Abstract
Purpose.
Post-operative pediatric cerebellar mutism syndrome (CMS), characterized by mutism, ataxia/hypotonia, and emotional lability, can result in long-term deficits following resection of posterior fossa (PF) tumors. This longitudinal study compared neuropsychological outcomes of pediatric patients with post-operative CMS to a matched control patient group without CMS.
Methods.
58 PF tumor patients received post-surgical proton radiation therapy (PRT) and testing at baseline and at ≥1-year post-PRT over a 10-year period. Of these, 18 (31%) had post-operative CMS with baseline and follow-up neuropsychological test data. Those participants were matched to 18 controls by tumor location, age, gender, and handedness; no significant group differences were found at baseline for clinical/demographic variables. Total mean age at baseline was 7.26 years (SD=4.42); mean follow-up interval was 3.26 years (SD=2.24). Areas assessed: overall intelligence, expressive and receptive vocabulary, visuomotor integration, fine motor speed, inhibition, emotional control, depression and anxiety.
Results.
Patients were 52% male; 86% medulloblastoma/14% ependymoma; 86% craniospinal irradiation/14% focal radiation; 86% chemotherapy. No group differences were found between most mean baseline scores; expressive vocabulary and fine motor speed were significantly lower in the post-operative CMS group (p<0.05). Mean change scores revealed no significant differences for the sample; scores were within the normal range except fine motor skills were impaired for both groups.
Conclusions.
Longitudinal neuropsychological outcomes for post-operative pediatric CMS patients did not differ significantly from matched controls without this condition. Patients were in the normal range in all areas except fine motor speed, which was impaired for both groups independent of CMS diagnosis.
Keywords: brain tumor, post-operative pediatric cerebellar mutism syndrome, posterior fossa syndrome, neuropsychological outcomes, proton radiation therapy
Introduction
Post-operative pediatric cerebellar mutism syndrome (CMS) is characterized by mutism, ataxia/hypotonia and emotional lability that develops following surgical resection of posterior fossa tumors. The symptoms associated with CMS have been classified differently by various investigators with synonymous terminologies that include: posterior fossa syndrome [21], cerebellar cognitive affective syndrome [26-27], cerebellar mutism and subsequent dysarthria [32], cerebellar mutism [6], cerebellar speech syndrome [12], mutism without behavioral impairment [3-4, 24], and pseudobulbar palsy [38]. More recently, an international consensus established the term “post-operative pediatric CMS” to refer to this constellation of neuropsychiatric and behavioral symptoms [10]; thus, this term is used throughout this paper to refer to this constellation of symptoms. Approximately 25% of patients diagnosed with medulloblastoma develop post-operative pediatric CMS following surgery [10, 13-14]. The speech, motor and emotional deficits that emerge can lead to both short- and long- term cognitive and psychosocial sequelae [10, 16, 20, 39].
One prior study found that post-operative pediatric CMS patients treated with photon radiation and evaluated at follow-up at least one year post diagnosis had significantly lower performance in processing speed, attention, working memory, executive function, cognitive efficiency, reading, spelling, and math than their age-matched controls who were treated for medulloblastoma but did not have post-operative pediatric CMS [20]. Similarly, longitudinal assessment of patients with post-operative pediatric CMS, when compared with matched controls, has found worse cognitive trajectories and minimal recovery, particularly in the domains of attention, working memory, and processing speed; these deficits may be secondary to greater disruption in the fronto-cerebellar pathways sustained during surgery in post-operative pediatric CMS patients [28].
Psychosocially, post-operative pediatric CMS patients are more likely to exhibit obsessive-compulsive type behaviors, withdrawal, social difficulties and internalizing problems (e.g., anxiety; depression) one to two years post treatment than patients who did not develop this condition [39]. Greater neurocognitive problems (e.g., intellectual ability, processing speed, attention) have also been reported by parents at follow-up [28]. Furthermore, mutism, which is generally thought to be transient, does not fully normalize for most post-operative pediatric CMS patients [5, 11, 29-30].
This longitudinal study compares the neuropsychological outcomes of a cohort of patients diagnosed with post-operative pediatric CMS to a matched patient control group without CMS, each treated with proton radiation therapy (PRT). It was hypothesized that participants with post-operative CMS would fare worse on neuropsychological, emotional, and behavioral measures than those who did not develop this post-operative complication. The findings may help inform treatment decisions and recommendations for the care of pediatric patients diagnosed with posterior fossa brain tumors.
Methods
Patient Selection
A total of 58 pediatric patients with posterior fossa tumors were treated with PRT and received neuropsychological testing at baseline (at initiation or during PRT) and at follow-up at least 1-year post-PRT at the Massachusetts General Hospital (MGH) Francis H. Burr Proton Center over a 10-year period. Patients received baseline and follow-up neuropsychological assessments at MGH as part of their routine clinical care. All patients underwent surgical resection in the posterior fossa region prior to PRT, were ≤ 16 years old at baseline assessment, and had no prior diagnosis of brain or central nervous system tumor. PRT was initiated within a median of 35 days following surgery, regardless of the presence of pediatric post-operative CMS.
Post-operative pediatric CMS was defined as the presence of a delayed onset mutism/reduced speech and emotional lability after cerebellar or fourth ventricle tumor surgery with additional common features that may include hypotonia, dysphagia, cerebellar motor syndrome, cerebellar cognitive affective syndrome, and/or brain stem dysfunction [10]. Of the 58 patients with baseline and follow-up testing, 18 patients (31%) were diagnosed with post-operative pediatric CMS by their treating physician. Four additional patients with post-operative pediatric CMS were unable to complete baseline testing due to scheduling conflicts and/or severity of symptoms and were therefore unable to be included as part of the longitudinal study.
The 18 patients with post-operative pediatric CMS were matched to 18 patients without CMS who also received PRT (control group) based on the following characteristics in order: tumor location in the posterior fossa; age at baseline testing; gender; and handedness. Of these, 16 controls could be matched for age; the two patients that did not have available matches had a one-year age difference. Fifteen could be matched for gender and 16 were matched for handedness (15 right handed in each group). Informed consent was obtained from all individual participants included in this study. Institutional Review Board approval was obtained for this study.
Radiation
Patients were treated with PRT, surgery, and chemotherapy appropriate for the diagnosis and according to the current standard of care. Chemotherapy agents used for treatment included: vincristine, cyclophosphamide, cisplatinum, cytoxan, carboplatinum, and etoposide. Proton radiation dose is reported in Gy (relative biological effectiveness [RBE]), using a RBE of 1.1 [17, 19]. PRT followed a standard protocol that includes CT- and MRI-based planning utilizing proton XiO software to generate passively scattered three-dimensional conformal proton treatment plans [17]. All radiation was delivered with standard fraction sizes of 1.8 Gy (RBE) per fraction in accordance with the national standard set by Children’s Oncology Group protocols for target coverage. All patients with medulloblastoma received craniospinal irradiation (CSI) followed by a boost to the tumor site, for a total dose of 54 Gy (RBE). Patients with ependymoma tumors received PRT to the tumor site alone (i.e., focal radiation). No surgical biopsies or resections were performed during or after PRT. Chemotherapy was administered concurrent or subsequent to PRT and was completed by the time of the follow-up assessment.
Neuropsychological Assessment
Neuropsychological assessment was conducted at initiation of or during PRT (baseline) and at one or more scheduled outpatient visits at least one year after completion of PRT (follow-up) (Table 1). When there were multiple follow-up assessments, the latest results were used for analysis. Direct assessment of patients was conducted using age-appropriate standardized measures to assess overall intelligence (not prorated; incorporating working memory and processing speed indices), receptive and expressive vocabulary, visuomotor integration/graphomotor skills, and fine motor speed and coordination. Parents completed two standardized rating scales at baseline and follow-up to assess behavioral inhibition, emotional control, depression and anxiety since these symptoms are commonly associated with post-operative pediatric CMS [16, 39]. Measures produce age-based standard scores with a mean of 100 and a standard deviation (SD) of 15. For those measures administered to the patients, lower scores indicate lower functioning or poorer performance, whereas higher scores on the parent rating scales indicate greater problems in those areas assessed.
TABLE 1.
Standardized neuropsychological assessment measures.
| Domain | Measure |
|---|---|
| Direct Assessment Measures | |
| Overall Intelligence (a) | Bayley Scales of Infant Development- 2nd Edition (MDI) [1] (< 2.5 years) Wechsler Preschool and Primary Scale of Intelligence- 3rd Edition (FSIQ) [35] (2.5 – 5 years) Wechsler Intelligence Scale for Children- 4th & 5th Editions (FSIQ) [34,37] (6 – 15 years) Wechsler Adult Intelligence Scale- 3rd & 4th Editions (FSIQ) [33, 36] (16 - 19 years) |
| Receptive Vocabulary | Peabody Picture Vocabulary Test- 4th Edition (PPVT-4) [7] |
| Expressive Vocabulary | Expressive One-Word Picture Vocabulary Test- 4th Edition (EOWPVT-4) [18] |
| Visuomotor integration/Graphomotor | Beery-Buktenica Developmental Test of Visual-Motor Integration- 5th & 6th Editions (VMI) [2-3] |
| Fine Motor Dominant/Nondominant | Purdue Pegboard Test [15] |
| Parent Rating Scales | |
| Inhibition Emotional Control | Behavior Rating Inventory of Executive Function (BRIEF) [8] |
| Depression Anxiety | Behavior Assessment System for Children- 2nd edition (BASC-2) [25] |
Note.
Intelligence scales yielded either a Mental Development Index (MDI) or Full Scale Intelligence Quotient (FSIQ). Intelligence measure administered was appropriate for patient’s age.
Statistical Analyses
Descriptive statistics were conducted to characterize the total sample at baseline and follow-up. Independent sample t tests and chi-squared analysis were conducted to determine if the two groups differed at baseline for the following demographic and clinical characteristics: gender, race, socioeconomic status (SES), histology, radiation field (CSI or focal radiation), treatment with chemotherapy, history of hydrocephalus, shunt placement, sensory problems, and rehabilitation therapy services received. Change scores between baseline and follow-up were calculated for each patient. One-way analysis of variance (ANOVA) and effect sizes were conducted to determine if there were significant differences in change scores between patient groups as well as to determine if clinical variables were associated with neuropsychological scores. Scores within the normal range were defined as a standard score of ≥ 85 for direct assessment measures. Median household income in community of residence served as a proxy for SES [31]. Statistical analyses were performed by using SPSS version 21.0. All reported p values used two-tailed significance with α (alpha) set at 0.05.
Results
Demographic and Clinical Characteristics
Demographic and clinical characteristics for patients with post-operative pediatric CMS and for matched controls are summarized in Table 2. For both groups, the mean age at baseline was 7 years old and the mean follow-up interval was 3 years. Half of the patients were under 6 years old at baseline. There were no significant differences between the two groups for gender, race, SES, histology, radiation field, treatment with chemotherapy, history of hydrocephalus or shunt placement. Nearly all of the patients with post-operative CMS (94.4%) had gross total resection, significantly more than the control group (66.7%) (p < 0.05).
TABLE 2.
Demographic and clinical characteristics of the total sample (N = 36)
| Variable | CMS (n = 18) Mean (± s.d.) or N (%) |
Matched Controls (n = 18) Mean (± s.d.) or N (%) |
p-value |
|---|---|---|---|
| Age at Baseline Testing (yr) | 7.1 (4.5) Range = 1.2- 15.2 |
7.4 (4.5) Range = 1.4-15.8 |
0.87 |
| Age at Follow-Up Testing (yr) | 10.2 (4.1) Range = 2.8- 17.1 |
10.9 (4.5) Range = 2.5- 18.2 |
0.65 |
| Interval to Follow-Up (yr) | 3.1 (2.2) | 3.5 (2.3) | 0.58 |
| Gender (male) | 8 (44.0) | 11 (61.1) | 0.31 |
| Race (Caucasian) | 17 (94.4) | 16 (88.9) | 0.59 |
| Socioeconomic Status(a) (median) | $89,399 ($41,742) | $82,813 ($36,335) | 0.70 |
| Histology | 0.63 | ||
| Medulloblastoma | 16 (88.9) | 15 (83.3) | |
| Ependymoma | 2 (11.1) | 3 (16.7) | |
| Radiation Field | 0.63 | ||
| Craniospinal | 16 (88.9) | 15 (83.3) | |
| Focal | 2 (11.1) | 3 (16.7) | |
| Surgery(b) | 0.04* | ||
| Gross Total Resection | 17 (94.4) | 12 (66.7) | |
| Subtotal Resection | 1 (5.6) | 6 (33.3) | |
| Chemotherapy(c) (yes) | 14 (77.8) | 17 (94.4) | 0.14 |
| Hydrocephalus (yes) | 14 (77.8) | 11 (61.1) | 0.27 |
| Shunt Placement (yes) | 2 (14.3) | 6 (54.6) | 0.10 |
Note.
p < 0.05 = statistically significant.
Median household income in community of residence used as a proxy indicator of socioeconomic status [31].
No surgical biopsies or resections were performed during or after proton radiation therapy.
Concurrent or subsequent to radiation therapy.
Patients received occupational therapy, physical therapy, and/or speech and language therapy as clinically indicated during and following PRT (Table 3). At baseline, significantly more patients with post-operative pediatric CMS experienced gross and fine motor problems (p < 0.01) and required inpatient rehabilitation, including physical, occupational, and speech/language therapies (p < 0.05). For those who did not require inpatient rehabilitation, more patients with post-operative pediatric CMS required outpatient occupational therapy (p = 0.05). At follow-up, patients with post-operative pediatric CMS continued to experience significantly more gross motor problems (p < 0.05) requiring outpatient physical therapy services (p < 0.05).
TABLE 3-.
Post-surgical sensory problems and rehabilitative therapy services at baseline and follow-up
| Sensory Problem/Services | CMS (n =18) N (%) |
Matched Controls (n = 18) N (%) |
p-value | |
|---|---|---|---|---|
| Baseline | Motor Problems | 16 (88.8) | 6 (33.3) | 0.00* |
| Hearing Problems | 0 (0.0) | 0 (0.0) | --- | |
| Vision Problems | 5 (27.7) | 6 (33.3) | 0.71 | |
| Inpatient Rehabilitation (PT/OT/SLT) | 8 (44.4) | 2 (11.1) | 0.02* | |
| Outpatient PT | 9 (50.0) | 7 (38.8) | 0.50 | |
| Outpatient OT | 7 (38.8) | 2 (11.1) | 0.05 | |
| Outpatient SLT | 1 (5.5) | 0 (0.0) | 0.26 | |
| Follow-Up | Motor Problems | 9 (50.0) | 3 (16.6) | 0.03* |
| Hearing Problems | 8 (44.4) | 11 (61.1) | 0.31 | |
| Vision Problems | 0 (0.0) | 3 (16.6) | 0.07 | |
| Inpatient Rehabilitation (PT/OT/SLT) | 0 (0.0) | 0 (0.0) | --- | |
| Outpatient PT | 8 (44.4) | 2 (11.1) | 0.02* | |
| Outpatient OT | 5 (27.7) | 4 (22.2) | 0.15 | |
| Outpatient SLT | 3 (16.6) | 3 (16.6) | --- |
Note.
p < 0.05 = statistically significant. PT= Physical Therapy; OT= Occupational Therapy; SLT= Speech-Language Therapy.
Neuropsychological Assessment
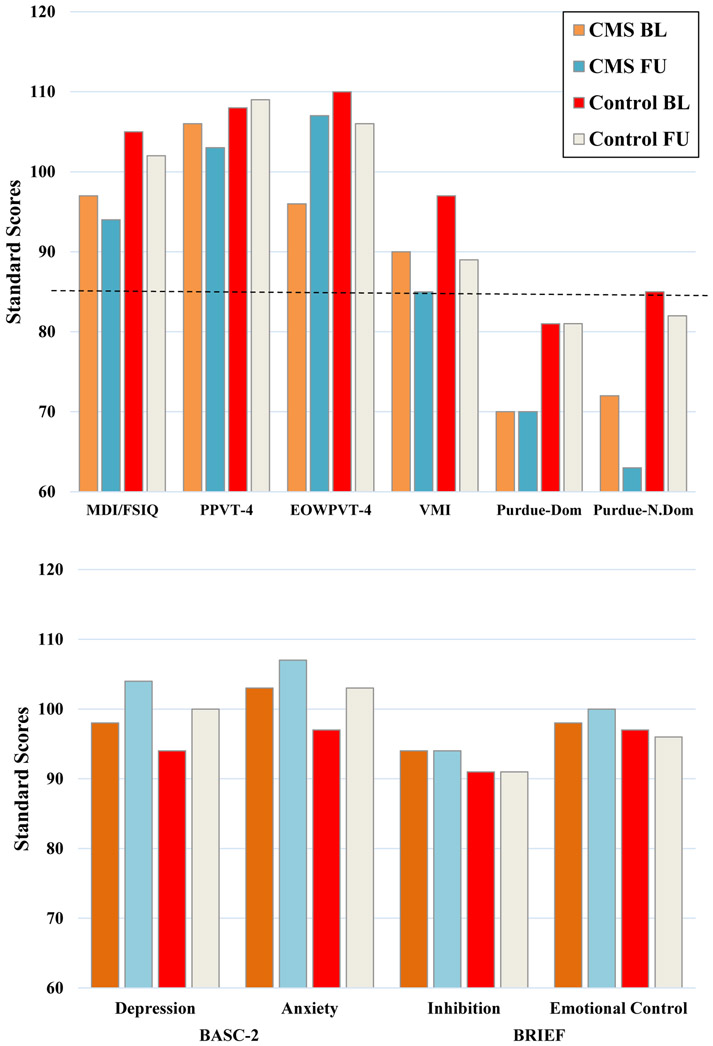
Results of neuropsychological assessment are shown in Figure 1. At baseline, mean scores in overall intelligence, receptive vocabulary, visuomotor integration/graphomotor skills, behavioral inhibition, emotional control, mood, and anxiety were in the normal range, and there was no significant difference between the two groups (p > 0.05). However, expressive vocabulary was significantly lower in the post-operative CMS group (mean = 95.58, SD = 20.76) compared to the control group at baseline (mean = 109.88; SD = 12.92) (p < 0.05), although both scores were within the normal range. Fine motor speed and coordination were impaired for the dominant and nondominant hands in both groups at baseline and no significant difference between groups was observed (p > 0.05).
Fig 1.
Mean test scores for neuropsychological measures and parent rating scales at baseline and follow-up. The dotted line in the upper chart indicates the minimum score within the normal range (standard score ≥ 85).
At follow-up, mean scores in overall intelligence, receptive and expressive vocabulary, behavioral inhibition, emotional control, mood, and anxiety were in the normal range and there were no significant differences between groups (p > 0.05). However, visuomotor integration/graphomotor skills were impaired in the post-operative CMS group at follow-up (mean = 84.57; SD = 17.41), but not in the control group (mean = 89.23; SD = 13.19), although the groups did not differ significantly (p > 0.05). Fine motor speed and coordination were also impaired at follow-up in both groups for the dominant and nondominant hands; there was a significant difference between groups (p = 0.05) in the nondominant hand with greater difficulty observed in the post-operative CMS group.
As shown in Table 4, the mean change scores between baseline and follow-up testing did not reveal significant differences between the two groups on any cognitive or emotional/behavioral domains assessed, including overall intelligence, expressive and receptive vocabulary, visuomotor integration/graphomotor skills, fine motor speed and coordination, behavioral inhibition, emotional control, mood, or anxiety.
TABLE 4.
Comparison of mean change scores from baseline and follow-up assessments between patients with post-operative pediatric cerebellar mutism syndrome and matched controls
| Measure | CMS (n = 18) Mean Change Score (Mean ± s.d.) |
Matched Controls (n = 18) Mean Change Score (Mean ± s.d.) |
p-value | ES |
|---|---|---|---|---|
| MDI/ FSIQ | −4.58 (8.47) | −2.87 (11.50) | 0.67 | 0.007 |
| PPVT-4 | −2.20 (9.36) | 0.62 (20.95) | 0.75 | 0.004 |
| EOWPVT-4 | 10.70 (34.05) | −4.44 (9.69) | 0.10 | 0.106 |
| VMI | −5.18 (9.25) | −8.40 (16.18) | 0.56 | 0.014 |
| Purdue Pegboard | ||||
| Dominant Hand | −8.40 (25.21) | −2.77 (20.10) | 0.63 | 0.015 |
| Nondominant Hand | −4.60 (5.73) | −7.00 (16.49) | 0.76 | 0.006 |
| BRIEF | ||||
| Inhibition | 0.62 (6.62) | −1.85 (5.24) | 0.41 | 0.005 |
| Emotional Control | −2.61 (15.11) | −2.85 (6.89) | 0.95 | 0.006 |
| BASC-2 | ||||
| Depression | 4.23 (13.69) | 2.57 (7.03) | 0.69 | 0.000 |
| Anxiety | 3.69 (15.58) | 2.07 (5.71) | 0.72 | 0.039 |
Note. CMS, cerebellar mutism syndrome; MDI, Mental Development Index & FSIQ, Full Scale Intelligence Quotient; PPVT-4, Peabody Picture Vocabulary Test- 4th Edition; EOWPVT-4, Expressive One-Word Picture Vocabulary Test- 4th Edition; VMI, Beery-Buktenica Developmental Test of Visual-Motor Integration- 5th & 6th Editions; BRIEF, Behavior Rating Inventory of Executive Function; BASC-2, Behavior Assessment System for Children- 2nd Edition; ES, effect size (.8 = large, .5 = moderate, .2 = small; Cohen, 1988).
Examination of the extent of tumor resection revealed that patients with subtotal resection showed notably greater decline in fine motor speed and coordination in their dominant hand across both the post-operative pediatric CMS and control groups (F(1, 16) = 4.68, p < 0.05); however, no other cognitive domains or aspects of emotional/behavioral functioning showed significant differences (p > 0.05).
Discussion
The present longitudinal study investigates neuropsychological outcomes in a cohort of posterior fossa tumor patients with post-operative pediatric CMS and matched controls all treated with PRT at an average of 3 years post-treatment. Between-group analyses revealed that patients with post-operative pediatric CMS did not significantly differ at baseline or at follow-up from patients who did not develop post-operative CMS in overall intelligence, receptive vocabulary, visuomotor integration/graphomotor skills, fine motor speed and coordination, behavioral inhibition, emotional control, depression and anxiety. Mean scores in these areas were within the normal range for both groups at each time point, although at baseline the post-operative CMS group performed consistently lower on direct neuropsychological measures and had more emotional and behavioral difficulties (although not statistically significant).
Expressive vocabulary was significantly lower in the post-operative CMS group at baseline, consistent with the symptom pattern of post-operative CMS. In addition, significantly more patients in this group experienced gross and fine motor problems at baseline requiring higher rates of service utilization of inpatient and outpatient rehabilitative services. The observed weaknesses in communication, motor functioning, and need for rehabilitative service provision are consistent with the expected findings among patients with post-operative pediatric CMS [10-11, 16, 20, 28, 30]. At follow-up, a significant difference in expressive vocabulary was no longer observed, but higher rates of persistent gross motor problems were observed, requiring greater use of physical therapy services, reflecting evidence of long-term sequelae at a greater rate than in controls.
When examining the longitudinal trajectory of patients within the post-operative CMS group, these patients showed significantly lower expressive vocabulary at baseline, consistent with features of post-operative CMS [5, 11, 29-30], yet scores normalized at follow-up such that there was no longer a significant difference between groups. This finding is favorable to that previously reported in the literature which describes persistent language deficits [5, 11, 29-30], although the severity of post-operative CMS was not well-defined among these studies.
Post-operative pediatric CMS is often associated with persistent problems with emotional and behavioral dysregulation [16, 28, 39]. In contrast, parents in this study did not report problems with those aspects of emotional and behavioral functioning at baseline or follow-up in either group; the patient group with post-operative pediatric CMS showed no significant problems regulating their emotions and behavior or symptoms of anxiety and depression at either time point. Given that significant difficulty was not endorsed in either group at baseline, one may deduce that these measures were either not sufficiently sensitive to detect symptoms that were present or that the severity of symptoms of this post-operative CMS group was not at a level that resulted in elevated scores.
At follow-up, fine motor speed and coordination were impaired bilaterally for all patients, independent of post-operative pediatric CMS diagnosis. Patients with post-operative pediatric CMS fared worse than matched controls, although not significantly so. Visuomotor integration/graphomotor skills, which require fine motor skills and coordination, were on average impaired at follow-up in the post-operative CMS group, whereas matched controls performed within the normal range. Given the frequency of vision problems was not significantly different between groups, it is unlikely that those impairments were vision-related.
Poor fine motor and visuomotor integration/graphomotor skills can negatively impact the ability to carry out daily living tasks, such as writing legibly, producing written output efficiently, tying shoes, manipulating buttons, and using keys. Disruption to the cerebellum and its associated connectivity is hypothesized to play a role in the presence of longstanding weaknesses in motor coordination and speed given the observation of weaknesses among all patients who received surgery and PRT [27-28], not unique to those who developed post-operative pediatric CMS.
Of note, both patient groups demonstrated follow-up outcomes that were favorable to that reported in the literature [20, 28, 39] where impairments in cognitive, language, emotional, social, and behavioral functioning have been reported. This may suggest that the role of dose-sparing PRT intervention [9, 17, 22-23, 40] may have played a role in cognitive preservation across groups. Future studies examining the neurocognitive outcomes of PRT among patients with post-operative pediatric CMS are indicated.
Limitations of this study include the potential representativeness of this group, as select demographic and clinical factors may not accurately represent the general population. This sample was predominantly Caucasian and from a middle to high SES, which may influence pre-morbid and post-PRT environmental factors potentially related to outcome. Because the study design required both pre- and post- comparison data, four patients were excluded who may have experienced severe post-operative pediatric CMS symptoms, which might limit the generalizability of the current results to more severely impacted patients. No specific data related to CMS severity (e.g., duration of symptoms following PRT) were available for the study patients. The time interval between baseline and follow-up assessment was, on average, 3 years post-PRT; continued follow-up with a longer interval since PRT is needed to more fully understand potential long-term sequelae. The small sample size and inclusion of a large range of ages in this study are limitations stemming from the rare occurrence of post-operative pediatric CMS; future studies should include larger cohorts with examination of various developmental levels of functioning to replicate findings and improve generalizability. Finally, it will be important for future neuropsychological research to include direct, independent assessment of attention and executive functioning due to the observation of specific impairments in these areas in the literature [20, 28]. While parents did not endorse the presence of significant difficulty with aspects of executive functioning examined in this study (inhibition; emotional control), the importance of more in-depth direct assessment cannot be overlooked.
In summary, the findings from this study are promising and demonstrate that long-term neuropsychological outcomes of patients with post-operative pediatric CMS who received PRT were more favorable than has generally been reported in the literature. In addition, there were no problems with mood or anxiety at 3-year follow-up. Poor fine motor speed and coordination were observed longitudinally in patients both with and without post-operative pediatric CMS, although patients with post-operative CMS fared worse as demonstrated by additionally compromised visuomotor integration/graphomotor skills. Ongoing rehabilitative services to support development of fine motor and visuomotor integration/graphomotor skills, such as occupational therapy, will be critical for many patients with post-operative CMS to prevent these skills from impacting quality of life. Overall, longitudinal cognitive, emotional, and behavioral results are reassuring. Further studies are needed to replicate these findings.
Acknowledgments:
The project described was supported by Award Number P01CA021239 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The project was supported by the Federal Share of program income earned by the Massachusetts General Hospital (MGH) on C06 CA059267 Proton Therapy Research and Treatment Center. The authors thank the MGH Francis Burr Proton Center pediatric patients and their families for participation in this study.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Bayley N (1993). Bayley scales of infant development, Second Edition: manual. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- 2.Beery KE, Buktenica NA, Beery NA. (2006). Beery-buktenica developmental test of visual-motor integration test (ed 5). San Antonio, TX: NCS Pearson. [Google Scholar]
- 3.Beery KE, Buktenica NA, Beery NA. (2010). Beery-buktenica developmental test of visual-motor integration test (ed 6). San Antonio, TX: NCS Pearson. [Google Scholar]
- 4.Catsman-Berrevoets CE, van Dongen HR, Zwetsloot CP. (1992). Transient loss of speech followed by dysarthria after removal of posterior fossa tumour. Dev Med Child Neurol. 34:1102–1109. [DOI] [PubMed] [Google Scholar]
- 5.De Smet HJ, Baillieux H, Catsman-Berrevoets C, De Deyn PP, Marien P, Paquier PF. (2007). Postoperative motor speech production in children with the syndrome of ‘cerebellar’ mutism and subsequent dysarthria: a critical review of the literature. Eur J Paediatr Neuro. 11(4): 193–207. [DOI] [PubMed] [Google Scholar]
- 6.Dietze DD Jr, Mickle JP. (1990). Cerebellar mutism after posterior fossa surgery. Pediatr Neurosurg. 16:25–31. [DOI] [PubMed] [Google Scholar]
- 7.Dunn L, Dunn D (2007). Peabody picture vocabulary test: Manual (ed 4). Minneapolis, Minnesota: Pearson. [Google Scholar]
- 8.Gioia GA, Isquith PK, Guy SC, Kenworthy L. (2000). Behavior rating inventory of executive function: Professional manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- 9.Greenberger BA, Pulsifer MB, Egg DH, MacDonald SM, Jones RM, Butler WE, Huang MS, Marcus KJ, Oberg JA, Tarbell NJ, Yock TI. (2014). Clinical outcomes and late endocrine, neurocognitive and visual profiles of proton radiation for pediatric low-grade gliomas. Int J Radiat Oncol Biol Phys. 89:1060–1068. [DOI] [PubMed] [Google Scholar]
- 10.Gudrunardottir T, Morgan AT, Lux AL, Walker DA, Walsh KS, Wells EM, Wisoff JH, Juhler M, Schmahmann JD, Keating RF, Catsman-Berrevoets C. (2016). Consensus paper on post-operative pediatric cerebellar mutism syndrome: the Iceland Delphi results. Childs Nerv Syst. 32(7): 1195–1203. [DOI] [PubMed] [Google Scholar]
- 11.Huber JF, Bradley K, Spiegler B, Dennis M. (2007). Long-term neuromotor speech deficits in survivors of childhood posterior fossa tumors: effects of tumor type, radiation, age at diagnosis and survival years. J Child Neurol. 22(7):848–854. [DOI] [PubMed] [Google Scholar]
- 12.Kingma A, Mooij JJ, Metzemaekers JD, Leeuw JA. (1994). Transient mutism and speech disorders after posterior fossa surgery in children with brain tumours. Acta Neurochir (Wien). 131:74–79. [DOI] [PubMed] [Google Scholar]
- 13.Korah MP, Esiashvili N, Mazewski CM, Hudgins RJ, Tighiouart M, Janss AJ, Schwaibold FP, Crocker IR, Curran WJ, Marcus RB. (2010). Incidence, risks, and sequelae of posterior fossa syndrome in pediatric medulloblastoma. Int J Radiat Oncol Biol Phys. 77(1):106–112. [DOI] [PubMed] [Google Scholar]
- 14.Kupeli S, Yalcin B, Bilginer B, Akalan N, Haksal P, Buyukpamukcu M. (2011). Posterior fossa syndrome after posterior fossa surgery in children with brain tumors. Pediatr Blood Cancer. 56(2):206–210. [DOI] [PubMed] [Google Scholar]
- 15.Lafayette Instrument. (2015). Purdue pegboard: Manual. Lafayette, IN; Lafayette Instrument Company. [Google Scholar]
- 16.Lanier JC, Abrams AN. (2017). Posterior fossa syndrome: review of the behavioral and emotional aspects in pediatric cancer patients. Cancer. 123(4):551–559. [DOI] [PubMed] [Google Scholar]
- 17.MacDonald SM, DeLaney TF, Loeffler JS. (2006). Proton beam radiation therapy. Cancer Invest. 24:199–208. [DOI] [PubMed] [Google Scholar]
- 18.Martin NA, Brownell R. (2011). Expressive one-word picture vocabulary test: Manual (ed 4). Novato, California: ATP Assessments. [Google Scholar]
- 19.Paganetti H, Niemierko A, Ancukiewicz M, Gerwek LD, Goitein M, Loeffler JS, Suit HD. (2002). Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 53:407–421. [DOI] [PubMed] [Google Scholar]
- 20.Palmer SL, Hassall T, Evankovich K, Mabbott DJ, Bonner DJ, Bonner M, Deluca C, Cohn R, Fisher MJ, Morris B, Broniscer A, Gajjar A. (2010). Neurocognitive outcome 12 months following cerebellar mutism syndrome in pediatric patients with medulloblastoma. Neuro-Oncology. 12:1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollack IF. (1997). Posterior fossa syndrome. Int Rev Neurobiol. 41:411–432. [DOI] [PubMed] [Google Scholar]
- 22.Pulsifer MB, Sethi RV, Kuhlthau KA, MacDonald SM, Tarbell NJ, Yock TI. (2015). Early cognitive outcomes following proton radiation in pediatric patients with brain and central nervous system tumors. Int J Radiat Oncol Biol Phys. 93:400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pulsifer MB, Duncanson H, Grieco J, Evans C, Tseretopoulous ID, MacDonald S, Tarbell NJ, Yock TI. (2018). Cognitive and adaptive outcomes after proton radiation for pediatric patients with brain tumors. Int J Radiat Oncol Biol Phys. 102:391–398. [DOI] [PubMed] [Google Scholar]
- 24.Rekate HL, Grub RL, Aram DM, et al. (1985). Muteness of cerebellar origin. Arch Neurol. 42:697–698. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds CR, Kamphaus RW. (2004). Behavior assessment system for children: Manual (ed 2). San Antonio, TX: NCS Pearson. [Google Scholar]
- 26.Sadeh M, Cohen I. (2001). Transient loss of speech after removal of posterior fossa tumors- one aspect of a larger neuropsychological entity: the cerebellar cognitive affective syndrome. Pediatr Hematol Oncol. 18:423–426. [DOI] [PubMed] [Google Scholar]
- 27.Schmahmann JD, Sherman JC. (1997). Cerebellar cognitive affective syndrome. Int Rev Neurobiol. 41:433–440. [DOI] [PubMed] [Google Scholar]
- 28.Schrieber JE, Palmer SL, Conklin HM, Mabbott DJ, Swain MA, Bonner MJ, Chapieski ML, Huang L, Zhang H, Gajjar A. (2017). Posterior fossa syndrome and long-term neuropsychological outcomes among children treated for medulloblastoma on a multi-institutional prospective study. Neuro-Oncology. 19(12): 1673–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinbok P, Cochrane DD, Perrin R, Price A. (2003). Mutism after posterior fossa tumour resection in children: incomplete recovery on long-term follow-up. Pediatr Neurosurg. 39:179–183. [DOI] [PubMed] [Google Scholar]
- 30.Tamburrini G, Frassanito P, Chieffo D, Massimi L, Caldarelli M, DiRocco C. (2015). Cerebellar mutism. Child Nerv Syst. 31:1841–1851. [DOI] [PubMed] [Google Scholar]
- 31.United States Census Bureau. 2012. American community survey, median income. From the 2008–2012 American community survey 5-year estimates. Available at http://factfinder2.census.gov/faces/nav/jsf/pages/community_facts.xhtml. Accessed 9 June 2014.
- 32.Van Dongen HR, Catsman-Berrevoets CE, van Mourik M. (1994). The syndrome of ‘cerebellar’ mutism and subsequent dysarthria. Neurology. 44:2040–2046. [DOI] [PubMed] [Google Scholar]
- 33.Wechsler D (1997). Wechsler adult intelligence scale (ed 3). San Antonio, Texas, A Harcourt Assessment Company. [Google Scholar]
- 34.Wechsler D (2003). Wechsler intelligence scale for children (ed 4). San Antonio, Texas, A Harcourt Assessment Company. [Google Scholar]
- 35.Wechsler D (2003). Wechsler preschool and primary scale of intelligence (ed 3). San Antonio, Texas, Psychological Corporation, A Harcourt Assessment Company. [Google Scholar]
- 36.Wechsler D (2008). Wechsler adult intelligence scale (ed 4). San Antonio, Texas, A Harcourt Assessment Company. [Google Scholar]
- 37.Wechsler D (2014). Wechsler intelligence scale for children (ed 5). San Antonio, Texas, A Harcourt Assessment Company. [Google Scholar]
- 38.Wisoff JH, Epstein FJ. (1984). Pseudobulbar palsy after posterior fossa operation n children. Neurosurgery. 15:707–709. [DOI] [PubMed] [Google Scholar]
- 39.Wolfe-Christensen C, Mullins LL, Scott JG, McNail-Knapp RY. (2007). Persistent psychosocial problems in children who develop posterior fossa syndrome after medulloblastoma resection. Pediatr Blood Cancer. 49(5):723–726. [DOI] [PubMed] [Google Scholar]
- 40.Yock T, Schneider R, Friedmann A, Adams J, Fullerton B, Tarbell N. (2005). Proton radiotherapy for orbital rhabdomyosarcoma: clinical outcome and a dosimetric comparison with photons. Int J Radiat Oncol Biol Phys. 63(4):1161–1168. [DOI] [PubMed] [Google Scholar]