Abstract
Objective:
To examine inflammatory mediators in 3 feto-maternal biological compartments to inform the theory that the fetal and maternal inflammatory contributions to parturition at term and preterm.
Methods:
We conducted a cross-sectional study of amniotic fluid, cord blood, and maternal plasma from women with singleton pregnancies. Women had 1 of 4 conditions: term labor (n = 11), term not in labor (n = 13), spontaneous preterm birth with intact membranes (preterm birth; n = 13), or preterm prelabor rupture of the membranes (PROM; n = 8). We measured 2 damage-associated molecular pattern markers (high-mobility group box 1 [HMGB1] and uric acid) and 2 acute phase response markers (interleukin [IL]-6 and C-reactive protein [CRP]) using enzyme-linked immunosorbent assay. The distribution of each analyte within amniotic fluid, cord blood, and maternal plasma across the 4 conditions (term not in labor, term labor, preterm birth, and preterm PROM) were calculated. To explore if there were distributional differences in each analyte across each of the 4 labor conditions, we used a nonparametric Kruskal Wallis test. For analytes that differed across groups, we further compared distributions by labor group (term labor vs term not in labor and preterm PROM vs preterm birth).
Results:
Fetal compartments (amniotic fluid and cord blood) showed higher HMGB1 in term labor vs. term not in labor and preterm PROM vs. preterm birth. Amniotic fluid IL-6, cord blood CRP and cord blood uric acid were higher in term vs term not in labor). Cord blood uric acid was higher in preterm PROM vs preterm birth. Only maternal plasma IL-6 was higher in term labor vs. term not in labor.
Conclusions:
Accumulation of HMGB1 and an overall increase in inflammation observed on the fetal side, but not the maternal side, may be signals of parturition. Understanding fetal-derived proparturition inflammatory signals at term and preterm, especially in preterm PROM, might provide fetal-specific biomarkers and identify underlying mechanisms and targets for interventions to reduce the risk of preterm birth and preterm PROM.
Précis:
Accumulation of uterotonic inflammatory markers in fetal, but not in maternal, biological compartments indicates fetal signaling of preterm and term parturition.
INTRODUCTION
Pregnancy is an inflammatory state, and a balance of pro- and anti-inflammatory factors is required for intrauterine tissue remodeling and feto-placental growth throughout gestation.1–9 Accumulation of proinflammatory mediators can transition quiescent uterine tissues to an active labor state.10–19 Spontaneous preterm birth and preterm prelabor rupture of the membranes (PROM) rates remain high, in part due to knowledge gaps of the origin and timing of generation of inflammatory labor triggers, impeding progress in their rate reduction.20 Filling this knowledge gap is essential to design targeted interventions to stop premature activation labor triggering inflammatory signals.
Inflammatory mediators arising from localized and endogenous cellular-level activities are referred as sterile (noninfectious) inflammation.16, 21–23 In preterm birth and preterm PROM, inflammation can be of a sterile or infectious etiology, depending on the type of risk exposure,24–26 and arise from both fetal and maternal uterine tissues. It is unclear if the mother or fetus determines the timing of inflammatory activation.10
We hypothesized that inflammatory markers vary in distinct feto-maternal biological compartments either at the time of term and preterm labor and with differing clinical scenarios. Additionally, their concentration in a given compartment may indicate inflammatory signaling by either the mother and or the fetus specific to the obstetrical situation. To test this, we examined 2 damage-associated molecular pattern markers (DAMPs), high-mobility group box-1 (HMGB1) and uric acid, and 2 acute phase immune mediators, IL-6 and CRP in amniotic fluid, in cord and maternal plasma from the same pregnancy either with term not in labor, term labor, preterm birth, and preterm PROM.
METHODS
We conducted a cross-sectional study where samples used were collected from pregnant participants as part of a larger cohort recruited at The Women’s Hospital at Centennial Medical Center, Nashville, TN, to study racial disparity in biomarker profiles that are associated with preterm birth. Samples used for this study were collected between September 1, 2008 and March 31, 2010 and included both preterm birth and preterm PROM. Informed consent was obtained for all participants. Descriptions of the cohort, collection of demographic and clinical data, inclusion and exclusion criteria, sample collection, processing, and storage conditions are detailed in our prior publications and hence briefly described in this manuscript.27–31 The Institutional Review Board (IRB) at TriStar Nashville, TN, approved the parent study, and the reuse of samples for this study was approved by the IRB at The University of Texas Medical Branch at Galveston, TX. All pregnancies resulted in singleton live births, and mothers were between the ages of 18 and 40. Gestational age was determined by last menstrual period and corroborated by ultrasound. Subjects were included only if the 2 dates had concordance. In our study, preterm births were defined as the presence of regular uterine contractions at a minimum frequency of 2 contractions every 10 minutes, followed by delivery at < 360/7 weeks’ gestation. The second group preterm birth preceded by preterm PROM was diagnosed by examination with a sterile speculum to verify either pooling of amniotic fluid in the vagina or a positive nitrazine test and, when necessary, confirmed by a positive AmniSure ROM test in the vagina (Qiagen). We excluded subjects with multiple gestations, preeclampsia, placental previa, fetal anomalies, gestational diabetes, poly- and oligohydramnios, and other complications, such as surgeries during pregnancies. Additional subjects were excluded if subjects reported clinical conditions, such as antibiotic treatment or surgeries during pregnancy for unspecified conditions in normal term deliveries and subjects presenting with spontaneous rupture of membranes at term. The term group was divided into a term-labor group and a repeat elective cesarean delivery group. In the former, delivery followed the onset of labor, defined as the presence of uterine contractions occurring at least twice in 10 minutes. Women in the repeat cesarean group were delivered prior to the onset of labor or rupture of membranes. Women at term were included if they had no medical or obstetrical complications and delivered at ≥ 370/7 weeks’ gestation. All women were nonsmokers and had singleton pregnancies without intrauterine infection (culture and PCR negative and negative for histologic chorioamnionitis). This choice of samples from nonsmokers without microbial invasion of the amniotic cavity was by design since our goal was to minimize any confounding factors that may not be adjustable in our limited number of samples.
Amniotic fluid samples were collected during delivery by transvaginal amniocentesis (either preterm or term) before rupture of the membranes. Samples were collected before cesarean deliveries or before preterm or term vaginal deliveries via a puncture of the intact membrane using a 22-gauge needle, prior to artificial rupture of the membranes, through the dilated cervical os. Approximately 30% of our samples were collected at the time of cesarean delivery, prior to artificial rupture of the membranes, by needle insertion through the uterine incision. In a few cases, where indicated for clinical reasons, samples were also collected by placement of an intrauterine pressure catheter (less than 2%). Direct sampling by puncturing the membranes avoided contamination of amniotic fluid from vaginal and cervical fluids and microbial colonizers. Amniotic fluid was centrifuged immediately after collection for 10 minutes at 2,000 g to remove cellular and particulate matter. Aliquots of amniotic fluid were stored at −80°C until analysis. Data are adjusted for sampling techniques.
Five cc of maternal blood were collected in EDTA tubes during clinically indicated phlebotomy upon admission for delivery. Ten cc of cord blood were drawn from the umbilical cord after delivery prior to placental detachment. Plasma was separated from both blood samples by centrifugation, and sample aliquots were stored at −80°C until analysis. Samples used for this study were never freeze thawed and always kept at −80°C.
All analyte assays (HMGB1, uric acid, IL-6, and CRP) were performed with standard enzyme-linked immunosorbent assay (ELISA) protocols, and we followed the manufacturer’s instructions with no modification. Detailed protocols are provided in Appendix 1.
Uric acid levels in the samples were detected using the Biovision Uric Acid Colorimetric and Fluorometric Assay Kit (cat# K608–100) according to the manufacturer’s instructions.
Descriptive statistics are reported for maternal characteristics, including maternal age, body mass index (BMI), race (white, black), marital status (married, single, or divorced), education (> high school, ≤ high school), employment (yes, no), income (≥ $30,000, < $30,000), gravidity (first pregnancy, multigravida), smoking status (yes, no), and cesarean delivery (yes, no) for each condition (term not in labor, term labor, preterm birth and preterm PROM).
Descriptive statistics were calculated, including mean, standard deviation, median, and range of each marker (HMGB1, uric acid, IL-6, and CRP) within each compartment (amniotic fluid, maternal plasma, and cord blood). There were 3 values that were determined to be outliers for amniotic fluid CRP, 3 for maternal plasma IL-6, and 2 for maternal plasma CRP, all of which were not biologically plausible. The proportion of nondetectable values and those below the limit of detection were also calculated. Values below the limit of detection are typically considered to be somewhere between true zeros and the limit of detection. For this analysis, values below the limit of detection were replaced with the limit of detection divided by the square root of 2.32 Amniotic fluid and cord blood CRP had 25% below the limit of detection and cord blood IL-6 had 50% below it. As a result, we have excluded cord blood IL6 from statistical analyses (for each of the 4 conditions) and considered the potential influence of the proportion below the limit of detection for amniotic fluid and cord blood CRP during the interpretation of our results.
For each compartment (amniotic fluid, cord blood, and maternal plasma), HMGB1, uric acid, IL-6, and CRP distributions are presented for each condition (term labor, term not in labor, spontaneous preterm birth, and preterm PROM; Appendix 2). A nonparametric Kruskal Wallis test was used to determine if the distribution of analytes within amniotic fluid, cord blood, and maternal plasma significantly differed across conditions (4-level variable for term not in labor, term labor, preterm birth, and preterm PROM). A P value of .05 determined if there was a difference in the distribution of each analyte between 2 or more conditions (term not in labor, term labor, preterm birth, and preterm PROM (Appendix 3). This omnibus test does not specify which groups differ in terms of analyte distribution. Thus, for analytes that statistically differed across groups, we compared the distribution of analytes between laboring groups at term and preterm (i.e. term vs. term not in labor and preterm PROM vs. preterm birth). Our selection of comparison groups was based on our objective to determine the site (fetal vs. maternal) where inflammation may accumulate to trigger parturition. Specifically, term labor was compared to term not in labor and preterm PROM was compared to preterm birth using the Wilcoxon-Mann-Whitney test. We then corrected all P values using the False Discovery Rate to correct for Type I error.33, 34 All statistical analyses were conducted using SAS V9.2 (Cary, NC). Figures were prepared using GraphPad software.
RESULTS
We used samples from 45 subjects that had an adequate volume of specimens available in all compartments (amniotic fluid, cord blood plasma, and maternal plasma) from each condition (late preterm or preterm PROM or at term;. Late preterm birth was chosen as this is a more homogeneous group than early and very early preterm birth. Similarly, early preterm PROM has multiple etiologies and underlying pathways that are heterogeneous and difficult to ascertain.
Women in this study had a median age of 27 years (95% CI 25%–29%) and median BMI of 26 (95% CI 23%–28%). The majority were white (87%; 95% CI 76%–97%), were married (60%; 95% CI 45%–75%), had a high-school education or lower (54%; 95% CI 39%–69%), had an income of < 30k per year (53%; 95% CI 38%–68%), were multigravida (77%; 95% CI 65%–90%), were nonsmokers (93%; 85%–100%), and had a cesarean delivery (60%, 95% CI 45%–76%). The majority of maternal characteristics were similar between term not in labor, term labor, preterm birth, and preterm PROM when compared using the Fishers exact test or Kruskal-Wallis test as appropriate (Table 1). However, unemployment (P = .029), income (P = .024), and cesarean delivery (P = .001) did significantly differ across conditions. Women with preterm PROM had a frequency of unemployment of 88% (n = 7, CI 58%–100%) compared to 18% in term labor (n = 2, CI 0%–45%), 46% among term not in labor (n = 6, CI 15%–78%), and 46% among preterm birth (n = 6, CI 15%–78%). The frequency of low income was 77% among women with preterm birth (n = 10, 50%–100%) and 75% in preterm PROM (n = 6, CI 36%–100%) compared to 46% in term labor (n = 5, CI 10%–81%) and 23% in term not in labor (n = 3, CI 0%–50%). As expected, all women with term not in labor had a cesarean delivery (100%). Frequency of cesarean deliveries was 63% in preterm PROM (n = 5, CI 19%–100%), 36% in term labor (n = 4, CI 2%–70%), and 39% in preterm birth (n = 5, CI 8%–69%).35
Table 1.
Descriptive statistics for maternal demographics by condition (all samples included).
| Term Not in Labor | Term Labor | Preterm Labor | PROM | |
|---|---|---|---|---|
| N=13 | N= 11 | N=13 | N=8 | |
| Maternal age | Median, CI (range) | Median, CI (range) | Median, CI (range) | Median, CI (range) |
| 28, 26–32 | 27, 22–36 | 27, 21–30 | 20, 19–34 | |
| (22–37) | 19–38 | 18–35 | (17–34) | |
| BMI | Median, CI (range) | Median, CI (range) | Median, CI (range) | Median, CI (range) |
| 24, 21–32 | 26, 23–37 | 27, 21–36 | 26, 20–31 | |
| (19–37) | (22–37) | (16–45) | (18–31) | |
| Race | n (%, CI) | n (%, CI) | n (%, CI) | n (%, CI) |
| White | 12 (92%, 76%–100%) | 9 (82%, 55%–100%) | 10 (77%, 50%–100%) | 8 (100%) |
| Black | 1 (8%, 0%–24%) | 2 (18%, 0%–45%) | 3 (23%, 0%50%) | 0 (0%) |
| Marital Status | n (%, CI) | n (%, CI) | n (%, CI) | n (%, CI) |
| Married | 11 (85%, 62%–100%) | 6 (55%, 19%–90%) | 6 (46%, 15%–78%) | 4 (50%, 5%–95%) |
| Single/divorced | 2 (15%, 0%–38%)) | 5 (45%, 10%–81%) | 7 (54%, 22%–85%) | 4 (50%, 5%–95%)) |
| Education | n (%, CI) | n (%, CI) | n (%, CI) | n (%, CI) |
| >HS | 8 (62%, 31%–92%) | 5 (50%, 12%–88%) | 5 (38%, 8%–69%) | 2 (25%, 0%–64%) |
| <=HS | 5 (38%, 8%–69%) | 5 (50%, 12%–88%) | 8 (62%, 31%–92%) | 6 (75%, 36%–100%) |
| Employed | n (%, CI) | n (%, CI) | n (%, CI) | n (%, CI) |
| Yes | 7 (54%, 22%–85%) | 9 (82%, 55%–100%) | 7 (54%, 22%–85%) | 1 (13%, 0%–42%) |
| No | 6 (46%, 15%–78%) | 2 (18%, 0%–45%) | 6 (46%, 15%–78%) | 7 (88%, 58%–100%) |
| Income | n (%, CI) | n (%, CI) | n (%, CI) | n (%, CI) |
| >=30k | 10 (77%, 50%–100%) | 6 (55%, 19%–90%) | 3 (23%, 0%–50%) | 2 (25%, 0%–64%) |
| <30k | 3 (23%, 0%–50%) | 5 (45%, 10%–81%) | 10 (77%, 50%–100%) | 6 (75%, 36%–100%) |
| Gravidity | n (%, CI) | n (%, CI) | n (%, CI) | n (%, CI) |
| 1st Pregnancy | 0 (0%) | 4 (36%, 2%–70%) | 4 (31%, 2%–60%) | 2 (25%, 0%–64%) |
| 1+ Pregnancies | 13 (100%) | 7 (64%, 30%–98%) | 9 (69%, 40%–98%) | 6 (75%, 36%–100%) |
| Smoker | n (%, CI) | n (%, CI) | n (%, CI) | n (%, CI) |
| No | 12 (92%, 76%–100%) | 11 (100%) | 11 (92%, 73%–100%) | 7 (88%, 58%–100%) |
| Yes | 1 (8%, 0%–24%) | 0 (0%) | 1 (8%, 0%–27%) | 1 (13%, 0%–42%) |
| Cesarean | n (%, CI) | n (%, CI) | n (%, CI) | n (%, CI) |
| No | 0 (0%) | 7 (64%, 30%–98%) | 8 (62%, 31%–92%) | 3 (38%, 0%–81%) |
| Yes | 13 (100%) | 4 (36%, 2%–70%) | 5 (38%, 8%–69%) | 5 (63%, 19%–100%) |
Continuous variables are presented as means, 95% confidence intervals (CI) and range. Binary/categorical variables are presented as frequency (n), 95% confidence intervals (CI) and percentage (%)
Concentration of analytes described in results below are median values. Figure 1A displays the distribution of HMGB1 in amniotic fluid, cord blood, and maternal plasma across term not in labor, term labor, preterm birth, and preterm PROM. The distribution of amniotic fluid HMGB1 (P < .001) and cord blood HMGB1 (P < .001) differs across term not in labor, term labor, preterm birth, and preterm PROM (Appendix 3). The distribution of maternal plasma HMGB1 does not differ across groups (P = .249). Amniotic fluid HMGB1 significantly differed in term labor (30.6 ng/mL) than term not in labor (4 ng/mL; P < .001), as well as in preterm PROM (32.5 ng/mL) and preterm birth (9.2 ng/mL; P < .01). Cord blood HMGB1 also significantly differed in term labor and term not in labor (97.4 ng/ml vs 23.8 ng/mL; P = .001) and in preterm PROM and preterm birth (65.5 and 13.1 ng/mL, respectively; P = .006).
Figure 1.

The raw distribution of HMGB1 in amniotic fluid (A), maternal plasma (B), and cord blood by each condition (C). The raw distribution of uric acid in amniotic fluid (D), maternal plasma (E), and cord blood (F) by each condition. Conditions include term not in labor, term labor, preterm birth, and preterm PROM. Statistical comparison of amniotic fluid, maternal plasma, and cord blood HMGB1 and uric acid are presented in Appendix 3 and in the text.
Figure 1B displays the distribution of uric acid in amniotic fluid, cord blood, and maternal plasma across term not in labor, term labor, preterm birth, and preterm PROM. Amniotic fluid uric acid (P = .001) and cord blood uric acid (P = .021), but not maternal plasma uric acid (P = .096), significantly differed across term not in labor, term labor, preterm birth, and preterm PROM. Uric acid concentrations were not different in amniotic fluids between term labor and term not in labor (144.9 vs 99.9 nMol/mL; P = .442), but distributions significantly differed in preterm birth and pPROM (294 vs 139.4 nMol/mL; P = .002). The distribution of cord blood uric acid significantly differed in term not in labor and term labor (128 vs 34 nMol/mL; P < .001) as well as in preterm birth and preterm PROM (204 vs 101 nMol/mL; P = .01).
Figure 2A displays the distribution of IL6 in amniotic fluid and maternal plasma across term not in labor, term labor, preterm birth, and preterm PROM. Cord blood levels were excluded due to ≥ 50% being below the limit of detection. Amniotic fluid IL6 (P = .018) and maternal plasma IL6 (P = .013) significantly differed across groups (Appendix 3). Amniotic fluid IL-6 significantly differed in term labor (2782 pg/mL) than term not in labor (402.5 pg/mL; P = .001). Although preterm PROM had higher median amniotic fluid IL-6 levels (6427 pg/mL), the distribution was not statistically different than preterm birth (1925 pg/mL; P = .2). The distribution of maternal plasma IL-6 did significantly differ in term labor than term not in labor (12.8 vs 3.7 pg/mL; P = .001); however, it did not differ between preterm birth and preterm PROM (10.3 pg/ml vs 3.9 pg/mL; P = .679).
Figure 2.
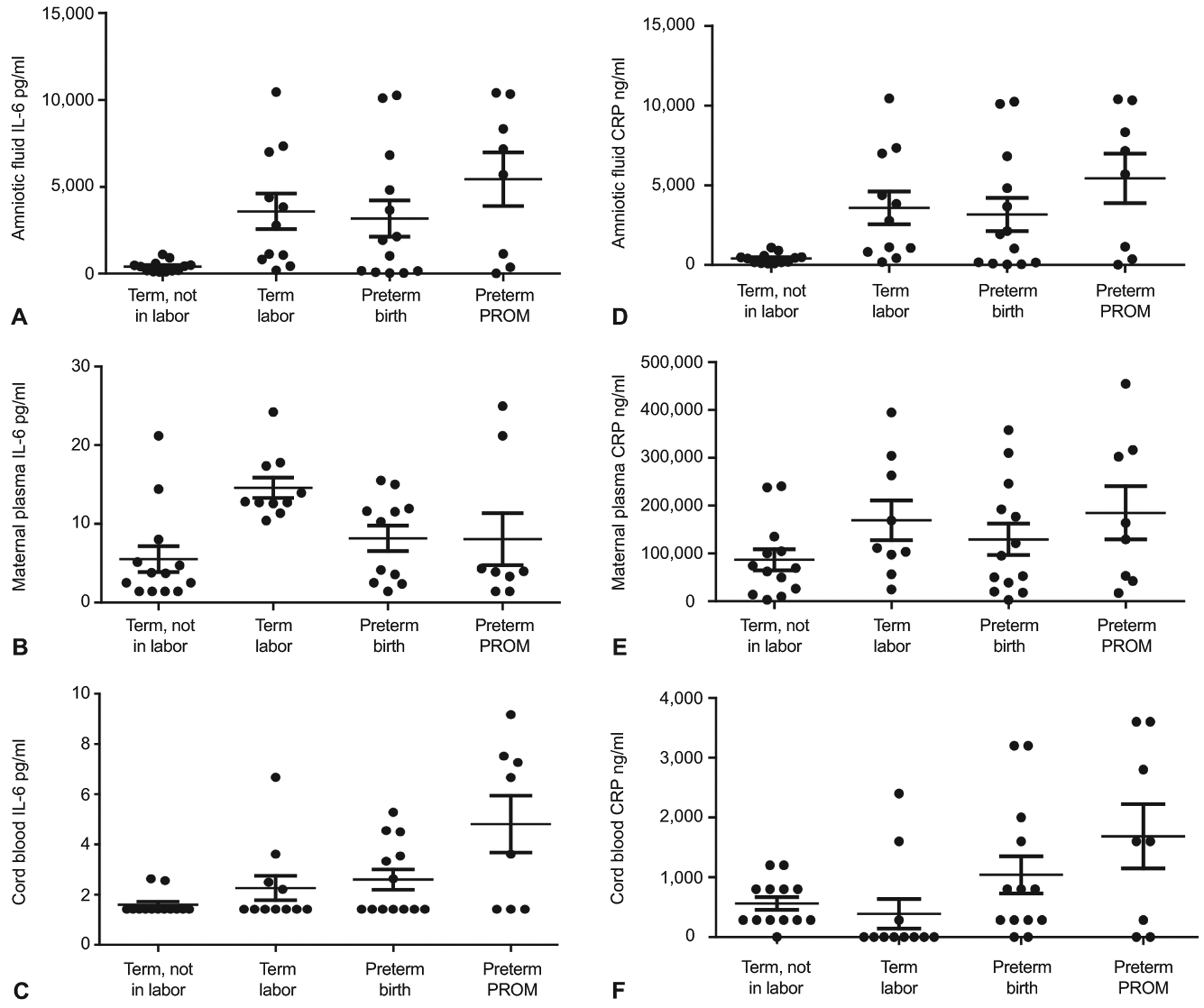
The raw distribution of interleukin (IL)-6 in amniotic fluid (A), maternal plasma (B), and cord blood (C) by each condition. The raw distribution of C-reactive protein (CRP) in amniotic fluid (D), maternal plasma (E), and cord blood (F) by each condition. Conditions include term not in labor, term labor, preterm birth, and preterm PROM. Statistical comparison of amniotic fluid, maternal plasma and cord blood IL-6 and CRP are presented in Appendix 3 and in the text.
Figure 2B displays the distribution of CRP in amniotic fluid, cord blood, and maternal plasma across term not in labor, term labor, preterm birth, and preterm PROM. The distribution of amniotic fluid CRP (P = .182) and maternal plasma CRP (P = .389) did not differ across groups (Appendix 3). However, the distribution of cord blood CRP (P = .036) differed across term not in labor, term labor, preterm birth, and preterm PROM. There was a significant difference in cord blood CRP in term labor compared to term not in labor (0 vs 283.6 ng/mL; P = .02) but not in preterm PROM and preterm birth (1600 vs. 800 ng/mL; P = .476).
To determine if multiple comparisons had any effect on our results, we used the false discovery rate to correct P values for error. The corrected P values can be found in Appendix 4. Overall, only one statistical test was no longer significant after correction for the false discovery rate. Specifically, the P value associated with the distribution of cord blood CRP across term not in labor, term labor, preterm birth, and preterm PROM was no longer significant (P = .053). All other results remained the same. Thus, multiple comparisons had little effect on our results.
DISCUSSION
Using 3 matching samples from the same pregnancy and 4 different conditions, our data support the hypothesis that fetal inflammatory signaling is one of the determinants of pregnancy outcome in humans. Women at term not in labor displayed low levels of inflammatory mediators in all 3 biologic samples, and proinflammatory mediators accumulate predominantly on the fetal side at term and preterm labor, irrespective of the membrane status. To note, HMGB1’s—an indicator of fetal tissue senescence, tissue damage, and injury—increase on the fetal side was dominant compared to other markers. Higher IL-6 concentrations were noted for term labor in maternal plasma, which is likely a secondary effect in response to a fetal inflammatory trigger. Both CRP and uric acid did not show differences or any specific pattern of changes between conditions in any of the fluids. The buildup of inflammatory mediators in the fetal biological compartment is an indication that the inflammatory overload required to force parturition is likely initiated on the fetal side to ensure fetal delivery irrespective of term or preterm status. This can be considered as fetal-derived biochemical signaling of parturition.
HMGB1 is a nonhistone chromatin-associated nuclear 25-kD protein required for various DNA functions.36–38 However, nuclear injury translocate to the cytoplasm, gets secreted and functions as a proinflammatory cytokine. HMGB1’s release from fetal cells can be considered a fetal-derived signaling mechanism whereby oxidative stress at term (physiologic) or preterm (pathophysiologic) promotes DNA damage and senescence (aging) of the fetal tissues.39 HMGB1 from fetal cells can reach maternal uterine tissues via extracellular vesicles released from these cells to cause inflammation and labor-related changes.40 Our data suggest that accumulation of HMGB1 in the amniotic fluid and cord blood provides indirect evidence that fetal-derived inflammatory mediators are likely signalers of parturition.
Our analysis supports the findings that increases in IL-6 and CRP are not an indication of any specific underlying physiologic or pathologic process, but an indication of generalized inflammation.26, 41 Although uric acid is implicated in adverse pregnancies,42, 43 our study did not reveal any differences worthy of discussion.
Transition from term not in labor to labor is an inflammatory process.44 HMGB1 and IL-6 is much more dominant in the fetal side than the maternal side. This finding supports the notion that the threshold required to shift the inflammatory balance during pregnancy to a proinflammatory, prolabor state may be accumulated on the fetal side and propagated to the maternal side to trigger changes required for delivery. A synergistic inflammatory activation in both the fetal and maternal systems is needed to facilitate an effective labor and it is likely triggered by fetal-derived paracrine inflammatory signals.
Term labor and preterm PROM are eerily similar.45, 46 As shown here, except CRP in cord blood, HMGB1, IL-6, and uric acid levels did not differ between the 2 conditions, irrespective of the compartment. Although overlap can be seen between preterm birth and preterm PROM in their etiologies and pathologies, they are clearly distinct phenotypes arising from different pathways.47 Marker analysis in this study also supports this concept since concentrations of markers in various compartments differed between the 2 conditions.
We limited our studies only to late preterm and did not include samples to perform a more stratified analysis to determine any risk-specific association between biomarkers and conditions in different compartments. The small sample size and resultant low power limit our ability to generalize any non-significant findings. As this is a descriptive study, we did not conduct multivariable tests of association. Significant differences in analyte distributions observed with the Kruskal Wallis test could be subject to confounding. Maternal characteristics were similar between groups. The term not in labor group consisted entirely of cesarean deliveries, and a potential limitation is the inclusion of both cesarean and vaginal deliveries for the labor groups (term labor, preterm birth, and preterm PROM). In our prior reports, we have not seen any analyte concentration differences irrespective of mode of delivery.35, 48 Additionally, there were < 1% below the limit of detection for the majority of our markers, including HMGB1. Future studies should also include risk-specific assessment, and confounding variables should include, but not be limited to, infection (microbial type and load),
We conclude that fetal inflammatory marker accumulation is associated with human parturition at term and preterm. Although this descriptive study does not provide functional explanations or mechanistic evidence, we postulate that fetal-derived inflammatory signals are likely initial triggers of human parturition. This knowledge may help us to design intervention strategies targeted to fetal tissues and fetal signals, not just focused on terminal inflammatory events in the maternal uterus and cervix.
Supplementary Material
Acknowledgement:
The authors thank Talar Kechichian, MS, lab manager, for conducting all the assays in The Menon laboratory; Poorna Ram Menon from Clear Falls High School, League City, TX, a summer intern in The Menon lab for preparing all the samples; and Hemi Park, MPH, student at Temple University, for assisting with preparing the figures.
Funding: This study is supported by the NICHD grant (1R01HD084532-01A1) awarded to R. Menon.
Footnotes
Financial Disclosure
Ramkumar Menon disclosed receiving money paid to their institution from NIH, AMAG Pharmaceuticals, Hologic Incorporated, and the Gates Foundation, ILIAS Therapeutics. The other author did not report any potential conflicts of interest.
REFERENCES
- 1.Peltier MR. Immunology of term and preterm labor. Reprod Biol Endocrinol 2003. December 2;1:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Negishi Y, Takahashi H, Kuwabara Y, Takeshita T. Innate immune cells in reproduction. J Obstet Gynaecol Res 2018. November;44(11):2025–36. [DOI] [PubMed] [Google Scholar]
- 3.Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M. Immune cells in term and preterm labor. Cell Mol Immunol 2014. November;11(6):571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev 2007. December;65(12 Pt 2):S194–202. [DOI] [PubMed] [Google Scholar]
- 5.Gomez-Lopez N, Romero R, Plazyo O, Panaitescu B, Furcron AE, Miller D, et al. Intra-Amniotic Administration of HMGB1 Induces Spontaneous Preterm Labor and Birth. Am J Reprod Immunol 2016. January;75(1):3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavan AR, Griffith OW, Wagner GP. The inflammation paradox in the evolution of mammalian pregnancy: turning a foe into a friend. Curr Opin Genet Dev 2017. December;47:24–32. [DOI] [PubMed] [Google Scholar]
- 7.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci 2011. March;1221:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pham J, Arul Nambi Rajan K, Li P, Parast MM. The role of Sirtuin1-PPARgamma axis in placental development and function. J Mol Endocrinol 2018. May;60(4):R201–R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garlanda C, Maina V, Martinez de la Torre Y, Nebuloni M, Locati M. Inflammatory reaction and implantation: the new entries PTX3 and D6. Placenta 2008. October;29 Suppl B:129–34. [DOI] [PubMed] [Google Scholar]
- 10.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014. August 15;345(6198):760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lockwood CJ, Kuczynski E. Markers of risk for preterm delivery. J Perinat Med 1999 1999;27(1):5–20. [DOI] [PubMed] [Google Scholar]
- 12.Kim SH, MacIntyre DA, Firmino Da Silva M, Blanks AM, Lee YS, Thornton S, et al. Oxytocin activates NF-kappaB-mediated inflammatory pathways in human gestational tissues. Mol Cell Endocrinol 2015. March 5;403:64–77. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton S, Oomomian Y, Stephen G, Shynlova O, Tower CL, Garrod A, et al. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol Reprod 2012. February;86(2):39. [DOI] [PubMed] [Google Scholar]
- 14.Yellon SM. Contributions to the dynamics of cervix remodeling prior to term and preterm birth. Biol Reprod 2017. January 1;96(1):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menon R Human fetal membranes at term: Dead tissue or signalers of parturition? Placenta 2016. August;44:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menon R, Mesiano S, Taylor RN. Programmed Fetal Membrane Senescence and Exosome-Mediated Signaling: A Mechanism Associated With Timing of Human Parturition. Front Endocrinol (Lausanne) 2017;8:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castillo-Castrejon M, Meraz-Cruz N, Gomez-Lopez N, Flores-Pliego A, Beltran-Montoya J, Viveros-Alcaraz M, et al. Choriodecidual cells from term human pregnancies show distinctive functional properties related to the induction of labor 2. Am J Reprod Immunol 2014 1/2014;71(1):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menon R, Richardson LS, Lappas M. Fetal membrane architecture, aging and inflammation in pregnancy and parturition. Placenta 2018. November 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lappas M NOD1 and NOD2 regulate proinflammatory and prolabor mediators in human fetal membranes and myometrium via nuclear factor-kappa B. Biol Reprod 2013. July;89(1):14. [DOI] [PubMed] [Google Scholar]
- 20.Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ 2010. January;88(1):31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plazyo O, Romero R, Unkel R, Balancio A, Mial TN, Xu Y, et al. HMGB1 Induces an Inflammatory Response in the Chorioamniotic Membranes That Is Partially Mediated by the Inflammasome. Biol Reprod 2016. December;95(6):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Behnia F, Sheller S, Menon R. Mechanistic Differences Leading to Infectious and Sterile Inflammation. Am J Reprod Immunol 2016. May;75(5):505–18. [DOI] [PubMed] [Google Scholar]
- 23.Romero R, Xu Y, Plazyo O, Chaemsaithong P, Chaiworapongsa T, Unkel R, et al. A Role for the Inflammasome in Spontaneous Labor at Term. Am J Reprod Immunol 2018. June;79(6):e12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2015. August;28(12):1394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol 2014. November;72(5):458–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menon R, Torloni MR, Voltolini C, Torricelli M, Merialdi M, Betran AP, et al. Biomarkers of spontaneous preterm birth: an overview of the literature in the last four decades. Reprod Sci 2011. November;18(11):1046–70. [DOI] [PubMed] [Google Scholar]
- 27.Menon R, Williams SM, Fortunato SJ. Amniotic fluid interleukin-1beta and interleukin-8 concentrations: racial disparity in preterm birth. Reprod Sci 2007. April;14(3):253–9. [DOI] [PubMed] [Google Scholar]
- 28.Fortunato SJ, Menon R, Velez DR, Thorsen P, Williams SM. Racial disparity in maternal-fetal genetic epistasis in spontaneous preterm birth. Am J Obstet Gynecol 2008. June;198(6):666e1–9; discussion e9–10. [DOI] [PubMed] [Google Scholar]
- 29.Menon R, Thorsen P, Vogel I, Jacobsson B, Williams SM, Fortunato SJ. Increased bioavailability of TNF-alpha in African Americans during in vitro infection: predisposing evidence for immune imbalance. Placenta 2007. Aug-Sep;28(8–9):946–50. [DOI] [PubMed] [Google Scholar]
- 30.R M, V DR, F SJ, W SM. Significant interaction between genetic variants in preterm birth associate with amniotic fluid protein concentrations and racial disparity. Ann Hum Genet 2011 2011. [Google Scholar]
- 31.Menon R, Pearce B, Velez DR, Merialdi M, Williams SM, Fortunato SJ, et al. Racial disparity in pathophysiologic pathways of preterm birth based on genetic variants. Reprod Biol Endocrinol 2009 2009;7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helsel DR. More than obvious: better methods for interpreting nondetect data. Environ Sci Technol 2005. October 15;39(20):419A–23A. [DOI] [PubMed] [Google Scholar]
- 33.Peltier MR, Wilcox CJ, Sharp DC. Technical note: Application of the Box-Cox data transformation to animal science experiments. J Anim Sci 1998. March;76(3):847–9. [DOI] [PubMed] [Google Scholar]
- 34.Elliott AC, Hynan LS. A SAS((R)) macro implementation of a multiple comparison post hoc test for a Kruskal-Wallis analysis. Comput Methods Programs Biomed 2011. April;102(1):75–80. [DOI] [PubMed] [Google Scholar]
- 35.Menon R, Fortunato SJ, Milne GL, Brou L, Carnevale C, Sanchez SC, et al. Amniotic fluid eicosanoids in preterm and term births: effects of risk factors for spontaneous preterm labor. Obstet Gynecol 2011. July;118(1):121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erlandsson HH, Andersson U. Mini-review: The nuclear protein HMGB1 as a proinflammatory mediator. Eur J Immunol 2004 6/2004;34(6):1503–12. [DOI] [PubMed] [Google Scholar]
- 37.Yang H, Wang H, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. J Leukoc Biol 2005. July;78(1):1–8. [DOI] [PubMed] [Google Scholar]
- 38.Wang HH, Yang H, Czura CJ, Sama AE, Tracey KJ. HMGB1 as a late mediator of lethal systemic inflammation. Am J Respir Crit Care Med 2001. November 15;164(10):1768–73. [DOI] [PubMed] [Google Scholar]
- 39.Menon R, Behnia F, Polettini J, Saade GR, Campisi J, Velarde M. Placental membrane aging and HMGB1 signaling associated with human parturition. Aging (Albany NY) 2016. February;8(2):216–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheller-Miller S, Urrabaz-Garza R, Saade G, Menon R. Damage-Associated molecular pattern markers HMGB1 and cell-Free fetal telomere fragments in oxidative-Stressed amnion epithelial cell-Derived exosomes. J Reprod Immunol 2017. September;123:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trochez-Martinez RD, Smith P, Lamont RF. Use of C-reactive protein as a predictor of chorioamnionitis in preterm prelabour rupture of membranes: a systematic review. BJOG 2007. July;114(7):796–801. [DOI] [PubMed] [Google Scholar]
- 42.Mulla MJ, Myrtolli K, Potter J, Boeras C, Kavathas PB, Sfakianaki AK, et al. Uric acid induces trophoblast IL-1beta production via the inflammasome: implications for the pathogenesis of preeclampsia. Am J Reprod Immunol 2011. June;65(6):542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Girard S, Heazell AE, Derricott H, Allan SM, Sibley CP, Abrahams VM, et al. Circulating cytokines and alarmins associated with placental inflammation in high-risk pregnancies. Am J Reprod Immunol 2014. October;72(4):422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med 2006. October;11(5):317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dutta EH, Behnia F, Boldogh I, Saade GR, Taylor BD, Kacerovsky M, et al. Oxidative stress damage-associated molecular signaling pathways differentiate spontaneous preterm birth and preterm premature rupture of the membranes. Mol Hum Reprod 2016. February;22(2):143–57. [DOI] [PubMed] [Google Scholar]
- 46.Menon R, Boldogh I, Hawkins HK, Woodson M, Polettini J, Syed TA, et al. Histological evidence of oxidative stress and premature senescence in preterm premature rupture of the human fetal membranes recapitulated in vitro. Am J Pathol 2014. June;184(6):1740–51. [DOI] [PubMed] [Google Scholar]
- 47.Menon R, Richardson LS. Preterm prelabor rupture of the membranes: A disease of the fetal membranes. Semin Perinatol 2017. November;41(7):409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009. July;20(4):488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


