Abstract
Context
Hepatitis C virus (HCV) infection is a prevalent disease worldwide. Thyroid dysfunction is one of the most common extrahepatic manifestations of HCV infection. We hypothesized that HCV can directly infect human thyrocytes thereby causing thyroid dysfunction.
Setting
Human thyrocytes in primary cell culture, ML-1 human thyroid cell line, and Huh7.5 human hepatocyte cell line were infected with HCV using the Huh7.5JFH1 cell line that releases infectious HCV virions. After infection, the release of new virions, production of proinflammatory cytokines, and expression of miR-122 were evaluated. Ribonucleic acid (RNA) extracted from HCV-infected cells and mock-infected cells was subjected to RNA sequencing and transcriptomic analysis. Ingenuity pathway analysis was used to detect up- and down-regulated pathways.
Results
Human thyrocytes express major HCV entry factors including CD81, occludin, claudin-1, and scavenger receptor class B1. Viral infection of thyroid cells was confirmed by detection of HCV core protein in supernatants and negative-sense HCV RNA in cell lysates. HCV infection of thyrocytes induced the production of the chemokine CXCL-8 and the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and significantly increased the expression of miR-122. Moreover, HCV infection of thyrocytes decreased expression of the thyroid peroxidase and thyroglobulin genes and increased expression of the deiodinase 2 gene. The top upregulated pathways in HCV-infected thyrocytes were immune pathways and metabolic pathways, while infected hepatocytes upregulated lipid and glucose metabolism pathways as previously reported.
Conclusions
HCV infection may induce thyroid dysfunction by different mechanisms including direct infection of thyrocytes leading to activation of inflammatory pathways and upregulation of miR-122. These findings support a general mechanism for viral induction of autoimmunity through direct infection of target tissues.
Keywords: hepatitis C virus, thyroiditis autoimmune, cytokine, RNA sequencing, miR-122, IL-8
There is growing evidence that viral infection may be involved in the development of thyroid autoimmunity as well as other autoimmune diseases such as type 1 diabetes. Hepatitis C virus (HCV) infection is a common disease worldwide that is frequently associated with extrahepatic autoimmune manifestations (1–3). This association raises the question of whether HCV infection induces autoimmunity through immune-related mechanisms or by target organ effects. HCV is a positive-sense, single-stranded ribonucleic acid (RNA) virus that belongs to the Flaviviridae family. HCV has specific tropism for hepatocytes through several entry factors; after entering the cell, HCV replicates and induces persistent infection in hepatocytes. This specific tropism to hepatocytes is also partly due to the high expression levels of miR-122 in hepatocytes that stabilizes HCV RNA and facilitates replication of viral RNA (4).
The involvement of extrahepatic organs in patients with HCV infection may suggest that other organs can be infected and serve as reservoirs of HCV virions. Thyroid involvement autoimmune and nonautoimmune is one of the most prevalent extrahepatic manifestations of HCV infection (5–7). While previously the majority of patients with HCV developing thyroid dysfunction were treated with interferon-α (IFN-α), the association of HCV with thyroiditis is also frequent in untreated patients and was recently reported in interferon-free regimes with direct-acting antiviral therapy (8). Indeed, postmortem histopathological data showed that thyroid disease was the only major endocrinopathy associated with HCV infection (13% of cases vs 7% of controls) (9).
HCV may induce autoimmune thyroiditis by direct effects on the thyroid. Indeed, studies have shown that HCV RNA can be detected in other organs including thyroid tissue in patients with HCV infection. Bartolome et al. detected positive-sense and negative-sense HCV RNA in 3 of 3 thyroid tissues from patients with hepatitis C (10). Autopsy material from patients with hepatitis C and acquired immunodeficiency syndrome demonstrated the presence of positive-sense HCV RNA in thyroid tissues from 8 of 8 subjects and negative-sense HCV RNA in 2 of 8 (11). HCV may also induce autoimmunity by bystander activation. We recently reported that thyrocytes express high levels of the major HCV receptor CD81 (12), and that HCV envelope protein E2 induced production of proinflammatory cytokines and activation of heat shock proteins in primary thyrocytes by binding to CD81 (13). Moreover, we recently demonstrated that HCV can infect a human thyroid cell line demonstrating that thyroid cells may serve as a reservoir for HCV (14). However, this cell line was obtained from a patient with follicular thyroid cancer (11); thus, definitive proof that HCV can infect normal human thyrocytes is limited.
The aim of the present study was to test whether HCV can infect human thyrocytes in primary cultures and to dissect the mechanisms by which HCV infection of thyrocytes induced thyroiditis. Our results show that HCV can infect primary human thyrocytes and that HCV infection induces a strong immune inflammatory response and increases the expression of miR-122 (Fig.1).
Figure 1.
HCV contains a single positive-strand RNA coding for 10 different proteins: core protein, envelope proteins (E1 and E2), and 7 nonstructural proteins (NS). Human thyrocytes are susceptible to HCV infection.
Human thyrocytes express HCV surface receptors [CD81, occludin (OCLN), claudin-1 (CLDN-1) and scavenger receptor B1 (SR-B1)]. By binding to its receptors, HCV enters the thyroid cells, replicates in the cell, and induces alteration in thyroid hormones synthesis and their transport molecules. Moreover, HCV infection induces activation of inflammatory signaling pathways and metabolic pathways (lipid and glucose) and increases the expression of miR-122. These findings may explain frequent association of thyroid dysfunction in patients with hepatitis C infection.
Abbreviations: ER, endoplasmic reticulum; HCV, hepatitis C virus; Tg, thyroglobulin; TPO, thyroid peroxidase; RNA, ribonucleic acid.
Methods
Cell cultures
Human thyroid primary cells.
The project was approved as exempt by the Icahn School of Medicine Institutional Review Board. Human primary thyrocytes were prepared from fresh, normal, deidentified thyroid tissues adjacent to a thyroid tumor collected during surgery (3 females and 3 males) as previously described by our group (15). Briefly, approximately 0.3 to 1.2 g of tissue was washed in phosphate-buffered saline, minced on ice, and incubated with 200 U/mL collagenase solution for 45 minutes at 37° C on a shaker 3 times. Cells were harvested from the tissue and cultured with medium E199/EBSS (Thermo Fischer Scientific, Waltham, MA, USA), supplemented with 10% fetal bovine serum (FBS), penicillin (100 ug/mL), and streptomycin (100 ug/mL). After overnight culture in 37°C humidified air at 5% CO2, cells were washed twice to remove mononuclear cells and kept in culture for 2 days. Each set of experiments was performed in duplicate or triplicate.
As the access to primary thyrocytes was limited, primary human thyrocytes were used in experiments detecting HCV virus replication, expression of MiR-122, and transcriptome analysis. Additional experiments were performed in ML-1 cells as described below.
Human thyroid cell line ML-1.
The ML-1 cell line (female origin; a gift from Dr Schönberger, University of Regensburg, Germany) was derived from a differentiated follicular thyroid carcinoma (16); ML-1 cells express thyroglobulin, and represent a suitable model for biological studies of thyrocyte interactions. ML-1 cells were maintained in Dulbecco’s modified Eagle’s medium, supplemented with 10% FBS, 1% penicillin and streptomycin. ML-1 cells were authenticated by measurement of their key phenotypic marker, thyroglobulin by polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA).
Human hepatocyte cell lines.
The human hepatocyte cell line Huh7.5 (male origin) was kindly provided by Apath LLC (St. Louis, MO) and maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS and 1% penicillin-streptomycin. Huh7.5JFH1 was authenticated by its ability to sustain and propagate HCV infection. Human hepatocellular carcinoma HepG2 cell line (male origin) was obtained and authenticated from the American Type Culture Collection and was cultured in Eagle’s minimum essential medium supplemented with 10% FBS and 1% penicillin-streptomycin.Cells were cultured at 37⁰ in 5% CO2, and medium was replaced every 48 hours until confluent.
Primary thyroid cells do not proliferate, and require time to acclimatize and during this time, some fibroblasts may grow in the cultures.
Infection of cells with HCV virions
Cells (4 x 105 ML-1 cells, 2 × 105 Huh7.5 cells and human primary thyrocytes in culture) were seeded into 24 well plates. Huh7.5JFH1, which releases HCV genotype 2a virions into cell culture supernatant was used to produce HCV virions (courtesy of Dr Guangxiang Luo) (17). Infectious virions were harvested as previously described and used to infect cells in cultures (14). Briefly, cells were infected with 50μL of virus (~11.6 ng/mL of HCV core protein), and virus solution was removed after 4 hours. Cells were washed with phosphate-buffered saline 3 times to remove unbound virus. Cells were further kept in culture for 6 or 24 hours, and for 3, 5, and 7 days for different experiments as indicated.
Cell surface receptors detection
HCV uses the cell surface receptors CD81, occludin (OCLN), claudin-1 (CLDN-1), and scavenger receptor class B1 (SR-B1) to enter cells (Fig. 1). In order to test whether human thyrocytes express these entry receptors, we evaluated ML-1 cells and primary human thyrocytes (3 different donors: 2 males and 1 female) for the presence of messenger RNA (mRNA) corresponding to CD81, OCL, CLDN-1, and SR-B1. Total RNA was isolated using TRIzol reagent (Thermo Fisher Scientific Inc., Waltham, Massachusettes, USA) in combination with the RNeasy Mini kit (Qiagen Inc., Germantown, Maryland, USA), followed by deoxyribonuclease treatment. Five hundred nanograms of total RNA were retrotranscribed using the SuperScript III kit (Thermo Scientific). The complementary deoxyribonucleic acids (cDNAs) obtained after retrotranscription were used as templates for semiquantitative PCR. The following forward (FW) and reverse (REV) primers were used for amplification: GAPDH FW: gtcagtggtggacctgacct, REV: aggggtctacatggcaactg; CD81 FW: gctgtcatgatgttcgttgg, REV: cctccttgaagaggttgctg; OCL FW: tggctgctgctgatgaatac, REV: gaagacatcgtctggggtgt; CLD-1 FW: tggctgctgctgatgaatac, REV: gaagacatcgtctggggtgt; SR-B1 FW: ggctgagcaaggttgacttc, REV: cagctgcagtttcacagagc. The results were quantified by 7300 Real-Time PCR Biosystem (Applied Biosystem, Foster City, CA).
ELISA for HCV core protein
To confirm that cells were infected by HCV, we quantified core protein levels in culture supernatants from infected and uninfected thyrocytes in primary culture and Huh7.5 cells in culture on day 3, 5, and 7, and ML-1 cells on day 3 using QuikTiter HCV Core Antigen ELISA Kit (Cell Biolabs, Inc., San Diego, CA), with a lower limit of detection of 1 ng/mL.
Quantitative detection of strand-specific HCV RNA
Mature HCV virions contain positive-sense RNA genome only, while negative-sense HCV RNA is present only in productively infected cells. Negative-sense HCV RNA was quantified in RNA extracted from human thyrocytes in primary culture and Huh7.5 cell culture supernatant (day 3 after infection) as previously described (14). For this experiment, The HCV-I antisense primer (5′-TGG ATG CAC GGT CTA CGA GAC CTC-3′, nt 342–320) was used, and PCR was performed with 30 cycles (94°C for 30 seconds, 58°C for 1 minute, and 72°C for 2 minutes). PCR products that were 295 base pairs in length were visualized by agarose gel electrophoresis.
Cytokine measurements
mRNA.
One µg of RNA extracted from cells at 24 hours was used to synthesize cDNA by SuperScript III first-strand kit (Invitrogen, Carlsbad, CA). mRNA levels of IL-6, CXCL8, and TNF-α were measured by quantitative PCR, using commercially available TaqMan gene expression specific primers (Applied Biosystem, Foster City, CA). The results were quantified by 7300 Real-Time PCR Biosystem (Applied Biosystem, Foster City, CA).
Proteins.
Supernatants of HCV-infected and uninfected cells in culture (ML-1 and Huh7.5) were collected after 6 hours and 24 hours of incubation. IL-6, CXCL8, TNF-α, IL-1β, IL-12(p40), IL-17, and IFN-γ were quantified using a Luminex multiplex assay according to the manufacturer’s instructions (EMD, Millipore Co, Billerica, MA).
Real-time quantitative PCR to quantify miRNA-122 levels
For analysis of microRNA (miRNA), total RNA was first extracted from ML-1 cells, primary thyroid cells, or Huh7.5 cells in culture. We then reverse transcribed the total RNA (at different concentrations: 40 ng, 200 ng, or 1 µg) using TaqMan miRNA reverse transcription kit (Applied Biosystems Foster City, CA) as previously described (18). Briefly, single-stranded cDNA was synthesized using commercially available, specific 5x RT primers for the target miRNA miR-122 and endogenous control miR-16 (Applied Biosystems Foster City, CA). The cDNA was amplified using the TaqMan Universal PCR Master Mix with UNG (Thermo Fisher Scientific, Waltham, MA), the miRNA-specific TaqMan probe hsa-miR-122-5p (UGGAGUGUGACAAUGGUGUUUG), and the hsa-miR-16-5p (UAGCAGCACGUAAAUAUUGGCG). The results were quantified by ABI7300 Real-Time PCR Biosystem (Applied Biosystem, Foster City, CA). RNA from Huh7.5 cells that express large amounts of miR-122 (kindly provided by Dr M.J. Evans, Icahn School of Medicine at Mount Sinai, NY) was included as positive control, and RNA from uninfected HepG2 cells served as a negative control (19).
Transcriptome and pathway analysis
Five hundred ng of RNA were extracted from infected and uninfected ML-1 and Huh7.5 cells (3 separate experiments performed for each cell line) at 6 hours and 24 hours and used for whole transcriptome RNA sequencing (RNA-seq). The early responses at 6 hours and 24 hours were limited to only a few genes. The experiments were repeated after 3 days in culture in ML-1, Huh7.5 (each 2 separate experiments for each cell line), and thyrocytes in primary cell cultures (from 3 different donors, 2 females and 1 male). RNA was isolated on day 3 after HCV infection. RNA-seq and transcriptome analysis were performed as previously described (20). Pathway analysis was performed to define functional networks and to identify genes that are up- or down-regulated by HCV infection in thyrocytes. The Ingenuity Pathway Analysis (IPA) (https://analysis.ingenuity.com/) (Qiagen Bioinformatics, Redwood, CA) was used to perform a core analysis on the dataset gene files generated by RNA-seq. Cut off of fold changes of >2 and >1.5, and P value < 0.05 were used for including genes in the pathway analysis.
Statistics
Data are expressed as the mean [standard error of the mean]. Differences in mean values were tested for statistical significance using the Student’s t test. A P value of < 0.05 was considered significant. All statistical analyses were performed using GraphPad Prism 5 software (San Diego, CA). For RNA-seq analyses, expressed transcripts were subjected to pathway analysis by Gene Ontology (GO) (geneontology.org) and IPA. The Fisher exact test was used to calculate P values for the probability that a pathway was significantly enriched in input genes compared to the genome, and the pathways/networks were ranked by fold change expressed in log2 ratio, and P values.
Results
Human primary thyrocytes express HCV cell surface receptors
Multiple surface receptors are involved in HCV entry in hepatocytes. CD81 is a transmembrane protein that mediates signal transduction and is involved in cell activation and growth. The large extracellular loop of CD81 binds to HCV envelope protein E2 and mediates the entry of HCV into cells (21, 22). OCLN is a plasma-membrane protein with an important role in tight junction function and is also required for the HCV infection (23). CLDN-1, another tight junction protein, is an HCV co-receptor required for the late entry of virus (23). Additionally, SR-B1 is a membrane protein that mediates the transfer of cholesterol and high-density lipoproteins into cells and serves as a cofactor for HCV entry (24). Our results show that human thyroid cells (both primary thyrocytes and ML-1 cells) express CD81, OCLN, CLDN-1, and SR-B1 as shown in Fig. 2.
Figure 2.
Expression of hepatitis C virus (HCV) cell receptors in human thyrocytes.
Agarose gel electrophoresis shows a robust expression of these major HCV surface receptors in thyrocytes. (2A) Human thyrocytes in primary cell culture (3 different donors). (2B) Human thyroid cell line ML-1 in cell culture.
HCV can infect human primary thyrocytes
We have previously shown that HCV can infect ML-1 cells (14); however, the ML-1 cell line was derived from a differentiated follicular thyroid cancer (16). Therefore, it is possible that the transformation of the thyrocytes might have rendered them susceptible to HCV infection. In order to test whether nontransformed thyrocytes can also support HCV infection and study the molecular consequences of HCV infection of thyrocytes, we examined whether HCV can infect thyrocytes in primary cell culture. HCV contains a single positive-strand RNA encoding 10 different proteins including the core protein, the envelope proteins E1 and E2, P7, and 7 nonstructural proteins (Fig. 1). Indeed, our results showed that HCV core protein was detectable in the supernatant of HCV-infected human primary thyrocytes on days 3, 5, and 7 postinfection, with levels that were comparable to those observed in Huh7.5 infected with HCV (Fig. 3A-C).
Figure 3.
Confirmation of hepatitis C virus (HCV) infection in human primary thyrocytes. HCV can infect primary thyrocytes with results comparable to that in hepatocytes. Core protein in cell culture supernatant of HCV-infected (3A) ML-1 and Huh7.5 on day 3 after HCV infection. (3B) Primary thyrocytes and Huh7.5 on day 5 and (3C) on day 7 after HCV infection. Huh7.5JFH1 cell line was used as a positive control. (3D) Negative-sense HCV RNA was detected in cell lysate from HCV-infected human thyrocytes in primary culture and HuH7.5. RNA extracted from Huh7.5JFH1 cell lysate was used as positive control.
To confirm productive viral infection, we tested the presence of negative-strand HCV RNA in cell lysates from HCV-infected and mock-infected cells. Because positive-sense HCV RNA resides within individual virions, the detection of positive-sense HCV RNA within cells may not represent bona fide viral infection and genome replication. Thus, the most important indicator of HCV genome replication is the production of negative-strand HCV RNA (25), which serves as a template for the production of new positive-strand viral genomes but is not present in virions. Figure 3D demonstrated the presence of negative-sense HCV RNA in cell lysates from infected primary thyrocytes and Huh7.5, but not in mock-infected cells. Huh7.5JFH1 cells were included as positive controls. Our group previously reported similar results in HCV-infected ML-1 cells. Huh7.5JFH1 cells were included as positive controls. Our group previously reported similar results in HCV-infected ML-1 cells (14).
HCV induces increased expression of CXCL8 and TNF-α in human thyrocytes
We evaluated cytokine responses in ML-1 and Huh7.5 cells upon infection with HCV. Protein and mRNA levels of 7 major cytokines/chemokines previously reported to be associated with autoimmunity were measured in the supernatants of cells in culture, both at 6 and 24 hours postinfection. The results for CXCL8, IL-6 and TNF-α are presented in Figure 4 (respectively Fig. 4A-C). A large increase in concentration of CXCL8 was observed both at the mRNA and protein levels (as measured in supernatants of ML-1 and Huh7.5 HCV-infected cells) at 6 and 24 hours compared with mock-infected cells. However, only the increase in CXCL8 protein levels reached statistical significance (Fig. 4A & 4D). Intriguingly, TNF-α protein levels were increased in HCV-infected ML-1 cells at 24 hours but not in HCV-infected Huh7.5 cells (Fig. 4C & 4F). The levels of interleukin 1 beta (IL-1β), IL-12(p40), IL-17, and IFN-γ were all lower than the detection limit (3.2 pmol/L) in treated and untreated cells. Except for an increase in IL-6 mRNA levels in ML-1–infected cells, no consistent changes in IL-6 levels were observed in ML-1 or Huh7.5 cells.
Figure 4.
Hepatitis C virus (HCV) infection increased the production of major cytokines at the protein levels in human thyrocytes compared to mock-infected cells.
Protein levels: Concentration of protein levels (pg/mL) in supernatant from cells in culture. Human thyroid cell line ML-1 cells: (A1) CXCL8, (B1) IL-6, and (C1) TNF-α; Human hepatocyte cell line Huh7.5 cells: (D1) CXCL8, (E1) IL-6, (F1) TNF-α.
Results are presented as mean [standard error of the mean]. ***P < 0.001. mRNA level: relative expression of mRNA by RT-qPCR corrected to GAPDH. ML-1 cells: (A2) CXCL8, (B2) IL-6, and (C2) TNF-α; Huh7.5 cells: (D2) CXCL8, (E2) IL-6, (F2) TNF-α. Results are presented in mean+/-SEM. *P < 0.05. Abbreviations: CXCL8, Chemokine 8; IL-6, Interleukin 6; TNF-α, Tummor necrosis alpha.
HCV infection increased expression of miR-122 in human thyrocytes
MiRNAs are small noncoding RNAs that are involved in a wide range of biological processes. miR-122 was shown to be highly expressed in hepatocytes (26, 27). Consistent with these previous reports, our result showed that miR-122 was highly expressed in the Huh7.5 human hepatocyte cell line. Our results also showed that miR-122 was expressed in human thyrocytes but at much lower levels (cycle threshold [CT]~30) than in Huh7.5 cells (CT~20). However, HCV infection significantly increased the expression of miR-122 in thyrocytes in primary cultures on day 3 (relative expression corrected to housekeeping miR-16: ~200-fold higher on day 3 compared to mock-infected cells) (CT~26). In contrast, no changes were observed in miR-122 levels in infected Huh7.5 cells, although expression remained high (CT~20). Similar results were seen in HCV-infected ML-1 cells at 6 hours, 24 hours, and days 3 postinfection (relative expression~200) (Fig. 5).
Figure 5.
Hepatitis C virus (HCV) infection increased the expression of miR-122 (microRNA) in human thyrocytes. The expression of miR-122 is low in human thyrocytes (ML-1 cells and thyrocytes in primary cell culture) compared with hepatocytes (Huh7.5), but increases significantly after HCV infection. This might partly explain frequent association of thyroid diseases with hepatitis C infection. miR-16 was used as housekeeping miR.
Transcriptome and pathway/network analyses
Transcriptome analyses were performed at 6 hours, 24 hours, and 3 days post infection; however, due to paucity of differentially expressed genes at the earlier time points, only the data at 3 days after infection are shown [GEO repository data accession number GSE 136 339 (28)]. We analyzed the enrichment of biological processes among the differentially expressed genes, using the GO database. Gene ontology analysis identified several GOs that were affected by HCV infection on day 3. The main pathways affected by HCV infection 3 days after infection are represented in Fig. 6 (Fig. 6A: primary thyrocytes; Fig. 6B: ML-1 cells; Fig. 6C: Huh7.5 cells).
Figure 6.
Top GOs induced by hepatitis C virus (HCV) infection.
Gene Ontology (GO) (geneontology.org) identified genes that were upregulated by HCV infection. Main identified upregulated pathway in human primary thyrocytes was inflammatory response. Genes involved in regulation of lipid and glucose metabolism pathways were common in thyrocytes and hepatocytes. (6A): Thyrocytes in primary culture cells, (6B): human thyroid cell line ML-1 cells, (6C): Human hepatocyte cell line Huh7.5 cells.
Human primary thyrocytes transcriptome changes
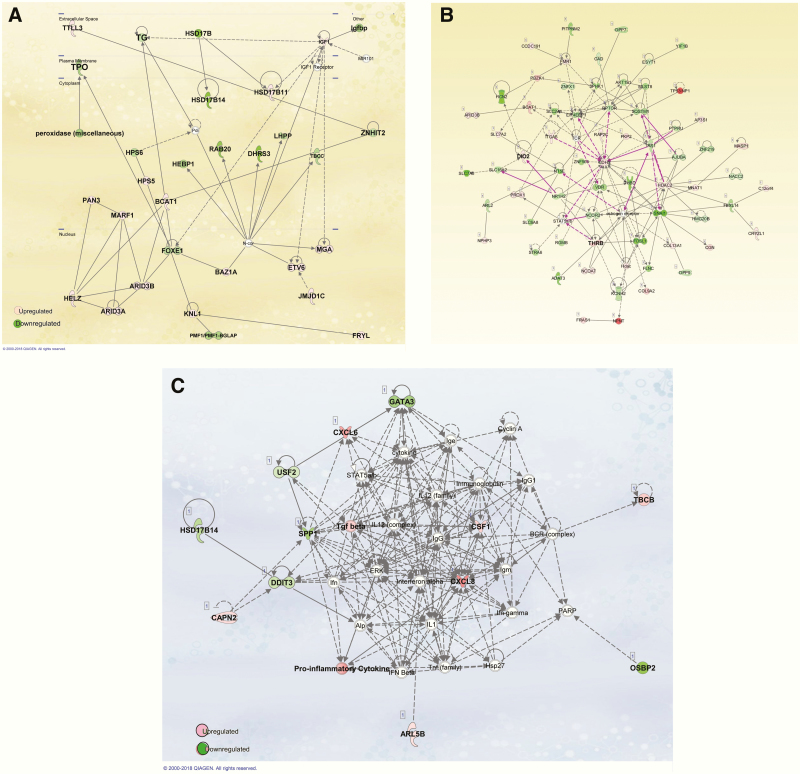
At 3 days postinfection, using a limma test and adjusted P< 0.05, 1185 genes were upregulated and 659 downregulated. GO mainly identified genes involved in (1) acute inflammatory response, (2) regulation of lipid metabolism process, (3) response to insulin and glucose stimulus, (4) activation of Jak/STAT, MAPK cascade and regulation of protein modification process and, (5) regulation of programmed cell death (Fig. 6A). HCV infection induced downregulation of several genes involved in thyroid hormone generation including thyroglobulin (Tg), thyroid peroxidase (TPO), deiodinase 1 (DIO1), FOXE1, and thyroid hormone receptor interactor 6 (Fig. 7A). The expression levels of key genes in antigen presentation, human leukocyte antigen C (HLA-C), HLA-E, HLA-H, HLA-L, B2M, FCGRT, and TAPBPL, were also differentially regulated by infection.
Figure 7.
. Ingenuity pathway analysis (IPA) (Qiagen Bioinformatics, Ca, USA) detected modification in expression of genes involved in thyroid hormone synthesis, activation, and transport in thyrocytes.
(7A): An example of response induced by hepatitis C virus (HCV) infection in human thyrocytes in primary culture. HCV infection induces downregulation of several molecules involved in thyroid hormone synthesis such as thyroid peroxidase (TPO), thyroglobulin (TG) and forkhead boxE1 (FOXE1). HCV infection in human thyrocytes induces downregulation of several genes involved in synthesis and transport of thyroid hormones: thyroid peroxidase (TPO), thyroglobulin (TG). (7B): An example of network of genes differentially modified by HCV infection in hepatocytes cell line Huh7.5.
HCV infection of hepatocyte cell line Huh7.5 induces activation of several proinflammatory cytokines such as CXCL8, CXCL-6 and other markers involved in hepatic fibrosis such as TGF beta (tissue growth factor beta), which is involved in organ fibrosis.
HCV infection activated other canonical pathways involved in CD40 signaling IL-6, IL-17A IL-7, IL-2 and CXCR4 signaling as well as IGF1 and β-cell receptor signaling. Other pathways detected by IPA were NF-κB and MAP kinase.
The 5 top canonical pathways detected by IPA were EIF2 signaling, acute phase response signaling, LXR/RXR activation, oxidative phosphorylation, and mammalian target of rapamycin signaling.
ML-1 cells transcriptome changes following HCV infection
At 3 days postinfection, the main upregulated pathways included inflammatory pathways such as acute inflammatory response, immune effector process and regulation of humoral immune responses, and negative regulation of apoptosis. In addition, several metabolic pathways were also upregulated including lipid homeostasis, response to insulin, and regulation of nicotinamide adenine dinucleotide phosphate oxidase (Fig. 6B).
At 24 hours there was differential regulation of several genes involved with thyroid hormone metabolism including decreased mRNA expression levels of deiodinase 1 (DIO1), increased expression of deiodinase 2 (DIO2), thyroid hormone receptor-β, transthyretin (TTR), and thyroid hormone responsive spot 14 (Fig. 7B).
Huh7.5 transcriptome changes following HCV infection
RNA-seq 3 days after infection of Huh7.5 cells mainly detected genes involved in the cell cycle, lipid metabolic process, cellular response to insulin stimulus, and oxidative phosphorylation. Other pathways of interest were immune system development, nicotinamide adenine dinucleotide dehydrogenase complex assembly, and positive regulation of ubiquitin-protein ligase activity. The main GOs are represented in Fig 6C.
Of particular interest, increased expression of inflammatory molecules CXCL8, CXCL6, CXCL5, PPAR-γ, and tissue growth factor beta were detected (Fig. 7C). The main canonical pathways detected by IPA were FXR/RXR activation and acute phase reaction pathways. These data are consistent with previous in vitro studies (29) and reports implicating these molecules in hepatic inflammatory reaction in patients with chronic hepatitis C [for review see (30)].
Discussion
This is the first study to show that human primary thyrocytes in culture can be infected by HCV (Fig. 1). We have used several complementary methods to prove productive HCV infection of human thyroid follicular cells. First, HCV core protein was detected in the supernatant of the cells, demonstrating production of new viral proteins (Fig. 3). Second, we demonstrated the presence of negative-strand HCV RNA in cell lysates, confirming that genome replication occurred within the thyrocytes (Fig. 3). These findings are consistent with studies reported by Laksus and Bartoleme in HCV-infected patients (10, 11). Third, we demonstrated that human thyrocytes express the necessary host factors that mediate attachment and entry of HCV virions into the cell (Fig. 2). Moreover, HCV infection of thyrocytes upregulated CLDN6 & 7 and miR-122, key host factors that facilitate productive infection. Taken together, these data show that HCV infection of thyrocytes may be enhanced by the virus through increased expression of viral receptors and miR-122.
Several of the changes we observed in thyrocytes infected with HCV can shed light on the association between HCV infection and thyroid autoimmunity.First, HCV infection of thyrocytes increased the expression of ICAM1, which has been shown to play a role in Hashimoto thyroiditis (31).
Moreover, HCV infection of human primary thyrocytes induced dysregulation of pathways involved with thyroid hormone synthesis and transport. HCV infection induced a decrease in expression of TPO, Tg (Fig. 7A), DIO1, and thyroid hormone receptor interactor 6. TPO is a membrane-based glycoprotein and acts as a central enzyme for thyroid hormone synthesis; the iodination of tyrosine residue in Tg generates the thyroid hormones thyroxin and triiodothyronine. We recently reported that treatment of human thyroid cells with IFN-α, a key innate immune response cytokine produced during viral infections, resulted in degradation of Tg (32). These data suggest that during viral infections, IFN-α–mediated degradation of Tg in the thyroid may release pathogenic peptides that can trigger autoimmune thyroid diseases (AITD) (32). Moreover, the reduction in levels of TPO and DIO1 can lead to accumulation of non-iodinated Tg in the cells causing endoplasmic reticulum (ER) stress, which can enhance its degradation into pathogenic peptides.
RNA-seq detected decreased expression of forkhead box E1 (FOXE1- TTF2), a thyroid transcription factor that binds to Tg and TPO gene promoters and plays a role in thyroid morphogenesis (33, 34). Intriguingly, mutations in this gene are associated with thyroid cancer and a rare form for congenital hypothyroidism Bamforth-Lazarus syndrome. We speculate that the reduction in FOXE1-TTF2 levels caused by HCV infection can also contribute to the accumulation of Tg and its degradation into immunogenic peptides that may induce autoimmunity.
In The ML-1thyroid cell line, HCV infection altered the expression of genes involved in the conversion of thyroid prohormone to active hormone, specifically a decrease in expression of DIO1 and increased DIO2 (Fig. 7B). DIO2 is also highly expressed in the thyroid of patients with Graves disease (35). An interesting common gene upregulated in ML-l cells, primary thyrocytes, and Huh7.5 cells was transthyretin (TTR), a transporter of thyroid hormone also involved in amyloid deposition. It was reported that environmental pollutants may disrupt thyroid function by interacting with TTR (36).
Viral infection may act as a trigger for thyroid autoimmunity by different mechanisms. As expected, HCV infection induced upregulation of CXCL8 in hepatocytes (Fig. 4D). In thyrocytes, an increase in production of CXCL8 and TNF-α was induced by HCV infection (Fig. 4A & 4C). These results are consistent with our previous studies showing that thyroid cells treated with HCV envelop protein E2 upregulate CXCL8 (13). HCV infection of thyrocytes induced a differential expression of antigen presentation-associated receptors, mostly decreasing HLA class I molecules. In contrast, upregulation of HLA-A, HLA-B, and HLA-C molecules was reported in thyroid tissues in autoimmune thyroid diseases (37), suggesting that the mechanisms by which HCV-induced thyroid autoimmunity may be unique. Similar to hepatocytes, HCV infection of thyrocytes had a significant effect on pathways of lipid and glucose metabolic processes (Fig. 6A-C). These findings may suggest that HCV infection has a dual effect, inducing pathways that trigger autoimmunity as well as metabolic pathways. Indeed, HCV infection has been shown to be strongly associated with type 2 diabetes [for review see (38)].
Taken together, our data show that HCV infection (and potentially other viral infections) may trigger thyroid dysfunction and autoimmunity by several mechanisms, including direct toxic effects on thyrocytes such as decreased expression of molecules involved in thyroid hormone synthesis and transport, production of pathogenic Tg peptides through increased degradation of Tg, and enhancement of immune responses (Fig. 1). Identifying the mechanisms by which infection triggers AITD will hopefully lead to new targets for therapy such as blocking autophagic degradation of Tg.
Conclusions
This is the first study dissecting directly the molecular effects of HCV infection on thyrocyte function and inflammation. Thyrocytes are susceptible to HCV infection because they express the main HCV receptors on their surface. In addition, thyrocytes express miR-122, a key cofactor facilitating HCV infection. Based on these findings, we conclude that HCV infection may induce thyroid dysfunction by influencing both immune and nonimmune thyroid-toxic mechanisms. These findings may explain frequent association of thyroid dysfunction in patients with hepatitis C infection. It remains to be seen whether the new antiviral therapies for HCV may also have beneficial effects on the thyroiditis associated with hepatitis C.
Acknowledgments
Financial Support: This work was supported in part by grants DK61659, and DK073681 from National institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (to Y.T.) and South-East Regional Health Authority Helse Sør Øst (HSØ) (to S.S.H.). The analysis was performed at Genomics, Epigenomics and Sequencing Core, Department of Environmental Health, University of Cincinnati.
Glossary
Abbreviations
- cDNA
complementary deoxyribonucleic acid
- CLDN-1
claudin-1
- DIO
deiodinase
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- FW
forward
- GO
Gene Ontology
- HCV
hepatitis C virus
- HLA
human leukocyte antigen
- IFN
interferon
- IL
interleukin
- IPA
Ingenuity Pathway Analysis
- miRNA
micro RNA
- mRNA
messenger RNA
- OCLN
occludin
- PCR
polymerase chain reaction
- REV
reverse
- RNA
ribonucleic acid
- RNA-seq
RNA sequencing
- Tg
thyroglobulin
- TNF-α
tumor necrosis alpha
- TPO
thyroid peroxidase
- TTR
transthyretin
Additional Information
Disclosure Summary: Yaron Tomer was previously (1/2015–6/2017) the PI on a basic research project jointly funded by the Juvenile Diabetes Research Foundation and Pfizer. The current manuscript is not related to that research project. All other authors have no potential conflict of interest to declare.
Data availability: Data generated by the authors or analyzed during the study are included in the published paper or available at repository data: (GEO Repository accession number GSE136339) (28).
References
- 1. Sherman AC, Sherman KE. Extrahepatic manifestations of hepatitis C infection: navigating CHASM. Curr HIV/AIDS Rep. 2015;12(3):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blackard JT, Kemmer N, Sherman KE. Extrahepatic replication of HCV: insights into clinical manifestations and biological consequences. Hepatology. 2006;44(1):15–22. [DOI] [PubMed] [Google Scholar]
- 3. Ferri C, Ramos-Casals M, Zignego AL, et al. ; ISG-EHCV coauthors International diagnostic guidelines for patients with HCV-related extrahepatic manifestations. A multidisciplinary expert statement. Autoimmun Rev. 2016;15(12):1145–1160. [DOI] [PubMed] [Google Scholar]
- 4. Cox EM, Sagan SM, Mortimer SA, Doudna JA, Sarnow P. Enhancement of hepatitis C viral RNA abundance by precursor miR-122 molecules. RNA. 2013;19(12):1825–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antonelli A, Ferri C, Pampana A, et al. Thyroid disorders in chronic hepatitis C. Am J Med. 2004;117(1):10–13. [DOI] [PubMed] [Google Scholar]
- 6. Menconi F, Hasham A, Tomer Y. Environmental triggers of thyroiditis: hepatitis C and interferon-α. J Endocrinol Invest. 2011;34(1):78–84. [DOI] [PubMed] [Google Scholar]
- 7. Kahloun A, Babba T, Fathallah B, et al. [Prevalence of extra-hepatic manifestations in infection with hepatitis C virus: study of 140 cases]. Tunis Med. 2011;89(6):557–560. [PubMed] [Google Scholar]
- 8. Wahid B, Waqar M, Rasool N, Wasim M, Khalid I, Idrees M. Prevalence of thyroid stimulating hormone dysfunction among sofosbuvir-treated HCV-infected patients: a real-world clinical experience. J Med Virol. 2019;91(3):514–517. [DOI] [PubMed] [Google Scholar]
- 9. Tran HA, Reeves GE, Lyons TJ, Attia JR. Histopathologic findings of autoimmunity in thyroid, pituitary, and adrenal diseases in chronic hepatitis C postmortem cases. Endocr Pract. 2010;16(4):566–569. [DOI] [PubMed] [Google Scholar]
- 10. Bartolomé J, Rodríguez-Iñigo E, Quadros P, et al. Detection of hepatitis C virus in thyroid tissue from patients with chronic HCV infection. J Med Virol. 2008;80(9):1588–1594. [DOI] [PubMed] [Google Scholar]
- 11. Laskus T, Radkowski M, Wang LF, Vargas H, Rakela J. Search for hepatitis C virus extrahepatic replication sites in patients with acquired immunodeficiency syndrome: specific detection of negative-strand viral RNA in various tissues. Hepatology. 1998;28(5):1398–1401. [DOI] [PubMed] [Google Scholar]
- 12. Akeno N, Blackard JT, Tomer Y. HCV E2 protein binds directly to thyroid cells and induces IL-8 production: a new mechanism for HCV induced thyroid autoimmunity. J Autoimmun. 2008;31(4):339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hammerstad SS, Stefan M, Blackard J et al. Hepatitis C virus E2 protein induces upregulation of IL-8 pathways and production of heat shock proteins in human thyroid cells. J Clin Endocrinol Metab. 2017;102(2):689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blackard JT, Kong L, Huber AK, Tomer Y. Hepatitis C virus infection of a thyroid cell line: implications for pathogenesis of hepatitis C virus and thyroiditis. Thyroid. 2013;23(7):863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lombardi A, Inabnet WB 3rd, Owen R, Farenholtz KE, Tomer Y. Endoplasmic reticulum stress as a novel mechanism in amiodarone-induced destructive thyroiditis. J Clin Endocrinol Metab. 2015;100(1):E1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schönberger J, Bauer J, Spruss T, et al. Establishment and characterization of the follicular thyroid carcinoma cell line ML-1. J Mol Med (Berl). 2000;78(2):102–110. [DOI] [PubMed] [Google Scholar]
- 17. Cai Z, Zhang C, Chang KS, et al. Robust production of infectious hepatitis C virus (HCV) from stably HCV cDNA-transfected human hepatoma cells. J Virol. 2005;79(22):13963–13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Israelow B, Mullokandov G, Agudo J, et al. Hepatitis C virus genetics affects miR-122 requirements and response to miR-122 inhibitors. Nat Commun. 2014;5:5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu X, Wu S, Tong L, et al. miR-122 affects the viability and apoptosis of hepatocellular carcinoma cells. Scand J Gastroenterol. 2009;44(11):1332–1339. [DOI] [PubMed] [Google Scholar]
- 20. Akeno N, Smith EP, Stefan M, et al. IFN-α mediates the development of autoimmunity both by direct tissue toxicity and through immune cell recruitment mechanisms. J Immunol. 2011;186(8):4693–4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flint M, Maidens C, Loomis-Price LD, et al. Characterization of hepatitis C virus E2 glycoprotein interaction with a putative cellular receptor, CD81. J Virol. 1999;73(8):6235–6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang J, Randall G, Higginbottom A, Monk P, Rice CM, McKeating JA. CD81 is required for hepatitis C virus glycoprotein-mediated viral infection. J Virol. 2004;78(3):1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Evans MJ, von Hahn T, Tscherne DM, et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446(7137):801–805. [DOI] [PubMed] [Google Scholar]
- 24. Bartosch B, Vitelli A, Granier C, et al. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J Biol Chem. 2003;278(43):41624–41630. [DOI] [PubMed] [Google Scholar]
- 25. Sangar DV, Carroll AR. A tale of two strands: reverse-transcriptase polymerase chain reaction detection of hepatitis C virus replication. Hepatology. 1998;28(5):1173–1176. [DOI] [PubMed] [Google Scholar]
- 26. Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309(5740):1577–1581. [DOI] [PubMed] [Google Scholar]
- 27. Schult P, Roth H, Adams RL, et al. microRNA-122 amplifies hepatitis C virus translation by shaping the structure of the internal ribosomal entry site. Nat Commun. 2018;9(1):2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. GEO repository: GSE accession #136339. Whole transcriptome RNA sequencing of human cells after HCV infection. 2019. [Google Scholar]
- 29. Woodhouse SD, Narayan R, Latham S, et al. Transcriptome sequencing, microarray, and proteomic analyses reveal cellular and metabolic impact of hepatitis C virus infection in vitro. Hepatology. 2010;52(2):443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li H, Huang MH, Jiang JD, Peng ZG. Hepatitis C: from inflammatory pathogenesis to anti-inflammatory/hepatoprotective therapy. World J Gastroenterol. 2018;24(47):5297–5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ozderya A, Aydin K, Temizkan S, Dogru Abbasoglu S, Vural P, Altuntas Y. High circulating levels of sICAM-1 and sVCAM-1 in the patients with Hashimoto’s thyroiditis. Endocr Res. 2017;42(2):110–116. [DOI] [PubMed] [Google Scholar]
- 32. Faustino LC, Lombardi A, Madrigal-Matute J, Owen RP, Libutti SK, Tomer Y. Interferon-α triggers autoimmune thyroid diseases via lysosomal-dependent degradation of thyroglobulin. J Clin Endocrinol Metab. 2018;103(10):3678–3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aza-Blanc P, Di Lauro R, Santisteban P. Identification of a cis-regulatory element and a thyroid-specific nuclear factor mediating the hormonal regulation of rat thyroid peroxidase promoter activity. Mol Endocrinol. 1993;7(10):1297–1306. [DOI] [PubMed] [Google Scholar]
- 34. Civitareale D, Lonigro R, Sinclair AJ, Di Lauro R. A thyroid-specific nuclear protein essential for tissue-specific expression of the thyroglobulin promoter. Embo J. 1989;8(9):2537–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ito M, Toyoda N, Nomura E, et al. Type 1 and type 2 iodothyronine deiodinases in the thyroid gland of patients with 3,5,3’-triiodothyronine-predominant Graves’ disease. Eur J Endocrinol. 2011;164(1):95–100. [DOI] [PubMed] [Google Scholar]
- 36. Duntas LH, Stathatos N. Toxic chemicals and thyroid function: hard facts and lateral thinking. Rev Endocr Metab Disord. 2015;16(4):311–318. [DOI] [PubMed] [Google Scholar]
- 37. Yin X, Sachidanandam R, Morshed S, Latif R, Shi R, Davies TF. mRNA-seq reveals novel molecular mechanisms and a robust fingerprint in Graves’ disease. J Clin Endocrinol Metab. 2014;99(10):E2076–E2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hammerstad SS, Grock SF, Lee HJ, Hasham A, Sundaram N, Tomer Y. Diabetes and hepatitis C: a two-way association. Front Endocrinol (Lausanne). 2015;6:134. [DOI] [PMC free article] [PubMed] [Google Scholar]