Abstract
Background
Crimean-Congo hemorrhagic fever (CCHF) is an emerging infectious disease caused by a Nairovirus. CCHF is a tick-borne disease that is predominantly associated with Hyalomma ticks and have a widespread distribution in Africa, Asia and Europe. CCHF usually presents as a subclinical disease, but in some cases, it may present as a hemorrhagic fever with a high mortality rate. This systematic review of the literature was performed to identify the available evidence on the prevalence of CCHF in the European Region of the World Health Organization, based on seroprevalence (IgG antibodies).
Methodology
A systematic review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement protocol. PubMed, Embase, and the Web of Science were used for the search (up to January 31, 2019), combining the following MeSH terms: [“Crimean-Congo haemorrhagic fever” OR “Crimean-Congo hemorrhagic fever virus” OR “Congo-Crimea” OR “Crimea-Congo”] AND [“Europe”] AND [“epidemiology” OR “seroprevalence”]. The abstracts were screened. Subsequently, full-text articles were selected and reviewed based on the PICOS (Population-Intervention-Comparison-Outcomes-Study type) criteria by two independent reviewers for inclusion in the final analysis. The data were qualitatively synthesized without quantitative pooling due to the heterogeneity in the study populations and methodologies.
Principal findings
Thirty articles (9 from western Europe, 18 from central Europe and 3 from eastern Europe) were included in the analysis. All articles were cross-sectional studies (descriptive studies).
Conclusions
The highest seroprevalence of CCHF is found in central and eastern European countries. Southern and western Europe countries, such as Greece and Spain, have low levels of endemicity, but the spread of the infection, which is associated with climate change, is a possibility that we should keep in mind. Further studies, especially larger seroprevalence studies in humans and animals, are needed to establish the current status of the CCHF epidemiology and to generate standardized guidelines for action in the region.
Author summary
Crimean Congo hemorrhagic fever (CCHF) is a widespread tick-borne viral disease caused by a Nairovirus of the Nairoviridae family. CCHF-virus (CCHFV) has been considered to be one of the eight priority emergent pathogens for the last 3 years by the World Health Organization (WHO), requiring urgent attention in Research, Development and Innovation (R&D&I) because of its epidemic potential in the near future In this systematic review, we aimed to describe the epidemiological impact of CCHFV (seroprevalence for IgG antibodies and the associated risk factors) in the WHO European Region (WHO/Europe). In this systematic review suggests the following conclusions. i) The highest values of CCHFV seroprevalence are found in Turkey, the Russian Federation, and Kazakhstan. ii) Greece has a high seroprevalence, though only one death associated with CCHFV has been reported. This fact contrasts with the neighboring countries, such as Balkan countries and Turkey, where the rate of severe infections seems to be higher. iii) Extensive studies should be developed in European countries to establish the actual epidemiological situation and to take additional preventive measures for the future.
Introduction
Crimean Congo hemorrhagic fever (CCHF) is a widespread tick-borne viral disease caused by CCHFV of the family Nairoviridae [1]. This disease was first described in 1944, during World War II, when an outbreak affected a group of Soviet soldiers in the Crimean Peninsula [2]. Twenty years later, in 1967, the virus was finally identified and was named Crimean-Congo virus, based on the similarities found with the virus that affected a febrile patient in the former Belgian Congo in 1956 [3].
Ninety percent of Crimean-Congo virus infections are oligosymptomatic or asymptomatic[4]. In the remaining 10%, the infections can present as a severe disease with a higher mortality rate [5–7]. Mortality is associated with different factors, such as age, viral strain, and endemicity [8–11].
The transmission of this virus to humans is mainly associated with the bite of hard-bodied ticks (Ixodidae family), predominantly those belonging to the genus Hyalomma, which are widely distributed in Asia, Africa and Europe. The infection can also be acquired through direct contact with blood and other bodily fluids from infected animals and humans, mainly those with a high viral load, including hospitalized patients with hemorrhagic fever. Thus, there is a high risk of transmission in healthcare environments [8,12–14]. Currently, the CCHF virus (CCHFV) is considered a level 4 biosecurity risk pathogen by the Centers for Disease Control and Prevention (CDC) [15,16]. CCHFV has been considered to be one of the eight priority emergent pathogens for the last 3 years by the World Health Organization (WHO), requiring urgent attention in Research, Development and Innovation (R&D&I) because of its epidemic potential in the near future [17,18].
Almost 1000 cases of CCHFV infection are reported in the Middle East and eastern European countries yearly [11,19]. In Europe, human cases have been reported in Albania, Bulgaria, Kosovo, Russia, Serbia, Turkey, Ukraine, Greece, Georgia and Spain [1,20,21].
The aim of this study was to identify the epidemiological impact of CCHFV (seroprevalence for IgG antibodies and the associated risk factors) in the WHO European Region, through a systematic review, to address the research question: what is the seroprevalence of CCHFV infection in the different geographic areas of Europe, and what are the possible associated risk factors?
Material and methods
Study design
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [22]. Study eligibility was defined according to the conventional PICOS (Population-Intervention-Comparison-Outcomes-Study type) criteria [23,24], which were determined a priori, including the following: Population (CCHFV-seropositive individuals); Intervention or Exposure, (risk factors, environmental determinants facilitating and inhibiting viral transmission); Comparators (three geographical regions of Europe, western/central/eastern Europe); Outcomes (seroprevalence data, IgG antibodies); and Study design (Observational studies, descriptive and analytical designs).
Search strategy and selection criteria
A systematic review of electronic bibliographic databases was performed for publications up to January 31, 2019. The following databases were searched for relevant studies to identify all the published studies about the seroprevalence of CCHFV in Europe: PubMed, Embase and the Web of Science, with the language restrictions of English, Spanish or French.
The electronic research was performed using the following Boolean operators and terms: ["Crimean Congo hemorrhagic fever" OR "Crimea Congo hemorrhagic fever" OR "Congo Crimea hemorrhagic fever" OR “Crimean Congo hemorrhagic fever virus” OR “Crimean Congo” OR “Crimea-Congo”] AND “Europe” AND [“seroprevalence” OR “epidemiology”].
Inclusion criteria: Published reports evaluating the epidemiology of CCHF in WHO/Europe were included when they fulfilled the following selection criteria, according to PRISMA guidelines. (1) Population: Human studies about the seroprevalence of CCHF. (2) Study design and interventions: We included observational studies. Randomized controlled trials were not included because these studies evaluate the efficacy of a treatment or an intervention. (3) Types of outcome measures: As the main outcome, we compared the seroprevalence of CCHF (defined as the number of individuals with evidence of IgG antibodies against CCHFV) in the 3 areas: western, central and eastern WHO/Europe.
Exclusion criteria: All nonhuman studies, intention-to-treat clinical trials, incidence data, editorial letters, letters to the editor, expert committees, author opinions and case reports (OCEBM Level of Evidence 5, Grade of Recommendation D) were excluded because they do not allow decision making or recommendation proposals and/or do not talk about an epidemiological observation. Finally, all studies about CCHF imported cases from an endemic to a nonendemic region were also excluded.
Selection of studies, data collection/extraction, and data synthesis/analysis
For the critical evaluation of the quality of the included studies, we applied a uniform checklist method to identify the internal validity and possible bias. To identify and select the studies, we classified them in a table following a systematic method. We evaluated the quality of each study, and the conclusions were based on the evidence levels according to the Oxford Centre for Evidence-Based Medicine (OCEBM) [25], which allow us to judge the strength of evidence. Also, we used the recommendations of the PRISMA declaration as a guide.
We prepared tables to make the systematic collection of the qualitative and quantitative data of each article. All the data were compiled by the last name of the first author, the year of publication, country, study design, objective, individuals or patients studied, population characteristics and other risk factors and the seroprevalence of anti-CCHFV IgG antibodies. First, the articles were evaluated based on the title and abstract, and afterwards they were evaluated based on the full text. All studies that did not fulfill the inclusion criteria were excluded from this systematic review.
All the articles identified were revised by two review team members (LMA and MAS), which followed the methodological standards recommended by the Committee on Standards for Systematic Reviews of Comparative Effectiveness Research for finding and assessing individual study: worked independently, screened and selected studies and extracted quantitative and other critical data from included studies. Each eligible study was systematically appraised for risk of bias; relevance to the study’s populations, and outcomes measures: seroprevalence. All the discrepancies were resolved by the rest of the study team.
We initially included all the countries of the WHO/European Region, and then we separated these studies into three different subgroups corresponding to three European regions, based on demographic and epidemiologic factors, and we made a qualitative comparison between these three European regions. The 53 countries of the WHO European Region were subdivided into three geographical areas, based on epidemiological considerations and in accordance with the division used by World Health Organization (WHO) and European Centre for Disease Control (ECDC) in others reports on surveillance in Europe: West (23 countries), Centre (15 countries) and East (15 countries).
Results
Summary of the included articles
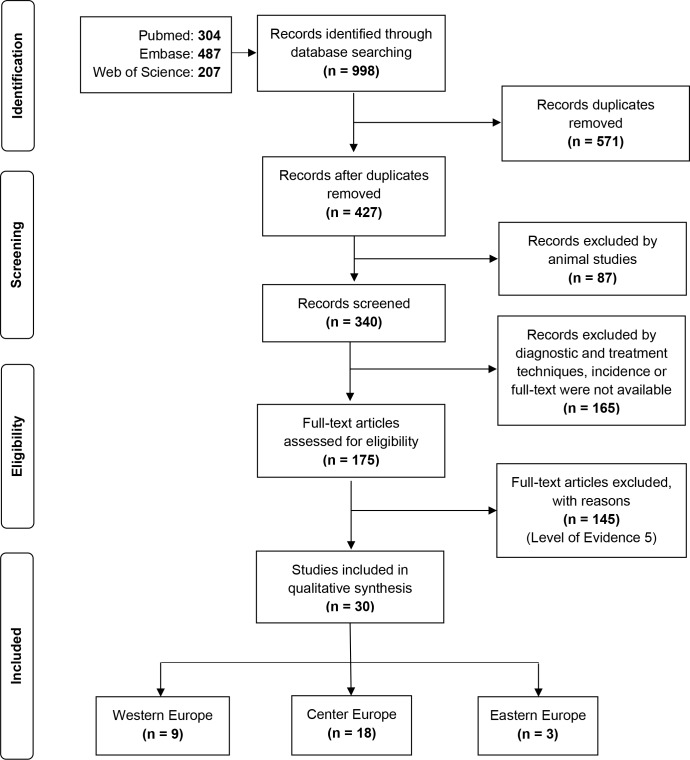
The initial search identified a total of 998 references that met the inclusion criteria: 304 on PubMed, 487 on Embase and 207 on the Web of Science. We first removed 571 duplicated records and 87 animal studies. Then, 165 articles about diagnostic and treatment techniques, incidence data or whose full-text papers were not available were excluded; and, 175 full-text articles were assessed for eligibility. A total of 145 of these papers were excluded because they were mainly case reports (OCEBM Level of Evidence 5, Grade of Recommendation D). Finally, 30 studies met our inclusion criteria and were included in the qualitative synthesis. Fig 1 shows the modified PRISMA flowchart with searching process.
Fig 1. PRISMA flowchart of identified and selected studies in the systematic review.
The articles were classified according to their origin in three European regions. The main characteristics of these studies are shown in Tables 1–3: Table 1, western Europe, 9 articles [6,26–33]; Table 2, central Europe, 18 articles [4, 34–50]; and Table 3, eastern Europe, 3 articles [51–53]. Even though the geographical origin was diverse, most studies were performed in Turkey (central Europe) (13 articles) [4,34,37–39,41–42,44–45,47–50] and in Greece (western Europe) (7 articles) [6,26,29–33].
Table 1. Principal results of CCHF studies in western Europe.
| Author | Publication year | Study years | Country | Objective (related to CCHF seroprevalence in humans) |
N | Risk factors | Seroprevalence of IgG: Value (%) |
|---|---|---|---|---|---|---|---|
| Antoniadis et al. [26] |
1982 | 1980–1981 | Greece (rural area, Northern Greece) |
To determine the prevalence of CCHFV antibodies in a rural population of Northern Greece | 65 | Farming Living in Northern Greece (CCHF isolated in this zone from Rhipicephalus bursa since 1978) |
4 (6.2) |
| Filipe et al. [27] |
1985 | 1980 | Portugal | To establish the seroprevalence of CCHFV virus in Southern Portugal | 190 | Living in certain areas of Southern Portugal | 2 (1.1) |
| Palomar et al. [28] |
2017 | 2010–2014 | Spain | To evaluate the presence of antibodies against the virus in individuals exposed to tick bites | 228 | No risk factors found | 0 (0.0) |
| Papa et al. [29] |
2014 | 2012 | Greece | To make a small-scale serologic survey in humans and animals in the area where CCHFV-positive tick had been detected | 100 | Ageing | 6 (6.0) |
| Papa et al. [30] |
2013 | 2010–2012 | Greece (Western, border to Albania and Ionian Sea Coast) |
To check in more detail the CCHFV situation in Thesprotia prefecture (western region, border with Albania) and find out any risk factors associated with seropositivity | 166 | Ruminants husbandry Slaughtering Ageing |
24 (14.4) |
| Papa et al. [31] |
2011 | 2008–2009 | Greece (Eastern, border to Bulgaria) |
To determine the prevalence of CCHFV antibodies in the human population of Northeastern Greece | 1178 | Female sex Ageing Ruminants husbandry Slaughtering Tick exposure |
37 (3.1) |
| Sargianou et al. [32] |
2013 | 2012 | Greece (Coast of the Gulf of Corinth) |
To estimate the seroprevalence of CCHFV in humans in Achaia Prefecture, Greece, and to assess risk factors in seropositivity | 207 | Agropastoral occupation Ruminants (especially with sheep) Living at an altitude of ≥400m |
7 (3.4) |
| Sidira et al. [33] |
2013 | 2010–2011 | Greece (Northern coast of the Aegean Sea) |
To estimate the CCHFV seroprevalence among humans residing in the prefecture of Imathia, and the neighbouring prefecture of Pella, and to investigate demographics and probable risk factors associated with the seropositivity | 277 | Tick exposure Residence in a hilly territory Ageing Agropastoral occupation |
6 (2.2) |
| Sidira et al. [6] |
2012 | 2009–2010 | Greece | To estimate endemic areas CCHF in Greece | 1611 | Slaughtering Agropastoral occupation Ruminants husbandry |
68 (4.2) |
Table 3. Main data of CCHF in studies in eastern Europe.
| Author | Publication year | Study years | Country | Objectives (related to CCHF seroprevalence in humans) |
N | Risk factors | Seroprevalence of IgG: Value (%) |
|---|---|---|---|---|---|---|---|
| Abdiyeva et al. [51] |
2019 | 2014–2015 | Kazakhstan | To detect the seroprevalence of CCHFV in patients with fever of unknown origin in endemic and non-endemic oblasts of Kazakhstan | 802 | Agro-pastoral occupation Ruminant and other livestock (except pigs) husbandry Living in rural areas |
102 (12.7) |
| Greiner et al. [52] |
2016 | 2014 | Georgia (12 rural affected communities) |
To determine CCHF seroprevalence, identify risk factors, and document CCHF-related knowledge, attitudes, and practices | 444 | Agro-pastoral occupation Animal husbandry Tick exposure |
12 (2.8) |
| Magnaval et al. [53] |
2011 | 2007 | Russian Federation (Northeastern Siberia) |
To determine the seroprevalence of nine zoonoses in Viljujsk, a Northern city in the Republic of Sakha (Eastern Siberia) | 90 | No risk factors found | 10 (11.1) |
Table 2. Main data of CCHF in studies in center Europe.
| Author | Publication year | Study years | Country | Objective (related to CCHF seroprevalence in humans) |
N | Risk factors | Seroprevalence of IgG: Value (%) |
|---|---|---|---|---|---|---|---|
| Bayram et al. [34] |
2017 | 2012 | Turkey (Eastern region, border with Iran) |
To determine the seroprevalence of CCHFV in individuals with a high risk of acquiring CCHF disease in Van province | 368 | No risk factors found | 53 (14.4) |
| Bodur et al. [4] |
2012 | 2009–2010 | Turkey | To investigate the seroprevalence of CCHFV infection in a sufficiently large sample representative of the region affected during the outbreak of 2011 in Turkey | 3557 | Ageing Low level of education Agropastoral occupation Tick exposure |
356 (10.0) |
| Christova et al. [35] |
2017 | 2015 | Bulgaria | To estimate the prevalence of IgG antibodies to CCHFV and hantaviruses, as stable and long persisting antibodies, in general human population of Bulgaria | 1500 | Living in southeastern Bulgaria (especially in the Haskovo district) Ageing Ruminant husbandry Tick exposure |
55 (3.7) |
| Christova et al. [36] |
2013 | 2011 | Bulgaria | To estimate the situation on CCHFV seroprevalence in both disease-endemic and -nonendemic areas in Bulgaria | 1018 | Tick exposure Ageing Living in the Black Sea Coast (Burgas District) |
28 (2.8) |
| Cikman et al. [37] |
2016 | Not specified | Turkey (North-Eastern region) |
To determine the seroprevalence and risk factors associated with Crimean-Congo hemorrhagic fever virus (CCHFV) in residents of North-Eastern, Turkey | 372 | Ruminant husbandry Living in rural areas Tick exposure |
59 (15.8) |
| Ergönül et al. [38] |
2006 | 2003 | Turkey | To determine the seroprevalence of CCHFV, among veterinarians in a highly endemic and a non-endemic region for these infections in Turkey | 83 | Percutaneous injuries in veterinarians | 1 (1.2) |
| Ertugrul et al. [39] |
2012 | Not specified | Turkey (Western region, Aegean Sea Coast) |
To determine the rate of specific IgG seropositivity against the virus and the contributory factors | 429 | Age <34 years Tick exposure Ruminant husbandry Female sex |
84 (19.6) |
| Fajs et al. [40] |
2014 | 2012 | Kosovo (Serbia for the WHO) |
To determine the prevalence of CCHF in Kosovo | 1105 | Living in the Southwestern Serbia (hyperendemic regions) Ageing Male sex |
44 (4.0) |
| Gargili et al. [41] |
2011 | 2008–2009 | Turkey (Western Turkey, border with Bulgaria) |
To estimate whether there is an immune-protection in the region as a result of a stablished infection and not a recent spread of infected ticks into the area | 193 | Male sex Ageing Living in the Black Sea Coast |
21 (10.9) |
| Gazi et al. [42] |
2016 | 2011–2013 | Turkey (rural part of Western Turkey) |
To determine the seroprevalence of CCHFV among the rural residents of Manisa region, Turkey, and to identify the associated risk factors | 324 | Ageing | 12 (3.7) |
| Gergova et al. [43] |
2014 | 2011–2012 | Bulgaria (Southeastern region) |
To determine the seroprevalence of CCHFV in endemic areas of Bulgaria | 751 | Tick exposure Ruminant husbandry |
24 (3.2) |
| Gozel et al. [44] |
2013 | 2012 | Turkey (Research Hospital, Centre-North Turkey) |
To analyze the serum seropositivity for CCHFV IgM and IgG of all healthcare workers at risk, and to determine the possible risk factors | 190 | Visiting an endemic region | 1 (0.5) |
| Gunes et al. [45] |
2009 | 2006 | Turkey (Centre-North region) |
To determine the seroprevalence of CCHFV in a high-risk population | 782 | Ageing Tick exposure Contact with livestock |
100 (12.8) |
| Horváth et al. [46] |
1976 | 1972–1975 | Hungary | To determine the seroprevalence of CCHFV in Hungary | 587 | Living in the Eastern Hungary, border to Rumania (Hajdú-Bihar county) | 17 (2.8) |
| Koksal et al. [47] |
2014 | 2004–2008 | Turkey (Eastern Black Sea regions) |
To determine the seroprevalence of CCHF infection and risk factors for disease in people living in the same environment with confirmed patients, either as household members or in the immediate neighbourhood, in the endemic area in the Black Sea region of Turkey | 625 | Ageing Ruminant husbandry Tick exposure Living rural areas |
85 (13.6) |
| Tekin et al. [48] |
2010 | Not specified | Turkey (Northern region) |
To determine the seroprevalence of CCHFV in humans in the province of Tokat (Centre-Northern region) | 715 | Contact with animals (not specified) Relatives of patients with CCHF (Airborne transmission?) |
69 (9.6) |
| Temocin et al. [49] |
2018 | 2016 | Turkey (Centre region) |
To determine the seroprevalence of CCHF disease among healthcare workers in a hospital in an endemic region, and to present the risk factors for healthcare workers | 112 | Percutaneous injuries in HCW | 2 (1.8) |
| Yagci-Caglayik et al. [50] | 2013 | Not specified | Turkey | To estimate the CCHFV seroprevalence in apparently healthy adult population living in urban and rural areas of seven geographically representative provinces of Turkey and to find out the risk factors associated with the seropositivity | 1066 | Ageing Low level of education Male sex Farming Living in a house of adobe |
25 (2.3) |
The oldest article was published by Horvath et al. in 1979 [46], and the most recent was published by Abdiyeva et al. in 2019 [51]. All articles were cross-sectional studies (descriptive studies). The study design that was generally used was to assess the prevalence of a disease in a population (prevalence study), which has an OCEBM Level of Evidence 4, Grade of Recommendation C.
Seroprevalence
Fig 2 shows in a Map the seroprevalence of CCHFV in the different European Regions.
Fig 2. Seroprevalence of CCHFV in Western Europe (yellow), Center Europe (blue) and Eastern Europe (orange).
Table 4 summarizes the main quantitative data collected from the studies that were analyzed: the sample sizes and IgG antibody levels (seroprevalence).
Table 4. Estimated seroprevalence of CCHFV in the European Regions*.
| Western Europe | ||
| Greece | 3604 | 2.2–14.4 ± 1 |
| Portugal | 258 | 0.8 ± 3 |
| Spain | 228 | 0.0 |
| Center Europe | ||
| Bulgaria | 3269 | 2.8–3.7 ± 1 |
| Hungary | 587 | 2.8 ± 1 |
| Serbia | 1105 | 4.0 ± 1 |
| Turkey | 8816 | 0.5–19.6 ± 1 |
| Eastern Europe | ||
| Georgia | 444 | 2.8 ± 2 |
| Kazakhstan | 802 | 12.7 ± 2 |
| Russian Federation | 90 | 11.1 ± 6 |
The sample sizes varied from 3557 individuals in a study conducted in Turkey [4] to 65 individuals in a study conducted in Greece by Antoniadis et al. [26]. The reported seroprevalence was between 0% in Spain [28] and 19.6% in Turkey [39]. We analyzed each European region independently as follows.
Western Europe
The lowest seroprevalence was observed in Spain 0% [28] and Portugal 1.1% [27]. The seven included studies that were performed in different geographical areas of Greece obtained seroprevalence ranging between 2.2% [33] and 14.4% [30]. Nevertheless, six of the seven studies included a reported prevalence between 2.2% and 6.2% (2.2% [33], 3.1% [31], 3.4% [32], 4.2% [6], 6% [29], and 6.2% [26]).
Central Europe
The studies conducted in Turkey also showed high variability. Most studies showed a seroprevalence that ranged from low values (0.5% [44], 1.2% [38], 1.8% [49], 2.3% [50], and 3.7% [42]) to moderate values (9.6% [48], 10% [4], 10.9% [41], 12.8% [45], 13.6% [47], 14.4% [34], and 15.8% [37]. One study showed a much higher prevalence (19.6% [39]), but this study was performed in an endemic area in southwestern Turkey. Bulgarian studies reported a similar seroprevalence: 2.8% [36], 3,2% [43], and 3.7% [35]. Additionally, Hungary (2.8% [46]) and Kosovo (4% [40]) reported a similar seroprevalence.
Russia and western Asia
Georgia shows a prevalence similar to that in eastern European countries (2.8%,[52]), while the Russian Federation (11.1%,[53]) and Kazakhstan (12.7%,[51]) show a prevalence closer to that found in Turkish areas, with a moderate/high prevalence.
Risk factors
Major risk factors, such as occupations associated with animal husbandry (especially of sheep and goats) [6,29,31,45,47–48,51–52] and agricultural and agropastoral activities [4,6,32,45,50,52] were identified in this study (no matter the geographical zone). Also another major risk factor was the tick exposure; tick exposure includes direct physical contact, tick bites, tick removal from people and animals and exposure to ticks around the working and home environments [12,31,33,35–37,39,43,45,47–48,52]. To a lesser extent, health care workers (physicians and nurses), veterinarians [38,44,48–49] and individuals with slaughtering-associated jobs [6,30,31] were also more likely to have the presence of CCHFV IgG antibodies.
Minor risk factors, such as gender and aging (risk markers) have been reported in some studies [4,29,31,33,35–36,39–42,45,47,50] in association with the presence of antibodies, but the heterogeneity of the studies and the populations that were evaluated prevent us from exhaustively affirming these results. Other risk factors that were evaluated were related to geographical aspects, such as residence in a hilly territory/living at an altitude [32–33], living in rural vs urban areas [26,37,47,50], in adobe houses [51], geographic regions as Black Sea Coast [36–41] and others [27,31,35,48] or endemic areas [40,43,47,49].
Some factors such as airborne transmission were also evaluated[48], but a causal association could not be determined, as the design of this study was not analytical.
Discussion
In the recent years, the epidemiology of vector-borne diseases is changing due to diverse factors, especially linked to the global warming phenomena [54–55]. In Europe, a rise on the prevalence of most important tick-borne infections are mainly due to tick-borne encephalitis and Lyme borreliosis in Central and Eastern Europe [56,57], and the emergence of the CCHFV in Southwestern Europe [21].
In this systematic review, we have realized an exhaustive and comparative analysis about the human seroprevalence of CCHFV in the WHO European Region countries (http://www.euro.who.int/en/countries). We have established three different areas (Center, Western and Eastern Europe) in order to evaluate the progression of tick-borne infection in the continent and to estimate which geographical regions are particularly at risk. This topic has been the subject of other systematic reviews based in specific subgroups of patients, such as travelers or pregnant women [58–59]. Likewise, a recently published systematic review, realized by Nasirian H. [60] discusses some of the CCHF seroprevalence studies that we also present in this systematic review. Nasirian H. study [60] collects global CCHFV seroprevalence data (no IgG—IgM antibodies difference) in humans and animals from different areas of the world, while our study is limited to CCHF seroprevalence for IgG antibodies at the WHO European Regions.
The eastern region of Europe is well known as the first site where this infection was reported [2]. Currently, CCHF continues to be endemic in the Russian Federation and in other countries of the former Soviet Union, though the real prevalence of CCHF is difficult to estimate because of the low number of seroprevalence studies. Nevertheless, the studies included in the analysis found values above 10% in Kazakhstan [51] and in the north of Russia [53].
In the central European region, human seroprevalences above 5% were also seen. The northeast of Turkey, especially the areas surrounding the Black Sea (eastern Anatolia), are classically described as highly endemic (high prevalence and incidence rates) for this infection [37,47]. However, we found the highest seroprevalence (19.6%) in a study performed on the Aegean Sea coast, which is not considered a CCHFV endemic zone. Balkan countries are also considered endemic [35,40,43,61], but their seroprevalence is lower than that in Turkey. Other countries in central Europe, such as Hungary, are not considered endemic for this infection, though seropositivity for CCHFV antibodies has been described since 1976 [46]. Recent studies have shown that this zoonosis is also circulating in animals in countries such as Romania and the former Yugoslav Republic of Macedonia [62–64].
The Western region the WHO/European Region had the lowest seroprevalence values for this infection. The infection has been documented in this area since the 1980s, when Antoniadis et al. [26] and Filipe et al. [27] demonstrated seropositivity in healthy humans in Greece and Portugal, respectively. Nevertheless, it was not until 2010 that the first and only autochthonous case was reported in Greece [20]. Additionally in 2010, the epidemiological alert in southwestern Europe increased after evidence of virus circulation in ticks belonging to the Hyalomma marginatum species were retrieved from a wild red deer in western Spain [65], near the Portuguese border. Six years later, first human autochthonous case was reported in Spain, a 62-year-old man that, after traveling to a little village at Central-Western Spain, presented at the Emergency Department with a severe viral hemorrhagic fever and died on the ninth day of illness. Four days later, a secondary (non-fatal) case due to a nosocomial transmission was also reported [21,66]. Since then, other two cases have also been reported in the Western Spain [67], reflecting probably only the visible part of the iceberg. Even though a recent study found no seroprevalence in humans in Spain [28], larger studies of seroprevalence need to be carried out in humans to corroborate the existence of an undetected circulation occurring in other areas of Spain and in neighboring countries, like Portugal, France, Italy or Malta where the vector tick exists and the weather conditions are favorable for the dissemination of this vector-borne disease.
Major risk factors have been well documented to be associated to tick exposure [12,31,33,35–37,39,43,45,47–48,52] in endemic regions, especially in the individuals involved in ungulate husbandry [6,29,31,45,47–48,51–52] and/or agropastoral activities [4,6,32,45,50,52]. The outbreaks emerge mainly in the spring and summer (May to October) [1]. Nevertheless, global migratory human movements, bird migrations from Africa, weather changes, global warming [55], and the presence of the main vector, Hyalomma marginatum ticks, in most countries of Europe might result in a situation in which this infection appears in other periods, spreads to new areas, increases in incidence, and becomes a diagnosis to be ruled-out in patients with hemorrhagic fever, even when a tick bite history cannot be documented.
The main limitation of this study was the heterogenicity of the studies and the lack of published seroprevalence investigations of some regions with elevated endemicity, especially at Northeastern Europe (Russia, Ukraine, between others). For this reason, a meta-analysis could not be performed. However, an extensive research in the main databases was performed, only excluding studies with low level of evidence. Nevertheless, a qualitative review improves the current lack of information. More studies are necessary to obtain conclusive evidence. The risks of bias (methodological and clinical) may have affected the results of our qualitative review. Despite these limitations, this systematic review sought to analyze the available information to date related to the epidemiology of CCHF in WHO/ European Region.
This systematic review suggests the following conclusions. i) The highest values of CCHFV seroprevalence are found in Turkey, the Russian Federation, and Kazakhstan. ii) Greece has a high seroprevalence, though only one death associated with CCHFV has been reported [20]. This fact contrasts with the neighboring countries, such as Balkan countries and Turkey, where the rate of severe infections seems to be higher. iii) Extensive studies should be developed in European countries to establish the actual epidemiological situation and to take additional preventive measures for the future.
Supporting information
(DOC)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by the Institute of Health Carlos III, ISCIII, Spain (www.isciii.es), grants: RICET RD16/0027/0018 (AM), DTS16/00207 (AM), PI16/01784 (PFS), European Union co-financing by FEDER (Fondo Europeo de Desarrollo Regional) ‘Una manera de hacer Europa’. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ergönül O. Crimean-Congo haemorrhagic fever. Lancet Infect Dis 2006;6:203–14. 10.1016/S1473-3099(06)70435-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoogstraal H. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol 1979;15:307–417. 10.1093/jmedent/15.4.307 [DOI] [PubMed] [Google Scholar]
- 3.Simpson DI, Knight EM, Courtois G, Williams MC, Weinbren MP, Kibukamusoke JW. Congo virus: a hitherto undescribed virus occurring in Africa. I. Human isolations—clinical notes. East Afr Med J 1967;44:86–92. [PubMed] [Google Scholar]
- 4.Bodur H, Akinci E, Ascioglu S, Öngürü P, Uyar Y. Subclinical infections with Crimean-Congo hemorrhagic fever virus, Turkey. Emerg Infect Dis 2012;18:640–2. 10.3201/eid1804.111374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res 2013;100:159–89. 10.1016/j.antiviral.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 6.Sidira P, Maltezou HCC, Haidich A-BA-B, Papa A. Seroepidemiological study of Crimean-Congo haemorrhagic fever in Greece, 2009–2010. Clin Microbiol Infect 2012;18:E16–9. 10.1111/j.1469-0691.2011.03718.x [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Crimean-Congo haemorrhagic fever WHO; 2016. http://www.who.int/mediacentre/factsheets/fs208/en/ (accessed February 23, 2017). [Google Scholar]
- 8.Akuffo R, Brandful JAM, Zayed A, Adjei A, Watany N, Fahmy NT, et al. Crimean-Congo hemorrhagic fever virus in livestock ticks and animal handler seroprevalence at an abattoir in Ghana. BMC Infect Dis 2016;16:324 10.1186/s12879-016-1660-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharifi-Mood B, Mardani M, Keshtkar-Jahromi M, Rahnavardi M, Hatami H, Metanat M. Clinical and epidemiologic features of Crimean-Congo hemorrhagic fever among children and adolescents from southeastern Iran. Pediatr Infect Dis J 2008;27:561–3. 10.1097/INF.0b013e3181673c28 [DOI] [PubMed] [Google Scholar]
- 10.Tezer H, Sucaklı IA, Saylı TR, Celikel E, Yakut I, Kara A, et al. Crimean-Congo hemorrhagic fever in children. J Clin Virol 2010;48:184–6. 10.1016/j.jcv.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leblebicioglu H. Crimean–Congo haemorrhagic fever in Eurasia. Int J Antimicrob Agents 2010;36:S43–6. 10.1016/j.ijantimicag.2010.06.020 [DOI] [PubMed] [Google Scholar]
- 12.Yadav PD, Patil DY, Shete AM, Kokate P, Goyal P, Jadhav S, et al. Nosocomial infection of CCHF among health care workers in Rajasthan, India 2016;16:624 10.1186/s12879-016-1971-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ftika L, Maltezou HC. Viral haemorrhagic fevers in healthcare settings. J Hosp Infect 2013;83:185–92. 10.1016/j.jhin.2012.10.013 [DOI] [PubMed] [Google Scholar]
- 14.Richards GA. Nososcomial transmission of viral haemorrhagic fever in South Africa. S Afr Med J 2015;105:709–12. 10.7196/SAMnew.8168 [DOI] [PubMed] [Google Scholar]
- 15.Vanhomwegen J, Alves M, Avšič T, Bino S, Chinikar S, Karlberg H, et al. Diagnostic Assays for Crimean-Congo Hemorrhagic Fever—Volume 18, Number 12—December 2012—Emerging Infectious Disease journal—CDC 2012;18:1958–65. 10.3201/eid1812.120710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appannanavar SB, Mishra B. An Update on Crimean Congo Hemorrhagic Fever. J Glob Infect Dis 2011;3:285–92. 10.4103/0974-777X.83537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Blueprint for R&D preparedness and response to public health emergencies due to highly infectious pathogens 2015.
- 18.World Health Organization. An R&D Blueprint for Action to Prevent Epidemics: Plan of Action [Internet]. Geneva, Switzerland; 2016. Available: http://www.who.int/csr/research-and-development/r_d_blueprint_plan_of_action.pdf?ua=1
- 19.Mertens M, Schmidt K, Ozkul A, Groschup MH. The impact of Crimean-Congo hemorrhagic fever virus on public health. Antiviral Res 2013;98:248–60. 10.1016/j.antiviral.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 20.Papa A, Dalla V, Papadimitriou E, Kartalis GNN, Antoniadis A. Emergence of Crimean–Congo haemorrhagic fever in Greece. Clin Microbiol Infect 2010;16:843–7. 10.1111/j.1469-0691.2009.02996.x [DOI] [PubMed] [Google Scholar]
- 21.Negredo A, de la Calle-Prieto F, Palencia-Herrejón E, Mora-Rillo M, Astray-Mochales J, Sánchez-Seco MP, et al. Autochthonous Crimean–Congo Hemorrhagic Fever in Spain. N Engl J Med 2017;377:154–61. 10.1056/NEJMoa1615162 [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg 2010;8:336–41. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 23.Centre for Reviews and Dissemination. Systematic Reviews: CRD’s guidance for undertaking reviews in health care [Internet]. York, United Kingdom: York Publishing Services Ltd; 2009. Available: https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf [Google Scholar]
- 24.Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res 2014;14:579 10.1186/s12913-014-0579-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oxford Centre for Evidence-Based Medicine. Levels of Evidence [Internet]. May 1 Oxford, England; 2011. p. 5653 Available: http://www.cebm.net/index.aspx?o=1025 [Google Scholar]
- 26.Antoniadis A, Casals J. Serological Evidence of Human Infection with Congo-Crimean Hemorrhagic Fever Virus in Greece *. Am J Trop Med Hyg 1982;31:1066–7. 10.4269/ajtmh.1982.31.1066 [DOI] [PubMed] [Google Scholar]
- 27.Filipe AR, Calisher CH, Lazuick J. Antibodies to Congo-Crimean haemorrhagic fever, Dhori, Thogoto and Bhanja viruses in southern Portugal. Acta Virol 1985;29:324–8. [PubMed] [Google Scholar]
- 28.Palomar AM, Portillo A, Santibáñez S, García-Álvarez L, Muñoz-Sanz A, Márquez FJ, et al. Molecular (ticks) and serological (humans) study of Crimean-Congo hemorrhagic fever virus in the Iberian Peninsula, 2013–2015. Enferm Infecc Microbiol Clin 2017;35:344–7. 10.1016/j.eimc.2017.01.009 [DOI] [PubMed] [Google Scholar]
- 29.Papa A, Chaligiannis I, Kontana N, Sourba T, Tsioka K, Tsatsaris A, et al. A novel AP92-like Crimean-Congo hemorrhagic fever virus strain, Greece. Ticks Tick Borne Dis 2014;5:590–3. 10.1016/j.ttbdis.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 30.Papa A, Sidira P, Kallia S, Ntouska M, Zotos N, Doumbali E, et al. Factors associated with IgG positivity to Crimean-Congo hemorrhagic fever virus in the area with the highest seroprevalence in Greece. Ticks Tick Borne Dis 2013;4:417–20. 10.1016/j.ttbdis.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 31.Papa A, Tzala E, Maltezou HC. Crimean-Congo hemorrhagic fever virus, northeastern Greece. Emerg Infect Dis 2011;17:141–3. 10.3201/eid1701.100073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sargianou M, Panos G, Tsatsaris A, Gogos C, Papa A. Crimean-Congo hemorrhagic fever: seroprevalence and risk factors among humans in Achaia, western Greece. Int J Infect Dis 2013;17:e1160–5. 10.1016/j.ijid.2013.07.015 [DOI] [PubMed] [Google Scholar]
- 33.Sidira P, Nikza P, Danis K, Panagiotopoulos T, Samara D, Maltezou H, et al. Prevalence of Crimean-Congo hemorrhagic fever virus antibodies in Greek residents in the area where the AP92 strain was isolated. Hippokratia 2013;17:322–5. [PMC free article] [PubMed] [Google Scholar]
- 34.Bayram Y, Parlak M, Ozkacmaz A, Cikman A, Guducuoglu H, Kilic S, et al. Seroprevalence of Crimean-Congo Hemorrhagic Fever in Turkey’s Van Province. Jpn J Infect Dis 2017;70:65–8. 10.7883/yoken.JJID.2015.675 [DOI] [PubMed] [Google Scholar]
- 35.Christova I, Panayotova E, Trifonova I, Taseva E, Hristova T, Ivanova V. Country-wide seroprevalence studies on Crimean-Congo hemorrhagic fever and hantavirus infections in general population of Bulgaria. J Med Virol 2017;89:1720–5. 10.1002/jmv.24868 [DOI] [PubMed] [Google Scholar]
- 36.Christova I, Gladnishka T, Taseva E, Kalvatchev N, Tsergouli K, Papa A. Seroprevalence of Crimean-Congo hemorrhagic fever virus, Bulgaria. Emerg Infect Dis 2013;19:177–9. 10.3201/eid1901.120299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cikman A, Aydin M, Gulhan B, Karakecili F, Kesik OA, Ozcicek A, et al. Seroprevalence of Crimean–Congo Hemorrhagic Fever Virus in Erzincan Province, Turkey, Relationship with Geographic Features and Risk Factors. Vector-Borne Zoonotic Dis 2016;16:199–204. 10.1089/vbz.2015.1879 [DOI] [PubMed] [Google Scholar]
- 38.Ergönül Ö, Zeller H, Kılıç S, Kutlu S, Kutlu M, Cavusoglu S, et al. Zoonotic infections among veterinarians in Turkey: Crimean-Congo hemorrhagic fever and beyond. Int J Infect Dis 2006;10:465–9. 10.1016/j.ijid.2006.06.005 [DOI] [PubMed] [Google Scholar]
- 39.Ertugrul B, Kirdar S, Ersoy OS, Ture M, Erol N, Ozturk B, et al. The seroprevalence of Crimean-Congo haemorrhagic fever among inhabitants living in the endemic regions of Western Anatolia. Scand J Infect Dis 2012;44:276–81. 10.3109/00365548.2011.621445 [DOI] [PubMed] [Google Scholar]
- 40.Fajs L, Humolli I, Saksida A, Knap N, Jelovšek M, Korva M, et al. Prevalence of Crimean-Congo hemorrhagic fever virus in healthy population, livestock and ticks in Kosovo. PLoS One 2014;9:e110982 10.1371/journal.pone.0110982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gargili A, Midilli K, Ergonul O, Ergin S, Alp HG, Vatansever Z, et al. Crimean-Congo Hemorrhagic Fever in European Part of Turkey: Genetic Analysis of the Virus Strains from Ticks and a Seroepidemiological Study in Humans. Vector-Borne Zoonotic Dis 2011;11:747–52. 10.1089/vbz.2010.0030 [DOI] [PubMed] [Google Scholar]
- 42.Gazi H, Özkütük N, Ecemis Ö, Atasoylu G, Köroglu G, Kurutepe S, et al. Seroprevalence of West Nile virus, Crimean-Congo hemorrhagic fever virus, Francisella tularensis and Borrelia burgdorferi in rural population of Manisa, western Turkey. J Vector Borne Dis 2016;53:112–7. [PubMed] [Google Scholar]
- 43.Gergova I, Kamarinchev B. Seroprevalence of Crimean-Congo hemorrhagic fever in southeastern Bulgaria. Jpn J Infect Dis 2014;67:397–8. 10.7883/yoken.67.397 [DOI] [PubMed] [Google Scholar]
- 44.Gozel MG, Dokmetas I, Oztop AY, Engin A, Elaldi N, Bakir M. Recommended precaution procedures protect healthcare workers from Crimean-Congo hemorrhagic fever virus. Int J Infect Dis 2013;17:e1046–50. 10.1016/j.ijid.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 45.Gunes T, Engin A, Poyraz O, Elaldi N, Kaya S, Dokmetas I, et al. Crimean-Congo hemorrhagic fever virus in high-risk population, Turkey. Emerg Infect Dis 2009;15:461–4. 10.3201/eid1503.080687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horváth LB. Precipitating antibodies to Crimean haemorrhagic fever virus in human sera collected in Hungary. Acta Microbiol Acad Sci Hung 1976;23:331–5. [PubMed] [Google Scholar]
- 47.Koksal I, Yilmaz G, Aksoy F, Erensoy S, Aydin H. The seroprevalance of Crimean-Congo haemorrhagic fever in people living in the same environment with Crimean-Congo haemorrhagic fever patients in an endemic region in Turkey. Epidemiol Infect 2014;142:239–45. 10.1017/S0950268813001155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tekin S, Barut S, Bursali A, Aydogan G, Yuce O, Demir F, et al. Seroprevalence of Crimean-Congo haemorrhagic fever (CCHF) in risk groups in Tokat Province of Turkey. African J Microbiol Res 2010;4:214–7. [Google Scholar]
- 49.Temocin F, Köse H, Sarı T, Duygu F, Şahin RO. Seroprevalence of Crimean–Congo hemorrhagic fever among health care workers in a hospital in an endemic region of Turkey. J Infect Dev Ctries 2018;12:587–91. 10.3855/jidc.9816 [DOI] [PubMed] [Google Scholar]
- 50.Yagci-Caglayik D, Korukluoglu G, Uyar Y. Seroprevalence and risk factors of Crimean-Congo hemorrhagic fever in selected seven provinces in Turkey. J Med Virol 2013;86:306–14. 10.1002/jmv.23699 [DOI] [PubMed] [Google Scholar]
- 51.Abdiyeva K, Turebekov N, Dmitrovsky A, Tukhanova N, Shin A, Yeraliyeva L, et al. Seroepidemiological and molecular investigations of infections with Crimean-Congo haemorrhagic fever virus in Kazakhstan. Int J Infect Dis 2019;78:121–7. 10.1016/j.ijid.2018.10.015 [DOI] [PubMed] [Google Scholar]
- 52.Greiner AL, Mamuchishvili N, Kakutia N, Stauffer K, Geleishvili M, Chitadze N, et al. Crimean-Congo Hemorrhagic Fever Knowledge, Attitudes, Practices, Risk Factors, and Seroprevalence in Rural Georgian Villages with Known Transmission in 2014 2016. 10.1371/journal.pone.0158049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magnaval J-F, Tolou H, Gibert M, Innokentiev V, Laborde M, Melnichuk O, et al. Seroepidemiology of Nine Zoonoses in Viljujsk, Republic of Sakha (Northeastern Siberia, Russian Federation). Vector-Borne Zoonotic Dis 2011;11:157–60. 10.1089/vbz.2009.0105 [DOI] [PubMed] [Google Scholar]
- 54.Estrada-Peña A, de la Fuente J. The ecology of ticks and epidemiology of tick-borne viral diseases. Antiviral Res. Elsevier; 2014;108: 104–128. 10.1016/J.ANTIVIRAL.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 55.Haines A, Ebi K. The Imperative for Climate Action to Protect Health. Solomon CG, editor. N Engl J Med. 2019;380: 263–273. 10.1056/NEJMra1807873 [DOI] [PubMed] [Google Scholar]
- 56.De la Fuente J, Estrada-Peña A. Ticks and tick-borne pathogens on the rise. Ticks and Tick-borne Diseases. 2012. pp. 115–116. 10.1016/j.ttbdis.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 57.Heyman P, Cochez C, Hofhuis A, Van Der Giessen J, Sprong H, Porter SR, et al. A clear and present danger: Tick-borne diseases in Europe. Expert Review of Anti-Infective Therapy. 2010. pp. 33–50. 10.1586/eri.09.118 [DOI] [PubMed] [Google Scholar]
- 58.Leblebicioglu H, Ozaras R, Fletcher TE, Beeching NJ. Crimean-Congo haemorrhagic fever in travellers: A systematic review. Travel Med Infect Dis 2016;14:73–80. 10.1016/j.tmaid.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pshenichnaya NY, Leblebicioglu H, Bozkurt I, Sannikova IV, Abuova GN, Zhuravlev AS, et al. Crimean-Congo hemorrhagic fever in pregnancy: A systematic review and case series from Russia, Kazakhstan and Turkey. Int J Infect Dis 2017;58:58–64. 10.1016/j.ijid.2017.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nasirian H. Crimean-Congo hemorrhagic fever (CCHF) seroprevalence: A systematic review and meta-analysis. Acta Trop. 2019;196: 102–120. 10.1016/j.actatropica.2019.05.019 [DOI] [PubMed] [Google Scholar]
- 61.Christova I, Younan R, Taseva E, Gladnishka T, Trifonova I, Ivanova V, et al. Hemorrhagic fever with renal syndrome and Crimean-Congo hemorrhagic fever as causes of acute undifferentiated febrile illness in Bulgaria. Vector Borne Zoonotic Dis 2013;13:188–92. 10.1089/vbz.2011.0938 [DOI] [PubMed] [Google Scholar]
- 62.Németh V, Oldal M, Egyed L, Gyuranecz M, Erdélyi K, Kvell K, et al. Serologic Evidence of Crimean-Congo Hemorrhagic Fever Virus Infection in Hungary. Vector-Borne Zoonotic Dis 2013;13:270–2. 10.1089/vbz.2012.1011 [DOI] [PubMed] [Google Scholar]
- 63.Ceianu CS, Panculescu-Gatej RI, Coudrier D, Bouloy M. First Serologic Evidence for the Circulation of Crimean-Congo Hemorrhagic Fever Virus in Romania. Vector-Borne Zoonotic Dis 2012;12:718–21. 10.1089/vbz.2011.0768 [DOI] [PubMed] [Google Scholar]
- 64.Mertens M, Vatansever Z, Mrenoshki S, Krstevski K, Stefanovska J, Djadjovski I, et al. Circulation of Crimean-Congo Hemorrhagic Fever Virus in the Former Yugoslav Republic of Macedonia Revealed by Screening of Cattle Sera Using a Novel Enzyme-linked Immunosorbent Assay. PLoS Negl Trop Dis 2015;9:e0003519 10.1371/journal.pntd.0003519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Estrada-Peña A, Palomar AM, Santibáñez P, Sánchez N, Habela MA, Portillo A, et al. Crimean-Congo Hemorrhagic Fever Virus in Ticks, Southwestern Europe, 2010. Emerg Infect Dis 2012;18:179–80. 10.3201/eid1801.111040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.García Rada A. First outbreak of Crimean-Congo haemorrhagic fever in western Europe kills one man in Spain. BMJ 2016;354:i4891 10.1136/bmj.i4891 [DOI] [PubMed] [Google Scholar]
- 67.Ministerio de Sanidad Consumo y Bienestar Social. Informe de situación y evaluación del riesgo de transmisión de Fiebre Hemorrágica de Crimea-Congo en España [Internet]. Spain: Dirección General de Salud Pública, Calidad e Innovación; 2019. Available: https://www.riojasalud.es/f/rs/docs/ACTUALIZACIÓN_ER_FHCC_Julio_2019.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.