Abstract
Objective
Coronary computed tomography angiography (CCTA) is a noninvasive diagnostic modality that remains underutilized compared to functional stress testing (ST) for investigating coronary artery disease (CAD). Several patients are misdiagnosed with noncardiac chest pain (CP) that eventually die from a cardiovascular event in subsequent years. We compared CCTA to ST to investigate CP.
Methods
We searched MEDLINE, PubMed, Cochrane Library, and Embase from January 1, 2007 to July 1, 2018 for randomized controlled trials (RCTs) comparing CCTA to ST in patients who presented with acute or stable CP. We used Review Manager (RevMan) [Computer program] Version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) for review and analysis.
Results
We included 16 RCTs enrolling 21,210 patients; there were more patients with hyperlipidemia and older patients in the ST arm compared to the CCTA arm. There was no difference in mortality: 103 in the CCTA arm vs. 110 in the ST arm (risk ratio [RR] = 0.93, 95% confidence interval [CI] = 0.71-1.21, P = .58, and I2 = 0%). A significant reduction was seen in myocardial infarctions (MIs) after CCTA compared to ST: 115 vs. 156 (RR = 0.71, CI = 0.56-0.91, P < .006, I2=0%). On subgroup analysis, the CCTA arm had fewer MIs vs. the ST with imaging subgroup (RR = 0.70, CI = 0.54-0.89, P = .004, I2 = 0%) and stable CP subgroup (RR = 0.66, CI = 0.50-0.88, P = .004, I2 = 0%). The CCTA arm showed significantly higher invasive coronary angiograms and revascularizations and significantly reduced follow-up testing and recurrent hospital visits. A trend towards increased unstable anginas was seen in the CCTA arm.
Conclusions
Our analysis showed a significant reduction in downstream MIs, hospital visits, and follow-up testing when CCTA is used to investigate CAD with no difference in mortality.
Keywords: angina, computed tomography angiography, cardiac imaging, coronary cta
Introduction
Coronary heart disease is one of the leading causes of death, globally. Annually, more than 20 million patients undergo workup for angina [1]. Patients misdiagnosed with noncardiac chest pain (CP) have died from a cardiovascular event five years from the misdiagnosis [2]. Therefore, it is essential to identify patients at the highest risk of coronary artery disease (CAD) who may benefit from a workup using invasive coronary angiography (ICA) and subsequent revascularization. Coronary computed tomography angiography (CCTA) is 89% sensitive and 96% specific for the diagnosis of CAD, and CCTA is becoming an alternative to ICA due to its comparatively high diagnostic accuracy and noninvasive approach [3-5]. In fact, current cardiology guidelines recommend using CCTA to diagnose CAD [6].
Materials and methods
We conducted a systematic review and meta-analysis to compare CCTA to ST with subgroup analyses of ST (with and without imaging which has never been done before) and CP (acute chest pain [ACP] or stable chest pain [SCP]). Over the years, few meta-analyses comparing CCTA to ST have been published, and the outcomes are variable; these are summarized in Table 1 [7-11].
Table 1. Characteristics of previously published meta-analyses.
ACS, acute coronary syndrome; CAD, coronary artery disease; CCTA, coronary computed tomography angiography; CP, chest pain; ED, emergency department; FST, functional stress testing; ICA, invasive coronary angiography; MI, myocardial infarction; UC, usual care.
| Meta-analysis | Studies (n) | Participants (n) | Results | Conclusion |
| D'Ascenzo et al. 2013 [7] | 4 | 2,567 | Patients in the CCTA group were more likely to undergo coronary revascularization in the future. Time to diagnosis was reduced along with the reduced cost of care in the ED. | CCTA proved to be cost-effective in limited data along with a higher number of invasive coronary revascularization procedures. |
| Hulten et al. 2013 [8] | 4 | 3,266 | CCTA did not show any mortality benefits, increased incidence of MI, and or rehospitalization after ED discharge. However, CCTA decreased the length of ED stay and ED cost. CCTA was associated with increased ICA and coronary revascularization. | The use of CCTA decreased the length of ED stay as well as ED cost but increased the incidence of ICA and revascularization. |
| El-Hayek et al. 2014 [9] | 7 | 6,058 | CCTA reduced the risk of ACS and repeat ED visits in the future but with higher rates of revascularization procedures. There was no difference in ICA. | CCTA use in the ED for patients with low to intermediate risk of CAD reduces the risk of future ACS and subsequent ED visits for CP. |
| Bittencourt et al. 2016 [10] | 4 | 14,817 | Compared to UC, the CCTA showed a reduced annual rate for MI and cardiac CP but no difference in all-cause mortality. A higher rate of ICA and revascularization were also seen among patients undergoing CCTA. | Although CCTA reduced the rate of MI, it increased the rate of ICA and revascularization in patients with stable CAD. |
| Foy et al. 2017 [11] | 13 | 20,092 | Compared to FST, CCTA showed reduced incidence of MI but a higher incidence of ICA and revascularization. CCTA use also increased the number of new CAD diagnosis and new prescription of aspirin and statins. However, despite all this, no mortality difference was noted between CCTA and FST. | CCTA increases the incidence of new CAD diagnosis with a higher number of invasive coronary angiography and revascularization but reduces the risk of MI in the future. |
Data sources and searches
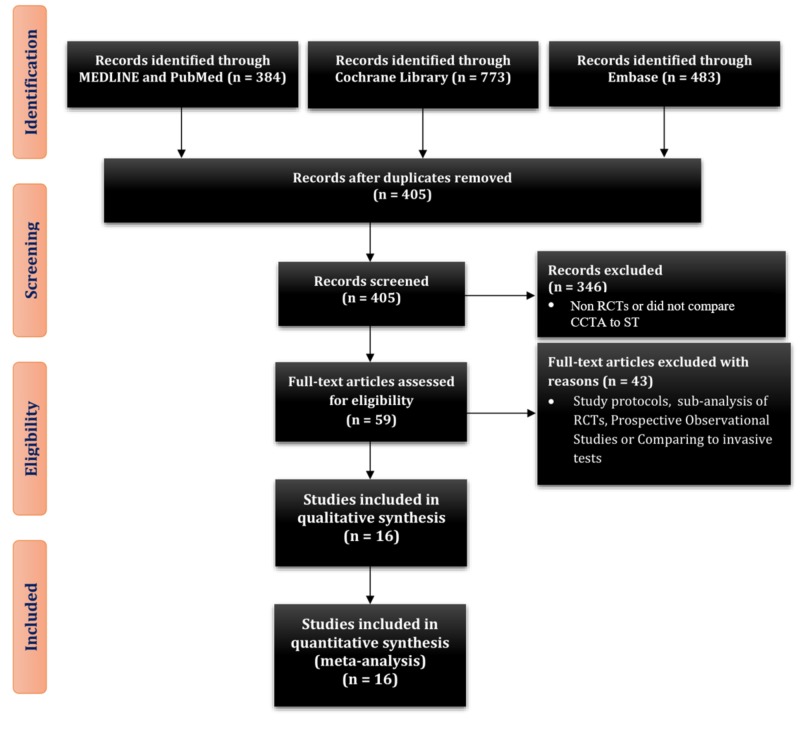
We completed a systematic review according to the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) guidelines [12]. We searched MEDLINE, PubMed, Cochrane Library, and Embase from January 1, 2007 to July 1, 2018 for RCTs, comparing CCTA to ST for suspected underlying CAD in patients who presented with CP. We combined search terms using the Boolean operator OR. Our search strategy included (Coronary Computed Tomography Angiography) OR (CCTA) OR (Coronary CTA) OR (Coronary CT Angiography). Due to the advancement in multislice CT technology, we only included studies performed after 2007. After duplicates were removed, a total of 405 studies were identified.
Study selection
Three reviewers (W.J.S., W.A., and M.S.R.) reviewed the abstracts and selected 59 articles for a full review. A total of 16 RCTs met the predefined inclusion criteria for qualitative and quantitative analysis comparing CCTA to ST: myocardial perfusion imaging or scan, stress electrocardiogram (bicycle or treadmill), stress echocardiogram, pharmacologic nuclear scan, graded exercise testing, and pharmacologic ST (Figure 1) [13-28].
Figure 1. PRISMA 2009 study flow diagram.
PRISMA, preferred reporting items for systematic reviews and meta-analyses; RCT, randomized control trial; CCTA, coronary computed tomography angiography; ST, stress testing.
Inclusion criteria
We used the following inclusion criteria: prospective RCTs, RCTs comparing CCTA to ST after CP, age ≥ 18 years, study population ≥ 50 patients, and follow-up ≥ four weeks.
Data extraction and quality assessment
W.J.S., M.S.R., and W.A. extracted data into predefined fields on a Microsoft Excel sheet for baseline characteristics and study outcomes. W.J.S. cross-checked the data and made the necessary corrections. All three reviewers discussed the revisions and agreed to the final entry.
Data synthesis and analysis
Statistical Method
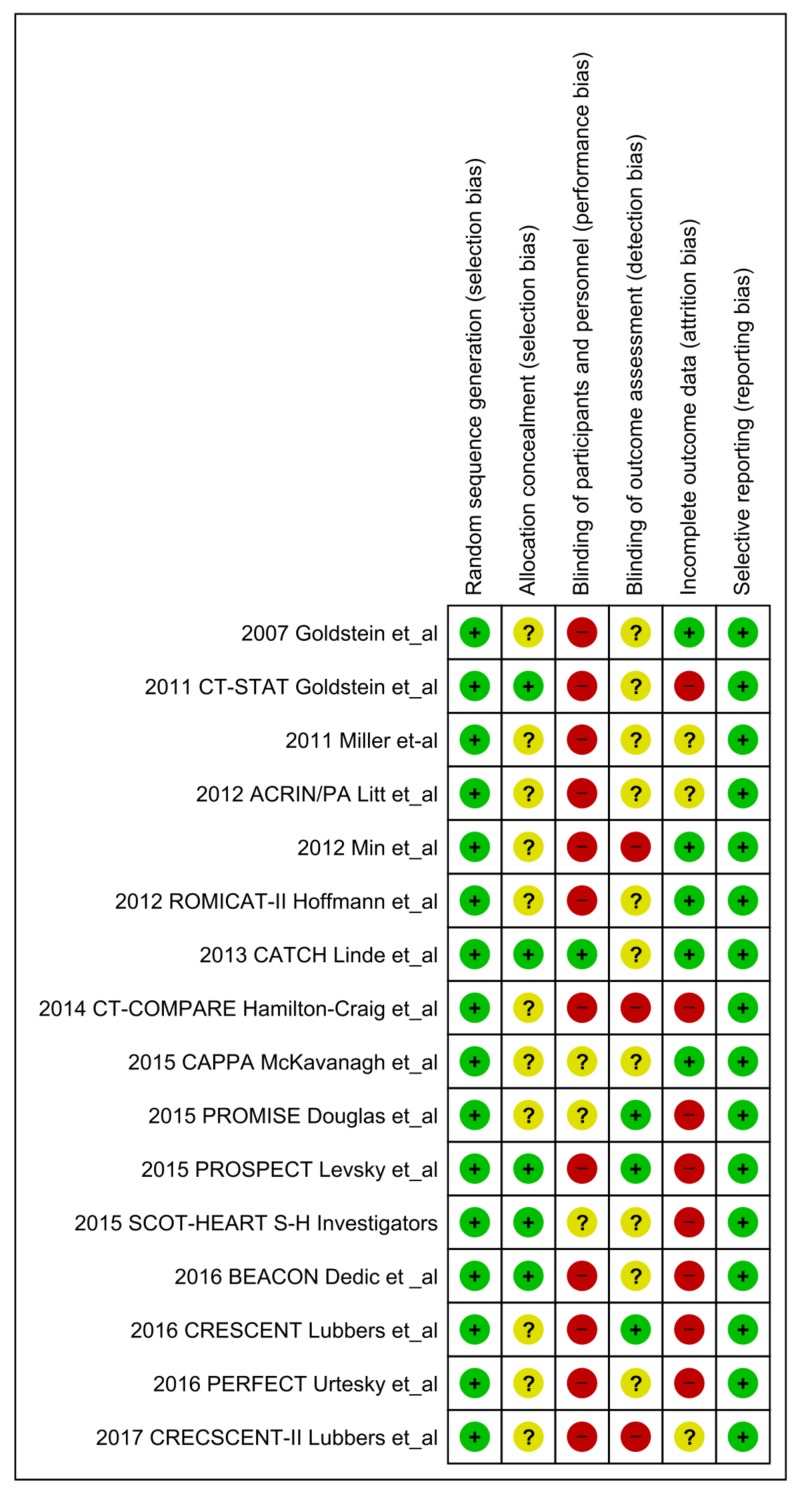
We used a random-effects model and Mantel-Haenszel method for dichotomous data to calculate the relative risk (RR) and odds ratio (OR), and inverse variance for the continuous data to estimate the standardized mean difference in Review Manager (RevMan) [Computer program] Version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). We reported results as forest plots. We used online GraphPad Online Version 8 (GraphPad Software, La Jolla, California, USA) to compare the baseline characteristics and to calculate the number needed to treat (NNT) to prevent one adverse event. A two-tailed P-value of < .05 was considered statistically significant. We assumed a 1:1 ratio in each arm except for the ACRIN/PA trial, which randomized patients in a 2:1 ratio where we used the same ratio for outcomes; this failed to uncover any event in both arms [19]. Baseline characteristics are summarized in Table 2 and Table 3 [13-28]. The salient features of each RCT are outlined in Table 4 [13-28]. We used the Cochrane Collaboration’s tool for the quality assessment of RCTs (Figure 2, Table 5) [13-28].
Table 2. Baseline characteristics.
BMI, body mass index; CCTA, coronary computed tomography angiography; DM, diabetes mellitus; HLD, hyperlipidemia; HTN, hypertension; N/A, not applicable; ST, stress testing.
| Intervention | n | Age | Male % | Female % | BMI (kg/m2) | HTN % | HLD % | DM % | Smoker % | Aspirin | |
| Goldstein et al. 2007 [15] | CCTA | 99 | 48±11 | 43 | 57 | 29±5 | 39 | 34 | 8.2 | 15 | 24 |
| ST | 98 | 51 ±12 | 57 | 43 | 29±5 | 38 | 38 | 12.2 | 20 | 29 | |
| CT-STAT Goldstein et al. 2011 [16] | CCTA | 361 | 50±10 | 45.2 | 54.8 | 28.1±4.7 | 35.5 | 31 | 5.5 | 25.2 | 24.9 |
| ST | 338 | 50±10 | 47 | 53 | 28.7±5.1 | 38.8 | 36.1 | 8.3 | 19.5 | 30.5 | |
| Miller et-al. 2011 [17] | CCTA | 30 | 51±10 | 43 | 57 | N/A | N/A | N/A | N/A | N/A | N/A |
| ST | 30 | 51±10 | 57 | 43 | N/A | N/A | N/A | N/A | N/A | N/A | |
| ACRIN Litt et al. 2012 [19] | CCTA | 908 | 49±9 | 49 | 51 | N/A | 51 | 27 | 14 | 32 | 22 |
| ST | 462 | 50±10 | 44 | 56 | N/A | 50 | 26 | 14 | 34 | 25 | |
| Min et al. 2012 [20] | CCTA | 91 | 55.9±10 | 58 | 42 | N/A | 62 | 53 | 23 | 58 | N/A |
| ST | 89 | 58.9±9.5 | 43 | 57 | N/A | 59 | 61 | 21 | 44 | N/A | |
| ROMICAT-II Hoffmann et al. 2012 [18] | CCTA | 501 | 54±8 | 52 | 48 | 29.4±5.3 | 54 | 46 | 17 | 50 | 23 |
| ST | 499 | 54±8 | 54 | 46 | 29.1±4.8 | 54 | 45 | 17 | 49 | 23 | |
| CATCH Linde et al. 2013 [21] | CCTA | 285 | 56.4±12.2 | 56.5 | 43.5 | 28 | 47.4 | 41.1 | 12.3 | 60.4 | N/A |
| ST | 291 | 54.9±12.2 | 57.7 | 42.3 | 28 | 36.4 | 34.7 | 10 | 67 | N/A | |
| CT-COMPARE Hamilton-Craig et al. 2014 [22] | CCTA | 322 | 52.2±10.7 | 59 | 41 | N/A | 31 | 25 | 7 | 24 | N/A |
| ST | 240 | 52.3±9.8 | 58 | 42 | N/A | 31 | 24 | 6 | 23 | N/A | |
| CAPPA McKavanagh et al. 2015 [26] | CCTA | 243 | 57.8±10.0 | 56.8 | 43.2 | 27.8±3.6 | 31.7 | N/A | 5.8 | 19% | N/A |
| ST | 245 | 58.9±10.2 | 53.5 | 46.5 | 28±3.6 | 29.8 | N/A | 4.9 | 19 | N/A | |
| PROMISE Douglas et al. 2015 [24] | CCTA | 4996 | 60.7±8.3 | 48.1 | 51.9 | 30.5±6.1 | 65 | 67.4 | 21.3 | 50.7 | 45.2 |
| ST | 5007 | 60.9±8.3 | 46.6 | 53.4 | 30.5±6.1 | 65 | 67.9 | 21.5 | 51.4 | 44.2 | |
| PROSPECT Levsky et al. 2015 [25] | CCTA | 200 | 56.8±11.8 | 37 | 63 | 30.5±6.2 | 70.5 | 49 | 33 | 17 | 39 |
| ST | 200 | 56.3±10.5 | 37.5 | 62.5 | 30.7±6.6 | 73.5 | 55 | 31 | 13 | 36 | |
| SCOT-HEART S-H Investigators 2015 [23] | CCTA | 2073 | 57.1±9.7 | N/A | N/A | 29.7±5.8 | 34 | 53 | 11 | 53 | 49 |
| ST | 2073 | 57.0±9.7 | N/A | N/A | 29.8±6 | 33 | 52 | 11 | 53 | 48 | |
| CRESCENT Lubbers et al. 2016 [14] | CCTA | 242 | 55±10 | 45 | 55 | 28±5 | 52 | 54 | 17 | 34 | 29 |
| ST | 108 | 55±10 | 44 | 56 | 28±5 | 52 | 61 | 16 | 36 | 29 | |
| BEACON Dedic et al. 2016 [27] | CCTA | 250 | 55±10 | 51 | 49 | N/A | 36 | 43 | 12 | 47 | 19 |
| ST | 250 | 53±9 | 55 | 45 | N/A | 35 | 45 | 13 | 40 | 14 | |
| PERFECT Uretsky et al. 2016 [28] | CCTA | 206 | 59 ±10 | 46 | 54 | N/A | 68 | 43 | 24 | 45 | 40 |
| ST | 205 | 60 ±10 | 47 | 53 | N/A | 69 | 53 | 33 | 46 | 44 | |
| CRECSCENT-II Lubbers et al. 2018 [13] | CCTA | 130 | 58±11 | 51 | 49 | 28±5 | 52 | 38 | 18 | 33 | N/A |
| ST | 138 | 58±11 | 44 | 56 | 28±5 | 52 | 40 | 18 | 42 | N/A |
Table 3. Comparing baseline characteristics.
BMI, body mass index; CCTA, coronary computed tomography angiography; DM, diabetes mellitus; HLD, hyperlipidemia; HTN, hypertension; N/A, not applicable; ST, stress testing.
| Intervention | CCTA | ST | Mean Difference | 95% Confidence Interval | P-value |
| n | 10,937 | 10,273 | |||
| Age | 57.4±10 | 58±9.8 | -0.600 | -0.867 to -0.333 | < .001 |
| BMI (kg/m2) | 30±5.9 (8,845) | 30.1±5.9 (8706) | -0.1 | -0.275 to 0.075 | .26 |
| Male % (n/total) | 49.4 (4,379/8,864) | 49.7 (4,075/8,200) | N/A | N/A | .71 |
| Female % (n/total) | 50.6 (4485/8864) | 50.3 (4,125/8,200) | N/A | N/A | .71 |
| HTN % (n/total) | 48.6 (5301/10,907) | 47.8 (4,896/10,243) | N/A | N/A | .2482 |
| HLD % (n/total) | 43.2 (4,607/10,664) | 45.6 (4,559/9,998) | N/A | N/A | .0006 |
| DM % (n/total) | 15.3 (1,669/10,907) | 15.8 (1,618/10,243) | N/A | N/A | .3310 |
| Smoker % (n/total) | 37.5 (4,090/10,907) | 37.1 (3,800/10,243) | N/A | N/A | .9070 |
| Aspirin % (n/total) | 31.5 (3,127/9,927) | 32.3 (3013/9,329) | N/A | N/A | .2417 |
Table 4. Characteristics of randomized control trials.
~ Traditional Care = Graded exercise testing/Pharmacologic stress testing
* Stress Test = Stress Echocardiography/MPI
# Functional testing = Exercise ECG, Exercise or Pharmacologic Nuclear Stress Testing, and Stress Echocardiography
Ѱ SOC = Standard Optimal Care
CCT, cardiac computerized tomography; CCTA, coronary computed tomography angiography; ECG, electrocardiography; EST, exercise stress electrocardiography test; F/u, follow up; JACC, Journal of American College of Cardiology; JCCT, Journal of Cardiovascular Computed Tomography; MPI, myocardial perfusion imaging; MPS, myocardial perfusion scan; MSCT, multi-slice computed tomographic angiography; NEJM, New England Journal of Medicine; NSTE-ACS, non-ST elevated acute coronary syndrome; RCT, randomized control trial; SC, standard care; SE, standard evaluation; SOC, standard of care; w/, with.
| Name | Design | Country | Publication Year | Journal | Enrollment | Population | Setting | Intervention vs Comparison | F/u Duration | CT Scanners |
| Goldstein et al. 2007 [15] | RCT | United States | 2007 | JACC | March 2005 – September 2005 | Acute chest pain | Emergency Department | MSCT vs rest-stress MPI | 6 months | 64-slice MSCT scanner (Sensation 64 Cardiac, Siemens Medical Systems, Forchheim, Germany) |
| CT-STAT Goldstein et al. 2011 [16] | Multicenter, comparative effectiveness RCT | United States | 2011 | JACC | June 2007 –November 2008 | Acute Chest pain | Emergency Department | CCTA vs rest-stress MPI | 6 months | 64-slice MSCT scanner (Sensation 64 Cardiac, Siemens Medical Systems, Forchheim, Germany) |
| Miller et al. 2011 [17] | Single-center RCT | United States | 2011 | Academic Emergency Medicine | October 20, 2008 – February 02, 2009 | Acute chest pain | Emergency Department | SC+CCTA vs SC | 3 months | 64-slice multidetector CT scanner (Toshiba America Medical Systems, Inc., Tustin, CA) |
| ACRIN/PA Litt et al. 2012 [19] | Multicenter RCT | United States | 2012 | NEJM | July 07, 2009 –November 03, 2011 | Acute Chest pain | Emergency Department | CCTA vs Traditional care~ | 1 month | 64-slice or greater multidetector CT scanner |
| Min et al. 2012 [20] | Multicenter (2 centers) RCT | United States | 2012 | JCCT | December 2008 – June 2009 | Stable chest pain | Outpatient | CCTA vs. MPS | 2 months | 64-detector row CT scanner (Lightspeed VCT; GE Healthcare, Milwaukee, WI) |
| ROMICAT-II Hoffmann et al. 2012 [18] | Multicenter RCT | United States | 2012 | NEJM | April 23, 2010 –January 30, 2012 | Acute chest pain | Emergency Department | CCTA vs. SE | 28 Days | 64-slice CT technology |
| CATCH Linde et al. 2013 [21] | Single-center RCT | Denmark | 2013 | International Journal of Cardiology | January 2010 –January 2013 | Acute chest pain | Hospitalized w/ suspicion of NSTE-ACS, d/c within 24 hours | CCTA vs. Bicycle exercise-ECG and/or MPI | 4 months | 320 multidetector scanner (Aquilion One, Toshiba Medical systems) |
| CT-COMPARE Hamilton-Craig et al. 2014 [22] | Single-center RCT | Australia | 2014 | International Journal of Cardiology | March 2010 –April 2011 | Acute chest pain | Emergency Department | CCTA vs Exercise ECG | 12 months | (Somaton Definition 64 detector, or Definition Flash 128-detector; Siemens, Erlangen, Germany) |
| CAPPA McKavanagh et al. 2015 [26] | Single-center RCT | Ireland | 2015 | European Heart Journal | September 2010 – November 2011 | Stable chest pain | Outpatient | CCT vs. EST | 12 months | 64-detector platform (Philips Brilliance 64 Cleveland, Ohio, USA) |
| PROMISE Douglas et al. 2015 [24] | Multicenter, comparative effectiveness RCT | United States | 2015 | NEJM | July 27, 2010 – September 19, 2013 | Stable chest pain | Outpatient | CCTA vs. Functional testing# | 25 months | 64-slice or greater multidetector CT scanner |
| PROSPECT Levsky et al. 2015 [25] | Single-center, comparative effectiveness RCT | United States | 2015 | Annals of Internal Medicine | July 2008 – March 2012 | Acute chest pain | Telemetry Inpatient Ward | CCTA vs. MPI | 12 months | 64 –detector-row scanners |
| SCOT-HEART S-H Investigators 2015 [23] | Open-label, parallel-group Multicenter RCT | Scotland | 2015 | Lancet | November 18, 2010 – September 24, 2014 | Stable chest pain | Outpatient | CCTA + SOC vs SOC | 20 months (1.7 Years) | 64-row scanners (Brilliance 64, Philips Medical Systems, Biograph mCT Siemens) and 320 detector row scanners (Aquilion ONE, Toshiba Medical Systems) |
| CRESCENT Lubbers et al. 2016 [14] | Multicenter RCT | Netherland | 2016 | European Heart Journal | April 2011 – July 2013 | Stable chest pain | Outpatient | CCT vs. Functional testing | 12 months | 64-slice or more advanced CT technology, with radiation minimizing measures |
| BEACON Dedic et al. 2016 [27] | Multicenter, Prospective, open-label, RCT | Netherland | 2016 | JACC | July 11, 2011 - January 30, 2014 | Acute chest pain | Emergency Department | CCTA vs. SOCѰ | 30 days | 64-slice or more advanced CT technology, using ECG-synchronized axial or spiral scan protocols |
| PERFECT Uretsky et al. 2016 [28] | Single-center, comparative effectiveness RCT | United States | 2016 | Journal of Nuclear Cardiology | July 2011 – December 2013 | Acute chest pain | Inpatient | CCTA vs. Stress Test * | 12 months | (Toshiba Aquilion 64-detector Toshiba America Medical Systems, Tustin, CA, or Siemens Somatoform Sensation 64-detector, Siemens Medical Solutions USA, Malvern, PA). |
| CRESCENT-II Lubbers et al. 2017 [13] | Multicenter RCT | Netherland | 2017 | JACC | July 2013 – November 2015 | Stable Angina | Outpatient | CCT vs. Functional testing | 6 months | Somatom Definition Flash and Force Siemens Healthineers, Forchheim, Germany |
Figure 2. Cochrane Collaboration’s tool for the quality assessment of randomized controlled trials .
Table 5. Cochrane risk of bias for quality assessment.
CTA, computed tomography angiogram.
| Name | Random Sequence | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Reporting Bias |
| Goldstein et al. 2007 [15] | Yes via SAS software version 9.1 | Not reported | No | Not reported | No | Low risk |
| Low risk | Unclear | High risk | Unclear | Low risk | ||
| CT-STAT Goldstein et al. 2011 [16] | 1:1 ratio, alternating block design | Randomization envelopes | No | Not reported | Yes | Low risk |
| Low risk | Low risk | High risk | Unclear | High risk | ||
| Miller et al. 2011 [17] | 1:1 ratio in an open-label fashion | Not reported | No | Not reported | Not reported | Low risk |
| Low risk | Unclear | High risk | Unclear | Unclear | ||
| ACRIN/PA Litt et al. 2012 [19] | Computer-based randomization, 2:1 ratio | Not reported | No | Not reported | Not reported | Low risk |
| Low risk | Unclear | High Risk | Unclear | Unclear | ||
| Min et al. 2012 [20] | 1:1 ratio, simple randomization stratified by site | Not reported | No | No | No | Low Risk |
| Low risk | Unclear | High risk | High risk | Low risk | ||
| ROMICAT-II Hoffmann et al. 2012 [18] | 1:1 ratio in the emergency department | Not reported | No | Not reported | No | Low risk |
| Low risk | Unclear | High risk | Unclear | Low risk | ||
| CATCH Linde et al. 2013 [21] | Computer-based block randomization, in a 1:1 ratio | Yes | Yes until tests were performed | Not reported | No | Low risk |
| Low risk | Low risk | Low risk | Unclear | Low risk | ||
| CT-COMPARE Hamilton-Craig et al. 2014 [22] | Computer-generated random sequence | Not reported | No | No | Yes | Low risk |
| Low risk | Unclear | High risk | High risk | High risk | ||
| CAPPA McKavanagh et al. 2015 [26] | Permuted block randomization at the clinic | Not reported | Not reported | Not reported | No | Low risk |
| Low risk | Unclear | Unclear | Unclear | Low risk | ||
| PROMISE Douglas et al. 2015 [24] | Yes | Not reported | Not reported | Independent clinical-events committee | Yes | Low risk |
| Low risk | Unclear | Unclear | Low risk | High risk | ||
| PROSPECT Levsky et al. 2015 [25] | SAS software-generated, blocked, 1:1 randomization | Sequentially numbered, sealed, opaque envelopes | No | Yes | Yes | Low risk |
| Low risk | Low risk | High risk | Low risk | High risk | ||
| SCOT-HEART S-H Investigators 2015 [23] | Web-based randomization in a 1:1 ratio | Yes | Not reported | Not reported | Yes | Low risk |
| Low risk | Low risk | Unclear | Unclear | High risk | ||
| CRESCENT Lubbers et al. 2016 [14] | Randomization in 2:1 ratio to CTA or functional testing | Not reported | No | Yes | Yes | Low risk |
| Low Risk | Unclear | High risk | Low risk | High risk | ||
| BEACON Dedic et al. 2016 [27] | 1:1 computer-generated block randomization | Sealed, sequentially numbered, opaque envelopes | No | Not reported | Yes | Low risk |
| Low risk | Low risk | High risk | Unclear | High risk | ||
| PERFECT Uretsky et al. 2016 [28] | Method of randomization not reported | Not reported | No | Not reported | Yes | Low risk |
| Low risk | Unclear | High risk | Unclear | High risk | ||
| CRESCENT-II Lubbers et al. 2017 [13] | Method of randomization not reported | Not reported | No | No | Not reported | Low risk |
| Low risk | Unclear | High risk | High risk | Unclear |
Heterogeneity
We used I2 statistics to calculate the heterogeneity. I2 > 50% was considered substantial heterogeneity, as explained in the Cochrane Handbook for Systematic Reviews [29]. We performed a sensitivity analysis for considerable heterogeneity.
Results
We included 16 RCTs with 21,210 patients (10,937 in the CCTA arm and 10,273 in the ST arm). Patients in the ST arm were older than those in the CCTA arm (57.9 ± 9.8 years vs. 57.4 ± 10 years, respectively; P = .0002) and had more hyperlipidemia (45.62% vs. 43.18%, respectively; P = .0004). There was no difference in baseline body mass index, hypertension, diabetes, smoking status, and baseline use of aspirin. Three studies used ST without imaging for a total of 1,110 patients (595 in the CCTA arm and 515 in the ST without imaging arm) [17,22,26].
Primary endpoints were all-cause mortality and new myocardial infarction (MI) during the follow-up period. Secondary endpoints included ICA after ST, true positive ICA, revascularizations, new unstable anginas, emergency room (ER) visits or hospital admissions during the follow-up period, follow-up tests, complications (stroke, bleeding, anaphylaxis, or renal failure) attributed to CCTA compared to ST, direct discharges from ER, ER cost and total cost, and radiation dose. The results are summarized in Table 6.
Table 6. Outcomes.
* Procedural complications include stroke, bleeding, anaphylaxis, or renal failure
Abbreviations: ER, emergency room; ICA, invasive coronary angiography; ST, stress testing.
| Outcome | CCTA | ST | Effect Estimate | Confidence Interval | P-value | I2 |
| Primary Outcomes | ||||||
| All-Cause Mortality | 103 | 110 | 0.93 | 0.71-1.21 | .58 | 0% |
| ST with Imaging | 100 | 108 | 0.92 | 0.70-1.21 | .55 | 0% |
| ST without Imaging | 3 | 2 | 1.26 | 0.21-7.71 | .8 | 0% |
| All-Cause Mortality | 103 | 110 | 0.93 | 0.71-1.21 | .58 | 0% |
| Acute Chest Pain | 9 | 12 | 0.75 | 0.30-1.89 | .54 | 0% |
| Stable Chest Pain | 103 | 110 | 0.95 | 0.71-1.25 | .7 | 0% |
| New Myocardial Infarction | 115 | 156 | 0.71 | 0.56-0.91 | .006 | 0% |
| ST with Imaging | 108 | 151 | 0.7 | 0.54-0.89 | .004 | 0% |
| ST without Imaging | 7 | 5 | 1.14 | 0.35-3.75 | .83 | 0% |
| New Myocardial Infarction | 115 | 156 | 0.71 | 0.56-0.91 | .006 | 0% |
| Acute Chest Pain | 35 | 36 | 0.88 | 0.54-1.44 | .61 | 0% |
| Stable Chest Pain | 80 | 20 | 0.66 | 0.5-0.88 | .004 | 0% |
| Secondary Outcomes | ||||||
| Cumulative ICA | 1,044 | 701 | 1.41 | 1.28-1.55 | < .00001 | 1% |
| ST with Imaging | 948 | 637 | 1.37 | 1.21-1.55 | < .00001 | 11% |
| ST without Imaging | 96 | 64 | 1.39 | 1.04-1.85 | .02 | 0% |
| Cumulative ICA | 1,044 | 701 | 1.41 | 1.28-1.55 | < .00001 | 1% |
| Acute Chest Pain | 311 | 205 | 1.35 | 1.13-1.62 | .001 | 8% |
| Stable Chest Pain | 733 | 496 | 1.44 | 1.30-1.61 | < .00001 | 0% |
| True Positive ICA | 629 | 270 | 2.85 | 2.28-3.56 | < .00001 | 0% |
| ST with Imaging | 565 | 246 | 2.84 | 2.25-3.59 | < .00001 | 0% |
| ST without Imaging | 64 | 24 | 4.67 | 1.15-18.91 | .03 | 48% |
| True Positive ICA | 629 | 270 | 2.85 | 2.28-3.56 | < .00001 | 0% |
| Acute Chest Pain | 117 | 41 | 3.2 | 1.83-5.60 | < .001 | 0% |
| Stable Chest Pain | 512 | 229 | 2.79 | 2.19-3.55 | < .00001 | 0% |
| Cumulative Revascularization | 789 | 472 | 1.84 | 1.44-2.35 | < .00001 | 53% |
| ST with Imaging | 737 | 450 | 1.77 | 1.34-2.33 | < .0001 | 60% |
| ST without Imaging | 52 | 22 | 2.36 | 1.40-3.98 | .001 | 0% |
| Cumulative Revascularization | 789 | 472 | 1.84 | 1.44-2.35 | < .00001 | 53% |
| Acute Chest Pain | 175 | 82 | 1.95 | 1.42-2.69 | < .0001 | 17% |
| Stable Chest Pain | 614 | 390 | 1.7 | 1.16-2.51 | .007 | 77% |
| New Unstable Anginas | 257 | 198 | 1.18 | 0.99-1.41 | .06 | 0% |
| ST with Imaging | 245 | 191 | 1.18 | 0.98-1.40 | .07 | 0% |
| ST without Imaging | 12 | 7 | 1.09 | 0.20-5.92 | .92 | 49% |
| New Unstable Anginas | 257 | 198 | 1.18 | 0.99-1.41 | .06 | 0% |
| Acute Chest Pain | 118 | 84 | 1.15 | 0.90-1.48 | .27 | 0% |
| Stable Chest Pain | 139 | 114 | 1.21 | 0.93-1.58 | .15 | 4% |
| ER visits or hospital admissions | 570 | 616 | 0.75 | 0.60-0.94 | .01 | 63% |
| ST with Imaging | 554 | 551 | 0.92 | 0.83-1.02 | .11 | 0% |
| ST without Imaging | 16 | 65 | 0.27 | 0.15-0.48 | < .0001 | 27% |
| ER visits or hospital admissions | 570 | 616 | 0.75 | 0.60-0.94 | .01 | 63% |
| Acute Chest Pain | 300 | 289 | 0.86 | 0.72-1.04 | .11 | 22% |
| Stable Chest Pain | 270 | 327 | 0.5 | 0.21-1.23 | .13 | 86% |
| Cumulative Follow up Testing | 242 | 342 | 0.45 | 0.22-0.90 | .02 | 86% |
| ST with Imaging | 159 | 197 | 0.43 | 0.16-1.14 | .09 | 86% |
| ST without Imaging | 83 | 145 | 0.39 | 0.28-0.56 | < .00001 | 0% |
| Cumulative Follow up Testing | 242 | 342 | 0.45 | 0.22-0.90 | .02 | 86% |
| Acute Chest Pain | 166 | 165 | 0.83 | 0.44-1.55 | .56 | 70% |
| Stable Chest Pain | 76 | 177 | 0.17 | 0.04-0.77 | .02 | 80% |
| Procedural Complications* | 7 | 7 | 0.98 | 0.35-2.74 | .96 | 0% |
| Direct ER Discharges | 936 | 421 | 1.45 | 0.63-3.30 | .38 | 94% |
| Cost in ER | - | - | -4.68 | (-10.38) - (1.01) | .11 | 100% |
| Total Downstream Cost | - | - | -0.01 | (-0.17) - (0.14) | .85 | 45% |
| Cumulative Radiation Dose | 7.3±6.6 | 2.6±6.5 | 0.47 | 0.08-0.86 | .02 | 97% |
Primary Endpoints
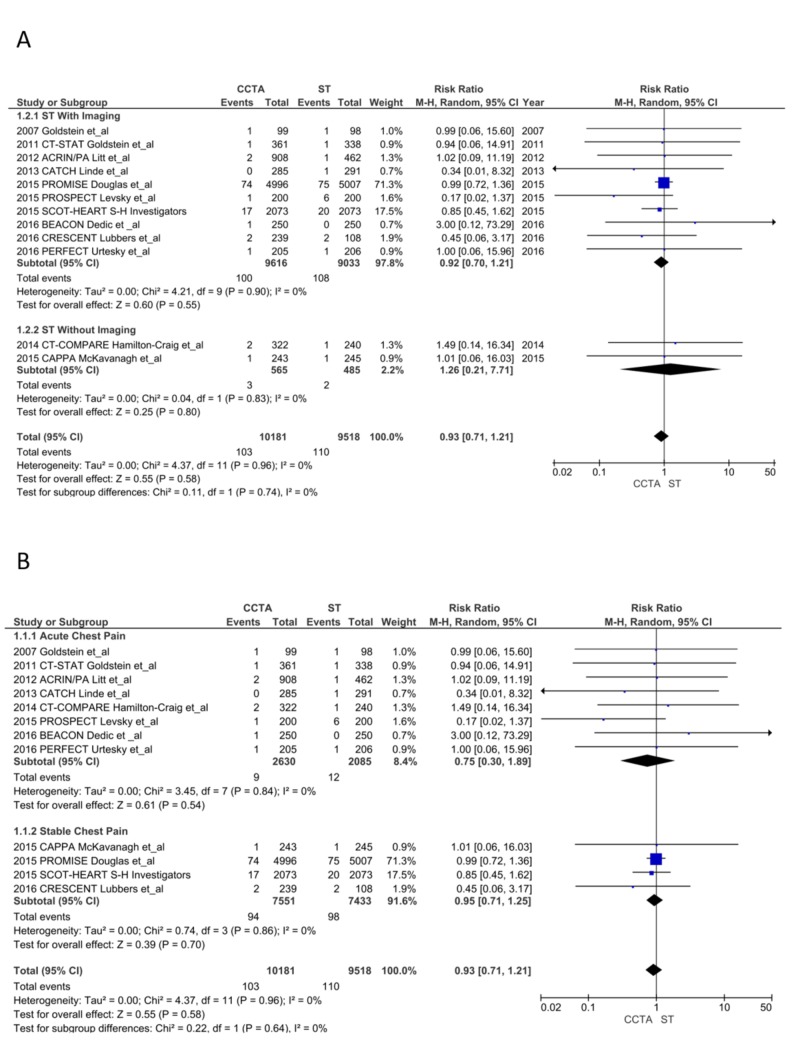
All-cause mortality: There was no difference in all-cause mortality (103 vs. 110; RR = 0.93, CI = 0.71-1.21; P = .58, I2 = 0%). The subgroup analyses for ST with imaging (RR = 0.92, CI = 0.70-1.21; P = .55, I2 = 0%), ST without imaging (RR = 1.26, CI = 0.21-7.71; P = .80, I2 = 0%), ACP (RR = 0.75, CI = 0.30-1.89; P = .54, I2 = 0%) and SCP (RR = 0.95, CI = 0.71-1.25; P = .70, I2 = 0%) found no differences (Figure 3A and 3B) [13-28].
Figure 3. All-cause mortality.
CCTA, coronary computed tomography angiography; ST, stress testing.
A. ST with imaging vs. ST without imaging [13-28]
B. Acute chest pain (ACP) vs. stable chest pain (SCP) [13-28]
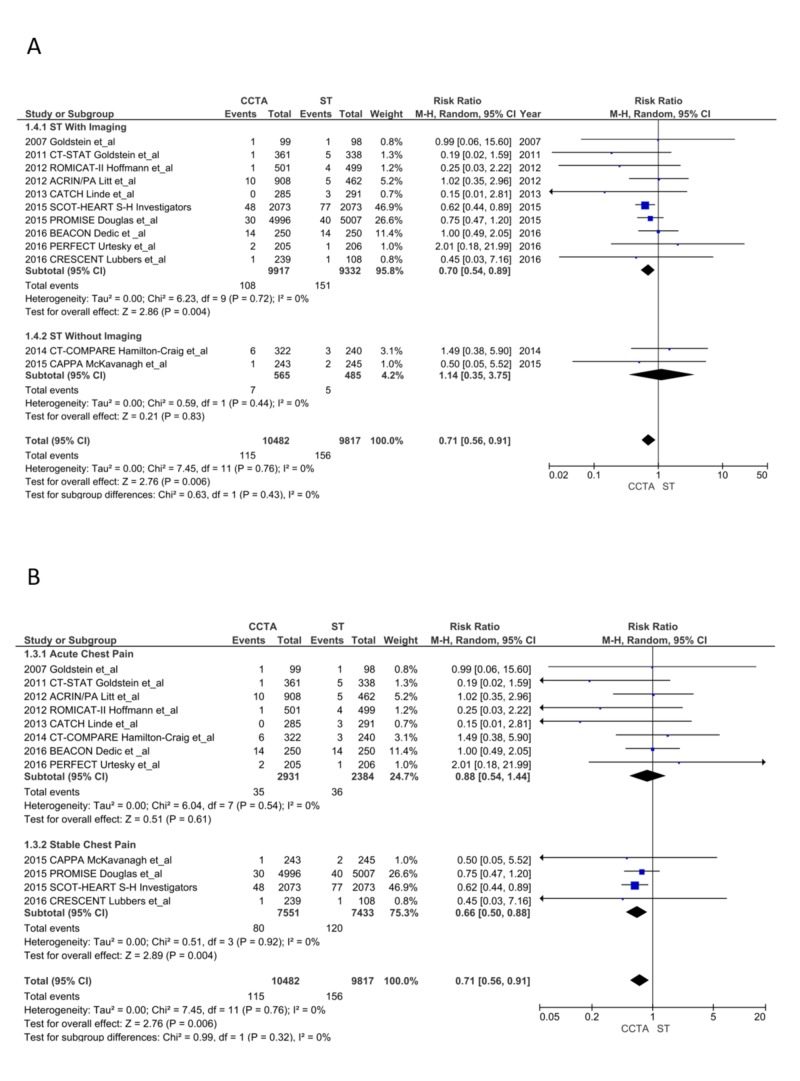
New MI during follow-up period: A significant reduction in the incidence of future MI was noticed in the CCTA arm (115 vs. 156; RR = 0.71, CI = 0.56-0.91; P < .006, I2 = 0%); this was mainly noted as a reduction in MI in the SCP subgroup patients (80 vs.120; RR = 0.66, CI = 0.50-0.88; P = .004, I2 = 0%) compared to the ACP subgroup that showed no difference (35 vs. 36; RR = 0.88, CI = 0.54-1.44; P = .61, I2 = 0%). The CCTA arm also had significantly reduced MIs compared to ST with imaging (RR = 0.70, CI = 0.54-0.89; P = .004, I2 = 0%) with no difference compared to ST without imaging (RR = 1.14, CI = 0.35-3.75; P = .83, I2 = 0%; Figure 4A and 4B) [13-28]. The NNT after CCTA to prevent one MI was 204 and NNT after ICA to prevent one MI was nine.
Figure 4. New myocardial infarction during the follow-up period.
CCTA, coronary computed tomography angiography; ST, stress testing; CI, confidence interval.
Secondary Endpoints
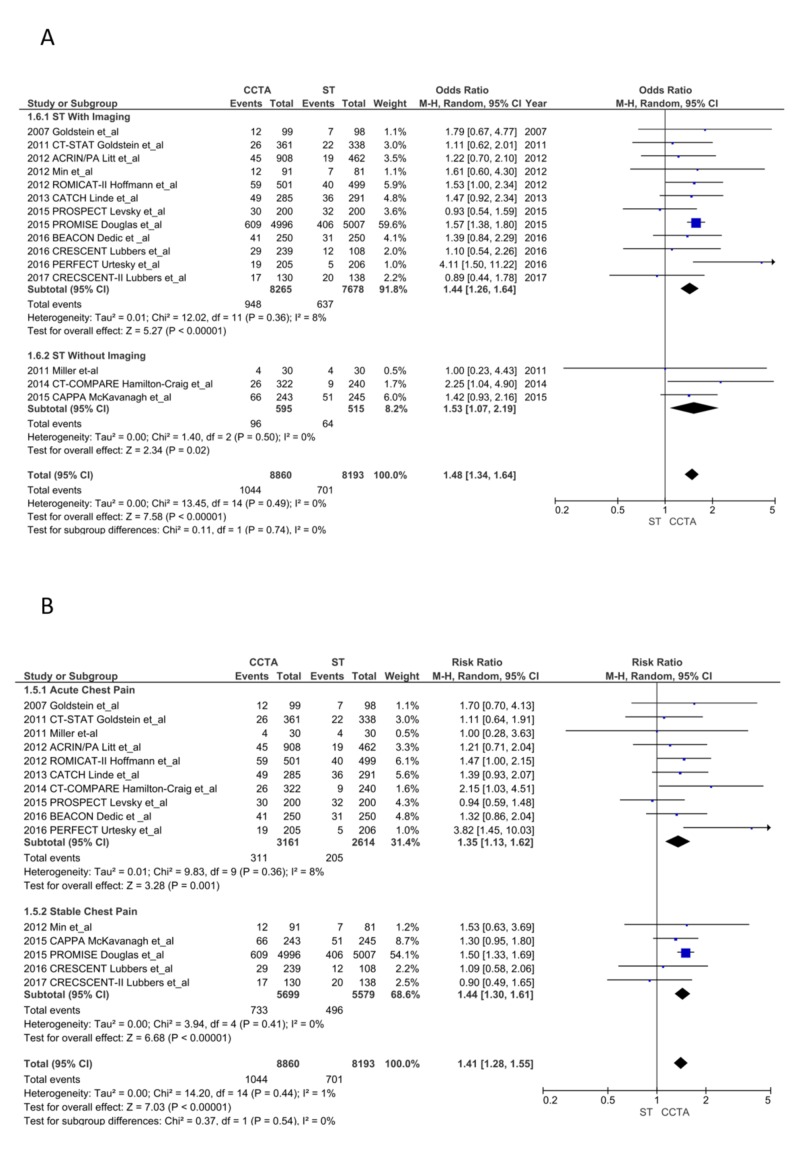
ICA after ST: The CCTA arm had significantly increased ICA (1,044 vs. 701; RR = 1.41, CI = 1.28-1.55; P < .00001, I2 = 1%). Both the ACP (311 vs. 205; RR = 1.35, CI = 1.13-1.62; P = .001, I2 = 8%) and SCP (733 vs. 496; RR = 1.44, CI = 1.30-1.61; P < .00001, I2 = 0%) subgroups had more ICA post-CCTA. ICA was common after CCTA compared to ST with imaging (RR = 1.37, CI = 1.21-1.55; P < .00001, I2 = 11%) and without imaging (RR = 1.37, CI = 1.21-1.55; P < .00001, I2 = 11%; Figure 5A and 5B) [13-28]. We did not include ICA from the SCOT-HEART study as they only reported new or canceled ICA in their manuscript and appendix [23].
Figure 5. Invasive coronary angiograms.
CCTA, coronary computed tomography angiography; ST, stress testing.
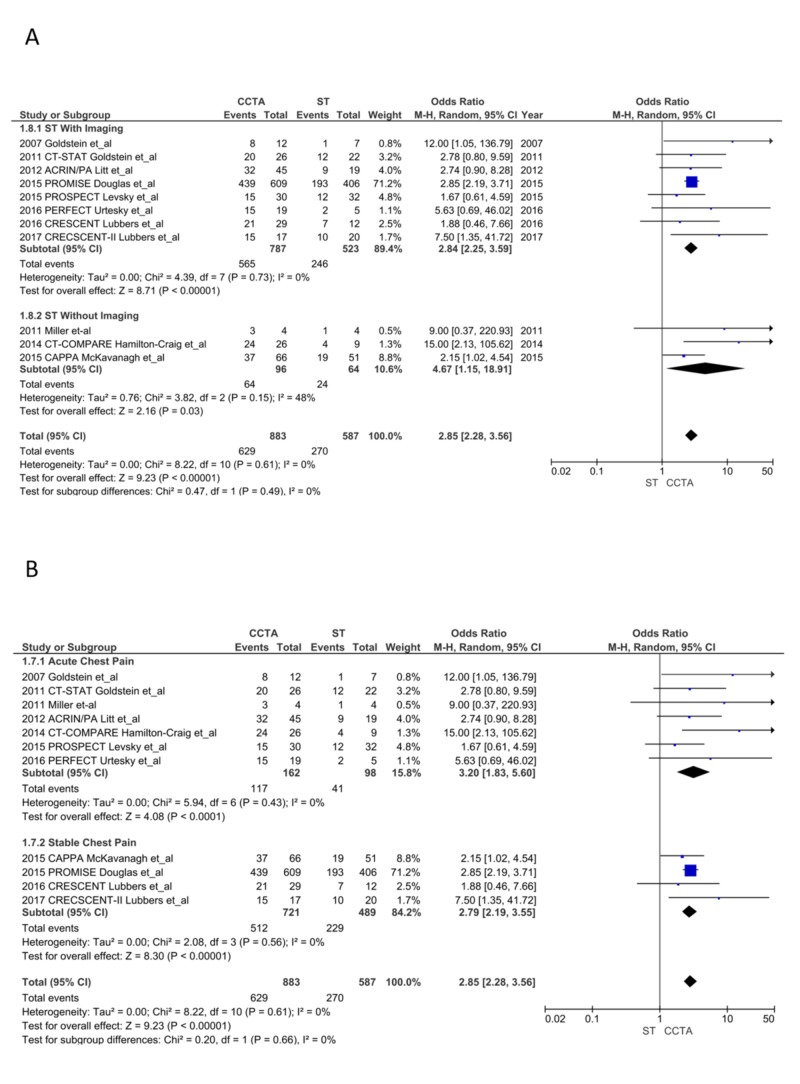
True positive ICA: CCTA lead to a significantly higher diagnosis of obstructive CAD (stenosis ≥ 50%) compared to ST (629/883 after CCTA vs. 270/587 after ST; OR = 2.85, CI = 2.28-3.56; P < 0.00001, I2 = 0%). This finding was consistent in both the ACP (OR = 3.20, CI = 1.83-5.60; P < .001, I2 = 0%) and SCP (OR = 2.79, CI = 2.19-3.55; P < .00001, I2 = 0%) subgroups and in ST with imaging (OR = 2.84, CI = 2.25-3.59; P < .00001, I2 = 0%) and without imaging (OR = 4.67, CI = 1.15-18.91; P = .03, I2 = 48%; Figure 6A and 6B) [13-28].
Figure 6. True positive invasive coronary angiograms.
CCTA, coronary computed tomography angiography; ST, stress testing.
Revascularization: Revascularization (percutaneous coronary intervention and coronary artery bypass grafting) was significantly higher after CCTA (789 vs. 472; OR = 1.84, CI = 1.44-2.35; P < .00001, I2 = 53%). Of note, I2 was reduced to 0% with exclusion of the SCOT-HEART trial and without affecting significance [24]. This trend was consistent on subgroup analysis of ST with imaging (RR = 1.77, CI = 1.34-2.33; P < .00001, I2 = 60%), ST without imaging (RR = 2.36, CI = 1.40-3.98; P = .001, I2 = 0%), ACP (175 vs. 82; OR = 1.95, CI = 1.42-2.69; P < .0001, I2 = 17%), and SCP (614 vs. 390; OR = 1.70, CI = 1.16-2.51; P = .007, I2 = 77% and 0% without inclusion of the SCOT-HEART trial [23].
New unstable angina: There was no difference in new unstable anginas in the CCTA group vs. ST group (257 vs. 198; RR = 1.18, CI = 0.99-1.41; P = .06, I2 = 0%). A similar trend was seen on subgroup analysis of ACP (118 vs. 84; RR = 1.15, CI = 0.90-1.48; P = .27, I2 = 0%), SCP (139 vs. 114; RR = 1.21, CI = 0.93-1.58; P = .15, I2 = 4%), ST with imaging (RR = 1.18, CI = 0.98-1.40; P = .07, I2 = 0%) and ST without imaging, (RR = 1.09, CI = 0.20-5.92; P = .92, I2 = 49%).
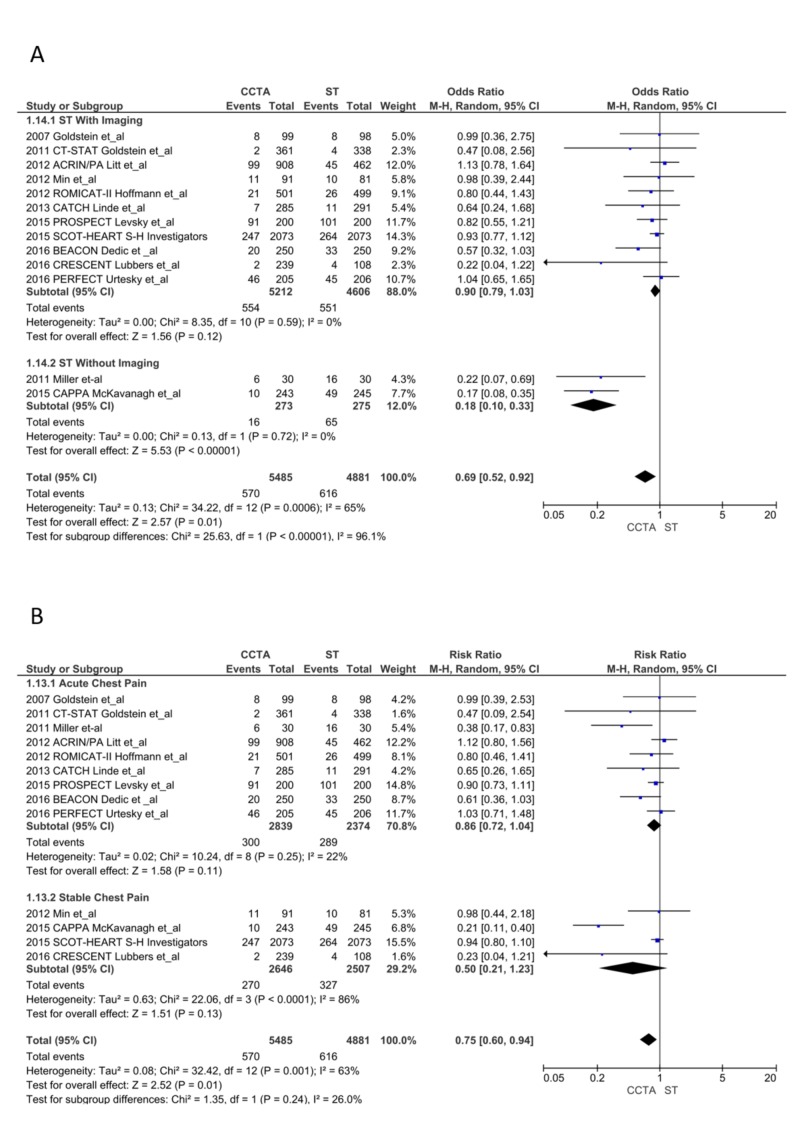
ER visits and/or hospital admissions during the follow-up period: ER visits and/or hospital admissions were reduced significantly in the CCTA arm (570 vs. 616; RR = 0.75, CI = 0.60-0.94; P = .01, I2 = 63%). I2 was reduced to 16% without the CAPPA trial, but the results became statistically insignificant. The subgroup analysis of ACP and SCP and ST with imaging revealed no difference between CCTA and ST, though there were significantly reduced ER visits or hospital admissions in the CCTA arm compared to ST without imaging (RR = 0.27, CI = 0.15-0.48; P < .0001; I2 = 27%; Figure 7A and 7B) [13-28].
Figure 7. Emergency room visits or hospital admissions during the follow-up period.
CCTA, coronary computed tomography angiography; ST, stress testing.
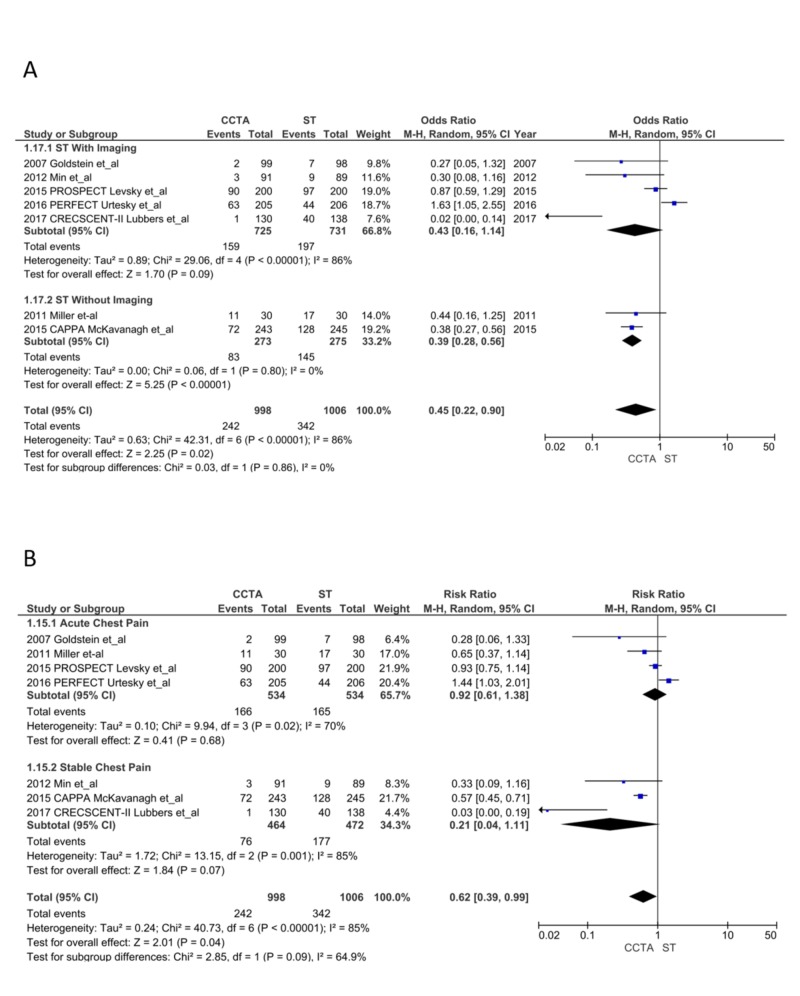
Follow-up tests: Patients in the CCTA arm had a significant reduction in downstream testing (242 vs. 342; OR = 0.45, CI = 0.22-0.90; P = .02, I2 = 86%); sensitivity analysis did not reduce the heterogeneity. The ST with imaging subgroup (RR = 0.43, CI = 0.16-1.14; P = .09, I2 = 86%) and ACP subgroup (RR = 0.83, CI = 0.44-1.55; P = .56, I2 = 70%) showed no difference in follow-up testing. ST without imaging (RR = 0.39, CI = 0.28-0.56; P < .00001, I2 = 0%) and the SCP subgroup (RR = 0.17, CI = 0.04-0.77; P = .02, I2 = 80%) had a significant reduction in follow-up testing after CCTA (Figure 8A and 8B) [13-28].
Figure 8. Follow-up tests.
CCTA, coronary computed tomography angiography; ST, stress testing.
Complications associated with CCTA vs. ST: Only four studies reported serious complications attributed to investigation modalities used in the trials. We did not identify any difference between the two arms (7 vs. 7; RR = 0.98, CI = 0.35-2.74; P = .96, I²=0%).
Direct discharge from the ER: Five studies reported direct ER discharges without admission to the hospital (CCTA arm = 936 vs. ST arm = 421; OR = 1.45, CI = 0.63-3.30; P = .38, I²=94%); sensitivity analysis did not reduce heterogeneity.
Cost analysis: Eight studies reported cost, but only five studies were usable as these reported mean cost and standard deviation.
Three studies reported the ER costs. There was a trend towards a decrease in ER costs in the CCTA arm (standardized mean difference [SMD] = -4.68, CI = -10.38 to 1.01; P = .11, I²=100%). Sensitivity analysis, without the CT-COMPARE trial, reduced the heterogeneity to 0% and the results became statistically significant (SMD = -0.38, CI = -0.51 to -0.26; P = .00001, I²=0%).
Five studies reported the total cost. There was no difference between the two arms (SMD = -0.64, CI = -1.75 to 0.46; P = .25, I²=99%). Sensitivity analysis without the CT-COMPARE reduced the heterogeneity to 45%; however, the results remained statistically insignificant. The subgroup analysis for the cost in the United States and cost elsewhere also had significant heterogeneity with no difference between the subgroups (chi-squared = 0.15, degrees of freedom = 1, P = .69, I²=0%).
Radiation dose: Four studies reported the cumulative radiation exposure usable for our analysis. The CCTA arm had significantly higher radiation exposure (SMD = 0.47, CI = 0.08-0.86; P = .02, I²=97%). Sensitivity analysis failed to reduce the heterogeneity.
Discussion
Our meta-analysis of 21,210 patients comparing CCTA to ST demonstrated a significant reduction in the primary endpoint of MIs in the CCTA group without any difference in mortality. The reduction in MI was secondary to a significantly reduced number of events in the SCP group. The reduction in MIs is likely due to the early diagnosis of obstructive CAD and subsequent early initiation of aggressive medical management and revascularizations. Recently published five-year outcomes of the SCOT-HEART trial, which enrolled patients with SCP, also showed a significant reduction in MIs over five years [30]. This discrepancy in downstream MIs between the ACP and SCP group calls for a novel assessment strategy to risk-stratify ACP patients who present to the ER regarding invasive versus conservative management. The lack of mortality benefit in our analysis may not be evident because of the short follow-up times of the individual studies (four weeks to 25 months) compared to the five-year outcomes of the SCOT-HEART trial which showed a significant reduction in mortality from coronary heart disease or nonfatal MI than standard care alone [30].
This analysis also showed increased ICA and revascularizations, which also lead to significantly reduced MIs (NNT of nine to prevent one MI for each ICA). This early difference in ICA and revascularization may be lost after an extended follow-up as suggested by the five-year outcomes of the SCOT-HEART study [23]. This indicates that CCTA use leads to early diagnosis of CAD and subsequent early intervention compared to the ST, where patients eventually needed ICA and revascularization at the cost of increased MIs and mortality. Due to the high sensitivity of CCTA (approximately 99%), a negative CCTA may reduce further testing whereas a positive CCTA leads to additional invasive procedures. In our analysis, there were significantly more ICA, true positive ICA, and revascularizations, with significantly reduced follow-up tests. The use of CCTA leads to a higher number of invasive procedures, including revascularization, ultimately leading to higher costs overall.
After the initial randomization and workup with either CCTA or ST, ER visits and rehospitalizations were significantly reduced in the CCTA arm; this differs from a previously published meta-analysis that showed no difference in ER visits and rehospitalizations [7-11]. A limitation of our analysis was the presence of substantial heterogeneity, making it difficult to generalize the results. The sensitivity analysis reduced heterogeneity with a trend towards reduced ER visits or rehospitalizations in the CCTA arm. Reduction in ER visits and rehospitalizations is promising, as earlier studies found that the reduced MIs after CCTA group was offset by increased future rehospitalizations and downstream costs.
The increased rates of angiographically confirmed CAD post-CCTA is another significant finding that suggests that CCTA has a better positive predictive value than ST (with or without imaging) to identify obstructive CAD at a time when current guidelines do not support the routine use of CCTA in intermediate-risk patients. Although our analysis showed an increasing trend towards unstable anginas in the CCTA arm, we hypothesize that this trend is likely the consequence of higher rates of revascularization in the CCTA group.
The cost analysis had substantial heterogeneity for both ER visits and downstream costs. The trials included in our analysis were conducted in different countries with different healthcare systems and cost structures [13-28]. In our analysis, even though a trend towards decreased ER costs was seen in the CCTA arm, there was no clear advantage of total downstream cost to either imaging strategy. In the absence of any significant mortality benefit, it is reassuring that whichever approach the provider offers will not adversely affect the patient. CCTA was associated with significantly higher cumulative radiation exposure; however, there was substantial heterogeneity, likely due to different scanners used in various trials.
Limitations
Our study had several significant limitations. First, a lack of long-term follow-up in the individual RCTs (≤25 months) that may not include events, hospitalizations, and revascularizations beyond 25 months would magnify the risks of ICA and revascularization and obscure potential long-term benefits. This may be true for ACP trials as short follow-up may have masked the advantage for either arm. Also, some outcomes were not reported by most studies, leading to substantial heterogeneity that persisted even after sensitivity analysis. In addition, we were unable to estimate radiation exposure from all studies between the two groups since they reported data in a variable form. Also, only three studies used ST without imaging, and the other studies used a combination of imaging and non-imaging ST; this leads to substantial overlap between the groups and has a risk to introduce bias in our results. Finally, these trials, although relatively modern, did not utilize high-sensitivity cardiac troponin tests. Their hypotheses must be tested again with the advent of these tests.
Conclusions
Our analysis is the largest to date of 16 RCTs and found a significant reduction in post-CCTA MIs with increased ICA and revascularizations. In the future, more RCTs are needed utilizing scoring methods to identify more robust downstream investigations, cost analysis, and radiation exposure.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Cardiovascular imaging research at the crossroads. Shaw LJ, Min JK, Hachamovitch R, et al. JACC Cardiovasc Imaging. 2010;3:316–324. doi: 10.1016/j.jcmg.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 2.How effective are rapid access chest pain clinics? Prognosis of incident angina and non-cardiac chest pain in 8762 consecutive patients. Sekhri N, Feder GS, Junghans C, Hemingway H, Timmis AD. Heart. 2007;93:458–463. doi: 10.1136/hrt.2006.090894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diagnostic performance of coronary angiography by 64-row CT. Miller JM, Rochitte CE, Dewey M, et al. N Engl J Med. 2008;359:2324–2336. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 4.Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. Budoff MJ, Dowe D, Jollis JG, et al. J Am Coll Cardiol. 2008;52:1724–1732. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 5.64-multislice detector computed tomography coronary angiography as potential alternative to conventional coronary angiography: a systematic review and meta-analysis. Abdulla J, Abildstrom SZ, Gotzsche O, Christensen E, Kober L, Torp-Pedersen C. Eur Heart J. 2007;28:3042–3050. doi: 10.1093/eurheartj/ehm466. [DOI] [PubMed] [Google Scholar]
- 6.Cardiac computed tomography in current cardiology guidelines. Al-Mallah MH, Aljizeeri A, Villines TC, Srichai MB, Alsaileek A. J Cardiovasc Comput Tomogr. 2015;9:514–523. doi: 10.1016/j.jcct.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Coronary computed tomographic angiography for detection of coronary artery disease in patients presenting to the emergency department with chest pain: a meta-analysis of randomized clinical trials. D'Ascenzo F, Cerrato E, Biondi-Zoccai G, et al. Eur Heart J Cardiovasc Imaging. 2013;14:782–789. doi: 10.1093/ehjci/jes287. [DOI] [PubMed] [Google Scholar]
- 8.Outcomes after coronary computed tomography angiography in the emergency department: a systematic review and meta-analysis of randomized, controlled trials. Hulten E, Pickett C, Bittencourt MS, Villines TC, Petrillo S, Di Carli MF, Blankstein R. J Am Coll Cardiol. 2013;61:880–892. doi: 10.1016/j.jacc.2012.11.061. [DOI] [PubMed] [Google Scholar]
- 9.Meta-analysis of coronary computed tomography angiography versus standard of care strategy for the evaluation of low risk chest pain: are randomized controlled trials and cohort studies showing the same evidence? El-Hayek G, Benjo A, Uretsky S, et al. Int J Cardiol. 2014;177:238–245. doi: 10.1016/j.ijcard.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Clinical outcomes after evaluation of stable chest pain by coronary computed tomographic angiography versus usual care: a meta-analysis. Bittencourt MS, Hulten EA, Murthy VL, Cheezum M, Rochitte CE, Di Carli MF, Blankstein R. Circ Cardiovasc Imaging. 2016;9:4419. doi: 10.1161/CIRCIMAGING.115.004419. [DOI] [PubMed] [Google Scholar]
- 11.Coronary computed tomography angiography vs functional stress testing for patients with suspected coronary artery disease: a systematic review and meta-analysis. Foy AJ, Dhruva SS, Peterson B, Mandrola JM, Morgan DJ, Redberg RF. JAMA Intern Med. 2017;177:1623–1631. doi: 10.1001/jamainternmed.2017.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Liberati A, Altman DG, Tetzlaff J, et al. BMJ. 2009;339:2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comprehensive cardiac CT with myocardial perfusion imaging versus functional testing in suspected coronary artery disease: the multicenter, randomized CRESCENT-II Trial. Lubbers M, Coenen A, Kofflard M, et al. JACC Cardiovasc Imaging. 2018;11:1625–1636. doi: 10.1016/j.jcmg.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Calcium imaging and selective computed tomography angiography in comparison to functional testing for suspected coronary artery disease: the multicentre, randomized CRESCENT trial. Lubbers M, Dedic A, Coenen A, et al. Eur Heart J. 2016;37:1232–1243. doi: 10.1093/eurheartj/ehv700. [DOI] [PubMed] [Google Scholar]
- 15.A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. Goldstein JA, Gallagher MJ, O'Neill WW, Ross MA, O'Neil BJ, Raff GL. J Am Coll Cardiol. 2007;49:863–871. doi: 10.1016/j.jacc.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 16.The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. Goldstein JA, Chinnaiyan KM, Abidov A, et al. J Am Coll Cardiol. 2011;58:1414–1422. doi: 10.1016/j.jacc.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 17.Is coronary computed tomography angiography a resource sparing strategy in the risk stratification and evaluation of acute chest pain? Results of a randomized controlled trial. Miller AH, Pepe PE, Peshock R, et al. Acad Emerg Med. 2011;18:458–467. doi: 10.1111/j.1553-2712.2011.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coronary CT angiography versus standard evaluation in acute chest pain. Hoffmann U, Truong QA, Schoenfeld DA, et al. N Engl J Med. 2012;367:299–308. doi: 10.1056/NEJMoa1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CT angiography for safe discharge of patients with possible acute coronary syndromes. Litt HI, Gatsonis C, Snyder B, et al. N Engl J Med. 2012;366:1393–1403. doi: 10.1056/NEJMoa1201163. [DOI] [PubMed] [Google Scholar]
- 20.Coronary CT angiography versus myocardial perfusion imaging for near-term quality of life, cost and radiation exposure: a prospective multicenter randomized pilot trial. Min JK, Koduru S, Dunning AM, et al. J Cardiovasc Comput Tomogr. 2012;6:274–283. doi: 10.1016/j.jcct.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Cardiac computed tomography guided treatment strategy in patients with recent acute-onset chest pain: results from the randomised, controlled trial: CArdiac cT in the treatment of acute CHest pain (CATCH) Linde JJ, Kofoed KF, Sorgaard M, Kelbaek H, Jensen GB, Nielsen WB, Hove JD. Int J Cardiol. 2013;168:5257–5262. doi: 10.1016/j.ijcard.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Diagnostic performance and cost of CT angiography versus stress ECG--a randomized prospective study of suspected acute coronary syndrome chest pain in the emergency department (CT-COMPARE) Hamilton-Craig C, Fifoot A, Hansen M, Pincus M, Chan J, Walters DL, Branch KR. Int J Cardiol. 2014;177:867–873. doi: 10.1016/j.ijcard.2014.10.090. [DOI] [PubMed] [Google Scholar]
- 23.SCOT-HEART investigators: CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383–2391. doi: 10.1016/S0140-6736(15)60291-4. [DOI] [PubMed] [Google Scholar]
- 24.Outcomes of anatomical versus functional testing for coronary artery disease. Douglas PS, Hoffmann U, Patel MR, et al. N Engl J Med. 2015;372:1291–1300. doi: 10.1056/NEJMoa1415516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coronary computed tomography angiography versus radionuclide myocardial perfusion imaging in patients with chest pain admitted to telemetry: a randomized trial. Levsky JM, Spevack DM, Travin MI, et al. Ann Intern Med. 2015;163:174–183. doi: 10.7326/M14-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.A comparison of cardiac computerized tomography and exercise stress electrocardiogram test for the investigation of stable chest pain: the clinical results of the CAPP randomized prospective trial. McKavanagh P, Lusk L, Ball PA, et al. Eur Heart J Cardiovasc Imaging. 2015;16:441–448. doi: 10.1093/ehjci/jeu284. [DOI] [PubMed] [Google Scholar]
- 27.Coronary CT angiography for suspected ACS in the era of high-sensitivity troponins: randomized multicenter study. Dedic A, Lubbers MM, Schaap J, et al. J Am Coll Cardiol. 2016;67:16–26. doi: 10.1016/j.jacc.2015.10.045. [DOI] [PubMed] [Google Scholar]
- 28.Comparative effectiveness of coronary CT angiography vs stress cardiac imaging in patients following hospital admission for chest pain work-up: The Prospective First Evaluation in. Uretsky S, Argulian E, Supariwala A, et al. Chest Pain (PERFECT) Trial. J Nucl Cardiol. 2017;24:1267–1278. doi: 10.1007/s12350-015-0354-6. [DOI] [PubMed] [Google Scholar]
- 29.The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Higgins JP, Altman DG, Gotzsche PC, et al. BMJ. 2011;343:5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. The. Newby DE, Adamson PD, Berry C, et al. N Engl J Med. 2018;379:924–933. doi: 10.1056/NEJMoa1805971. [DOI] [PubMed] [Google Scholar]