Abstract
The neurodevelopmental trajectory in individuals with fetal alcohol spectrum disorders (FASD) has not been well characterized. We examined age-related differences in the volume of the corpus callosum, basal ganglia, and cerebellum across adolescence and young adulthood, due to the sensitivity of these regions to prenatal alcohol exposure. T1-weighted anatomical magnetic resonance images (MRI) were acquired from a cross-sectional sample of subjects 13–30 years old who had received an alcohol-related diagnosis (FASD, n=107) and typically developing controls (CON, n=56). FreeSurfer v5.3 was used to obtain volumetric data for the corpus callosum, caudate, putamen, pallidum, and cerebellum. Analysis of variance (ANOVA) was used to examine the effects of group (FASD, CON), sex, and age on region volume. Data were analyzed with and without correction for intracranial volume (ICV). All subregions were significantly smaller in the FASD group compared to controls, and these findings persisted even after ICV correction. Furthermore, the FASD and control groups differed in their relationship between age and total volume of the corpus callosum, caudate, and cerebellum. Specifically, older FASD individuals had smaller total volume in these regions; this relationship was not seen in the control group. Control males demonstrated larger volumes than control females in all regions prior to ICV correction; however, sex differences were attenuated in the FASD group in both the pallidum and cerebellum. Sex differences remained after ICV correction in the pallidum and cerebellum. These cross-sectional findings suggest that at least some brain regions may become smaller at an earlier than expected age in individuals with FASD, and that sex is an important factor to consider when examining neural structures in FASD. Further evaluation is necessary using longitudinal methods and including older ages.
Keywords: prenatal alcohol exposure, fetal alcohol spectrum disorders (FASD), neurodevelopment, neuroimaging
1. Introduction
The consequences of prenatal alcohol exposure are wide-ranging, and include physical characteristics, cognitive deficits, and behavioral problems (Riley et al., 2011). The term fetal alcohol spectrum disorder (FASD) encompasses the full range of the effects of prenatal alcohol exposure, including the diagnoses of fetal alcohol syndrome (FAS), partial fetal alcohol syndrome (pFAS), alcohol-related neurodevelopmental disorder (ARND), neurobehavioral disorder associated with prenatal alcohol exposure (ND-PAE), and alcohol-related birth defects (ARBD). Prenatal alcohol exposure is a leading preventable cause of birth defects, intellectual disability, and developmental disorders. Recent estimates from active case ascertainment studies indicate that FASD affects 1.1–5.0% of children in the United States (May et al., 2018). There is limited research examining the consequences of prenatal alcohol exposure in adulthood; however, the results to date indicate that detrimental effects can last into young adulthood (Coles et al., 2010; Moore and Riley, 2015; Santhanam et al., 2011; Spohr et al., 2007; Streissguth, 2007).
The developmental trajectory of the brain has not been well studied beyond childhood in individuals with prenatal alcohol exposure. Human brain development is a dynamic, protracted process in which different structures, tissues, and neural circuits have particular developmental trajectories (Giedd et al., 2015). In general, white matter volumes increase through middle age, whereas gray matter volumes decrease after childhood, with different central nervous system (CNS) regions reaching peak volumes at different points in development (Coupe et al., 2017; Giedd et al., 2015; Mills et al., 2016). Longitudinal studies up to age 17 have demonstrated atypical patterns of brain maturation in children with FASD, as well as differential relationships between developmental trajectory and cognitive function, compared to controls (Gautam et al., 2014; Lebel et al., 2012; Moore and Riley, 2015; Treit et al., 2013). However, the extent to which these trajectories differ into adulthood in individuals with prenatal alcohol exposure has not been studied.
1.1. Corpus Callosum
The corpus callosum comprises a tract of fibers that connects the left and right cerebral hemispheres, and allows for the transmission and coordination of information between homologous areas on either side of the brain. Sex differences have been reported in this structure (Shiino et al., 2017; Steinmetz et al., 1995; Sullivan et al., 2001), although some have reported that these differences are due to general brain size differences between the sexes rather than a localized sexual dimorphism (Giedd et al., 1999; Luders et al., 2014). This white matter structure is important to the integration of motor and sensory information, as well as cognitive processing, and is selectively affected by prenatal alcohol exposure (Bookstein et al., 2002a; Riley et al., 1995). Compared to typically developing controls, the volume of the corpus callosum is smaller, and its shape is more variable in individuals exposed to alcohol in utero, with the mid- and posterior areas being the most severely affected (Bookstein et al., 2002a; Riley et al., 1995). Importantly, the shape and size of the corpus callosum are predictive of neuropsychological functioning (Biffen et al., 2017; Bookstein et al., 2002b). In typical development, corpus callosum volume demonstrates a curvilinear relationship with age: its volume increases into early adulthood, plateaus around age thirty, and then volume declines occur with aging (Westlye et al., 2010). Interestingly, for children and adolescents with FASD, increases in corpus callosum volume over the course of development are related to improved cognitive outcomes (Gautam et al., 2014).
1.2. Basal Ganglia
The basal ganglia are subcortical structures that include the caudate, putamen, and pallidum, all of which are regions sensitive to insult by prenatal alcohol (Mattson et al., 1994; Mattson et al., 1996). Through its connections with frontal lobe areas, the basal ganglia are involved in regulating motor skills and cognitive function, including attention and affective control (Giedd et al., 2015). Normal development of the basal ganglia tends to follow an inverted U-shaped trajectory, with peak volume occurring in early adolescence (i.e., age 12 in females and age 14 in males) (Giedd et al., 2015; Raznahan et al., 2014). This set of structures has also been reported to be sexually dimorphic, possibly related to the high density of sex steroid receptors (Peper et al., 2011). After accounting for sex differences in brain size, males have been reported to have larger volume of the pallidum and putamen (Ahsan et al., 2007; Rijpkema et al., 2012; Ruigrok et al., 2014), whereas females reportedly have larger caudate (Filipek et al., 1994; Luders et al., 2009). The basal ganglia are disproportionately smaller in both children and adolescents with FASD, even after accounting for smaller overall brain size (Archibald et al., 2001; Mattson et al., 1994; Mattson et al., 1996). The reduced volume of the basal ganglia may be related to the profile of neurobehavioral deficits seen in FASD, including impaired visuospatial abilities and executive functioning (Mattson et al., 1996; Riley and McGee, 2005).
1.3. Cerebellum
The cerebellum plays an important role in motor skills, balance, and coordination, as well as cognitive function and emotional processing (Tiemeier et al., 2010). Total cerebellum volume also generally follows an inverted U-shaped developmental trajectory, though subdivisions of this structure mature in distinct patterns (Tiemeier et al., 2010). The hemispheres of the cerebellum continue to grow into late adolescence, whereas the volume of the cerebellar vermis does not change with age (Giedd et al., 2015). Males tend to have larger cerebellar volumes than females even after accounting for sex differences in brain size (Carne et al., 2006; Ruigrok et al., 2014; Tiemeier et al., 2010; Wierenga et al., 2014), although some have not found this effect (Nopoulos et al., 2000). The cerebellum is often implicated in neurodevelopmental disorders (Tiemeier et al., 2010), and its protracted development may make it especially vulnerable to the effects of environmental factors and toxins (Ciesielski and Knight, 1994), such as alcohol. Cerebellar abnormalities are a common finding in children with prenatal alcohol exposure (Archibald et al., 2001; Bookstein et al., 2006; Johnson et al., 1996), including smaller total volume, particularly in the anterior vermis (Riley and McGee, 2005). Such effects may be associated with deficits in attention shifting (Courchesne et al., 1994), balance (Roebuck et al., 1998), and poorer short-delay eyeblink conditioning (Jacobson et al., 2008; Jacobson et al., 2011; Spottiswoode et al., 2011) seen in individuals with FASD.
1.4. Study Rationale
Because there is little information about the impact of prenatal alcohol exposure on the brain beyond adolescence, we examined age-related differences in the volumes of the corpus callosum, structures that comprise the basal ganglia (i.e., caudate, putamen, pallidum), and cerebellum in a relatively large sample of adolescents and young adults with FASD and typically developing controls. We chose to examine the corpus callosum, basal ganglia, and cerebellum because these regions have been consistently reported to be particularly sensitive to the teratogenic effects of alcohol in children. Indeed, numerous MRI studies have reported that these brain regions are disproportionately smaller in those with FASD as compared to controls (Archibald et al., 2001; Bookstein et al., 2001; Bookstein et al., 2002a; Bookstein et al., 2006; Jacobson et al., 2017; Mattson et al., 1994; Mattson et al., 1996). Furthermore, previous findings support that reductions in total brain volume and several subcortical regions persist into adulthood (Chen et al., 2012; Treit et al., 2017). Many diffusion tensor imaging (DTI) studies of children and adolescents have found that white matter is sensitive to prenatal alcohol exposure (reviewed in (Ghazi Sherbaf et al., 2019; Wozniak and Muetzel, 2011)); thus, it is not surprising that young adults with FASD have also demonstrated poorer white matter microstructural integrity in the corpus callosum (Li et al., 2009; Ma et al., 2005). However, it remains unclear the extent to which the trajectory of development of the corpus callosum, caudate, putamen, pallidum, and cerebellum may differ from that of typically developing individuals. We aimed to take the first step in addressing this question by determining whether these regions with known sensitivity to alcohol consistently showed age-related changes in a cross-sectional sample of adolescents and young adults with FASD. We hypothesized that the volume of these regions would be reduced in adulthood in the FASD group. In addition, because individuals with other neurodevelopmental disorders exhibit altered patterns of brain growth (e.g., ADHD, Down syndrome) (Shaw et al., 2007; Zigman, 2013), we hypothesized that individuals with FASD would also demonstrate an association between older age and smaller volumes. For example, individuals with ADHD exhibit a cortical maturation delay of approximately three years in childhood (Shaw et al., 2007); on the other hand, the majority of individuals with Down syndrome show neuropathological changes consistent with Alzheimer’s disease after age 35 to 40 (Zigman, 2013). We have previously published our hypotheses regarding developmental trajectories in adults with FASD (Moore and Riley, 2015). In support of these hypotheses, we predicted that individuals with FASD would show increased brain region volume across adolescence and into young adulthood, similar to ADHD, but that we may also see a pattern suggestive of volumetric decline at an earlier age, similar to other populations with facial dysmorphology (e.g., Down syndrome, Prader-Willi syndrome, Williams syndrome) (Dykens, 2013; Zigman, 2013) and congenital abnormalities, such as spina bifida (Ware et al., 2017). Attenuated sex differences have also been reported in FASD (Chen et al., 2012; Moore et al., 2016; Treit et al., 2017); therefore, we hypothesized that the FASD group would demonstrate reduced sexual dimorphism in our regions of interest.
2. Results
2.1. Subject Characteristics
The FASD and control (CON) groups did not differ on sex, race, or age (ps > .160). However, the FASD group had significantly lower FSIQ scores and years of education compared to the CON group. More individuals with FASD were left-handed compared to controls. Visual inspection and statistical analysis revealed the same overall pattern between right and left-handed subjects; therefore, we chose to retain left-handed individuals in our analysis to increase power. Demographics are reported in Table 1.
Table 1.
Subject characteristics.
| CON | FASD | Group difference | |
|---|---|---|---|
| Total Sample | n = 55 | n = 106 | |
| Age in Years [M (SD)] | 19.1 (4.1) | 19 (4.52) | ns |
| Sex [n (% female)] | 28 (50.9) | 54 (50.9) | ns |
| Race [n (%)] | ns | ||
| White | 33 (60) | 67 (63.2) | |
| American Indian/Alaska Native | 14 (25.5) | 32 (30.2) | |
| Black/African American | 8 (14.5) | 6 (5.7) | |
| Unknown/Not Reported | 0 (0) | 1 (0.9) | |
| FAS [n (%)] | - | 57 (53.8) | - |
| Handedness [n (% right)] | 54 (98.2) | 87 (82.1) | p = 0.004 |
| Years Education [M (SD)] | 11.6 (2.65) | 10 (2.03) | p < .001 |
| WAIS FSIQ [M (SD)] | 108.2 (14.47) | 82.3 (13.71) | p < .001 |
| WAIS Verbal IQ [M (SD)] | 107.5 (12.5) | 82.1 (12.74) | p < .001 |
| WAIS Performance IQ [M (SD)] | 107.5 (16.15) | 86.4 (15.9) | p < .001 |
FASD = individuals with prenatal alcohol exposure, CON = non-exposed control subjects, FAS = fetal alcohol syndrome, IQ = Wechsler Adult Intelligence Scale (WAIS-III) Full Scale IQ (FSIQ).
ns = No significant difference, p > .05.
2.2. Region of Interest Analyses
Table 2 reports group differences in ROI volume and ICV. See Table 3 for ANOVA results. Supplementary Figure 1 shows graphical representations of sex differences, with Column 1 (graphs A/D/G/J/M) depicting differences in raw values, and Column 3 (graphs C/F/I/L/O) depicting differences in residuals. Column 3 graphs represent the values that were used as the dependent variable in the ICV-adjusted analyses described below.
Table 2.
Average region volumes and intracranial volume (ICV) in mm3 for the alcohol-exposed (FASD) and control (CON) groups.
| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| CON | FASD | CON | FASD | |||||
| Variable | n | Volume [M(SD)] | n | Volume [M(SD)] | n | Volume [M(SD)] | n | Volume [M(SD)] |
| Corpus Callosum | 26 | 3043 (317) | 50 | 2655 (514) | 26 | 3036 (476) | 48 | 2580 (432) |
| Caudate | 24 | 8118 (1029) | 50 | 6853 (1352) | 26 | 7459 (78) | 48 | 6479 (1253) |
| Putamen | 26 | 11725 (1147) | 50 | 9858 (1578) | 26 | 10222 (1054) | 49 | 9210 (1380) |
| Pallidum | 26 | 4220 (469) | 50 | 3466 (607) | 26 | 3462 (404) | 48 | 3218 (583) |
| Cerebellum | 26 | 152882 (12389) | 51 | 131806 (16080) | 26 | 134084 (12653) | 47 | 123895 (11697) |
| ICV | 27 | 1732705 (121953) | 52 | 1550700 (192448) | 28 | 1548705 (172022) | 54 | 1392326 (165905) |
Note: Sample size n indicates number of subjects included for each analysis.
FASD = individuals with prenatal alcohol exposure, CON = non-exposed control subjects
Table 3.
ANOVA results for Age Model and Sex Model for both raw and ICV-controlled region volumes.
| Region | Age Model | Sex Model | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Age | Age2 | Group x Age interaction | Group x Age2 interaction | Group | Sex | Group x Sex interaction | ||
| Corpus Callosum | |||||||||
| Raw Volume | <.001 (0.168) | .006 (0.051) | .007 (0.05) | .018 (0.038) | .014 (0.041) | Raw Volume | <.001 (0.168) | .60 (0.002) | .663 (0.001) |
| F (1,144) | 29.10 | 7.78 | 7.51 | 5.70 | 6.16 | F (1,146) | 29.58 | 0.28 | 0.19 |
| ICV Controlled | <.001 (0.101) | .01 (0.046) | .014 (0.041) | .045 (0.028) | .041 (0.029) | ICV Controlled | <.001 (0.104) | .167 (0.013) | .473 (0.004) |
| F (1,144) | 16.17 | 6.87 | 6.23 | 4.11 | 4.25 | F (1,146) | 16.94 | 1.93 | 0.52 |
| Caudate | |||||||||
| Raw Volume | <.001 (0.169) | .06 (0.025) | .032 (0.032) | .062 (0.024) | .041 (0.029) | Raw Volume | <.001 (0.17) | .013 (0.042) | .491 (0.003) |
| F (1,142) | 28.78 | 3.59 | 4.68 | 3.54 | 4.25 | F (1,144) | 29.57 | 6.26 | 0.48 |
| ICV Controlled | .001 (0.079) | .053 (0.026) | .034 (0.031) | .256 (0.009) | .214 (0.011) | ICV Controlled | <.001 (0.081) | .384 (0.005) | .629 (0.002) |
| F (1,142) | 12.18 | 3.80 | 4.59 | 1.30 | 1.56 | F (1,144) | 12.69 | 0.76 | 0.24 |
| Putamen | |||||||||
| Raw Volume | <.001 (0.187) | .965 (<0.001) | .916 (<0.001) | .462 (0.004) | .37 (0.006) | Raw Volume | <.001 (0.205) | <.001 (0.126) | .069 (0.022) |
| F (1, 145) | 33.31 | 0.002 | 0.01 | 0.55 | 0.81 | F (1,147) | 37.90 | 21.16 | 3.35 |
| ICV Controlled | .013 (0.042) | 0.337 (0.006) | .356 (0.006) | .709 (0.001) | .758 (0.001) | ICV Controlled | .013 (0.041) | .841 (0) | .083 (0.02) |
| F (1, 145) | 6.39 | 0.93 | 0.86 | 0.14 | 0.10 | F (1,147) | 6.27 | 0.04 | 3.04 |
| Pallidum | |||||||||
| Raw Volume | <.001 (0.143) | 0.213 (0.011) | .12 (0.017) | .697 (0.001) | .622 (0.002) | Raw Volume | <.001 (0.163) | <.001 (0.165) | .007 (0.048) |
| F (1, 144) | 24.04 | 1.56 | 2.44 | 0.15 | 0.25 | F (1,146) | 28.35 | 28.81 | 7.40 |
| ICV Controlled | .087 (0.02) | 0.366 (0.006) | .231 (0.01) | .534 (0.003) | .558 (0.002) | ICV Controlled | .073 (0.022) | .184 (0.012) | .009 (0.045) |
| F (1, 144) | 2.97 | 0.82 | 1.45 | 0.39 | 0.35 | F (1,146) | 3.25 | 1.79 | 6.92 |
| Cerebellum | |||||||||
| Raw Volume | <.001 (0.195) | 0.849 (<.001) | .859 (<0.001) | .75 (0.001) | .591 (0.002) | Raw Volume | <.001 (0.235) | <.001 (0.183) | .021 (0.036) |
| F (1, 144) | 34.94 | 0.04 | 0.03 | 0.10 | 0.29 | F (1, 146) | 44.77 | 32.67 | 5.43 |
| ICV Controlled | .008 (0.049) | 0.607 (0.002) | .485 (0.003) | .38 (0.005) | .48 (0.003) | ICV Controlled | .005 (0.052) | .598 (0.002) | .023 (0.035) |
| F (1, 144) | 7.35 | 0.27 | 0.49 | 0.78 | 0.50 | F (1, 146) | 8.04 | 0.28 | 5.26 |
All values are presented as p (η2). ICV = intracranial volume
2.2.1. Corpus Callosum
2.2.1.1. Age model
The CON group showed a linear increase in corpus callosum volume across age, whereas volumes in the FASD group had a quadratic relationship with age, in which corpus callosum volume demonstrated an initial increase, peaking in early adulthood, followed by a decrease in volume (see Figure 1a). There were also significant simple main effects of group (CON > FASD) and age2. Results were similar after adjusting corpus callosum volume for ICV.
Figure 1.
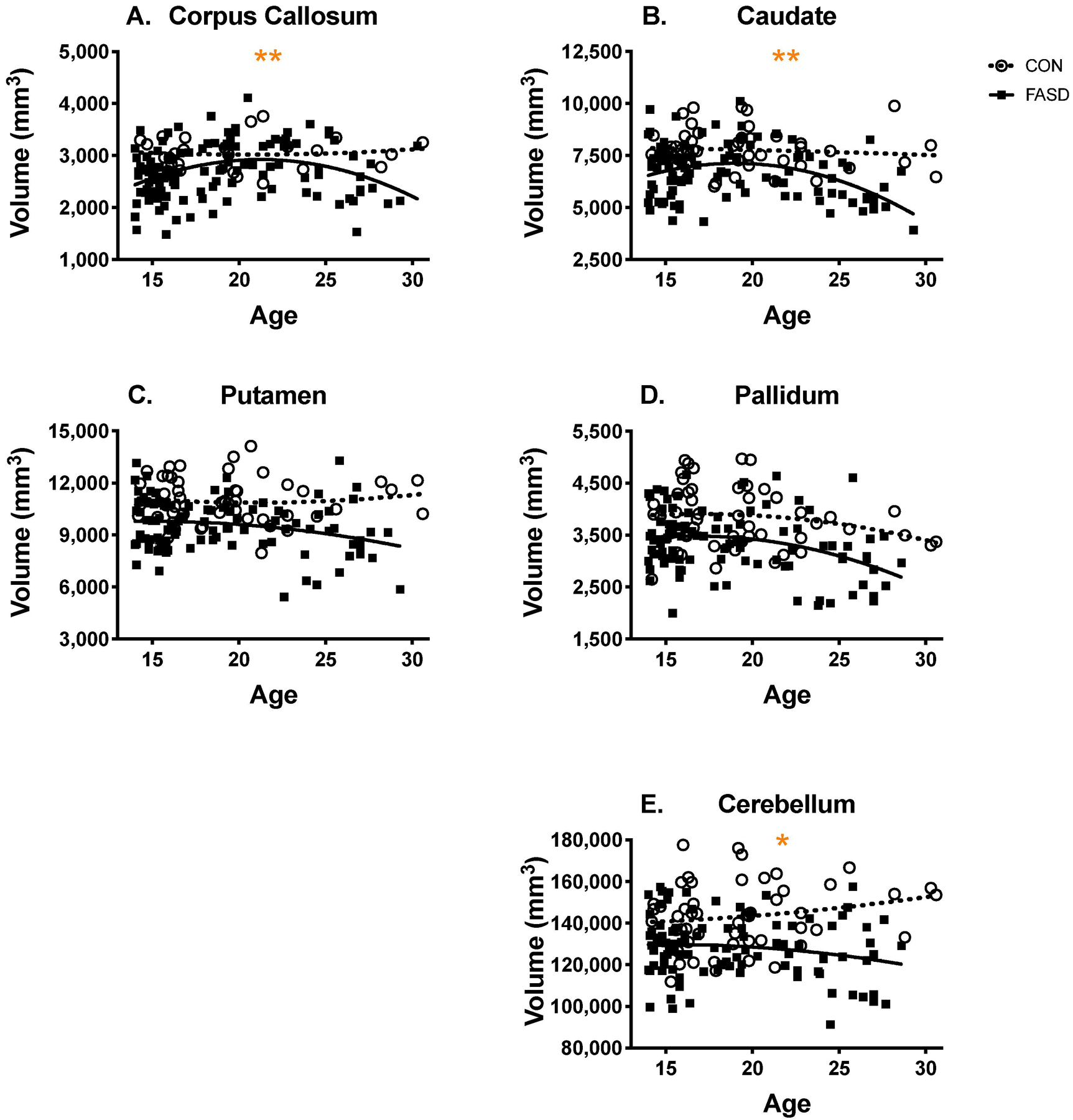
Raw region volumes across age in the FASD and control group. Raw volumes in all regions were significantly smaller across age in the FASD group, compared to controls. The corpus callosum (a), caudate (b), and cerebellum (e) demonstrated an altered trajectory of development in the FASD group, suggesting that prenatal alcohol exposure is associated with premature volumetric decline in these regions.
*group x age interaction (linear)
**group x age2 interaction (quadratic)
2.2.1.2. Sex model
There was no significant group x sex interaction or main effect of sex for the corpus callosum volume. However, there was a significant main effect of group, in which the CON group had greater volumes than the FASD group. Modeling ICV-adjusted corpus callosum volume revealed similar results.
2.2.2. Caudate
2.2.2.1. Age model
For the caudate, the CON group showed a linear decrease in volume across age, whereas volumes in the FASD group demonstrated a quadratic relationship with age: caudate volume demonstrated an initial increase, peaking in early adulthood, followed by a decrease in volume up to age 30 (see Figure 1b). The caudate also demonstrated a significant simple main effect of group (CON > FASD), as well as age2. ICV-adjusted caudate volume no longer demonstrated a group x age2 interaction; however, the quadratic effect of age remained significant, as did the main effect of group (CON > FASD).
2.2.2.2. Sex model
There was no significant group x sex interaction in the caudate. However, there were significant main effects of group (CON > FASD) and sex (male > female). For ICV-adjusted caudate volume, there was no group x sex interaction or main effect of sex. The main effect of group persisted, in which CON had larger ICV-adjusted caudate volume than FASD.
2.2.3. Putamen
2.2.3.1. Age model
There was no significant group x age or group x age2 interactions in the putamen, nor was there a significant linear or quadratic effect of age (see Fig. 1c). However, there was a significant main effect of group (CON > FASD). To further probe potential linear effects of age we removed the age2 term from the model; however, this did not reveal any age effects in the putamen. Results were similar for the ICV-adjusted putamen volume.
2.2.3.2. Sex model
There was no significant group x sex interaction in the putamen. However, there were significant main effects of group (CON > FASD) and sex (male > female). For ICV-adjusted putamen volume, the main effect of group persisted (CON > FASD), although there was no group x sex interaction or main effect of sex.
2.2.4. Pallidum
2.2.4.1. Age model
In the pallidum, there was no significant group x age interaction or quadratic main effect of age, although there was a significant main effect of group (see Figure 1d; CON > FASD). To further probe the linear effects of age, the age2 term was subsequently removed from the model. This analysis revealed that there was a significant linear effect of age on pallidum volume [F(1, 146) = 8.12, p = .005, η2 = 0.053]. For ICV-adjusted pallidum volume, there were no significant group x age interactions or quadratic effects of age. When only the linear effect of age was included in the model, both groups demonstrated a negative linear relationship between ICV-adjusted pallidum volume and age [F(1, 146) = 9.08, p = .003, η2 = 0.059]. Additionally, the main effect of group persisted at the trend level (p = .055, η2 = 0.025; CON > FASD).
2.2.4.2. Sex model
The pallidum demonstrated a significant group x sex interaction, and follow-up simple effects pairwise comparisons revealed that CON males displayed greater pallidum volume than FASD males (p < .001, η2 = 0.183); however, CON females did not significantly differ from FASD females (p = .069, η2 = 0.023). Results were similar following adjustment for ICV; CON males had larger ICV-adjusted pallidum volumes than FASD males (p = .002, η2 = 0.064), but there was no difference in ICV-adjusted pallidum volumes for CON females compared to FASD females (p = .561, η2 = 0.002). With regard to sex, males had significantly greater volume than females in both groups (CON male vs. female: p < .001, η2 = 0.15; FASD male vs. female: p = .026, η2 = 0.033). ICV-adjusted pallidum volume was significantly greater in CON males compared to CON females (p = .015, η2 = 0.04), but this sex effect was not observed in the FASD group (p = .273, η2 = 0.008). There was no significant simple main effect of group or sex after accounting for ICV.
2.2.5. Cerebellum
2.2.5.1. Age model
The volume of the cerebellum did not demonstrate a quadratic relationship across age, and when the age2 term was removed from the model, analyses revealed a significant group x age interaction [F(1, 146) = 4.37, p = .038, η2 = 0.029]. The CON group demonstrated a positive linear relationship between cerebellum volume and age, whereas there was a negative linear relationship between cerebellum volume and age in the FASD group (see Figure 1e). There was also a simple main effect of group [CON > FASD; F(1, 146) = 35.78, p < .001, η2 = 0.197].
Following adjustment for ICV, there were no significant quadratic effects of age. However, when only the linear effect of age was included in the model, ICV-adjusted cerebellum volume demonstrated a trending group x age interaction [F(1, 146) = 3.78, p = .054, η2 = 0.025], in which volumes for CON were larger across age, and FASD volume demonstrated no relationship to age. There was also a significant simple main effect of group [CON > FASD; F(1, 146) = 7.49, p = .007, η2 = 0.049].
2.2.5.2. Sex model
The cerebellum demonstrated a significant group x sex interaction, and follow-up simple effects pairwise comparisons indicated that CON had greater cerebellum volumes than FASD for both sexes (CON vs. FASD males: p < .001, η2 = 0.22; CON vs. FASD females: p = .003, η2 = 0.06). After adjusting for ICV, the group x sex interaction persisted: CON males had larger ICV-adjusted cerebellum volumes than FASD males (p < .001, η2 = 0.084), but there was no difference in ICV-adjusted cerebellum volumes in CON females compared to FASD females (p = .704, η2 = 0.001). With regard to sex, males had significantly greater volume than females in both groups (CON male vs. female: p < .001, η2 = 0.145; FASD male vs. female: p = .005, η2 = 0.054). ICV-adjusted cerebellum volume did not significantly differ in CON males compared to CON females (p = .083, η2 = 0.02), or in FASD males compared to FASD females (p = .136, η2 = 0.015). After controlling for ICV, there was a simple main effect of group (CON > FASD), but not sex.
2.3. Post-hoc Analyses: Diagnostic Subtype
Given the uniquely large number of individuals with FAS in this sample, we conducted a post-hoc analysis to examine the effects of sex and age across the three diagnostic categories: CON, FAE, and FAS. With regard to demographic characteristics of the FAS group, the mean age was 19.3, 37 (65%) were White, 28 (49%) were female, and 44 (77%) were right-handed. The FAS group completed an average of 9.9 years of education, and had a mean FSIQ of 80.5, verbal IQ of 80.5, and performance IQ of 84.3. The three groups did not significantly differ on age, sex, or race, although there were significant group differences in education level, handedness, FSIQ, verbal IQ, and performance IQ. Follow-up analyses indicated that CON had a higher level of education, fewer left-handers, and higher FSIQ, verbal IQ, and performance IQ, compared to both FAE and FAS groups. FAE and FAS groups did not significantly differ on any demographic variables.
It is important to note that findings from these 3-group analyses are limited by power, and thus, certain effects may not have been detectable. There were no statistically significant age-related differences across the groups for any of the regions examined. The pallidum was the only region that demonstrated a significant group x sex interaction [F(2,144) = 7.238, p = .001, η2 = .091). Follow-up simple effects pairwise comparisons revealed that CON males displayed greater pallidum volume than both FAE (p < .001, η2 = 0.131) and FAS males (p < .001, η2 = 0.183). In contrast, the volume of the pallidum in CON females did not differ from FAE females (p = .577, η2 = 0.002), and both CON and FAE females had significantly larger volumes compared to FAS females (CON vs. FAS: p < .001, η2 = 0.096; FAE vs. FAS: p < .001, η2 = 0.117). Additionally, males had significantly greater volume than females in the CON and FAS groups (CON male vs. female: p < .001, η2 = 0.163; FAS male vs. female: p < .001, η2 = 0.086); however, pallidum volume did not differ between FAE males and females (FAE male vs. female: p = .892, η2 < 0.001).
3. Discussion
We examined age- and sex-related differences in the volume of the corpus callosum, caudate, putamen, pallidum, and cerebellum in adolescents and young adults with FASD. These regions are especially sensitive to prenatal alcohol exposure, and other studies have found disproportionately smaller volumes in these areas in individuals prenatally exposed to alcohol (Archibald et al., 2001; Bookstein et al., 2006; Jacobson et al., 2017; Mattson et al., 1994; Mattson et al., 1996; Riley and McGee, 2005). However, few studies have examined the effects of prenatal alcohol exposure on brain development into adulthood. Chen et al. (2012) investigated volumetric differences in cortical and subcortical structures in young adults with prenatal alcohol exposure in their early 20s, and found that whole brain volumes were smaller at this age, and volume of the cerebellum was negatively related to level of prenatal alcohol exposure. We expanded upon these findings by examining whether there were consistent alcohol-related volumetric differences in the corpus callosum, basal ganglia, and cerebellum, and the extent to which these changes in volume were associated with age in an adolescent and adult sample of individuals with FASD.
We hypothesized that the volume of the corpus callosum, caudate, putamen, pallidum, and cerebellum would continue to be smaller in adulthood in alcohol-exposed individuals compared to controls, as has been found in infant and pediatric FASD samples (Archibald et al., 2001; Biffen et al., 2017; Jacobson et al., 2017; Roussotte et al., 2012). Our results were consistent with this hypothesis, and the volumes of these regions were smaller in the FASD group, compared to typically developing controls, even when adjusting for ICV. In particular, the volumes of the corpus callosum, caudate, putamen, and cerebellum were smaller; a finding that supports that these areas are especially sensitive to alcohol and that alcohol’s impact on the brain persists into early adulthood.
The developmental trajectory of the brain has not been well studied beyond childhood in individuals with prenatal alcohol exposure. Children with other developmental disorders exhibit patterns of brain development that deviate from typical brain maturation (Giedd et al., 2015). Understanding the neurodevelopmental pattern of FASD into adulthood could offer additional insights regarding long-term outcomes of prenatal alcohol exposure in the context of cognition, behavior, adaptive functioning, and secondary disabilities and problems (e.g., substance use, incarceration). Thus, we had hypothesized that the developmental trajectories of the corpus callosum, basal ganglia, and cerebellum would differ between alcohol-exposed individuals and typically developing controls.
In the corpus callosum and caudate, the FASD group displayed a positive relationship between volume and age across adolescence but a negative relationship with age across young adulthood. In contrast, the control group showed a tendency for a positive linear relationship between age and volume in the corpus callosum, and a negative linear relationship between age and volume in the caudate throughout the age span. In the cerebellum, FASD demonstrated a negative linear relationship across age, whereas volumes had a positive linear relationship with age in controls. These findings are supportive of an altered trajectory of development in the corpus callosum, caudate, and cerebellum, and indicate that those with FASD may undergo a premature volumetric decline in these regions. Other populations with facial dysmorphology and cognitive impairment due to genetic disorders (e.g., Prader-Willi syndrome, Williams syndrome (Dykens, 2013), Down Syndrome (Zigman, 2013)) also have been found to have early decreases in brain volume. This pattern may suggest a premature aging or potentially an accelerated cognitive decline process; however, further research with longitudinal samples is necessary to characterize the brain maturation pattern for individuals with FASD.
Elucidating the developmental trajectories of regions that have demonstrated sensitivity to prenatal alcohol exposure is an important step in understanding how neurobehavioral functioning and clinical outcomes of individuals with FASD may change over time. These data indicate that in adolescents and adults with FASD, volumes of the corpus callosum, caudate, putamen, pallidum, and cerebellum are significantly smaller, compared to controls. Furthermore, this reduction in volume is disproportionate in all regions examined, with the exception of the pallidum, suggesting that the observed effects are specifically related to alcohol, rather than a secondary effect of smaller brain size associated with FASD. These findings are consistent with reduced cortical volumes shown in adolescent and adult mice with developmental ethanol exposure (Leigland et al., 2013), and provide further evidence that the detrimental effects of prenatal alcohol exposure persist into adulthood. The corpus callosum, caudate, and cerebellum demonstrate a differential trajectory of development, in which FASD volumes initially appear to catch up to those of controls, but in young adulthood, seem to show a premature decline. This premature decline is consistent with the developmental trajectories of other populations with reduced cognitive reserve (e.g., Down syndrome, adult survivors of childhood cancer, childhood maltreatment, traumatic brain injury, spina bifida) (Moretti et al., 2012; Ness et al., 2013; Tyrka et al., 2010; Ware et al., 2017; Zigman, 2013).
While the FASD group also had smaller putamen and pallidum volumes compared to controls, the developmental trajectory of these regions was similar in both groups. Given the variability in developmental patterns for different neural structures, it may also be the case that other parts of the alcohol-exposed brain converge toward a normal developmental trajectory. Another possibility is that individuals with prenatal alcohol exposure exhibit altered developmental timing in certain regions relative to other regions. In the first 20 years of life, cortical gray matter volume decreases significantly, likely related to the high rate of pruning and the myelination of subcortical white matter fibers, whereas white matter volume follows an inverted U-shaped trajectory, peaking in middle life (Coupe et al., 2017). Neurobiological changes (e.g., pruning, myelination) that accompany neurodevelopment may be differentially affected by prenatal alcohol exposure (Lebel et al., 2012), and could underlie the consistent volumetric reductions and altered developmental trajectories observed in this study. Longitudinal DTI has already shown that children with FASD have delayed white matter development (Treit et al., 2013). Differences in developmental timing of gray matter versus white matter could potentially impact clinical outcomes in individuals with FASD. For example, there are differential relationships between neural developmental trajectory and cognitive function, compared to controls (Gautam et al., 2014; Lebel et al., 2012; Treit et al., 2013). Children with FASD and typically developing controls demonstrate similar patterns of increasing white matter volume across childhood and adolescence, but only in alcohol-exposed children is this increase significantly associated with cognitive improvement over time (Gautam et al., 2014). Children with FASD also show differences in the shape of neural structures, even in the absence of overall volumetric differences (Joseph et al., 2014). Indeed, Bookstein et al. (2002b) previously analyzed the three-dimensional shape of the corpus callosum in a subset of individuals in this sample, and found greater shape variability in the alcohol-exposed group, which in turn was associated with different profiles of neurobehavioral deficits. One could therefore speculate that, even if gross volumetric trajectories did not differ based on prenatal exposure to alcohol, the relationship between these trajectories, shape of the structure, and neurobehavioral function could still be altered in affected individuals.
Few studies have examined the corresponding neurobehavioral outcomes of adults with FASD, but the limited findings in adults ranging from ages 18 to 41 years indicate that in addition to having more behavior problems than controls (Day et al., 2013), adults with FASD perform worse on measures of balance, motor functioning (Connor et al., 2006), sustaining and shifting attention (Connor et al., 1999), cognitive flexibility, planning, and learning and memory (Coles et al., 2010; Rangmar et al., 2015). In particular, arithmetic skills and adaptive behavior remain prominent areas of deficit in affected adults (Streissguth et al., 2004). The corpus callosum, basal ganglia, and cerebellum are crucial to proper motor and cognitive functioning (Tiemeier et al., 2010), particularly in the domains of attention, visuospatial ability, and executive function (Mattson et al., 1996; Riley and McGee, 2005). Thus, the persistence of such deficits into adulthood may be directly related to the robust volumetric reduction, and it will be important to know if a premature decline of corpus callosum, caudate, and cerebellum volumes may also be related to an age-related functional decline in these individuals.
Sex differences have also been described in numerous brain regions, and smaller overall brain volume in females compared to males has been one of the most consistent findings in typically developing samples (Giedd et al., 2012). However, few neuroimaging studies have examined sex differences in brain structure in FASD, and even fewer have done so in adolescents and adults with FASD. Based on the existing MRI evidence of attenuated sex differences in FASD (Chen et al., 2012; Moore et al., 2016; Treit et al., 2017), we hypothesized that the FASD group would demonstrate reduced sexual dimorphism in the selected ROIs. The group by sex interaction observed in both the pallidum and cerebellum indicated that sex differences were attenuated in the FASD group, compared to controls. This attenuated sexual dimorphism was particularly evident when ICV was considered, and alcohol-exposed males and females no longer significantly differed from each other. In contrast, studies of healthy young adults have demonstrated sexual dimorphism of the pallidum and cerebellum, in which male volumes are disproportionately larger compared to females (Fan et al., 2010; Rijpkema et al., 2012; Ruigrok et al., 2014). Results from our control group are consistent with this finding. Others have also reported attenuated sexual dimorphism of neural structures, including the pallidum, caudate, putamen, and thalamus (Treit et al., 2017). Furthermore, prenatal alcohol exposure has been associated with significantly smaller volumes of several cortical regions in males, but not females (Chen et al., 2012). Results from our post-hoc 3-group analyses suggest that this attenuation is particularly evident in the pallidum in males with FAE. The lack of sex differences observed in the alcohol-exposed group could be related to alterations in the hypothalamic-pituitary-gonadal axis. Testosterone plays an important role in increasing neuronal density, size, and synaptogenesis (Goldstein et al., 2001), and in humans with prenatal alcohol exposure, testosterone levels are lower in both males and females (Carter et al., 2014). Furthermore, preclinical studies have shown that male rats with prenatal alcohol exposure are less sensitive to the neurophysiological effects of testosterone (Lan et al., 2009); it is possible that the effects of alcohol on the hormonal system are an underlying mechanism for the smaller region volumes observed in males with FASD (Chen et al., 2012). Regardless, our findings suggest that the relationship between prenatal alcohol exposure and brain structure is dependent on sex, making it an important factor to consider when examining the brain structure of individuals with FASD.
This study is limited by its use of cross-sectional data, particularly regarding conclusions drawn about developmental trajectories. Longitudinal data are essential to understanding within-subject changes over time, and future studies should aim to assess individuals with FASD at multiple points across their lifespan. Of note, some of the older adults in the FASD group were born prior to the characterization of FAS and subsequent public awareness of the consequences of prenatal alcohol exposure. Thus, these older adults may have been exposed to greater amounts of alcohol compared to younger adults with FASD. In addition, we had a limited number of female controls over the age of 25, which prevented us from adequately probing the impact of sex on development in the alcohol-exposed and control individuals. Given the differential relationship between region volume and sex in this older sample, it will be crucial for future studies to include an equal distribution of males and females across the entire age range so that these effects may be thoroughly examined. Pubertal status also was not available for this study, but would be an important factor to consider in future studies of neurodevelopment in FASD. While there were no group differences in age, sex, or race, the FASD group had fewer years of education than the control group; information on income level was not available for this sample, but its relationship to neurodevelopment in FASD should be considered in future research. Additionally, although this sample included a large number of individuals with FAS, our power to detect significant differences across all three diagnostic groups may have been limited by sample size. This study also examined relatively young adults; it remains unknown how the brains of individuals with prenatal alcohol exposure change into middle and older adulthood, and the extent to which they exhibit cognitive or behavioral changes with age. With longitudinal information, we will be better able to characterize the developmental trajectories of the brain in adults with FASD, and thereby improve our understanding of neurobehavioral outcomes across age, and implement interventions that are developmentally appropriate.
3.1. Conclusions
Adults with FASD show consistently smaller volumes in the corpus callosum, basal ganglia, and cerebellum, compared to controls; these findings persist even after controlling for total brain size. Based on these cross-sectional data, it would appear that individuals with FASD may also follow a differential pattern of brain maturation in the corpus callosum, caudate, and cerebellum, such that the volumes of these regions tend to decline at an earlier age as compared to controls. Future studies are needed to examine how these brain abnormalities manifest in adulthood, particularly in neurobehavioral domains related to the corpus callosum, caudate, putamen, and cerebellar white matter (e.g., learning, attention, memory, motor skills, balance, coordination). Other neuroimaging techniques (e.g., tensor-based morphometry) and analytical methods (e.g., independent components analysis) (Meintjes et al., 2014) may further elucidate patterns of structural deformation that are not easily observed from gross volumetric analysis. Longitudinal studies are also crucial to better characterizing the developmental pattern of these and other brain regions, and to determining the factors that affect neurobehavioral outcomes in adulthood, such as environment and education. Furthermore, the extent to which the brain maintains plasticity across age in this population remains unclear; examining the cognitive and behavioral functioning of adults with FASD along with trajectories of underlying neuronal structures could point to areas of the brain that are more resistant to prenatal alcohol exposure, or that may have the potential to recover with appropriate intervention (Lebel et al., 2012). Understanding the effects of prenatal alcohol exposure across age will help to determine the extent to which the features seen in children with FASD are still present in adulthood, and will ultimately aid in the creation of interventions tailored to individuals identified after childhood (e.g., educational, vocational, social, physical, occupational). Such information will increase our understanding of the high rates of incarceration, substance abuse, and psychiatric disorders in adults with FASD (Streissguth et al., 2004), and will lay the groundwork to most effectively address FASD as a public health issue across the lifespan.
4. Experimental Procedure
4.1. Subjects
Participants included 161 individuals enrolled in the FAS Follow-up Study conducted by Streissguth and colleagues at the University of Washington, which began over 40 years ago (Bookstein et al., 2002a; Streissguth et al., 1991). The University of Washington Institutional Review Board approved all study procedures, and informed consent was obtained from each participant. Participants in the alcohol-exposed group were recruited from the Seattle FAS Follow-up Database, which was accrued from over 30 years of referrals from dysmorphologists. The diagnoses were made by Dr. David Smith or one of his fellows, most often Dr. Sterling Clarren, after a dysmorphology exam based on the clinical guidelines of the time; however, it is important to note that this occurred before the Institute of Medicine diagnostic changes (Bookstein et al., 2001; Streissguth et al., 1991; Streissguth et al., 2004). We analyzed archival demographic information (i.e., age, sex, race, years of education, handedness), full scale IQ, and de-identified magnetic resonance imaging data collected from subjects age 13 to 30 years, including 106 individuals with FASD and 55 healthy control subjects (CON). Fifty-four percent of the individuals in the FASD group were diagnosed with FAS based on the guidelines described in Streissguth et al. (1991): evidence of a compromised CNS (e.g., microcephaly, history of delayed development, hyperactivity, attention deficits, learning disabilities, intellectual disability, seizures), growth deficiency of prenatal origin, and dysmorphic features unique to heavy prenatal alcohol exposure (i.e., short palpebral fissures, flat philtrum, thin upper vermilion, flat midface). Those who did not meet criteria for FAS were diagnosed with fetal alcohol effects (FAE), a term that has since been replaced, and would likely be most similar to a current diagnosis of alcohol-related neurodevelopmental disorder (ARND) or perhaps partial fetal alcohol syndrome (pFAS). The individuals categorized as FAE may have lacked sufficient facial dysmorphology for an FAS diagnosis, but they demonstrated CNS dysfunction, as described above, and had a confirmed history of prenatal alcohol exposure. These two groups of subjects were combined into a single FASD group based on the literature showing that, even in the absence of facial dysmorphology, individuals with prenatal alcohol exposure are similar with regard to IQ (Mattson et al., 1997), neuropsychological deficits (Mattson et al., 1998; Mattson et al., 1999), behavior problems (Roebuck et al., 1999), and adaptive functioning (Whaley et al., 2001).
Age- and ethnicity-matched typically developing controls were recruited from employees and their children at local healthcare and educational institutions in Washington state (Bookstein et al., 2002a). Individuals were excluded if they had HIV, were taking neurotoxic medications, were legally blind, wore dental braces, had psychological testing within the past year, or were not primary English speakers. Typically developing controls were also excluded if they had alcohol or drug problems, neurological problems, birth defects involving the brain, reported seeing visions or hearing voices, had a bachelor’s degree or higher education, or if their biological mother had a history of alcohol problems, drug problems, or binge drinking around the time of pregnancy with the participant (Bookstein et al., 2002a; Streissguth et al., 1991).
4.2. MRI Data Acquisition
T1-weighted anatomical magnetic resonance images (MRI) were collected using the same 1.5-Tesla General Electric Signa scanner at the University of Washington over three years (1997–2000). De-identified images were shared with our group for analysis, as approved by the University of Washington Institutional Review Board. Scan parameters were as follows: TE=8ms, TR=29ms, flip angle=45°, field of view=220×220mm2, producing images containing 2562 x 124 voxels of size 0.862 x 1.50 mm 3 (Bookstein et al., 2002b). Volumetric data were obtained for intracranial volume [ICV], corpus callosum, caudate, putamen, pallidum, and cerebellum using automatic segmentation in FreeSurfer v5.3 (http://surfer.nmr.mgh.harvard.edu/) (Fischl et al., 2002). Left and right hemisphere volumes for each ROI were summed to obtain estimates of bilateral volume and to reduce the number of hypotheses tested. All images were visually inspected for quality control and edited, if necessary, by an individual blind to subject group. Six subjects were excluded for poor scan quality (e.g., excessive motion), and 72 scans (44 FASD, 28 CON) were manually edited to ensure accurate segmentation. The most common error was inclusion of the fornix within the corpus callosum segmentation. Two subjects were excluded due to unknown group membership.
4.3. Statistical Analyses
Statistical analyses were conducted using SPSS 25. Results were considered significant at p < .05. The Holm-Bonferroni method was used to correct for multiple comparisons. Continuous independent variables were tested for normality using skewness and kurtosis statistics, and were centered to reduce multicollinearity. Data were assessed for outliers using box plots, and were removed from an analysis if they had an absolute value greater than 1.5 times the interquartile range (IQR) on the observed variable. In total, six subjects from the FASD group and three subjects from the CON group were deemed to be outliers on one or more regions of interest. Final sample sizes for each analysis are included in Table 2. Predictors of interest were exposure group, sex, and age; however, there were a limited number of CON females over the age of 25. Therefore, we conducted two separate 2 x 2 analyses of variance (ANOVAs) to probe the effects of age and sex on the following ROIs: corpus callosum, caudate, putamen, pallidum, and cerebellum. The first model included group, age, age2, group x age, and group x age2, to evaluate the linear and quadratic effects of age. The second model included group (FASD vs. CON), sex (male vs. female), and the group x sex interaction. Significant interactions were followed up with simple effects pairwise comparisons.
4.3.1. Subject Characteristics.
Group differences in age, years of education, and FSIQ were evaluated with independent samples t-tests. Separate chi-square analyses were conducted to evaluate group differences in sex, race, and handedness.
4.3.2. Evaluation of Potential Covariates.
ICV was evaluated as a potential covariate for follow-up analysis of variance (ANOVA) tests. ICV did not interact with independent variables group or sex, meeting the assumptions of homogeneity of regression. ICV demonstrated significant associations with all region of interest (ROI) volumes (rs ≥ .481, ps < .001). Therefore, ROI volumes were analyzed with and without adjustment for ICV (explained below).
Sex was also examined as a potential covariate for inclusion within the overarching age model. The corpus callosum and pallidum demonstrated a significant interaction effect of sex and age, indicating the homogeneity of regression assumption was not met for these regions. Unfortunately, there were too few CON females over the age of 25 to adequately assess these relationships. Therefore, we chose to probe the effects of sex in a separate model as an independent variable.
4.3.3. Correction for ICV.
Data were analyzed with and without correction for ICV. Adjustment for ICV was conducted following the residual approach discussed in Mathalon et al. (Mathalon et al., 1993) and O’Brien et al. (O’Brien et al., 2011). Data from the control group were analyzed using a regression model that included the ROI volume as the dependent variable and estimated ICV as the independent variable. Estimates from the model were then used to generate residuals for each group, representing the discrepancy between the subject’s observed ROI volume and predicted ROI volume. For all analyses correcting for ICV, the ROI residual was used as the dependent variable.
Supplementary Material
Highlights.
Corpus callosum, caudate, and cerebellar volumes smaller with increased age in FASD
Corpus callosum and caudate volumes had quadratic relationship with age in FASD
Cerebellar volume was smaller in older FASD but larger in older controls
Volumetric findings persisted in FASD even after intracranial volume adjustment
Sexual dimorphism of pallidum and cerebellum volume was attenuated in FASD
Acknowledgements
We acknowledge the important contributions of Drs. Ann P. Streissguth, Fred L. Bookstein, Paul D. Sampson, and Paul D. Connor for their critical role in collecting these digital images twenty years ago under research supported by U.S. Public Health Service Grants AA01455, AA10836, AA11037 (AP Streissguth) and GM37251 (FL Bookstein). We would also like to acknowledge Dr. Therese Grant, Dr. Janet Huggins, and Timothy Smith-Stuart for their assistance providing us with access to these images and demographic information about the subjects; Dr. Adolf Pfefferbaum for his assistance converting the older images to an analyzable format; Dr. Jay Giedd for his valuable recommendations for statistical analyses; and Dr. Jennifer Thomas for her thoughtful comments on a previous version of this manuscript. Additionally, our secondary analyses were supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA) grants T32 AA013525, F31 AA027148, U24 AA014811, K99/R00 AA022661, and R01 AA026994.
Funding:
This work was supported by National Institute on Alcohol Abuse and Alcoholism grants T32 AA013525, F31 AA027148, U24 AA014811, K99/R00 AA022661, and R01 AA026994.
Abbreviations:
- ARND
alcohol-related neurodevelopmental disorder
- CON
control
- FAE
fetal alcohol effects
- FASD
fetal alcohol spectrum disorders
- FAS
fetal alcohol syndrome
- pFAS
partial fetal alcohol syndrome
- ICV
intracranial volume
- ND-PAE
neurobehavioral disorder associated with prenatal alcohol exposure
- MRI
magnetic resonance imaging
- ROI
region of interest
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
The authors declare that they have no conflict of interest.
References
- Ahsan RL, Allom R, Gousias IS, Habib H, Turkheimer FE, Free S, Lemieux L, Myers R, Duncan JS, Brooks DJ, Koepp MJ, Hammers A, 2007. Volumes, spatial extents and a probabilistic atlas of the human basal ganglia and thalamus. Neuroimage. 38, 261–70. [DOI] [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL, 2001. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 43, 148–54. [PubMed] [Google Scholar]
- Biffen SC, Warton CMR, Lindinger NM, Randall SR, Lewis CE, Molteno CD, Jacobson JL, Jacobson SW, Meintjes EM, 2017. Reductions in Corpus Callosum Volume Partially Mediate Effects of Prenatal Alcohol Exposure on IQ. Front Neuroanat. 11, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein FL, Sampson PD, Streissguth AP, Connor PD, 2001. Geometric morphometrics of corpus callosum and subcortical structures in the fetal-alcohol-affected brain. Teratology. 64, 4–32. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Sampson PD, Connor PD, Streissguth AP, 2002a. Midline corpus callosum is a neuroanatomical focus of fetal alcohol damage. Anat Rec. 269, 162–74. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Streissguth AP, Sampson PD, Connor PD, Barr HM, 2002b. Corpus callosum shape and neuropsychological deficits in adult males with heavy fetal alcohol exposure. Neuroimage. 15, 233–51. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Streissguth AP, Connor PD, Sampson PD, 2006. Damage to the human cerebellum from prenatal alcohol exposure: the anatomy of a simple biometrical explanation. Anat Rec B New Anat. 289, 195–209. [DOI] [PubMed] [Google Scholar]
- Carne RP, Vogrin S, Litewka L, Cook MJ, 2006. Cerebral cortex: an MRI-based study of volume and variance with age and sex. J Clin Neurosci. 13, 60–72. [DOI] [PubMed] [Google Scholar]
- Carter RC, Jacobson JL, Dodge NC, Granger DA, Jacobson SW, 2014. Effects of prenatal alcohol exposure on testosterone and pubertal development. Alcohol Clin Exp Res. 38, 1671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Coles CD, Lynch ME, Hu X, 2012. Understanding specific effects of prenatal alcohol exposure on brain structure in young adults. Hum Brain Mapp. 33, 1663–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielski KT, Knight JE, 1994. Cerebellar abnormality in autism: a nonspecific effect of early brain damage? Acta Neurobiol Exp (Wars). 54, 151–4. [PubMed] [Google Scholar]
- Coles CD, Lynch ME, Kable JA, Johnson KC, Goldstein FC, 2010. Verbal and nonverbal memory in adults prenatally exposed to alcohol. Alcohol Clin Exp Res. 34, 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor PD, Streissguth AP, Sampson PD, Bookstein FL, Barr HM, 1999. Individual differences in auditory and visual attention among fetal alcohol-affected adults. Alcohol Clin Exp Res. 23, 1395–402. [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Streissguth AP, Bookstein FL, Barr HM, 2006. Effects of prenatal alcohol exposure on fine motor coordination and balance: A study of two adult samples. Neuropsychologia. 44, 744–51. [DOI] [PubMed] [Google Scholar]
- Coupe P, Catheline G, Lanuza E, Manjon JV, Alzheimer’s Disease Neuroimaging I, 2017. Towards a unified analysis of brain maturation and aging across the entire lifespan: A MRI analysis. Hum Brain Mapp. 38, 5501–5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Townsend J, Akshoomoff NA, Saitoh O, Yeung-Courchesne R, Lincoln AJ, James HE, Haas RH, Schreibman L, Lau L, 1994. Impairment in shifting attention in autistic and cerebellar patients. Behav Neurosci. 108, 848–65. [DOI] [PubMed] [Google Scholar]
- Day NL, Helsel A, Sonon K, Goldschmidt L, 2013. The association between prenatal alcohol exposure and behavior at 22 years of age. Alcohol Clin Exp Res. 37, 1171–8. [DOI] [PubMed] [Google Scholar]
- Dykens EM, 2013. Aging in rare intellectual disability syndromes. Dev Disabil Res Rev. 18, 75–83. [DOI] [PubMed] [Google Scholar]
- Fan L, Tang Y, Sun B, Gong G, Chen ZJ, Lin X, Yu T, Li Z, Evans AC, Liu S, 2010. Sexual dimorphism and asymmetry in human cerebellum: an MRI-based morphometric study. Brain Res. 1353, 60–73. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS Jr., 1994. The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex. 4, 344–60. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM, 2002. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 33, 341–55. [DOI] [PubMed] [Google Scholar]
- Gautam P, Nunez SC, Narr KL, Kan EC, Sowell ER, 2014. Effects of prenatal alcohol exposure on the development of white matter volume and change in executive function. Neuroimage Clin. 5, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazi Sherbaf F, Aarabi MH, Hosein Yazdi M, Haghshomar M, 2019. White matter microstructure in fetal alcohol spectrum disorders: A systematic review of diffusion tensor imaging studies. Hum Brain Mapp. 40, 1017–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL, 1999. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 2, 861–3. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Mills KL, Lenroot RK, 2012. Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol Sex Differ. 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Alexander-Bloch A, Schmitt E, Gogtay N, Rapoport JL, 2015. Child psychiatry branch of the National Institute of Mental Health longitudinal structural magnetic resonance imaging study of human brain development. Neuropsychopharmacology. 40, 43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS Jr., Faraone SV, Tsuang MT, 2001. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 11, 490–7. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL, 2008. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 32, 365–72. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Dodge NC, Pienaar M, Fuller DS, Molteno CD, Meintjes EM, Hoyme HE, Robinson LK, Khaole N, Jacobson JL, 2011. Impaired delay and trace eyeblink conditioning in school-age children with fetal alcohol syndrome. Alcohol Clin Exp Res. 35, 250–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Molteno CD, Warton CMR, Wintermark P, Hoyme HE, De Jong G, Taylor P, Warton F, Lindinger NM, Carter RC, Dodge NC, Grant E, Warfield SK, Zollei L, van der Kouwe AJW, Meintjes EM, 2017. Heavy Prenatal Alcohol Exposure is Related to Smaller Corpus Callosum in Newborn MRI Scans. Alcohol Clin Exp Res. 41, 965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VP, Swayze VW II, Sato Y, Andreasen NC, 1996. Fetal alcohol syndrome: craniofacial and central nervous system manifestations. Am J Med Genet. 61, 329–39. [DOI] [PubMed] [Google Scholar]
- Joseph J, Warton C, Jacobson SW, Jacobson JL, Molteno CD, Eicher A, Marais P, Phillips OR, Narr KL, Meintjes EM, 2014. Three-dimensional surface deformation-based shape analysis of hippocampus and caudate nucleus in children with fetal alcohol spectrum disorders. Hum Brain Mapp. 35, 659–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan N, Hellemans KG, Ellis L, Viau V, Weinberg J, 2009. Role of testosterone in mediating prenatal ethanol effects on hypothalamic-pituitary-adrenal activity in male rats. Psychoneuroendocrinology. 34, 1314–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, Bookheimer SY, O’Connor MJ, Narr KL, Kan E, Abaryan Z, Sowell ER, 2012. A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal processes of brain development. J Neurosci. 32, 15243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigland LA, Ford MM, Lerch JP, Kroenke CD, 2013. The influence of fetal ethanol exposure on subsequent development of the cerebral cortex as revealed by magnetic resonance imaging. Alcohol Clin Exp Res. 37, 924–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Coles CD, Lynch ME, Hu X, 2009. Voxelwise and skeleton-based region of interest analysis of fetal alcohol syndrome and fetal alcohol spectrum disorders in young adults. Hum Brain Mapp. 30, 3265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Gaser C, Narr KL, Toga AW, 2009. Why sex matters: brain size independent differences in gray matter distributions between men and women. J Neurosci. 29, 14265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Toga AW, Thompson PM, 2014. Why size matters: differences in brain volume account for apparent sex differences in callosal anatomy: the sexual dimorphism of the corpus callosum. Neuroimage. 84, 820–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Coles CD, Lynch ME, Laconte SM, Zurkiya O, Wang D, Hu X, 2005. Evaluation of corpus callosum anisotropy in young adults with fetal alcohol syndrome according to diffusion tensor imaging. Alcohol Clin Exp Res. 29, 1214–22. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Sullivan EV, Rawles JM, Pfefferbaum A, 1993. Correction for head size in brain-imaging measurements. Psychiatry Res. 50, 121–39. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Jernigan TL, Garcia A, Kaneko WM, Ehlers CL, Jones KL, 1994. A decrease in the size of the basal ganglia following prenatal alcohol exposure: a preliminary report. Neurotoxicol Teratol. 16, 283–9. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Sowell ER, Jernigan TL, Sobel DF, Jones KL, 1996. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 20, 1088–93. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL, 1997. Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. J Pediatr. 131, 718–21. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL, 1998. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 12, 146–53. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP, 1999. Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 23, 1808–15. [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, Kopald D, Hasken JM, Xu R, Honerkamp-Smith G, Taras H, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Vaux K, Jewett T, Elliott AJ, Kable JA, Akshoomoff N, Falk D, Arroyo JA, Hereld D, Riley EP, Charness ME, Coles CD, Warren KR, Jones KL, Hoyme HE, 2018. Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities. JAMA. 319, 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintjes EM, Narr KL, van der Kouwe AJ, Molteno CD, Pirnia T, Gutman B, Woods RP, Thompson PM, Jacobson JL, Jacobson SW, 2014. A tensor-based morphometry analysis of regional differences in brain volume in relation to prenatal alcohol exposure. Neuroimage Clin. 5, 152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Goddings AL, Herting MM, Meuwese R, Blakemore SJ, Crone EA, Dahl RE, Guroglu B, Raznahan A, Sowell ER, Tamnes CK, 2016. Structural brain development between childhood and adulthood: Convergence across four longitudinal samples. Neuroimage. 141, 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Riley EP, 2015. What Happens When Children with Fetal Alcohol Spectrum Disorders Become Adults? Curr Dev Disord Rep. 2, 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Infante MA, Migliorini R, Mattson SN, Riley EP, 2016. Pituitary lacks sexual dimorphism and displays reduced signal intensity on T1-weighted MRI in adolescents with histories of heavy prenatal alcohol exposure. Neurotoxicol Teratol. 57, 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti L, Cristofori I, Weaver SM, Chau A, Portelli JN, Grafman J, 2012. Cognitive decline in older adults with a history of traumatic brain injury. Lancet Neurol. 11, 1103–12. [DOI] [PubMed] [Google Scholar]
- Ness KK, Krull KR, Jones KE, Mulrooney DA, Armstrong GT, Green DM, Chemaitilly W, Smith WA, Wilson CL, Sklar CA, Shelton K, Srivastava DK, Ali S, Robison LL, Hudson MM, 2013. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol. 31, 4496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopoulos P, Flaum M, O’Leary D, Andreasen NC, 2000. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res. 98, 1–13. [DOI] [PubMed] [Google Scholar]
- O’Brien LM, Ziegler DA, Deutsch CK, Frazier JA, Herbert MR, Locascio JJ, 2011. Statistical adjustments for brain size in volumetric neuroimaging studies: some practical implications in methods. Psychiatry Res. 193, 113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, van den Heuvel MP, Mandl RC, Hulshoff Pol HE, van Honk J, 2011. Sex steroids and connectivity in the human brain: a review of neuroimaging studies. Psychoneuroendocrinology. 36, 1101–13. [DOI] [PubMed] [Google Scholar]
- Rangmar J, Sandberg AD, Aronson M, Fahlke C, 2015. Cognitive and executive functions, social cognition and sense of coherence in adults with fetal alcohol syndrome. Nord J Psychiatry. 69, 472–8. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R, Pipitone J, Chakravarty MM, Giedd JN, 2014. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proc Natl Acad Sci U S A. 111, 1592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijpkema M, Everaerd D, van der Pol C, Franke B, Tendolkar I, Fernandez G, 2012. Normal sexual dimorphism in the human basal ganglia. Hum Brain Mapp. 33, 1246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL, 1995. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res. 19, 1198–202. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL, 2005. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood). 230, 357–65. [DOI] [PubMed] [Google Scholar]
- Riley EP, Infante MA, Warren KR, 2011. Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev. 21, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebuck TM, Simmons RW, Mattson SN, Riley EP, 1998. Prenatal exposure to alcohol affects the ability to maintain postural balance. Alcohol Clin Exp Res. 22, 252–8. [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP, 1999. Behavioral and psychosocial profiles of alcohol-exposed children. Alcohol Clin Exp Res. 23, 1070–6. [PubMed] [Google Scholar]
- Roussotte FF, Sulik KK, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, O’Connor MJ, Narr KL, Sowell ER, 2012. Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Hum Brain Mapp. 33, 920–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok AN, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J, 2014. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 39, 34–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam P, Coles CD, Li Z, Li L, Lynch ME, Hu X, 2011. Default mode network dysfunction in adults with prenatal alcohol exposure. Psychiatry Res. 194, 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL, 2007. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 104, 19649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiino A, Chen YW, Tanigaki K, Yamada A, Vigers P, Watanabe T, Tooyama I, Akiguchi I, 2017. Sex-related difference in human white matter volumes studied: Inspection of the corpus callosum and other white matter by VBM. Sci Rep. 7, 39818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spohr HL, Willms J, Steinhausen HC, 2007. Fetal alcohol spectrum disorders in young adulthood. J Pediatr. 150, 175–9, 179 e1. [DOI] [PubMed] [Google Scholar]
- Spottiswoode BS, Meintjes EM, Anderson AW, Molteno CD, Stanton ME, Dodge NC, Gore JC, Peterson BS, Jacobson JL, Jacobson SW, 2011. Diffusion tensor imaging of the cerebellum and eyeblink conditioning in fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 35, 2174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz H, Staiger JF, Schlaug G, Huang Y, Jancke L, 1995. Corpus callosum and brain volume in women and men. Neuroreport. 6, 1002–4. [DOI] [PubMed] [Google Scholar]
- Streissguth A, 2007. Offspring Effects of Prenatal Alcohol Exposure from Birth to 25 Years: The Seattle Prospective Longitudinal Study. Journal of Clinical Psychology in Medical Settings. 14, 81–101. [Google Scholar]
- Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF, 1991. Fetal alcohol syndrome in adolescents and adults. JAMA. 265, 1961–7. [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK, 2004. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 25, 228–38. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Desmond JE, Pfefferbaum A, 2001. Sex differences in corpus callosum size: relationship to age and intracranial size. Neurobiol Aging. 22, 603–11. [DOI] [PubMed] [Google Scholar]
- Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN, 2010. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage. 49, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit S, Lebel C, Baugh L, Rasmussen C, Andrew G, Beaulieu C, 2013. Longitudinal MRI reveals altered trajectory of brain development during childhood and adolescence in fetal alcohol spectrum disorders. J Neurosci. 33, 10098–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit S, Chen Z, Zhou D, Baugh L, Rasmussen C, Andrew G, Pei J, Beaulieu C, 2017. Sexual dimorphism of volume reduction but not cognitive deficit in fetal alcohol spectrum disorders: A combined diffusion tensor imaging, cortical thickness and brain volume study. Neuroimage Clin. 15, 284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL, 2010. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biol Psychiatry. 67, 531–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware AL, Kulesz PA, Juranek J, Cirino PT, Fletcher JM, 2017. Cognitive control and associated neural correlates in adults with spina bifida myelomeningocele. Neuropsychology. 31, 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjornerud A, Due-Tonnessen P, Engvig A, Grydeland H, Tamnes CK, Ostby Y, Fjell AM, 2010. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 20, 2055–68. [DOI] [PubMed] [Google Scholar]
- Whaley SE, O’Connor Mj, Gunderson B, 2001. Comparison of the adaptive functioning of children prenatally exposed to alcohol to a nonexposed clinical sample. Alcohol Clin Exp Res. 25, 1018–24. [PubMed] [Google Scholar]
- Wierenga L, Langen M, Ambrosino S, van Dijk S, Oranje B, Durston S, 2014. Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. Neuroimage. 96, 67–72. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Muetzel RL, 2011. What does diffusion tensor imaging reveal about the brain and cognition in fetal alcohol spectrum disorders? Neuropsychol Rev. 21, 133–47. [DOI] [PubMed] [Google Scholar]
- Zigman WB, 2013. Atypical aging in Down syndrome. Dev Disabil Res Rev. 18, 51–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


