Abstract
BACKGROUND/OBJECTIVES
The cellular and extracellular matrix (ECM) interactions that regulate adipose tissue homeostasis are incompletely understood. Proteoglycans (PGs) and their sulfated glycosaminoglycans (GAGs) provide spatial and temporal signals for ECM organization and interactions with resident cells by impacting growth factor and cytokine activity. Therefore, PGs and their GAGs could be significant to adipose tissue homeostasis. The purpose of this study was to determine the role of ECM sulfated GAGs in adipose tissue homeostasis.
METHODS
Adipose tissue and metabolic homeostasis in mice deficient in xylosyltransferase 2 (Xylt2−/−) were examined by histologic analyses, gene expression analyses, whole body fat composition measurements, and glucose tolerance test. Adipose tissue inflammation and adipocyte precursors were characterized by flow cytometry and in vitro culture of mesenchymal stem cells.
RESULTS
Xylt2−/− mice have low body weight due to overall reductions in abdominal fat deposition. Histologically, the adipocytes are reduced in size and number in both gonadal and mesenteric fat depots of Xylt2−/− mice. In addition, these mice are glucose intolerant, insulin resistant, and have increased serum triglycerides as compared to Xylt2+/+ control mice. Furthermore, the adipose tissue niche has increased inflammatory cells and enrichment of pro-inflammatory factors IL6 and IL1β, and these mice also have a loss of adipose tissue vascular endothelial cells. Lastly, xylosyltransferease-2 (XylT2) deficient mesenchymal stem cells from gonadal adipose tissue and bone marrow exhibit impaired adipogenic differentiation in vitro.
CONCLUSIONS
Decreased GAGs due to the loss of the key GAG assembly enzyme XylT2 causes reduced steady state adipose tissue stores leading to a unique lipodystrophic model. Accumulation of an adipocytic precursor pool of cells is discovered indicating an interruption in differentiation. Therefore, adipose tissue GAGs are important in the homeostasis of adipose tissue by mediating control of adipose precursor development, tissue inflammation, and vascular development.
Keywords: Xylosyltransferase II, Adipogenesis, Angiogenesis, Lipodystrophy, Adipose Stem Cells
Introduction
Adipose tissue maintenance and accumulation in obesity is a highly regulated process. Adipogenic development is regulated by growth factors, adipokines, and cellular-cross talk between the adipose tissue vasculature, mesenchymal stem cells, and adipocytes. All these processes depend on a dynamic and responsive extracellular matrix (ECM) to maintain adipose tissue development and homeostasis1. For instance, when adipocytes accumulate lipid during development or during caloric excess they proteolytically remodel the ECM to accommodate their dramatic size increase2, 3, and blocking of MMP activity inhibits maturation of committed preadipocytes in vitro and impairs adipose tissue development in vivo 4. The ECM proteins can both enhance and inhibit adipogenesis. Collagens enhance attachment of preadipocytes to the ECM leading to increased lipid accumulation and differentiation 5, and genetically removing collagen VI in the type II diabetic model ob/ob mice led to reduced body weight, and improved glucose and lipid metabolism 6. However, fibronectin inhibits adipogenic differentiation by inducing stress fiber formation 4, 7 and by binding to α5 integrin and activating GTPase RAC8.
Angiogenesis and adipogenesis are interdependent processes. Preadipocytes and endothelial cells arise from a common progenitor within the adipose tissue niche 9. During adipocyte maturation, adipogenic precursor stem cells commit to the adipogenic lineage in response to as yet unknown environmental cues; these precursor cells are mainly located in the adipogenic-vascular niche (e.g. stromal vascular fraction) and are typically characterized by many protein markers including Lin−, CD34+, CD24+, Sca1+, CD29+, and CD105+10, 11. This common progenitor and its close proximity to the vasculature during subsequent developing stages suggests interdependent signals are critical to the development and homeostasis of adipose tissue12. One such signal could be VEGF since blockage of VEGF signaling prevents adipose tissue development13. ECM PGs in the endothelial basement membrane form capillary-like structures aiding capillary development 14 and they act as a source of angiogenic factors such as VEGF 15.
Lipodystrophy is a collection of rare disorders that can be acquired or inherited. For example, in familial partial lipodystrophy there is progressive loss of adipose tissue from various body adipose tissue niches. In addition, these patients develop a variety of metabolic abnormalities some of which can be life threatening and include glucose intolerance, hypertriglyceridemia, leptin deficiency, and diabetes. But not all patients share all clinical abnormalities. For example, patients with congenital generalize lipodystrophy develop hepatic steatosis whereas the other predominant form familial partial lipodystrophy do not. Furthermore, the extent of adipose tissue loss usually determines the severity of the associated metabolic complications. Current treatment is quite limited, but is aimed at controlling the unique and specific symptoms of each patient16, 17.
Increased inflammation in the adipose tissue niche is a hallmark of obesity and type II diabetes18. Tissue ECM components such as collagen I19, chondroitin sulfate PGs (CSPG)(e.g. biglycan20 and decorin21), and heparan sulfate (HS) GAGs22, 23 can stimulate inflammatory cells and initiate inflammation. Genetically removing collagen VI in ob/ob mice decreased the diabetic profile in these mice including reduced adipose tissue inflammation 6. Therefore, in addition to adipogenesis and angiogenesis, PGs may be important to adipocyte homeostasis by modulating tissue inflammation.
Sulfated PGs are a diverse group of glycoproteins due to their varied core proteins and glycosaminoglycan (GAG) chains. They are found in the ECM and at the cell surface. The GAG chains can facilitate ligand receptor interactions of several important signaling pathways 24 and their loss predictably would significantly alter adipose tissue homeostasis. GAG assembly requires a complex series of reactions that initiates with attachment of a xylose to designated serine(s) of the core protein 25. Specifically, this step is catalyzed by two xylosyltransferases where xylosyltransferase 2 (XylT2) tissue expression is more ubiquitous 26–29. The small leucine-rich PGs biglycan and decorin are highly expressed in adipose tissue and are increased in obesity implying they are significant to growth of the fat niche and the development of Type-II diabetes 30, 31. However, the role that sulfated GAGs have in adipose tissue development, maintenance, and metabolism is unknown.
In previous studies, we had identified in the XylT2 deficient mice an unexpected liver biliary cystic phenotype initiating with biliary hyperplasia that progresses to fibrosis and cyst development. In addition, these mice develop renal hydronephrosis, fibrosis, and tubule dilation. These findings demonstrated that XylT2-dependent GAGs are important to liver and renal homeostasis. Other abnormalities were not readily observed.
Our present study shows that adipose tissue homeostasis is highly dependent on sulfated GAGs. We show that XylT2-dependent GAGs are critical to maintaining the adipose tissue. Furthermore, our studies show that severe metabolic dysfunction occurs as a result of decreased adipose tissue GAGs that lead to increased inflammation, reduced angiogenesis, and attenuation of the adipose stem/progenitor population.
MATERIALS AND METHODS
Animals
Xylt2−/− mice were generated as previously described27. All mice were housed in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. The Institutional Animal Care and Use Committees of all institutions approved all animal procedures and experiments. Numbers of animals used for each analyses are indicated in figure legends. In regard to samples for gonadal fat tissue (GAT), mesenteric adipose tissue (MAT), and other samples please see supplemental methods online.
Isolation of Stromal Vascular Fraction (SVF) and Adipose Tissue Derived Stem Cells (AdSC)
GAT SVF isolation and cell analyses were as described10 and in supplementary methods. AdSCs were isolated from GAT as described32 with additional details in supplementary methods. Briefly, gonadal white adipose tissue (GAT) was dissected and collected from 6–8 week-old male mice digested with Type I collagenase. The digested tissue was filtered and pelleted followed by resuspension in DMEM growth media. The cells were grown until 80–90% confluence (designated Passage 0) then trypsinized and then depleted using CD11b and CD45 microbeads (Miltenyi Biotech, USA) and replated. Passage 2 cells were used for the studies.
Bone Marrow Mesenchymal Stem Cells (BMMSC) - Isolation
Cells were isolated as per33 and described in supplemental data. Femur and humeri from 8–10 week-old mice were collected under sterile conditions; the bone marrow was flushed using a complete medium and single-cell suspension was used to isolate BMMSC by repeat pipetting. After centrifugation, the pellet was resuspended in the complete medium and plated; thereafter all steps were followed as per AdSCs isolation.
Flow Cytometry
To characterize isolated stem cells, 80% confluent passage 2 cells were washed with a 1% BSA buffer and stained with the following antibodies for 30 minutes on ice: Sca1-FITC, CD9-PE, CD11b-FITC, CD29-PE, CD45-PE, CD71-PE, and CD81-biotin and streptavidin-PerCep. Corresponding isotype controls were used for comparison. The stained cells were analyzed with a C6 Accuri flow cytometer. The raw data was analyzed using Flowjo software (BD San Jose, CA, USA). For SVF flow cytometry analysis, the antibodies used were CD11b-FITC, CD45-PE, F4/80-FITC, CD144, CD31, CD11c, and CD34. For adipocyte precursor characterization we used Mouse Hematopoietic Lineage eFluor® 450 Cocktail, CD29-APC, CD34-PE, and Sca1-FITC (see supplementary Table 1 for all antibodies used and their sources).
Adipogenic Differentiation and Oil Red O Staining Protocol
For adipogenic differentiation, 3×104 second-passage cells were plated on a coverslip in a 24-well plate. After overnight incubation, the stimulatory α-MEM medium containing 10% FBS, dexamethasone (10−7M), insulin (10 μg/ml), and isobutylmethylxanthine (0.5 μM) (all from Sigma Aldrich) was added and incubated for 10 days. The medium was changed every 2–3 days for 10 days. Cells were fixed in 4% PFA, and stained with Oil Red O according to the manufactures’ protocol (Poly Scientific, Bay Shore, NY, USA).
Dual Energy X-ray Absorptiometry (DEXA) Analysis
Five-month-old female mice were used for body composition that was measured in isoflurane-anesthetized mice using a DEXA Scanner from Lunar/GE Medical System (PIXImus2). With each use, the DEXA scanner was calibrated for bone mineral density and percent fat content using the mouse phantom supplied with the machine. Mice were scanned in sternal recumbency to include the entire body.
H&E Staining and Adipocyte Quantification
GAT from two-month-old Xylt2+/+ or Xylt2−/− male mice was fixed with 4% paraformaldehyde, paraffin-embedded, sectioned and stained with hematoxylin-eosin stain. Mesenteric adipose tissue (MAT) was also processed accordingly. Histological quantification of adipocytes was performed using ImageJ software. Cells on the image edges were excluded to avoid partial estimations. Adipocyte size was measured on >200 cells per mouse (3–4 mice/group).
Intraperitoneal Glucose Tolerance Test (IPGTT)
Five to six-month-old female mice were fasted for 6 hours and then received an intraperitoneal injection of 1.5 g glucose per kilogram of body weight. Blood was collected from the tail vein at 0, 30,60, 90, and 120 minutes post-injection. Glucose was measured using One Touch Ultra2 blood glucose monitoring systems (Johnson & Johnson, Langhorne, PA). For insulin measurement, plasma levels were measured at 0, 60, and 120 minute time points from the same mice followed by measurement with Ultra Sensitive Mouse Insulin ELISA kits from CrystalChem, USA.
Measurement of Serum Adipokines, Total Cholesterol (TC), High-Density Lipoprotein cholesterol (HDL), and Triglycerides (TGs)
Serum from nonfasting 4–5 month old female mice were assayed with adiponectin and leptin ELISA (Raybiotech, Norcross, GA, USA). For plasma lipids, blood from 5 hour fasted, 5 month old female mice was collected into EDTA, aprotinin, and gentamicin. TC, HDL, and TG were evaluated based on a methods described in supplemental data.
Quantitative Real Time RT PCR (qPCR)
GAT collected in RNAlater was used for RNA isolation using a RNeasy Lipid Tissue Mini Kit (Qiagen, USA, inclusive of DNase treatment). One μg of purified RNA was used to synthesize complementary DNA, and real-time quantitative PCR was performed using SYBR Green I in an Applied Biosystem 7500 Fast Real Time PCR system. Relative gene expression of genes listed was evaluated based on the ddCT method34. Data were expressed as fold changes relative to Xylt2+/+. The list of primers is described in supplemental Table 2.
Western blot analyses
Westerns were performed with protein isolates from at least three animals of each genotype. Decorin was detected as previously described27. TGFβ activity in tissues was assessed by detection of Smad2/3 and Smad4 interaction by Smad2/3 immunoprecipitation followed by Smad4 immunodetection on western blots as described35. Quantitation was performed using Image J software.
Data Analysis
The results are shown as means ± S.D. The statistical significance between mouse groups was determined using unpaired student’s t tests; p value < 0.05 was considered significant (*p < 0.05, **p < 0.01, ***p < 0.001).
RESULTS
Xylt2−/− mice have reduced body weight and adipose tissue
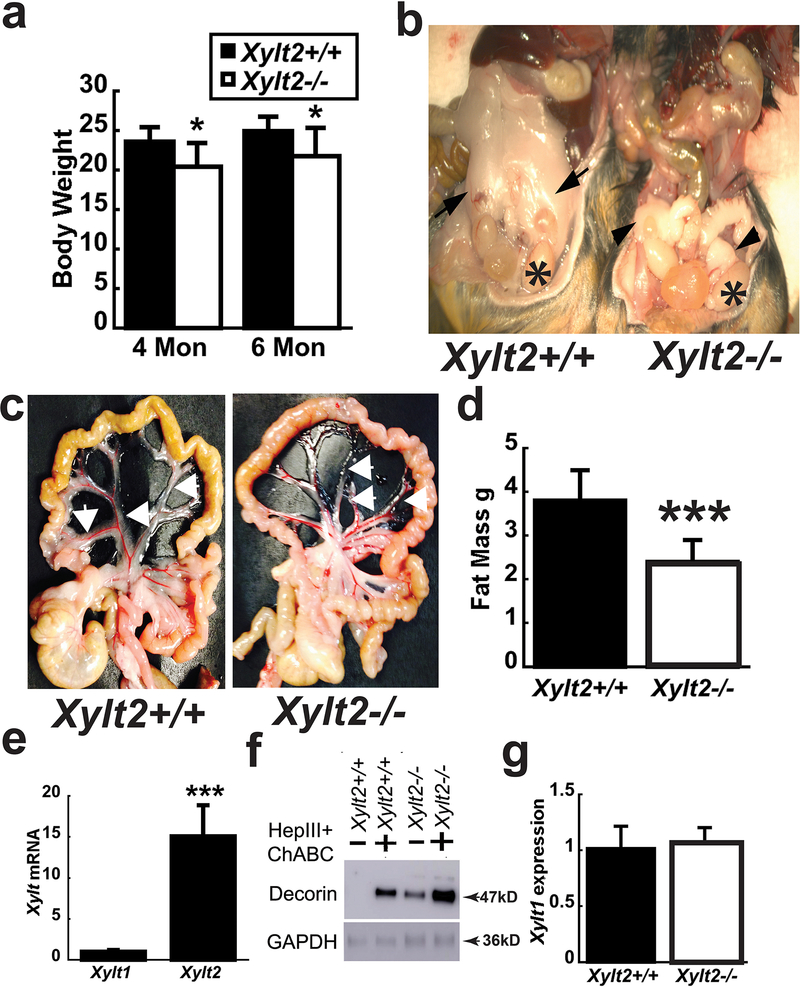
The Xylt2−/− mice are null for Xylt2 activity and have been previously described27. Body weights at 8 weeks of age in female mice were no different from Xylt2+/+ mice, but at 4 and 6 months, Xylt2−/− mice were significantly decreased (Figure 1a). Gross examination of Xylt2+/+ and Xylt2−/− mice revealed severe reductions of white adipose tissue in the gonadal (GAT) (Figures 1b) and mesenteric (MAT) (Figure 1c) areas. Oil red O staining of liver tissue was negative for steatosis (data not sown). Whole body fat mass composition was assessed using dual energy X-ray absorptiometry (DEXA). This showed the Xylt2−/− female mice had significantly decreased fat mass (Figure 1d) suggesting that loss of XylT2 dependent glycosaminoglycans (GAGs) greatly impacts white adipocyte homeostasis. The significant role of XylT2 in adipose tissue is reinforced by expression analyses showing that XylT2 has a relative level of mRNA 15-fold (p < 0.001) higher than Xylt1 mRNA (Figure 1e). Furthermore, reduced adipose tissue GAG is demonstrated by high levels of GAG-free decorin core protein in undigested samples by western blot (Figure 1f). Xylt2−/− GAT Xylt1 mRNA transcript was unchanged indicating a lack of a compensatory increased Xylt1 expression in the Xylt2−/− mice GAT (Figure 1g). These results indicate that Xylt2−/− adipocytes are abnormal in size, number, and/or function due to loss of GAGs.
Figure 1.
Body weight, adipose tissue levels, and XylT2 expression in Xylt2−/− mice. (a) Female Xylt2−/− mice on normal chow diet have reduced body weight at 4 and 6 months of age (n=5–8 of each genotype) and (b) gonadal adipose tissue is reduced in 3 month old male Xylt2−/− mice. Arrows in Xylt2+/+ are gonadal adipose tissue and arrowheads in Xylt2−/− show remnants of gonadal adipose tissue. Asterisks indicate testicles. (c) Perivascular mesenteric adipose tissue also reduced in 4-month-old Xylt2−/− females. (d) DEXA scanning analyses shows reduced total body fat in female 4–5 month Xylt2−/−mice, (n=10 of each genotype). (e) Xylt2 is predominant expressed isoform in white adipose gonadal tissue by real time RT-PCR. (f) GAT western from both mice showing free decorin in Xylt2−/− mice. (g) Xylt1 expression did not increase in white gonadal adipose tissue in Xylt2−/− mice, n= 3–4, 2–3 months of age. All analyses are student’s t test where * p<0.05, ***p<0.001.
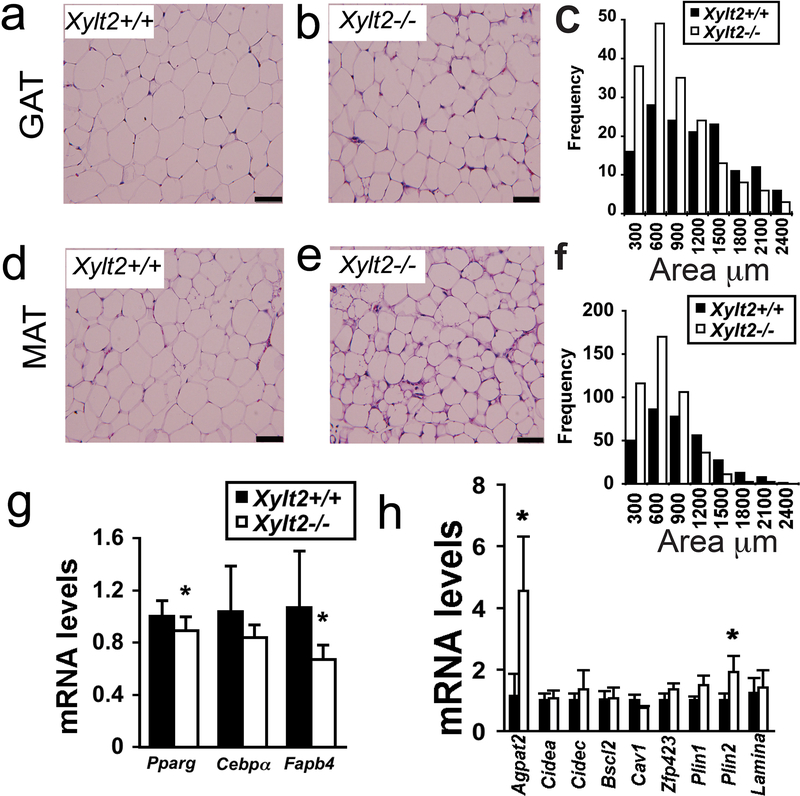
Morphological analyses of adipocyte size in 2–3 month old male Xylt2−/− mice found that the Xylt2−/− adipocytes are abnormal in GAT and MAT niches. Consistently, instead of the typical large single lipid vacuole as seen in the Xylt2+/+ adipocytes, the Xylt2−/− adipocytes possessed either a small single vacuole or multiple small vacuoles (Figure 2a versus b, d versus e). Furthermore, the Xylt2−/− adipocytes were reduced in surface area in both niches (Figure 2c and f). Since these cellular changes are apparent in the younger mice before the obvious body weight differences observed in the 4-month-old animals, defective metabolism adipogenesis, or adipocyte differentiation must be present that prevents maintenance of normal adipocyte size. To investigate the latter, we measured the expression of the adipogenic factors of peroxisomal proliferation activator receptor-γ, CCAAT/enhancer binding protein alpha, and fatty acid binding protein four in the GAT of the 2–3 month-old mice and found that peroxisomal proliferator activating receptor-γ and fatty acid binding protein-4 were significantly decreased in Xylt2−/− adipose (Figure 2g). These changes, although small, are significant and suggest that adipogenesis is decreased in the older Xylt2−/− mice. Since adipogenesis is decreased in many forms of lipodystrophy and to identify candidate factors for the decrease in adipose tissue, we investigated the GAT expression of those loci with mutations known to cause lipodystrophy. The expression profile for most genes were unchanged but the profiles for Agpat2 and Pln2 were significantly increased (Figure 2h). Next, we speculated the decreased white adipose tissue levels would have an impact on glucose metabolism similar to lipodystrophy where increased insulin resistance and glucose intolerance occurs.
Figure 2.
Adipocyte morphology, gene expression, and GAG content with XylT2 deficiency in 2–3 month male mice. Adipocyte morphology was assessed by hematoxylin and eosin staining of GAT. (a) Xylt2+/+ and (b) Xylt2−/− mice followed by (c) adipocyte size distribution measurements, n=4 of each genotype. (d)(e)(f) Similar analyses in MAT, n=4 of each genotype, scale bars = 100μm. (g) GAT expression of adipogenic differentiation factors peroxisomal proliferation activator receptor-γ (Pparg), CCAAT/enhancer binding protein-α (Cebpa), and fatty acid binding protein four (Fabp4) in GAT (n=4 of each genotype). (h) GAT expression analyses of known lipodystrophic genes in Xylt2+/+ and Xylt2−/− mice. * p<0.05.
Glucose intolerance, insulin resistance, and reduced GAT fatty acid metabolism in Xylt2−/− mice
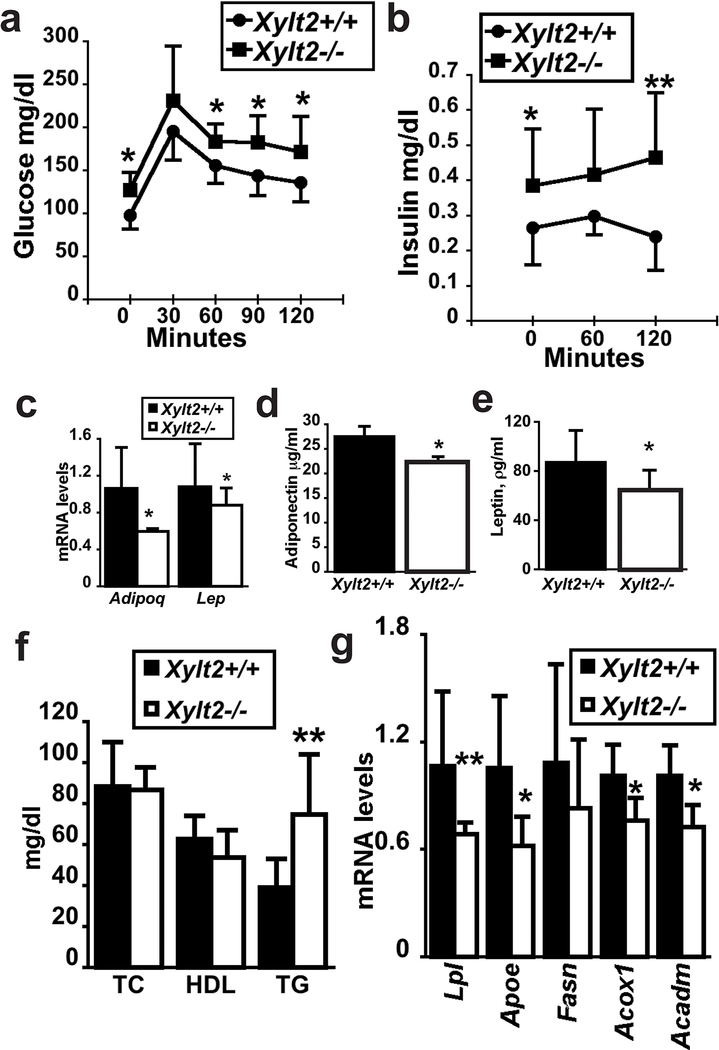
We performed a glucose tolerance test in the Xylt2−/− mice. Interestingly, we found that the Xylt2−/− mice were glucose intolerant throughout the test (Figure 3a). This was further supported by significant increases in insulin levels in these Xylt2−/− mice (Figure 3b). Adipose tissue is a significant source for plasma adiponectin and leptin, and in lipodystrophic patients both these adipokines are decreased16. Therefore, with such a reduction of adipose tissue in the Xyt2−/− mice, we expected decreased expression of these factors in the mice. The analyses confirmed this with reduced GAT mRNA and serum levels for these adipokines in the Xylt2−/− mice (Figure 3c,d,e). Since the significant reduction in adipose tissue suggests the mice may also have a disturbance in lipid metabolism, we measured fasting serum lipid levels, and these assays found unchanged total cholesterol and high-density lipoprotein cholesterol. However, triglyceride levels were nearly 2 times higher in the Xylt2−/− mice (Figure 3f). Furthermore, since fat associated lipoprotein lipase is rate limiting for fatty acid uptake by adipocytes36 and apolipoprotein E is important for receptor-mediated binding and uptake of fatty acid laden VLDL and in transport of free fatty acids by adipocytes37, changes in these factors could indicate reduced fatty acid uptake. We checked GAT expression of these two genes in the 2–3 month mice and found them both to be significantly reduced (Figure 3g) in the Xylt2−/− mice. Fatty acids and the TGs they subsequently become are the major constituents of the lipid droplet in adipocytes. Therefore, these findings prompted us to measure GAT expression levels of genes important in TG metabolism. Measurements of the TG catabolic enzymes medium chain acyl-coenzyme A dehydrogenase and acetyl-Coenzyme A oxidase palmitoyl showed significant (p<0.05) overall reductions. Conversely, fatty acid synthase expression was not significantly different (Figure 3g). Therefore, reduced GAGs decrease the expression of the adipogenic factor PPARγ, and genes important for fatty acid uptake, and fatty acid catabolism. Since the loss of adipose tissue, development of insulin resistance, and development of hypertriglyceridemia in the Xylt2−/− mice are findings similar to those seen in lipodystrophic patients17, we speculated similar to these patients that the adipocyte niche in the mice harbors an inflammatory component.
Figure 3.
Glucose challenge, adipokine secretion, serum lipids, and GAT fatty acid gene expression in XylT2 deficiency. (a) Serum glucose levels of glucose tolerance test (n=7–8, female mice). (b) Serum insulin levels in glucose tolerance test. (c) GAT expression of adiponectin and leptin mRNAs in 3month old male mice (n=7–8, each genotype). Serum levels of (d) adiponectin and (e) leptin in 4–5 month old mice (n=7–8, each genotype). (f) Plasma lipid measurements in 4–5 month mice (n=7–8). TC, serum total cholesterol, HDL, serum high density lipoprotein cholesterol, TG, serum triglycerides. (g) Fatty acid metabolic gene expression in GAT (n=4, each genotype, 2–3 months old). LPL, lipoprotein lipase, APOE, apolipoprotein E, FASN, fatty acid synthase, ACOX1, acetyl-Coenzyme A oxidase palmitoyl, ACADM, medium chain acyl-coenzyme A dehydrogenase. * p<0.05, **p<0.01.
XylT2 Deficiency Increases Adipose Tissue Inflammation
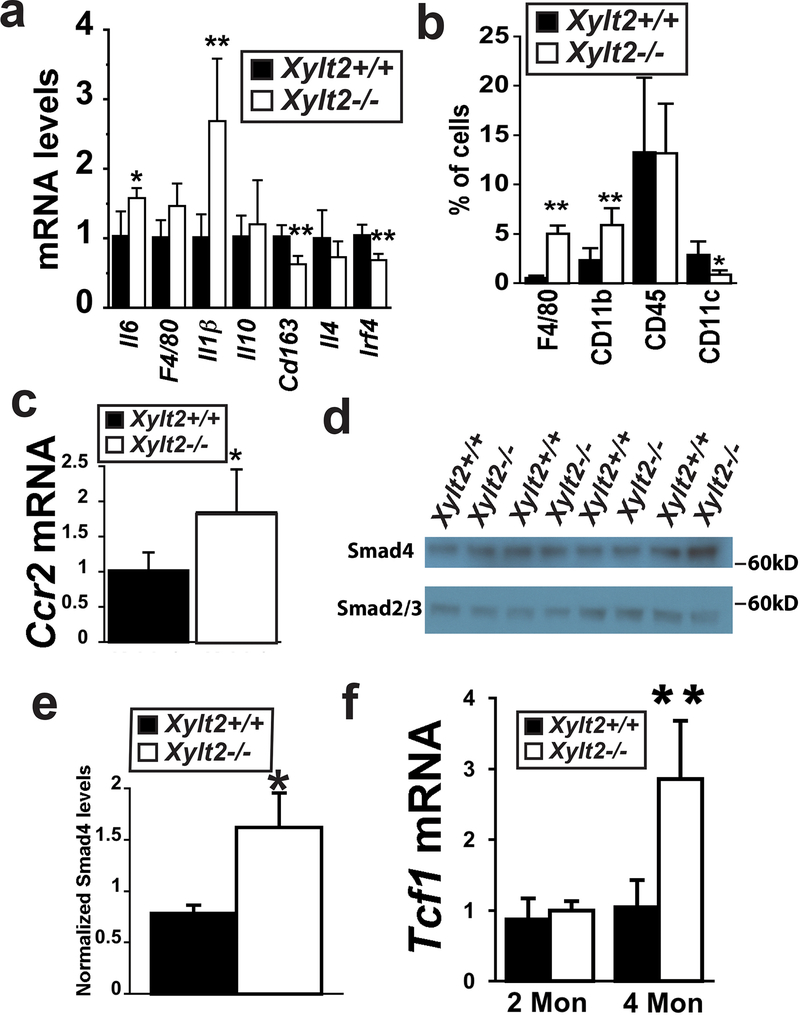
To assess adipose tissue inflammation, we measured GAT expression of the inflammatory markers and cytokines associated with macrophage activation and GAT inflammatory cellular content. We found an upward trend for macrophage marker F4/80, and significant elevations in IL6 and IL1β proinflammatory M1 cytokines (Figure 4a). In contrast, the expression of prorepair M2 macrophage marker Cd163, and cytokine Il4, and interferon regulatory factor 4 were significantly down-regulated (Figure 4a). An increased macrophage infiltrate in the Xylt2−/− GAT was demonstrated with five-fold higher levels of F4/80 positive cells and six-fold higher levels of CD11b positive macrophages in the Xylt2−/− mice (Figure 4b). This macrophage M1 type pro-inflammatory infiltrate could possibly be mediated in part through the cytokine receptor Ccr2, so we investigated its expression in the GAT and found it to be twice as high in the Xylt2−/− mice as in control Xylt2+/+ mice (Figure 4c) thus suggesting a role for Ccl2 in Xylt2−/− adipose tissue inflammation. Since proinflammatory cytokines can reduced adipogenesis and induce development of insulin resistance 38, inflammation is likely key to the adipose tissue loss in the Xylt2−/− mice.
Figure 4.
GAT inflammation, TGFβ−1 activity, and Wnt activity. (a) GAT expression of M1 and M2 markers (n=4 of each genotype, 2–3 months old males). (b) Flow cytometry of GAT inflammatory cells (n=3–4 of each genotype, 3 months old). (c) GAT Ccr2 receptor expression. (d) Western blot analysis of TGFβ signaling proteins, Smad2/3 and Smad4 after immunoprecipitation, and (e) histogram shows band quantitation and normalization to Smad2/3, n=3 of each group, 3 months of age. (f) GAT expression of Wnt induced Tcf1 expression, n=4 of each genotype. * p<0.05, ***p<0.001.
We further investigated for additional negative molecular regulators of adipogenesis in the adipose tissue of the Xylt2−/− mice. TGFβ1 has been shown to inhibit adipogenesis in pre-adipocyte cells of the 3T3-F442A cell line and many other model systems and this inhibition is mediated directly by SMAD3/4 but counter acted by SMAD7 activities39. GAT expression of Tgfβ−1 was increased at 2 and 4 months of age and was matched with a decrease in Smad7 expression (see supplemental figure1). Furthermore, increased TGFβ1 GAT activity in the Xylt2−/− mice was confirmed using immunoblots for active SMAD4 complexed with SMAD2/3 (Figure 4d,e). Therefore, the Xylt2−/− adipose tissue niche is inflammatory and has high TGFβ activity. TGFβ activity has also been shown to inhibit human mesenchymal stem cell adipogenesis through activation of Wnt40, and in vivo Wnt inhibits adipogenesis 41, 42. Consequently we investigated GAT Wnt activity.
XylT2 Deficiency Increases Adipose Tissue Wnt activity
PGs are important regulators of Wnt signaling24 tying together Wnt and PGs with adipogenesis. We investigated the expression of Wnt induced genes in GAT. Although TCF1 in the GAT of Xylt2−/− mice was not significantly up-regulated at two months, it was significantly elevated at 4 months as compared to Xylt2+/+ mice (Figure 4f) whereas LEF1 expression was unchanged (data not shown). In all, these results illustrate that the Xylt2−/− GAT has increases in three major anti-adipogenic pathways of proinflammatory cytokines, TGFβ1, and Wnt. Since there could be a primary defect in adipocyte differentiation, we investigated how well Xylt2−/− adipogenesis occurs.
Xylt2−/− adipose derived stem cells (AdSCs) Show Impaired Adipogenic Differentiation
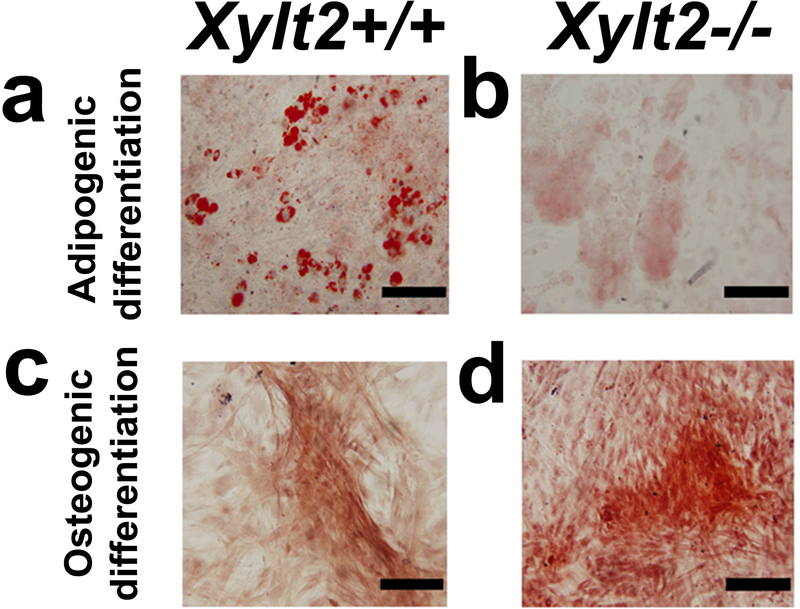
To investigate a primary effect of reduced GAGs on adipogenesis in vitro we isolated and induced GAT AdSCs to undergo adipogenesis in vitro. These stem/progenitor cells located in the adipocytic-vasculature niche are essential to adipose tissue homeostasis especially for the normal adipocytic hyperplasia needed for metabolic homeostasis and during the development of obesity. This cellular population also includes what are typically called mesenchymal stem cells (MSCs). AdSCs from both Xylt2+/+ and Xylt2−/− mice do not have any morphological or growth rate differences (data not shown). FACS analysis of AdSCs from both Xylt2+/+ and Xylt2−/− mice showed both AdSCs isolates were more than 95% positive for typical MSC markers such as Sca1, CD9, CD29, CD44, and CD81 and less than 5% positive for CD11b and CD45. In addition to adipogenesis, to assess overall differentiation potential of the AdSCs, they were challenged to also undergo osteogenic differentiation, and notably, osteogenic differentiation was not reduced. However, with adipogenic induction, the Xylt2−/− AdSCs, as compared to those from the Xylt2+/+ mice, failed to differentiate into equivalent numbers of mature adipocytes (Figure 5b). Although some oil red O positive cells were observed in the Xylt2−/− cultures, the distribution of the intracellular lipid droplets was small and the droplets were dispersed. In contrast, Xylt2+/+ AdSCs promptly differentiated into matured adipocytes with more than 60% of the cells accumulating cytoplasmic large coalesced lipid droplets (Figure 5a). We speculated that regardless of the mesenchymal stem cell niche, the reduced in vitro adipogenesis could be a primary cellular defect caused by reduced GAGs biosynthesis. To investigate this, we isolated bone marrow mesenchymal cells (BMMSC) and challenged them to undergo adipogenesis. Similar to the AdSCs, the MSC marker expression and distribution did not differ between Xylt2+/+ and Xylt2−/− BMMSC (supplement Table 3). Also, similar to the AdSCs, these Xylt2−/− MSCs were similarly defective in adipogenesis. Therefore, regardless of niche, reduced GAGs decrease MSC in vitro adipogenesis. Based on these findings, we hypothesized that the reduced GAGs in the Xylt2−/− mice reduces the number of adipocyte progenitor cells in this niche leading to reduced in vitro adipogenesis. In addition, since adipose tissue vasculature development is also dependent on this population of stem cells43, we thought this niche may also subsequently have reduced angiogenesis.
Figure 5. Xylt2+/+ and Xylt2−/− SVF MSC differentiation.
(a) Representative panels for in vitro adipogenic differentiation of AdSCs from male Xylt2+/+ mice and that of (b) Xylt2−/− mice. (c) Representative figure for in vitro osteogenic differentiation of AdSCs from male Xylt2+/+ mice and (d) that of Xylt2−/− mice. Scale bar is 100μm.
Reduced mature endothelium and increased adipogenic precursors in Xylt2−/− GAT
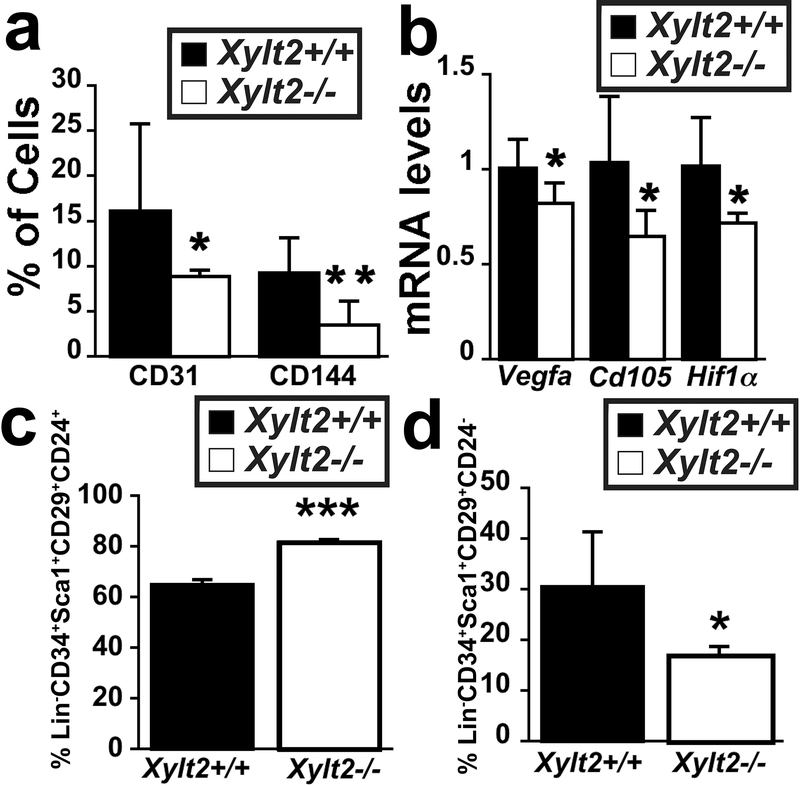
There is close interplay between stromal cells, adipocytes, and blood vessels during adipogenesis where angiogenesis precedes adipogenesis in vivo 43. In addition, endothelial cells and adipocytes may share a common precursor44, 45, and CD34, Sca1, and CD105 positive populations in the adipocyte niche can give rise to committed endothelial and adipocyte progenitor cells 44, 45. Rodeheffer et al., functionally identified the adipocyte precursor within the Lin−CD34+Sca1+CD29+CD24+ population of cells in the SVF46. Furthermore, the SVF is also known to contain endothelial cells and their progenitors for the adipocytic niche9. Given the potential for the ECM to impact all these processes, we investigated for a progenitor defect in the Xylt2−/− mice. Flow cytometry analyses of SVF for the mature endothelial markers of CD31 and CD144 showed significant decreases in the Xylt2−/− GAT (Figure 6a) and GAT expression of endothelial specific genes Vegfa and Cd105 was decreased (Figure 6b) indicating reduced mature endothelium in the Xylt2−/− GAT. Endothelial differentiation depends on stimulation by hypoxia and Hif1 expression47, hence we predicted that these changes might be linked to a lack of HIF1α expression. This was confirmed by a significant decrease in SVF HIF1α expression in the Xylt2−/− mice (Figure 6b). Since there is a close association between vascular structures and adipogenic precursors, we next investigated if this reduced vascularity is also associated with reduce adipogenic precursor numbers. Using FACS we assessed GAT adipocyte precursor levels in the Xylt2−/− GAT, and found, most interestingly, an increase in Lin−CD34+Sca1+CD29+CD24+ cells (Figure 6c) and a decrease in the Lin−CD34+Sca1+CD29+CD24− population (Figure 6d). Therefore, there is a shift in distributions of adipocytic precursor populations in the Xylt2−/− GAT niche as compared to Xylt2+/+ mice.
Figure 6.
GAT vascular and adipocytic progenitor cells. (a) Flow cytometry for endothelial progenitor markers CD144 and CD31 in SVF show significant decreases in the Xylt2−/− mice. (b) GAT gene expression of angiogenic regulators. (c) Flow cytometry of SVF Lin−Sca1+CD34+CD29+CD24+ population. (d) Flow cytometry of SVF Lin−Sca1+CD34+CD29+CD24− population. For all each genotype is n=3–4, 3 months old. * p<0.05, **p<0.01, ***p<0.001.
DISCUSSION
XylT2 is responsible for the initial step in assembly of GAGs onto core proteins during PG biosynthesis. In this study we determine that adipocytic niche homeostasis depends on XylT2 since its deficiency results in an age-associated loss of adipose tissue and subsequent glucose intolerance and insulin resistance similar to lipodystrophic patients48. A defect in adipogenesis leading to lipodystrophy as result of reduced GAGs to our knowledge has never been described before.
Lipodystrophy is a collection of heterologous diseases that ultimately result in complete or partial loss of fat. Although not all causative etiologies are known, most patients with congenital or partial lipodystrophy have loss of function mutations in lipid and triglyceride biosynthetic factors, lipid droplet formation, or in signaling factors important in adipocyte differentiation16, 17, 48. When we looked for candidate lipodystrophic associated loci expression in the Xylt2−/− mice, we did not see any decreases but in contrast did see increases in Agpat2 and Plin2. The explanation for this is not completely clear, but expression of both increases with increasing embryonic age and decreases precipitously in postnatal white adipose tissue 49, 50. Therefore, we speculate that the adipocytes in the Xylt2−/− mice might be arrested in an earlier stage of differentiation that expresses higher levels of Agpat2 and Plin2.
Adipogenesis and maintenance of the adipose tissue niche is thought to proceed through a precursor clonal expansion followed by differentiation 9. Adipogenesis can be inhibited experimentally by increases in Wnt signaling, inflammation, Hedgehog signaling, TGFβ signaling, retinoic acid, and changes in specific master regulatory transcription factors important in the process such as PPARγ (reviewed in51). Inflammation in the expanded adipose tissue niche in insulin resistant and type II diabetic individuals decreases adipogenesis52, 53 by predominately impacting the differentiation of the adipocyte precursors. However, adipose tissue niche inflammation is also an important finding in the reduced adipose tissue environment of lipodystrophy 54,50. Thus, these observations indicate that a balance of adipose tissue levels impacts adipose tissue inflammation with subsequent effects on adipogenesis. The Xylt2−/− adipose tissue niche has inflammation. Furthermore, angiogenesis is critical to adipogenesis 9, 12. Given the role that GAGs have as co-receptors55 and as an ECM source for growth factor ligands15, we speculate that reduced GAGs result in decreased angiogenic growth factor receptor activation reducing adipogenesis in the XylT2 deficient mice. This in conjunction with the increased inflammation, TGFβ, and Wnt signaling all have a negative impact on adipogenesis causing the loss of mature adipocytes in the Xylt2−/− mice through modification of adipocyte progenitor differentiation in vivo. Similarly, loss of GAGs and their effects on ligand-receptor activity are responsible for the significant reduction of in vitro adipogenesis of the Xylt2−/− SVF since the SVF is a heterogeneous mixture of adipocyte precursors, adipocytes, and stromal cells such as fibroblasts, endothelium, and vascular smooth muscle cells, some of which are responsible for secreting adipogenic factors whose activity likely depends on GAG concentrations. This is currently under investigation.
A significant change in adipocyte progenitor populations as a result of XylT2 deficiency demonstrates that decreased GAG levels disrupt progenitor differentiation leading to loss of adipocytic niche homeostasis. In regard to adipogenic precursors, the increase in Lin−CD34+Sca1+CD29+CD24+ and decrease in Lin−CD34+Sca1+CD29+CD24− suggests that the Lin−CD34+Sca1+CD29+CD24− cells are developmentally downstream of the CD24+ population of cells. Lineage maker analyses support this conclusion that the CD24− population does indeed derive from the CD24+ population56. Therefore, loss of GAGs selectively impacts this particular step in adipocyte precursor differentiation.
The exact core proteins involved in this process are unknown. Aikio et. al., showed that selective loss of the PG Col18a1 core protein and its domain with structural identity to Wnt binding frizzle receptors results in increased Wnt10a signaling, increased adipocyte Lin−CD32+Sca1+CD29+CD24+ precursors, and reduced adipocyte size in 4–7 week old mice57. This suggests that one candidate PG for the adipose tissue loss in the Xylt2−/− mice could be Col18a1. However, this is not likely the only mediator given the contrasting observations in these mice compared to those in the Xylt2−/− mice. In the Col18a1 mutant mice as opposed to the Xylt2−/− mice, the Col18a1 GAG sites were intact and there was no progressive change in body weight on a standard diet with age. In fact the only difference in body weight was in 4 week old animals and this difference disappeared once the animals were 7 weeks of age. Similarly, there was a change in adipocyte size morphology in 4 week old animals that disappeared by the time the animals were 7 weeks old. Lastly, there was no change in GAT vasculature 57. These results do suggest changes in Col18a1 disrupt adipocyte differentiation but due exclusively to excessive Wnt signaling and in a very temporal manner. GAT inflammation was not assessed in the Col18a1 mutant mice. So although there could be some overlap in phenotype between the mutant Col18a1 and Xylt2−/− mice, the loss of XylT2 activity is impacting adipose tissue homeostasis in a previously unknown way mediated by, as yet to be identified, core proteins located at the cell surface or in the ECM. In regards to XylT1 dependent GAGs and adipogenesis, early analyses in targeted deletion of the Xylt1 locus found considerable neonatal loss precluding study of adipogenesis (unpublished data).
The endocrine and metabolic impact of adipose tissue is complex. Investigations of adipose tissue’s role in the pathogenesis of diabetes and lipodystrophy are giving great insight into the critical functions this tissue has in these diseases by revealing that adipocyte stem cell expansion and differentiation are critical to adipose tissue homeostasis which greatly impacts metabolism. This current study reveals a novel role of GAGs in adipocyte stem cell differentiation. We show that loss of sulfated GAGs in the adipose tissue niche results in a lipodystrophic condition due to a disruption of a specific transition in adipocyte precursor differentiation thus identifying a role of proteoglycans in adipose tissue homeostasis.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by funds from the Oklahoma Center for Adult Stem Cell Research a program of TSET, NIH Center of Biomedical Research Excellence (COBRE) (P20 GM103648), NIH P20GM103441, P30GM114731.
Oklahoma State University Research Activity funding. We also thank the COBRE Animal Models Core for assistance. We also thank Huan Song for expert technical assistance.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supplementary information is available at the International Journal of Obesity website (http://www.nature.com/ijo).
References
- 1.Huang G, Greenspan DS. ECM roles in the function of metabolic tissues. Trends Endocrinol Metab 2012; 23: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kubo Y, Kaidzu S, Nakajima I, Takenouchi K, Nakamura F. Organization of extracellular matrix components during differentiation of adipocytes in long-term culture. In Vitro Cell Dev Biol Anim 2000; 36: 38–44. [DOI] [PubMed] [Google Scholar]
- 3.Bouloumie A, Sengenes C, Portolan G, Galitzky J, Lafontan M. Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes 2001; 50: 2080–2086. [DOI] [PubMed] [Google Scholar]
- 4.Lijnen HR, Maquoi E, Hansen LB, Van Hoef B, Frederix L, Collen D. Matrix metalloproteinase inhibition impairs adipose tissue development in mice. Arterioscler Thromb Vasc Biol 2002; 22: 374–379. [DOI] [PubMed] [Google Scholar]
- 5.Nakajima I, Muroya S, Tanabe R, Chikuni K. Positive effect of collagen V and VI on triglyceride accumulation during differentiation in cultures of bovine intramuscular adipocytes. Differentiation 2002; 70: 84–91. [DOI] [PubMed] [Google Scholar]
- 6.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, et al. Metabolic Dysregulation and Adipose Tissue Fibrosis: Role of Collagen VI. Molecular and Cellular Biology 2009; 29: 1575–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiegelman BM, Ginty CA. Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell 1983; 35: 657–666. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, DeYoung SM, Zhang M, Zhang M, Cheng A, Saltiel AR. Changes in integrin expression during adipocyte differentiation. Cell Metab 2005; 2: 165–177. [DOI] [PubMed] [Google Scholar]
- 9.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, et al. White fat progenitor cells reside in the adipose vasculature. Science 2008; 322: 583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Church CD, Berry R, Rodeheffer MS. Isolation and study of adipocyte precursors. Methods Enzymol 2014; 537: 31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lv XJ, Zhou GD, Liu Y, Liu X, Chen JN, Luo XS, et al. In vitro proliferation and differentiation of adipose-derived stem cells isolated using anti-CD105 magnetic beads. Int J Mol Med 2012; 30: 826–834. [DOI] [PubMed] [Google Scholar]
- 12.Cao Y Angiogenesis modulates adipogenesis and obesity. The Journal of Clinical Investigation 2007; 117: 2362–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, et al. Paracrine Regulation of Angiogenesis and Adipocyte Differentiation During In Vivo Adipogenesis. Circulation Research 2003; 93: e88–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnan L, Hoying JB, Nguyen H, Song H, Weiss JA. Interaction of angiogenic microvessels with the extracellular matrix 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iozzo RV, San Antonio JD. Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. The Journal of Clinical Investigation 2001; 108: 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussain I, Garg A. Lipodystrophy Syndromes. Endocrinol Metab Clin North Am 2016; 45: 783–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nolis T Exploring the pathophysiology behind the more common genetic and acquired lipodystrophies. J Hum Genet 2014; 59: 16–23. [DOI] [PubMed] [Google Scholar]
- 18.Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol 2017. [DOI] [PubMed] [Google Scholar]
- 19.O’Reilly PJ, Hardison MT, Jackson PL, Xu X, Snelgrove RJ, Gaggar A, et al. Neutrophils contain prolyl endopeptidase and generate the chemotactic peptide, PGP, from collagen. J Neuroimmunol 2009; 217: 51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest 2005; 115: 2223–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merline R, Moreth K, Beckmann J, Nastase MV, Zeng-Brouwers J, Tralhao JG, et al. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and MicroRNA-21. Sci Signal 2011; 4: ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol 2002; 168: 5233–5239. [DOI] [PubMed] [Google Scholar]
- 23.Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J 2006; 20: 9–22. [DOI] [PubMed] [Google Scholar]
- 24.Lin X Functions of heparan sulfate proteoglycans in cell signaling during development. Development 2004; 131: 6009–6021. [DOI] [PubMed] [Google Scholar]
- 25.Esko JD, Kimata K, and Lindahl U Proteoglycans and Sulfated Glycosaminoglycans Essentials of glycobiology. 2nd ed Cold Spring Harbor Laboratory Press: Cold Spring Harbor, N.Y: 2009:xxix, 784 p. [PubMed] [Google Scholar]
- 26.Cuellar K, Chuong H, Hubbell SM, Hinsdale ME. Biosynthesis of Chondroitin and Heparan Sulfate in Chinese Hamster Ovary Cells Depends on Xylosyltransferase II. Journal of Biological Chemistry 2007; 282: 5195–5200. [DOI] [PubMed] [Google Scholar]
- 27.Condac E, Silasi-Mansat R, Kosanke S, Schoeb T, Towner R, Lupu F, et al. Polycystic disease caused by deficiency in xylosyltransferase 2, an initiating enzyme of glycosaminoglycan biosynthesis. Proc Natl Acad Sci U S A 2007; 104: 9416–9421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gotting C, Kuhn J, Zahn R, Brinkmann T, Kleesiek K. Molecular cloning and expression of human UDP-d-Xylose:proteoglycan core protein beta-d-xylosyltransferase and its first isoform XT-II. J Mol Biol 2000; 304: 517–528. [DOI] [PubMed] [Google Scholar]
- 29.Roch C, Kuhn J, Kleesiek K, Gotting C. Differences in gene expression of human xylosyltransferases and determination of acceptor specificities for various proteoglycans. Biochem Biophys Res Commun 2010; 391: 685–691. [DOI] [PubMed] [Google Scholar]
- 30.Bolton K, Segal D, McMillan J, Jowett J, Heilbronn L, Abberton K, et al. Decorin is a secreted protein associated with obesity and type 2 diabetes. Int J Obes (Lond) 2008; 32: 1113–1121. [DOI] [PubMed] [Google Scholar]
- 31.Bolton K, Segal D, Walder K. The small leucine-rich proteoglycan, biglycan, is highly expressed in adipose tissue of Psammomys obesus and is associated with obesity and type 2 diabetes. Biologics 2012; 6: 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 2007; 117: 2621–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc 2009; 4: 102–106. [DOI] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 35.Liu D, Wang CJ, Judge DP, Halushka MK, Ni J, Habashi JP, et al. A Pkd1-Fbn1 genetic interaction implicates TGF-beta signaling in the pathogenesis of vascular complications in autosomal dominant polycystic kidney disease. Journal of the American Society of Nephrology 2014; 25: 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldberg IJ, Eckel RH, Abumrad NA. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. Journal of Lipid Research 2009; 50: S86–S90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang ZH, Minshall RD, Mazzone T. Mechanism for endogenously expressed ApoE modulation of adipocyte very low density lipoprotein metabolism: role in endocytic and lipase-mediated metabolic pathways. J Biol Chem 2009; 284: 31512–31522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc 2001; 60: 349–356. [DOI] [PubMed] [Google Scholar]
- 39.Zamani N, Brown CW. Emerging roles for the transforming growth factor-{beta} superfamily in regulating adiposity and energy expenditure. Endocr Rev 2011; 32: 387–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou S, Eid K, Glowacki J. Cooperation between TGF-beta and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J Bone Miner Res 2004; 19: 463–470. [DOI] [PubMed] [Google Scholar]
- 41.Longo KA, Wright WS, Kang S, Gerin I, Chiang SH, Lucas PC, et al. Wnt10b inhibits development of white and brown adipose tissues. J Biol Chem 2004; 279: 35503–35509. [DOI] [PubMed] [Google Scholar]
- 42.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, et al. Inhibition of Adipogenesis by Wnt Signaling. Science 2000; 289: 950–953. [DOI] [PubMed] [Google Scholar]
- 43.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes 2007; 56: 1517–1526. [DOI] [PubMed] [Google Scholar]
- 44.Hager G, Holnthoner W, Wolbank S, Husa AM, Godthardt K, Redl H, et al. Three specific antigens to isolate endothelial progenitor cells from human liposuction material. Cytotherapy 2013; 15: 1426–1435. [DOI] [PubMed] [Google Scholar]
- 45.Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 2004; 109: 656–663. [DOI] [PubMed] [Google Scholar]
- 46.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell 2008; 135: 240–249. [DOI] [PubMed] [Google Scholar]
- 47.Lee SW, Jeong HK, Lee JY, Yang J, Lee EJ, Kim SY, et al. Hypoxic priming of mESCs accelerates vascular-lineage differentiation through HIF1-mediated inverse regulation of Oct4 and VEGF. EMBO Mol Med 2012; 4: 924–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fiorenza CG, Chou SH, Mantzoros CS. Lipodystrophy: pathophysiology and advances in treatment. Nat Rev Endocrinol 2011; 7: 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Birsoy K, Berry R, Wang T, Ceyhan O, Tavazoie S, Friedman JM, et al. Analysis of gene networks in white adipose tissue development reveals a role for ETS2 in adipogenesis. Development 2011; 138: 4709–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cautivo KM, Lizama CO, Tapia PJ, Agarwal AK, Garg A, Horton JD, et al. AGPAT2 is essential for postnatal development and maintenance of white and brown adipose tissue. Mol Metab 2016; 5: 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berry DC, Stenesen D, Zeve D, Graff JM. The developmental origins of adipose tissue. Development 2013; 140: 3939–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dubois SG, Heilbronn LK, Smith SR, Albu JB, Kelley DE, Ravussin E, et al. Decreased expression of adipogenic genes in obese subjects with type 2 diabetes. Obesity (Silver Spring) 2006; 14: 1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang X, Jansson PA, Nagaev I, Jack MM, Carvalho E, Sunnerhagen KS, et al. Evidence of impaired adipogenesis in insulin resistance. Biochem Biophys Res Commun 2004; 317: 1045–1051. [DOI] [PubMed] [Google Scholar]
- 54.Herrero L, Shapiro H, Nayer A, Lee J, Shoelson SE. Inflammation and adipose tissue macrophages in lipodystrophic mice. Proceedings of the National Academy of Sciences 2010; 107: 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teran M, Nugent MA. Synergistic Binding of Vascular Endothelial Growth Factor-A and Its Receptors to Heparin Selectively Modulates Complex Affinity. J Biol Chem 2015; 290: 16451–16462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol 2013; 15: 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aikio M, Elamaa H, Vicente D, Izzi V, Kaur I, Seppinen L, et al. Specific collagen XVIII isoforms promote adipose tissue accrual via mechanisms determining adipocyte number and affect fat deposition. Proc Natl Acad Sci U S A 2014; 111: E3043–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.