was first prepared on the surface of DDW water. Next, the sample was transferred onto a separate graphene TEM grid either by pipetting, or by touching the grid surface with the sample solution. The graphene TEM grid with the sample solution on the surface was then placed upside down with the sample surface contacting a second piece of floating graphene to sandwich the sample solution between two layers of graphene.31 Samples were then imaged using our aberration corrected STEM (JEOL ARM-200CF).
Freeze fracture studies
Postnatal mouse molar enamel samples from 3 days postnatal mice were dissected into small pieces with a razor blade and placed in the recess of a sample carrier (Type “B”, #242–200, Technotrade) for the high-pressure freezer (HPM100, Leica). 0.1 M PIPES buffer (#P8203, Sigma) with 20% Dextran (#31389, Sigma) was used as a filler, and the flat side of another sample carrier was placed on top of the sample before the assembly was high-pressure frozen. Samples were stored in liquid nitrogen until further processing for cryo-SEM. For cryo-SEM, the frozen carrier sandwiches were separated, and samples were cryo-planed in a cryo-ultramicrotome (UC7-FC7, Leica). Approximately 20 micrometers of the samples were removed with a glass knife at a temperature of −160° C. The cryo-planed carriers with samples were kept in liquid nitrogen, transferred to a sample loading dock, and mounted under liquid nitrogen onto a cryo-SEM sample holder. A precooled cryo-shuttle (VCT100, Leica) was used to transfer the cryo-SEM sample holder with the mounted samples into a cryo-etching/coating system (ACE600, Leica). For etching, the temperature of the cryo-SEM sample holder in the ACE600 was raised to −105° C for 5 minutes in a vacuum of 2×10–6 mbar. After etching, the temperature was decreased to −120° C and the samples were coated with 5 nm platinum followed by 8 nm of carbon. The cryo-SEM sample holder with the coated samples was loaded into the precooled cryo-shuttle and inserted into the SEM (S-4800-II, Hitachi) with a pre-cooled cryo-stage (Leica). The samples were observed at a temperature of −120° C with an acceleration voltage of 0.5 kV or 1 kV.
Supplementary Material
Acknowledgements
Generous funding for these studies was provided by the National Institute for Dental and Craniofacial Grant DE018900. The use of the JEOL JEM-ARM200CF was supported by the UIC Department of Physics and the Scientific Imaging and Nanotechnology Division of the UIC Research Resources Center. Our cryo-fracture studies made use of the EPIC facility of Northwestern University’s NUANCE Center, which has received support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-1542205); the MRSEC program (NSF DMR-1720139) at the Materials Research Center; the International Institute for Nanotechnology (IIN); the Keck Foundation; and the State of Illinois, through the IIN. The authors would like to thank E. Beniash at the University of Pittsburgh for helpful suggestions that have improved the quality of this manuscript.
Footnotes
Supporting Information Available: Figures illustrating sample preparation, lattice measurement, and EDX analysis. This information is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Gopinathan G; Jin T; Liu M, Li S; Atsawasuwan P; Galang MT; Allen M; Luan X; Diekwisch TGH The Expanded Amelogenin Polyproline Region Preferentially Binds to Apatite versus Carbonate and Promotes Apatite Crystal Elongation. Front. Physiol 2014, 5, 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowenstam HA Minerals Formed by Organisms. Science 1981, 211, 1126–1131. [DOI] [PubMed] [Google Scholar]
- 3.Mann S Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry Oxford University Press: Oxford, UK, 2001; pp 3. [Google Scholar]
- 4.Diekwisch T; David S; Bringas P Jr; Santos V; Slavkin HC Antisense Inhibition of AMEL Translation Demonstrates Supramolecular Controls for Enamel HAP Crystal Growth during Embryonic Mouse Molar Development. Development 1993, 117, 471–482. [DOI] [PubMed] [Google Scholar]
- 5.Diekwisch TG; Berman BJ; Gentner S; Slavkin HC Initial Enamel Crystals are not Spatially Associated with Mineralized Dentine. Cell Tissue Res 1995, 279, 49–67. [DOI] [PubMed] [Google Scholar]
- 6.Diekwisch TG Subunit Compartments of Secretory Stage Enamel Matrix. Connect. Tissue Res 1998, 38, 101–111. [DOI] [PubMed] [Google Scholar]
- 7.Jin T; Ito Y; Luan X; Dangaria S; Walker C; Allen M; Kulkarni A; Gibson C; Braatz R; Liao X; Diekwisch TG Elongated Polyproline Motifs Facilitate Enamel Evolution through Matrix Subunit Compaction. PLoS Biol 2009, 7, e1000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandya M; Tiffani Lin T, Li L; Allen MJ; Jin T; Luan X; Diekwisch TGH Posttranslational Amelogenin Processing and Changes in Matrix Assembly during Enamel Development. Front. Physiol 2017, 8, 790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang PA; Lam RS; Beniash E Relationships between Dentin and Enamel Mineral at the Dentino-enamel Boundary: Electron Tomography and High-Resolution Transmission Electron Microscopy Study. Eur. J. Oral Sci 2011,119 Suppl 1,120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moradian-Oldak J; Goldberg M Amelogenin Supra-Molecular Assembly in vitro compared with the Architecture of the Forming Enamel Matrix. Cells Tissues Organs 2005, 181, 202–128. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Avila O; Wu S; Kim SJ; Cheng Y;Khan F; Samudrala R; Sali A; Horst JA; Habelitz S Self-Assembly of Filamentous Amelogenin Requires Calcium and Phosphate: From Dimers via Nanoribbons to Fibrils. Biomacromolecules 2012, 13, 3494–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan TX; Chow SK; Zhang D Biomorphic Mineralization: From Biology to Materials. Prog. Mater. Sci 2009, 54, 542–659. [Google Scholar]
- 13.De Yoreo JJ; Gilbert PU; Sommerdijk NA; Penn RL; Whitelam S; Joester D; Zhang H; Rimer JD; Navrotsky A; Banfield JF; Wallace AF; Michel FM; Meldrum FC; Cölfen H; Dove PM CRYSTAL GROWTH. Crystallization by Particle Attachment in Synthetic, Biogenic, and Geologic Environments. Science 2015, 349, aaa6760. [DOI] [PubMed] [Google Scholar]
- 14.Smeets PJM; Finney AR; Habraken WJEM; Nudelman F; Friedrich H; Laven J; De Yoreo JJ; Rodger PM; Sommerdijk NA J.M. A Classical View on Nonclassical Nucleation. PNAS 2017, 114, E7882–E7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandya M; Diekwisch TGH Enamel Biomimetics – Fiction or Future of Dentistry? Int. J. Oral Science 2019, 11:8; 10.1038/s41368-018-0038-6,1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie B; Halter TJ; Borah BM; Nancollas GH Tracking Amorphous Precursor Formation and Transformation during Induction Stages of Nucleation. J. Cryst. Growth Des 2014, 14, 1659–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uskokovic V; Tang S; Wu V On Grounds of the Memory Effect in Amorphous and Crystalline Apatite: Kinetics of Crystallization and Biological Response. ACS Appl. Mater. Interfaces 2018, 10, 14491–14508. [DOI] [PubMed] [Google Scholar]
- 18.Dey A; Bomans PHH; Müller FA; Will J; Frederik PM; de With G; Sommerdijk NAJM The Role of Prenucleation Clusters in Surface-Induced Calcium Phosphate Crystallization. Nat. Mater 2010, 9, 1010–1014. [DOI] [PubMed] [Google Scholar]
- 19.Robinson C; Kirkham J; Hallsworth AS Volume Distribution and Concentration of Protein Mineral and Water in Developing Dental Enamel. Arch. Oral Biol 1988, 33, 159–162. [DOI] [PubMed] [Google Scholar]
- 20.Cuisinier F; Bres EF; Hemmerle J; Voegel JC; Frank RM Transmission Electron Microscopy of Lattice Planes in Human Alveolar Bone Apatite Crystals. Calcif. Tissue Int 1987, 40, 332–328. [DOI] [PubMed] [Google Scholar]
- 21.Hong SI; Lee KH; Outslay ME; Kohn DH Ultrastructural Analyses of Ananoscale Apatite Biomimetically Grown on Organic Template. J. Mater. Res 2008, 23, 478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanmaekelbergh D; van Vugt LK; Bakker HE; Rabouw FT; de Nijs B; van Dijk-Moes RJA; van Huis MA;, Baesjou PJ; van Blaaderen A Shape-Dependent Multiexciton Emission and Whispering Gallery Modes in Supraparticles of CdSe/Multishell Quantum Dots. ACS Nano 2015, 9, 3942–3950. [DOI] [PubMed] [Google Scholar]
- 23.Zhang P; To AC Point Group Symmetry and Deformation-Induced Symmetry Breaking of Superlattice Materials. Proc. R. Soc. A 2015, 471, 20150125. [Google Scholar]
- 24.Weiner S; Addadi L Crystallization Pathways in Biomineralization. Ann. Rev. Mate. Res 2011, 41, 21–40. [Google Scholar]
- 25.Addadi L; Weiner S Biomineralization: Mineral Formation by Organisms. Phys. Scr 2014, 89, 098003. [Google Scholar]
- 26.Robinson C; Brookes SJ; Shore RC; Kirkham J The Developing Enamel Matrix: Nature and Function. Eur. J. Oral Sci 1998,106 Suppl 1, 282–291. [DOI] [PubMed] [Google Scholar]
- 27.Robinson C Self-Oriented Assembly of Nano-Apatite Particles: A Subunit Mechanism for Building Biological Mineral Crystals. J. Dent. Res 2007, 86, 677–679. [DOI] [PubMed] [Google Scholar]
- 28.Margolis HC; Kwak SY; Yamazaki H Role of Mineralization Inhibitors in the Regulation of Hard Tissue Biomineralization: Relevance to Initial Enamel Formation and Maturation. Front. Physiol 2014, 5, 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang PA; Lam RSK; Beniash E Relationships between Dentin and Enamel Mineral at the Dentino–Enamel Boundary: Electron Tomography and High-Resolution Transmission Electron Microscopy Study. Eur. J. Oral Sci 2011, 119(Suppl 1), 120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lupulescu AI; Rimer JD In situ Imaging of Silicalite-1 Surface Growth Reveals the Mechanism of Crystallization. Science 2014, 344, 729–732. [DOI] [PubMed] [Google Scholar]
- 31.Wang C; Qiao Q; Shokuhfar T; Klie RF High-Resolution Electron Microscopy and Spectroscopy of Ferritin in Biocompatible Graphene Liquid Cells and Graphene Sandwiches. Adv. Mater 2014, 26, 3410–3414. [DOI] [PubMed] [Google Scholar]
- 32.Beniash E; Simmer JP; Margolis HC The Effect of Recombinant Mouse Amelogenins on the Formation and Organization of Hydroxyapatite Crystals in vitro. J. Struct. Biol 2005, 149, 182–190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


