Abstract
Background
Hypercalcemia of malignancy (HCM) is a common complication of advanced cancer. PTH-independent HCM may be mediated through different mechanisms: (1) humoral HCM, caused by the secretion of PTH-related peptide (PTHrP), (2) local osteolysis resulting from metastatic lesions, and (3) calcitriol-mediated hypercalcemia. Calcitriol-mediated HCM in patients with nonlymphomatous solid tumors is thought to be rare.
Methods
We performed a retrospective chart review from 2008 to 2017 to characterize further patients at our institution with solid tumors who had HCM with concomitant elevations in calcitriol. Patients with PTH-dependent hypercalcemia and patients with evidence of granulomatous disease were excluded, as were patients with hematologic malignancies. We hypothesized that patients with HCM and elevated calcitriol levels would respond less favorably to treatment with antiresorptive therapy compared with patients with HCM but without calcitriol elevation. We also aimed to assess mortality and determine if PTHrP and phosphorus levels correlate with calcitriol because both factors may alter calcitriol levels.
Results
Of 101 eligible patients, calcitriol was elevated in 45 (45%). PTHrP was elevated in 76% of patients with elevated calcitriol compared with 52% of patients without calcitriol elevation. The mean PTHrP value did not differ between patients with HCM and elevated calcitriol (36.3 ± 22 pg/mL) and those without calcitriol elevation (37.4 ± 19 pg/mL). Those with elevated calcitriol levels generally did not respond completely to antiresorptive treatment (80% incomplete response rate), whereas most patients without an elevation in calcitriol responded well to antiresorptive treatment (78% response rate: P < .001). There was no significant difference in the percentage of patients with metastatic bone disease among the 2 groups (49% vs. 55%, respectively). There was no difference in mortality between the 2 groups (P = .14). A weak but significant negative correlation was found between phosphorus and calcitriol (Pearson r = -0.261, P = .016). This correlation was only significant in patients without calcitriol elevation (Pearson r = -0.4, P = .0082). Also, a significant negative correlation was found between PTHrP and phosphorus, again only in patients without calcitriol elevation.
Discussion
In the setting of HCM, patients with calcitriol elevation are much less likely to respond to antiresorptive therapy than patients without calcitriol elevation. Because calcitriol elevation did not appear to be correlated with hypophosphatemia or elevated PTHrP, it would appear that calcitriol production under these conditions is autonomous, and not subject to normal physiological controls. These observations indicate that calcitriol elevations in patients with HCM have clinical significance.
Keywords: hypercalcemia, parathyroid hormone-related peptide, calcitriol, vitamin D, hypercalcemia of malignancy
Hypercalcemia of malignancy (HCM) is a common complication of advanced cancer, with an estimated incidence of 20% to 30% (1). Clinical presentations are variable and depend on the degree of hypercalcemia. Primary hyperparathyroidism resulting from parathyroid adenoma(s) is characterized by nonsuppressed PTH values and may occur in the setting of malignancy with or without HCM (2, 3). PTH-independent HCM is characterized by suppressed PTH values and may be mediated through various mechanisms, including the secretion of PTH-related peptide (PTHrP), also known as humoral HCM; development of local osteolytic lesions; and overproduction of 1,25-dihydroxyvitamin D (calcitriol).
Overproduction of calcitriol is a well-recognized complication of lymphomas and granulomatous diseases and is thought to be driven by increased activity of 1-alpha-hydroxylase in macrophages (4–6). Dysregulated calcitriol production in patients with solid tumors, however, has seldom been reported. Its pathophysiology and prognostic significance are unclear (7). Several factors are involved in regulating calcitriol homeostasis. For example, in states of calcium and/or phosphorus deficiency, the activity of 1-alpha-hydroxylase is increased via a PTH-dependent process to mediate the renal conversion of 25-hydroxyvitamin D to calcitriol. Conversely, in states of hypercalcemia and hyperphosphatemia, calcitriol levels are reduced (8). Hypophosphatemia may also increase calcitriol levels through a PTH-independent process. Circulating fibroblast growth factor 23 (FGF23), a potent regulator of vitamin D metabolism, is significantly reduced in the setting of hypophosphatemia, which subsequently results in a significant increase in the level of calcitriol (9, 10). Unlike PTH, the effect of PTHrP on calcitriol production is controversial. PTHrP was first described in the 1980s, and PTHrP-mediated hypercalcemia is a well-established complication of malignancy (11, 12). PTHrP-mediated HCM has been associated with limited survival, specifically in patients with solid tumors (13). In addition to its direct effect on bone resorption, PTHrP can have a paracrine function by stimulating osteolysis. This process is mediated by the release of osteoclast-activating cytokines, such as IL-1, IL-6, receptor activator of nuclear factor-ĸB ligand, and macrophage inflammatory protein-1α (14). PTHrP-mediated hypercalcemia has been associated with suppressed serum calcitriol concentrations (15). However, clinical studies in healthy humans and animal studies revealed that PTHrP administration is associated with an increase in serum calcitriol (16, 17).
The prevalence and clinical significance of elevated calcitriol in patients with PTH-independent HCM and solid tumors have not been described. Some patients with HCM have refractory and/or relapsing hypercalcemia after treatment with antiresorptive therapy. The risk factors associated with failure to normalize and maintain calcium levels after antiresorptive therapy have not been identified. Elevations in calcitriol are known to directly increase gastrointestinal calcium absorption and bone resorption. Therefore, we hypothesized that elevated calcitriol in the setting of HCM may have clinical significance.
We conducted a retrospective chart review to further characterize patients at Memorial Sloan Kettering Cancer Center with solid tumors who had HCM with concomitant elevations in calcitriol. We sought to determine differences in their demographics and response to treatment with antiresorptive therapy compared with those of patients with solid tumors who had HCM without calcitriol elevation. We also aimed to assess mortality and discern the mechanism of calcitriol elevation by determining if PTHrP and phosphorus levels correlate with calcitriol levels in these patients.
Methods
After obtaining approval from our institutional review board, a retrospective review of medical records from patients at Memorial Sloan Kettering Cancer Center was conducted between January 11, 2008, and August 7, 2017.
Patients were included in the study if they had hypercalcemia, defined as an albumin-corrected calcium value greater than the upper limit of normal (>10.5 mg/dL); a suppressed PTH concentration (<10 pg/mL [normal: 12 to 88 pg/mL]); recorded measurements of calcitriol, PTHrP, and phosphorus levels measured within 1 week of the onset of hypercalcemia; and treatment with bisphosphonates and/or denosumab in addition to standard of care (IV fluid ± calcitonin). Patients were excluded from the study for the following reasons: hematologic malignancies, insufficient medical records, evidence of granulomatous disease, current treatment with vitamin D supplements, and/or a treatment regimen that did not include antiresorptive therapy.
Eligible patients with HCM were classified into 2 groups based on whether they had elevated calcitriol levels or not. Elevated calcitriol was defined as a level greater than the upper limit of normal, whereas an inappropriately elevated calcitriol level was defined as within the upper third of normal for the assay. The collected data included patient demographics, cancer diagnosis, prior treatment with antiresorptive therapy, and levels of serum albumin-corrected calcium, serum phosphate, 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D (calcitriol) (18 to 72 pg/mL), PTH, PTHrP, serum creatinine, and estimated glomerular filtration rate (eGFR). The presence of bone metastases was determined by computed tomography, magnetic resonance imaging, positron emission tomography, or bone scan reports. Evidence of granulomatous disease, an exclusion criterion, was determined by searching medical records, imaging, or pathology reports. Modalities of antiresorptive treatment with either IV bisphosphonate or denosumab were identified, and patient response to treatment was determined. After 7 to 30 days of treatment with antiresorptive therapy, patients were considered to have a “complete response” if their hypercalcemia resolved or an “incomplete response” if their hypercalcemia was refractory or relapsing. Refractory hypercalcemia was defined as the failure of an albumin-corrected calcium to return to a normal level within 7 to 30 days after antiresorptive treatment. Patients were considered to have relapsing hypercalcemia if their hypercalcemia initially resolved with treatment but recurred within 7 to 30 days. The total number of bisphosphonate doses administered within 1 month and 3 months of HCM diagnosis was compared between the 2 main groups. Response to denosumab was assessed in patients who received denosumab after an incomplete response to bisphosphonate. The time from diagnosis to death was also measured to determine survival outcomes.
Statistical analysis
Descriptive statistical analyses of basic patient demographics and biochemical laboratory assessments were performed. Continuous variables of different groups were compared using Student t tests, whereas categorical variables were compared using χ 2 tests. Kaplan-Meier curves were used to present the survival outcomes of patients. Pearson’s correlation coefficient (r) analysis was performed to assess whether there was a linear correlation between calcitriol, PTHrP, and/or phosphorus levels. Univariate logistic regression models were constructed to calculate the odds ratio (OR) for incomplete responses based on the independent variables, including PTHrP, calcitriol, albumin, phosphorus, and bone metastasis. Statistical analysis was performed using R, version 3.5.1 (R Foundation for Statistical Computing, University of Auckland, New Zealand).
Results
A total of 136 patients were assessed for eligibility. Thirty-five patients were excluded for the following reasons: diagnosis of a hematologic malignancy, managed without an antiresorptive therapy, or insufficient medical records. A total of 101 patients were eligible and included in the study. Table 1 outlines the overall characteristics of all patients evaluated. Approximately 40% of patients had either renal cell carcinoma or breast cancer. Of all patients included, PTHrP was elevated in 62% and bone metastases were identified in 53%. Twenty-six percent of patients received IV bisphosphonates within 1 year before their hypercalcemia diagnosis for treatment of a different indication, such as osteoporosis or bone metastasis (to prevent skeletal-related events).
Table 1.
Overall Baseline Characteristics of All Patients (N = 101)
| Characteristic | No. of Patients | No. of Patients Analyzed for Each Characteristic |
|---|---|---|
| Gender | 101 | |
| Female | 49 (49%) | |
| Male | 52 (52%) | |
| Age, ya | 57 (15) | 101 |
| Tumor type | 101 | |
| Renal cell carcinoma | 22 (22%) | |
| Breast cancer | 19 (19%) | |
| Head and neck SCC | 9 (9%) | |
| Bladder cancer | 9 (9%) | |
| Pancreatic cancer | 6 (6%) | |
| Sarcoma | 5 (5%) | |
| Ovarian cancer | 4 (4%) | |
| Neuroendocrine tumor | 4 (4%) | |
| Lung SCC | 3 (3%) | |
| Prostate cancer | 3 (3%) | |
| Melanoma | 2 (2%) | |
| Others | 15 (15%) | |
| Race | 101 | |
| White | 75 (74%) | |
| Black | 11 (11%) | |
| Unknown | 15 (15%) | |
| Bone metastasis | 53 (53%) | 101 |
| Prior BP | 26 (26%) | 101 |
| High PTHrP | 63 (62%) | 101 |
| Baseline laboratory assessmentsa | ||
| Calcium (8.5-10.5 mg/dL) | 13.0 (1.46) | 101 |
| Albumin (3.8-5 g/dL) | 3.57 (0.59) | 101 |
| Albumin-corrected calcium, mg/dL | 13.4 (1.51) | 101 |
| 25(OH)D, ng/mL | 23.4 (15.4) | 94 |
| Calcitriol, 18-72 pg/mL | 61.4 (53.5) | 92 |
| PTH (12-65 pg/mL | 4.22 (2.86) | 101 |
| PTHrP, pg/mL | 36.9 (20.3) | 90 |
| Creatinine, 0.6-1.3 mg/dL | 1.05 (0.44) | 101 |
| Phosphorus, 2.5-4.2 mg/dL | 2.69 (0.90) | 94 |
| eGFR, mL/min/1.73 m2 | 78.1 (30.9) | 101 |
aValues reported as the mean (SD).
25(OH)D, 25-hydroxyvitamin D; BP, bisphosphonate; eGFR, estimated glomerular filtration rate; PTHrP, PTH-related peptide; SCC, squamous cell carcinoma.
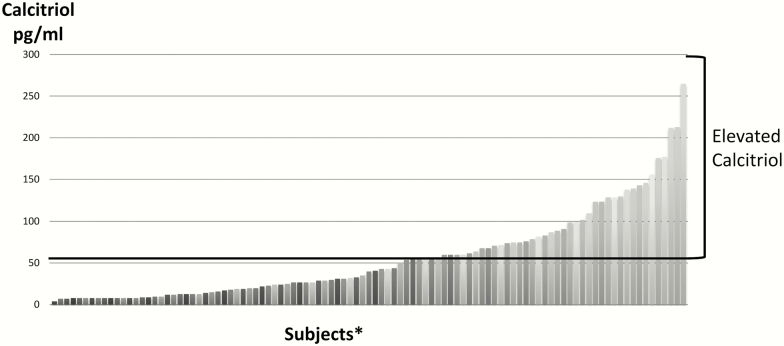
The demographics and baseline laboratory assessments of patients without calcitriol elevation (n = 56) compared with those with calcitriol elevation (n = 45) were analyzed (Table 2). The mean calcitriol value for the elevated calcitriol group was 102 ± 49.2 pg/mL compared with 22.2 ± 11.7 pg/mL for the group without calcitriol elevation. Figure 1 shows the calcitriol distribution in the entire cohort. PTHrP was elevated in 76% of patients in the elevated calcitriol group compared with 52% of patients in the group without elevated calcitriol (P = .025). The mean PTHrP value did not differ between the 2 groups (36.3 ± 22.0 pg/mL vs. 37.4 ± 19.0 pg/mL, respectively). Eleven patients whose PTHrP was measured in a different assay were not included in the mean calculation. There was no significant difference (P = .655) in the percentage of patients with metastatic bone disease between the elevated calcitriol (49%) and nonelevated calcitriol (55%) groups. There was a small but statistically significant difference (P = 0009) in eGFR between patients with calcitriol elevation (87.1 ± 31.0 mL/min/1.73 m2) and those without (70.8 ± 29.1 mL/min/1.73 m2). Approximately 50% of patients with elevated calcitriol received bisphosphonates within 1 year before their hypercalcemia diagnosis compared with 5% of patients without elevated calcitriol.
Table 2.
Patient Demographics and Baseline Laboratory Assessments from the Without Elevated Calcitriol Group (N = 56) and Elevated Calcitriol Group (N = 45)
| Characteristic | No. Without Elevated Calcitriol | No. With Elevated Calcitriol | P Value |
|---|---|---|---|
| Gender | 1 | ||
| Female | 28 (50%) | 21 (47%) | |
| Male | 28 (50%) | 24 (52%) | |
| Age, ya | 59.4 (14) | 54.87 (15) | .120 |
| Tumor type | .360 | ||
| Renal cell carcinoma | 11 (20%) | 11 (24%) | |
| Breast cancer | 9 (16%) | 10 (22%) | |
| Head and neck SCC | 5 (9%) | 4 (9%) | |
| Bladder cancer | 8 (14%) | 1 (2%) | |
| Pancreatic cancer | 2 (4%) | 4 (9%) | |
| Sarcoma | 3 (5%) | 2 (4%) | |
| Ovarian cancer | 1 (2%) | 3 (7%) | |
| Neuroendocrine tumor | 2 (4%) | 2 (4%) | |
| Lung SCC | 3 (5%) | 0 (0%) | |
| Prostate cancer | 1 (2%) | 2 (4%) | |
| Melanoma | 2 (4%) | 0 (0%) | |
| Others | 9 (16%) | 6 (13%) | |
| Race | .759 | ||
| White | 43 (77%) | 32 (71%) | |
| Black | 6 (11%) | 5 (11%) | |
| Unknown | 7 (13%) | 8 (18%) | |
| Bone metastasis | .655 | ||
| Yes | 31 (55%) | 22 (49%) | |
| Prior BP | <.001 | ||
| Yes | 3 (5%) | 23 (51%) | |
| PTHrP | .025 | ||
| High | 29 (52%) | 34 (76%) | |
| Baseline laboratory assessments a | Absolute Values (SD) | Absolute Values (SD) | |
| Calcium, mg/dL | 12.7 (1.25) | 13.4 (1.62) | .021 |
| Albumin, g/dL | 3.47 (0.58) | 3.69 (0.58) | .055 |
| Albumin-corrected calcium, mg/dL | 13.2 (1.29) | 13.7 (1.73) | .106 |
| 25(OH)D, ng/mL | 24.8 (13.9) | 21.6 (17.2) | .340 |
| Calcitriol, pg/mL | 22.2 (11.7) | 102 (49.2) | <.001 |
| PTH, pg,mL | 3.94 (2.84) | 4.58 (2.88) | .259 |
| PTHrP, pg,mL | 37.4 (19.0) | 36.3 (22.0) | .782 |
| Creatinine, mg/dL | 1.14 (0.44) | 0.93 (0.42) | .014 |
| Phosphorus, mg/dL | 2.98 (0.91) | 2.35 (0.78) | <.001 |
| eGFR, mL/min/1.73 m2 | 70.8 (29.1) | 87.1 (31.0) | .009 |
aCharacteristics of age and baseline assessments with values, except for the P value column, are reported as the mean (SD).
25(OH)D, 25-hydroxyvitamin D; BP, bisphosphonate; eGFR, estimated glomerular filtration rate; PTHrP, parathyroid hormone-related peptide; SCC, squamous cell carcinoma.
Figure 1.
Calcitriol distribution in the entire cohort. *Each vertical bar represents 1 patient.
Table 3 outlines the types of antiresorptive therapy administered, response to treatment, as well as the number of bisphosphonates received within 1 and 3 months of hypercalcemia diagnosis for patients in this study. All patients with elevated calcitriol (n = 45) and 89% of patients without elevated calcitriol (n = 50) survived 30 days after treatment and were included in the analysis of response to treatment. Patients with elevated calcitriol had a significantly higher risk of an incomplete response (80%) compared with patients without calcitriol elevation (22%), despite receiving more bisphosphonates within 1 and 3 months of their hypercalcemia diagnosis (P < .001). On subgroup analysis, a higher risk of incomplete response was observed in the high calcitriol group (n = 30, 83%) and the inappropriately elevated calcitriol group (n = 15, 73%) compared with the group without calcitriol elevation (n = 56, 22%) (P < .001). In the group without calcitriol elevation, denosumab was administered to 3 of 11 patients with an incomplete response to bisphosphonate, and all 3 subsequently achieved a complete response. In the group with elevated calcitriol, denosumab achieved a complete response in 5 of 13 patients (39%) with an incomplete response to bisphosphonates. The tumor type did not differ between the low- and high-calcitriol groups. Looking at the entire cohort, breast cancer was the only tumor type demonstrating a disproportionately high percentage (83%) of patients with incomplete response to treatment (in general, tumor type was not associated with response).
Table 3.
Patient Responses to Antiresorptive Therapy for the Nonelevated (N = 55) and Elevated Calcitriol (N = 46) Groups
| Characteristic | No. of Patients Without Elevated Calcitriola | No. of Patients With Elevated Calcitriola | P Value |
|---|---|---|---|
| Response to treatment | n = 50 | n = 45 | <.001 |
| Complete response | 39 (78%) | 9 (20%) | |
| Incomplete response | 11 (22%) | 36 (80%) | |
| Refractory | 1 (2%) | 10 (22%) | |
| Relapsed | 10 (20%) | 26 (58%) | |
| Antiresorptive treatment type | N = 55 | N = 46 | .002 |
| Zoledronic acid | 43 (77%) | 27 (61%) | |
| Pamidronate | 10 (18%) | 4 (9%) | |
| BP + denosumab | 3 (5%) | 14 (31%) | |
| No. of BP administered within 1 month of HCM dx | 1.18 (0.43) | 1.71 (0.82) | <.001 |
| No. of BP administered within 3 months of HCM dx | 1.41 (0.76) | 2.33 (1.51) | .001 |
| Response postdenosumab | n = 3 | n = 13 | .200 |
| Complete | 3 (100%) | 5 (39%) | |
| Incomplete | 0 (0%) | 8 (62%) |
aPercentages are based on the total number of patients evaluated in each characteristic in that specific column.
BP, bisphosphonate; HCM dx, hypercalcemia of malignancy diagnosis.
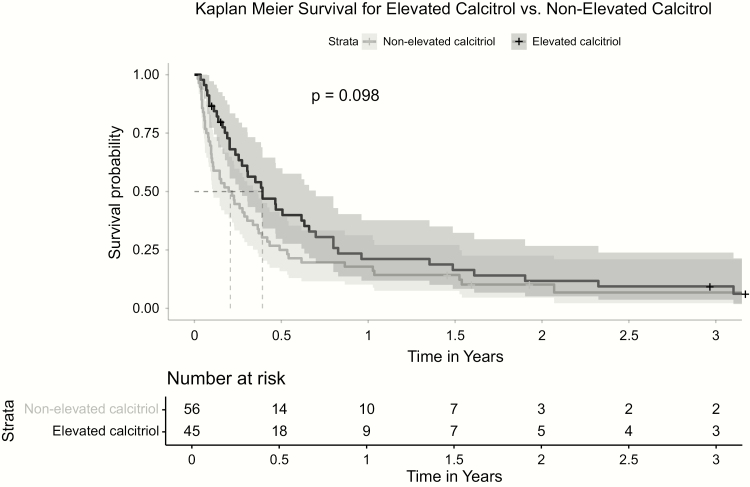
Fifty percent of patients died within 6 months of their hypercalcemia diagnosis. There was no difference (P = .14) in mortality between patients with elevated calcitriol and those without calcitriol elevation (Figure 2).
Figure 2.
Kaplan-Meier survival for elevated calcitriol versus nonelevated calcitriol.
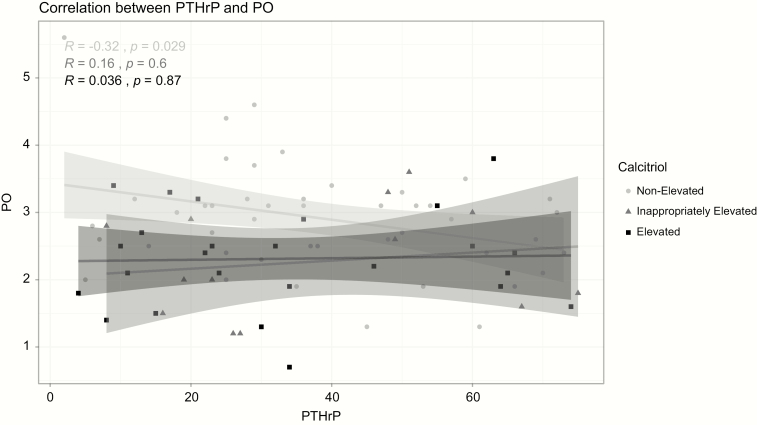
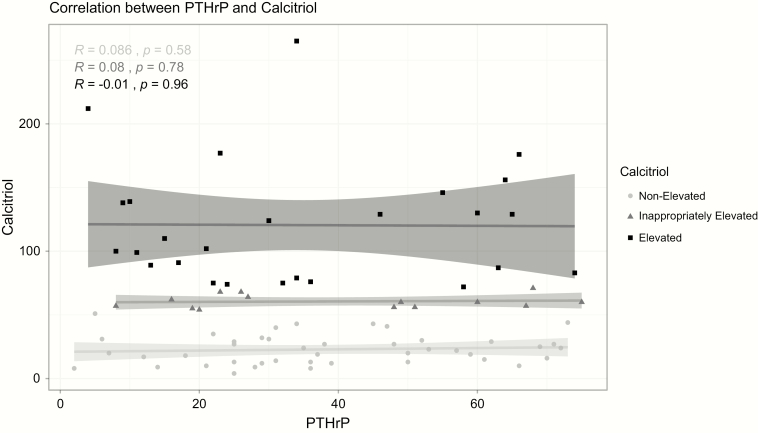
A weak but significant negative correlation (r = -0.261; P = .016) was observed between phosphorus and calcitriol. This correlation was only significant in the group without calcitriol elevation (Pearson r = -0.4, P = .008) and the group with inappropriately elevated calcitriol (Pearson r = -0.62, P = .018). A significant negative correlation was also found between PTHrP and phosphorus only in the group without calcitriol elevation (Figure 3). There was no significant correlation between PTHrP and calcitriol both in the entire cohort and on subgroup analysis based on calcitriol status (Figure 4). There was no correlation between the degree of hypercalcemia and calcitriol (data not shown).
Figure 3.
Correlation between PTHrP and phosphorus. PO, phosphorus; PTHrP, PTH-related peptide.
Figure 4.
Correlation between PTHrP and calcitriol. PTHrP, PTH-related peptide.
The OR for incomplete response to antiresorptive therapy (Table 4) was increased in patients with elevated calcitriol (OR, 15.22; 95% confidence interval [CI], 5.12-52.58, P < .001), but not with high PTHrP values (OR, 3.05; 95% CI, 0.95-10.58, P = .066), bone metastasis (OR, 1.22; 95% CI, 0.39-3.96, P = .734), or low phosphorous (OR, 1.11; 95% CI, 0.57-2.13, P = .758).
Table 4.
Logistic Regression Model for Patients with Incomplete Response to Antiresorptive Therapy
| Variable | Odds Ratio | Confidence Interval | P Value |
|---|---|---|---|
| High calcitriol | 15.22 | 5.12–52.58 | <.001 |
| High PTHrP | 3.05 | 0.95–10.58 | .066 |
| High albumin | 1.18 | 0.47–3.08 | .723 |
| Low phosphorous | 1.11 | 0.57–2.13 | .758 |
| Bone metastasis | 1.22 | 0.39–3.96 | .734 |
PTHrP, PTH-related peptide.
Discussion
To our knowledge, this is the first study to demonstrate that, in the setting of HCM, calcitriol elevation is associated with a higher risk of incomplete response to antiresorptive therapy. Overall, our cohort reflected patients with advanced cancer and a poor prognosis. Thus, a relatively high percentage of our cohort (49%) had hypercalcemia refractory to antiresorptive therapy.
The mechanism by which calcitriol is produced in patients with solid tumors remains unclear and requires further investigation. In our study, patients with elevated calcitriol had a significantly lower phosphorus level compared with patients without calcitriol elevation. However, a significant negative linear correlation between phosphorus and calcitriol was only seen in the group without calcitriol elevation and in the group with inappropriately elevated calcitriol but was not seen in the elevated calcitriol group. This finding suggests that, in the elevated calcitriol group, hypophosphatemia was not the driver of calcitriol elevation. Hypophosphatemia is commonly observed in patients with malignancy who present with excess PTHrP, malnutrition, a history of treatment with antineoplastic agents (eg, cisplatin, tyrosine kinase inhibitors), and rarely from paraneoplastic FGF23 production (18–21). Antiresorptive therapy may also cause hypophosphatemia in patients with cancer, but this is usually from reductions in serum calcium and resulting secondary hyperparathyroidism.
Our study found no correlation between PTHrP and calcitriol both when all patients together, or when the 2 calcitriol status subgroups, were analyzed separately. There was a significant number of patients who had elevated PTHrP levels without calcitriol elevation (n = 29). We could not determine a “threshold” PTHrP level that was associated with calcitriol elevation. Further studies are needed to clarify the relationship between PTHrP and calcitriol in HCM and whether tumor associated factors have a modulatory effect on calcitriol levels (22–24). The negative correlation between PTHrP and phosphorus (only seen in patients without calcitriol elevation) is not surprising as PTHrP causes phosphaturia.
Inappropriately elevated calcitriol in patients with malignancy (eg, lymphoma, a few case reports in solid tumors) has been attributed to extrarenal expression and activation of 1-alpha-hydroxylase in some immunohistochemistry studies, which demonstrated that 1-alpha-hydroxylase is expressed in nonmalignant cells (eg, normal colonic cells, tumor-associated macrophages) as well as malignant cells (eg, prostate, breast, renal cell, colon cancers) (25–27). Our study had 11 patients (24%) in the elevated calcitriol group who did not have high PTHrP values, indicating PTHrP was clearly not a driver of calcitriol elevation in these patients. Seven of these 11 patients had normal phosphorus levels, whereas the remaining 4 were hypophosphatemic. Staining for 1-alpha-hydroxylase was not feasible in this study because of the unavailability of pathology slides. Although the small difference in renal function between the 2 groups was statistically significant, it is unlikely to have a clinical impact on calcitriol levels. Given a lack of other viable mechanisms, we believe that ectopic production of calcitriol contributed to the calcitriol elevation in our study population. Because expression of 1-alpha-hydroxylase is seen in certain benign and cancerous tissue without causing hypercalcemia, future studies should evaluate whether the degree of 1-alpha-hydroxylase mRNA expression or other factors correlate with calcium levels (28).
Elevated calcitriol significantly increased the odds of incomplete response to antiresorptive therapy, whereas elevated PTHrP did not. This finding suggests that hypercalcemia is likely mediated through an additional process independent of bone resorption. The most prominent mechanism whereby calcitriol elevations mediate hypercalcemia is thought to be via increasing the intestinal absorption of calcium; other mechanisms include an increase in bone resorption and possibly an inhibition of bone mineralization (25, 29, 30). Therefore, appropriate therapies targeting intestinal absorption of calcium as well as bone resorption might be useful in controlling hypercalcemia. Avoidance of calcium- and vitamin D-fortified foods and supplements should be recommended, and dietary restriction should be considered on a case-by-case basis. Patients with HCM and calcitriol elevation in the setting of hematologic malignancy are often treated with glucocorticoids to inhibit extrarenal 1-alpha-hydroxylase; analogous data in solid tumor patients with calcitriol elevation are limited to case reports, but it is a reasonable option (6). In our study, a very small number of patients were treated with steroids after the diagnosis was made; hence, we were not able to evaluate the response to treatment in this group. In patients who do not tolerate or fail treatment with corticosteroids, ketoconazole or other inhibitors of 1-alpha-hydroyxlase may be considered because they have been used successfully to improve hypercalcemia in patients with sarcoidosis by decreasing the activity of 1-alpha-hydroxylase (31).
Treatment of hypophosphatemia may also improve hypercalcemia of malignancy. Before the advent of bisphosphonates, IV and/or oral phosphate supplementations demonstrated improvements in treating HCM (32, 33). Calcium and phosphate are thought to complex in the blood and get eliminated via the reticuloendothelial system.
The limitations of this study include its retrospective design, which lacked a standardized protocol for the workup and management of HCM, and the exclusion of patients with incomplete medical records as well as additional analyses (eg, FGF23). Calcitriol levels may have been tested more frequently in complex cases when endocrinology or renal services were consulted. Therefore, the study was subject to selection bias, resulting in a study population that may not truly represent the general HCM population. Nevertheless, given the rarity of calcitriol-mediated hypercalcemia, a retrospective design is the most appropriate approach for this investigation. Finally, the clinical utility of our definition of incomplete response could be questioned because mild hypercalcemia may be tolerated without intervention and normalization is not an imperative. However, achieving a normal calcium level is clinically desirable given the propensity for recurrent hypercalcemia as the clinical scenario evolves. Future prospective studies may evaluate the role of adjunctive measures, such as treatment with steroids in the management of hypercalcemia in the setting of calcitriol elevations.
Acknowledgments
Financial Support: This research was supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748.
Glossary
Abbreviations
- eGFR
estimated glomerular filtration rate
- FGF23
fibroblast growth factor 23
- HCM
hypercalcemia of malignancy
- OR
odds ratio
- PTHrP
PTH-related peptide
Additional Information
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Stewart AF. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352(4):373–379. [DOI] [PubMed] [Google Scholar]
- 2. Gomes Lda S, Kulak CA, Costa TM, Vasconcelos EC, Carvalho Md, Borba VZ. Association of primary hyperparathyroidism and humoral hypercalcemia of malignancy in a patient with clear cell renal carcinoma. Arch Endocrinol Metab. 2015;59(1):84–88. [DOI] [PubMed] [Google Scholar]
- 3. Axelrod DM, Bockman RS, Wong GY, Osborne MP, Kinne DW, Brennan MF. Distinguishing features of primary hyperparathyroidism in patients with breast cancer. Cancer. 1987;60(7):1620–1624. [DOI] [PubMed] [Google Scholar]
- 4. Fuss M, Pepersack T, Gillet C, Karmali R, Corvilain J. Calcium and vitamin D metabolism in granulomatous diseases. Clin Rheumatol. 1992;11(1):28–36. [DOI] [PubMed] [Google Scholar]
- 5. Hewison M, Kantorovich V, Liker HR, et al. . Vitamin D-mediated hypercalcemia in lymphoma: evidence for hormone production by tumor-adjacent macrophages. J Bone Miner Res. 2003;18(3):579–582. [DOI] [PubMed] [Google Scholar]
- 6. Seymour JF, Gagel RF. Calcitriol: the major humoral mediator of hypercalcemia in Hodgkin’s disease and non-Hodgkin’s lymphomas. Blood. 1993;82(5):1383–1394. [PubMed] [Google Scholar]
- 7. Donovan PJ, Sundac L, Pretorius CJ, d’Emden MC, McLeod DS. Calcitriol-mediated hypercalcemia: causes and course in 101 patients. J Clin Endocrinol Metab. 2013;98(10):4023–4029. [DOI] [PubMed] [Google Scholar]
- 8. Tebben PJ, Singh RJ, Kumar R. Vitamin D-mediated hypercalcemia: mechanisms, diagnosis, and treatment. Endocr Rev. 2016;37(5):521–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91(8): 3144–3149. [DOI] [PubMed] [Google Scholar]
- 10. Perwad F, Portale AA. Vitamin D metabolism in the kidney: regulation by phosphorus and fibroblast growth factor 23. Mol Cell Endocrinol. 2011;347(1-2):17–24. [DOI] [PubMed] [Google Scholar]
- 11. Burtis WJ, Wu T, Bunch C, et al. . Identification of a novel 17,000-dalton parathyroid hormone-like adenylate cyclase-stimulating protein from a tumor associated with humoral hypercalcemia of malignancy. J Biol Chem. 1987;262(15):7151–7156. [PubMed] [Google Scholar]
- 12. Donovan PJ, Achong N, Griffin K, Galligan J, Pretorius CJ, McLeod DS. PTHrP-mediated hypercalcemia: causes and survival in 138 patients. J Clin Endocrinol Metab. 2015;100(5):2024–2029. [DOI] [PubMed] [Google Scholar]
- 13. Mundy GR, Edwards JR. PTH-related peptide (PTHrP) in hypercalcemia. J Am Soc Nephrol. 2008;19(4):672–675. [DOI] [PubMed] [Google Scholar]
- 14. Schilling T, Pecherstorfer M, Blind E, Leidig G, Ziegler R, Raue F. Parathyroid hormone-related protein (PTHrP) does not regulate 1,25-dihydroxyvitamin D serum levels in hypercalcemia of malignancy. J Clin Endocrinol Metab. 1993;76(3):801–803. [DOI] [PubMed] [Google Scholar]
- 15. Rosol TJ, Capen CC, Horst RL. Effects of infusion of human parathyroid hormone-related protein-(1-40) in nude mice: histomorphometric and biochemical investigations. J Bone Miner Res. 1988;3(6):699–706. [DOI] [PubMed] [Google Scholar]
- 16. Horwitz MJ, Tedesco MB, Sereika SM, et al. . Continuous PTH and PTHrP infusion causes suppression of bone formation and discordant effects on 1,25(OH)2 vitamin D. J Bone Miner Res. 2005;20(10):1792–1803. [DOI] [PubMed] [Google Scholar]
- 17. Hu MI, Glezerman IG, Leboulleux S, et al. . Denosumab for treatment of hypercalcemia of malignancy. J Clin Endocrinol Metab. 2014;99(9):3144–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liamis G, Milionis HJ, Elisaf M. Medication-induced hypophosphatemia: a review. QJM. 2010;103(7):449–459. [DOI] [PubMed] [Google Scholar]
- 19. Rosner MH, Dalkin AC. Electrolyte disorders associated with cancer. Adv Chronic Kidney Dis. 2014;21(1):7–17. [DOI] [PubMed] [Google Scholar]
- 20. Latifyan SB, Vanhaeverbeek M, Klastersky J. Tumour-associated osteomalacia and hypoglycaemia in a patient with prostate cancer: is Klotho involved? BMJ Case Rep. 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chong WH, Molinolo AA, Chen CC, Collins MT. Tumor-induced osteomalacia. Endocr Relat Cancer. 2011;18(3):R53–R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de la Mata J, Uy HL, Guise TA, et al. . Interleukin-6 enhances hypercalcemia and bone resorption mediated by parathyroid hormone-related protein in vivo. J Clin Invest. 1995;95(6):2846–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tørring O, Turner RT, Carter WB, Firek AF, Jacobs CA, Heath H 3rd. Inhibition by human interleukin-1 alpha of parathyroid hormone-related peptide effects on renal calcium and phosphorus metabolism in the rat. Endocrinology. 1992;131(1):5–13. [DOI] [PubMed] [Google Scholar]
- 24. Uy HL, Mundy GR, Boyce BF, et al. . Tumor necrosis factor enhances parathyroid hormone-related protein-induced hypercalcemia and bone resorption without inhibiting bone formation in vivo. Cancer Res. 1997;57(15):3194–3199. [PubMed] [Google Scholar]
- 25. Akai PS, Wong T, Chang-Poon V, Green F, Whitelaw WA, Hanley DA. Resectable bronchogenic carcinoma presenting with hypercalcemia: tumor-associated granulomatous reaction and probable production of 1,25-dihydroxyvitamin D. Clin Invest Med. 1989;12(3):212–216. [PubMed] [Google Scholar]
- 26. Seymour JF, Gagel RF, Hagemeister FB, Dimopoulos MA, Cabanillas F. Calcitriol production in hypercalcemic and normocalcemic patients with non-Hodgkin lymphoma. Ann Intern Med. 1994;121(9):633–640. [DOI] [PubMed] [Google Scholar]
- 27. Tangpricha V, Flanagan JN, Whitlatch LW, et al. . 25-hydroxyvitamin D-1alpha-hydroxylase in normal and malignant colon tissue. Lancet. 2001;357(9269):1673–1674. [DOI] [PubMed] [Google Scholar]
- 28. McCarthy K, Laban C, Bustin SA, et al. . Expression of 25-hydroxyvitamin D-1-alpha-hydroxylase, and vitamin D receptor mRNA in normal and malignant breast tissue. Anticancer Res. 2009;29(1):155–157. [PubMed] [Google Scholar]
- 29. Lieben L, Masuyama R, Torrekens S, et al. . Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J Clin Invest. 2012;122(5):1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lieben L, Masuyama R, Torrekens S, et al. . Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J Clin Invest. 2012;122(5):1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Conron M, Beynon HL. Ketoconazole for the treatment of refractory hypercalcemic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17(3):277–280. [PubMed] [Google Scholar]
- 32. Goldsmith RS, Ingbar SH. Inorganic phosphate treatment of hypercalcemia of diverse etiologies. N Engl J Med. 1966;274(1):1–7. [DOI] [PubMed] [Google Scholar]
- 33. Thalassinos N, Joplin GF. Phosphate treatment of hypercalcaemia due to carcinoma. Br Med J. 1968;4(5622):14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]