Mental disorders often involve difficulty with complex cognitive functions, such as decision making, emotional self-regulation, or behavioral adaptation to changing circumstances. Those functions arise from the carefully timed and orchestrated synchrony of activity across multiple structures1–in other words, from network activity. It should therefore be possible to treat mental disorders by identifying abnormalities in that network activity and restoring them to healthy patterns.
We should be able to restore that healthy network activity through brain stimulation. In some sense, this is what evidence-based psychotherapies do, although they act indirectly and depend on the patient having sufficient self-regulatory capacity to achieve the desired network change. Thus far, both invasive2 and noninvasive3 neuromodulation trials have yielded inconsistent results. We argue that this inconsistency occurs because existing neuromodulation therapies are not sufficiently specific (Figure, A). They have spatial specificity, delivering energy to specific parts of the brain, but they lack temporal specificity, failing to deliver that energy in tune with the brain’s endogenous activity.
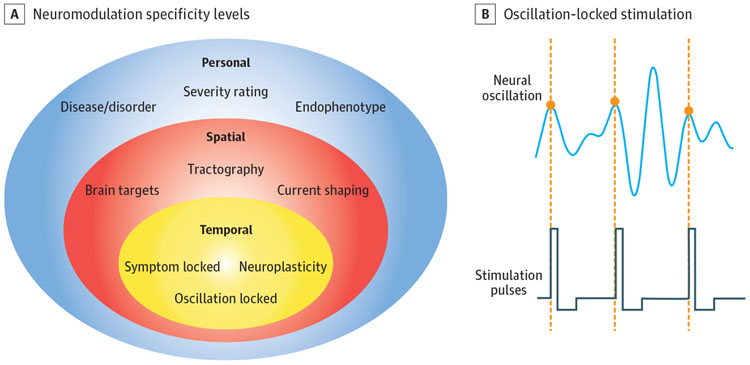
Figure. Levels of Specificity and Methods for Enhancing the Specificity of Neurostimulation.
A, Conceptual diagram of neuromodulation specificity levels and methods/concepts for implementation. Personal specificity involves delivering neuromodulation to the appropriate patients: those with a specific diagnosis, level of severity, or phenotype (eg, the exclusion of patients with rapid-cycling bipolar disorder from trials in which mania is a risk). Spatial specificity refines brain targets within an individual, moving beyond average stereotactic coordinates to a precise application of energy to key brain structures. Temporal specificity extends this application to stimulate targets only at key moments, such as times when symptoms are high or when neural activity is especially vulnerable to change. B, Conceptual diagram of oscillation-locked stimulation, one variant of temporal specificity. A neural oscillation shows peaks and troughs (phases) at a defined frequency. Advanced modeling may permit stimulation pulses to be delivered only at specific phases of the oscillation, acting in tune with changes in tissue excitability.
Large populations—sometimes millions—of neurons often fire together rhythmically. This coordinated activity generates electrical oscillations at frequencies ranging from less than 1 Hz to greater than 100 Hz. These oscillations can be detected in local field potentials or electroencephalographic recordings inside and outside the skull. Mounting evidence suggests that these oscillations play a role in neural communication. When 2 populations of neurons are phase synchronized (ie, in lock step), they can influence each other because they are in an excited state at the same time. Conversely, if neurons are out of sync or anticorrelated, their communication will be weakened.
Different aspects of cognition appear to be encoded in different oscillatory frequencies.1 For example, bottom-up processing of incoming sensory information generates bursts of gamma (>30 Hz) activity throughout the cortex. In contrast, top-down processing, used to redirect attention when certain sensory inputs need to be suppressed or ignored, generates alpha (8-15 Hz) or beta (15-30 Hz) activity. These frequencies seem to suppress gamma activity and filter out bottom-up inputs. Gamma activity may also be necessary for ordering thought; it may keep items held “in mind” separate and in sequence by coding them on different phases of the gamma cycle.4 In a sense, these frequencies can be thought of as individual “radio stations,” each carrying a different type of information.
These same oscillatory dynamics are potentially disrupted in patients with mental illness. For instance, overattention to bottom-up sensory inputs in attention-deficit/hyperactivity disorder may reflect overly strong bottom-up gamma activity. In contrast, gamma rhythms are frequently diminished in schizophrenia,5 potentially reflecting a failure of bottom-up information gating into cognitive awareness. In another example, the slower theta (5-8 Hz) rhythm appears necessary for prefrontal influence over limbic structures, such as the amygdala. In both rodents and primates, successful top-down or self-regulatory behavior requires synchronization between prefrontal and limbic system theta rhythms.6
Neurostimulation might normalize these oscillatory relationships and, in doing so, restore normal neural communication. We posit that normalization would be best achieved by applying stimulation with precise timing relative to the underlying brain oscillations (Figure, B). Stimulation in phase with a rhythm can amplify it, whereas stimulation not in phase can cancel or attenuate it. In contrast, stimulation not locked to an oscillation may be ineffective, like throwing a part into an engine and hoping it lands in the correct place. Methods that modulate overall excitability, such as direct current stimulation, may be able to amplify an existing strong oscillation. Such methods might, however, be more useful as research tools in healthy individuals because patients with mental illness may not have the substrate necessary for amplification.
Failure to consider oscillatory dynamics may explain the heterogeneous outcomes of recent trials; stimulation may have matched brain activity in only a fraction of patients. In contrast, specific efforts have been made in Parkinson disease to develop phase-related stimulation in the subthalamic nucleus. Stimulating in phase with a tremor oscillation was reported to control symptoms and use half the energy of standard clinical stimulation. A “coordinated reset” approach based on oscillatory cycles relieved tremor for hours after the stimulation ended.7 Psychiatric illnesses might also be susceptible to this phase-based disruption, albeit through the use of a more complex approach. The networks underlying mental illness are more distributed than the basal ganglia loops of Parkinson disease and may require recording and/or modulation at multiple sites simultaneously.8
One challenge to implementing in-phase stimulation is that ongoing brain activity needs to be analyzed rapidly so that corresponding stimulation can be generated on the fly. Many existing systems have lags of 100 ms or more, which limit their use to slower frequencies. Advanced signal processing can compensate for this lag, allowing precise phase locking to theta (5-8 Hz) oscillations.8 Such approaches may be powerful methods for altering brain circuit communication. Another challenge is that clinical neurostimulators produce large artifacts that overwhelm delicate recording equipment. However, neural recording amplifiers can be designed to interoperate with stimulation circuits, allowing removal of artifacts. Those systems have already been used in movement disorders, revealing pathologic oscillations that were then targeted with phase-locked stimulation.9 The Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) Initiative is funding studies regarding the use of these sensing neurostimulators in depression and obsessive-compulsive disorder (eg, NCT03184454, NCT03457675, and NCT01984710).
If those studies are able to show us how stimulation can interact with and change circuit oscillations, they may aid the design of next-generation, physiologically informed psychiatric neurostimulation. Consider the examples described above. Stimulating the amygdala and prefrontal cortex in tune with their theta oscillations may improve the fear regulation processes that are deficient in posttraumatic stress disorder and some anxiety disorders. Transcranial stimulation locked to the prefrontal gamma oscillation might amplify gamma activity, remediating cognitive deficits in schizophrenia.10Advances in phase-locked stimulation might also address the problem of canceling pathologic oscillations, which appears substantially more difficult than amplifying an existing signal. Further development of these technologies combined with our growing understanding of the neural basis of mental disorders has the potential to greatly expand the reach and effectiveness of neurostimulation.
Footnotes
Conflict of Interest Disclosures: Dr Widge reported receiving grants from the National Institutes of Health during the conduct of the study, receiving personal fees and nonfinancial support from Medtronic outside the submitted work, and having patents pending for applications of deep brain stimulation and neural oscillations and for applications of neural oscillations for cognitive remediation. Dr Miller reported having patents pending in applications of neural oscillations for cognitive remediation.
Contributor Information
Alik S. Widge, Department of Psychiatry, University of Minnesota, Minneapolis..
Earl K. Miller, The Picower Institute for Learning and Memory, Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology, Cambridge..
REFERENCES
- 1.Miller EK, Lundqvist M, Bastos AM. Working Memory 2.0. Neuron. 2018;100(2):463–475. doi: 10.1016/j.neuron.2018.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Widge AS, Malone DAJ Jr, Dougherty DD. Closing the loop on deep brain stimulation for treatment-resistant depression. Front Neurosci. 2018;12:175. doi: 10.3389/fnins.2018.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Philip NS, Nelson BG, Frohlich F, Lim KO, Widge AS, Carpenter LL. Low-intensity transcranial current stimulation in psychiatry. Am J Psychiatry. 2017;174 (7):628–639. doi: 10.1176/appi.ajp.2017.16090996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel M,Warden MR,Miller EK. Phase-dependent neuronal coding of objects in short-term memory. Proc Natl Acad Sci U S A. 2009; 106(50):21341–21346. doi: 10.1073/pnas.0908193106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathalon DH, Sohal VS. Neural oscillations and synchrony in brain dysfunction and neuropsychiatric disorders. JAMA Psychiatry. 2015; 72(8):840–844. doi: 10.1001/jamapsychiatry.2015.0483 [DOI] [PubMed] [Google Scholar]
- 6.Likhtik E, Paz R. Amygdala-prefrontal interactions in (mal)adaptive learning. Trends Neurosci. 2015;38(3):158–166. doi: 10.1016/j.tins.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meidahl AC, Tinkhauser G, Herz DM, Cagnan H, Debarros J, Brown P. Adaptive deep brain stimulation for movement disorders. Mov Disord. 2017;32(6):810–819. doi: 10.1002/mds.27022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackwood E, Lo M-C, Widge AS. Continuous phase estimation for phase-locked neural stimulation using an autoregressive model for signal prediction. In: 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) Piscataway, NJ: IEEE; 2018: 4736–4739. doi: 10.1109/EMBC.2018.8513232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramirez-Zamora A, Giordano JJ, Gunduz A, et al. Evolving applications, technological challenges and future opportunities in neuromodulation. Front Neurosci. 2018;11:734. doi: 10.3389/fnins.2017.00734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoy KE, Bailey NW, Arnold SL, Fitzgerald PB. The effect of transcranial direct current stimulation on gamma activity and working memory in schizophrenia. Psychiatry Res. 2015;228(2):191–196. doi: 10.1016/j.psychres.2015.04.032 [DOI] [PubMed] [Google Scholar]