Abstract
Context
Bone turnover increases rapidly during the menopause transition (MT) and plateaus above premenopausal levels in early postmenopause. It is uncertain whether higher bone turnover is associated with fracture in midlife women with near-normal bone mineral density (BMD).
Objective
Examine whether faster increases in bone turnover during the MT (2 years before to 2 years after the final menstrual period [FMP]), and greater bone turnover during early postmenopause (≥2 years after the FMP) are risk factors for subsequent fracture, accounting for BMD.
Design and Setting
The Study of Women’s Health Across the Nation, a longitudinal cohort study of the MT.
Participants
A total of 484 women (initially pre- or early perimenopausal, who transitioned to postmenopause) with bone turnover (urine collagen type I N-telopeptide), BMD, and fracture data.
Main Outcome Measure
Incident fracture after the MT.
Results
Adjusting for age, race/ethnicity, fracture before the MT, cigarette use, body mass index, and study site in Cox proportional hazards regression, each SD increment in the rate of increase in bone turnover during the MT was associated with 24% greater hazard of incident fracture in postmenopause (P = .008). Accounting for the same covariates, each SD increment in bone turnover during early postmenopause was associated with a 27% greater hazard of fracture (P = .01). Associations remained significant after controlling for MT rate of change and early postmenopausal level of BMD.
Conclusion
Faster increases in bone turnover during the MT and greater bone turnover in early postmenopause forecast future fractures.
Keywords: biochemical markers of bone turnover, menopause, osteoporosis, general population studies
Bone turnover increases rapidly during the menopause transition (MT), and plateaus above premenopausal levels in early postmenopause (1–4). Using a time relative to final menstrual period (FMP) approach, the Study of Women’s Health Across the Nation (SWAN) reported that bone turnover begins to increase 2 years before the FMP, rises rapidly for the next 4 years, peaks 2 years after the FMP, and plateaus thereafter (1). Prior longitudinal studies of women demonstrated that bone turnover (assessed by bone histomorphometry) doubles from before to after the FMP (2), leading to alterations in bone microarchitecture that diminish bone strength (eg, decreased trabecular number, increased trabecular spacing, conversion of trabecular plates to rods, decreased connectivity) (5).
Although it is widely appreciated that increased bone turnover and the resulting damage to bone microarchitecture is associated with fracture in older postmenopausal women, there is less certainty about whether faster increases in bone turnover during the MT, and greater bone turnover in early postmenopause are similarly associated with fracture in midlife women (6–14). One reason for this uncertainty is that although older postmenopausal women have already lost a substantial amount of bone mineral density (BMD) (15), younger women in their 40s and 50s still have near-normal BMD. For example, by the ages of 60 to 69 and 70 to 79, 12.3% and 25.7% of women will have developed densitometric osteoporosis, respectively (15). In contrast, BMD values in healthy, community-based samples of early postmenopausal women do not warrant consideration of pharmacologic treatment despite average cumulative declines of 7.5% and 5.3% in the lumbar spine (LS) and femoral neck (FN) BMD during the MT (16).
The objective of this study was therefore to examine whether increased bone turnover assessed during the MT or early postmenopause is directly linked to subsequent fracture. We had 2 research questions: (1) Are faster increases in bone turnover during the MT (operationalized as 2 years before to 2 years after the FMP, when bone turnover increases rapidly) associated with incident fracture in postmenopause; and (2) Does greater bone turnover, assessed once in early postmenopause (2 or more years after the FMP, when MT-related bone turnover has plateaued above premenopausal levels) predict subsequent fracture? If, indeed, faster increases in bone turnover during the MT and greater bone turnover in early postmenopause are risk factors for fracture, the clinical implications could be substantial: early, short-term antiresorptive therapy during these critical periods might prevent permanent damage to bone microarchitecture and thereby decrease the risk of future fracture (17).
This study was conducted in SWAN, a longitudinal study of the MT in a multiracial/ethnic cohort of community-dwelling women. We used previously collected bone turnover marker, BMD, and fracture data from SWAN to address our questions. In SWAN, the bone turnover marker that was available to us was urine collagen type I N-telopeptide (U-NTX), a bone resorption marker that is correlated with histomorphometric indices of bone turnover (18).
Materials and Methods
SWAN is a multicenter, longitudinal cohort study of the MT in an ambulatory cohort of multiracial/ethnic women (19). At study baseline, participants were between 42 and 52 years of age; in premenopause (regular menstrual bleeding in the past year) or early perimenopause (less predictable menstrual bleeding at least once every 3 months); had an intact uterus with ≥1 ovary; and were not taking exogenous sex steroid hormones. The entire SWAN cohort consisted of 3,302 participants recruited from seven clinical sites (Boston, MA; Chicago, IL; Detroit, MI; Pittsburgh, PA; Los Angeles, CA; Newark, NJ; Oakland CA). The SWAN Bone Cohort was a subset of 2407 participants from 5 sites, which excludes Chicago and Newark (where bone assessments were not performed). Participants provided written informed consent, and each site obtained institutional review board approval.
Study sample derivation
To test the rate of increase in bone turnover during the MT and bone turnover level in early postmenopause as risk factors for incident fracture in postmenopause, participants had to have a known FMP date, and bone turnover marker measurements at 2 time points: once around the start of the MT (when bone turnover begins to increase, 2 years before the FMP), and again in early postmenopause (just after bone turnover peaks at plateaus 2 years following the FMP). To allow for observation of incident fracture after the second bone turnover marker measurement, women additionally needed to have at least 1 additional follow-up visit after that measurement. From the 2407 SWAN Bone Cohort participants, we excluded participants if they: (1) did not have a known FMP date (N = 1214); (2) did not have the requisite 2 bone turnover marker measurements (at the start of the MT and in early postmenopause) (N = 588); (3) initiated a bone-modifying medication before early postmenopause (sex steroid hormones, oral glucocorticoids, aromatase inhibitors, chemotherapy for breast cancer, and osteoporosis medications [bisphosphonates, selective estrogen receptor modulators, calcitonin, parathyroid hormone]) (N = 79); (4) sustained a fracture during the MT (N = 28); and (5) did not have follow-up after the second bone turnover assessment (N = 14). This left us with a study sample of 484 women.
Outcome: time to first fracture after the MT
During each SWAN follow-up visit, fractures since the previous visit were self-reported using standardized questionnaires. The number of fractures, body site(s) affected, and how fractures occurred were also recorded. Fractures were considered to be minimum trauma if they did not occur after a fall from a height of 6 inches or more, a motor vehicle accident, moving fast (eg, skating), playing sports, or from impact with heavy or fast-moving projectiles. SWAN did not collect dates of fractures during the first 6 follow-up visits; dates were thus imputed using the midpoints between the participants’ previous and index visits. SWAN began collecting the date of fracture at visit 7. Also starting at visit 7, medical records were obtained to adjudicate fractures; since inauguration of adjudication, 95% of self-reports were confirmed. We did not include craniofacial and digital fractures in our analyses. To optimize power, however, we included minimum and nonminimum trauma fractures as both fracture types are associated with low BMD (20).
Primary exposure: bone turnover
The primary exposures in our analyses were (1) rate of increase in bone turnover during the MT and (2) first available bone turnover assessment in early postmenopause. Bone turnover was assessed using the bone resorption marker, U-NTX. Participants provided fasting, nonfirst-voided urine samples before 10 am. Specimens were then stored at local study sites at -20 to -80°C for up to 1 month, after which they were shipped to the Central Lab (Medical Research Laboratories, Highland Heights, KY) and stored at -80°C. SWAN measured U-NTX using the Osteomark competitive inhibition enzyme immunoassay (nM BCE; Osteomark, Ostex International Inc., Seattle WA; inter-assay coefficient of variation [CV] <12%; intra-assay CV <8%). Urinary creatinine was measured using the Cobas Mira autoanalyzer (mM; Horiba ABX, Montpellier, France; inter-assay CV 4.1%; intra-assay CV 0.6%). U-NTX was expressed as a ratio to urinary creatinine (nM BCE/mM Cr).
Covariates
Risk factors for fracture that are similar to those in the FRAX Fracture Assessment Tool, and also recorded in SWAN, were included as covariates in analyses. These included age (years), race/ethnicity, body mass index (BMI, calculated as weight in kilograms/[height in meters]2), current cigarette use (yes/no), prior fracture (in this case, before the MT: yes/no), and BMD. Each of these variables was collected at every SWAN visit using standardized self-report or interview forms. Anthropometrics were ascertained using standardized and quality-controlled protocols (19). At the SWAN baseline visit, participants reported prior fractures in adult life, along with their age at the time of the fractures. This information was combined with interim fractures reported in follow-up visits to create the “fracture before the MT: yes/no” variable.
BMD at the LS and FN BMD was measured by dual-energy X-ray absorptiometry. At study inception, the Pittsburgh and Oakland sites used the Hologic QDR 2000 machine, and the Boston, Los Angeles, and Michigan sites used the Hologic QDR 4500A model. At follow-up visit 8, Pittsburgh and Oakland upgraded to the 4500A models. To develop cross-calibration regression equations, each site obtained duplicate scans using the old and new hardware in 40 volunteers within a maximum of 90 days. To determine the short-term in vivo precision error, each study site measured LS and FN BMD twice in 5 women with complete subject repositioning between duplicate scans. Using the root-mean-square SD approach, the precision error in SWAN was 1.4% at the LS and 2.2% at the FN. An anthropomorphic spine phantom was circulated between sites for cross-site calibration. Standard quality control phantom scans were conducted before each BMD measurement session. If necessary, these were used to adjust for longitudinal machine drift.
Statistical analysis
Descriptive statistics for all variables were generated and distributions of continuous variables were assessed for normality.
Our first set of analyses examined whether faster increase in bone turnover during the MT, defined as the 4-year period starting 2 years before the FMP and ending 2 years after the FMP, was associated with greater hazard of incident fracture in postmenopause (Fig. 1). In women who entered SWAN before their MT (ie, at least 2 years before their FMP), the rate of increase in bone turnover during the MT was calculated as the total increase in U-NTX from the last visit before the MT to the first visit after the MT (early postmenopause) divided by the number of years spanning the MT. Based on prior analyses, bone turnover does not increase significantly before or after the MT (1). In participants who were already within 2 years of their FMP at SWAN inception (ie, entered SWAN during their MT), the rate of increase in bone turnover during the MT was calculated as the increase in U-NTX from the baseline visit to early postmenopause, divided by the number of years from baseline to the end of the MT. We then used Cox proportional hazards regression with time to first fracture (clock starting at the time of the U-NTX measurement in early postmenopause) as the outcome, and rate of increase in U-NTX during the MT as the primary predictor. Covariates were obtained with the second U-NTX measurement. Our base model included the following covariates: age (years), race/ethnicity, BMI (kg/cm2), history of fracture before the start of the MT (yes/no), smoking status (yes/no), and study site. We then assessed fracture prediction independent of early postmenopausal (LS or FN) BMD and rate of decline in (LS or FN) BMD during the MT (g/cm2/y), by individually adding each of the four BMD variables in separate models. If participants entered SWAN before 1 year before the FMP, the rate of decline in BMD during the MT was estimated as the absolute decrease in BMD from the last visit before the start of the MT to early postmenopause divided by 1 plus the number of years post-FMP in early postmenopause. Based on prior data, areal BMD is stable until 1 year before the FMP (16). In participants who were already within 1 year of their FMP at SWAN inception, the rate of MT-related decline in BMD was calculated as the decrease in BMD from the baseline visit to early postmenopause, divided by the number of intervening years.
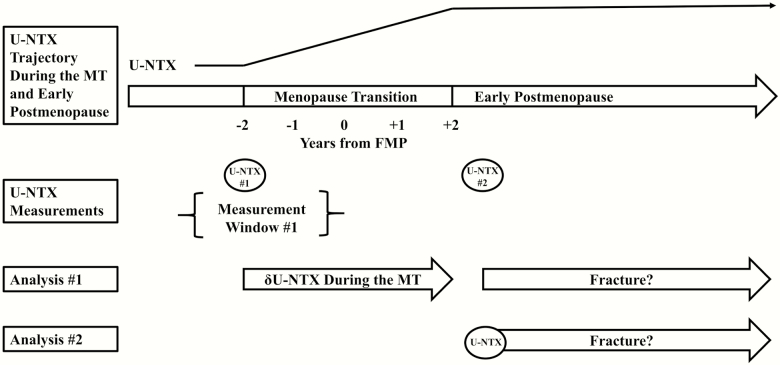
Figure 1.
Visual representation of urine collagen type I N-telopeptide (U-NTX) measurement time points and analyses. The menopause transition (MT) was operationalized as the period spanning 2 years before to 2 years after the FMP. In all participants, U-NTX was measured at 2 time points: (1) once around the start of the MT, indicated by #1; and (2) once at the first visit after the MT (early postmenopause), shown as #2. In most women, the first U-NTX measurement was from their last visit before the MT. Some women entered the study after the MT had started; the first U-NTX measurement in these women was their SWAN baseline visit. The rate of increase in U-NTX over the MT was calculated differently in these 2 groups and is detailed in the text. In our first analysis, we examined whether rate of increase in U-NTX over the MT was associated with incident fracture after early postmenopause. In our second analysis, we assessed whether greater U-NTX in early postmenopause was associated with subsequent fracture.
Our second set of analyses examined whether absolute bone turnover level, assessed once in early postmenopause, was associated with subsequent fracture (Fig. 1). We used Cox proportional hazards regression with time to incident fracture in postmenopause (with observation period starting at the U-NTX measurement in early postmenopause) as the outcome, and U-NTX in early postmenopause as the primary predictor, adjusted for covariates measured with the bone turnover assessment. The base model included age, race/ethnicity, BMI, history of fracture before the start of the MT, smoking status, and study site as covariates. We additionally assessed fracture prediction independent of LS or FN BMD in early postmenopause, by adding each of the 2 variables in separate model runs.
The objective of our third set of analyses was to compare the contributions of rate of bone turnover increase during the MT and absolute bone turnover level in early postmenopause to the hazard of incident fracture in postmenopause. First, we classified the rate of increase in bone turnover during the MT as fast or slow based on whether the rate was greater or less than the median rate, and classified bone turnover in early postmenopause as high or low based on whether the measured level was greater or less than the early postmenopausal median. We then examined membership in the 4 groups (slow increase and low turnover level, fast increase and low turnover level, slow increase and high turnover level, fast increase and high turnover level) as predictor of time to fracture in postmenopause (with clock staring at the second U-NTX measurement) using Cox proportional hazards regression, and controlled for study site and race/ethnicity. We limited the number of covariates in this exploratory analysis because the 2 discordant groups (fast increase and low turnover level; slow increase and high turnover level) were small, reflecting the correlation between rate of increase in bone turnover and bone turnover level in early postmenopause. Also because of this correlation, and the modest number of fractures in this younger cohort, we did not have sufficient power to examine the rate of increase in bone turnover during the MT and bone turnover level in early postmenopause as continuous predictors in the same model.
Results
Participant characteristics
The study sample was composed of 484 women, 23.3% of whom were black, 16.9% Chinese, 17.6% Japanese, and 42.2% white. At the time of the first measurement of U-NTX close to the start of the MT, participants were on average 48.7 years of age and 2.2 years before their FMP (this visit occurred within 2 years of the FMP in 139 women). At the first early postmenopausal visit when the second U-NTX was measured, participants were on average 53.4 years of age and 2.5 years after their FMP. A total of 5.6% of participants had sustained a fracture (traumatic or fragility) before the start of the MT. All of these fractures occurred at least more than 1 year before the start of the MT (defined as 2 years before the FMP). BMI, smoking status, and BMD at the start of the MT and at the visit in early postmenopause are summarized in Table 1.
Table 1.
Descriptive Statisticsa for Analytic Sample: Study of Women’s Health Across the Nation (SWAN)
| Study Sample N = 484 | |
|---|---|
| Race/ethnicity | |
| Black | 113 (23.3) |
| Chinese | 82 (16.9) |
| Japanese | 85 (17.6) |
| White | 204 (42.2) |
| At SWAN visit close to start of the menopause transition b | |
| Age, y | 48.7 (2.3) |
| Time before the final menstrual period, y | 2.2 (0.9) |
| Body mass index, kg/m2 | 26.9 (6.9) |
| Smoking, yes | 70 (14.4) |
| N-telopeptide, urine, nM BCE/mM Cr | 36.8 (17.5) |
| Lumbar spine bone mineral density, g/cm2 | 1.056 (0.131) |
| Femoral neck bone mineral density, g/cm2 | 0.823 (0.129) |
| At SWAN visit in early postmenopause c | |
| Age, y | 53.4 (2.4) |
| Time after the final menstrual period, y | 2.5 (0.6) |
| Body mass index, kg/m2 | 27.5 (7.1) |
| Smoking, yes | 69 (14.4) |
| N-telopeptide, urine, nM BCE/mM Cr | 48.8 (22.5) |
| Lumbar spine bone mineral density, g/cm2 | 0.979 (0.148) |
| Femoral neck bone mineral density, g/cm2 | 0.776 (0.128) |
aCount (percentage) for categorical variables; mean (SD) for continuous variables.
bThe start of the menopause transition (MT) was operationalized as follows: For women who were more than 2 years from their final menstrual period (FMP) at SWAN inception, the start of the MT was operationalized as the last study visit before 2 years before the FMP (ie, just before bone turnover expected to begin increasing). For women who were already within 2 years of their FMP at SWAN inception, the start of the MT was operationalized as the SWAN baseline visit.
cThe visit in early postmenopause corresponded to the first follow-up visit after 2 years following the FMP (ie, just after bone turnover was expected to reach its peak and plateau).
Rate of increase in bone turnover during the MT and subsequent fracture
Bone turnover marker (U-NTX) measurements were normally distributed at both the start of the MT and in early postmenopause. Mean (SD) U-NTX was 36.8 (17.5) and 48.8 (22.5) nM BCE/nM Cr at the start of the MT and in early postmenopause, respectively. This corresponded to a mean rate of increase in U-NTX during the MT of +3.3 nM BCE/mM Cr per year. The Spearman rank correlation between the U-NTX measurements at the start of the MT and in early postmenopause was 0.31 (P < .0001).
Among the 484 participants, a total of 65 incident fractures occurred after the early postmenopausal visit (the visit at which the early postmenopausal bone turnover marker was obtained). Thirty-eight (58.5%) of these fractures were categorized as minimum trauma. Fractures occurred at the following sites: foot (N = 14); ankle (N = 13); wrist (N = 11); leg above the ankle (N = 10); arm above the wrist (N = 8); ribs (N = 7); spine (N = 1); and hand (N = 1). Mean (SD) duration of the observation period was 8.5 (2.9) years; therefore, on average, this analysis followed participants into their early 60s.
After adjustment for age, race/ethnicity, BMI, current cigarette use, fracture before the MT, and study site in Cox proportional hazards regression, each SD increment in rate of increase in U-NTX during the MT was associated with a 24% greater hazard of incident fracture in postmenopause (P = .008) (Table 2). The Spearman rank correlations between the rate of increase in U-NTX during the MT with LS and FN BMD in early postmenopause were -0.12 (P = .005) and -0.11 (P = .01), respectively. The respective correlations between rate of increase in U-NTX with concurrent rates of decrease in LS and FN BMD were 0.28 (P < .0001) and 0.17 (P = .0003). The rate of increase in U-NTX remained a significant predictor even after additional adjustment (in separate models) for absolute BMD at the LS or FN in early postmenopause, and rate of change in BMD during the MT at the LS or FN (Table 2).
Table 2.
Associations of Rate of Increase in Bone Turnovera During the Menopause Transition (MT)b with Incident Fracture in Postmenopause
| HR per SD Increment in Rate of Increase in U-NTX During the MT (95% CI) | P Value | |
|---|---|---|
| Base modelc | 1.24 (1.05-1.45) | .008 |
| + LS BMD in early postmenopause | 1.24 (1.05-1.46) | .009 |
| + FN BMD in early postmenopause | 1.25 (1.05-1.50) | .01 |
| + Rate of decrease in LS BMD during the MT | 1.22 (1.03-1.45) | .02 |
| + Rate of decrease in FN BMD during the MT | 1.20 (1.00-1.42) | .03 |
aBone turnover assessed by U-NTX.
bThe MT was operationalized at 2 years before to 2 years after the final menstrual period,
cBase model controlled for the following covariates measured in early postmenopause (at the end of the MT, defined as the first study visit after 2 years following the final menstrual period): age, body mass index, race/ethnicity, fracture before the MT, current cigarette use (yes/no), and Study of Women’s Health Across the Nation study site.
CI, confidence interval; FN, femoral neck; LS, lumbar spine; U-NTX, urinary collagen type I N-telopeptide.
First available assessment of bone turnover in early postmenopause and subsequent fracture
Greater U-NTX in early postmenopause (after U-NTX peaks and plateaus) was also associated with future fracture (Table 3). Each SD increment in the first available U-NTX measurement in early postmenopause was associated with a 27% greater hazard for incident fracture in postmenopause (P = .01), accounting for age, race/ethnicity, BMI, fracture before the MT, and smoking status. The Spearman rank correlations between U-NTX in early postmenopause and concurrently measured LS and FN BMD were -0.24 (P < .0001) and -0.17 (P = .0002), respectively. The association between early postmenopausal U-NTX and fracture persisted after controlling for BMD at the LS (P = .04) or FN (P = .04) in early postmenopause.
Table 3.
Associations of Bone Turnover Levela in Early Postmenopauseb with Incident Fracture in Postmenopause
| HR per SD Increment in Peak Level of U-NTX in Early Postmenopause (95% CI) | P Value | |
|---|---|---|
| Base modelc | 1.27 (1.04-1.54) | .01 |
| + LS BMD in early postmenopause | 1.24 (1.00-1.54) | .03 |
| + FN BMD in early postmenopause | 1.25 (1.00-1.56) | .03 |
aBone turnover assessed by U-NTX.
bEarly postmenopause was operationalized as 2 years following the final menstrual period. The bone turnover assessment was made at the first available visit in early postmenopause.
cBase model controlled for the following covariates obtained in early postmenopause: age, body mass index, race/ethnicity, fracture before the MT, current cigarette use (yes/no), and Study of Women’s Health Across the Nation study site.
BMD, bone mineral density; CI, confidence interval; FN, femoral neck; HR, hazard ratio; LS, lumbar spine; U-NTX, urinary collagen type I N-telopeptide.
Rate of increase in bone turnover during the MT versus absolute bone turnover level in early postmenopause and subsequent fracture (exploratory analyses)
Of the 484 women in the sample, 181 had both slow increase in U-NTX during the MT (rate less than +2.9 nM BCE/nmM Cr per year) and low early postmenopausal U-NTX (early postmenopausal U-NTX less than 45.6 nM BCE/mM Cr), and served as the reference group. The 3 comparator groups had 61 participants in the fast increase/low turnover group, another 61 in the slow increase/high turnover group, and 181 in the fast increase/high turnover group. Compared with the referent group (slow increase/low turnover), women in the fast increase/high turnover group had 80% greater hazard for incident fracture in postmenopause (P = .04) after adjusting for race/ethnicity and study site. Women in the fast increase/low turnover group had 54% greater hazard for fracture compared to the referent group, but the association did not reach statistical significance (P = .2). Women with slow increase/high turnover did not have greater hazards for fracture compared with referent women (Table 4).
Table 4.
Contributions of Fast Increase in Bone Turnovera During the Menopause Transition (MT)b versus High Bone Turnover Level in Early Postmenopause as Predictors of Incident Fracture in Postmenopause
| Relative Fracture Hazardd (95% CI) | P Value | |
|---|---|---|
| Fast MT increase and low early postmenopausal peake | 1.54 (0.68-3.47) | .2 |
| Slow MT increase and high early postmenopausal peake | 1.04 (0.43-2.50) | .8 |
| Fast MT increase and high early postmenopausal peake | 1.80 (1.00-3.22) | .04 |
aBone turnover assessed by urinary collagen type I N-telopeptide (U-NTX).
bThe MT was operationalized at 2 years before to 2 years after the final menstrual period, corresponding to when bone turnover increases most rapidly.
cEarly postmenopause was operationalized as 2 years following the final menstrual period (corresponding to when U-NTX reaches its peak and plateaus). The bone turnover assessment was obtained at the first available visit in early postmenopause.
dHazard for fracture compared with women with slow increase in bone turnover during the MT and low bone turnover in early postmenopause. Slow vs. fast increase in bone turnover during the MT was defined as a rate of increase in U-NTX during the MT that was less vs. more than the sample median (+2.9 nM BCE/nmM Cr per year). Low vs. high bone turnover level in early postmenopause was defined as an U-NTX level (obtained at the first available visit in early postmenopause) that was less vs. more than the sample median (45.6 nM BCE/mM Cr).
eAll models adjusted for race/ethnicity and SWAN study site.
CI, confidence interval.
Discussion
The objective of this study was to determine if faster increases in bone turnover during the MT or greater bone turnover assessed once in early postmenopause are risk factors for fracture in the years after early postmenopause, when BMD remains relatively preserved (compared with older women). We report that faster increases in bone turnover during the MT and greater bone turnover in early postmenopause are both associated with fracture, even after accounting for change in BMD during the MT or absolute BMD in early postmenopause. An association between increased bone turnover and fracture in older, postmenopausal women is well established (6–13). One prior study in SWAN reported that greater bone turnover in pre- and early perimenopause was associated with incident fracture (14). This current study extends that prior work to directly link the rate of increase in bone turnover during the MT and bone turnover in early postmenopause to subsequent fracture. The clinical implication of these findings is that the MT and early postmenopause could be opportune times for early, short-term antiresorptive therapy to lessen a woman’s risk of future fracture (17).
Our finding that faster increases in bone turnover during the MT (and greater bone turnover in early postmenopause) are significant risk factors for incident fracture, even after adjustment for rate of BMD decline, supports the thesis that non-BMD factors contribute to loss of bone strength during the MT (2, 3, 21). Indeed, prior bone histomorphometry and epidemiologic studies have demonstrated that bone remodeling activation frequency doubles during the MT (2) and is associated with changes in bone microarchitecture that are associated with diminished fracture resistance (eg, decreased trabecular number, increased trabecular spacing, conversion of trabecular plates to rods, decreased trabecular connectivity, trabecular perforation, greater number of stress risers) (2, 5, 7, 22). Increased bone turnover may also contribute to suboptimal bone material properties (7) and decreased bone size (23). Our study contributes to the current body of literature by directly linking faster increases in bone turnover during the MT and greater bone turnover in early postmenopause to fracture despite BMD not being near osteoporosis levels. Of note, some conditions characterized by greater bone turnover (eg, after cessation of denosumab (24) or pregnancy (25)) are commonly associated with vertebral fractures. Whether increased bone turnover contributes to vertebral and appendicular fractures through the same mechanisms is uncertain.
On an exploratory basis, we also examined the relative contributions of rate of increase in bone turnover during the MT versus absolute bone turnover level in early postmenopause with subsequent fracture in postmenopause. We found that women with the greatest hazard for fracture had both a high rate of increase in bone turnover and high bone turnover in early postmenopause, suggesting that both are risk factors for fracture. However, the hazard ratio for fracture in these women (hazard ratio, 1.80) was similar in magnitude to that in women with a high rate of increase in bone turnover but low early postmenopausal bone turnover (hazard ratio, 1.54). Based on these findings, we hypothesize that the rate of increase in bone turnover may be more important than absolute bone turnover level in early postmenopause. Because the rate of increase in bone turnover and bone turnover level were correlated, and the number of fractures in this young study cohort was low, we did not have sufficient power to discern whether each predictor was associated with fracture independent of the other.
Although some investigators have long proposed that the MT and early postmenopause may be time-limited windows for early antiresorptive therapy (17), this approach has not gained widespread acceptance, in part, because women in their 40s and 50s have near-normal BMD (16). Up to this point, it has been uncertain whether the increase in bone turnover, loss of BMD, and deterioration in bone quality during the MT is sufficient to affect risk of future fracture and warrant early intervention. Our study suggests that, faster increases in bone turnover during the MT and greater bone turnover in early postmenopause do indeed increase a woman’s risk for fracture. Although clinical trials to test the efficacy of early intervention are still multiple research steps away, it is worthwhile to consider whether there are interventions that could prevent rapid increases in bone turnover during the MT and early postmenopause. Rapid-offset agents, such as denosumab (24, 26), are not suitable because stopping these medications leads to rapid loss of BMD that can exceed prior gains. Bisphosphonates, however, can prevent BMD decline in perimenopausal women (27); zoledronic acid, in particular, is intriguing because a single dose maintains BMD for at least 36 months (28).
This study has several limitations that warrant mention. The first is that we used U-NTX as a marker of bone turnover because it was available to us in the SWAN dataset. Although U-NTX was commonly measured in 1996 when SWAN was initiated, serum collagen type I C-telopeptide and procollagen type I N-terminal propeptide are now recommended as reference bone turnover markers for clinical studies (18). However, this recommendation is principally based on the need to create international reference databases and universal standards for use in clinical applications (18). U-NTX measurements using the Osteomark assay yield different values from those obtained by automated immunoassay analyzers (29). Despite these constraints, we consider our bone turnover assessments to be acceptable because our study’s objective was not to create clinical thresholds or tools, but to demonstrate the physiologic importance of bone turnover during the MT on future fracture risk. A second limitation is that although fractures of the spine and hip are associated with the greatest morbidity and mortality (30, 31), the majority of fractures sustained in our younger study cohort occurred at appendicular sites. Identifying risk factors for these fractures is clinically important, as these fractures are associated with 2-fold greater odds of osteoporotic fractures in older age (32). Last, we did not have a sufficient number of fractures to examine the association between bone turnover and each fracture type.
To summarize, to our knowledge, this is the first study to report that faster increases in bone turnover during the MT and greater bone turnover during early postmenopause are risk factors for fracture, even in women with relatively preserved BMD. To further delineate the clinical implications of these findings, future studies must (1) determine whether it is possible to identify women who are likely to have the fastest increases in bone turnover during the MT and the highest levels of bone turnover in early postmenopause and (2) examine whether early, short-term antiresorptive therapy in these women can indeed prevent the rapid increase in bone turnover during the MT.
Acknowledgments
The authors thank the study staff at each site and all the women who participated in SWAN.
Financial Support: The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH. Additional support for this project provided by NIA through P30-AG028748; UCLA Claude Pepper Older Adults Independence Center (principal investigator [PI]: Reuben) funded by the National Institute of Aging (5P30AG028748); National Institutes of Health/National Center for Advancing Translational Sciences UCLA CTSI Grant Number UL1TR000124; NIH Grant Number R01AG033067 (PI: Karlamangla). Dr Shieh was supported by the UCLA Specialty Training and Advanced Research Program and the Iris Cantor-UCLA Women’s Health Center Executive Advisory Board.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
Clinical Centers: University of Michigan, Ann Arbor—Siobán Harlow, PI 2011–present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA—Joel Finkelstein, PI 1999–present; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL—Howard Kravitz, PI 2009–present; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser—Ellen Gold, PI; University of California, Los Angeles—Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI 2011–present, Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry—New Jersey Medical School, Newark—Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA—Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD—Chhanda Dutta 2016– present; Winifred Rossi 2012–2016; Sherry Sherman 1994–2012; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD—Program Officers.
Central Laboratory: University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA—Maria Mori Brooks, PI 2012–present; Kim Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995–2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
Author Contributions: Study design: A.S., G.A.G., and A.S.K. Data analysis: A.S. Data interpretation: A.S., G.A.G., J.A.C., C.K.G., J.C.L., and A.S.K. Drafting manuscript: A.S., G.A.G., and A.S.K. Revising manuscript content: A.S., G.A.G., J.A.C., C.K.G., J.C.L., and A.S.K. Approving final version of manuscript: A.S., G.A.G., J.A.C., C.K.G., J.C.L., and A.S.K. A.S. and A.S.K. take responsibility for the integrity of the data analysis.
Glossary
Abbreviations
- BMD
bone mineral density
- BMI
body mass index
- CV
coefficient of variation
- FMP
final menstrual period
- FN
femoral neck
- LS
lumbar spine
- MT
menopause transition
- SWAN
Study of Women’s Health Across the Nation
- U-NTX
urine collagen type I N-telopeptide
Additional Information
Disclosure Statement: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References and Notes
- 1. Sowers MR, Zheng H, Greendale GA, et al. . Changes in bone resorption across the menopause transition: effects of reproductive hormones, body size, and ethnicity. J Clin Endocrinol Metab. 2013;98(7):2854–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Recker R, Lappe J, Davies KM, Heaney R. Bone remodeling increases substantially in the years after menopause and remains increased in older osteoporosis patients. J Bone Miner Res. 2004;19(10):1628–1633. [DOI] [PubMed] [Google Scholar]
- 3. Gossiel F, Altaher H, Reid DM, et al. . Bone turnover markers after the menopause: T-score approach. Bone. 2018;111:44–48. [DOI] [PubMed] [Google Scholar]
- 4. Rogers A, Hannon RA, Eastell R. Biochemical markers as predictors of rates of bone loss after menopause. J Bone Miner Res. 2000;15(7):1398–1404. [DOI] [PubMed] [Google Scholar]
- 5. Akhter MP, Lappe JM, Davies KM, Recker RR. Transmenopausal changes in the trabecular bone structure. Bone. 2007;41(1):111–116. [DOI] [PubMed] [Google Scholar]
- 6. Garnero P, Hausherr E, Chapuy MC, et al. . Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996;11(10):1531–1538. [DOI] [PubMed] [Google Scholar]
- 7. Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res. 1996;11(3):337–349. [DOI] [PubMed] [Google Scholar]
- 8. Akesson K, Ljunghall S, Jonsson B, et al. . Assessment of biochemical markers of bone metabolism in relation to the occurrence of fracture: a retrospective and prospective population-based study of women. J Bone Miner Res. 1995;10(11):1823–1829. [DOI] [PubMed] [Google Scholar]
- 9. Sornay-Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas PD. Identification of osteopenic women at high risk of fracture: the OFELY study. J Bone Miner Res. 2005;20(10):1813–1819. [DOI] [PubMed] [Google Scholar]
- 10. Vergnaud P, Garnero P, Meunier PJ, Bréart G, Kamihagi K, Delmas PD. Undercarboxylated osteocalcin measured with a specific immunoassay predicts hip fracture in elderly women: the EPIDOS Study. J Clin Endocrinol Metab. 1997;82(3):719–724. [DOI] [PubMed] [Google Scholar]
- 11. Szulc P, Chapuy MC, Meunier PJ, Delmas PD. Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture in elderly women. J Clin Invest. 1993;91(4):1769–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chapurlat RD, Garnero P, Sornay-Rendu E, Arlot ME, Claustrat B, Delmas PD. Longitudinal study of bone loss in pre- and perimenopausal women: evidence for bone loss in perimenopausal women. Osteoporos Int. 2000;11(6):493–498. [DOI] [PubMed] [Google Scholar]
- 13. Garnero P, Cloos P, Sornay-Rendu E, Qvist P, Delmas PD. Type I collagen racemization and isomerization and the risk of fracture in postmenopausal women: the OFELY prospective study. J Bone Miner Res. 2002;17(5):826–833. [DOI] [PubMed] [Google Scholar]
- 14. Cauley JA, Danielson ME, Greendale GA, et al. . Bone resorption and fracture across the menopausal transition: the Study of Women’s Health Across the Nation. Menopause. 2012;19(11):1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wright NC, Looker AC, Saag KG, et al. . The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greendale GA, Sowers M, Han W, et al. . Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the Study of Women’s Health Across the Nation (SWAN). J Bone Miner Res. 2012;27(1):111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zaidi M, Turner CH, Canalis E, et al. . Bone loss or lost bone: rationale and recommendations for the diagnosis and treatment of early postmenopausal bone loss. Curr Osteoporos Rep. 2009;7(4):118–126. [DOI] [PubMed] [Google Scholar]
- 18. Vasikaran S, Eastell R, Bruyère O, et al. ; IOF-IFCC Bone Marker Standards Working Group Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22(2):391–420. [DOI] [PubMed] [Google Scholar]
- 19. Sowers M, Crawford S, Sternfeld B, et al. . SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo R, Kelsey J, Marcus R, eds. Menopause: Biology and Pathobiology. San Diego: Academic Press; 2000:175–188. [Google Scholar]
- 20. Mackey DC, Lui LY, Cawthon PM, et al. ; Study of Osteoporotic Fractures (SOF) and Osteoporotic Fractures in Men Study (MrOS) Research Groups High-trauma fractures and low bone mineral density in older women and men. Jama. 2007;298(20):2381–2388. [DOI] [PubMed] [Google Scholar]
- 21. Shieh A, Ishii S, Greendale GA, Cauley JA, Lo JC, Karlamangla AS. Urinary N-telopeptide and rate of bone loss over the menopause transition and early postmenopause. J Bone Miner Res. 2016;31(11):2057–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hernandez CJ. How can bone turnover modify bone strength independent of bone mass? Bone. 2008;42(6):1014–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shieh A, Ishii S, Greendale GA, Cauley JA, Karvonen-Gutierrez C, Karlamangla AS. A bone resorption marker as predictor of rate of change in femoral neck size and strength during the menopause transition. Osteoporos Int. 2019;30(12):2449–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cummings SR, Ferrari S, Eastell R, et al. . Vertebral fractures after discontinuation of denosumab: a Post Hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res. 2018;33(2):190–198. [DOI] [PubMed] [Google Scholar]
- 25. Sanz-Salvador L, García-Pérez MÁ, Tarín JJ, Cano A. Bone metabolic changes during pregnancy: a period of vulnerability to osteoporosis and fracture. Eur J Endocrinol. 2015;172(2):R53–R65. [DOI] [PubMed] [Google Scholar]
- 26. Bone HG, Bolognese MA, Yuen CK, et al. . Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96(4):972–980. [DOI] [PubMed] [Google Scholar]
- 27. Hosking D, Chilvers CE, Christiansen C, et al. . Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. Early Postmenopausal Intervention Cohort Study Group. N Engl J Med. 1998;338(8):485–492. [DOI] [PubMed] [Google Scholar]
- 28. Reid IR, Black DM, Eastell R, et al. ; HORIZON Pivotal Fracture Trial and HORIZON Recurrent Fracture Trial Steering Committees Reduction in the risk of clinical fractures after a single dose of zoledronic acid 5 milligrams. J Clin Endocrinol Metab. 2013;98(2):557–563. [DOI] [PubMed] [Google Scholar]
- 29. Eastell R, Garnero P, Audebert C, Cahall DL. Reference intervals of bone turnover markers in healthy premenopausal women: results from a cross-sectional European study. Bone. 2012;50(5):1141–1147. [DOI] [PubMed] [Google Scholar]
- 30. Forsén L, Sogaard AJ, Meyer HE, Edna T, Kopjar B. Survival after hip fracture: short- and long-term excess mortality according to age and gender. Osteoporos Int. 1999;10(1):73–78. [DOI] [PubMed] [Google Scholar]
- 31. Gehlbach SH, Burge RT, Puleo E, Klar J. Hospital care of osteoporosis-related vertebral fractures. Osteoporos Int. 2003;14(1):53–60. [DOI] [PubMed] [Google Scholar]
- 32. Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15(4):721–739. [DOI] [PubMed] [Google Scholar]