Abstract
Tacrolimus (TAC) is the cornerstone of immunosuppressive therapy in liver transplantation. This study aimed at elucidating the interplay between pharmacogenetic determinants of TAC whole blood and intracellular exposures as well as the pharmacokinetic-pharmacodynamic relationship of TAC in both compartments. Complete pharmacokinetic profiles (Predose, and 20 min, 40 min, 1h, 2h, 3h, 4h, 6h, 8h, 12h post drug intake) of twice daily TAC in whole blood and peripheral blood mononuclear cells (PBMC) were collected in 32 liver transplanted patients in the first ten days post transplantation. A non-parametric population pharmacokinetic model was applied to explore TAC pharmacokinetics in blood and PBMC. Concurrently, calcineurin activity was measured in PBMC. Influence of donor and recipient genetic polymorphisms of ABCB1, CYP3A4 and CYP3A5 on TAC exposure was assessed. Recipient ABCB1 polymorphisms 1199G>A could influence TAC whole blood and intracellular exposure (p<0.05). No association was found between CYP3A4 or CYP3A5 genotypes and TAC whole blood or intracellular concentrations. Finally, intra-PBMC calcineurin activity appeared incompletely inhibited by TAC and less than 50% of patients were expected to achieve intracellular IC50 concentration (100 pg/millions of cells) at therapeutic whole blood concentration (i.e.: 4–10 ng/mL). Together, these data suggest that personalized medicine regarding TAC therapy might be optimized by ABCB1 pharmacogenetic biomarkers and by monitoring intracellular concentration whereas the relationship between intracellular TAC exposure and pharmacodynamics biomarkers more specific than calcineurin activity should be further investigated.
1. Introduction
Tacrolimus (TAC) is an immunosuppressive drug widely prescribed in solid organ transplant patients. Its effect is mediated through the inhibition of intracellular calcineurin (CaN), a serine-threonine phosphatase enzyme, which results in the inhibition of interleukine-2 (IL-2) synthesis by T-lymphocytes [1].
TAC pharmacological response exhibits substantial inter-individual variability. This variability can be partially managed by performing therapeutic drug monitoring (TDM) of TAC whole blood concentrations [2]. Indeed, TDM of TAC is mandatory since it was evidenced that lower whole blood concentrations increase risk of acute rejection (ACR) and that some toxicity are induced by high whole blood concentrations [3].
Despite this personalized approach, some patients experience ACR or toxicity while having blood concentration within the therapeutic range [2]. These observations emphasize the need to look for alternative biomarkers of TAC response.
Measuring TAC directly into its site of effect (i.e. the lymphocyte or, for more practical reason, peripheral blood mononuclear cells (PBMC) a fraction that is enriched in lymphocytes) appears to be a promising strategy to refine TAC TDM. The proof of concept of the clinical interest of TAC measurement in PBMC was established in liver transplant recipients by Capron et al. [4]. The authors reported a good correlation between intrahepatic or intra-PBMC concentrations of TAC, and an histological rejection score determined at day-7 post-transplantation whereas no association was found with whole blood TAC concentrations. Besides, the relationship between TAC whole blood concentrations and TAC concentrations in PBMC have been studied in various organ transplant patients [5–11]. These works were concordant to report a poor correlation between trough whole blood and trough intracellular concentrations, meaning that monitoring TAC in its target cell could be a better surrogate marker of its pharmacological effect.
The study of TAC pharmacokinetics in PBMC may be of interest, but it is worthwhile to investigate factors determining drug intracellular disposition. TAC is known to be a substrate of membrane transporters, in particular the efflux transporter ABCB1 (P-glycoprotein or P-gp) expressed in lymphocytes [3,12]. Thus, genetic factors that alter P-gp activity could influence TAC disposition into its target cell. Many single nucleotide polymorphisms (SNP) of ABCB1 gene (coding for P-gp) have been described. The most extensively studied regarding TAC pharmacokinetics are 1236C>T (rs1128503), 2677G>T/A (rs2032582), and 3435C>T (rs1045642) which are in strong linkage disequilibrium. The influence of these SNPs on TAC whole blood concentrations has been widely investigated and led to conflicting results [13–17]. Nevertheless, some data suggest that ABCB1 genotype could impact TAC intracellular pharmacokinetics rather than whole blood pharmacokinetics [4,18–20]. In addition, the SNP 1199G>A (rs2229109) was associated with TAC intra-PBMC or intra-hepatic concentrations [18,20,21], and the variant 1199A was associated with an increased risk of kidney allograft loss [22]. Data regarding this SNP and TAC pharmacokinetics are sparse and should be further explored. Besides, CYP3A5 is the major metabolism enzymes of TAC. To date, CYP3A5 polymorphism is the only genetic marker used in clinical practice because of a clear relationship between CYP3A5 expression and TAC dose requirement to reach whole blood therapeutic range [23]. In addition, CYP3A4 genotype could influence TAC pharmacokinetics in particular in CYP3A5 non-expresser [23]. Nevertheless, it remains to be elucidated whether the most relevant SNP in CYP3A4 and CYP3A5 could impact TAC intracellular concentration in comparison to whole blood.
Finally, drug disposition in PBMC could determine the level of inhibition of CaN, the target enzyme. CaN activity in PBMC is intuitively an interesting pharmacodynamic biomarker of the immunosuppressive effect since an increase of CaN activity was suggested to occur before acute cellular rejection [24–26]. A few studies, conducted in liver transplantation, described the pharmacokinetic-pharmacodynamic relationship of TAC by studying the link between whole blood concentrations and CaN activity in PBMC [27–29]. However, the complete relationship between TAC whole blood concentration, TAC intra-PBMC concentration and TAC-induced CaN inhibition, have only been reported in a preliminary work by our team [30], and remains to be widely explored and emphasized with the drug pharmacogenetics.
The aims of the present study was then i) to develop a population pharmacokinetic model using a non-parametric modeling approach to describe whole blood and intracellular pharmacokinetics of tacrolimus, ii) to explore the pharmacogenetic-whole blood and intracellular pharmacokinetic-pharmacodynamic (PG-PK-PK-PD or PG-PK2-PD) relationships of TAC in liver transplant recipients in the early period post transplantation.
2. Material and methods
2.1. Study design
2.1.1. Patients
De novo Caucasian liver transplant recipients transplanted between November 2015 and September 2017 in Rennes University Hospital were included in the study which is an ancillary study of the CYPTAC’H protocol (“Pharmacogenetic study of tacrolimus in hepatic transplants » Clinical trial number: NCT01388387). The study protocol was approved by the local ethical committee (Rennes University Hospital). Adult patients, treated with TAC and who gave their written consent to participate in the study, were suitable for inclusion. Nevertheless, patient could not be included if its donor of graft was entered in the national register of refusals (all donors were deceased donors). Patients receiving induction treatment (i.e. anti-lymphocyte or anti-interleukine-2) were excluded because of PBMC (lymphocytes) depletion for several days after transplantation.
2.1.2. Immunosuppressive regimen
TAC treatment was started on post-operative day 0 or 1 (either at 8:00 AM or 8:00 PM, depending on the time the surgical procedure was completed). Patients received initially a dose of 0.04 to 0.05 mg/kg per 12 h or a dose of 0.02 to 0.03 mg/kg per 12 h in case of concomitant administration of fluconazole prophylaxis. TAC whole blood concentrations were monitored daily, then three times a week to maintain trough TAC whole-blood concentrations between 4 and 10 ng/mL. From day-1 post-transplantation, patients concomitantly received oral mycophenolate mofetil 1.5 g twice daily and 20 mg of prednisone once daily. They also received a 500 mg methylprednisolone infusion as an induction and one other 500 mg infusion at portal vein clamp removal. Other co-medications were recorded in the case report form to be sure that patients were not concomitantly treated by CYP450 or ABCB1 inducer or inhibitor at the time of the study.
2.1.3. Data collection
For each patient, 5 milliliters of peripheral venous blood were collected in EDTA tubes at 0, 20, 40, 60, 120, 180, 240, 360, 540 and 720 min after the morning oral dose of twice daily TAC, between the seventh and the tenth day of treatment. No dosage modification occurred within 3 days before sampling to measure concentrations as close as possible to steady state. After whole blood concentrations measurement, the remaining blood was used to obtain complete pharmacokinetic profiles of TAC in PBMC and to measure CaN activity in PBMC at each sampling time. Additional biological parameters were prospectively collected on the day of blood sampling such as albumin, blood count and hematocrit.
2.2. Isolation of PBMC
Beforehand measuring TAC concentration and CaN activity in PBMC, cells were isolated from whole blood at each sampling time by density gradient centrifugation according to the procedure previously described [31].
2.3. Pharmacokinetic modeling
Whole blood and intracellular pharmacokinetics of TAC were investigated using a non-parametric modeling approach (Pmetrics, version v. 1.5.2) [32]. The model was derived from the structural model previously developed by Robertsen et al. to describe everolimus concentrations in whole blood and PBMC [33]. Influence of covariates on individual pharmacokinetic parameters were investigated by multiple linear regression and non-parametric Kruskal-Wallis or Mann-Whitney comparison after graphical inspection. Thus, age, sex, body weight, albumin, hematocrit and count of PBMC in whole blood, were investigated as biological covariates. Additionally, SNPs in genes of ABCB1, CYP3A4 and CYP3A5 of donor and recipient were evaluated as genetic covariates of pharmacokinetic parameters. After graphical inspection, if significant association was found (p<0.01), covariates were individually introduced in the model. Decision to keep a covariate in the final model was based on the comparison of the Akaike information criterion (AIC), improvement of the bias and precision of the model. The model performance was assessed by diagnostic plots. An internal validation was performed using the visual predictive check (VPC) based on 1000 Monte-Carlo simulations.
2.4. Genotyping analysis
Genomic DNA was extracted from whole blood using a Janus automated workstation varispan (PerkinElmer, Courtaboeuf, France). Donor DNA was obtained from a DNA bank from the French Blood Agency. Genotyping were performed using Taqman® allelic discrimination assays (Thermofisher Waltham, MA, USA) on ABI 7900HT instrument (Applied Biosystems, Foster City, CA, USA).
Recipients and donors genotypes were determined for CYP3A4 rs35599367 C>T (CYP3A4*22allele), CYP3A5 rs776746 A>G (CYP3A5*3 allele). In addition, recipients and donor DNA were analyzed for the four SNPs of ABCB1: c.3435C>T (exon 26, rs1045642), ABCB1 c.1236 C>T (exon 12, rs1128503), ABCB1 c.2677 G>T/A (exon 21, rs2032582), and c.1199 G>A (exon 11, rs2229109).
2.5. Assessment of tacrolimus exposure in whole blood and PBMC and calcineurin activity assay
Whole-blood and intra-PBMC TAC concentrations were measured with a fully validated liquid chromatography tandem mass spectrometry procedure adapted from previously published methods [34,6]. Exposure to TAC was assessed in whole blood and PBMC by the areas under the curve of concentrations (AUC0-12h). AUC0-12h were derived from the population pharmacokinetic model developed. Additionally, trough concentrations (C0), TAC peak concentrations (Cmax) and the times to peak concentration (Tmax) in whole blood and PBMC were extracted from the data set.
The activity of CaN in PBMC was measured by HPLC-Ultraviolet according to Blanchet et al. [35]. For the pharmacokinetic-pharmacodynamic analysis, CaN activity was expressed by the area under the activity (AUA0-12h) calculated by the trapezoidal method. Maximal inhibition of CaN on the inter-dose was calculated relatively to the basal CaN activity of the patient measured in PBMC on a blood sample collected just before the first TAC intake.
2.6. Probability of target attainment
The population pharmacokinetic model was applied to further investigate the pharmacokinetic-pharmacodynamic relationship of TAC by simulations. The probability of attainment of a target intracellular concentration of TAC inhibiting CaN activity (ICtarget), was explored for several ranges of TAC trough concentrations in whole blood. Ranges were selected according to TDM recommendations in liver transplanted patients. Multimodal Monte Carlo simulations were performed to generate 1,000 concentration time profiles per subject of the dataset. For each profile, trough whole blood concentrations (C0WB) were extracted and categorized in one of the C0WB concentration range groups: 0–4 ng/mL (very low exposure), 4–6 ng/mL (low exposure), 6–10 ng/mL (recommended exposure). For each C0WB, the corresponding intra-PBMC Cmax was estimated with the model. Then, the probability of achieving an intra-PBMC Cmax above the ICtarget was assessed for each C0WB range.
2.7. Statistical analysis
Statistical analysis was performed using R software (version 3.2.5). Results are reported as means +/- standard error of the mean (SEM) or median and range. Shapiro-test was used to check variable distribution normality. Correlations between whole blood and intracellular pharmacokinetic parameters were performed using Pearson or Spearman test as appropriate. Coefficient of determination (r2) were reported and calculated from the corresponding coefficient of correlation when relationships were assessed by linear regression. The « SNPassoc package » was used to assess the Hardy-Weinberg equilibrium and “haplo.stat” to infer the most probable haplotype of ABCB1 (3435/1236/2677) (Package available on https://cran.r-project.org). Associations between TAC exposure parameters and genotypes were investigated using the non-parametric Mann-Whitney test or the Kruskal-Wallis test as appropriate. When a post-hoc test was required, the Bonferroni correction was applied.
Relationships between TAC concentrations in blood or PBMC and CaN activity were explored using linear regression. When needed, data were log-transformed and Shapiro-Wilk-test was applied to check normality of the residual of the linear model. Additionally, influence of TAC concentrations on CaN activity was analyzed with an inhibition model computed in Graphpad Prim (Version 8). A p-value< 0.05 was considered significant.
3. Results
3.1. Patients’ characteristics
A total of 32 liver transplant recipients were included in the study. Patients’ demographic and clinical characteristics are summarized in Table 1.
Table 1. Patients characteristics (n = 32).
| Demographic characteristics | Median [range] or n (%) |
| Sex (M) | 30 (94) |
| Age (years) | 62 [51–70] |
| Body weight (kg) | 97 [50–121] |
| Delay since transplantation (days) | 9 [7–11] |
| Biological characteristics (the day of the study) | |
| Albumin (g/L) | 23.8 [23.0–39.6] |
| Hematocrite (%) | 30.5 [22.8–39.1] |
| PBMC count in whole blood (G/L) | 2.3 [1.3–3.6] |
M: masculine, PBMC: peripheral blood mononuclear cells
Delay since transplantation means time between transplantation and the day of the study.
3.2. Model development and validation
The model developed was a two compartments model, with two absorption phases described by a double gamma distribution in the first compartment. Other absorption models including lag time and two mean absorption times in the structural model were also assessed but lead to worse concentration predictions. Several polynomial error models were tested. The standard deviation (SD) of the TAC concentrations (C) representing the assay error was calculated by the formula SD = 1+0.1 x C for whole blood concentrations corresponding to an additive (in μg/L) and proportional (%) part of the assay error, respectively, and SD = 5+0.12 x C for PBMC concentrations. In addition, both concentrations were weighted by an additional noise term λ = 1, according to the formula: 1/(SD+λ)2. The population pharmacokinetic parameters are presented in S1 Table.
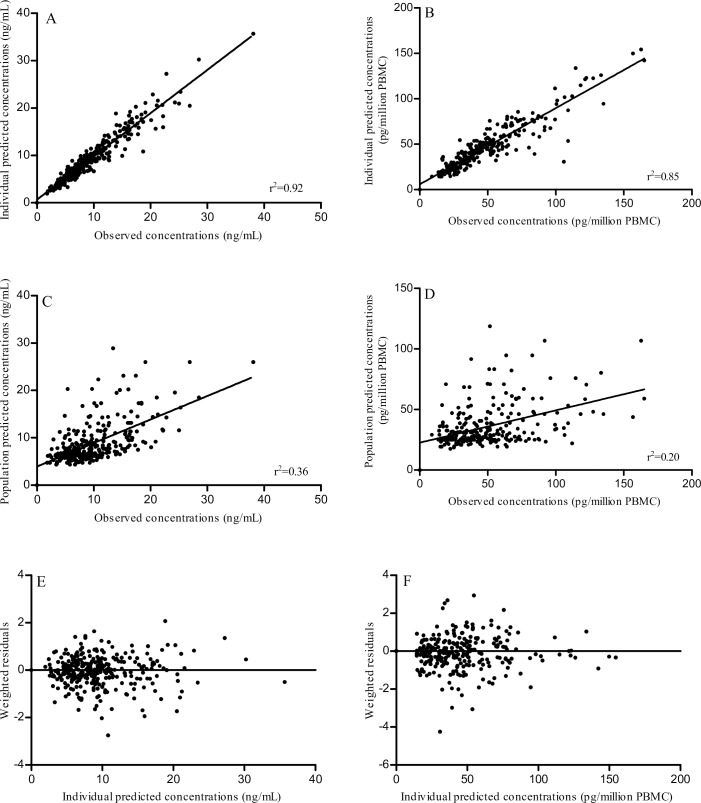
Influences of covariates on model parameters were assessed. Significant associations were found between the count of PBMC in whole blood and the inter-compartmental rate constant k21 (p = 0.00354) and, between genotype 1199GA for rs2229109 (ABCB1 exon 11) and C01 (model estimated whole blood trough concentration) (p = 0.00082) (S2 Table). Introduction of these covariates in the model did not improve the AIC neither the model precision. Plots of the model performances, for TAC in whole blood and in PBMC, are presented in Fig 1 as individual predicted versus observed plots, population predicted versus observed plots, weighted residuals versus predicted concentrations plots. The best and the worst fit of individual pharmacokinetic profiles with all-time points are reported in S1A and S1B Fig. Goodness of fit plots did not show any major bias in whole blood whereas a slight under-estimation was observed in PBMC. The weighted residuals were homogeneously distributed over the concentration ranges. For whole blood and PBMC, the VPC demonstrated that the majority of the normalized observed data fell within the 90% prediction intervals of the simulations and that the median tracks the middle of the observed data (S1C and S1D Fig).
Fig 1. Model performances-diagnostic plots.
Individual predicted versus observed concentrations of tacrolimus in whole blood (A) and in PBMC (B), population predicted versus observed concentrations of tacrolimus in whole blood (C) and in PBMC (D), weighted residuals versus individual predicted concentrations of tacrolimus in whole blood (E) and in PBMC (F).
3.3. Tacrolimus whole blood and intracellular pharmacokinetics
Time course profiles of TAC concentrations in whole blood and PBMC over 12h are presented in S2 Fig. Pharmacokinetic parameters of TAC in whole blood and PBMC are reported in Table 2.
Table 2. Tacrolimus pharmacokinetics parameters in whole blood and PBMC (n = 32).
| Median [range] | Mean (SD) | |
|---|---|---|
| Dose (mg/12 h) | 1.5 [0.5–4] | 1.8 (1.0) |
| Dose (mg/kg/12h) | 0.017 [0.005–0.048] | 0.021 (0.012) |
| Whole blood pharmacokinetics (WB) | ||
| Cmax (ng/mL) | 17.7 [3.5–36.3] | 16.4 (6.9) |
| Cmax/dose (ng/mL/mg) | 9.5 [3.0–21.4] | 10.8 (5.2) |
| Tmax (h) | 1.6 [0.2–6] | 1.9 (1.4) |
| C0 (ng/mL)a | 6.2 [2.5–10.0] | 6.4 (2.2) |
| C0/dose (ng/mL/mg) | 3.9[1.1–17.7] | 5.1 (4.2) |
| AUC0–12h (ng∙h/mL) | 102.3 [35.0–215.5] | 108.9 (38.9) |
| Cl/F (L.h-1) | 16.2 [5.0–39.2] | 17.8 (9.0) |
| Intracellular pharmacokinetics (PBMC) | ||
| Cmax (pg/million PBMC) | 71.3 [25.7–156.0] | 78.1 (37.1) |
| Cmax/dose (pg/million PBMC/mg) | 44.2[17.1–258.7] | 56.1 (46.1) |
| Tmax (h) | 1.6 [0.3–6] | 1.9 (1.2) |
| C0 (pg/million PBMC) | 28.4 [9.6–80.4] | 37.2 (17.7) |
| C0/dose (pg/million PBMC/mg) | 20.0 [3.2–67.2] | 24.8 (16.8) |
| AUC0–12h (pg∙h/million PBMC) | 491.6 [223.0–1127.2] | |
| Whole blood—intracellular relationships | ||
| Intracellular diffusion ratio (AUC0–12h PBMC / AUC0–12h WB) |
23.6 [14.8–39.7] | 24.9 (6.9) |
| r2 (p) | ||
| Correlation AUC0–12h PBMC & AUC0–12h WB | 0.51 (<0.001) | |
| Correlation C0PBMC & C0WB | 0.39 (<0.001) | |
| Correlation CmaxPBMC & CmaxWB | 0.53 (<0.001) | |
| Correlation CmaxPBMC & C0WB | 0.17 (0.02) | |
| Correlation AUC0–12h PBMC & C0WB | 0.53 (<0.001) |
Cmax: maximum concentration; Tmax: time when Cmax is achieved; C0: predose concentration; AUC0-12h: area under the concentration–time curve from 0h to 12h; Cl/F: apparent clearance; PBMC: peripheral blood mononuclear cells; WB: whole blood; SD: standard deviation; r2: coefficient of determination; p: p-value
a: the day of the study, 81.2% of patients had tacrolimus whole blood concentration between 4–10 ng/mL.
Relationships between TAC AUC0-12h and C0 or Cmax were explored within each compartment. Significant but poor correlations were found between C0 and AUC in whole blood (r2 = 0.42, p<0.001) and in PBMC (r2 = 0.61, p<0.001). A weak correlation was found between Cmax and AUC in whole blood (r2 = 0.52, p<0.001) and in PBMC as well (r2 = 0.55, p<0.001).
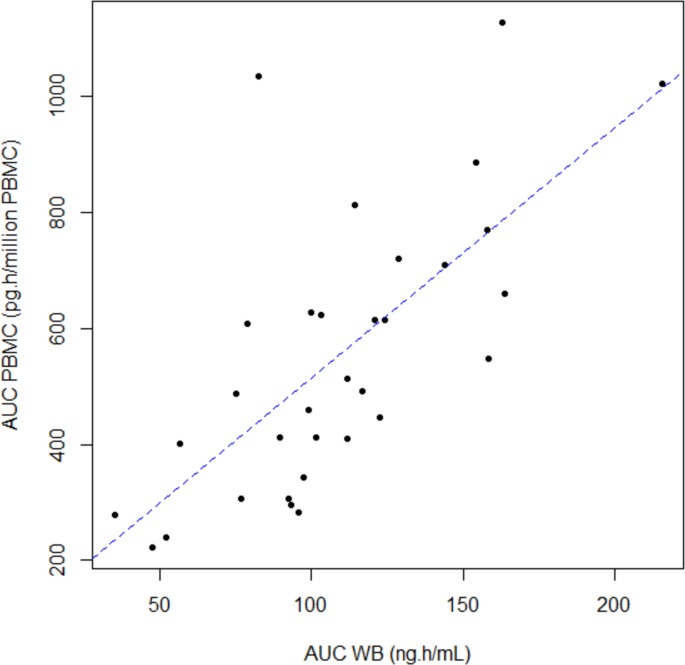
Median intracellular distribution ratio of TAC (AUCPBMC/AUCWB) was 23.6. TAC exposure in PBMC was correlated to TAC exposure in whole blood. However, the strength of the linear association was weak, whatever the exposure parameters studied (r2< 0.53) (Table 2). Fig 2 displays the correlation between AUC0-12h in whole blood and in AUC0-12h in PBMC compartments.
Fig 2. Relationship between area under the concentration–time curve from 0 to 12 h (AUC) of tacrolimus in whole blood (WB) and in peripheral mononuclear blood cells (PBMC).
The dotted line is the linear regression curve. (n = 32) (r2 = 0.51, p<0.001).
No significant association was found between whole blood or PBMC pharmacokinetics parameters (AUC0-12h and C0 or Cmax, AUCPBMC/AUCWB), and patients’ demographic characteristics (age, sex weight and time since transplantation). In addition, intra-PBMC exposure and intracellular distribution ratio were not correlated to serum albumin nor PBMC count in blood (p>0.05). Hematocrit appeared to have a slight influence on intra-PBMC exposure (AUC0-12h) and the distribution ratio (r = -0.31, p = 0.047 and r = -0.34, p = 0.036 respectively). Nevertheless, hematocrit value was not influenced by the time post transplantation (p = 0.13, Anova- test).
3.4. TAC pharmacokinetic-pharmacogenetic relationship
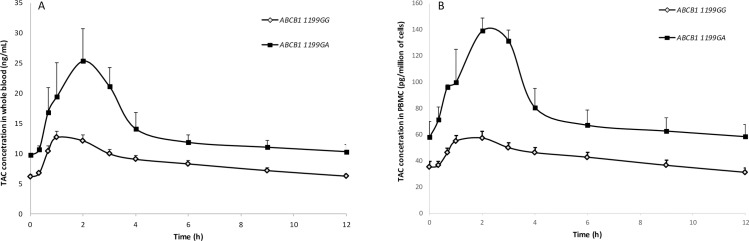
Although integration of genetic covariates did not improve the pharmacokinetic model, the influences of SNPs on TAC exposure parameters in whole blood and PBMC were investigated. Genotypes frequencies for ABCB1, CYP3A4 and CYP3A5 (donor and recipient) genes are shown in Table 3. Hardy–Weinberg equilibrium was verified for each genotype except recipient ABCB1 3435C>T (exon 26, rs1045642) and donor CYP3A5 rs776746 A>G. This deviation was attributed to the small size of our dataset since genotyping analysis of this SNP was double-checked by another external laboratory. Effect of each SNP on TAC pharmacokinetics in whole blood and PBMC are presented in Table 3. No significant association was found between ABCB1 3435C>T SNP and TAC pharmacokinetics in whole blood or PBMC. Recipients heterozygous CT for ABCB1 1236C>T polymorphism seemed to have lower AUC0-12h and Cmax in whole blood than wild type CC (p = 0.045 and p = 0.035 respectively), however these associations were not significant after Bonferroni correction (p = 0.092 and p = 0,107 respectively). Similarly, a lower whole blood Cmax was observed in recipients heterozygous CT for ABCB1 2677G>T SNP (p = 0.046) but the difference compared to GG genotype was not significant after post-hoc analysis. Intracellular distribution ratio was lower in recipients homozygous for ABCB1 2677TT compared to 2677GT (p = 0.026). The ratio was also influenced by ABCB1 haplotype (3435/1236/2677) since homozygous TTT/TTT had lower ratio than subject carrying only one TTT allele (p = 0.001). The SNP ABCB11199G>A in recipient influenced both whole blood and intra-PBMC exposure. Median AUC0-12h, C0 and Cmax were significantly higher among subjects carrying the “A” allele (p = 0.009, p = 0.009, p = 0.03 in whole blood, and p = 0.006, p = 0.04, p = 0.0008 in PBMC, respectively). This is illustrated in Fig 3 by whole blood and PBMC concentration versus time profiles according to recipient 1199G/A genotype. No influence of donor (graft) ABCB1 genotype was found on TAC whole blood or PBMC exposures. A non-significant trend to lower C0 was observed in whole blood and PBMC for subject with a graft expresser of CYP3A5 (carrier of at least one 1 allele *1). Neither donor nor recipient CYP3A4*22 polymorphism had any impact on TAC pharmacokinetics in whole blood or PBMC.
Table 3. Influence of single nucleotide polymorphisms on TAC pharmacokinetics in whole blood and in PBMC.
| Genotype | Allelic status | n | (%) | Tac AUC0-12h WB | Tac AUC0-12h PBMC | AUC0-12h PBMC/ | Tac C0 WB | Tac C0 PBMC | Tac Cmax WB | Tac Cmax PBMC |
|---|---|---|---|---|---|---|---|---|---|---|
| (ng.h/mL) | (pg.h/million of PBMC) | AUC0-12h WB | (ng/mL) | (pg/million of cells) | (ng/mL) | (pg/million PBMC) | ||||
| Recipient ABCB1 3435C>T (rs1045642) | CC | 2 | (6) | 132.2 | 769.1 | 27.4 | 6.7 | 49.9 | 19.4 | 101.2 |
| [101.4–162.9] | [411.1–1127.2] | [20.2–34.6] | [3.7–9.8] | [19.4–80.4] | [15.9–22.9] | [60.1–142.3] | ||||
| CT | 21 | (66) | 99.7 | 548.8 | 24.3 | 6.2 | 30 | 15.3 | 71.3 | |
| [35.0–215.5] | [223.0–1022.0] | [14.8–39.7] | [2.5–10.0] | [9.6–76.9] | [3.5–36.3] | [25.7–158.0] | ||||
| TT | 9 | (28) | 103.1 | 486.6 | 19.2 | 6.2 | 23.2 | 18.3 | 74.9 | |
| [75.1–163.8] | [295.7–1034.2] | [15.9–32.4] | [3.7–9.3] | [19.9–43.6] | [7.0–24.9] | [34.2–152.5] | ||||
| Recipient ABCB1 1236 C>T (rs1128503) | CC | 10 | (31) | 128.9 | 642 | 23.6 | 8.1 | 31.4 | 19.3 | 77.9 |
| [47.3–215.5] | [223.0–1127.2] | [14.8–35.6] | [3.7–9.8] | [9.6–80.4] | [5.9–36.3] | [25.7–156.8] | ||||
| CT | 15 | (47) | 89.7 | 491.6 | 26.7 | 6.5 | 29.2 | 11 | 62.8 | |
| [35.0–154.2] | [238.9–1034.2] | [17.6–39.7] | [2.5–10.0] | [17.7–60.9] | [3.5–22.4] | [28.9–158.0] | ||||
| TT | 7 | (22) | 111.8 | 447.4 | 18.3 | 5.4 | 23.9 | 18.1 | 71.3 | |
| [92.4–158.1] | [295.7–769.5] | [15.9–24.8] | [3.7–10.0] | [19.9–58.2] | [14.9–24.9] | [34.2–101.7] | ||||
| Recipient ABCB1 2677 G>T/A (rs2032582) | GG | 11 | (34) | 114 | 623.9 | 23.6 | 7.8 | 23.2 | 18.3 | 77 |
| [47.3–215.5] | [223.0–1127.2] | [14.8–35.6] | [3.7–9.8] | [9.6–80.4] | [5.9–36.3] | [25.7–156.8] | ||||
| GT | 15 | (47) | 89.7 | 514.2 | 26.7 | 6.5 | 32.1 | 11.2 | 69.4 | |
| [35.0–158.1] | [238.9–1034.2] | [20.0–39.7] | [2.5–10.0] | [17.7–60.9] | [3.5–22.4] | [28.9–158.0] | ||||
| TT | 6 | (19) | 105.4 | 428.2 | 18.3 | 5.3 | 23.1 | 19.6 | 70 | |
| [92.4–123.9] | [295.7–613.6] | [15.9–24.8] * | [3.7–6.2] | [19.9–43.9] | [17.5–24.9] | [34.2–101.7] | ||||
| Recipient ABCB1 Haplotype 3435/1236/2177 | Het TTT | 17 | (53) | 99.1 | 514.2 | 25.1 | 6.2 | 32.1 | 14.9 | 69.4 |
| [35.0–158.1] | [238.9–1034.2] | [20.0–39.7] | [2.5–10.0] | [17.7–60.9] | [3.5–22.4] | [29.0–158.0] | ||||
| HomTTT | 4 | (13) | 102.4 | 357.4 | 17.4 | 4.5 | 21.6 | 20.4 | 64.3 | |
| [92.4–122.6] | [295.7–447.4] | [15.9–18.3] * | [3.7–6.2] | [19.9–23.9] | [17.5–24.9] | [34.2–82] | ||||
| Other | 11 | (34) | 114 | 623.9 | 23.6 | 7.8 | 23.2 | 18.3 | 77.0 | |
| [47.3–215.5] | [223.0–1127.2] | [14.8–35.6] | [3.7–9.8] | [9.6–80.4] | [5.9–36.3] | [25.7–156.8] | ||||
| Recipient ABCB1 1199 G>A (rs2229109) | GG | 29 | (91) | 99.7 | 486.6 | 23.1 | 6 | 23.9 | 17.5 | 69.4 |
| [35.0–163.8] | [223.0–1034.2] | [14.8–39.7] | [2.5–10.0] | [9.6–76.9] | [3.5–24.9] | [25.7–152.5] | ||||
| GA | 3 | (9) | 162.9 | 1022 | 28 | 9.8 | 54.6 | 22.9 | 156.8 | |
| [128.5–215.5]* | [720.4–1127.2]* | [23.7–34.6] | [9.6–10.0]* | [39.3–80.4]* | [19.9–36.3]* | [142.3–158.0]* | ||||
| Recipient CYP3A4 (C>T) (*22, rs35599367) | CC | 30 | (94) | 102.3 | 531.5 | 23.6 | 6.3 | 29.6 | 17.7 | 70.3 |
| [35.0–215.5] | [223.0–1127.2] | [14.8–39.7] | [2.5–10.0] | [9.6–80.4] | [3.5–36.3] | [25.7–158.0] | ||||
| CT | 2 | (6) | 84.2 | 405.7 | 26.9 | 4.2 | 22.7 | 20.1 | 76.6 | |
| [56.7–111.8] | [402.2–409.1] | [18.3–35.5] | [3.7–4.7] | [22.0–23.3] | [15.3–24.9] | [71.3–82.0] | ||||
| Recipient CYP3A5 6986 G>A (*3, rs776746) | AA | 32 | (100) | 102.3 | 502.9 | 23.6 | 6.2 | 28.4 | 17.7 | 71.3 |
| [35.0–215.5] | [223.0–1127.2] | [14.8–39.7] | [2.5–10.0] | [9.6–80.4] | [3.5–36.3] | [25.7–158.0] | ||||
| Donor ABCB1 3435C>T (rs1045642) | CC | 8 | (25) | 97.7 | 357.9 | 20.1 | 5.9 | 22.3 | 17.9 | 72.1 |
| [52.0–163.8] | [238.9–720.4] | [14.8–31.5] | [2.9–10.0] | [9.6–39.3] | [8.6–24.9] | [33.4–158.0] | ||||
| CT | 12 | (38) | 96.1 | 453.7 | 24.3 | 5.1 | 28.5 | 15.6 | 71.3 | |
| [35.0–158.1] | [277.6–1034.2] | [15.9–39.7] | [2.5–10.0] | [17.7–76.9] | [3.5–23.2] | [28.9–129.4] | ||||
| TT | 12 | (38) | 120.3 | 581.2 | 24.2 | 7.2 | 34.8 | 19.9 | 74.2 | |
| [47.3–215.5] | [223.0 1127.1] | [17.4–34.6] | [3.7–9.8] | [18.1–80.4] | [5.9–36.3] | [25.7–156.8] | ||||
| Donor ABCB1 1236 C>T (rs1128503) | CC | 11 | (34) | 111.7 | 514.2 | 23.0 | 5.8 | 23.9 | 18.3 | 74.9 |
| [35.0–163.8] | [238.9–813.0] | [14.8–39.7] | [2.5–10.0] | [9.6–76.9] | [3.5–24.9] | [28.9 158.0] | ||||
| CT | 13 | (41) | 97.5 | 447.4 | 21.6 | 6.5 | 23.3 | 15.9 | 71.3 | |
| [56.7–158.2] | [295.7–1034.2] | [15.9–35.5] | [3.7–10.0] | [18.1–58.2] | [7.0–23.3] | [40.5–152.5] | ||||
| TT | 8 | (25) | 120.3 | 610.7 | 24.2 | 6.1 | 37.0 | 19.4 | 83.5 | |
| [47.3–215.5] | [223.0–1127.2] | [21.1–38.6] | [3.7–9.8] | [19.2–80.4] | [5.9–36.3] | [25.7–156.8] | ||||
| Donor ABCB1 2677 G>T/A (rs2032582) | GG | 10 | (31) | 105.6 | 564.4 | 24.2 | 6.4 | 28.0 | 17.9 | 72.1 |
| [35.0–163.8] | [238.9–813.0] | [14.8–39.7] | [2.5–10.0] | [9.6–76.9] | [3.5–22.4] | [28.9–158.0] | ||||
| GT/A | 11 | (34) | 97.48 | 411.1 | 20.1 | 4.9 | 22.2 | 17.6 | 74.9 | |
| [56.7–143.8] | [295.7–1034.2] | [15.9–35.5] | [3.7–9.2] | [18.1–51.5] | [8.9–24.9] | [40.5–152.5] | ||||
| TT/A | 11 | (34) | 123.9 | 607.8 | 24.3 | 7.0 | 43.6 | 18.1 | 65.4 | |
| [47.3–215.5] | [223.0–1127.2] | [17.4–38.6] | [3.7–10.0] | [19.2–80.4] | [5.9–36.3] | [25.7–156.8] | ||||
| Donor ABCB1 Haplotype 3435/1236/2177 | HetTTT | 14 | (44) | 98.3 | 473.3 | 23.2 | 5.8 | 28.5 | 16.8 | 68.3 |
| [56.7–158.2] | [295.7–1034.2] | [15.9–38.6] | [3.7–10.0] | [18.1–58.2] | [7.0–23.3] | [40.5–152.5] | ||||
| HomTTT | 6 | (19) | 139.1 | 750.2 | 24.2 | 7.7 | 29.6 | 21.0 | 114.0 | |
| [47.3–215.5] | [223.0–1127.2] | [21.1–34.6] | [3.7–9.8] | [19.2–80.4] | [5.9–36.3] | [25.7–156.8] | ||||
| other | 12 | (38) | 105.7 | 461.7 | 23.0 | 6.1 | 23.2 | 17.9 | 72.1 | |
| [35.0–163.8] | [238.7–813.0] | [14.8–39.7] | [2.5–10.0] | [9.6–76.9] | [3.5–24.9] | [28.9–158.0] | ||||
| Donor ABCB1 1199 G>A (rs2229109) | GG | 31 | (97) | 103.1 | 514.2 | 23.4 | 6.2 | 29.2 | 17.7 | 71.3 |
| [47.3–215.5] | [223.0–1127.2] | [14.8–38.6] | [3.0–10.0] | [9.6–80.4] | [5.9–36.3] | [25.7–158.0] | ||||
| GA | 1 | (3) | 35.0 [n/a] | 277.6 [n/a] | 39.7 [n/a] | 2.5 [n/a] | 17.7 [n/a] | 3.5 [n/a] | 28.9 [n/a] | |
| Donor CYP3A4 (C>T) (*22, rs35599367) | CC | 27 | (84) | 99.7 | 491.6 | 24.5 | 6.2 | 29.2 | 17.5 | 71.3 |
| [35.0–162.9] | [223.0–1127.2] | [14.8–39.7] | [2.5–10.0] | [9.6–80.4] | [3.5–24.9] | [25.7–158.0] | ||||
| CT | 5 | (16) | 158.2 | 548.8 | 20.3 | 8.5 | 23.4 | 18.3 | 74.9 | |
| [89.7–215.5] | [411.1–1022.0] | [17.4–23.7] | [3.7–10.0] | [19.4–54.6] | [15.9–36.3] | [60.1–156.8] | ||||
| Donor CYP3A5 6986 G>A (*3, rs776746) | Expressor GG and GA | 2-Jan | (3)/(6) | 103.1 | 447.4 | 18.3 | 4.8 | 19.9 | 23.2 | 77 |
| [95.8–122.6] | [283.7–623.9] | [14.8–30.3] | [3.8–4.9] | [9.6–23.2] | [20.2–23.3] | [74.9–152.5] | ||||
| Non expressor AA | 29 | (91) | 101.44 | 514.2 | 23.6 | 6.5 | 30 | 17.5 | 69.4 | |
| [35.0–215.5] | [223.0–1127.2] | [15.9–39.7] | [2.5–10.0] | [17.7–80.4] | [3.4–36.2] | [25.72–158.0] |
AUC0-12h: area under the concentration–time curve from 0h to 12h; PBMC: peripheral blood mononuclear cells; WB: whole blood; C0: predose concentration; Cmax: maximum concentration; Het: heterozygote; Hom: homozygote. Data are expressed as median [range]
* p<0.05.
Fig 3. Influence of recipient ABCB1 1199G>A on whole blood and on intracellular (PBMC) areas under the tacrolimus (TAC) concentrations–time curve from 0 to 12 h (AUC).
Each symbol represents mean ± standard deviation of the mean. (n = 29 ABCB1 1199GG, n = 3 ABCB1 1199GA).
3.5. Tacrolimus pharmacokinetic-pharmacodynamic relationship
The time course profiles of CaN activity between two TAC intakes is shown in S2 Fig. Pharmacodynamic parameters related to CaN activity in PBMC are reported in Table 4. Median minimal CaN activity (CaNmin) was achieved 2h post TAC intake (Tmin) which is slightly delayed after the Tmax of TAC in whole blood and PBMC (1.6h). Coefficients of variation of pharmacodynamic parameters were higher than 30% which reflects a high inter-patient variability of CaN activity. No correlation was found between AUA0-12h and TAC AUC0-12h in whole blood or PBMC. Inhibition of CaN compared to its basal value (i.e. before TAC treatment) was never complete and the median CaNImax was only -37%.
Table 4. Pharmacodynamic parameters (n = 32).
| Median [range] | CV (%) | |
|---|---|---|
| AUA0-12h (pmol.h/min/million PBMC) | 4864 [2903–8598] | 36 |
| CaN basal (pmol/min/million PBMC) | 371.6 [151.6–1550.1] | 66 |
| CaNmin (0-12h) (pmol/min/million PBMC) | 317.3 [96–547.8] | 39 |
| CaNmoy (pmol/min/million PBMC) | 412.2 [229.9–791.96] | 32 |
| % Inhibition max | -37 [–74–50] | 45 |
| Tmin (h) | 2 [0.33–12] | n/a |
AUA0-12h: area under the calcineurin-time curve from 0 to12h; CaN basal: calcineurin activity at baseline before the first administration of tacrolimus; CaNmin(0-12h): calcineurin activity when inhibition is maximal between 0 and 12h post tacrolimus intake; CaNmoy: mean calcineurin activity between 0 and 12h post tacrolimus intake; Tmin: Time corresponding to CaNmin (0-12h). PBMC: peripheral blood mononuclear cells CaN activity assay was based on the detection of the product formed by CaN incubated with a peptide substrate. CaN activity was expressed as picomoles of dephosphorylated peptides per minute per 106 cells
Using a linear model, an association was found between CaNImax in PBMC and Cmax of TAC (log-transformed) in PBMC (r2 = 0.21, p = 0.019) or in whole blood (r2 = 0.27, p = 0.007). The trend of the concentration dependent inhibition of CaN activity by TAC was better described using an equation like CaNImax = Imin+(Imin-Imax)/(1+(Cmax/IC50)) where CaNImax is the maximal inhibition of CaN activity observed on the 0-12h period for a concentration Cmax of TAC, Imin is the highest inhibitory effect and Imax is the lowest inhibitory effect. IC50 is TAC Cmax which gives a 50% inhibition of CaN compared to basal activity.
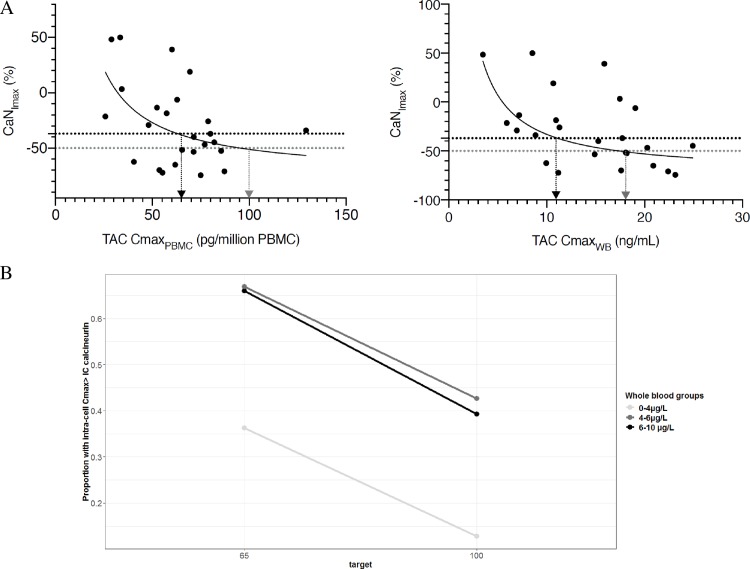
Graphically, the IC50 of TAC for CaN was 18 ng/mL in whole blood and 100 pg/million of cells in PBMC. In addition, the IC37 (TAC Cmax which gives the median CaNImax of 37% in the study) was 11 ng/mL in whole blood and 65 pg/millions of cells in PBMC (Fig 4A).
Fig 4. Tacrolimus (TAC) pharmacokinetic-pharmacodynamic relationship.
(A): Relationship between calcineurin maximum inhibition (CaNImax) and TAC maximum concentration (Cmax) in peripheral mononuclear cells (PBMC) or whole blood (WB). Black arrows show tacrolimus concentration inhibiting 37% (IC37) of calcineurin activity (65 pg/million of cells in PBMC and 11 ng/mL in whole blood) and greys arrows show tacrolimus concentration inhibiting 50% (IC50) of calcineurin activity (100 pg/million of cells in PBMC and 18 ng/mL in whole blood). (n = 32). Probability of intracellular target attainement (B). Targets are IC37 and IC50 in PBMC. IC: Inhibitory concentration.
Intra-PBMC inhibitory concentrations of CaN where used as ICtarget of TAC Cmax to explore the probability of target attainment of these intracellular concentrations for several ranges of trough whole blood concentrations. Less than 50% of patients were expected to achieve intra-PBMC IC50 whatever their C0WB group (13%, 39% and 42% for the low, medium and high exposure groups respectively). The probability to achieve the intra-PBMC IC37 was 36% for the low exposure group, 66% for the medium exposure group and 67% for the group with highest C0WB (Fig 4B).
4. Discussion
To the best of our knowledge, this is the first study aiming at exploring the complete pharmacogenetic-whole blood/intracellular pharmacokinetic-pharmacodynamic (PG-PK2-PD) relationship of TAC in liver transplant recipients. Most of the studies published to date aimed at exploring part of this relationship (i.e. PG-PK or PK-PD) but none had given a complete overview of this relationship in a quite large number of rich pharmacological profiles. These full profiles were used to model TAC concentrations in whole blood and, for the first time, in PBMC. The model quite well described the drug concentrations in both compartments. As observed on individual predicted versus observed plots, intra-PBMC concentrations were slightly under-estimated for a few patients. One can raise the hypothesis that it might be due to the normalization of TAC intra-PBMC concentration in quantity per number of cells instead of volumetric unit. Indeed, as suggested by Pensi et al., it would be more accurate to normalize intracellular concentration by the mean cell volume of each patient [7,36]. Unfortunately, measurement of cells volume was not available on the instrument use in our study and we could not check whether the underestimated concentrations were due to extreme values in PBMC volumes in these individuals.
Although it should be further validated in an independent cohort, the model appears to be a useful tool to assess TAC intracellular exposure. This is of particular interest since the experimental process to quantify TAC in cells is time-consuming and can be challenging for an application in routine practice.
One of the most point open for criticism of this study is our choice to assess TAC exposure in the early postoperative period which is thought to be unstable. In particular, hematocrit is assumed to change in the early phase post transplantation which could alter equilibrium between whole blood and intracellular TAC concentrations. However we consider that the study was performed in a relative steady state. Indeed, concentrations were measured beyond 7 days post-transplantation and TAC dose was unchanged since at least 3 days before blood collection. In addition, we showed that hematocrit was not significantly different within to the range of times post transplantation in our cohort. The poor correlation between hematocrit and intracellular exposure explains that the influence of this parameter was not sufficient to retain it as a significant covariate in the model developed. Moreover, we chose to focus on this early period post transplantation because acute rejection in liver recipients is more frequent within the first 10 postoperative days. It appears then much more relevant to study biomarkers related to TAC pharmacokinetics variability during this critical period.
In the present work, we provided a pharmacogenetics analysis to explore the influence of SNPs in both donors and recipients, on TAC pharmacokinetics in the early period post-transplantation. We focused on the most relevant polymorphisms which have been associated with TAC pharmacokinetics in the literature [13].
Interestingly, we found an impact of recipient ABCB1 polymorphisms 1199G>A on whole blood but also on intracellular exposure of the drug. Despite the actual relevance of this result could suffer from the small sample size and should be confirmed further, it is consistent with previous reports. Indeed, it is assumed that the variant 1199A lead to a lower activity of ABCB1 protein. In a functional in-vitro study in recombinant cell line, Dessilly et al. showed that TAC efflux was strongly lower in cells expressing the variant allele (1199A) compared to wild type cells [21]. Thus, 1199A genotype in recipient could lead to increase whole blood exposure by increasing its absorption through the intestinal barrier (due to decreased TAC efflux), and to increase TAC accumulation in PBMC due to a less active efflux from the cells. This mechanism was confirmed in a clinical study from the same group showing that TAC PBMC concentration at day 7 post kidney transplantation was 1.4 fold higher in recipient carrier of the 1199A allele [18]. Besides, recipient ABCB1 2677TT genotype and haplotype (3435/1236/2677) influenced TAC blood to PBMC diffusion ratio leading to lower ratio in patient homozygous TTT. Conflicting results have been reported regarding ABCB1 2677G>T polymorphism or corresponding haplotype and TAC dose requirement [12,13,37,38]. In liver transplant recipient ABCB1 haplotype is not expected to have a strong impact on TAC whole blood pharmacokinetics but it could significantly influence leukocytes concentration since P-gp is expressed in the cell membrane. Contrary to observations of TAC accumulation in hepatocytes for T variant carrier [20], in PBMC, presence of T-allele seemed to lower intracellular exposure. The molecular mechanism remains to be elucidated. Nevertheless, a limit of our study is the size of our data set which was likely not large enough to evidence accurately all genetic associations for SNP with low allele frequency. Then these results should be further confirmed in larger cohorts. Besides, we did not assess the influence of corticosteroids dosages on tacrolimus concentrations in whole blood and PBMC. It is unfortunate since steroids are known to induce CYP3A and ABCB1 expression so it could have been valuable to look for the influence of this covariate as well.
Besides, whole-blood and intracellular concentrations seems to be correlated contrary to what has been initially reported in a previous study [30]. This relationship legitimates TAC TDM in whole blood since whole blood concentrations could roughly reflect concentration in the target compartment. A work from Han et al. in kidney transplantation, exploring the relationship between whole blood and intracellular TAC concentrations, ended to the same conclusion [10]. However, whole blood concentrations are only a partial reflection of intracellular concentrations as highlighted by the mild to moderate correlations found between different time points (r2 ranging from 0.17 to 0.53). This poor association might not be due to the physiologic instability in the early period post transplantation. Indeed, a work from Klaasen et al. refutes the hypothesis of the lack of correlation linked to de novo status of the liver, since the authors reported remaining poor correlations between whole blood and intra-PBMC tacrolimus concentrations from 1 week to 1 year post transplantation [39]. As confirmed by the pharmacogenetic association study, drug transporters such as P-gp may at least partially explain these variabilities. This might also explain why some patients exhibit adverse events (rejection or toxicity) while having whole blood concentrations within the therapeutic range. New strategies aiming at evidencing this sup-population of patients displaying inconsistencies between whole blood concentrations and clinical outcome are needed. While confirmation of its value is required, measuring TAC intracellular concentrations might help detecting this at-risk population. Moreover, consistently with previous reports in the literature, poor correlations were observed between AUC of tacrolimus and trough concentrations within the same matrix, which shows that trough concentration is an imperfect surrogate marker of AUC [2,40].
Considering pharmacodynamic parameters, we report a low and relatively flat calcineurin inhibition in the study population. In addition, a relatively high inter-patient variability of CaN activities was observed. These results are consistent with previous published work on that topic [27–29]. We tried to develop a pharmacokinetic-pharmacodynamic model to describe the relationship between TAC exposure and inhibition of its molecular target but all modeling approaches tested failed. It was attributed to the size of our data set and the flat profile of CaN activity over time within patients. Moreover, another determinant of the complex relationship between CaN activity and exposure to TAC could be SNPs in the promoter region of the catalytic subunit of CaN (e.g. PPP3CA, rs45441997), as highlighted by the work of Noceti et al. [41]. Unfortunately, genetic analysis of gene involved in the pharmacodynamic pathway of TAC could not be performed in our work.
A non-complete inhibition of CaN was observed since the median CaN inhibition was 37%. High TAC whole blood concentrations have been previously reported as CaN IC50 (i.e 26.4 ng/mL for Fukudo et al. and 20.9 ng/mL for Yano et al.). These concentrations are hardly reached with modern TAC minimizing strategy [27,42]. In our study, we found an IC50 of 18 ng/mL for whole blood TAC concentration, a value slightly below but close to previous findings. More interestingly, we also highlighted an intracellular concentration of 100 pg/million cells as in vivo intracellular CaN IC50. This value is slightly lower than the one previously determined in vitro by our group (160 pg/million cells) [31]. Again, this threshold is rarely reached with current drug regimen in liver transplantation. When simulating 1,000 concentrations profiles and determining their intracellular Cmax, we observed that a few patients reached the concentration threshold corresponding to the IC50. When categorizing simulated patients according to their whole blood trough concentrations, patients with a C0WB lower than 4 ng/mL almost never reach the intracellular concentration threshold for IC50 while roughly the same proportion of patients (but less than 50%) with C0WB between 4 and 6 ng/mL or 6 and 10 ng/mL (i.e. the actual target recommendation in liver transplantation) reach the target. Despite not consistently attaining the threshold even with the current recommended trough whole blood concentrations (6–10 ng/mL), TAC based treatments seems to present sufficient efficacy in term of rate of rejection and graft survival. This means that, in the era of a combined immunosuppressive therapy associating TAC, mycophenolic acid or m-TOR inhibitor and corticosteroids, a high level of CaN inhibition may not be necessary. In agreement with this statement, Daher Abdi et al. reported that immunosuppressive efficacy (in renal transplant recipients) was not associated with calcineurin inhibitor exposure while mycophonolic acid exposure significantly mattered [43]. This hypothesis is emphasized by the median maximal CaN inhibition found in our study (37%). Considering this median CaN inhibition as a threshold, only 36% of patients in the very low exposure group of simulated patients would reach the corresponding intracellular Cmax (65 pg/million cells) while difference in the proportion of patients reaching it in the low-exposure or recommended exposures groups were again similar (66% versus 67%).
The main limitation of our study is the lack of clinical endpoint to link with the PG-PK2-PD analysis. In particular, it should have been relevant to confront our results to toxicity or rejection rate. However, ACR monitoring could not be included in the design of the study since in our center liver biopsy is not systematically performed in the patient’s follow-up to diagnose ACR. Moreover, data regarding associations between genetic biomarkers and graft or patient survival are lacking but will be obtained from the larger CYPTAC’H study on going in our center.
5. Conclusion
In conclusion, a population pharmacokinetic model was successfully developed and applied to the first global investigation of the pharmacogenetic-whole blood/intracellular pharmacokinetic-pharmacodynamic (PG-PK2-PD) relationship of TAC in liver transplant recipients. Recipient ABCB1 polymorphisms 1199G>A could influence whole blood but also intracellular exposure of TAC but the clinical relevance of this genetic variant remains to be investigated. In addition, CaN activity appeared incompletely inhibited by TAC and only few patients were expected to reach intracellular IC50 concentrations at therapeutic whole blood concentration suggesting alternative pharmacodynamic effects of TAC than CaN inhibition. These results should be confirmed in a larger cohort. Further studies are required to clarify the relationship between intracellular TAC exposure and clinical outcomes in order to find whether TAC intracellular concentration could be useful to tailor the immunosuppressive therapy.
Supporting information
Worst (A) and best (B) individual predicted profiles for tacrolimus (TAC) in whole blood (bottom curve) and in peripheral blood mononuclear cells (PBMC) (upper curve). Green line represents observed tacrolimus concentrations in PBMC, red line represents fitting of the model for PBMC concentrations. Blue line represents observed tacrolimus concentrations in whole blood, black line represents fitting of the model for whole blood concentrations. Visual predictive checks for whole blood (C) and PBMC (D) concentration of tacrolimus. Grey zones are confidence intervals at 95% of 5th, 50th and 95th percentiles of predictions obtained from 1000 Monte-Carlo simulations from the model. Curves are percentiles of the observed data. These curves must be included within the confidence interval above mentioned.
(PDF)
Time course profiles of tacrolimus (TAC) concentrations in whole blood and PBMC (left axis) and calcineurin activity in PBMC (right axis). Each symbol represents mean ± standard deviation of the mean. TACWB: tacrolimus concentration in whole blood, TACPBMC: tacrolimus concentration in PBMC, CaN: calcineurin, PBMC: peripheral blood mononuclear cells. (n = 32).
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors thank Marie-Jose Ferrand-Sorre and Julie Querzerho-Raguideau (Laboratory of Experimental and Clinical Pharmacology, Rennes 1 University) for their precious involvement in the analytical measurement of tacrolimus in clinical samples. We would like to thank Regis Bouvet (Department of molecular genetics and genomics, Rennes University Hospital) and Gwendal Coste (Pharmacology department, Rennes University Hospital) for their technical assistance in genotyping analysis.
Abbreviations
- ABCB1
ATP-binding cassette subfamily B member 1
- ACR
acute cellular rejection
- AUA
area under the calcineurin -time curve from 0 to12h
- AUC0-12h
area under the concentration–time curve from 0h to 12h
- C0
trough or predose concentration
- CaN
calcineurin
- CaN min
median minimal CaN activity
- CaNImax
calcineurin maximum inhibition
- Cmax
maximum concentration
- CYP3A4/5
cytochrome P450 isoform 3A4/5
- DNA
deoxyribonucleic acid
- EDTA
ethylene diamine tetracetique
- HPLC
high performance liquid chromatography
- IC50
concentration inhibiting 50% of the maximal activity of calcineurin
- IC37
concentration inhibiting 37% of the maximal activity of calcineurin
- ICtarget
target intracellular concentration of TAC inhibiting CaN activity
- IL2
interleukin-2
- PBMC
peripheral blood mononuclear cells
- PD
pharmacodynamic
- PG
pharmacogenetics
- P-pg
P-glycoprotein
- PK
pharmacokinetic
- SD
standard deviation
- SEM
standard deviation of the mean
- SNP
single nucleotide polymorphism
- TAC
tacrolimus
- TDM
therapeutic drug monitoring
- Tmax
time when Cmax is achieved
- Tmin
Time corresponding to CaNmin (0-12h).
- VPC
visual predictive check
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Thomson AW, Bonham CA, Zeevi A. Mode of action of tacrolimus (FK506): molecular and cellular mechanisms. Ther Drug Monit. déc 1995;17(6):584–91. [DOI] [PubMed] [Google Scholar]
- 2.Brunet M, van Gelder T, Åsberg A, Haufroid V, Hesselink DA, Langman L, et al. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy: Second Consensus Report. Ther Drug Monit. juin 2019;41(3):261–307. [DOI] [PubMed] [Google Scholar]
- 3.Staatz CE, Tett SE. Clinical Pharmacokinetics and Pharmacodynamics of Tacrolimus in Solid Organ Transplantation: Clinical Pharmacokinetics. 2004;43(10):623–53. 10.2165/00003088-200443100-00001 [DOI] [PubMed] [Google Scholar]
- 4.Capron A, Lerut J, Latinne D, Rahier J, Haufroid V, Wallemacq P. Correlation of tacrolimus levels in peripheral blood mononuclear cells with histological staging of rejection after liver transplantation: preliminary results of a prospective study: PBMCs tacrolimus levels and graft rejection. Transplant International. janv 2012;25(1):41–7. [DOI] [PubMed] [Google Scholar]
- 5.Capron A, Musuamba F, Latinne D, Mourad M, Lerut J, Haufroid V, et al. Validation of a liquid chromatography-mass spectrometric assay for tacrolimus in peripheral blood mononuclear cells. Therapeutic drug monitoring. 2009;31(2):178–186. 10.1097/FTD.0b013e3181905aaa [DOI] [PubMed] [Google Scholar]
- 6.Lemaitre F, Antignac M, Fernandez C. Monitoring of tacrolimus concentrations in peripheral blood mononuclear cells: Application to cardiac transplant recipients. Clinical Biochemistry. oct 2013;46(15):1538–41. [DOI] [PubMed] [Google Scholar]
- 7.Pensi D, De Nicolò A, Pinon M, Calvo PL, Nonnato A, Brunati A, et al. An UPLC–MS/MS method coupled with automated on-line SPE for quantification of tacrolimus in peripheral blood mononuclear cells. Journal of Pharmaceutical and Biomedical Analysis. mars 2015;107:512–7. 10.1016/j.jpba.2015.01.054 [DOI] [PubMed] [Google Scholar]
- 8.Capron A, Haufroid V, Wallemacq P. Intra-cellular immunosuppressive drugs monitoring: A step forward towards better therapeutic efficacy after organ transplantation? Pharmacol Res. sept 2016;111:610–8. [DOI] [PubMed] [Google Scholar]
- 9.Lemaitre F, Antignac M, Verdier M-C, Bellissant E, Fernandez C. Opportunity to monitor immunosuppressive drugs in peripheral blood mononuclear cells: Where are we and where are we going? Pharmacological Research. août 2013;74:109–12. [DOI] [PubMed] [Google Scholar]
- 10.Han SS, Yang SH, Kim MC, Cho J-Y, Min S-I, Lee JP, et al. Monitoring the Intracellular Tacrolimus Concentration in Kidney Transplant Recipients with Stable Graft Function. PLoS ONE. 2016;11(4):e0153491 10.1371/journal.pone.0153491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahmany S, de Wit LEA, Hesselink DA, van Gelder T, Shuker NM, Baan C, et al. Highly sensitive and rapid determination of tacrolimus in peripheral blood mononuclear cells by liquid chromatography-tandem mass spectrometry. Biomed Chromatogr. janv 2019;33(1):e4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goto M, Masuda S, Kiuchi T, Ogura Y, Oike F, Tanaka K, et al. Relation between mRNA expression level of multidrug resistance 1/ABCB1 in blood cells and required level of tacrolimus in pediatric living-donor liver transplantation. J Pharmacol Exp Ther. mai 2008;325(2):610–6. 10.1124/jpet.107.135665 [DOI] [PubMed] [Google Scholar]
- 13.Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I. Clin Pharmacokinet. Mars 2010;49(3):141–75. 10.2165/11317350-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 14.Picard N, Bergan S, Marquet P, van Gelder T, Wallemacq P, Hesselink DA, et al. Pharmacogenetic biomarkers predictive of the pharmacokinetics and pharmacodynamics of immunosuppressive drugs. Therapeutic drug monitoring. 2016;38:S57–S69. 10.1097/FTD.0000000000000255 [DOI] [PubMed] [Google Scholar]
- 15.Hesselink DA, Bouamar R, Elens L, van Schaik RHN, van Gelder T. The Role of Pharmacogenetics in the Disposition of and Response to Tacrolimus in Solid Organ Transplantation. Clinical Pharmacokinetics. Févr 2014;53(2):123–39. 10.1007/s40262-013-0120-3 [DOI] [PubMed] [Google Scholar]
- 16.Haufroid V, Mourad M, Van Kerckhove V, Wawrzyniak J, De Meyer M, Eddour DC, et al. The effect of CYP3A5 and MDR1 (ABCB1) polymorphisms on cyclosporine and tacrolimus dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenetics. mars 2004;14(3):147–54. [DOI] [PubMed] [Google Scholar]
- 17.Tron C, Lemaitre F, Verstuyft C, Petitcollin A, Verdier M-C, Bellissant E. Pharmacogenetics of Membrane Transporters of Tacrolimus in Solid Organ Transplantation. Clin Pharmacokinet. mai 2019;58(5):593–613. [DOI] [PubMed] [Google Scholar]
- 18.Capron A, Mourad M, De Meyer M, De Pauw L, Eddour DC, Latinne D, et al. CYP3A5 and ABCB1 polymorphisms influence tacrolimus concentrations in peripheral blood mononuclear cells after renal transplantation. Pharmacogenomics. Mai 2010;11(5):703–14. 10.2217/pgs.10.43 [DOI] [PubMed] [Google Scholar]
- 19.Vafadari R, Bouamar R, Hesselink DA, Kraaijeveld R, van Schaik RH, Weimar W, et al. Genetic polymorphisms in ABCB1 influence the pharmacodynamics of tacrolimus. Therapeutic drug monitoring. 2013;35(4):459–465. 10.1097/FTD.0b013e31828c1581 [DOI] [PubMed] [Google Scholar]
- 20.Elens L, Capron A, Kerckhove VV, Lerut J, Mourad M, Lison D, et al. 1199G>A and 2677G>T/A polymorphisms of ABCB1 independently affect tacrolimus concentration in hepatic tissue after liver transplantation: Pharmacogenetics and Genomics. October 2007;17(10):873–83. 10.1097/FPC.0b013e3282e9a533 [DOI] [PubMed] [Google Scholar]
- 21.Dessilly G, Elens L, Panin N, Capron A, Decottignies A, Demoulin J-B, et al. ABCB1 1199G>A Genetic Polymorphism (Rs2229109) Influences the Intracellular Accumulation of Tacrolimus in HEK293 and K562 Recombinant Cell Lines. Zhang J-T, éditeur. PLoS ONE. 12 Mars 2014;9(3):e91555 10.1371/journal.pone.0091555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woillard J-B, Gatault P, Picard N, Arnion H, Anglicheau D, Marquet P. A donor and recipient candidate gene association study of allograft loss in renal transplant recipients receiving a tacrolimus-based regimen. Am J Transplant. Déc 2018;18(12):2905–13. 10.1111/ajt.14894 [DOI] [PubMed] [Google Scholar]
- 23.Birdwell KA, Decker B, Barbarino JM, Peterson JF, Stein CM, Sadee W, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clin Pharmacol Ther. Juill 2015;98(1):19–24. 10.1002/cpt.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunet M, Shipkova M, van Gelder T, Wieland E, Sommerer C, Budde K, et al. Barcelona consensus on biomarker-based immunosuppressive drugs management in solid organ transplantation. Therapeutic drug monitoring. 2016;38:S1–S20. 10.1097/FTD.0000000000000287 [DOI] [PubMed] [Google Scholar]
- 25.Sanquer S, Amrein C, Grenet D, Guillemain R, Philippe B, Boussaud V, et al. Expression of Calcineurin Activity after Lung Transplantation: A 2-Year Follow-Up. Gregson A, éditeur. PLoS ONE. 25 Mars 2013;8(3):e59634 10.1371/journal.pone.0059634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukudo M, Yano I, Katsura T, Ito N, Yamamoto S, Kamoto T, et al. A transient increase of calcineurin phosphatase activity in living-donor kidney transplant recipients with acute rejection. Drug Metab Pharmacokinet. 2010;25(5):411–7. 10.2133/dmpk.dmpk-10-rg-026 [DOI] [PubMed] [Google Scholar]
- 27.Yano I, Masuda S, Egawa H, Sugimoto M, Fukudo M, Yoshida Y, et al. Significance of trough monitoring for tacrolimus blood concentration and calcineurin activity in adult patients undergoing primary living-donor liver transplantation. European Journal of Clinical Pharmacology. Mars 2012;68(3):259–66. 10.1007/s00228-011-1129-x [DOI] [PubMed] [Google Scholar]
- 28.Blanchet B, Duvoux C, Costentin CE, Barrault C, Ghaleh B, Salvat A, et al. Pharmacokinetic-pharmacodynamic assessment of tacrolimus in liver-transplant recipients during the early post-transplantation period. Therapeutic drug monitoring. 2008;30(4):412–418. 10.1097/FTD.0b013e318178e31b [DOI] [PubMed] [Google Scholar]
- 29.Iwasaki M, Yano I, Fukatsu S, Hashi S, Yamamoto Y, Sugimoto M, et al. Pharmacokinetics and Pharmacodynamics of Once-Daily Tacrolimus Compared With Twice-Daily Tacrolimus in the Early Stage After Living Donor Liver Transplantation. Ther Drug Monit. 2018;40(6):675–81. 10.1097/FTD.0000000000000551 [DOI] [PubMed] [Google Scholar]
- 30.Lemaitre F, Blanchet B, Latournerie M, Antignac M, Houssel-Debry P, Verdier M-C, et al. Pharmacokinetics and pharmacodynamics of tacrolimus in liver transplant recipients: inside the white blood cells. Clinical Biochemistry. Avr 2015;48(6):406–11. 10.1016/j.clinbiochem.2014.12.018 [DOI] [PubMed] [Google Scholar]
- 31.Tron C, Allard M, Petitcollin A, Ferrand-Sorre M-J, Verdier M-C, Querzerho-Raguideau J, et al. Tacrolimus diffusion across the peripheral mononuclear blood cell membrane: impact of drug transporters. Fundam Clin Pharmacol. Févr 2019;33(1):113–21. 10.1111/fcp.12412 [DOI] [PubMed] [Google Scholar]
- 32.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit. Août 2012;34(4):467–76. 10.1097/FTD.0b013e31825c4ba6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robertsen I, Debord J, Åsberg A, Marquet P, Woillard J-B. A Limited Sampling Strategy to Estimate Exposure of Everolimus in Whole Blood and Peripheral Blood Mononuclear Cells in Renal Transplant Recipients Using Population Pharmacokinetic Modeling and Bayesian Estimators. Clin Pharmacokinet. nov 2018;57(11):1459–69. [DOI] [PubMed] [Google Scholar]
- 34.Bogusz MJ, Enazi EA, Hassan H, Abdel-Jawaad J, Ruwaily JA, Tufail MA. Simultaneous LC-MS-MS determination of cyclosporine A, tacrolimus, and sirolimus in whole blood as well as mycophenolic acid in plasma using common pretreatment procedure. J Chromatogr B Analyt Technol Biomed Life Sci. 1 Mai 2007;850(1‑2):471–80. [DOI] [PubMed] [Google Scholar]
- 35.Blanchet B, Hulin A, Duvoux C, Astier A. Determination of serine/threonine protein phosphatase type 2B (PP2B) in lymphocytes by HPLC. Analytical biochemistry. 2003;312(1):1–6. 10.1016/s0003-2697(02)00214-2 [DOI] [PubMed] [Google Scholar]
- 36.Pensi D, De Nicolò A, Pinon M, Pisciotta C, Calvo PL, Nonnato A, et al. First UHPLC-MS/MS method coupled with automated online SPE for quantification both of tacrolimus and everolimus in peripheral blood mononuclear cells and its application on samples from co-treated pediatric patients. J Mass Spectrom. 2017;52(3):187–95. 10.1002/jms.3909 [DOI] [PubMed] [Google Scholar]
- 37.Hawwa AF, McElnay JC. Impact of ATP-binding cassette, subfamily B, member 1 pharmacogenetics on tacrolimus-associated nephrotoxicity and dosage requirements in paediatric patients with liver transplant. Expert Opin Drug Saf. janv 2011;10(1):9–22. [DOI] [PubMed] [Google Scholar]
- 38.Kravljaca M, Perovic V, Pravica V, Brkovic V, Milinkovic M, Lausevic M, et al. The importance of MDR1 gene polymorphisms for tacrolimus dosage. Eur J Pharm Sci. 15 Févr 2016;83:109–13. 10.1016/j.ejps.2015.12.020 [DOI] [PubMed] [Google Scholar]
- 39.Klaasen RA, Bergan S, Bremer S, Daleq L, Andersen AM, Midtvedt K, et al. Longitudinal Study of Tacrolimus in Lymphocytes During the First Year After Kidney Transplantation. Ther Drug Monit. 2018;40(5):558–66. 10.1097/FTD.0000000000000539 [DOI] [PubMed] [Google Scholar]
- 40.Marquet P, Albano L, Woillard J-B, Rostaing L, Kamar N, Sakarovitch C, et al. Comparative clinical trial of the variability factors of the exposure indices used for the drug monitoring of two tacrolimus formulations in kidney transplant recipients. Pharmacol Res. 2018;129:84–94. 10.1016/j.phrs.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 41.Noceti OM, Woillard J-B, Boumediene A, Esperon P, Taupin J-L, Gerona S, et al. Tacrolimus Pharmacodynamics and Pharmacogenetics along the Calcineurin Pathway in Human Lymphocytes. Clinical Chemistry. 1 October 2014;60(10):1336–45. 10.1373/clinchem.2014.223511 [DOI] [PubMed] [Google Scholar]
- 42.Fukudo M, Yano I, Masuda S, Fukatsu S, Katsura T, Ogura Y, et al. Pharmacodynamic analysis of tacrolimus and cyclosporine in living-donor liver transplant patients. Clinical Pharmacology & Therapeutics. Août 2005;78(2):168–81. [DOI] [PubMed] [Google Scholar]
- 43.Daher Abdi Z, Prémaud A, Essig M, Alain S, Munteanu E, Garnier F, et al. Exposure to mycophenolic acid better predicts immunosuppressive efficacy than exposure to calcineurin inhibitors in renal transplant patients. Clin Pharmacol Ther. October 2014;96(4):508–15. 10.1038/clpt.2014.140 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Worst (A) and best (B) individual predicted profiles for tacrolimus (TAC) in whole blood (bottom curve) and in peripheral blood mononuclear cells (PBMC) (upper curve). Green line represents observed tacrolimus concentrations in PBMC, red line represents fitting of the model for PBMC concentrations. Blue line represents observed tacrolimus concentrations in whole blood, black line represents fitting of the model for whole blood concentrations. Visual predictive checks for whole blood (C) and PBMC (D) concentration of tacrolimus. Grey zones are confidence intervals at 95% of 5th, 50th and 95th percentiles of predictions obtained from 1000 Monte-Carlo simulations from the model. Curves are percentiles of the observed data. These curves must be included within the confidence interval above mentioned.
(PDF)
Time course profiles of tacrolimus (TAC) concentrations in whole blood and PBMC (left axis) and calcineurin activity in PBMC (right axis). Each symbol represents mean ± standard deviation of the mean. TACWB: tacrolimus concentration in whole blood, TACPBMC: tacrolimus concentration in PBMC, CaN: calcineurin, PBMC: peripheral blood mononuclear cells. (n = 32).
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.