The continuous increase in long-distance travel and recent large migratory movements have changed the epidemiological characteristics of imported malaria in countries where malaria is not endemic (here termed non-malaria-endemic countries). While malaria was primarily imported to nonendemic countries by returning travelers, the proportion of immigrants from malaria-endemic regions and travelers visiting friends and relatives (VFRs) in malaria-endemic countries has continued to increase. VFRs and immigrants from malaria-endemic countries now make up the majority of malaria patients in many nonendemic countries.
KEYWORDS: Europe, VFR, clinical characteristics, diagnosis, imported, malaria, nonimmune, prophylaxis, semi-immune, treatment
SUMMARY
The continuous increase in long-distance travel and recent large migratory movements have changed the epidemiological characteristics of imported malaria in countries where malaria is not endemic (here termed non-malaria-endemic countries). While malaria was primarily imported to nonendemic countries by returning travelers, the proportion of immigrants from malaria-endemic regions and travelers visiting friends and relatives (VFRs) in malaria-endemic countries has continued to increase. VFRs and immigrants from malaria-endemic countries now make up the majority of malaria patients in many nonendemic countries. Importantly, this group is characterized by various degrees of semi-immunity to malaria, resulting from repeated exposure to infection and a gradual decline of protection as a result of prolonged residence in non-malaria-endemic regions. Most studies indicate an effect of naturally acquired immunity in VFRs, leading to differences in the parasitological features, clinical manifestation, and odds for severe malaria and clinical complications between immune VFRs and nonimmune returning travelers. There are no valid data indicating evidence for differing algorithms for chemoprophylaxis or antimalarial treatment in semi-immune versus nonimmune malaria patients. So far, no robust biomarkers exist that properly reflect anti-parasite or clinical immunity. Until they are found, researchers should rigorously stratify their study results using surrogate markers, such as duration of time spent outside a malaria-endemic country.
INTRODUCTION
Malaria is a common disease, endemic to tropical and subtropical regions (1). In regions where malaria is not endemic (here termed nonendemic regions), malaria is an imported disease, acquired by exposure to Plasmodium parasites in endemic regions during travel undertaken for professional or tourism-related reasons (2, 3). Historically, malaria was mainly imported to nonendemic countries by citizens of that country. However, this pattern has significantly changed over the last few decades (2, 4). Currently, migrants from endemic countries constitute a high proportion of imported malaria cases in nonendemic countries (4). Furthermore, migrants originating from endemic areas who have permanently settled in a nonendemic country make up an important group of travelers, called “visiting friends and relatives” (VFRs), to their countries of origin (4–7). These groups of malaria patients may be different from classical travel-related malaria patients based on differences in risk perception, preexisting semi-immunity against malaria, adherence to recommendations for prophylaxis against malaria, and clinical presentation, diagnosis, and management of malaria. Whereas some of the above-cited aspects of malaria patients in nonendemic regions have been explored in epidemiological research, there is a lack of a comprehensive perspective on the similarities and potential differences of malaria in nonimmune versus semi-immune travelers. Therefore, the aim of this review is to provide a summary of available evidence on clinically relevant peculiarities of these patient populations.
EPIDEMIOLOGY OF IMPORTED MALARIA
Global Epidemiology
Between 2010 and 2015, malaria decreased from 237 million cases globally to 211 million and then rose to 219 million again in 2017 (1). The vast majority of cases in 2017 were reported by the WHO African Region (92%), followed by 5% in the WHO South-East Asia Region and 2% in the WHO Eastern Mediterranean Region. Among the 87 states reporting autochthonous cases of malaria in 2017, 15 states, 14 from sub-Saharan Africa and India, sustained 80% of the worldwide malaria burden (1). The largest decrease in malaria incidence was observed in the WHO South-East Asia Region (48%), followed by the WHO Region of the Americas (22%) and the WHO African Region (20%). Plasmodium falciparum was the most prevalent parasite species in sub-Saharan Africa, the WHO Western Pacific Region, the WHO Eastern Mediterranean region, and the WHO South-East Asia Region, where it was responsible for 99%, 72%, 69%, and 63% of malaria cases in 2017, respectively. In the WHO Region of the Americas, P. vivax is the most prevalent parasite species, accounting for 74% of malaria cases in 2017 (1). However, despite the global decline of malaria since 2010, the global number of cases per 1,000 inhabitants at risk has remained largely constant for the past 3 years (1).
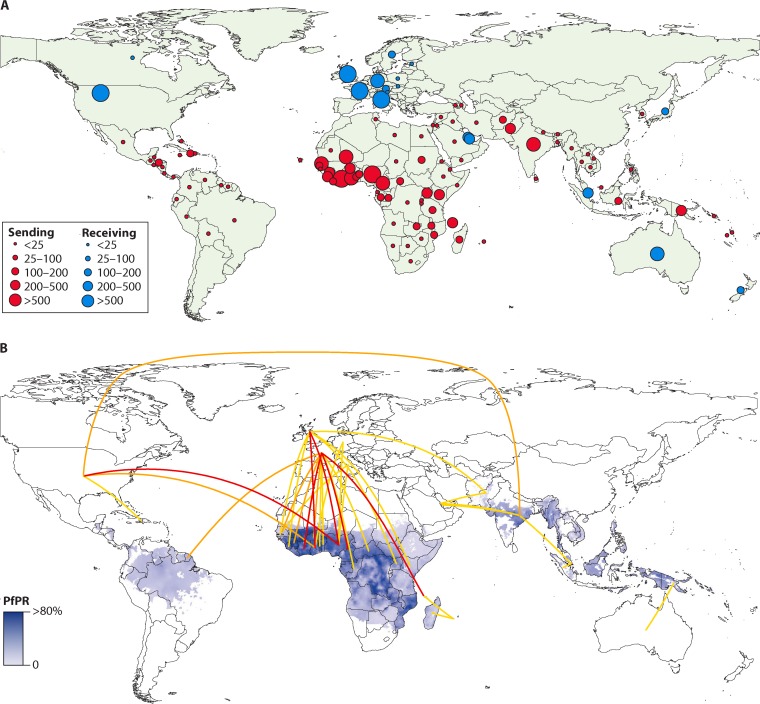
A specific definition of imported malaria is used throughout this work, denoting that infection is acquired in a malaria-endemic area and that clinical manifestation, diagnosis, and management take place in a nonendemic country. In 2017, a meta-analysis was published that included official country-level data on imported malaria between 2005 and 2015 (8, 9). The results demonstrated that European countries carried the highest global burden of imported malaria, measured in absolute case numbers per year and in cases of imported malaria relative to the population size of the nonendemic country (Fig. 1). France carried the highest average number of cases per year, reporting 2,169 cases, followed by the United Kingdom (1,898 cases), Italy (637 cases), Germany (401 cases), Spain (374 cases), The Netherlands (366 cases), Belgium (227 cases), and Switzerland (225 cases). Other nonendemic countries, outside Europe, with significant average numbers of annual cases, are the United States (1,511 cases), Australia (222 cases), Bahrain (158 cases), Singapore (148 cases), and Qatar (146 cases). On the other hand, nonendemic countries, such as Canada, Japan, and New Zealand, as well as the city of Hong Kong, have reported a lower average number of cases per year, 20, 45, 44, and 40, respectively. Special mention should be given to China, as the country may have been misrepresented in previous studies on imported malaria, because it is not yet considered a malaria-free country despite the recent interruption of transmission (8, 10). It was in 2017 that China reported zero indigenous cases, and the disease is on course for elimination by 2020 (1). However, imported malaria in China has probably been and may still be underreported. Imported malaria in China is increasing, with growing Chinese overseas investment and international travel to tropical settings (11, 12). Most described cases of imported malaria in China had a self-reported work-related travel history (94%) and were of male sex (88%); 90% of them acquired falciparum malaria in Africa, and 77% acquired vivax malaria in Asia (12). These imported cases present a new challenge to malaria elimination in China, highlighting the importance of prevention and control of cases of imported malaria (11, 13–16).
FIG 1.
Number and movement of imported and exported cases of malaria between 2005 and 2015. (A) Average annual number of malaria cases (all species) exported from endemic to nonendemic countries (red) and imported to nonendemic from endemic countries (blue). (B) P. falciparum prevalence overlaid with flow lines showing country connectivity by average annual flows of >50 (>200 [red], 100 to 200 [orange], and 50 to 100 [yellow]) cases. (Reproduced from reference 8.)
As indicated earlier, official country-level data indicate that Europe carries approximately 70% of the global burden of imported malaria, followed by the United States, with approximately 15% of the global burden (8). This is explained by historical, language, and travel ties (8). Therefore, the majority of literature published to date on imported malaria comes from Europe; this is particularly true for papers in the field of clinical research (1, 8).
The malaria program of the WHO European Region compiles data on malaria cases from 51 states of the region on a yearly basis. Between 1972 and 1988, the annual case count of imported malaria rose from 1,500 to 12,000, constituting an 8-fold increase, and in the year 2000, 15,500 cases were reported, the majority by Western European countries; France, the United Kingdom, and Germany accounted for more than 70% of total cases. P. falciparum is responsible for the majority of cases of imported malaria and is mostly acquired during travel to sub-Saharan Africa, particularly to West Africa (1, 8). With regard to P. vivax, large proportions of imported infections are acquired in India and Pakistan. This reflects the high burden of P. vivax malaria in South Asia and the increased travel of immigrants between these regions and Europe (17). The incidence rates of imported malaria attributable to P. ovale and P. malaria and mixed infections are comparable to the incidence of infection in West Africa; it is argued that this reflects the high proportion of immigrants from these regions (2, 6, 8, 18, 19).
In the United States, a similar picture has been described by the Centers for Disease Control and Prevention. Between 1974 and 2014, the number of malaria cases diagnosed in the United States has been steadily rising. Between 2014 and 2015, a decrease of malaria cases diagnosed was observed, which was explained by a decrease in international travel, potentially associated with the Ebola outbreak (20). This was followed by an increase in cases in 2016 (21). In 2016, malaria was mainly acquired in Africa (91%), with West Africa accounting for the majority of cases. The remaining cases were imported from the Caribbean, South America, Oceania, and the Middle East. Of the cases in 2016, 58.5% were U.S. civilians, 28% were non-U.S. residents, and 11.5% of individuals had unknown resident status; only 2% of cases were members of the U.S. military (21). Like the situation in Europe, most imported cases in the United States were due to P. falciparum (77%), followed by P. vivax (14%), P. ovale (5%), P. malariae (3%), and mixed infections (1%).
The percentage of imported malaria attributable to immigrant populations has risen significantly. In Europe, this percentage increased from 14% to 86% throughout the last decade (4). Immigrants represented about 43% of cases of imported malaria reported by European tertiary centers (4, 22). Furthermore, settled immigrants from malaria-endemic countries who travel to visit friends and relatives in their countries of origin constituted a high proportion of imported malaria cases. Those VFRs comprise about 70% of cases in some European settings and constitute the main risk group for imported malaria in Europe (18, 23). This is particularly true for France and the UK, where the highest risk for imported malaria is carried by West African VFRs (5, 6). Imported malaria in France accounts for 50% of all imported malaria in Europe, and trends show a rise in the proportion of malaria patients with origins in sub-Saharan Africa who live in France and visit family and friends in their country of origin (7). Similar reports come from the United States, indicating that VFRs are among those most affected by imported malaria, which was acquired mostly in West Africa (24, 25). Such findings are reflected by the intense malaria transmission in sub-Saharan African countries and patterns of travel between Africa and nonendemic settings (1, 8, 26).
Several factors are assumed to be responsible for the higher risk for acquiring malaria in VFRs. First, they travel to high-risk destinations for longer periods of time. Second, they have a lack of risk awareness due to previous residence in a malaria-endemic area (27). Third, VFRs often do not seek pretravel advice, precluding prophylactic medication. Factors for not accessing pretravel care may be related to cultural or language barriers and a different risk perception when traveling in tropical regions (28, 29).
Apart from West African VFRs, migrants from the Horn of Africa, specifically Eritrea, have changed the profile of imported malaria in various European states, such as Germany, Sweden, and Switzerland, in recent years (30–32). Since 2013, the arrival of Eritrean immigrants and asylum seekers to Europe has caused a shift toward increasing numbers of Plasmodium vivax malaria cases (31). Prior to 2013, P. falciparum was the most prevalent Plasmodium species imported to Europe, primarily due to VFRs and tourists returning from sub-Saharan Africa (27).
A recent study on imported malaria in pregnant women was conducted in Europe, Japan, and the United States. Six hundred thirty-one pregnant women with imported malaria were included, and it was demonstrated that pregnant VFRs were the main at-risk population (58%). West Africa was the most common origin of malaria infections in this population (33).
ACQUISITION OF SEMI-IMMUNITY TO MALARIA
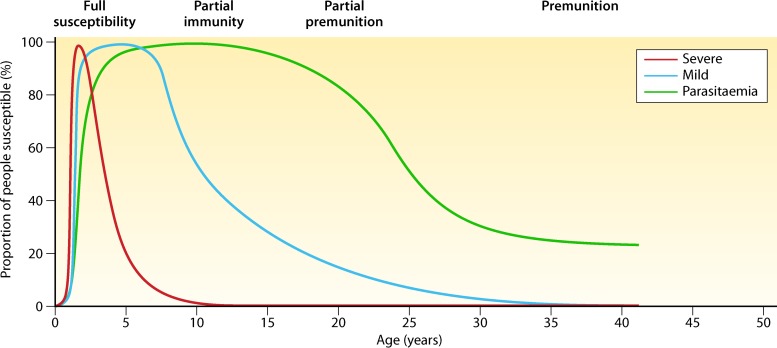
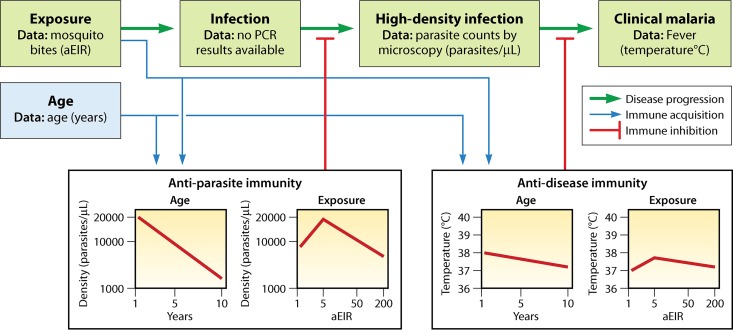
It is a well-known phenomenon that individuals residing in malaria-endemic regions progressively acquire a certain degree of immunity against clinical complications and high parasitemia. However, immunity to malaria cannot be considered a sterile immunity, as it does not prevent infection by the parasite but rather prevents clinical manifestation and complications of disease (i.e., anti-disease immunity or clinical immunity). Anti-parasite immunity is the ability to control parasite density upon infection. Because immunity against malaria is not sterile, it is referred to as a semi-immune state (34–36). It is believed that both anti-parasite and anti-disease immunity develop gradually and in parallel, but anti-parasite immunity does take longer to develop (37, 38) (Fig. 2). The acquisition of semi-immunity against malaria is of clinical importance, as it may affect the clinical presentation, associated performance of diagnostic tools, and ultimately the clinical management. Therefore, the following sections of this review focus on the development of semi-immunity against malaria in respective patient populations and its durability and natural decline in the absence of exposure to malaria.
FIG 2.
Relationship between age and malaria severity. Protection from malaria is acquired gradually with repeated exposure, first against severe disease and then against clinical symptoms of disease, and at a lower rate against high levels of parasitemia. Premunition means protection from illness but not from infection. (Adapted from reference 38 with permission from Elsevier.)
Acquisition of Naturally Acquired Immunity to Malaria
Naturally acquired immunity (NAI) to malaria is usually developed by individuals living in a malaria-endemic area and results from being subjected to the parasite repeatedly over several years. The acquisition of immunity is slow and requires repeated inoculations of the Plasmodium parasite by the Anopheles mosquito vector. Moreover, protection from disease appears to wane in the absence of continuous exposure. The rate of acquisition of naturally acquired immunity predominantly depends on transmission intensity and age (34, 35). Classically, spleen rate (the proportion of children with an enlarged spleen in a sample of the population) has been used to estimate malaria endemicity, although a more accurate measure of transmission intensity seems to be the entomological inoculation rate (the number of infectious mosquito bites received per person per unit of time) (39). However, both measurements have limitations, as spleen rate is nonspecific due to non-malaria etiologies causing enlargement of the spleen, and establishing the entomological inoculation rate requires repeated measurements in representative sentinel sites, making this a resource-intense endeavor. Parasite prevalence in children is a suboptimal estimate of disease burden for reasons such as various levels of endemicity, long incubation time, complex pathogenicity, and parasite multiclonality. More recently, a mathematical model of transmission intensity using age-stratified antibody titers was introduced (40, 41).
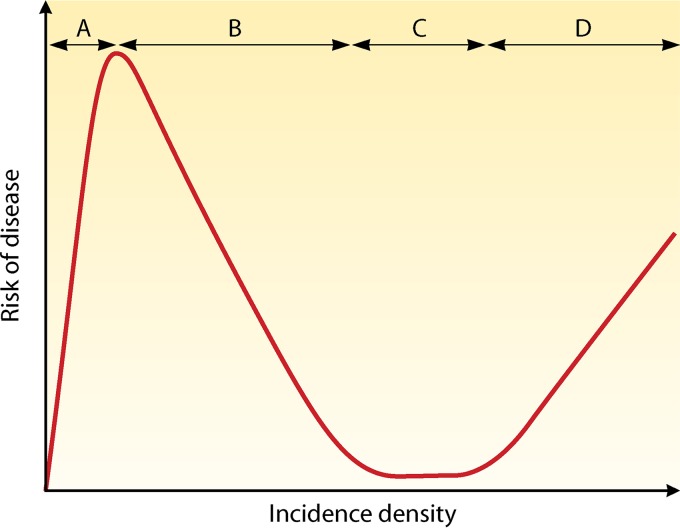
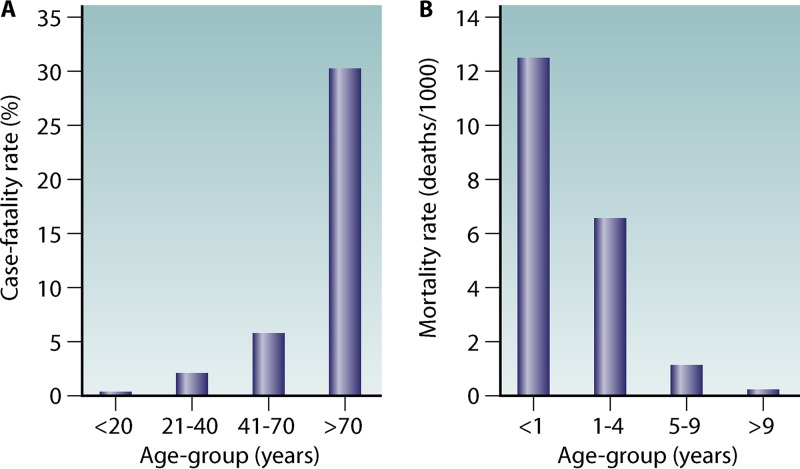
Traditionally, transmission intensity is classified into hypo-, meso-, hyper-, and holoendemicity. There is a negative correlation between the individual risk of adults for severe disease and the risk of infection in a region (Fig. 3). Furthermore, intense transmission drives the susceptibility to disease down the age range (34). Thus, in areas of high transmission, the risk of severe disease is predominantly confined to small children, pregnant women, and malaria-naive visitors. It has been shown that in areas of low or unstable transmission (e.g., highland areas or the Sahel), there was still an association between the development of anti-disease immunity and age (42). A study conducted in Indonesian Papua compared age-dependent parasite prevalence in malaria-naive migrants at an average of 8 months of risk for malaria infection (N = 689) and at an average of 20 months of risk for malaria infection (N = 553) (Fig. 4). It was shown that infection prevalence was similar across all age groups at 8 months of risk. However, at 20 months of risk, a distinct age-specific infection pattern can be seen that parallels infection patterns in endemic populations (43). In endemic areas, significant correlations were demonstrated between the intensity of entomological inoculation rate and incidence and density of parasitemia in young children (44–47). Furthermore, an inverted age pattern for fatal clinical outcomes of acute and chronic malaria episodes is demonstrated on a population level (34, 43) (Fig. 5).
FIG 3.
Hypothetical relationship between risk of disease and exposure. (A) A low but increasing incidence density equates to a high risk of disease. (B) Further increase in incidence induces NAI, resulting in decreasing risk of disease. (C) Sustained high incidence maintains the risk of disease at a low level. (D) High risk of disease in those with inadequate NAI, such as small children. (Adapted from reference 49.)
FIG 4.
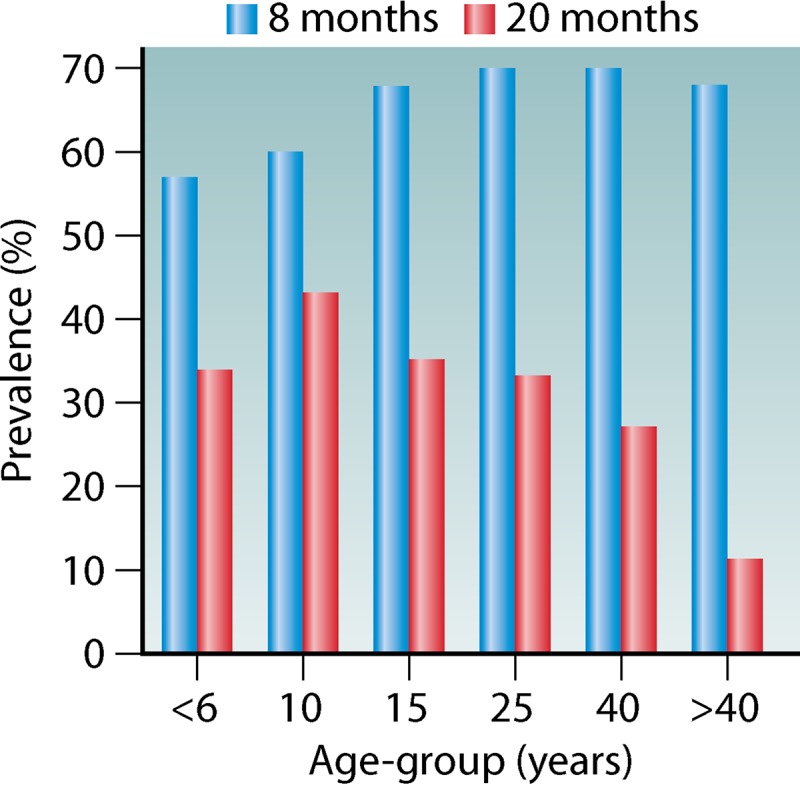
Prevalence of parasitemia across age groups in malaria-naive migrants to a malaria-endemic area. Prevalence is largely uniform after 8 months of exposure, while at 20 months of exposure an age-distinct pattern has developed. (Adapted from reference 43.)
FIG 5.
Age-related susceptibility to death in relation to acute or chronic exposure. (A) Case fatality rates for malaria-naive American civilians (n = 1,111) traveling to malaria-endemic areas. (B) Mortality rates for populations living in holoendemic West Africa show an inverse trend. (Adapted from reference 43.)
It is known that interventions aimed at reducing the transmission intensity do reduce the prevalence of malaria and, thus, morbidity and mortality (44, 46–48). The notion that interventions reducing malaria transmission result in a subsequent loss of population immunity, with all its potential negative impact in the case of malaria resurgence, has been concerning researchers for some time (34). As Doolan et al. (34) put it,
An intervention that pushes the attack rate below the threshold of risk of severe disease does not necessarily cross below the threshold of exposure needed to sustain acquired clinical immunity. However, there must be a threshold of exposure to sustain clinical immunity, and it can be crossed by interventions that diminish the risk of infection. If this threshold is crossed, an increase in susceptibility to less frequent episodes of infection may occur.
This was also demonstrated in the Garki Project, investigating the interruption of malaria in a region of high transmission (50, 51). This is further supported by pediatric hospital surveillance data in Kenya from 1991 to 2006, where it was shown that the average age of malaria patients rose as transmission intensity declined; however, the absolute number of annual malaria episodes remained comparable until transmission crossed below a critical level (52). In an age-structured mathematical malaria transmission model, the best model fit to reproduce real-life epidemiological age prevalence data was gained by a model that incorporated clinical immunity (i.e., reducing susceptibility to malaria) developing with age and parasite exposure. It was shown that such modeled clinical immunity had a half-life of less than 5 years, while a form of anti-parasite immunity (i.e., reducing parasitemia) had a half-life of above 20 years (53).
A recent modeling study investigated clinical (i.e., anti-disease) immunity and anti-parasite immunity using data from three Ugandan pediatric cohorts (N = 1,021) (37). Participants were recruited based on household surveys using random sampling methods. The authors concluded that age and individual exposure (as measured by the household annual entomological inoculation rate [aEIR]) were best at explaining both types of immunity. Figure 6 demonstrates how infection progresses to disease and at what stages of this progress immunity plays a role (37, 54). However, only high-parasite-density infections were included, and active disease was defined only by the presence of fever. Therefore, study results may be influenced by selection bias.
FIG 6.
Network of factors used to model immunity to malaria. Progression from infection to disease develops through stages (green arrows). Exposure to malaria by mosquito bites leads to low-level infections (only detectable by highly sensitive methods, like PCR) that can progress into high-level infections (only those were included in the figure), some of which can lead to clinical malaria (here defined as the presence of fever). Red lines indicate the inhibitory influence of immunity, and boxes demonstrate how immunity depends on age and exposure (annual entomological inoculation rate [aEIR]). (Adapted from reference 54.)
Naturally Acquired Immunity to Malaria within Certain Populations
Children.
Surviving past the fifth year of life in a region of holo- or hyperendemic malaria transmission is the result of repeated P. falciparum infections leading to a considerable level of accumulated clinical immunity. Protection from symptomatic disease occurs at an early age in areas of high transmission but rarely manifests before 2 years of age. The risk of severe disease in childhood has been shown to be lowest for individuals residing in areas of heavy transmission (55), and immunity to noncerebral severe malaria may be acquired only after repeated episodes of malaria (56). It has been demonstrated that antibody titers to parasite-specific merozoite proteins have a short half-life during early infancy (57, 58). In a randomized controlled trial, Guinovart et al. found no evidence that the age of first exposure to malaria parasites during childhood determines the build-up of immunity. Interestingly, malaria incidence did not rise significantly in the second year of children’s lives after exposure to P. falciparum antigens had been considerably reduced throughout the first year of life. The authors stated that the development of naturally acquired immunity would only be impaired in the face of substantial reductions of parasite exposure (59). Later, in another randomized controlled trial, the age of the first P. falciparum infection was shown not to influence the intensity of IgG responses, while earlier exposure was essential for antibody production (60).
Pregnant women.
The characteristics of naturally acquired immunity against malaria substantially change during pregnancy. Despite accumulated immunity from growing up in an endemic area, pregnancy seems to offset protection from malaria, once again leaving women susceptible to severe illness, particularly during first and second pregnancies (61–63). This loss of protection from malaria is likely due to the general suppression of cell-mediated immunity during pregnancy and the lack of immunity to pregnancy-specific isolates that sequester in the placenta (64–66). Gradual clinical immunity is achieved with each subsequent pregnancy at least in part due to the boosting of humoral immunity against P. falciparum VAR2CSA (PfVAR2CSA) antigens (64, 65, 67). In the context of decreasing malaria prevalence, the decline in immunity in pregnant women was shown to be modest over a 7-year period, with differing antibody levels depending on the setting and use of insecticide-treated bed nets (ITN) (68). Importantly, Mayor and colleagues found substantial declines in the prevalence of malaria and a reduction of antimalarial antibodies, while the adverse consequences of P. falciparum infections increased, in pregnant Mozambican women who delivered infants between 2003 and 2012 (69).
Ethnic differences.
There are also differences in susceptibility to malaria between different ethnic groups, such as the Fulani and Dogon in Africa. Although it is well established that the Fulani are better protected against clinical malaria than sympatric ethnic groups, the immunological basis of this protection is not understood (70).
How Long Does Specific Immunity Last after Migration to a Nonendemic Country?
The question of the longevity of semi-immunity is important for multiple reasons. First, the proportion of VFR travelers has consistently risen among all patients presenting with malaria in nonendemic countries (2, 4). Moreover, individuals with acquired semi-immunity may be protected from severe disease, but this protection wanes over time, again rendering these individuals at high risk for clinical disease and adverse outcome. Generally, to maintain semi-immunity over time, regular exposure to malaria parasites is required. Individuals from endemic countries residing in nonendemic areas therefore progressively lose their semi-immunity (71). However, observations from an area of Madagascar, where malaria returned after prolonged absence, suggest that even after 20 years individuals previously exposed retained some immunity compared to persons infected for the first time (72). Furthermore, an experimental study indicated long-term persistence (>13 years) of the cellular and humoral response to P. falciparum peptide antigens in West African migrants residing in France who had not returned to Africa (73). The longevity of antibody responses has been shown to differ between previously exposed and malaria-naive individuals (74, 75), although anti-PfEMP1 antibody responses in previously malaria-naïve adults experiencing a single P. falciparum infection have been detected 20 weeks after infection (76). While levels of antibodies are known to wane, Ndungu et al. showed that P. falciparum can induce long‐lasting memory B cells that are maintained for up to 16 years in the absence of reexposure (77). Mugyenyi and colleagues found that in areas of declining malaria transmission, loss of humoral immunity is differential in a population of children. Over a 3-year period, while AMA1 and MSP2 antibodies were found to drop substantially, other key functional responses and antibodies to infected erythrocytes were better maintained (78). In a prospective study in a nonendemic setting monitoring individuals diagnosed with acute malaria over the course of a year, the magnitude of the antibody response to whole lysed P. falciparum parasites was consistently higher in previously malaria-exposed individuals than in individuals infected for the first time (79). In the same study, previously malaria-exposed individuals also showed a larger expansion of a subpopulation of switched memory B cells than individuals with a primary infection.
A study on the Thailand-Myanmar border between 2001 and 2004 showed a simultaneous decline in P. falciparum-specific antibody levels in participants and a drop in P. falciparum transmission in the study area. Between 2007 and 2011, in the face of emerging artemisinin resistance, the variability of individual antibody responses was high, and high levels of antibody titers were correlated with faster parasite clearance (80). There are several other studies suggesting that declining malaria transmission results in declining antibody levels (81). In a 3-year study in age-matched children in Mali, Portugal and colleagues determined that P. falciparum-specific antibody responses were more pronounced in participants carrying their infection throughout the dry season. There was an observable decline of antibody levels during the dry season in all study participants that was comparable in infected and uninfected individuals. Arguably, this indicates that chronic asymptomatic parasite carriage in itself does not maintain humoral immunity against malaria (82). However, its effect over the long term has not yet been determined.
Despite having lived in nonendemic countries, often for multiple decades, P. falciparum malaria was reportedly less common in VFRs from sub-Saharan Africa than in nonimmune Caucasian travelers (7, 22, 83–85). Therefore, in this review we consider the population of VFRs as having existing but declining immunity. As this specific population is of major interest for this review, a more detailed discussion will take place in various sections of this paper.
Need for Biomarkers Reflecting Semi-immunity
The fact that individuals engaging in VFR travels have often spent years to decades in nonendemic countries is closely linked to the question of to what degree they are still protected by semi-immunity to malaria. To better understand this phenomenon, a robust biomarker reflecting the degree of exposure to Plasmodium spp. or the degree of anti-disease and anti-parasite immunity would be required. Therefore, the scientific community has been engaged in the search for modern biomarkers for some time. Individuals living in malaria-endemic areas show an array of different Plasmodium species proteins, which may be protective or serve as serologic indicators of parasite exposure. In the following paragraphs, we try to highlight past endeavors in the search for biomarkers reflecting exposure to Plasmodium parasites.
In a large study conducted in a Papua New Guinean population, more than 30 recombinant merozoite proteins of P. vivax were studied for their potential value in acquired immunity. Out of those 30 proteins, the 12 with the highest immunoreactivity were selected for further analysis. The data indicate that the antibodies are good markers of cumulative exposure, increasing with age (reflecting the number of infections acquired over time), and that some of them can also be valuable indicators of current P. vivax infection. High levels of IgG to PVX_081550, P41, and P12 were associated with protective immunity. Additionally, children with elevated titers of IgG to PVX_081550 and P41 had a significantly lower risk of clinical P. vivax malaria. Unfortunately, high levels of IgG to PVX_081550, P41, and P12, associated with protection against P. vivax, evidently were not correlated with a risk of clinical episodes caused by P. falciparum (86). Screening for specific IgG antibodies to a large number of different recombinant Plasmodium proteins in an exposed population at risk may be a useful approach in identifying new targets of natural malaria immunity. An extension of this approach in Papua New Guinea detected levels of total and IgG subclasses against six P. vivax proteins of the reticulocyte-binding protein family (PvRBPs), revealing that antibodies to the reticulocyte-specific binder PvRBP2b and the nonspecific binder PvRBP1a were strongly associated with a lower risk of clinical malaria in young children (87).
A study describing the seroprevalence of antibodies to P. vivax CelTOS (cell-traversal protein for ookinetes and sporozoites) and two major circumsporozoite proteins (CSP; predominant sporozoite-coating antigen) variants, CSP210 and CSP247, in inhabitants from the Thailand-Myanmar border showed that IgG positivity was persistent over a 1-year period. There was no significant difference in mean antibody titers against preerythrocytic antigens in patients with or without quantitative PCR-detectable blood-stage parasites, but the magnitude of the response significantly declined over time (88). Responses to CSP antigen have been assessed frequently in populations exposed to both P. falciparum and P. vivax (89).
In a subsequent cross-sectional survey conducted in a low-transmission region of western Thailand, a strong IgG reaction to 11 different proteins, including CSP and molecules involved in binding to or invasion of reticulocytes, was documented during concurrent P. vivax infection in asymptomatic residents with only a low parasite density. Arguably, this indicates the potential utility of such antigens to be used as biomarkers reflecting exposure to parasites or specific immunity (90, 91).
An immunoserological survey of rural Amazonians from the equatorial rain forest located in northwestern Brazil documented that naturally acquired strong anti-PvDBP (P. vivax Duffy binding protein) IgG response is positively correlated with clinical immunity to malaria in a population exposed to low levels of parasite transmission. It was suggested that long-lasting PvDBP-specific antibodies elicited by a vaccination is promising in the prevention of P. vivax infection and clinical disease (92). A systematic literature review involving populations from the Brazilian Amazon Basin underlines the role of specific IgGs against the PvMSP119 molecule as a predictor of exposure to malaria (89). Several reports from Turkey, Papua New Guinea, and Brazil indicate an elevation of the levels of PvMSP-119 molecules during P. vivax infections. However, other reports highlight significant heterogeneity within immune responses to the PvMSP-119 molecule; this even includes a study reporting decreased levels of PvMSP-119 molecule during P. vivax infection (93).
The question of whether merozoite antigens of P. falciparum can serve as markers for naturally acquired protective immunity has been addressed by various authors. Doolan and colleagues developed an original algorithm that explains the individual profile of specific antibody responses following either vaccination or infection by natural or experimental exposure to malaria parasites to identify potential vaccine candidates. Using a new DNA microarray approach, the authors were able to evaluate 250 P. falciparum recombinant proteins associated with partial immunity in naturally exposed subjects. Protection developed in volunteers immunized with radiation-attenuated sporozoites and in malaria-naive individuals, and they selected 72 of them with the highest immunological reactivity. Antigens expressed solely during the preerythrocytic stage of infection, including liver-stage-specific antigens, were dominant in naturally exposed individuals (94).
In Senegalese villagers, the presence of specific IgG3 in response to MSP3 (merozoite surface protein 3) demonstrated a strong association with clinical immunity against malaria independently of the age group. Naturally developed anti-MSP3 IgG3 antibodies in young children also could be elicited by vaccination in naive volunteers, providing long-term clinical protection for at least 6 years (95). In the highly P. falciparum endemic region of Vietnam, elevated levels of specific antibodies to MSP4 (merozoite surface protein 4) were detected in inhabitants of Khanh-Hoa province, supporting the lysis of free merozoites by means of opsonization and the complement system. Several immunoepidemiological investigations showed correlations between protective immunity against P. falciparum infection and the presence of MSP1 (merozoite surface protein 1) antibodies, a well-studied protein and important vaccine candidate. According to Wang et al., when IgG antibody responses to MSP1 were measured in Vietnamese residents, no correlation with protective immunity was observed, as indicated by the presence of P. falciparum parasitemia during follow-up (96).
Boyle and colleagues underlined a role of the first factor of the classical complement cascade on the surface of merozoites (C1q), which is responsible for lysis and inhibition of invasion, in a synthesis of protective IgG of cytophilic IgG1 and IgG3 subclasses against P. falciparum (90).
In a recent study, performed in the central provinces of Vietnam, the authors observed an association between IgG seroprevalence and increasing age, with only minimal variation of antibody responses to PfAMA1 (P. falciparum apical membrane antigen 1) and PvAMA1 (P. vivax apical membrane antigen 1) in a representative population of residents. The authors argued that such antigens could be better suited for investigations of long-term changes of parasite exposure in a population. It was concluded that the close to 100% seroprevalence of P. falciparum in persons of higher age is explained by the long-term exposure to high levels of P. falciparum transmission and the presence of specific IgG against the PfAMA1 antigen, which has a long half-life. However, specific IgG antibodies against PfGLURP-R2 (P. falciparum glutamate-rich protein R2) were considered a favorable indicator of recent exposure due to their shorter half-life of approximately 6 months (97). In another immunodiagnostic survey conducted in Cambodia, IgG-specific antibodies against LSA3-RE (liver stage recombinant antigen 3), as well as GLURP and PfGLURP-R2 antigens, characterized by a short half-life, were considered good clinical markers of a recent infection with P. falciparum (98).
Multicenter studies involving all Plasmodium species, including mixed infections, in larger populations from diverse geographical areas of the world reflecting different levels of malaria endemicity now are required to validate scientific laboratory findings and select proteins that could be valuable practical biomarkers of immune status of an individual patient and his/her exposure to infection.
Evidence on Development of Clinical Malaria According to Immune Status
Studying the natural course of clinical malaria in nonimmune individuals has been problematic, as it is unethical not to treat somebody who is symptomatic. Therefore, much evidence on the topic comes from historic studies on iatrogenic malaria infections for the treatment of neurosyphilis (99–103). Infection was induced either by exposure to malaria-infected mosquitos or by directly applying parasites via intravenous or intramuscular injections of blood freshly obtained from patients harboring malaria parasites. These studies indicate that primary infection with P. falciparum or P. vivax in nonimmune individuals leads to a high peak parasitemia. Clinical symptoms, including fever, occur at a relatively low parasitemia in this patient population (99–101, 104). This is consistent with recent results from controlled human malaria infection (CHMI) trials, in which nonimmune individuals experience symptoms at low, and at times submicroscopic, levels of parasitemia (105). Subsequent infections are progressively associated with lower levels of parasitemia, and fever occurs at comparatively higher parasitemia thresholds, indicating the development of specific immunity in response to parasite exposure. For P. ovale or P. malariae, initial parasitemia rarely reaches above 10,000 parasites per microliter, even during primary infection in a malaria-naive individual, and tend to stay at low levels during subsequent infections (102, 103). In the previous century, no similar research was conducted on P. knowlesi infections in humans; however, P. knowlesi is known to be associated with high parasitemia and clinical complications (106, 107).
CLINICAL AND LABORATORY CHARACTERISTICS
As mentioned above, the epidemiological picture of patients with imported malaria has changed over recent decades. This raises the important question of whether clinically important variables differ between nonimmune persons and persons with various degrees of semi-immunity. Owing to the lack of a robust marker of immunity, which is easy to measure and reflects individual immunity against malaria in a patient, the duration of residency in a malaria-endemic versus nonendemic country is most commonly used as the only available surrogate (108, 109). Individuals growing up in a malaria-endemic country and traveling to a nonendemic country are considered semi-immune. The population of VFRs is characterized by existing but declining semi-immunity. However, due to the difficulty of adjusting for individual risk and travel patterns, some residual bias is certainly inherent to this classification system. The following section compares clinical outcomes between nonimmune patients and patients with various degrees of semi-immunity (Table 1).
TABLE 1.
Comparison of clinical outcomes between (partially) semi-immune and nonimmune travelers with imported malaria based on studies stratified by immunity status
| Reference | Country of study | Study designa | Population(s) | Outcome | Findingsb |
|---|---|---|---|---|---|
| Farnert et al. (110) | Sweden | Retrosp. | (i) Africans, lived in Sweden for <1 year (n = 57); (ii) Africans, lived in Sweden for 1–4 years (n = 52); (iii) Africans, lived in Sweden for 5–9 years (n = 67); (iv) Africans, lived in Sweden for 10–14 years (n = 50); (v) Africans, lived in Sweden for ≥15 years (n = 74); (vi) Swedes (n = 186) | Presence of WHO-defined criterion for severe malaria | Africans having lived in Sweden for 10–14 years and ≥15 years were similar to Swedes concerning severe malaria, defined according to WHO criteria (9.7% and 10.0% vs 9.5%); Africans having lived in Sweden <10 years had lower proportions of WHO-defined severe malaria (range of proportions, 4.2% to 8.8%) |
| Presence of severe clinical signs | Africans having lived in Sweden for ≥15 years had a proportion of severe signs similar to those of Swedes (8.1% and 6.5%, respectively); Africans having lived in Sweden <15 years had lower proportions of severe signs (range of proportions, 3.2% to 3.9%) | ||||
| ICU admission | Proportion of Africans having lived in Sweden for ≥15 years and being admitted to ICU similar to that of Swedes (9.2% and 9.7%, respectively); Africans having lived in Sweden <15 years were admitted to ICU less often (range of proportions, 3.2% to 6%) | ||||
| Pistone et al. (7) | France | Retrosp. | (i) Africans, lived in France for ≥15 years (n = 106); (ii) Europeans (n = 240) | Presence of WHO-defined criterion for severe malaria | Adjusted OR, 0.25 (95% CI, 0.074 to 0.846), indicating less frequent occurrence of severe malaria in Africans |
| Bouchaud et al. (83) | France | Prosp. | (i) Africans, lived in France for a median of 14 (4 min, 45 max) years (n = 252); (ii) Europeans (n = 99) | Presence of WHO-defined criterion for severe malaria | Crude OR, 4.3 (95% CI, 1.6 to 11.9), indicating more frequent occurrence of severe malaria in Europeans |
| Percent parasitemia | Mean parasitemia was lower in Africans than in Europeans (0.8% vs 1.4%; P = 0.007) | ||||
| Fever clearance time | Mean fever clearance time was shorter in Africans than in Europeans (40.1 ± 24.6 vs. 54.6 ± 24 h; P < 0.0001) | ||||
| Parasite clearance time | Mean parasite clearance time was shorter in Africans than in Europeans (56.1 ± 31.2 vs 62.5 ± 30.5 h; P = 0.03) | ||||
| Phillips et al. (85) | UK | Prosp. | (i) Africans with main residency in the UK for preceding 12 months (n = 309); (ii) Asians (n = 94); (iii) Europeans (n = 79) | Presence of WHO-defined criterion for severe malaria | Compared with Africans, the adjusted OR for severe malaria was 8.05 (95% CI, 2.93 to 22.1) and 8.2 (95% CI, 2.94 to 22.9) for Asians and Europeans, respectively |
| Presence of unfavorable outcomes | Compared with Africans, the adjusted OR for an unfavorable outcome was 4.78 (95% CI, 2.47 to 9.23) and 3.88 (95% CI, 1.96 to 7.71) for Asians and Europeans, respectively | ||||
| Length of hospitalization | Compared with Africans, hospitalization was, on average, 40% (95% CI, 19% to 64%) and 34% (95% CI, 14% to 57%) longer for Asians and Europeans, respectively (P < 0.001) | ||||
| Koopmans et al. (112) | Netherlands | Prosp. | (i) Nonimmune patients (n = 246); (ii) partially immune patients (n = 198); (iii) semi-immune patients (n = 9) | Acute kidney injury (AKI) | Proportion of nonimmune patients with AKI (74%) was higher than proportion of nonimmune patients without AKI (55%) (P = 0.007); concordantly, the proportion of partially immune (26%) and semi-immune patients (0%) with AKI was lower than the proportion of partially immune (45%) and semi-immune patients (2%) without AKI (P = 0.06 and P value not applicable, respectively) |
Retrosp., retrospective; Prosp., prospective.
Proportions were extracted geometrically from figures, as they were not presented in the main text.
Comparison of Clinical and Parasitological Outcomes between Populations with Declining Semi-immunity and Nonimmune Travelers
The degree of clinical and parasitological outcomes of malaria-infected persons is a function of various factors related to the parasite, the environment, and the individual. Parasite-related factors include the infective Plasmodium species, the specific parasite strain, multiplicity of infection, and potentially other factors, such as specific drug resistance of the parasite. Patient-related factors include acquired immunity, natural resistance against infection by genetic mutations, and potential drug intake for chemoprophylaxis or treatment, whereas environmental factors are mostly associated with the potential of parasite transmission by the Anopheles vector.
A retrospective study from Sweden assessed the clinical outcome of malaria in African immigrants (n = 315) according to their duration of residency in Sweden (110). The authors demonstrated an association between duration of residency in this nonendemic region and poor clinical outcomes. Immigrants who had lived in Sweden for less than 10 years had better clinical outcomes than those residing in Sweden for 10 years and more. Those with residency of 15 or more years (n = 74) had outcomes similarly as poor as those for nonimmune travelers (n = 186). This includes comparable proportions of both groups experiencing severe malaria, admission to intensive care units (ICU), or presenting with a risk factor for adverse outcome, such as impaired consciousness, acidosis, circulatory collapse, convulsions, pulmonary edema, or abnormal bleeding (110).
A similar retrospective study from France compared clinical characteristics of immigrants from sub-Saharan Africa who presented with malaria. They included only individuals who had lived in France for 15 years and longer and had recently traveled to visit friends and relatives (VFRs; n = 106). As a comparison, the same outcomes were investigated in nonimmune travelers of European origin (n = 240) (7). This study demonstrates that rates of severe malaria among all patients with P. falciparum malaria were 3% for the VFR group and 11% for the nonimmune European group (P = 0.02). Moreover, in this study, Europeans had less favorable parasitological and laboratory parameters than VFRs, despite the fact that 43% of Europeans showed good compliance with antimalarial prophylaxis compared with only 15% of VFRs. Such parameters comprise initial parasitemia above 2% in 24% (56/233) of Europeans versus 16% (15/96) of VFRs (P = 0.09), hemoglobin below 10 g/dl in 13% (30/232) of Europeans versus 6% (6/104) of VFRs (P = 0.06), and thrombocytopenia below 50,000/mm3 in 19% (45/232) of Europeans versus 10% (10/104) of VFRs (P = 0.03). However, after adjusting for various confounders in a multivariable model, the only factor that was still independently associated with VFRs was that this group was 75% less likely to experience severe malaria than Europeans (adjusted odds ratio [OR], 0.25; 95% confidence interval [CI], 0.074 to 0.846) (7).
A prospective hospital-based study on P. falciparum malaria from France compared clinical characteristics of nonimmune European travelers (n = 99) and VFRs (n = 252) who had lived in France for a minimum of 4 years (83). The median duration of VFRs’ residence in France was 14 years (range, 4 to 45 years), and the authors did not stratify clinical characteristics for various intervals of VFRs’ length of stay but presented summary measures for the whole VFR group. They demonstrated that the mean parasitemia was lower in VFRs than in nonimmune Europeans (0.8% versus 1.4%; P = 0.007). Additionally, among all Europeans (n = 99), 15.2% experienced severe malaria versus 4.4% among all VFRs (n = 252) (P = 0.0005), so that the odds for severe malaria was 4.3-fold higher in Europeans than in VFRs (OR, 4.3 [95% CI, 1.6 to 11.9]). Thrombocyte numbers were higher in VFRs than in Europeans, with 119.6 ± 55.7 (109/liter) and 105.7 ± 59.2 (109/liter), respectively (P = 0.04), while hemoglobin was lower in VFRs than in Europeans (12.8 ± 1.7 g/dl versus 13.6 ± 1.6 g/dl; P = 0.0003). Furthermore, fever clearance and parasite clearance times were more favorable for VFRs than for Europeans: VFR fever clearance time was 40.1 ± 24.6 h compared with 56.1 ± 31.2 h in Europeans (P < 0.0001), and the VFR parasite clearance time was 54.6 ± 24 h compared with 62.5 ± 30.5 h in Europeans (P = 0.03). Additionally, the authors measured antibody titers against P. falciparum and demonstrated that these were higher in VFRs than in Europeans, which corresponds with the clinical findings.
In London, UK, a prospective study looked at returning travelers with malaria who had their main residency in the UK for the preceding 12 months (85). Among all who were diagnosed with falciparum malaria (N = 482) over a period of 16 years, 309 self-identified to be of African, 94 of Asian, and 79 of Caucasian ethnicity. Compared with Africans, the confounder-adjusted odds for severe malaria was 8.2-fold (95% CI, 2.94 to 22.9) higher for Caucasians and 8.05-fold (95% CI, 2.93 to 22.1) higher for Asians. This potentially reflects a previous high parasite exposure in the individuals’ African country of origin. Reporting a previous malaria episode led to 65% reduced odds for severe P. falciparum malaria (adjusted OR, 0.35; 95% CI, 0.15 to 0.80). Unfavorable outcomes, defined as “death,” “admission to intensive care unit,” and “length of stay in the hospital of 5 days or more,” were much more common in Caucasians (adjusted OR, 3.88; 95% CI, 1.96 to 7.71) and Asians (adjusted OR, 4.78; 95% CI, 2.47 to 9.23) than in Africans. The authors did not present parasite density stratified by ethnicity but reported that patients with a parasite density of 2% and above had an increased odds ratio of experiencing severe malaria (adjusted OR, 4.93; 95% CI, 2.22 to 11.0) and an unfavorable outcome (adjusted OR, 4.38; 95% CI, 2.46 to 7.79) compared with those of patients having a parasite density lower than 2%. Lastly, the duration of hospitalization was, on average, 40% (95% CI, 19% to 64%) and 34% (95% CI, 14% to 57%) longer for Asians and Caucasians, respectively, than for Africans (P < 0.001).
All of the above-mentioned findings are somewhat in discordance with a study from the Hospital of Tropical Diseases in London, UK, which prospectively recruited returning travelers (n = 99) with P. falciparum malaria over a 2-year period (111). The authors concluded that there is no robust marker reflecting individual immunity to malaria; therefore, they classified all patients into two groups of mild and severe disease according to modified WHO criteria. Among patients with mild disease (n = 74), 28% were of European, 69% of African, and 3% of other ethnicity, while among patients with severe disease (n = 25), 28% were of European, 60% of African, and 12% of other ethnicity (P = 0.18). As this study had an exploratory study design, lacking formal a priori sample size calculations, it may have been underpowered for detecting differences in the proportion of ethnicities between groups of mild and severe malaria disease. The data suggest that the proportion of Africans (those with potential semi-immunity) was higher in those with mild disease (69%) than in the group with severe disease (60%), even though the proportion of Europeans (potentially nonimmune) was the same in both groups (28% and 28%). However, those of other ethnicities (potentially nonimmune) were 4-fold more common in the severe disease group (12%) than in the mild disease group (3%).
A prospective hospital-based study from Rotterdam, Netherlands, investigated the role of immunity against malaria and acute kidney injury (AKI) in malaria patients (112). They demonstrated that the proportion of nonimmune patients with acute kidney injury was higher than the proportion of nonimmune patients without acute kidney injury (74% [28/38] versus 53% [218/415]; P = 0.007). Concordantly, the proportion of patients with partial immunity and semi-immunity among all patients with acute kidney injury was lower than that among those without acute kidney injury (26% [10/38] versus 45% [188/415], P = 0.06, and 0% [0/38] versus 2% [9/415], P value not applicable, respectively). Partial immunity was defined as having been born in a malaria-endemic country with current residence in the Netherlands.
Adherence to antimalarial chemoprophylaxis was associated with a reduced risk for severe disease in several studies (84, 113, 114). Many of these studies did not have detailed information on the use of chemoprophylaxis, and it may be hypothesized that most returning travelers who presented to respective hospitals with malaria were not adherent to prophylaxis.
In a cross-sectional study from Israel, which focused on treatment outcomes of nonimmune malaria patients (n = 135), increasing age was identified as an independent risk factor for mortality (115). Subjects of 40 years or older had 4.29-fold elevated odds of experiencing a fatal malaria episode compared with those of younger subjects (95% CI, 1.25 to 14.74). There were no differences with regard to severity of disease between males and females. A meta-analysis presented in the same study concluded that the overall odds ratio for the above-mentioned association was 3.97 (95% CI, 2.62 to 6.02) (115). No such data exist for VFRs; however, data from malaria-endemic countries indicate that mortality rates are highest for young children (group of no/low immunity) and lowest for adults (group with semi-immunity) (34). Given that young and old VFRs have a similar degree of semi-immunity, case fatality rates may also be higher in old VFRs than in young VFRs.
In summary, these findings support the hypothesis of long-term persistence of anti-disease immunity against malaria to a certain degree. Large observational studies included in this review have almost consistently reported some degree of protection for at least a 10-year period postarrival in nonendemic countries. Evidence of protection largely concerned milder clinical presentation in VFRs and smaller proportions of VFR patients with severe malaria than presentation in nonimmune patients. To a lesser extent, there was evidence of remaining anti-parasite immunity in populations with declining semi-immunity.
Diagnostic Performance of Clinical Symptoms, Signs, and Laboratory Values in Semi-immune and Nonimmune Patients
Textbooks on tropical medicine denote malaria as a tropical disease that involves episodes of fever (116). However, it is believed that malaria symptoms are nonspecific regardless of immune status. In the past, few studies investigated the prognostic value of clinical symptoms in imported malaria using gold-standard methodologies to ascertain diagnostic validity, such as likelihood ratios or sensitivity and specificity. A systematic review and meta-analysis by Taylor et al. investigated a total of 14 variables (clinical symptoms, signs, and laboratory characteristics) for their predictive value to diagnose malaria (117). They demonstrated that for nonimmune returning travelers, no diagnostic item was considerably more frequent in patients with malaria than in patients without malaria, as would be indicated by a positive likelihood ratio (LR+) above 10. The most favorable positive LRs were associated with splenomegaly (LR+, 6.5; 95% CI, 3.9 to 11.0) and hyperbilirubinemia above 1.2 mg/dl (LR+, 7.3 [95% CI, 5.5 to 9.6]). Other favorable positive LRs were 4.5 (95% CI, 1.7 to 12.0) for jaundice and 5.6 (95% CI, 4.1 to 7.5) for thrombocytopenia. Fever, a clinical sign that is regarded by many authors to be closely associated with malaria, had an LR+ of 5.1 (95% CI, 4.9 to 5.3). While this is doubtlessly valuable supplementary information for determining malaria status, focusing solely on fever as the main symptom is misleading, which is well demonstrated by its specificity as a predictive sign for malaria of 82.3% (95% CI, 81.9 to 82.7). A specificity of 82.3% indicates that fever was also present in 17.7% of patients with conditions other than malaria, which is not surprising in a population of returning travelers from tropical or subtropical regions. Furthermore, the most favorable negative likelihood ratio (LR−) was associated with fever (LR−, 0.12; 95% CI, 0.10 to 0.15), indicating that the absence of fever was almost 10-fold less common in malaria cases than in patients without malaria. This is also well demonstrated by the sensitivity of fever, i.e., 90% (95% CI, 88 to 92), indicating that 10% of those who actually had malaria did not have a fever at presentation. Due to the replication cycle of malaria blood stages of 24 to 72 h depending on the Plasmodium species, a history of fever in the last 48 or 72 h may have an even more favorable LR− due to potentially increased sensitivity, although systematic evaluation of this hypothesis is still lacking (38, 105). It is important to note that most studies had fever as a (co-)criterion for patient inclusion, and meta-analyses for fever are based on a single study (n = 30,221). Therefore, due to the paucity of high-quality studies, the diagnostic performance of fever, as indicated by LRs, may be limited (117). It should be noted that the presence of cough was 25-fold less common in malaria patients than in other patients (LR+, 0.04 [95% CI, 0 to 0.56]), constituting a potential sign to rule out malaria in nonimmune returning travelers.
To the best of our knowledge, there are no studies that investigated the predictive value of clinical symptoms in semi-immune returning travelers with similar methodological quality. However, Taylor et al. also provided diagnostic performance indicators of symptoms and signs for patients with semi-immunity in malaria-endemic countries demonstrating that associations were much weaker (117). The most favorable positive LRs were splenomegaly for both adults and children, with an LR+ of 3.1 (95% CI, 1.9 to 4.4) and 3.4 (95% CI, 1.2 to 9.8), respectively. The most favorable negative LRs were chills/rigors for adults with an LR− of 0.69 (95% CI, 0.56 to 0.84) and headache for children with an LR− of 0.67 (95% CI, 0.52 to 0.87).
Differential Blood Count and Laboratory Characteristics (Differences between Populations with Declining Semi-immunity and Nonimmune Travelers)
Malaria can cause nonspecific alterations in the differential blood count. An observational study from Munich recruited 210 malaria patients and 210 healthy controls (matched for age and sex) to investigate alterations of differential blood count and stratified results by individual immune status (Table 2) (118). They demonstrated a positive correlation of parasitemia with neutrophil counts and a negative correlation of parasitemia with thrombocyte, lymphocyte, and leukocyte counts in all patients. Furthermore, the neutrophil-to-lymphocyte count ratio (NLCR) and monocyte-to-lymphocyte count ratio (MLCR) were evaluated. While NLCR constitutes a proposed parameter reflecting the severity of illness and inflammation in intensive care patients, the MLCR was described as a potential parameter to assess the risk of clinical manifestation of malaria (119, 120). Both median (interquartile range [IQR]) NLCR and MLCR were higher in nonimmune malaria cases than in controls (2.88 [1.66 to 5.19] versus 1.7 [1.2 to 2.4], P < 0.001, and 0.32 [0.17 to 0.52] versus 0.16 [0.1 to 0.25], P < 0.001, respectively). On the contrary, for semi-immune patients there was weak evidence for such differences; median (IQR) MLCR was 0.2 (0.1 to 0.35) in cases and 0.1 (0.07 to 0.21) in controls (P = 0.19), while median (IQR) NLCR was 1.86 (1.31 to 3.19) in cases and 1.69 (0.77 to 2.83) in controls (P = 0.53). However, despite a potentially smaller effect size for semi-immune patients, the authors partly attributed nonsignificant P values to a very small sample size of semi-immune control subjects (n = 5). Furthermore, the NLCR was higher in severe malaria cases than in cases with uncomplicated malaria; however, MLCR was lower in severe malaria than in uncomplicated malaria (118, 121). Additionally, the effect of modification of immune status was revealed. NLCR and MLCR were consistently higher in nonimmune patients than in semi-immune patients. A logistic regression model adjusting for age and sex showed the following associations, stratified for nonimmune and semi-immune patients. MLCR and NLCR cutoffs were set to reflect the lower bound of the 95% CI in malaria cases. Nonimmune patients with an MLCR of ≥0.27 had 5.92-fold higher adjusted odds (95% CI, 3.66 to 9.59) of being a malaria case than a control. This equaled a sensitivity and specificity of an MLCR of ≥0.27 to detect malaria of 59% and 80%, respectively, in nonimmune patients. For semi-immune patients, there was insufficient evidence for a similar effect (OR, 3.9 [95% CI, 0.5 to 27.43], P = 0.20); however, the MLCR cutoff was set at ≥0.15. Concordantly, nonimmune patients with an NLCR of ≥0.25 had an adjusted OR of 5.24 (95% CI, 3.25 to 8.45) to be malaria positive rather than malaria negative, while for semi-immune patients with an NLCR of ≥0.15, there again was no sufficient evidence for a similar effect (P = 0.98). However, the sensitivity and specificity of an NLCR of ≥0.25 to detect severe malaria in semi-immune patients were 63.6% and 80%, respectively.
TABLE 2.
Comparison of laboratory characteristics between (partially) semi-immune and nonimmune travelers with imported malaria based on studies stratified by immunity status
| Reference | Country | Study design | Population(s) | Outcome(s)a | Findings |
|---|---|---|---|---|---|
| Behrens-Riha et al. (118) | Germany | Matched case-control study | (i) Semi-immune malaria patients and controls (n = 71); (ii) nonimmune malaria patients and controls (n = 349) | Peripheral blood MLCR | Both median (IQR) NLCR and MLCR were higher in nonimmune malaria cases than in controls (2.88 [1.66 to 5.19] versus 1.7 [1.2 to 2.4], P < 0.001, and 0.32 [0.17 to 0.52] versus 0.16 [0.1 to 0.25], P < 0.001); for semi-immune patients there was weak evidence for such differences; median (IQR) MLCR was 0.2 (0.1 to 0.35) in cases and 0.1 (0.07 to 0.21) in controls (P = 0.19), while median (IQR) NLCR was 1.86 (1.31 to 3.19) in cases and 1.69 (0.77 to 2.83) in controls (P = 0.53) |
| NLCR | Nonimmune patients with an MLCR of ≥0.27 had 5.92-fold higher adjusted odds (95% CI, 3.66 to 9.59) to be a malaria case than a control; this equaled a sensitivity and specificity of an MLCR of ≥0.27 to detect malaria of 59% and 80%, respectively, in nonimmune patients; for semi-immune patients there was insufficient evidence for a similar effect (OR, 3.9 [95% CI, 0.5 to 27.43]; P = 0.20); however, the MLCR cutoff was set at ≥0.15; concordantly, nonimmune patients with an NLCR of ≥0.25 had an adjusted OR of 5.24 (95% CI, 3.25 to 8.45) to be malaria positive than malaria negative, while for semi-immune patients with an NLCR of ≥0.15 there again was no sufficient evidence for a similar effect (P = 0.98); however, the sensitivity and specificity of an NLCR of ≥0.25 to detect severe malaria in semi-immune patients were 63.6% and 80%, respectively | ||||
| Chiwakata et al. (126) | Germany | Prospective | (i) Nonimmune patients with uncomplicated malaria (n = 20); (ii) semi-immune patients with uncomplicated malaria (n = 16); (iii) patients with severe malaria (n = 30) | Procalcitonin | Nonimmune patients with uncomplicated malaria had a geometric mean concentration of 1.1 ng/ml, semi-immune patients with uncomplicated malaria of 2.4 ng/ml, and those with severe malaria of 10.7 ng/ml (P < 0.001) |
MLCR, monocyte-to-lymphocyte count ratio; NLCR, neutrophil-to-lymphocyte count ratio.
A study group from the Netherlands investigated the differential leukocyte counts of nonimmune malaria-infected individuals (n = 39) as part of controlled human malaria infection trials (CHMI) (122). During the hepatocytic parasite cycle, there was a statistically significant increase of total white blood cell count, lymphocyte count, and monocyte count but at levels that may not be clinically relevant (thus, they are not reported here). During the subsequent erythrocytic parasite cycle, clinically relevant leukocytopenia (nadir median of 3,300/μl; P = 0.0001), lymphocytopenia (nadir median of 700/μl; P = 0.0001), and borderline neutropenia (nadir median of 1,500/μl; P = 0.0001) occurred. Parasite density negatively correlated with absolute lymphocyte count (Spearman’s rho, −0.46; P < 0.001) and positively correlated with NLCR (Spearman’s rho, +0.5; P < 0.001), which reached a maximum at 4.0. After parasite clearance, differential blood count changes normalized.
Various studies have investigated the role of procalcitonin (PCT) in human malaria infection and concluded that it is elevated during clinical episodes of malaria (123–125). PCT has been shown to correlate with malarial parasite density (r = 0.53; P = 0.027) (125). However, few studies reported results stratified by immune status. A prospective clinical study from Hamburg compared serum levels of PCT among nonimmune patients with uncomplicated falciparum malaria (n = 20), semi-immune patients with uncomplicated falciparum malaria (n = 16), and patients with severe falciparum malaria (n = 30) (126). While normal values of PCT are expected to be below 0.5 ng/ml, nonimmune patients with uncomplicated malaria had a geometric mean concentration of 1.1 ng/ml, semi-immune patients with uncomplicated malaria of 2.4 ng/ml, and those with severe malaria of 10.7 ng/ml. The authors performed t tests for hypothesis testing among the three groups, all yielding results at a P value of <0.001. Among those with severe malaria, 6 had a fatal outcome and their PCT levels had a range of 25.6 to 132.1 ng/ml. A case report of two pediatric patients from Italy with severe cerebral malaria suggests that nonimmune children with severe disease also have extremely high levels of PCT at admission. PCT at baseline for the two patients were 6.3 ng/ml and 100 ng/ml, respectively, falling after treatment initiation with PCT of 4.4 ng/ml and 25.6 ng/ml at day 1 posttreatment and PCT of 3.2 ng/ml and 4 ng/ml at day 2 posttreatment (127).
Additionally, three studies from Rotterdam evaluated the role of plasma lactate and hyponatremia and the presence of schizonts in nonimmune travel medicine patients with malaria but not in semi-immune travelers (128–130). First, if plasma lactate was above the upper range, the odds for severe malaria were 31-fold (OR, 31; 95% CI, 6 to 164) increased, which is in line with the current definition of severe malaria; its sensitivity to detect severe malaria was 67%, and specificity was 94% (128). Second, for nonimmune malaria patients with serum sodium values of below 133 mmol/liter at admission, the confounder-adjusted odds for severe malaria was 10.4-fold (adjusted OR, 10.4; 95% CI, 3.1 to 34.9) elevated above those with normal serum sodium values (129). Concordantly, hyponatremia with values below 133 mmol/liter had a sensitivity of 69% and specificity of 76% to predict severe malaria (129). Third, out of a cohort of 401 malaria patients, 49 had P. falciparum schizonts in the peripheral blood by microscopy, with a 23-fold elevation in the odds for severe malaria (95% CI, 11.3 to 46.6) compared with those without P. falciparum schizonts (130). Furthermore, the odds for other unfavorable clinical outcomes were also much higher in the presence of P. falciparum schizonts (130). A hospital-based study from London did not determine the odds of severe malaria in the presence of schizonts in the peripheral blood but found weak evidence of a trend that more nonimmune patients showed P. falciparum schizonts in their peripheral blood film than semi-immune patients (14% [24/167] versus 8% [7/93], respectively; P = 0.1) (131). Lastly, a hospital-based study on nonimmune travelers (n = 94) from Israel indicated that at admission, D-dimer levels were higher in patients with falciparum malaria than in patients with non-falciparum malaria and were higher in patients with severe falciparum malaria than in patients with uncomplicated falciparum malaria (132). The authors concluded that elevated levels in falciparum malaria reflect increased endothelial damage.
It worth mentioning that severe malaria can behave as disseminated intravascular coagulation in bacterial sepsis (133). Therefore, it is crucial that the interpretation of results of studies on severe malaria, particularly those studies focusing on laboratory results, takes into account the composition of participants in the control group.
DIAGNOSIS OF PLASMODIUM INFECTIONS IN NON-MALARIA-ENDEMIC COUNTRIES
As demonstrated in the previous section, clinical signs and symptoms of malaria are nonspecific; therefore, the presence of Plasmodium parasites in blood must be demonstrated for the proper establishment of the diagnosis of malaria. Blood is almost exclusively used for diagnostic sample preparation, although new approaches are under investigation, testing the validity of other biological material for detection of malaria, e.g., saliva (134). Their development process, however, is not sufficiently advanced for routine care; thus, here the focus will be on the diagnosis of malaria in blood samples.
A variety of diagnostic tools exists for the detection of malaria in blood (135–143). Depending on the available resources, it may be feasible to use highly sensitive and specific methods, such as PCR, loop-mediated amplification (LAMP), or enzyme-linked immunosorbent assay (ELISA) (137, 141, 143–146). However, these techniques are still restricted to a small number of specialized centers (143, 147). The first reason is the cost and time constraints of such methods and the necessity of having highly trained personnel to operate specific tools. The second reason is malaria is a rather uncommon condition in many nonendemic settings; therefore, the implementation of high-throughput testing for Plasmodium parasites in clinical samples in daily routine is not required. Third, clinical malaria can rapidly progress to severe disease; thus, waiting for the arrival of diagnostic results stemming from time-intensive highly sensitive and specific methods often is not indicated. Therefore, malaria light microscopy and rapid diagnostic tests (RDTs) are the most commonly applied methods for rapid ascertainment of malaria in clinical routine. Both can yield diagnostic results quickly and at high validity, implying that assays have high values in diagnostic sensitivity and specificity (135, 138, 148, 149). However, due to its versatility, favorable diagnostic performance, and rapidity with which diagnostic results can be obtained, expert light microscopy is still regarded the gold standard in clinical routines (135, 149, 150).
Diagnostic Challenges in Nonendemic Areas
While sensitivity and specificity are specific properties of the respective diagnostic method, the concepts of positive (PPV) and negative (NPV) predictive values also take into account the condition of interest (e.g., disease) in the target population (151–154). Such predictive values denote the probabilities to which given PPV and NPV are correct in a certain setting; both strongly depend on the prevalence of the condition that they aim to detect (151). Given constant values of sensitivity and specificity of a diagnostic method, PPVs drop the lower the prevalence (equal to pretest probability) in a given setting, while NPVs drop the higher the prevalence gets (151, 154). While both PPV and NPV are dependent on sensitivity and specificity, PPVs are particularly affected by low specificities and NPVs by low sensitivities (151, 154). Regardless of patients’ potential immunity, malaria prevalence among patient populations in nonendemic countries may be low even in many specialized centers. This means that particularly positive test results of non-gold standard tools need to be treated with caution due to unfavorable PPVs. This highlights the importance of collecting detailed medical and travel histories so accurate pretest probabilities of malaria can be assigned prior to testing an individual for malaria. A clinician thereby may interpret a positive malaria test result with much more diagnostic certainty in the presence of conclusive evidence in favor of a potential malaria episode, as ascertained by individual patient history, than in the absence of such evidence. For that, structured algorithms for the management of individuals coming from tropical and subtropical regions may be helpful (155). NPVs, on the other hand, may mostly be favorably high in nonendemic settings; this is convenient in the context of clinical management of malaria cases, as it is more dangerous to miss a malaria episode in nonendemic settings than give a treatment course of the up-to-date, well-tolerable, and efficient antimalarial medicines to someone with a false-positive test result (156).
Potential Diagnostic Differences between Populations of Semi-immunity and Nonimmune Travelers
Semi-immune and nonimmune patients presenting themselves to a health facility with symptoms constitute two considerably heterogenous groups. While nonimmune patients may present with a high parasitemia due to an absence of parasite-clearing immunity, they also may have a low parasitemia, as they can become symptomatic already at a low parasite density, which may prompt them to seek health care early (99–103). On the other hand, semi-immune patients tend to experience malaria-attributable symptoms at a higher parasite threshold; therefore, it is likely that semi-immune individuals actively seeking care have high parasitemia (99–101, 104). However, parasite density may seldomly reach very high levels (e.g., above 100,000 parasites per microliter) in individuals with (partial) semi-immunity (103). Two hospital-based studies from Munich (n = 210) and London (n = 260) demonstrated that there was not sufficient evidence to conclude that the parasite density of nonimmune malaria patients was considerably different from that of semi-immune malaria patients (P = 0.224 and P = 0.8, respectively) (118, 131). In the UK study, the mean parasite density at admission was 0.32% (range, 0.001% to 45%) for nonimmune patients and 0.4% (range, 0.001% to 7%) for semi-immune patients (P = 0.8). There was a similar proportion of nonimmune and semi-immune patients in the category of parasite density, <2% (119/167 [71%] versus 70/93 [75%]), and 7.8% (13/167) of the nonimmune patients had a parasitemia of >10%, with no semi-immune patients in this category (131). Overall, nonimmune patients had 4.5-fold (OR, 4.5; 95% CI, 1.5 to 13.2) higher odds of having a parasite density above 5% than semi-immune patients (131).
It is possible that semi-immune patients (for instance, migrants from sub-Saharan Africa) have an asymptomatic malaria parasitemia, reflecting the long and continuous parasite exposure in their malaria-endemic countries of origin (157). This may be more true for recently arrived immigrants than VFRs who have lived in nonendemic areas for decades. Therefore, PPVs are probably higher in this patient population due to a higher malaria prevalence than that of particularly short-term travelers. A study from Spain demonstrated that submicroscopically low-level parasitemia was much more common in patients with potential semi-immunity than in nonimmune travelers (158). This may highlight the need to implement more sensitive diagnostic methods (e.g., PCR) for screening such patient populations in clinical routines, as asymptomatic malaria infections may also have negative long-term health implications (e.g., potential development of anemia and thrombocytopenia) (159, 160). A hospital-based study from Ghana has demonstrated that the lower the parasitemia in a symptomatic semi-immune patient, the less likely it is that malaria is the causal factor of the symptoms that urged the individual to seek health care (161). Thus, in a semi-immune symptomatic patient with a low parasitemia, it is important that alternative causes of the presenting symptoms are considered.
Challenges in Detecting Infections of Very Low and Very High Parasite Densities
Sensitive diagnostic methods should be applied in order not to miss a patient with malaria. Due to the differing parasitological characteristics of presenting patients, it is important that routine diagnostic tools are capable of detecting a high parasite density as well as a (very) low one. For light microscopy, diagnostic sensitivity has a correlation with parasite density, with a theoretical limit of detection of ten parasites per microliter, while in reality the limit of detection is higher depending on the skill and training of the microscopist (135). To enhance diagnostic sensitivity, repeated testing of blood samples being sampled in 12- to 24-h intervals has been recommended (116). This is based on the thought that a parasite density that had previously been too low to be detected may surpass the limit of detection at the time of second testing. Therefore, hospitalization of symptomatic, suspected malaria patients with (initially) negative test results may be considered to cover the interval needed for the maturation of an asexual parasite generation, which may take a minimum of 24 h (P. knowlesi), 48 h (P. falciparum, P. ovale wallikeri, P. ovale curtisi, and P. vivax), or 72 h (P. malariae) (38, 105). However, a diagnostic study from Australia suggests that only as much as 3.5% of initially negative blood films were revealed to be positive upon subsequent testing (162). To avert the potentially life-threatening consequences of malaria, particularly in nonimmune patients, some authors suggested that despite a negative RDT result, antimalarial treatment should be administered if no other valid and rapid diagnostic method is available and there is high suspicion of malaria (163).
Modern RDT performance was demonstrated to be highly favorable for a parasite density of above 100 per microliter (164). However, for HRP-2-based RDTs, the so-called prozone effect was described, which can lead to false-negative test results in patients with moderate to (very) high parasitemia (>20,000 parasites per microliter) (165–167). It has been hypothesized that due to a high concentration of antigens and/or antibodies, RDT-specific immunological reactions are impaired, thereby preventing the emergence of a positive band. Prozone effect-attributable false-negative RDT results recently were linked to fatal malaria episodes in returning travelers (166). To rule out prozone effects in a presumably false-negative RDT test result, one can dilute the blood sample with an isotonic agent and perform another (aldolase-based) RDT; however, another diagnostic method should also be applied (e.g., microscopy) (165).
A meta-analysis on RDTs in nonimmune travelers used the negative likelihood ratio (LR−) as the main measure of diagnostic performance in nonendemic settings (168). The LR− demonstrates how much less likely it is to have a negative test result in a malaria patient than in a person without malaria (154). Thus, the LR− is similar to the NPV in terms of being helpful in ruling out malaria independent from prevalence. Overall RDT performance in nonimmune malaria patients was best for HRP-2-based assays for detecting P. falciparum, as reflected by an LR− of 0.08 (95% CI, 0.06 to 0.1), followed by lactate dehydrogenase (LDH)-based tests, with an LR− of 0.13 (95% CI, 0.07 to 0.22). Given that HRP-2 deletions may become more frequent, LR− values may rise for HRP-2-based tests, while performance of LDH-based tests is believed to be independent of HRP-2 deletions. Overall performance of RDTs to detect P. vivax was better for LDH-based assays than for HRP-2-based assays, with an LR− of 0.13 (95% CI, 0.06 to 0.27) and 0.24 (95% CI, 0.11 to 0.54), respectively. The overall performance of RDTs for detecting P. ovale and P. malariae was unfavorable, with negative LRs being close to 1 (null value). When stratifying outcomes by different levels of parasite density, RDT performance was unsatisfactory at detecting a parasite density of P. falciparum in the range of 0 to 100 parasites per microliter (mean LR−, 0.33) but was good for the range of 101 to 1,000 parasites per microliter (mean LR−, 0.07). For P. vivax, one study found that RDT performance was unsatisfactory for a parasite density of up to 1,000 parasites per microliter (168).
TREATMENT OF IMPORTED MALARIA
To date, artemisinin-based combination therapies (ACTs) are recommended for the treatment of uncomplicated malaria in endemic and nonendemic areas, and they are administered orally (156). The recommendation applies both to nonimmune patients and to semi-immune patients. Possible differences and susceptibility patterns between these two populations could be important for clinical management, and different management scenarios are conceivable based on immune status. For those lacking semi-immunity, inpatient management may be favorable due to higher risk for adverse outcomes, while those with potential semi-immunity may be managed safely on an outpatient basis. However, such differential therapeutic management based on immune status has yet to be evaluated and should be guided by robust biomarkers reflecting anti-disease immunity. Therefore, differential therapeutic management based on immune status is currently not supported by firm evidence.
For the treatment of severe and complicated malaria, intravenous (i.v.) artesunate has been the recommendation by the WHO since 2010, replacing quinine as the first-line agent (169–171). This decision was largely based on the AQUAMAT and SEQUAMAT trials, two large multicenter studies in Africa and Asia, respectively, demonstrating more favorable survival rates in adults and children who were treated with i.v. artesunate than in those who were treated with i.v. quinine (172, 173). As for uncomplicated malaria, to the best of our knowledge there is no evidence available supporting differential management approaches for patients with severe malaria based on individual immune status. However, based on the potentially unfavorable clinical outcomes of severe malaria, differential management approaches most likely would not be indicated.
Recently, a large hospital-based study from France investigated several clinical outcomes among patients with severe malaria receiving either i.v. artesunate or i.v. quinine (174). The authors analyzed a historical cohort of patients with imported falciparum malaria who presented to 110 hospitals in France between 2011 and 2017, using propensity scores to account for systematic differences between the two treatment arms. Interestingly, they did not demonstrate the superiority of artesunate over quinine in most of the outcomes, but the retrospective design is a limitation of this study. Among 1,544 total cases, the proportion of fatal outcomes on day 28 posttreatment was 2.9% (32/1,084) in the artesunate group and 3.9% (18/460) in the quinine group (weighted adjusted hazard ratio [HR], 1.03; 95% CI, 0.47 to 2.25; P = 0.923). Additionally, there was no evidence for different hospitalization times between the treatment arms, with a median (IQR) length of hospital stay of 6 (4 to 10) days in the artesunate group and 5 (4 to 7) days in the quinine group (weighted-adjusted HR, 1.12; 95% CI, 0.94 to 1.34; P = 0.212). Patients in the artesunate group had a faster discharge rate from intensive care unit (ICU) than those in the quinine group, with a median (IQR) of 2 (1 to 3) days in the ICU in the artesunate group and a median of 3 (2 to 5) days in the quinine group (weighted-adjusted HR, 1.18; 95% CI, 1.02 to 1.36; P = 0.03).
These findings highlight the importance of high-level care, which is often available in non-malaria-endemic high-resource settings. Given the common side effects of treatment with quinine, including hypoglycemia, cardiotoxicity, and hearing disturbance, along with the longer stay in the ICU of patients in the quinine treatment arm, artesunate remains the drug of choice (175). The authors conclude that these findings should not be seen as favoring a reintroduction of quinine as a first-line treatment choice for severe malaria in high-resource settings, as shortened ICU rates also hold both individual and economic benefits.
Furthermore, a recently published multicenter study (n = 984) investigated the complementary role of semi-immunity on malaria parasite blood clearance during treatment with artemisinin derivates (176). The authors concluded that the prevalence of IgG3, the presence of complement-fixing antibodies, and opsonic phagocytosis seropositivity were associated with faster parasite clearance rates (range of the mean reduction of parasite clearance, 0.47 to 1.16 h; P value range, 0.001 to 0.03) and reduced odds of having parasitemia 3 days posttreatment.
PROPHYLAXIS IN POPULATIONS OF SEMI-IMMUNITY AND NONIMMUNE TRAVELERS
The currently recommended options for chemoprophylaxis are atovaquone-proguanil, doxycycline, or mefloquine in semi-immune and nonimmune travelers. Recently, tafenoquine received regulatory approval in the United States as a chemoprophylactic agent against malaria (177). The choice of chemoprophylaxis is influenced by, among other factors, destination of travel, duration of stay, preexisting diseases (e.g., renal impairment, hepatic diseases, and psychiatric disorders), preferred intake regimen, common or previously experienced side effects, and cost.
A study evaluating prophylactic regimens for malaria in risk groups traveling to sub-Saharan Africa concluded that mefloquine is the drug of choice, particularly for those traveling for longer periods, for pregnant/breastfeeding women, for small children, and/or for those with a limited budget (178). However, solid data on differences in chemoprophylaxis regimens between semi-immune and nonimmune travelers are scarce.
Several aspects will impact the selection of chemoprophylaxis for semi-immune (usually VFR) travelers versus nonimmune travelers, one of them being financial constraints (29). Generally, mefloquine and doxycycline are less costly options than atovaquone-proguanil, although this may change due to the availability of generic products. However, mefloquine is contraindicated in individuals with psychiatric disorders or predisposing factors for psychiatric diseases (179). Doxycycline can increase skin sensitivity to sunlight and is contraindicated in pregnant women and (in many countries) in young children (180). One explanation for the increased skin sensitivity is that UVA radiation can penetrate deeper into the skin during doxycycline intake (181). Melanin is a protecting factor for UV exposure; thus, doxycycline may be better tolerated by individuals with pigmented skin (182). Solid data supporting this hypothesis, however, are lacking. Another advantage of doxycycline is its antibiotic activity. Thus, it also may have a preventive effect against bacterial infections, such as rickettsiosis, sexually transmitted infections, or infection by Yersinia pestis (116). However, the protective efficacy of this low dose taken for malaria prophylaxis is unknown for the prevention of these diseases.
It has been demonstrated that VFR travelers have a low acceptance rate for malaria chemoprophylaxis (6, 28). The low uptake is probably influenced by a variety of factors. Importantly, the VFRs’ risk awareness may be lower, as they are visiting countries to which they are accustomed. Considering that VFRs are at higher risk to acquire malaria than travelers from nonendemic regions, the adequate intake of correct chemoprophylactic regimens is of particular importance (183).
In the UK, the most recent malaria prevention guidelines highlight the importance of access to medication for malaria prevention, particularly for those who are unlikely to seek regular pretravel advice (184). In UK pharmacies, atovaquone-proguanil chemoprophylaxis now can be purchased without a prescription (185). Whether this improved access to chemoprophylaxis will lead to lower levels of imported malaria remains to be seen, but it is certainly an innovative step. Further strategies to raise awareness for and reduce barriers to effective antimalarial chemoprophylaxis are most needed for this high-risk group (186).
One approach is to address financial barriers with innovative approaches. Earlier studies proposed that pretravel counseling and malaria chemoprophylaxis should be subsidized for VFRs traveling to areas of high malaria endemicity, such as West Africa. These studies have shown that such an approach is cost-effective; indeed, it is less expensive to prevent than to treat imported P. falciparum malaria (187, 188). However, currently there is a lack of political support for such initiatives to subsidize malaria chemoprophylaxis for VFR travelers.
For nonimmune travelers, the current options for malaria chemoprophylaxis are the same as those cited above for semi-immune travelers. Several studies in nonimmune individuals traveling to sub-Saharan Africa demonstrated that mefloquine was less well tolerated than atovaquone-proguanil or doxycycline (189, 190). A Cochrane Review published in 2017 found higher incidences of psychological problems in mefloquine users than in travelers with either atovaquone-proguanil or doxycycline. However, it did not identify more frequent serious side effects during mefloquine chemoprophylaxis than chemoprophylaxis with either atovaquone-proguanil or doxycycline (191). Nevertheless, evidence was of low to very low certainty.
In the same review, a comparable efficacy of mefloquine versus atovaquone-proguanil or doxycycline was reported in nonimmune travelers engaging in short-term international trips. Four studies were identified that directly compared the efficacy of mefloquine, atovaquone-proguanil, and doxycycline in this group of travelers, and only one clinical case of malaria occurred among 1,822 participants (191).
In some countries, such as the United States and Canada, primaquine may be considered an option for chemoprophylaxis (after G6PD deficiency has been excluded). A new drug of this class of antimalarials, tafenoquine, recently was licensed in the United States and Australia for chemoprophylaxis (192). Tafenoquine has important advantages over classical malaria chemoprophylactic medications. First, the regimen is rather simple, with a loading dose of two tablets per day for 3 days within 1 week of travel, only 2 tablets once per week during travel, and 2 tablets once within 1 week of return from the trip. This simple regimen most likely increases adherence and traveler compliance, improving the overall effectiveness of this prophylaxis. Second, classical chemoprophylactic drugs in use in travel medicine suppress blood schizont stages but not the dormant liver stages and therefore are essentially suppressing blood-stage development (193). Due to the hypnozoite stages in Plasmodium ovale and Plasmodium vivax malaria, relapses can occur after termination of prophylaxis. Tafenoquine, in contrast, has efficacy against all Plasmodium species and activity against the liver stages (schizonts, latent hypnozoites, or their respective early postinvasion forms) and gametocytes of all plasmodial species (194–196).
Treatment with tafenoquine in persons with G6PD can lead to hemolytic anemia. To prevent this, prior to treatment with tafenoquine, quantitative G6PD functional testing is required; however, this testing is not routinely available. G6PD deficiency is a genetic disorder that occurs frequently, but often to a moderate phenotypic extent, in sub-Saharan African populations (197). In a geostatistical model, G6PD deficiency prevalences have been estimated to be higher than 20% in some parts of Africa (198). Thus, it remains to be seen if tafenoquine can be a valuable option for semi-immune travelers originating from these malaria-endemic areas.
AREAS OF FUTURE RESEARCH
Notwithstanding the enormous progress in biotechnology, immunology, bioengineering, molecular biology, genetics, and vaccinology in recent years in tropical medicine research, there is still a large malaria burden in populations in areas where it is endemic and in migrants and travelers infected during journeys to malaria-endemic regions. Resolving current problems related to the elaboration of more effective methods of prevention and control of the disease, as well as to advance in eradicating the parasite in different populations with a variable level of semi-immunity against Plasmodium spp., is of paramount importance.
A profound understanding of the nature and pathophysiology of naturally acquired immunity in people constantly exposed to Anopheles species infective bites in tropical regions could play an essential role in the selection of effective tools for the long-term process of the global eradication of malaria in the future.
Despite efforts to identify biomarkers that reflect individual immunity and exposure to malaria, robust biomarkers that indicate clinical and/or anti-parasite immunity are pending. During patient management, such markers could be a key parameter in differential management algorithms and could further give way to future assessment of personalized recommendations for chemoprophylaxis. It may be conceivable that at one time, with the help of a robust biomarker for semi-immunity, antimalarial chemoprophylaxis may still be universally recommended to all below a critical semi-immunity threshold, while those above this threshold might travel without chemoprophylaxis. Biomarkers may also be important for prognostic reasons during clinical courses of malaria episodes. Furthermore, clinical studies describing imported cases of malaria should distinguish among patients with semi-immunity, declining semi-immunity, and nonimmunity to malaria because of evidently variable features in diagnostic parameters, clinical course, potential complications, and prognosis of the disease.
The importance of semi-immunity biomarkers for research is manifold. In trials on antimalarial chemotherapy, it is important to determine the efficacy of the study drug, particularly in nonimmune populations, as no synergistic therapeutic effect of semi-immunity is anticipated in such populations. For this reason, complex multicenter trials need to be conducted in which the immune status of the various participating study populations is mostly based on transmission intensity of the respective study settings (199). In vaccine trials, such biomarkers also could become surrogate parameters, if not primary or secondary study end points. Likewise, diagnostics are known to yield various results on performance characteristics depending on an individual’s immune status. Finally, a better and more accurate classification of immune status would be facilitated by a solid semi-immunity marker given the suboptimal current proxy measures of time spent in host country; this would be of crucial importance especially for studies on imported malaria in nonendemic settings.
Among clinicians working in infectious disease departments in nonendemic countries, there are various beliefs about naturally acquired immunity in immigrants returning from tropical or subtropical regions. While some believe in rapidly waning immunity in this population, some tend to generalize potential immunity onto virtually all of the population. These variable opinions can have important and potentially dangerous implications for patient management.
CONCLUSIONS
The continuous increase in long-distance travel and recent large migratory movements has importantly changed the epidemiological characteristics of imported malaria. While malaria was primarily imported to nonendemic countries by returning travelers, the proportion of immigrants from malaria-endemic regions and VFR travelers in malaria-endemic countries has consistently risen. This shifted epidemiology urges health care systems of non-malaria-endemic countries to develop strategies to identify, diagnose, and treat those with imported malaria for their benefit and for the prevention of the reintroduction of malaria and subsequent autochthonous malaria transmission. Travelers belonging to vulnerable populations and those who are at high risk to acquire malaria should receive increased attention to ensure reliable access to recommended chemoprophylaxis and pretravel counseling. Most importantly, further research is needed to better understand the mechanisms of individual semi-immunity against the Plasmodium parasite and clinical malaria.
Differences between patients with existing but declining semi-immunity and those lacking immunity were described in this work. Most large studies indicate at least a decades-long effect of a certain degree of protection from naturally acquired immunity in VFRs. This manifests in decreased odds for severe malaria and clinical complications compared with those for nonimmune returning travelers. Whereas this longevity of some degree of immunity is of interest, it must not be confused with individual protection, and it should be stressed that all travelers require identical tools to minimize the risk of malaria during travel. Diagnostic features and clinical signs are more pronounced in nonimmune individuals with imported malaria, the most prominent ones being splenomegaly, hyperbilirubinemia, thrombocytopenia, and fever. For semi-immune individuals with imported malaria, no such data could be identified; however, the diagnostic utility of clinical and laboratory features of semi-immune malaria patients in endemic settings seems much less favorable, indicating that indirect predictors of malaria are not of much use in semi-immune travelers. Furthermore, due to the multitude of factors related to the manifestation of individual malaria episodes, both semi-immune and nonimmune patients may present with highly varying parasite densities. This has important implications for diagnostic algorithms, as false-negative test results may be inherent to both low parasite density (e.g., submicroscopic parasitemia) and high parasite density (e.g., prozone effect).
So far, no robust biomarkers exist that properly reflect anti-parasite or clinical immunity. Identification of such a biomarker would be helpful for clinical management and risk stratification as well as serving as guidance for immune correlates in vaccine and drug development. Until then, researchers should rigorously stratify their results by immune status using surrogate markers such as duration spent outside of a malaria-endemic country.
ACKNOWLEDGMENTS
This work involved the ESCMID Study Group on Clinical Parasitology and the ESCMID Study Group on travel and migrations.
We have no relevant interests to declare.
Biographies

Johannes Mischlinger completed his studies in medicine at the Medical University of Graz, Austria, and obtained an M.Sc. in Tropical Medicine and International Health by the London School of Hygiene and Tropical Medicine (LSHTM), UK. Afterwards he worked at the Centre de Recherches Médicales de Lambaréné in Gabon as a research physician in clinical trials on antimalarial chemotherapy. As part of these activities, he completed a Ph.D. in Public Health at the Medical University of Vienna, Austria, and a distance-learning M.Sc. in Epidemiology at LSHTM is currently ongoing. His research interests comprise research methodology, epidemiology, and statistical analysis in the broad field of clinical tropical medicine. Since 2018, he has been working at the Bernhard Nocht Institute for Tropical Medicine in Hamburg, Germany, in the Department of Tropical Medicine, headed by Michael Ramharter.

Caroline Rönnberg obtained her medical degree in 2007 from the Medical University of Warsaw, Poland followed by 1.5 years of internship in Sandnessjøen District Hospital on Alsten island in north Norway. In 2010, she received a Diploma in Tropical Medicine and Hygiene at the Liverpool School of Tropical Medicine, UK. Specialist training in the field of clinical microbiology was undertaken at Karolinska University Hospital in Stockholm, Sweden, and finalized in 2017. She defended her Ph.D. thesis on B cells and Plasmodium falciparum malaria at Karolinska Institutet in November 2019. She currently holds a position as analyst and clinical microbiology consultant in the parasitology unit of the Department of Microbiology at the Public Health Agency in Stockholm, Sweden.

Míriam J. Álvarez-Martínez, M.D., Ph.D., is a Medical Microbiologist and Clinical Parasitologist at Hospital Clinic-Isglobal, Barcelona, and Associated Professor at the University of Barcelona, Spain. Her main research interest is the molecular parasitology applied to clinical diagnostics and molecular characterization of drug resistance in parasitic diseases, as well as the study of new diagnostic methods for neglected tropical diseases. She has been a visiting scholar at the University of North Carolina, Chapel Hill, NC, and holds an M.Sc. from the London School of Hygiene & Tropical Medicine. She has been involved in research projects in Bolivia, Brazil, Congo DR, Malawi, Morocco, Mozambique, the United States, and South Africa. Currently, she is the Chair of ESGCP (European Study Group of Clinical Parasitology) of the European Society of Clinical Microbiology & Infectious Diseases.

Silja Bühler worked in clinical medicine for four years and completed her master’s degrees (Public Health in 2008 and Epidemiology in 2011, both from the London School of Hygiene and Tropical Medicine), and subsequently worked from 2011 to 2018 as a senior research scientist at the Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Switzerland. In 2018, she joined the Bernhard Nocht Institute/University Medical Center Hamburg-Eppendorf, Hamburg, Germany, as head of the Travel Clinic. Her research includes projects in diverse fields of travel medicine (prophylaxis of infectious and noninfectious diseases, travel characteristics, influence of travel style on health outcomes, and travel patterns of individuals with chronic diseases and the elderly population) as well as vaccinology (new vaccination schemes, vaccination of the immunocompromised, and vaccination knowledge and attitudes). She maintains close collaborations with international research groups, such as the European Network for Tropical Medicine and Travel Health (TropNet).

Małgorzata Paul is an associate professor in the Department and Clinic of Tropical and Parasitic Diseases, University of Medical Sciences in Poznan (Poland). She is a specialist of tropical diseases and clinical parasitology and also a regional consultant on tropical and maritime medicine. She has received a Diploma of Tropical Medicine and Hygiene (DTM&H) at the Instituto de Medicina Tropical Alexander von Humboldt, Universidad Peruana Cayetano Heredia, Lima (Peru), awarded by the Gorgas Memorial Institute of Tropical and Preventive Medicine, the University of Alabama, Birmingham, Alabama. She is a Polish coordinator of the European Network for Tropical Medicine and Travel Health, Member of the Board of the Study Group on Clinical Parasitology in European Society for Clinical Microbiology and Infectious Diseases, expert of the European Centers for Disease Control and Prevention on infectious diseases (ECDC), and Editor of the Annals of Parasitology, in the field of medical parasitology.

Patricia Schlagenhauf is a Professor and Senior Scientist at the University of Zürich. She is a Codirector of the WHO Collaborating Centre for Travellers' Health, Zürich GeoSentinel Site Director, and EuroTravNet Steering Committee member. Her research focus areas are travellers’ malaria, epidemiology and prevention of travel-associated disease, travellers’ vaccines and medications, and gender issues in travel medicine. She has published widely, with more than 160 papers and the books Travelers’ Malaria (BC Decker 2001, 2nd edition 2008), Infectious Diseases—a Geographic Guide (Wiley 2011, 2nd edition 2017), and the handbook PDQ Handbook of Travelers’ Malaria (BC Decker 2005). Since 2011, she serves as Editor-in-Chief of Travel Medicine and Infectious Disease.

Eskild Petersen is a Professor of Tropical Medicine, Institute of Clinical Medicine, Aarhus University, Denmark, and Consultant, Directorate General for Disease Surveillance and Control, Ministry of Health, Oman. He received a Specialist Degree in Infectious Diseases in 1985, a Specialist Degree in Tropical Medicine in 1988, and a D.M.Sc. in 2005 from Karolinska Institute, Stockholm, Sweden. He was, Associate Professor, Aarhus University, from 2005 to 2013 and was a Professor in 2014. He was a Consultant, Department of Infectious Diseases, Aarhus University Hospital, Denmark, from 2003 to 2015 and at Statens Serum Institut (National Public Health Institute), Copenhagen, Denmark, from 1989 to 2003. He performed field research on malaria in Liberia under the Department of Immunology, University of Stockholm, from 1985 to 1987. He is a Co-Editor (Moderator) on parasitic diseases, ProMED (www.promedmail.org), he has edited several textbooks, including Infectious Disease: a Geographic Guide (2nd edition, Wiley, 2017) and coauthored the malaria chapter in Keystone et al. Travel Medicine (4th edition, Elsevier, 2019). He was a member of the ECCMID Study Group for Clinical Parasitology from 2010 to 2016, responsible for malaria. Presently, he is cochair of the ESCMID Emerging Infections Task Force.

Michael Ramharter was trained as an M.D. at the Medical University of Vienna, Austria, specializing in Internal Medicine, Infectious Diseases, and Tropical Medicine. His research interest focuses on the clinical development of antimalarial drugs in Africa, where he is involved clinical phase I to IV drug and vaccine development programs. Clinical research in the fields of neglected tropical diseases and emerging infectious diseases are further key aspects of his work. A common theme in these scientific projects is the focus of improving health care for the most vulnerable populations in sub-Saharan Africa, such as young children and pregnant women. He heads the scientific working group Infectious Disease Control at the Centre de Recherches Médicales de Lambaréné, Gabon, and is Professor in Tropical Medicine, heading the Department of Tropical Medicine at the Medical Faculty of Hamburg and the Department of Clinical Research at the Bernhard Nocht Institute for Tropical Medicine in Hamburg, Germany.
APPENDIX
GLOSSARY
- anti-parasite immunity
Immunity that may prevent high malaria parasite blood densities.
- clinical immunity
Used synonymously with “anti-disease immunity” here. Immunity against malaria disease (note that the individual may still be infected).
- elimination
Interruption of local mosquito-borne malaria transmission in a defined geographical area, usually a country.
- eradication
Permanent reduction to zero of the worldwide incidence of malaria.
- immigrant
Person living in a country other than his or her birthplace.
- imported malaria
Plasmodium infection is acquired in a malaria-endemic area, and clinical manifestation, diagnosis, and management take place in a non-malaria-endemic country.
- indigenous malaria
Acquired and diagnosed malaria in malaria-endemic area.
- likelihood ratios
Measure of diagnostic performance. The higher the positive likelihood ratio (LR+), the better the diagnostic performance. The lower the negative likelihood ratio (LR−), the better the diagnostic performance.
- naturally acquired immunity (NAI)
(Semi-)Immunity to Plasmodium parasites due to sustained and years-long exposure in malaria-endemic countries.
- nonimmune person
Person born and living most of the time in a country without malaria transmission.
- person with declining semi-immunity to malaria
Person born and living in a country of moderate to high malaria endemicity who migrated to a non-malaria-endemic country and therefore has a declining level of semi-immunity. This includes the majority of VFR travelers.
- person with semi-immunity to malaria
Person born and living in a country of moderate to high malaria endemicity and still residing in a malaria-endemic country.
- Plasmodium infection
Infection with Plasmodium parasites.
- predictive values
Positive (PPV) and negative (NPV) predictive values denote the probability that one can trust that a positive/negative test result truly corresponds to the presence/absence of disease.
- returning travelers
Conventional international tourists returning from travel.
- VFRs (visiting friends and relatives)
Immigrants from malaria-endemic regions often with permanent residence in non-malaria-endemic regions traveling to the country of birth mostly for visits to friends or relatives.
REFERENCES
- 1.World Health Organization. 2018. World malaria report 2018. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Thierfelder C, Schill C, Hatz C, Nuesch R. 2008. Trends in imported malaria to Basel, Switzerland. J Travel Med 15:432–436. doi: 10.1111/j.1708-8305.2008.00251.x. [DOI] [PubMed] [Google Scholar]
- 3.Huang Z, Tatem AJ. 2013. Global malaria connectivity through air travel. Malar J 12:269. doi: 10.1186/1475-2875-12-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monge-Maillo B, López-Vélez R. 2012. Migration and malaria in Europe. Mediterr J Hematol Infect Dis 4:e2012014. doi: 10.4084/MJHID.2012.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Checkley AM, Smith A, Smith V, Blaze M, Bradley D, Chiodini PL, Whitty CJ. 2012. Risk factors for mortality from imported falciparum malaria in the United Kingdom over 20 years: an observational study. BMJ 344:e2116. doi: 10.1136/bmj.e2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith AD, Bradley DJ, Smith V, Blaze M, Behrens RH, Chiodini PL, Whitty CJ. 2008. Imported malaria and high risk groups: observational study using UK surveillance data 1987–2006. BMJ 337:a120. doi: 10.1136/bmj.a120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pistone T, Diallo A, Mechain M, Receveur MC, Malvy D. 2014. Epidemiology of imported malaria give support to the hypothesis of “long-term” semi-immunity to malaria in sub-Saharan African migrants living in France. Travel Med Infect Dis 12:48–53. doi: 10.1016/j.tmaid.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Tatem AJ, Jia P, Ordanovich D, Falkner M, Huang Z, Howes R, Hay SI, Gething PW, Smith DL. 2017. The geography of imported malaria to non-endemic countries: a meta-analysis of nationally reported statistics. Lancet Infect Dis 17:98–107. doi: 10.1016/S1473-3099(16)30326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tatem AJ. 2017. Underestimate of annual malaria imports to Canada–author reply. Lancet Infect Dis 17:142–143. doi: 10.1016/S1473-3099(17)30025-7. [DOI] [PubMed] [Google Scholar]
- 10.Lai S, Li Z, Wardrop NA, Sun J, Head MG, Huang Z, Zhou S, Yu J, Zhang Z, Zhou SS, Xia Z, Wang R, Zheng B, Ruan Y, Zhang L, Zhou XN, Tatem AJ, Yu H. 2017. Malaria in China, 2011–2015: an observational study. Bull World Health Organ 95:564–573. doi: 10.2471/BLT.17.191668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang SS, Feng J, Zhang L, Ren X, Geoffroy E, Manguin S, Frutos R, Zhou SS. 2019. Imported malaria cases in former endemic and non-malaria endemic areas in China: are there differences in case profile and time to response? Infect Dis Poverty 8:61. doi: 10.1186/s40249-019-0571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou S, Li Z, Cotter C, Zheng C, Zhang Q, Li H, Zhou S, Zhou X, Yu H, Yang W. 2016. Trends of imported malaria in China 2010–2014: analysis of surveillance data. Malar J 15:39. doi: 10.1186/s12936-016-1093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai S, Wardrop NA, Huang Z, Bosco C, Sun J, Bird T, Wesolowski A, Zhou S, Zhang Q, Zheng C, Li Z, Tatem AJ, Yu H. 2016. Plasmodium falciparum malaria importation from Africa to China and its mortality: an analysis of driving factors. Sci Rep 6:39524. doi: 10.1038/srep39524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Yang Y, Xiao N, Zhou S, Lin K, Wang D, Zhang Q, Jiang W, Li M, Feng X, Yu J, Ren X, Lai S, Sun J, Fang Z, Hu W, Clements ACA, Zhou X, Yu H, Yang W. 2015. Malaria imported from ghana by returning gold miners, China, 2013. Emerg Infect Dis 21:864–867. doi: 10.3201/eid2105.141712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong X, Yang J, Lou L, Zhu L, Feng X, Yao L. 2017. Once malaria is eliminated, more attention should be paid to imported malaria: data from five years of surveillance in the city of Yiwu in eastern China. Biosci Trends 11:360–362. doi: 10.5582/bst.2017.01113. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Sturrock HJW, Yang H, Gosling RD, Cao J. 2017. The challenge of imported malaria to eliminating countries. Lancet Infect Dis 17:141. doi: 10.1016/S1473-3099(17)30006-3. [DOI] [PubMed] [Google Scholar]
- 17.Broderick C, Nadjm B, Smith V, Blaze M, Checkley A, Chiodini PL, Whitty CJ. 2015. Clinical, geographical, and temporal risk factors associated with presentation and outcome of vivax malaria imported into the United Kingdom over 27 years: observational study. BMJ 350:h1703. doi: 10.1136/bmj.h1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mascarello M, Gobbi F, Angheben A, Concia E, Marocco S, Anselmi M, Monteiro G, Rossanese A, Bisoffi Z. 2009. Imported malaria in immigrants to Italy: a changing pattern observed in north eastern Italy. J Travel Med 16:317–321. doi: 10.1111/j.1708-8305.2009.00321.x. [DOI] [PubMed] [Google Scholar]
- 19.Norman FF, +REDIVI Study Group, López-Polín A, Salvador F, Treviño B, Calabuig E, Torrús D, Soriano-Arandes A, Ruíz-Giardín J-M, Monge-Maillo B, Pérez-Molina J-A, Perez-Ayala A, García M, Rodríguez A, Martínez-Serrano M, Zubero M, López-Vélez R. 2017. Imported malaria in Spain (2009–2016): results from the +REDIVI Collaborative Network. Malar J 16:407. doi: 10.1186/s12936-017-2057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. 2018. Morbidity and mortality weekly report malaria surveillance–United States 2015. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/mmwr/volumes/67/ss/pdfs/ss6707a1-H.pdf. [Google Scholar]
- 21.Mace KE, Arguin PM, Lucchi NW, Tan KR. 2019. Malaria surveillance–United States, 2016. MMWR Surveill Summ 68:1–35. doi: 10.15585/mmwr.ss6805a1. [DOI] [PubMed] [Google Scholar]
- 22.Jelinek T, Schulte C, Behrens R, Grobusch MP, Coulaud JP, Bisoffi Z, Matteelli A, Clerinx J, Corachan M, Puente S, Gjorup I, Harms G, Kollaritsch H, Kotlowski A, Bjorkmann A, Delmont JP, Knobloch J, Nielsen LN, Cuadros J, Hatz C, Beran J, Schmid ML, Schulze M, Lopez-Velez R, Fleischer K, Kapaun A, McWhinney P, Kern P, Atougia J, Fry G, da Cunha S, Boecken G. 2002. Imported Falciparum malaria in Europe: sentinel surveillance data from the European network on surveillance of imported infectious diseases. Clin Infect Dis 34:572–576. doi: 10.1086/338235. [DOI] [PubMed] [Google Scholar]
- 23.Romi R, Sabatinelli G, Majori G. 2001. Malaria epidemiological situation in Italy and evaluation of malaria incidence in Italian travelers. J Travel Med 8:6–11. doi: 10.2310/7060.2001.5140. [DOI] [PubMed] [Google Scholar]
- 24.Hickey PW, Cape KE, Masuoka P, Campos JM, Pastor W, Wong EC, Singh N. 2011. A local, regional, and national assessment of pediatric malaria in the United States. J Travel Med 18:153–160. doi: 10.1111/j.1708-8305.2011.00514.x. [DOI] [PubMed] [Google Scholar]
- 25.Mathai S, Bishburg E, Slim J, Nalmas S. 2010. Severe malaria in immigrant population: a retrospective review. J Immigr Minor Health 12:921–924. doi: 10.1007/s10903-009-9256-5. [DOI] [PubMed] [Google Scholar]
- 26.Smith DL, McKenzie FE, Snow RW, Hay SI. 2007. Revisiting the basic reproductive number for malaria and its implications for malaria control. PLoS Biol 5:e42. doi: 10.1371/journal.pbio.0050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pavli A, Maltezou HC. 2010. Malaria and travellers visiting friends and relatives. Travel Med Infect Dis 8:161–168. doi: 10.1016/j.tmaid.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Schlagenhauf P, Patel D. 2018. “Who,” “where,” and “why”: moves to checkmate imported malaria? Clin Infect Dis 69:1163–1164. doi: 10.1093/cid/ciy1044. [DOI] [PubMed] [Google Scholar]
- 29.Neave PE, Jones CO, Behrens RH. 2014. Challenges facing providers of imported malaria-related healthcare services for Africans visiting friends and relatives (VFRs). Malar J 13:17. doi: 10.1186/1475-2875-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlagenhauf P, Grobusch MP, Hamer DH, Asgeirsson H, Jensenius M, Eperon G, Rothe C, Isenring E, Fehr J, Schwartz E, Bottieau E, Barnett ED, McCarthy A, Kelly P, Schade Larsen C, van Genderen P, Stauffer W, Libman M, Gautret P. 2018. Area of exposure and treatment challenges of malaria in Eritrean migrants: a GeoSentinel analysis. Malar J 17:443. doi: 10.1186/s12936-018-2586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roggelin L, Tappe D, Noack B, Addo MM, Tannich E, Rothe C. 2016. Sharp increase of imported Plasmodium vivax malaria seen in migrants from Eritrea in Hamburg, Germany. Malar J 15:325. doi: 10.1186/s12936-016-1366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonden K, Castro E, Tornnberg L, Stenstrom C, Tegnell A, Farnert A. 2014. High incidence of Plasmodium vivax malaria in newly arrived Eritrean refugees in Sweden since May 2014. Euro Surveill 19:20890. doi: 10.2807/1560-7917.es2014.19.35.20890. [DOI] [PubMed] [Google Scholar]
- 33.Kaser AK, Arguin PM, Chiodini PL, Smith V, Delmont J, Jimenez BC, Farnert A, Kimura M, Ramharter M, Grobusch MP, Schlagenhauf P. 2015. Imported malaria in pregnant women: a retrospective pooled analysis. Travel Med Infect Dis 13:300–310. doi: 10.1016/j.tmaid.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doolan DL, Dobano C, Baird JK. 2009. Acquired immunity to malaria. Clin Microbiol Rev 22:13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Struik SS, Riley EM. 2004. Does malaria suffer from lack of memory? Immunol Rev 201:268–290. doi: 10.1111/j.0105-2896.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 36.Tran TM, Li S, Doumbo S, Doumtabe D, Huang CY, Dia S, Bathily A, Sangala J, Kone Y, Traore A, Niangaly M, Dara C, Kayentao K, Ongoiba A, Doumbo OK, Traore B, Crompton PD. 2013. An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clin Infect Dis 57:40–47. doi: 10.1093/cid/cit174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez-Barraquer I, Arinaitwe E, Jagannathan P, Kamya MR, Rosenthal PJ, Rek J, Dorsey G, Nankabirwa J, Staedke SG, Kilama M, Drakeley C, Ssewanyana I, Smith DL, Greenhouse B. 2018. Quantification of anti-parasite and anti-disease immunity to malaria as a function of age and exposure. Elife 7:e35832. doi: 10.7554/eLife.35832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM. 2014. Malaria. Lancet 383:723–735. doi: 10.1016/S0140-6736(13)60024-0. [DOI] [PubMed] [Google Scholar]
- 39.Beier JC, Killeen GF, Githure JI. 1999. Short report: entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am J Trop Med Hyg 61:109–113. doi: 10.4269/ajtmh.1999.61.109. [DOI] [PubMed] [Google Scholar]
- 40.Pothin E, Ferguson NM, Drakeley CJ, Ghani AC. 2016. Estimating malaria transmission intensity from Plasmodium falciparum serological data using antibody density models. Malar J 15:79. doi: 10.1186/s12936-016-1121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yman V, White MT, Rono J, Arcà B, Osier FH, Troye-Blomberg M, Boström S, Ronca R, Rooth I, Färnert A. 2016. Antibody acquisition models: A new tool for serological surveillance of malaria transmission intensity. Sci Rep 6:19472. doi: 10.1038/srep19472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rolfes MA, McCarra M, Magak NG, Ernst KC, Dent AE, Lindblade KA, John CC. 2012. Development of clinical immunity to malaria in highland areas of low and unstable transmission. Am J Trop Med Hyg 87:806–812. doi: 10.4269/ajtmh.2012.11-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baird JK. 1998. Age-dependent characteristics of protection v. susceptibility to Plasmodium falciparum. Ann Trop Med Parasitol 92:367–390. doi: 10.1080/00034989859366. [DOI] [PubMed] [Google Scholar]
- 44.Beadle C, McElroy PD, Oster CN, Beier JC, Oloo AJ, Onyango FK, Chumo DK, Bales JD, Sherwood JA, Hoffman SL. 1995. Impact of transmission intensity and age on Plasmodium falciparum density and associated fever: implications for malaria vaccine trial design. J Infect Dis 172:1047–1054. doi: 10.1093/infdis/172.4.1047. [DOI] [PubMed] [Google Scholar]
- 45.Beier JC, Oster CN, Onyango FK, Bales JD, Sherwood JA, Perkins PV, Chumo DK, Koech DV, Whitmire RE, Roberts CR. 1994. Plasmodium falciparum incidence relative to entomologic inoculation rates at a site proposed for testing malaria vaccines in western Kenya. Am J Trop Med Hyg 50:529–536. doi: 10.4269/ajtmh.1994.50.529. [DOI] [PubMed] [Google Scholar]
- 46.McElroy PD, Beier JC, Oster CN, Beadle C, Sherwood JA, Oloo AJ, Hoffman SL. 1994. Predicting outcome in malaria: correlation between rate of exposure to infected mosquitoes and level of Plasmodium falciparum parasitemia. Am J Trop Med Hyg 51:523–532. doi: 10.4269/ajtmh.1994.51.523. [DOI] [PubMed] [Google Scholar]
- 47.McElroy PD, Beier JC, Oster CN, Onyango FK, Oloo AJ, Lin X, Beadle C, Hoffman SL. 1997. Dose- and time-dependent relations between infective Anopheles inoculation and outcomes of Plasmodium falciparum parasitemia among children in western Kenya. Am J Epidemiol 145:945–956. doi: 10.1093/oxfordjournals.aje.a009054. [DOI] [PubMed] [Google Scholar]
- 48.Trape JF, Tall A, Sokhna C, Ly AB, Diagne N, Ndiath O, Mazenot C, Richard V, Badiane A, Dieye-Ba F, Faye J, Ndiaye G, Diene Sarr F, Roucher C, Bouganali C, Bassene H, Toure-Balde A, Roussilhon C, Perraut R, Spiegel A, Sarthou JL, da Silva LP, Mercereau-Puijalon O, Druilhe P, Rogier C. 2014. The rise and fall of malaria in a West African rural community, Dielmo, Senegal, from 1990 to 2012: a 22 year longitudinal study. Lancet Infect Dis 14:476–488. doi: 10.1016/S1473-3099(14)70712-1. [DOI] [PubMed] [Google Scholar]
- 49.Baird JK. 2000. Resurgent malaria at the millennium: control strategies in crisis. Drugs 59:719–743. doi: 10.2165/00003495-200059040-00001. [DOI] [PubMed] [Google Scholar]
- 50.Molineaux L, Dietz K, Thomas A. 1978. Further epidemiological evaluation of a malaria model. Bull World Health Organ 56:565–571. [PMC free article] [PubMed] [Google Scholar]
- 51.Bruce-Chwatt LJ. 1981. Molineaux, L. and Gramiccia, G. (1980). The Garki Project. Research on the epidemiology and control of malaria in the Sudan savanna of West Africa. Geneva: World Health Organization, 311 pp, 33 tables, 83 figs. Price: Sw. Fr. 33. Trans R Soc Trop Med Hyg 75:190–191. doi: 10.1016/0035-9203(81)90085-7. [DOI] [Google Scholar]
- 52.O'Meara WP, Mwangi TW, Williams TN, McKenzie FE, Snow RW, Marsh K. 2008. Relationship between exposure, clinical malaria, and age in an area of changing transmission intensity. Am J Trop Med Hyg 79:185–191. doi: 10.4269/ajtmh.2008.79.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Filipe JA, Riley EM, Drakeley CJ, Sutherland CJ, Ghani AC. 2007. Determination of the processes driving the acquisition of immunity to malaria using a mathematical transmission model. PLoS Comput Biol 3:e255. doi: 10.1371/journal.pcbi.0030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White M, Watson J. 2018. Age, exposure and immunity. Elife 7:e40150. doi: 10.7554/eLife.40150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Snow RW, Omumbo JA, Lowe B, Molyneux CS, Obiero JO, Palmer A, Weber MW, Pinder M, Nahlen B, Obonyo C, Newbold C, Gupta S, Marsh K. 1997. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet 349:1650–1654. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- 56.Gupta S, Snow RW, Donnelly CA, Marsh K, Newbold C. 1999. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med 5:340–343. doi: 10.1038/6560. [DOI] [PubMed] [Google Scholar]
- 57.Akpogheneta OJ, Duah NO, Tetteh KK, Dunyo S, Lanar DE, Pinder M, Conway DJ. 2008. Duration of naturally acquired antibody responses to blood-stage Plasmodium falciparum is age dependent and antigen specific. Infect Immun 76:1748–1755. doi: 10.1128/IAI.01333-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kinyanjui SM, Conway DJ, Lanar DE, Marsh K. 2007. IgG antibody responses to Plasmodium falciparum merozoite antigens in Kenyan children have a short half-life. Malar J 6:82. doi: 10.1186/1475-2875-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guinovart C, Dobano C, Bassat Q, Nhabomba A, Quinto L, Manaca MN, Aguilar R, Rodriguez MH, Barbosa A, Aponte JJ, Mayor AG, Renom M, Moraleda C, Roberts DJ, Schwarzer E, Le Souef PN, Schofield L, Chitnis CE, Doolan DL, Alonso PL. 2012. The role of age and exposure to Plasmodium falciparum in the rate of acquisition of naturally acquired immunity: a randomized controlled trial. PLoS One 7:e32362. doi: 10.1371/journal.pone.0032362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nhabomba AJ, Guinovart C, Jimenez A, Manaca MN, Quinto L, Cistero P, Aguilar R, Barbosa A, Rodriguez MH, Bassat Q, Aponte JJ, Mayor A, Chitnis CE, Alonso PL, Dobano C. 2014. Impact of age of first exposure to Plasmodium falciparum on antibody responses to malaria in children: a randomized, controlled trial in Mozambique. Malar J 13:121. doi: 10.1186/1475-2875-13-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. 2007. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 62.McLean AR, Ataide R, Simpson JA, Beeson JG, Fowkes FJ. 2015. Malaria and immunity during pregnancy and postpartum: a tale of two species. Parasitology 142:999–1015. doi: 10.1017/S0031182015000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Menendez C. 2006. Malaria during pregnancy. Curr Mol Med 6:269–273. doi: 10.2174/156652406776055186. [DOI] [PubMed] [Google Scholar]
- 64.Hviid L, Salanti A. 2007. VAR2CSA and protective immunity against pregnancy-associated Plasmodium falciparum malaria. Parasitology 134:1871–1876. doi: 10.1017/S0031182007000121. [DOI] [PubMed] [Google Scholar]
- 65.Rogerson SJ. 2010. Malaria in pregnancy and the newborn. Adv Exp Med Biol 659:139–152. doi: 10.1007/978-1-4419-0981-7_12. [DOI] [PubMed] [Google Scholar]
- 66.Umbers AJ, Aitken EH, Rogerson SJ. 2011. Malaria in pregnancy: small babies, big problem. Trends Parasitol 27:168–175. doi: 10.1016/j.pt.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 67.Ataide R, Mayor A, Rogerson SJ. 2014. Malaria, primigravidae, and antibodies: knowledge gained and future perspectives. Trends Parasitol 30:85–94. doi: 10.1016/j.pt.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 68.Teo A, Hasang W, Randall LM, Feng G, Bell L, Unger H, Langer C, Beeson JG, Siba PM, Mueller I, Molyneux ME, Brown GV, Rogerson SJ. 2014. Decreasing malaria prevalence and its potential consequences for immunity in pregnant women. J Infect Dis 210:1444–1455. doi: 10.1093/infdis/jiu264. [DOI] [PubMed] [Google Scholar]
- 69.Mayor A, Bardají A, Macete E, Nhampossa T, Fonseca AM, González R, Maculuve S, Cisteró P, Rupérez M, Campo J, Vala A, Sigaúque B, Jiménez A, Machevo S, de la Fuente L, Nhama A, Luis L, Aponte JJ, Acácio S, Nhacolo A, Chitnis C, Dobaño C, Sevene E, Alonso PL, Menéndez C. 2015. Changing trends in P. falciparum burden, immunity, and disease in pregnancy. N Engl J Med 373:1607–1617. doi: 10.1056/NEJMoa1406459. [DOI] [PubMed] [Google Scholar]
- 70.Arama C, Maiga B, Dolo A, Kouriba B, Traore B, Crompton PD, Pierce SK, Troye-Blomberg M, Miller LH, Doumbo OK. 2015. Ethnic differences in susceptibility to malaria: what have we learned from immuno-epidemiological studies in West Africa? Acta Trop 146:152–156. doi: 10.1016/j.actatropica.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 71.Colbourne MJ. 1955. Malaria in Gold Coast students on their return from the United Kingdom. Trans R Soc Trop Med Hyg 49:483–487. doi: 10.1016/0035-9203(55)90017-1. [DOI] [PubMed] [Google Scholar]
- 72.Deloron P, Chougnet C. 1992. Is immunity to malaria really short-lived? Parasitol Today 8:375–378. doi: 10.1016/0169-4758(92)90174-z. [DOI] [PubMed] [Google Scholar]
- 73.Chougnet C, Deloron P, Savel J. 1991. Persistence of cellular and humoral response to synthetic peptides from defined Plasmodium falciparum antigens. Ann Trop Med Parasitol 85:357–363. doi: 10.1080/00034983.1991.11812574. [DOI] [PubMed] [Google Scholar]
- 74.Eisen DP, Wang L, Jouin H, Murhandarwati EE, Black CG, Mercereau-Puijalon O, Coppel RL. 2007. Antibodies elicited in adults by a primary Plasmodium falciparum blood-stage infection recognize different epitopes compared with immune individuals. Malar J 6:86. doi: 10.1186/1475-2875-6-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yman V, White MT, Asghar M, Sundling C, Sonden K, Draper SJ, Osier FHA, Farnert A. 2019. Antibody responses to merozoite antigens after natural Plasmodium falciparum infection: kinetics and longevity in absence of re-exposure. BMC Med 17:22. doi: 10.1186/s12916-019-1255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elliott SR, Payne PD, Duffy MF, Byrne TJ, Tham WH, Rogerson SJ, Brown GV, Eisen DP. 2007. Antibody recognition of heterologous variant surface antigens after a single Plasmodium falciparum infection in previously naive adults. Am J Trop Med Hyg 76:860–864. doi: 10.4269/ajtmh.2007.76.860. [DOI] [PubMed] [Google Scholar]
- 77.Ndungu FM, Lundblom K, Rono J, Illingworth J, Eriksson S, Farnert A. 2013. Long-lived Plasmodium falciparum specific memory B cells in naturally exposed Swedish travelers. Eur J Immunol 43:2919–2929. doi: 10.1002/eji.201343630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mugyenyi CK, Elliott SR, Yap XZ, Feng G, Boeuf P, Fegan G, Osier FFH, Fowkes FJI, Avril M, Williams TN, Marsh K, Beeson JG. 2017. Declining malaria transmission differentially impacts the maintenance of humoral immunity to Plasmodium falciparum in children. J Infect Dis 216:887–898. doi: 10.1093/infdis/jix370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sundling C, Ronnberg C, Yman V, Asghar M, Jahnmatz P, Lakshmikanth T, Chen Y, Mikes J, Forsell MN, Sonden K, Achour A, Brodin P, Persson KE, Farnert A. 2019. B cell profiling in malaria reveals expansion and remodelling of CD11c+ B cell subsets. JCI Insight 4:126492. doi: 10.1172/jci.insight.126492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ataide R, Powell R, Moore K, McLean A, Phyo AP, Nair S, White M, Anderson TJ, Beeson JG, Simpson JA, Nosten F, Fowkes F. 2017. Declining transmission and immunity to malaria and emerging artemisinin resistance in Thailand: a longitudinal study. J Infect Dis 216:723–731. doi: 10.1093/infdis/jix371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Niang M, Niass O, Diagne N, Sarr FD, Faye MM, Diop F, Diouf B, Faye J, Badiane A, Perraut R, Sokhna C, Trape JF, Tall A, Toure-Balde A. 2017. Temporal analysis of IgG antibody responses to Plasmodium falciparum antigens in relation to changing malaria epidemiology in a West African setting. Malar J 16:283. doi: 10.1186/s12936-017-1928-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Portugal S, Tran TM, Ongoiba A, Bathily A, Li S, Doumbo S, Skinner J, Doumtabe D, Kone Y, Sangala J, Jain A, Davies DH, Hung C, Liang L, Ricklefs S, Homann MV, Felgner PL, Porcella SF, Farnert A, Doumbo OK, Kayentao K, Greenwood BM, Traore B, Crompton PD. 2017. Treatment of chronic asymptomatic Plasmodium falciparum infection does not increase the risk of clinical malaria upon reinfection. Clin Infect Dis 64:645–653. doi: 10.1093/cid/ciw849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bouchaud O, Cot M, Kony S, Durand R, Schiemann R, Ralaimazava P, Coulaud JP, Le Bras J, Deloron P. 2005. Do African immigrants living in France have long-term malarial immunity? Am J Trop Med Hyg 72:21–25. doi: 10.4269/ajtmh.2005.72.21. [DOI] [PubMed] [Google Scholar]
- 84.Castelli F, Matteelli A, Caligaris S, Gulletta M, el-Hamad I, Scolari C, Chatel G, Carosi G. 1999. Malaria in migrants. Parassitologia 41:261–265. [PubMed] [Google Scholar]
- 85.Phillips A, Bassett P, Zeki S, Newman S, Pasvol G. 2009. Risk factors for severe disease in adults with falciparum malaria. Clin Infect Dis 48:871–878. doi: 10.1086/597258. [DOI] [PubMed] [Google Scholar]
- 86.Franca CT, Hostetler JB, Sharma S, White MT, Lin E, Kiniboro B, Waltmann A, Darcy AW, Li Wai Suen CS, Siba P, King CL, Rayner JC, Fairhurst RM, Mueller I. 2016. An antibody screen of a Plasmodium vivax antigen library identifies novel merozoite proteins associated with clinical protection. PLoS Negl Trop Dis 10:e0004639. doi: 10.1371/journal.pntd.0004639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Franca CT, He WQ, Gruszczyk J, Lim NT, Lin E, Kiniboro B, Siba PM, Tham WH, Mueller I. 2016. Plasmodium vivax reticulocyte binding proteins are key targets of naturally acquired immunity in young Papua New Guinean children. PLoS Negl Trop Dis 10:e0005014. doi: 10.1371/journal.pntd.0005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Longley RJ, Reyes-Sandoval A, Montoya-Diaz E, Dunachie S, Kumpitak C, Nguitragool W, Mueller I, Sattabongkot J. 2016. Acquisition and longevity of antibodies to Plasmodium vivax preerythrocytic antigens in western Thailand. Clin Vaccine Immunol 23:117–124. doi: 10.1128/CVI.00501-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Folegatti PM, Siqueira AM, Monteiro WM, Lacerda MV, Drakeley CJ, Braga EM. 2017. A systematic review on malaria sero-epidemiology studies in the Brazilian Amazon: insights into immunological markers for exposure and protection. Malar J 16:107. doi: 10.1186/s12936-017-1762-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boyle MJ, Reiling L, Osier FH, Fowkes FJ. 2017. Recent insights into humoral immunity targeting Plasmodium falciparum and Plasmodium vivax malaria. Int J Parasitol 47:99–104. doi: 10.1016/j.ijpara.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Longley RJ, Franca CT, White MT, Kumpitak C, Sa-Angchai P, Gruszczyk J, Hostetler JB, Yadava A, King CL, Fairhurst RM, Rayner JC, Tham WH, Nguitragool W, Sattabongkot J, Mueller I. 2017. Asymptomatic Plasmodium vivax infections induce robust IgG responses to multiple blood-stage proteins in a low-transmission region of western Thailand. Malar J 16:178. doi: 10.1186/s12936-017-1826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nicolete VC, Frischmann S, Barbosa S, King CL, Ferreira MU. 2016. Naturally acquired binding-inhibitory antibodies to Plasmodium vivax duffy binding protein and clinical immunity to malaria in rural Amazonians. J Infect Dis 214:1539–1546. doi: 10.1093/infdis/jiw407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cutts JC, Powell R, Agius PA, Beeson JG, Simpson JA, Fowkes FJ. 2014. Immunological markers of Plasmodium vivax exposure and immunity: a systematic review and meta-analysis. BMC Med 12:150. doi: 10.1186/s12916-014-0150-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Doolan DL, Mu Y, Unal B, Sundaresh S, Hirst S, Valdez C, Randall A, Molina D, Liang X, Freilich DA, Oloo JA, Blair PL, Aguiar JC, Baldi P, Davies DH, Felgner PL. 2008. Profiling humoral immune responses to P falciparum infection with protein microarrays. Proteomics 8:4680–4694. doi: 10.1002/pmic.200800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roussilhon C, Oeuvray C, Müller-Graf C, Tall A, Rogier C, Trape J-F, Theisen M, Balde A, Pérignon J-L, Druilhe P. 2007. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med 4:e320. doi: 10.1371/journal.pmed.0040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang L, Richie TL, Stowers A, Nhan DH, Coppel RL. 2001. Naturally acquired antibody responses to Plasmodium falciparum merozoite surface protein 4 in a population living in an area of endemicity in Vietnam. Infect Immun 69:4390–4397. doi: 10.1128/IAI.69.7.4390-4397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kattenberg JH, Erhart A, Truong MH, Rovira-Vallbona E, Vu KAD, Nguyen THN, Nguyen VH, Nguyen VV, Bannister-Tyrrell M, Theisen M, Bennet A, Lover AA, Tran TD, Nguyen XX, Rosanas-Urgell A. 2018. Characterization of Plasmodium falciparum and Plasmodium vivax recent exposure in an area of significantly decreased transmission intensity in Central Vietnam. Malar J 17:180. doi: 10.1186/s12936-018-2326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kerkhof K, Sluydts V, Willen L, Kim S, Canier L, Heng S, Tsuboi T, Sochantha T, Sovannaroth S, Menard D, Coosemans M, Durnez L. 2016. Serological markers to measure recent changes in malaria at population level in Cambodia. Malar J 15:529. doi: 10.1186/s12936-016-1576-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rogier C, Commenges D, Trape JF. 1996. Evidence for an age-dependent pyrogenic threshold of Plasmodium falciparum parasitemia in highly endemic populations. Am J Trop Med Hyg 54:613–619. doi: 10.4269/ajtmh.1996.54.613. [DOI] [PubMed] [Google Scholar]
- 100.Gatton ML, Cheng Q. 2002. Evaluation of the pyrogenic threshold for Plasmodium falciparum malaria in naive individuals. Am J Trop Med Hyg 66:467–473. doi: 10.4269/ajtmh.2002.66.467. [DOI] [PubMed] [Google Scholar]
- 101.Collins WE, Jeffery GM, Roberts JM. 2004. A retrospective examination of reinfection of humans with Plasmodium vivax. Am J Trop Med Hyg 70:642–644. doi: 10.4269/ajtmh.2004.70.642. [DOI] [PubMed] [Google Scholar]
- 102.Collins WE, Jeffery GM. 2002. A retrospective examination of sporozoite-induced and trophozoite-induced infections with Plasmodium ovale: development of parasitologic and clinical immunity during primary infection. Am J Trop Med Hyg 66:492–502. doi: 10.4269/ajtmh.2002.66.492. [DOI] [PubMed] [Google Scholar]
- 103.Collins WE, Jeffery GM. 1999. A retrospective examination of secondary sporozoite- and trophozoite-induced infections with Plasmodium falciparum: development of parasitologic and clinical immunity following secondary infection. Am J Trop Med Hyg 61:20–35. doi: 10.4269/tropmed.1999.61-020. [DOI] [PubMed] [Google Scholar]
- 104.Boisier P, Jambou R, Raharimalala L, Roux J. 2002. Relationship between parasite density and fever risk in a community exposed to a low level of malaria transmission in Madagascar highlands. Am J Trop Med Hyg 67:137–140. doi: 10.4269/ajtmh.2002.67.137. [DOI] [PubMed] [Google Scholar]
- 105.Walk J, Schats R, Langenberg MCC, Reuling IJ, Teelen K, Roestenberg M, Hermsen CC, Visser LG, Sauerwein RW. 2016. Diagnosis and treatment based on quantitative PCR after controlled human malaria infection. Malar J 15:398. doi: 10.1186/s12936-016-1571-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.William T, Menon J, Rajahram G, Chan L, Ma G, Donaldson S, Khoo S, Frederick C, Jelip J, Anstey NM, Yeo TW. 2011. Severe Plasmodium knowlesi malaria in a tertiary care hospital, Sabah, Malaysia. Emerg Infect Dis 17:1248–1255. doi: 10.3201/eid1707.101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Daneshvar C, Davis TME, Cox-Singh J, Rafa'ee MZ, Zakaria SK, Divis PCS, Singh B. 2009. Clinical and laboratory features of human Plasmodium knowlesi infection. Clin Infect Dis 49:852–860. doi: 10.1086/605439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Campo JJ, Whitman TJ, Freilich D, Burgess TH, Martin GJ, Doolan DL. 2011. Toward a surrogate marker of malaria exposure: modeling longitudinal antibody measurements under outbreak conditions. PLoS One 6:e21826. doi: 10.1371/journal.pone.0021826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marsh K. 2002. Immunology of malaria. Essential Malariology, 4th ed Arnold, London, United Kingdom. [Google Scholar]
- 110.Farnert A, Wyss K, Dashti S, Naucler P. 2015. Duration of residency in a non-endemic area and risk of severe malaria in African immigrants. Clin Microbiol Infect 21:494–501. doi: 10.1016/j.cmi.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 111.Jennings RM, DE Souza JB, Todd JE, Armstrong M, Flanagan KL, Riley EM, Doherty JF. 2006. Imported Plasmodium falciparum malaria: are patients originating from disease-endemic areas less likely to develop severe disease? A prospective, observational study. Am J Trop Med Hyg 75:1195–1199. doi: 10.4269/ajtmh.2006.75.1195. [DOI] [PubMed] [Google Scholar]
- 112.Koopmans LC, van Wolfswinkel ME, Hesselink DA, Hoorn EJ, Koelewijn R, van Hellemond JJ, van Genderen PJ. 2015. Acute kidney injury in imported Plasmodium falciparum malaria. Malar J 14:523. doi: 10.1186/s12936-015-1057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vliegenthart-Jongbloed K, de Mendonca Melo M, van Wolfswinkel ME, Koelewijn R, van Hellemond JJ, van Genderen PJ. 2013. Severity of imported malaria: protective effect of taking malaria chemoprophylaxis. Malar J 12:265. doi: 10.1186/1475-2875-12-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wetsteyn JC, de Geus A. 1995. Falciparum malaria, imported into The Netherlands, 1979–1988. II. Clinical features. Trop Geogr Med 47:97–102. [PubMed] [Google Scholar]
- 115.Schwartz E, Sadetzki S, Murad H, Raveh D. 2001. Age as a risk factor for severe Plasmodium falciparum malaria in nonimmune patients. Clin Infect Dis 33:1774–1777. doi: 10.1086/322522. [DOI] [PubMed] [Google Scholar]
- 116.Magill A, Ryan E, Hill D, Solomon T. 2012. Hunter’s tropical medicine and emerging infectious diseases, 9 ed. Elsevier, San Diego, CA. [Google Scholar]
- 117.Taylor SM, Molyneux ME, Simel DL, Meshnick SR, Juliano JJ. 2010. Does this patient have malaria? JAMA 304:2048–2056. doi: 10.1001/jama.2010.1578. [DOI] [PubMed] [Google Scholar]
- 118.Berens-Riha N, Kroidl I, Schunk M, Alberer M, Beissner M, Pritsch M, Kroidl A, Fröschl G, Hanus I, Bretzel G, von Sonnenburg F, Nothdurft HD, Löscher T, Herbinger K-H. 2014. Evidence for significant influence of host immunity on changes in differential blood count during malaria. Malar J 13:155. doi: 10.1186/1475-2875-13-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Warimwe GM, Murungi LM, Kamuyu G, Nyangweso GM, Wambua J, Naranbhai V, Fletcher HA, Hill AV, Bejon P, Osier FH, Marsh K. 2013. The ratio of monocytes to lymphocytes in peripheral blood correlates with increased susceptibility to clinical malaria in Kenyan children. PLoS One 8:e57320. doi: 10.1371/journal.pone.0057320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zahorec R. 2001. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy 102:5–14. [PubMed] [Google Scholar]
- 121.van Wolfswinkel ME, Vliegenthart-Jongbloed K, de Mendonca Melo M, Wever PC, McCall MB, Koelewijn R, van Hellemond JJ, van Genderen PJ. 2013. Predictive value of lymphocytopenia and the neutrophil-lymphocyte count ratio for severe imported malaria. Malar J 12:101. doi: 10.1186/1475-2875-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van Wolfswinkel ME, Langenberg MCC, Wammes LJ, Sauerwein RW, Koelewijn R, Hermsen CC, van Hellemond JJ, van Genderen PJ. 2017. Changes in total and differential leukocyte counts during the clinically silent liver phase in a controlled human malaria infection in malaria-naive Dutch volunteers. Malar J 16:457. doi: 10.1186/s12936-017-2108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hesselink DA, Burgerhart JS, Bosmans-Timmerarends H, Petit P, van Genderen PJ. 2009. Procalcitonin as a biomarker for severe Plasmodium falciparum disease: a critical appraisal of a semi-quantitative point-of-care test in a cohort of travellers with imported malaria. Malar J 8:206. doi: 10.1186/1475-2875-8-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Righi E, Merelli M, Arzese A, Siega PD, Scarparo C, Bassetti M. 2016. Determination of PCT on admission is a useful tool for the assessment of disease severity in travelers with imported Plasmodium falciparum malaria. Acta Parasitol 61:412–418. doi: 10.1515/ap-2016-0055. [DOI] [PubMed] [Google Scholar]
- 125.Uzzan B, Izri A, Durand R, Deniau M, Bouchaud O, Perret GY. 2006. Serum procalcitonin in uncomplicated falciparum malaria: a preliminary study. Travel Med Infect Dis 4:77–80. doi: 10.1016/j.tmaid.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 126.Chiwakata CB, Manegold C, Bonicke L, Waase I, Julch C, Dietrich M. 2001. Procalcitonin as a parameter of disease severity and risk of mortality in patients with Plasmodium falciparum malaria. J Infect Dis 183:1161–1164. doi: 10.1086/319283. [DOI] [PubMed] [Google Scholar]
- 127.Carannante N, Rossi M, Fraganza F, Coppola G, Chiesa D, Attanasio V, Sbrana F, Corcione A, Tascini C. 2017. A high PCT level correlates with disease severity in Plasmodium falciparum malaria in children. New Microbiol 40:72–74. [PubMed] [Google Scholar]
- 128.van Genderen PJ, van der Meer IM, Consten J, Petit PL, van Gool T, Overbosch D. 2005. Evaluation of plasma lactate as a parameter for disease severity on admission in travelers with Plasmodium falciparum malaria. J Travel Med 12:261–264. doi: 10.2310/7060.2005.12504. [DOI] [PubMed] [Google Scholar]
- 129.van Wolfswinkel ME, Hesselink DA, Zietse R, Hoorn EJ, van Genderen PJ. 2010. Hyponatraemia in imported malaria is common and associated with disease severity. Malar J 9:140. doi: 10.1186/1475-2875-9-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van Wolfswinkel ME, de Mendonca Melo M, Vliegenthart-Jongbloed K, Koelewijn R, van Hellemond JJ, van Genderen PJ. 2012. The prognostic value of schizontaemia in imported Plasmodium falciparum malaria. Malar J 11:301. doi: 10.1186/1475-2875-11-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bunn A, Escombe R, Armstrong M, Whitty CJ, Doherty JF. 2004. Falciparum malaria in malaria-naive travellers and African visitors. QJM 97:645–649. doi: 10.1093/qjmed/hch113. [DOI] [PubMed] [Google Scholar]
- 132.Meltzer E, Keller S, Shmuel S, Schwartz E. 2018. D-dimer levels in non-immune travelers with malaria. Travel Med Infect Dis 27:104–106. doi: 10.1016/j.tmaid.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 133.Krishnan A, Karnad DR. 2003. Severe falciparum malaria: an important cause of multiple organ failure in Indian intensive care unit patients. Crit Care Med 31:2278–2284. doi: 10.1097/01.CCM.0000079603.82822.69. [DOI] [PubMed] [Google Scholar]
- 134.Hede MS, Fjelstrup S, Lotsch F, Zoleko RM, Klicpera A, Groger M, Mischlinger J, Endame L, Veletzky L, Neher R, Simonsen AKW, Petersen E, Mombo-Ngoma G, Stougaard M, Ho YP, Labouriau R, Ramharter M, Knudsen BR. 2018. Detection of the malaria causing Plasmodium parasite in saliva from infected patients using topoisomerase I activity as a biomarker. Sci Rep 8:4122. doi: 10.1038/s41598-018-22378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mischlinger J, Pitzinger P, Veletzky L, Groger M, Zoleko-Manego R, Adegnika AA, Agnandji ST, Lell B, Kremsner PG, Mombo-Ngoma G, Mordmüller B, Ramharter M. 2018. Validity and reliability of methods to microscopically detect and quantify malaria parasitaemia. Trop Med Int Health 23:980–991. doi: 10.1111/tmi.13124. [DOI] [PubMed] [Google Scholar]
- 136.Hede M, Okorie P, Fruekilde S, Fjelstrup S, Thomsen J, Franch O, Tesauro C, Bugge M, Christiansen M, Picot S, Lötsch F, Mombo-Ngoma G, Mischlinger J, Adegnika A, Pedersen F, Ho Y-P, Petersen E, Stougaard M, Ramharter M, Knudsen B. 2015. Refined method for droplet microfluidics-enabled detection of Plasmodium falciparum encoded topoisomerase I in blood from malaria patients. Micromachines 6:1505–1513. doi: 10.3390/mi6101432. [DOI] [Google Scholar]
- 137.Marti H, Stalder C, Gonzalez IJ. 2015. Diagnostic accuracy of a LAMP kit for diagnosis of imported malaria in Switzerland. Travel Med Infect Dis 13:167–171. doi: 10.1016/j.tmaid.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 138.Pham NM, Karlen W, Beck H-P, Delamarche E. 2018. Malaria and the “last” parasite: how can technology help? Malar J 17:260. doi: 10.1186/s12936-018-2408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tran TM, Aghili A, Li S, Ongoiba A, Kayentao K, Doumbo S, Traore B, Crompton PD. 2014. A nested real-time PCR assay for the quantification of Plasmodium falciparum DNA extracted from dried blood spots. Malar J 13:393. doi: 10.1186/1475-2875-13-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.UNITAID. 2016. Malaria diagnostics technology and market landscape. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 141.Berry A, Benoit-Vical F, Fabre R, Cassaing S, Magnaval JF. 2008. PCR-based methods to the diagnosis of imported malaria. Parasite 15:484–488. doi: 10.1051/parasite/2008153484. [DOI] [PubMed] [Google Scholar]
- 142.Berry A, Fabre R, Benoit-Vical F, Cassaing S, Magnaval JF. 2005. Contribution of PCR-based methods to diagnosis and management of imported malaria. Med Trop 65:176–183. [PubMed] [Google Scholar]
- 143.Calderaro A, Piccolo G, Gorrini C, Rossi S, Montecchini S, Dell'Anna ML, De Conto F, Medici MC, Chezzi C, Arcangeletti MC. 2013. Accurate identification of the six human Plasmodium spp. causing imported malaria, including Plasmodium ovale wallikeri and Plasmodium knowlesi. Malar J 12:321. doi: 10.1186/1475-2875-12-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Noedl H, Bronnert J, Yingyuen K, Attlmayr B, Kollaritsch H, Fukuda M. 2005. Simple histidine-rich protein 2 double-site sandwich enzyme-linked immunosorbent assay for use in malaria drug sensitivity testing. Antimicrob Agents Chemother 49:3575–3577. doi: 10.1128/AAC.49.8.3575-3577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Noedl H, Yingyuen K, Laoboonchai A, Fukuda M, Sirichaisinthop J, Miller RS. 2006. Sensitivity and specificity of an antigen detection ELISA for malaria diagnosis. Am J Trop Med Hyg 75:1205–1208. doi: 10.4269/ajtmh.2006.75.1205. [DOI] [PubMed] [Google Scholar]
- 146.Frickmann H, Hinz R, Rojak S, Bonow I, Ruben S, Wegner C, Zielke I, Hagen RM, Tannich E. 2018. Evaluation of automated loop-mediated amplification (LAMP) for routine malaria detection in blood samples of German travelers–a cross-sectional study. Travel Med Infect Dis 24:25–30. doi: 10.1016/j.tmaid.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 147.Dakic Z, Ivovic V, Pavlovic M, Lavadinovic L, Markovic M, Djurkovic-Djakovic O. 2014. Clinical significance of molecular methods in the diagnosis of imported malaria in returning travelers in Serbia. Int J Infect Dis 29:24–30. doi: 10.1016/j.ijid.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 148.Murray CK, Gasser RA Jr, Magill AJ, Miller RS. 2008. Update on rapid diagnostic testing for malaria. Clin Microbiol Rev 21:97–110. doi: 10.1128/CMR.00035-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chiodini PL. 2014. Malaria diagnostics: now and the future. Parasitology 141:1873–1879. doi: 10.1017/S0031182014001371. [DOI] [PubMed] [Google Scholar]
- 150.Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH. 2007. A review of malaria diagnostic tools: microscopy and rapid diagnostic test (RDT). Am J Trop Med Hyg 77:119–127. doi: 10.4269/ajtmh.2007.77.119. [DOI] [PubMed] [Google Scholar]
- 151.Mischlinger J, Schernhammer E. 2017. A common trap of diagnostic tests: disease prevalence and positive predictive value. Wien Klin Wochenschr 129:583–584. doi: 10.1007/s00508-017-1204-0. [DOI] [PubMed] [Google Scholar]
- 152.Akobeng AK. 2007. Understanding diagnostic tests 1: sensitivity, specificity and predictive values. Acta Paediatr 96:338–341. doi: 10.1111/j.1651-2227.2006.00180.x. [DOI] [PubMed] [Google Scholar]
- 153.Akobeng AK. 2007. Understanding diagnostic tests 2: likelihood ratios, pre- and post-test probabilities and their use in clinical practice. Acta Paediatr 96:487–491. doi: 10.1111/j.1651-2227.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 154.Kent P, Hancock MJ. 2016. Interpretation of dichotomous outcomes: sensitivity, specificity, likelihood ratios, and pre-test and post-test probability. J Physiother 62:231–233. doi: 10.1016/j.jphys.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 155.Bhargava A, Ralph R, Chatterjee B, Bottieau E. 2018. Assessment and initial management of acute undifferentiated fever in tropical and subtropical regions. BMJ 363:k4766. doi: 10.1136/bmj.k4766. [DOI] [PubMed] [Google Scholar]
- 156.World Health Organization. 2015. Guidelines for the treatment of malaria, 3rd ed WHO, Geneva, Switzerland. [Google Scholar]
- 157.Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. 2013. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther 11:623–639. doi: 10.1586/eri.13.45. [DOI] [PubMed] [Google Scholar]
- 158.Ramirez-Olivencia G, Rubio JM, Rivas P, Subirats M, Herrero MD, Lago M, Puente S. 2012. Imported submicroscopic malaria in Madrid. Malar J 11:324. doi: 10.1186/1475-2875-11-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jeremiah ZA, Uko EK. 2007. Depression of platelet counts in apparently healthy children with asymptomatic malaria infection in a Nigerian metropolitan city. Platelets 18:469–471. doi: 10.1080/09537100701194871. [DOI] [PubMed] [Google Scholar]
- 160.Kurtzhals JA, Addae MM, Akanmori BD, Dunyo S, Koram KA, Appawu MA, Nkrumah FK, Hviid L. 1999. Anaemia caused by asymptomatic Plasmodium falciparum infection in semi-immune African schoolchildren. Trans R Soc Trop Med Hyg 93:623–627. doi: 10.1016/s0035-9203(99)90073-1. [DOI] [PubMed] [Google Scholar]
- 161.Hogan B, Fever Without Source (FWS) Study Group, Eibach D, Krumkamp R, Sarpong N, Dekker D, Kreuels B, Maiga-Ascofare O, Gyau Boahen K, Wiafe Akenten C, Adu-Sarkodie Y, Owusu-Dabo E, May J. 2018. Malaria coinfections in febrile pediatric inpatients: a hospital-based study from Ghana. Clin Infect Dis 66:1838–1845. doi: 10.1093/cid/cix1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pasricha JM, Juneja S, Manitta J, Whitehead S, Maxwell E, Goh WK, Pasricha SR, Eisen DP. 2013. Is serial testing required to diagnose imported malaria in the era of rapid diagnostic tests? Am J Trop Med Hyg 88:20–23. doi: 10.4269/ajtmh.2012.11-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Askling HH, European Society for Clinical Microbiology and Infectious Diseases Study Group on Clinical Parasitology, Bruneel F, Burchard G, Castelli F, Chiodini PL, Grobusch MP, Lopez-Velez R, Paul M, Petersen E, Popescu C, Ramharter M, Schlagenhauf P. 2012. Management of imported malaria in Europe. Malar J 11:328. doi: 10.1186/1475-2875-11-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Poti KE, Sullivan DJ, Dondorp AM, Woodrow CJ. 2020. HRP2: transforming malaria diagnosis, but with caveats. Trends Parasitol 36:112–126. doi: 10.1016/j.pt.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 165.Gillet P, Mori M, Van Esbroeck M, Van den Ende J, Jacobs J. 2009. Assessment of the prozone effect in malaria rapid diagnostic tests. Malar J 8:271. doi: 10.1186/1475-2875-8-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Santos L, Pereira NR, Andrade P, Dias PF, Alves CL, Abreu C, Serrao R, Ribeiro M, Sarmento A. 2015. Prozone-like phenomenon in travellers with fatal malaria: report of two cases. J Infect Dev Ctries 9:321–324. doi: 10.3855/jidc.5454. [DOI] [PubMed] [Google Scholar]
- 167.Falade CO, Ajayi IO, Nsungwa-Sabiiti J, Siribie M, Diarra A, Serme L, Afonne C, Yusuf OB, Gansane Z, Jegede AS, Singlovic J, Gomes M. 2016. Malaria rapid diagnostic tests and malaria microscopy for guiding malaria treatment of uncomplicated fevers in Nigeria and prereferral cases in 3 African countries. Clin Infect Dis 63:S290–S297. doi: 10.1093/cid/ciw628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Marx A, Pewsner D, Egger M, Nuesch R, Bucher HC, Genton B, Hatz C, Juni P. 2005. Meta-analysis: accuracy of rapid tests for malaria in travelers returning from endemic areas. Ann Intern Med 142:836–846. doi: 10.7326/0003-4819-142-10-200505170-00009. [DOI] [PubMed] [Google Scholar]
- 169.World Health Organization. 2014. Severe malaria. Trop Med Int Health 19:7–131. doi: 10.1111/tmi.12313_2. [DOI] [PubMed] [Google Scholar]
- 170.World Health Organization. 2013. Management of severe malaria: a practical handbook, 3rd ed. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 171.World Health Organization. 2010. The treatment of malaria, 2nd ed. WHO, Geneva, Switzerland. [Google Scholar]
- 172.Dondorp A, South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) Group, Nosten F, Stepniewska K, Day N, White N. 2005. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 366:717–725. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 173.Dondorp AM, AQUAMAT Group, Fanello CI, Hendriksen IC, Gomes E, Seni A, Chhaganlal KD, Bojang K, Olaosebikan R, Anunobi N, Maitland K, Kivaya E, Agbenyega T, Nguah SB, Evans J, Gesase S, Kahabuka C, Mtove G, Nadjm B, Deen J, Mwanga-Amumpaire J, Nansumba M, Karema C, Umulisa N, Uwimana A, Mokuolu OA, Adedoyin OT, Johnson WB, Tshefu AK, Onyamboko MA, Sakulthaew T, Ngum WP, Silamut K, Stepniewska K, Woodrow CJ, Bethell D, Wills B, Oneko M, Peto TE, von Seidlein L, Day NP, White NJ. 2010. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet 376:1647–1657. doi: 10.1016/S0140-6736(10)61924-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Elket N, Kendjo E, Thellier M, Assoumou L, Potard V, Taieb A, Tantaoui I, Caumes E, Piarroux R, Roussel C, Buffet P, Costagliola D, Jaureguiberry S. 2019. Propensity score analysis of artesunate versus quinine for severe imported Plasmodium falciparum malaria in France. Clin Infect Dis 70:280–287. doi: 10.1093/cid/ciz206. [DOI] [PubMed] [Google Scholar]
- 175.Rolling T, Wichmann D, Schmiedel S, Burchard GD, Kluge S, Cramer JP. 2013. Artesunate versus quinine in the treatment of severe imported malaria: comparative analysis of adverse events focussing on delayed haemolysis. Malar J 12:241. doi: 10.1186/1475-2875-12-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.O’Flaherty K, Ataide R, Zaloumis SG, Ashley EA, Powell R, Feng G, Reiling L, Dondorp AM, Day NP, Dhorda M, Fairhurst RM, Lim P, Amaratunga C, Pukrittayakamee S, Hien TT, Htut Y, Mayxay M, Faiz MA, Beeson JG, Nosten F, Simpson JA, White NJ, Fowkes F. 2019. The contribution of functional antimalarial immunity to measures of parasite clearance in therapeutic efficacy studies of artemisinin derivatives. J Infect Dis 220:1178–1187. doi: 10.1093/infdis/jiz247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tan KR, Hwang J. 2018. Tafenoquine receives regulatory approval in USA for prophylaxis of malaria and radical cure of Plasmodium vivax. J Travel Med 25:tay071. doi: 10.1093/jtm/tay071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shellvarajah M, Hatz C, Schlagenhauf P. 2017. Malaria prevention recommendations for risk groups visiting sub-Saharan Africa: a survey of European expert opinion and international recommendations. Travel Med Infect Dis 19:49–55. doi: 10.1016/j.tmaid.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 179.Wooltorton E. 2002. Mefloquine: contraindicated in patients with mood, psychotic or seizure disorders. CMAJ 167:1147. [PMC free article] [PubMed] [Google Scholar]
- 180.Simman R, Raynolds D. 2012. Skin hypersensitivity to sun light due to doxycycline ingestion causing hand partial-thickness burn. J Am Coll Clin Wound Spec 4:16–17. doi: 10.1016/j.jccw.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Glette J, Sandberg S, Haneberg B, Solberg CO. 1984. Effect of tetracyclines and UV light on oxygen consumption by human leukocytes. Antimicrob Agents Chemother 26:489–492. doi: 10.1128/aac.26.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.D'Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. 2013. UV radiation and the skin. Int J Mol Sci 14:12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schlagenhauf P, EuroTravNet, Weld L, Goorhuis A, Gautret P, Weber R, von Sonnenburg F, Lopez-Velez R, Jensenius M, Cramer JP, Field VK, Odolini S, Gkrania-Klotsas E, Chappuis F, Malvy D, van Genderen PJ, Mockenhaupt F, Jaureguiberry S, Smith C, Beeching NJ, Ursing J, Rapp C, Parola P, Grobusch MP. 2015. Travel-associated infection presenting in Europe (2008–12): an analysis of EuroTravNet longitudinal, surveillance data, and evaluation of the effect of the pre-travel consultation. Lancet Infect Dis 15:55–64. doi: 10.1016/S1473-3099(14)71000-X. [DOI] [PubMed] [Google Scholar]
- 184.Public Health England. 2016. Malaria imported into the United Kingdom: 2016 Implications for those advising travellers. Public Health England, London, United Kingdom: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/686034/Malaria_imported_into_the_UK_2016_FINAL_REVISED.pdf. Accessed 20 January 2019. [Google Scholar]
- 185.Simons H, Patel D. 2017. Atovaquone/proguanil (Maloff Protect) is now available without prescription in UK pharmacies. Travel Med Infect Dis 20:67. doi: 10.1016/j.tmaid.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 186.Savelkoel J, Binnendijk KH, Spijker R, van Vugt M, Tan K, Hanscheid T, Schlagenhauf P, Grobusch MP. 2018. Abbreviated atovaquone-proguanil prophylaxis regimens in travellers after leaving malaria-endemic areas: a systematic review. Travel Med Infect Dis 21:3–20. doi: 10.1016/j.tmaid.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pistone T, Schwarzinger M, Chauvin P, Ezzedine K, Receveur MC, Djossou F, Siriwardana M, Larouze B, Malvy D. 2008. Reimbursement of malaria chemoprophylaxis for travellers from Europe to Sub-Saharan Africa: cost-effectiveness analysis from the perspective of the French national health insurance system. Health Policy 88:186–199. doi: 10.1016/j.healthpol.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 188.Widmer LL, Blank PR, Van Herck K, Hatz C, Schlagenhauf P. 2010. Cost-effectiveness analysis of malaria chemoprophylaxis for travellers to West-Africa. BMC Infect Dis 10:279. doi: 10.1186/1471-2334-10-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schlagenhauf P, Tschopp A, Johnson R, Nothdurft HD, Beck B, Schwartz E, Herold M, Krebs B, Veit O, Allwinn R, Steffen R. 2003. Tolerability of malaria chemoprophylaxis in non-immune travellers to sub-Saharan Africa: multicentre, randomised, double blind, four arm study. BMJ 327:1078. doi: 10.1136/bmj.327.7423.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Overbosch D, Malarone International Study Team, Schilthuis H, Bienzle U, Behrens RH, Kain KC, Clarke PD, Toovey S, Knobloch J, Nothdurft HD, Shaw D, Roskell NS, Chulay JD. 2001. Atovaquone-proguanil versus mefloquine for malaria prophylaxis in nonimmune travelers: results from a randomized, double-blind study. Clin Infect Dis 33:1015–1021. doi: 10.1086/322694. [DOI] [PubMed] [Google Scholar]
- 191.Tickell-Painter M, Maayan N, Saunders R, Pace C, Sinclair D. 2017. Mefloquine for preventing malaria during travel to endemic areas. Cochrane Database Syst Rev 10:CD006491. doi: 10.1002/14651858.CD006491.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sixty Degrees Pharma, Biocelect. 2018. KODATEF (tafenoquine) approved in Australia; first malaria prevention drug in more than two decades. Sixty Degrees Pharma, Biocelect, Sydney, Australia: https://60degreespharma.com/wp-content/uploads/2018/09/Biocelect-Welcomes-TGA-Approval-of-KODATEF-Press-Release-18092018.pdf. Accessed 05 February 2018. [Google Scholar]
- 193.Baird JK. 2017. Management of Plasmodium vivax risk and illness in travelers. Trop Dis Travel Med Vaccines 3:7. doi: 10.1186/s40794-017-0049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Baird JK. 2018. Tafenoquine for travelers’ malaria: evidence, rationale and recommendations. J Travel Med 25 doi: 10.1093/jtm/tay110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mischlinger J, Agnandji ST, Ramharter M. 2016. Single dose treatment of malaria–current status and perspectives. Expert Rev Anti Infect Ther 14:669–678. doi: 10.1080/14787210.2016.1192462. [DOI] [PubMed] [Google Scholar]
- 196.Ramharter M, Noedl H, Thimasarn K, Wiedermann G, Wernsdorfer G, Wernsdorfer WH. 2002. In vitro activity of tafenoquine alone and in combination with artemisinin against Plasmodium falciparum. Am J Trop Med Hyg 67:39–43. doi: 10.4269/ajtmh.2002.67.39. [DOI] [PubMed] [Google Scholar]
- 197.U.S. National Library of Science. 2018. Glucose-6-phosphate dehydrogenase deficiency. https://ghr.nlm.nih.gov/condition/glucose-6-phosphate-dehydrogenase-deficiency#resources. Accessed 05 February 2019.
- 198.Howes RE, Piel FB, Patil AP, Nyangiri OA, Gething PW, Dewi M, Hogg MM, Battle KE, Padilla CD, Baird JK, Hay SI. 2012. G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. PLoS Med 9:e1001339. doi: 10.1371/journal.pmed.1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Macintyre F, OZ-Piperaquine Study Group, Adoke Y, Tiono AB, Duong TT, Mombo-Ngoma G, Bouyou-Akotet M, Tinto H, Bassat Q, Issifou S, Adamy M, Demarest H, Duparc S, Leroy D, Laurijssens BE, Biguenet S, Kibuuka A, Tshefu AK, Smith M, Foster C, Leipoldt I, Kremsner PG, Phuc BQ, Ouedraogo A, Ramharter M. 2017. A randomised, double-blind clinical phase II trial of the efficacy, safety, tolerability and pharmacokinetics of a single dose combination treatment with artefenomel and piperaquine in adults and children with uncomplicated Plasmodium falciparum malaria. BMC Med 15:181. doi: 10.1186/s12916-017-0940-3. [DOI] [PMC free article] [PubMed] [Google Scholar]