Abstract
Our purpose was to determine the relationship between serum prostate-specific antigen (PSA) level categories (<5, 5–10, 10–20, and >20 ng/mL) and the incidence of bone metastases detected by total-body 68Ga-prostate-specific membrane antigen (PSMA)-11 PET/CT and to assess if expanding the 68Ga-PSMA-11 PET/CT imaging field to include the vertex and lower extremities (total-body acquisition) affects bone metastasis detection rates and patient management. Methods: This was a retrospective analysis of 388 prostate cancer patients enrolled in 5 prospective studies (NCT02940262, NCT03368547, NCT03042312, NCT04050215, and NCT03515577). All underwent 68Ga-PSMA-11 PET/CT scans acquired from vertex to toes for primary staging (n = 93/388, 24%), biochemical recurrence (BCR) localization (n = 225/388, 58%), or restaging metastatic disease (M1) before or during systemic therapy (n = 70/388, 18%) between September 2017 and May 2018. Results: In total, 321 of 388 patients (83%) had a positive 68Ga-PSMA-11 study. PSMA-positive bone lesions were found in 105 of 388 (27%) patients, with an incidence that was positively associated with serum PSA level (<10 ng/mL, 21%; 10–20 ng/mL, 41%; ≥20 ng/mL, 41%; P < 0.001). This association was maintained for all 3 indications: initial staging, BCR, and restaging M1. Bone metastases occurred most frequently in restaging M1, followed by BCR and initial staging. Bone metastasis incidence was not significantly associated with National Comprehensive Cancer Network risk score (P = 0.22). The average number of PSMA-positive regions also increased with serum PSA level (P < 0.001). Eighteen of 388 (5%) and 18 of 388 (5%) had lesions above the superior orbital ridge and below the proximal third of the femur, respectively. There was only 1 of 388 patients (0.26%) in whom the total-body PET acquisition had an impact on management. Conclusion: Bone metastases as assessed with 68Ga-PSMA-11 PET/CT are prevalent even in patients with low serum PSA levels. Therefore, current guidelines for bone assessments in prostate cancer patients should be revisited because 68Ga-PSMA-11 PET/CT may provide additional information for accurate bone staging at low serum PSA levels. Including the total body (from vertex to toes) in 68Ga-PSMA-11 PET/CT imaging revealed additional bone lesions in 6% of patients, but without significantly affecting patient management.
Keywords: PSMA, PET/CT, prostate cancer, bone metastasis, field of view, guidelines
CT, MRI, conventional bone scintigraphy, and 18F-NaF PET or PET/CT bone imaging have been used to stage skeletal involvement in prostate cancer patients. Guidelines for detecting prostate cancer bone metastases devised by different expert panels and professional associations can vary. The Society of Nuclear Medicine and Molecular Imaging, the American College of Radiology, the National Comprehensive Cancer Network (NCCN), the Society of Urologic Oncology, the American Urological Association, and the American Society for Radiation Oncology recommend bone scintigraphy for initial staging of high-risk disease. They also suggest “considering” bone scintigraphy for initial staging of intermediate-risk disease or for restaging (1–4). There are no serum prostate-specific antigen (PSA) level criteria for the restaging indication. These guidelines vary depending on the expert committee and contain differences in lexicon, including “should consider staging with bone scan [expert opinion],” “should stage with bone scan [clinical principle],” “may be appropriate,” “may be considered (Option; Grade C),” “usually appropriate,” or “appropriate.” Table 1 summarizes current guidelines (1–4).
TABLE 1.
Guidelines
| Indication | Organization | Year | Wording | Population | Gleason | PSA | T stage | Other |
| Initial staging | AUA, ASTRO, SUO | 2017 | “Should consider staging with bone scan” (expert opinion) | Intermediate-risk | 7 or | 10–20 or | T2b–T2c | — |
| “Should stage with bone scan” (clinical principle) | High-risk | ≥8 or | >20 or | T3–T4 | — | |||
| NCCN | 2018 | “Staging work-up with bone scan if” | Intermediate-risk | — | >10 and | T2 | — | |
| “Staging work-up with bone scan if” | High-risk | ≥8 or | >20 or | T3–T4 | — | |||
| “Staging work-up with bone scan if” | — | — | — | — | Bone symptom | |||
| ACR | 2017 | “May be appropriate” | Intermediate-risk | — | >10 and | T2 | — | |
| “Usually appropriate” | High-risk | ≥8 or | >20 or | T3–T4 | — | |||
| “Usually appropriate” | — | — | — | — | Bone symptom | |||
| SNMMI | 2017 | “May be appropriate” | Low-risk | — | — | — | Elevated PAL | |
| “May be appropriate” | Intermediate-risk | 7 or | 10–20 or | T2b–T2c | — | |||
| “Appropriate” | Intermediate-risk | 7 and | 10–20 and | T2b–T2c | — | |||
| “Appropriate” | High-risk | ≥8 or | >20 or | T3–T4 | — | |||
| “Appropriate” | — | — | — | — | Bone symptom | |||
| Biochemical recurrence localization, restaging, posttreatment follow-up | AUA, ASTRO | 2013 | “Restaging may be considered (Option; Grade C)” | After radical prostatectomy | — | — | — | — |
| NCCN | 2018 | “Consider restaging with bone imaging” | After radical prostatectomy | — | — | — | — | |
| “Restaging with bone imaging if” | After definitive radiation therapy | — | — | — | — | |||
| “Monitoring with bone scan every 6–12 mo if” | Localized under observation | — | — | — | Bone symptom | |||
| “Monitoring with bone scan every 6–12 mo if” | N1/M1 under systemic therapy | — | — | — | Bone symptom | |||
| ACR | 2017 | “May be appropriate” | After radical prostatectomy | — | — | — | — | |
| 2017 | “May be appropriate” | After nonsurgical treatment | — | — | — | — | ||
| “Usually appropriate” | N1/M1 under systemic therapy | — | — | — | — | |||
| SNMMI | 2017 | “Appropriate” | — | — | — | — | Treatment change is planned | |
| “Appropriate” | — | — | — | — | Bone symptom | |||
| “Appropriate” | — | — | — | — | Before bone RNT | |||
| “Appropriate” | — | — | — | — | Equivocal imaging findings |
SNMMI = Society of Nuclear Medicine and Molecular Imaging; ACR = American College of Radiology; SUO = Society of Urologic Oncology; AUA =American Urological Association; ASTRO = American Society for Radiation Oncology; PAL = phosphatase alkaline; RNT = radionuclide therapy.
Imaging the expression of the prostate-specific membrane antigen (PSMA) using various radiolabeled PSMA ligands has been introduced fairly recently (5). PSMA is a transmembrane glycoprotein enzyme that can be up to 1,000-fold overexpressed in prostate cancer cells (6). 68Ga-PSMA-11 PET/CT assists in disease localization at biochemical recurrence (BCR) (7), at staging and restaging (8), and to select patients for PSMA-targeted molecular radiotherapy (9).
The ability of 68Ga-PSMA-11 PET/CT imaging to detect bone metastases is well established (10). However, it is unknown whether and how bone lesion incidence varies among different indications such as initial staging, BCR localization, or restaging of known metastatic disease. Moreover, the relationship between serum PSA level or the NCCN risk score (11), among others—including pathologic stage, serum PSA level, Gleason score, and bone involvement—is unknown.
Consistent with standard 18F-FDG protocols, PSMA PET/CT images are most frequently acquired from the base of the skull to the proximal third of the femur in the United States and from the top of the head to the proximal third of the femur in most of Europe (12,13). By contrast, bone planar scintigraphy and PET for prostate cancer assessments encompass the whole body from the top of the head to the feet.
In this study, we determined the incidence of bone metastases stratified by serum PSA level, NCCN risk score, and scan indication. This information is relevant because 68Ga-PSMA-11 imaging can have a profound impact on patient management (14,15) and may inform about necessary adaptations of current guidelines. We also determined if a total-body acquisition protocol (from vertex to toes) reveals lesions outside the standard whole-body field (base of the skull to mid thigh) that may affect patient management.
MATERIALS AND METHODS
Patients and Data Source
This was a retrospective analysis of 388 prostate cancer patients included in 5 prospective single-center studies from September 2017 to May 2018 (NCT02940262, NCT03368547, NCT03042312, NCT04050215, and NCT03515577) who underwent a total-body 68Ga-PSMA-11 PET/CT scan (from vertex to toes).
68Ga-PSMA-11 PET/CT Image Acquisition and Reconstruction
68Ga-PSMA-11 (Glu-NH-CO-NH-Lys-(Ahx)-[68Ga(HBED-CC)]) was used as the PSMA ligand (16). The median injected activity was 196.1 MBq (5.3 mCi) (range, 107.3–233.1 MBq [2.9–6.3 mCi]). The median tracer uptake period was 60 min (range, 48–113 min). We acquired images using a 64-detector PET/CT scanner (2007 Biograph 64 TruePoint or 2010 Biograph mCT 64; Siemens). A diagnostic CT scan (200–240 mAs, 120 kV) was performed after administration of oral contrast medium (600 mL of barium sulfate, 2.1% [Readi-Cat 2; Bracco]) and intravenous contrast medium (115 mL of iohexol [Omnipaque; GE Healthcare], 350 mg iodine/mL, injection speed of 2 mL/s, portal venous phase +80 s after injection) unless contraindicated. The PET image acquisition included a whole-body scan (pelvis to vertex, 2–4 min/bed position depending on the patient weight (17)), 1 dedicated pelvic scan after voiding (same acquisition time/bed position time as used for the whole body), and 1 dedicated scan of the lower extremities (pelvis to toes, 1 min/bed position for a total of 8–12 min). All PET images were reconstructed using attenuation, dead-time, random-event, and scatter corrections. PET images were reconstructed with an iterative algorithm (ordered-subset expectation maximization) in an axial 168 × 168 matrix on the Biograph 64 TruePoint (2-dimensional, 2 iterations, 8 subsets, gaussian filter 5.0) and in a 200 × 200 matrix on the Biograph mCT 64 (3-dimensional, 2 iterations, 24 subsets, gaussian filter 5.0).
68Ga-PSMA-11 PET/CT Image Analysis
Total-body 68Ga-PSMA-11 PET/CT images were analyzed according to guidelines during clinical readouts by experienced nuclear medicine physicians with unlimited access to all medical records (12,13,18–20). Any focal uptake of 68Ga-PSMA-11 above the background level and not associated with physiologic uptake or known pitfalls (18,19,21,22) was considered PSMA-positive. The PROMISE criteria were applied (20). Based on TNM staging, the following regions were analyzed systematically: prostate fossa (T), pelvic lymph nodes (N) (internal iliac, obturator, external iliac, perirectal, presacral, and common iliac), extrapelvic lymph nodes (M1a) (abdominal, inguinal, and above the diaphragm), bone (M1b), and other visceral organs (M1c). PSMA-positive bone lesion localization was categorized as follows: skull above the superior orbit ridge, skull below the superior orbital ridge, ribs/clavicles/sternum, humerus, spine, pelvis, the proximal third of the femur, and the lower extremities below the proximal third of the femur.
Statistical Analysis
Patient characteristics and study variables were summarized using frequency (%) or median and range. We determined associations between serum PSA categories and PSMA-positive bone lesion incidence using the Spearman correlation coefficient. The association between PSMA-positive bone lesions and NCCN risk score (1, 2, or 3) or study indication and PSA level were assessed using the χ2 test. Statistical analyses were performed using SPSS (version 25; IBM), and P values of less than 0.05 were considered statistically significant.
RESULTS
Patient Population
Table 2 lists patient demographics. The indication for 68Ga-PSMA-11 PET/CT was primary staging in 93 of 388 (24%), localization of the source of BCR in 225 of 388 (58%), or restaging metastatic disease (M1) before or during systemic therapy in 70 of 388 (18%). Median serum PSA level at inclusion was 3.66 ng/mL (range, 0.04–1,776.79 ng/mL), with a median of 41 d between the scan and the PSA blood test. Table 3 shows the scan indication broken down by serum PSA level.
TABLE 2.
Patient Characteristics (n = 388)
| Characteristic | Data |
| Initial PSA at diagnosis | |
| Median (ng/mL) | 8.9 |
| Range (ng/mL) | 0.7–2,086 |
| <10 ng/mL (n) | 144 (37%) |
| ≥10 to < 20 ng/mL (n) | 56 (14%) |
| ≥20 ng/mL (n) | 55 (14%) |
| Unknown (n) | 133 (34%) |
| Gleason score (n) | |
| ≤7 | 163 (42%) |
| ≥8 | 150 (39%) |
| Unknown | 75 (19%) |
| Primary tumor stage (n) | |
| T1–T2 | 79 (20%) |
| T3–T4 | 96 (25%) |
| Unknown | 213 (55%) |
| Initial NCCN risk group (n) | |
| Low | 29 (7%) |
| Intermediate | 102 (26%) |
| High | 194 (50%) |
| N1 | 18 (5%) |
| Unknown | 45 (12%) |
| Prior treatment (n) | |
| Primary surgery | 227 (59%) |
| Surgery only | 126 (32%) |
| Surgery + ADT | 13 (3%) |
| Surgery + SRT ± ADT | 52 (13%) |
| Surgery + PLND ± ADT | 3 (1%) |
| Surgery + SRT + PLND + ADT | 1 (0%) |
| Surgery + chemotherapy ± ADT | 32 (8%) |
| Primary RT | 55 (14%) |
| RT only | 35 (9%) |
| RT + ADT | 13 (3%) |
| RT + chemotherapy ± ADT | 7 (2%) |
| Age at PET/CT (y) | |
| Median | 69 |
| Range | 39–95 |
| Time between primary treatment and PET/CT (mo) | |
| Median | 161 |
| Range | 0.75–794.50 |
| Ongoing systemic therapy (n) | 70 (18%) |
| Last serum PSA before PET/CT (ng/mL) | |
| Median | 3.66 |
| Range | 0.04–1,776.79 |
| Indication for PET/CT (n) | |
| Initial staging | 93 (24%) |
| Biochemical recurrence localization | 225 (58%) |
| Restaging M1 | 70 (18%) |
ADT = androgen deprivation therapy; SRT = salvage radiation therapy; PLND = pelvic lymph node dissection; RT = radiation therapy.
TABLE 3.
Study Indication Stratified by Serum PSA Level
| Indication | 0.2−5.0 ng/mL | 5.1–10.0 ng/mL | 10.1–20.0 ng/mL | >20.1 ng/mL |
| Initial staging (n = 93) | 12 (13%) | 26 (28%) | 22 (24%) | 33 (35%) |
| Biochemical recurrence localization (n = 225) | 175 (78%) | 28 (12%) | 17 (8%) | 5 (2%) |
| Restaging M1 disease (n = 70) | 29 (41%) | 10 (14%) | 11 (16%) | 20 (29%) |
68Ga-PSMA-11 PET/CT Findings
Scan Positivity and Disease Distribution
In total, 321 of 388 patients (83%) had positive scans: 176 of 388 (45%) had PSMA-positive lesions in the prostate or prostate bed, 157 of 388 (40%) had positive pelvic lymph nodes (N1), 79 of 388 (20%) had positive extrapelvic lymph nodes (M1a), 105 of 388 (27%) had positive bone lesions (M1b), and 27 of 388 (7%) had positive visceral lesions (M1c) (Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org). Of note, 53 of 388 (14%) had both lymph node and bone metastases. Of the 105 patients with PSMA-positive bone lesions (M1b), 18 (17%) had a solitary metastatic lesion, 19 (18%) had 2–3 metastatic lesions, 14 (13%) had 4–5 metastatic lesions, and 54 (52%) had more than 5 metastatic lesions (Table 4).
TABLE 4.
Patients with PSMA-Positive Bone Lesions (M1b, n = 105) Stratified by Number of Suspected Metastatic Lesions (N, M1a, M1b, or M1c)
| Parameter | Solitary (1) | Oligometastatic (2–3) | Oligometastatic (4–5) | Polymetastatic (5+) | Total |
| All patients with bone lesions | 18 (17%) | 19 (18%) | 14 (13%) | 54 (52%) | 105 |
| T0N0M1 | 14 | 8 | 4 | 21 | 47 (45%) |
| T0N1M1 | 0 | 3 | 2 | 14 | 19 (18%) |
| T+N0M1 | 4 | 7 | 3 | 6 | 20 (19%) |
| T+N1M1 | 0 | 1 | 5 | 13 | 19 (18%) |
| Median PSA (ng/mL) | 6.85 | 6.85 | 7.54 | 8.25 | 8.25 |
Bone Lesion Regions Stratified by Indication
Bone metastases occurred in 12 of 93 patients (13%) who underwent initial staging, 44 of 225 patients (20%) with BCR, and 49 of 70 patients (70%) who underwent restaging of M1 disease (Supplemental Table 2). Thus, as expected, the incidence of metastases increased with more advance disease (P < 0.001).
Bone Lesion Incidence as Stratified by Serum PSA and NCCN Risk Score
The incidence of bone metastases increased with serum PSA level (Spearman correlation, 0.26; P < 0.001). Levels of less than 5, 5–10, 10–20, and more than 20 ng/mL were associated with bone metastases in 17.6%, 34.4%, 40.8%, and 41.4% of patients, respectively (P < 0.001) (Table 5). Thirty-eight of 216 patients (17.6%) with serum PSA levels of less than 5 ng/mL had evidence of bone metastases. These occurred in 2 of 12 patients (17%) who underwent initial staging, 24 of 175 (14%) with BCR, and 12 of 29 (41%) who underwent restaging of M1 disease (Fig. 1). Bone metastasis incidence was not significantly associated with NCCN risk score (P = 0.22).
TABLE 5.
Characteristics of Positive Bone Lesions Stratified by PSA Value
| PSA value before PET/CT (ng/mL) | Average PSA (ng/mL) | Percentage with positive bone lesions | Average PSA of patients with positive bone lesions (ng/mL) | Average number of positive bone lesion areas |
| <10 (n = 281) | 2.6 | 21% (n = 59) | 3.8 | 2.0 |
| ≥10 to <20 (n = 49) | 14.1 | 41% (n = 20) | 14.8 | 3.9 |
| ≥20 (n = 58) | 122.5 | 41% (n = 24) | 211.4 | 4.4 |
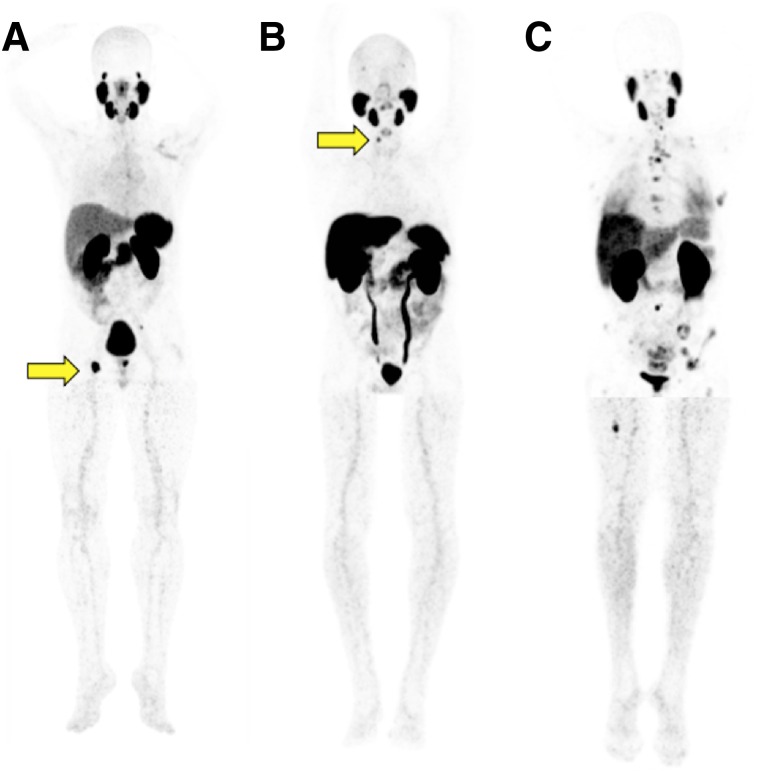
FIGURE 1.
68Ga-PSMA-11 PET maximum-intensity projections of patients with PSA level of less than 5 ng/mL and positive bone lesions. (A) Patient undergoing initial staging with PSA of 0.7 mg/mL and positive right ischial tuberosity bone lesion (arrow). (B) Patient with biochemical recurrence, PSA of 0.7 mg/mL, and positive right cervical vertebral body lamina lesion (arrow). (C) Patient undergoing restaging with PSA of 0.19 mg/mL and multifocal positive bone lesions.
Serum PSA Level and Number of Involved Bone Regions
The average number of PSMA-positive bone lesion areas increased with serum PSA level. Patients with a serum PSA level of less than 10 ng/mL, 10.1−20 ng/mL, and more than 20 ng/mL averaged 2.0, 3.9, and 4.4 positive bone lesion areas, respectively (Spearman correlation, 0.46; P < 0.001). Bone lesions were most frequently located in the pelvis (73/105, 70%); ribs, clavicle, and sternum (62/105, 59%); and spine (60/105, 57%). This distribution was consistent for all 3 indications.
Potential Impact of Total-Body PET/CT Scanning from Vertex to Toes
Eighteen patients had lesions below the proximal third of the femur, and all but one of these had extensive bone disease elsewhere. Similarly, 18 patients had lesions above the skull base, and all of these had extensive bone involvement elsewhere. Twenty-five patients (6%) had lesions outside the standard PET/CT imaging field. However, all but one had extensive disease elsewhere and were referred for 177Lu-PSMA treatment eligibility assessments. Therefore, discovering metastases outside the standard whole-body field of view would not have changed patient management. The remaining patient had BCR of unknown localization, and total-body PSMA PET/CT detected 2 solitary bone metastases in the right distal femur and in the left clivus (Fig. 2). After stereotactic radiation therapy of the 2 lesions, his serum PSA level declined from 7.1 to 0.19 ng/mL. Figure 3 displays additional examples of patients with PSMA-positive bone lesions both within and excluded from the standard field of view.
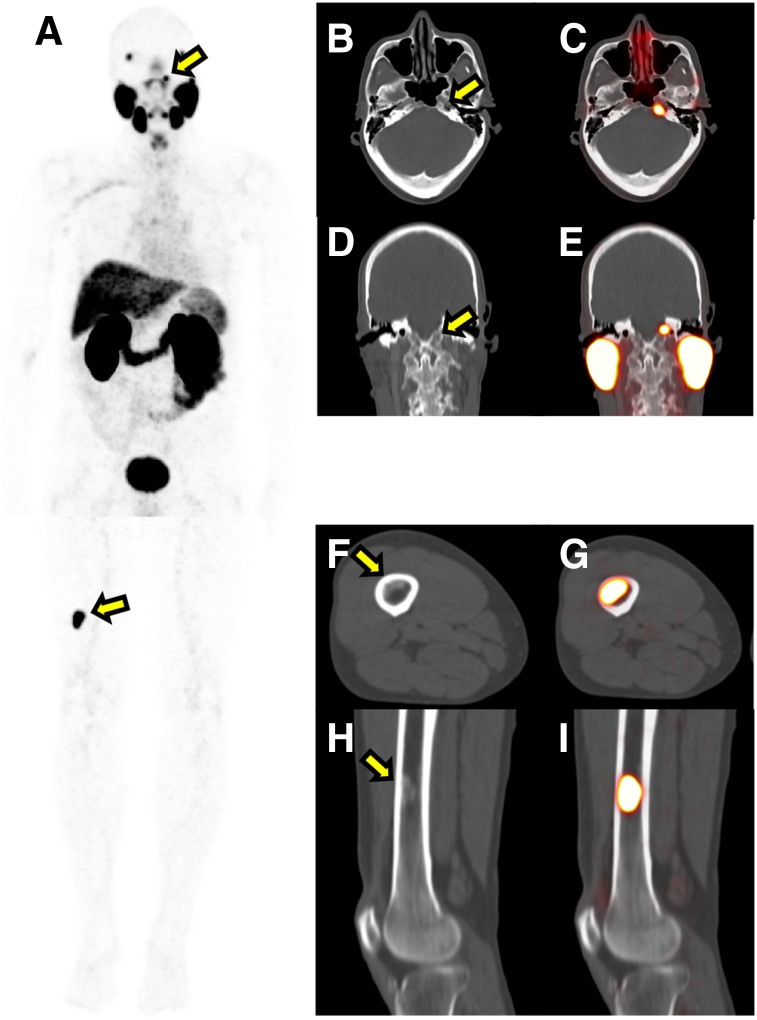
FIGURE 2.
Total-body 68Ga-PSMA-11 PET/CT imaging affected management of only 1 of 388 patients. That patient had biochemical recurrence of unknown localization 5 y after primary radiation therapy. He underwent multiple 18F-NaF and 18F-fluciclovine PET/CT studies, which were negative. His last PSA level before 68Ga-PSMA-11 PET was 7 ng/mL. Total-body 68Ga-PSMA-11 PET/CT detected 1 lesion in the right distal femur and 1 in the left clivus. Patient subsequently underwent ablative stereotactic body radiation therapy of the 2 lesions, resulting in PSA decrease from 7.1 to 0.19 ng/mL. (A) 68Ga-PSMA-11 PET maximum-intensity projection. (B and C) Axial CT (B) and PET/CT (C) images of the left clivus lesion. (D and E) Coronal CT (D) and PET/CT (E) images of the left clivus lesion. (F and G) Axial CT (F) and PET/CT (G) images of the right femur lesion. (H and I) Coronal CT (H) and PET/CT (I) images of the right femur lesion. Arrows indicate lesions.
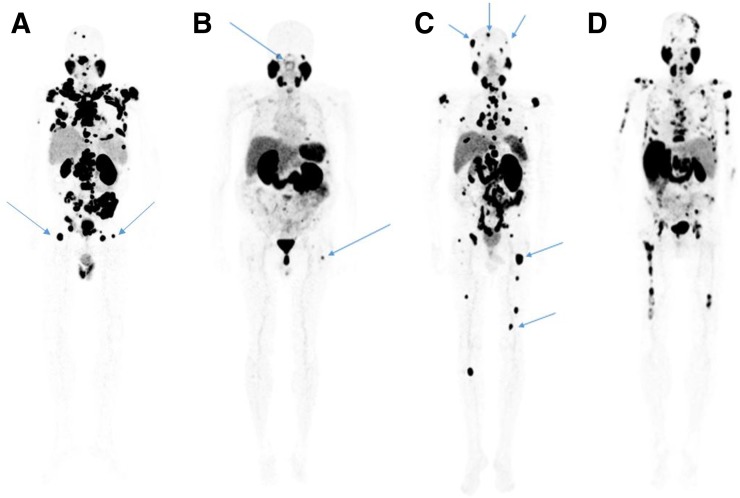
FIGURE 3.
68Ga-PSMA-11 PET maximum-intensity projections of patients with multiple positive bone lesions. (A) Patient with lesions in the proximal third of femur but not below that point. (B) Patient with lesions in the proximal third of femur and the skull base. (C) Patient with lesions in the superior skull and both above and below the proximal third of femur. (D) Patient with bone lesions in the superior skull, skull base, and both above and below the proximal third of femur. Arrows indicate lesions.
DISCUSSION
Here, we used a database of prospectively enrolled patients who underwent total-body 68Ga-PSMA-11 PET/CT to determine the incidence of bone metastases. We stratified patients by study indication (initial staging, BCR, or restaging M1), serum PSA level, and NCCN risk score. Presence of PSMA-positive bone lesions was associated with serum PSA level and disease progression (from initial diagnosis through BCR to restaging M1). Surprisingly, 38 of 216 patients (17.6%) with serum PSA levels of less than 5.0 ng/mL had PSMA-positive lesions suggestive of bone metastases. These occurred in 2 of 12 patients (17%) who underwent initial staging, 24 of 175 (14%) with BCR, and 12 of 27 (41%) who underwent restaging of known M1 disease before or during systemic therapy.
The initial NCCN risk score was not significantly associated with presence of PSMA-positive bone lesions. The reason is likely the long interval between initial diagnosis and the PET/CT study (median, 161 mo).
The considerable number of patients with PSMA-positive bone lesions at low PSA levels suggests that indications for bone staging require adaptation. Various expert opinions from professional organizations have been proposed and promoted. For high-risk patients and those with bone pain, initial staging usually includes bone imaging as recommended by most organizations and experts. On the other hand, intermediate-risk patients “may be” appropriately staged with bone imaging (Table 1). Bone imaging can also be suggested in patients with BCR of unknown localization and in patients who are undergoing restaging whereby clear PSA thresholds or risk scores are not provided. The current incidence of bone metastases in these groups suggests that bone assessments may be critically important to optimize management of these patients.
A metaanalysis of bone scan detection rates in patients undergoing initial staging revealed the presence of bone lesions in 2.3%, 5.3%, and 16.2% of patients with serum PSA levels of less than 10, 10.1–19.9, and 20–49.9 ng/mL, respectively (23). In this analysis, detection rates were 5.6% and 29.9% for Gleason scores ≤ 7 and ≥ 8, respectively. We cannot draw clear conclusions from the current study regarding recommendations and guidelines. However, detection rates reported here suggest that 68Ga-PSMA-11 PET/CT imaging detects bone involvement in a higher fraction than anticipated from conventional bone scan data.
Systematic comparisons between planar, SPECT, or PET and PSMA-targeted PET/CT imaging for detecting prostate cancer bone metastases are sparse. In a small study of 28 patients, 18F-NaF PET/CT detected more bone lesions than 18F-DCFPyL (24). In another direct comparison, Lengana et al. showed superiority of PSMA imaging over bone scanning (25). Lastly, a third study showed that PSMA and 18F-NaF PET/CT were similarly accurate (26).
Here, we were not focusing on comparing 68Ga-PSMA-11 with other imaging modalities. Rather, we established the incidence of PSMA-positive bone lesions suggestive of metastasis for various PSA levels, indications, and risk scores. The current data support that even patients with low serum PSA values can be at risk for bone metastases. Of course, metastasizing derives from many more factors than just the most recent PSA level—among many others, the effectiveness of the primary definitive therapy and biologic features of aggressiveness (high Gleason grade, PSA kinetics). A shorter PSA doubling time has been independently associated with scan positivity and extrapelvic metastases (27,28).
As a secondary aim, we evaluated whether total-body imaging, from vertex to toes, provides diagnostic information that may affect patient management. Thirty-six patients with lesions above the base of the skull and below the proximal femur were identified. All but one of these patients had already known extensive bone disease, and planned management was changed in this only one patient. Simsek et al. also evaluated the clinical impact of including the lower limbs in 68Ga-PSMA-11 PET/CT scans for prostate cancer patients (29). They similarly found that lower-limb imaging did not change the metastatic status of disease or significantly affect the therapeutic approach. Obtaining the PET images of the legs can add up to a dozen minutes. Thus, a significant and useful impact of total-body versus whole-body imaging appears unlikely. Of note, in a symptomatic patient, it could be beneficial to include lower-limb imaging for possible palliative therapies (29).
This study had some limitations. Although all patients were enrolled in prospective 68Ga-PSMA-11 imaging trials, the current data were extracted retrospectively. Thus, the study is subject to all known biases of retrospective studies.
Another limitation is the absence of lesion verification. This limitation cannot be overcome, even in prospective studies, because bone biopsies are infrequently done, and because many patients had oligometastatic to extensive bone involvement. Thus, false-positive findings cannot be ruled out and therefore may affect specificity. When considering the specificity of 88% for 68Ga-PSMA-11 PET for bone lesions found in a study by Pyka et al. (30), up to 13 of our 105 patients (12%) with PSMA-positive bone lesions may be false-positives. Additionally, false-negatives cannot be ruled out, which may affect sensitivity. Although Pyka et al. found a sensitivity of nearly 100% for bone metastases for 68Ga-PSMA-11 PET, our study may contain false-negative scans.
CONCLUSION
We demonstrate a higher than expected rate of bone involvement as determined by 68Ga-PSMA-11 PET/CT imaging: 18%, 34%, and 41% of patients with serum PSA levels of less than 5.0 ng/mL, 5–10 ng/mL, and 10–20 ng/mL, respectively, had PSMA-positive lesions suggestive of bone metastases. Therefore, current guidelines for bone assessments in prostate cancer patients should be revisited because 68Ga-PSMA-11 PET/CT may provide additional information for accurate bone staging at low serum PSA levels. The current study demonstrates the importance of initiating a prospective direct comparison between conventional imaging (bone scans, CT, or whole-body MRI, which has replaced bone scans in some institutions) and 68Ga-PSMA-11 PET/CT imaging for bone metastasis staging and suggests the need to adapt bone imaging guidelines in prostate cancer.
DISCLOSURE
This was an investigator-initiated study with institutional funding. The study was funded by the Ahmanson Translational Theranostics Division (UCLA). Jeremie Calais receives fees from Progenics Pharmaceuticals and RadioMedix and is a consultant for Blue Earth Diagnostics, outside the submitted work. He is the recipient of a grant from the Philippe Foundation Inc., and the ERF-SNMMI. Johannes Czernin is the recipient of a grant from the U.S. Department of Energy (DE SC0012353), from the Prostate Cancer Foundation (2017 challenge award 17CHAL02), and from the Johnson Comprehensive Cancer Center NIH-NCI Cancer Center (support grant P30 CA016042). He is a founder and board member and holds equity in Sofie Biosciences and Trethera Therapeutics, outside the submitted work. Intellectual property patented by the University of California is licensed to Sofie Biosciences and Trethera Therapeutics. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the relationship between serum PSA level and the incidence of bone metastases detected by total-body 68Ga-PSMA-11 PET/CT, and does expanding the imaging field to include the vertex and lower extremities affect bone metastasis detection rates and patient management?
PERTINENT FINDINGS: This was a retrospective analysis of 388 prostate cancer patients enrolled in 5 prospective studies who underwent 68Ga-PSMA-11 PET/CT scans acquired from vertex to toes for primary staging, biochemical recurrence localization, or restaging M1 disease. PSMA-positive bone lesion incidence was positively associated with serum PSA level (P < 0.001). There was only 1 of 388 patients (0.26%) in whom the total-body PET acquisition had an impact on management.
IMPLICATIONS FOR PATIENT CARE: Bone metastases as assessed with 68Ga-PSMA-11 PET/CT are prevalent even in patients with low serum PSA levels; therefore, current guidelines for bone assessments in prostate cancer patients should be revisited, as 68Ga-PSMA-11 PET/CT may provide additional information for accurate bone staging at low serum PSA levels.
Supplementary Material
REFERENCES
- 1.Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO Guideline. Part I: risk stratification, shared decision making, and care options. J Urol. 2018;199:683–690. [DOI] [PubMed] [Google Scholar]
- 2.Donohoe KJ, Cohen EJ, Giammarile F, et al. Appropriate use criteria for bone scintigraphy in prostate and breast cancer: summary and excerpts. J Nucl Med. 2017;58(4):14N–17N. [PubMed] [Google Scholar]
- 3.Expert Panel on Urologic Imaging Coakley FV, Oto A, et al. ACR Appropriateness Criteria® prostate cancer-pretreatment detection, surveillance, and staging. J Am Coll Radiol. 2017;14(suppl):S245–S257. [DOI] [PubMed] [Google Scholar]
- 4.Carroll PH, Mohler JL. NCCN guidelines updates: prostate cancer and prostate cancer early detection. J Natl Compr Canc Netw. 2018;16:620–623. [DOI] [PubMed] [Google Scholar]
- 5.Eiber M, Fendler WP, Rowe SP, et al. Prostate-specific membrane antigen ligands for imaging and therapy. J Nucl Med. 2017;58(suppl):67S–76S. [DOI] [PubMed] [Google Scholar]
- 6.Hofman MS, Hicks RJ, Maurer T, Eiber M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. Radiographics. 2018;38:200–217. [DOI] [PubMed] [Google Scholar]
- 7.Sathianathen NJ, Butaney M, Konety BR. The utility of PET-based imaging for prostate cancer biochemical recurrence: a systematic review and meta-analysis. World J Urol. 2019;37:1239–1249. [DOI] [PubMed] [Google Scholar]
- 8.Kim S-J, Lee S-W, Ha HK. Diagnostic performance of radiolabeled prostate-specific membrane antigen positron emission tomography/computed tomography for primary lymph node staging in newly diagnosed intermediate to high-risk prostate cancer patients: a systematic review and meta-analysis. Urol Int. 2019;102:27–36. [DOI] [PubMed] [Google Scholar]
- 9.Barrio M, Fendler WP, Czernin J, Herrmann K. Prostate specific membrane antigen (PSMA) ligands for diagnosis and therapy of prostate cancer. Expert Rev Mol Diagn. 2016;16:1177–1188. [DOI] [PubMed] [Google Scholar]
- 10.Zacho HD, Nielsen JB, Haberkorn U, Stenholt L, Petersen LJ. 68Ga-PSMA PET/CT for the detection of bone metastases in prostate cancer: a systematic review of the published literature. Clin Physiol Funct Imaging. October 29, 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11.Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:479–505. [DOI] [PubMed] [Google Scholar]
- 12.Fendler WP, Eiber M, Beheshti M, et al. 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017;44:1014–1024. [DOI] [PubMed] [Google Scholar]
- 13.Rauscher I, Maurer T, Fendler WP, Sommer WH, Schwaiger M, Eiber M. 68Ga-PSMA ligand PET/CT in patients with prostate cancer: how we review and report. Cancer Imaging. 2016;16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calais J, Fendler WP, Eiber M, et al. Impact of 68Ga-PSMA-11 PET/CT on the management of prostate cancer patients with biochemical recurrence. J Nucl Med. 2018;59:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calais J, Kishan AU, Cao M, et al. Potential impact of 68Ga-PSMA-11 PET/CT on the planning of definitive radiation therapy for prostate cancer. J Nucl Med. 2018;59:1714–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eder M, Schäfer M, Bauder-Wüst U, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012;23:688–697. [DOI] [PubMed] [Google Scholar]
- 17.Halpern BS, Dahlbom M, Auerbach MA, et al. Optimizing imaging protocols for overweight and obese patients: a lutetium orthosilicate PET/CT study. J Nucl Med. 2005;46:603–607. [PubMed] [Google Scholar]
- 18.Schwarzenboeck SM, Rauscher I, Bluemel C, et al. PSMA ligands for PET imaging of prostate cancer. J Nucl Med. 2017;58:1545–1552. [DOI] [PubMed] [Google Scholar]
- 19.Hofman MS, Hicks RJ, Maurer T, Eiber M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. Radiographics. 2018;38:200–217. [DOI] [PubMed] [Google Scholar]
- 20.Eiber M, Herrmann K, Calais J, et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med. 2018;59:469–478. [DOI] [PubMed] [Google Scholar]
- 21.Fendler WP, Calais J, Allen-Auerbach M, et al. 68Ga-PSMA-11 PET/CT interobserver agreement for prostate cancer assessments: an international multicenter prospective study. J Nucl Med. 2017;58:1617–1623. [DOI] [PubMed] [Google Scholar]
- 22.Rischpler C, Beck TI, Okamoto S, et al. 68Ga-PSMA-HBED-CC uptake in cervical, celiac, and sacral ganglia as an important pitfall in prostate cancer PET imaging. J Nucl Med. 2018;59:1406–1411. [DOI] [PubMed] [Google Scholar]
- 23.Abuzallouf S, Dayes I, Lukka H. Baseline staging of newly diagnosed prostate cancer: a summary of the literature. J Urol. 2004;171:2122–2127. [DOI] [PubMed] [Google Scholar]
- 24.Harmon SA, Bergvall E, Mena E, et al. A prospective comparison of 18F-sodium fluoride PET/CT and PSMA-targeted 18F-DCFBC PET/CT in metastatic prostate cancer. J Nucl Med. 2018;59:1665–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lengana T, Lawal IO, Boshomane TG, et al. 68Ga-PSMA PET/CT replacing bone scan in the initial staging of skeletal metastasis in prostate cancer: a fait accompli? Clin Genitourin Cancer. 2018;16:392–401. [DOI] [PubMed] [Google Scholar]
- 26.Dyrberg E, Hendel HW, Huynh THV, et al. 68Ga-PSMA-PET/CT in comparison with 18F-fluoride-PET/CT and whole-body MRI for the detection of bone metastases in patients with prostate cancer: a prospective diagnostic accuracy study. Eur Radiol. 2019;29:1221–1230. [DOI] [PubMed] [Google Scholar]
- 27.Ceci F, Uprimny C, Nilica B, et al. 68Ga-PSMA PET/CT for restaging recurrent prostate cancer: which factors are associated with PET/CT detection rate? Eur J Nucl Med Mol Imaging. 2015;42:1284–1294. [DOI] [PubMed] [Google Scholar]
- 28.Kakhki VRD, Anvari K, Sadeghi R, Mahmoudian A-S, Torabian-Kakhki M. Pattern and distribution of bone metastases in common malignant tumors. Nucl Med Rev Cent East Eur. 2013;16:66–69. [DOI] [PubMed] [Google Scholar]
- 29.Simsek DH, Sanli Y, Kuyumcu S, Engin MN, Buyukkaya F, Demirci E. Clinical impact of lower-limb imaging in 68Ga-PSMA PET/CT for patients with prostate cancer. J Nucl Med Technol. 2019;47:233–237. [DOI] [PubMed] [Google Scholar]
- 30.Pyka T, Okamoto S, Dahlbender M, et al. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:2114–2121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.