During infection, pathogenic organisms must acquire essential transition metals from the host environment. Through the process of nutritional immunity, the host employs numerous strategies to restrict these key nutrients from invading pathogens. In this study, we describe a mechanism by which the important human pathogen Clostridioides difficile resists transition-metal limitation by the host. We report that C. difficile utilizes a zinc transporter, ZupT, to compete with the host protein calprotectin for nutrient zinc. Inactivation of this transporter in C. difficile renders this important pathogen sensitive to host-mediated metal restriction and confers a fitness disadvantage during infection. Our study demonstrates that targeting nutrient metal transport proteins in C. difficile is a potential avenue for therapeutic development.
KEYWORDS: Clostridium difficile, host-pathogen interactions, infectious disease, nutrient transport
ABSTRACT
Clostridioides difficile is a spore-forming bacterium that causes severe colitis and is a major public health threat. During infection, C. difficile toxin production results in damage to the epithelium and a hyperinflammatory response. The immune response to CDI leads to robust neutrophil infiltration at the sight of infection and the deployment of numerous antimicrobials. One of the most abundant host immune factors associated with CDI is calprotectin, a metal-chelating protein with potent antimicrobial activity. Calprotectin is essential to the innate immune response to C. difficile and increasing levels of calprotectin correlate with disease severity in both adults and children with CDI. The fact that C. difficile persists in the presence of high levels of calprotectin suggests that this organism may deploy strategies to compete with this potent antimicrobial factor for essential nutrient metals during infection. In this report, we demonstrate that a putative zinc (Zn) transporter, ZupT, is employed by C. difficile to survive calprotectin-mediated metal limitation. ZupT is highly expressed in the presence of calprotectin and is required to protect C. difficile against calprotectin-dependent growth inhibition. When competing against wild-type C. difficile, zupT mutants show a defect in colonization and persistence in a murine model of infection. Together these data demonstrate that C. difficile utilizes a metal import system to combat nutritional immunity during CDI and suggest that strategies targeting nutrient acquisition in C. difficile may have therapeutic potential.
IMPORTANCE During infection, pathogenic organisms must acquire essential transition metals from the host environment. Through the process of nutritional immunity, the host employs numerous strategies to restrict these key nutrients from invading pathogens. In this study, we describe a mechanism by which the important human pathogen Clostridioides difficile resists transition-metal limitation by the host. We report that C. difficile utilizes a zinc transporter, ZupT, to compete with the host protein calprotectin for nutrient zinc. Inactivation of this transporter in C. difficile renders this important pathogen sensitive to host-mediated metal restriction and confers a fitness disadvantage during infection. Our study demonstrates that targeting nutrient metal transport proteins in C. difficile is a potential avenue for therapeutic development.
INTRODUCTION
Transition metals, including iron (Fe), manganese (Mn), and zinc (Zn), are essential micronutrients for all living organisms. These metals are thus key resources in the battle between a host and pathogen during infection. Upon colonization of the mammalian host, pathogens must acquire transition metals from complex, diverse, and largely metal-restricted environments (1, 2). Pathogens employ numerous strategies to scavenge metals from the host, including pirating metal from host proteins, releasing metal-chelating metallophores, and expressing high-affinity metal import systems (2). To combat this, vertebrates exploit the pathogen requirement for transition metals by producing factors that deplete the environment of free transition metals in a process termed nutritional immunity (3, 4). One such factor is calprotectin, a heterodimer of S100A8 and S100A9 proteins. Calprotectin is abundant in the cytoplasm of neutrophils and chelates multiple transition metals, including Fe, Mn, and Zn (5). Calprotectin-mediated metal limitation is antimicrobial to numerous pathogens and is critical to combating infection (6–9). Defining the battle for transition metals between host and pathogen is important to understanding the factors that impact the outcome of infection, and has the potential to uncover novel therapeutic strategies for targeting pathogens.
The spore-forming bacterium Clostridioides difficile is the most commonly reported health care-associated pathogen in the United States and a global public health threat (10). The primary risk factor for C. difficile infection (CDI) is antibiotic treatment, which disturbs the resident microbial community in the gastrointestinal tract and reduces colonization resistance against the pathogen (11). Notably, the first-line therapy for C. difficile is also antibiotic treatment, which further perturbs the gut microbiota and increases risk for recurrent infection. Over the past decade, the rate, severity, and economic cost of CDI have risen dramatically in both children and adults (10, 12). This highlights the urgent need for new antibiotic targets and novel therapeutic strategies for treating CDI.
Recent work from our group and others has demonstrated that fecal calprotectin is associated with CDI in humans and high levels of calprotectin are correlated with increased disease severity (7, 13–15). We have further demonstrated that calprotectin is antimicrobial against C. difficile and calprotectin-mediated metal limitation is an essential host immune response during CDI (7). Despite calprotectin’s antimicrobial properties and high concentrations in the gastrointestinal tract during severe infections, C. difficile persists in this metal-limited environment. This suggests that C. difficile employs strategies to compete with the host for essential transition metals during infection, allowing for persistence. The mechanisms by which C. difficile combats host nutritional immunity have not been explored and may represent a new therapeutic target for treatment of CDI.
In this study, we investigated the role for a putative Zn transporter in C. difficile during host-mediated metal limitation. ZupT homologs in Salmonella enterica and Escherichia coli are important for metal scavenging in these organisms, but the role for ZupT in C. difficile has not been experimentally explored (16–18). We demonstrate that zupT is highly upregulated in the presence of recombinant calprotectin and is required for survival of calprotectin-mediated metal limitation in vitro. Furthermore, we show that ZupT imports Zn when C. difficile is metal starved and ZupT-deficient strains of C. difficile are less fit in a mouse model of infection. Together these results show that ZupT is an important factor used by C. difficile to combat host nutritional immunity during CDI.
RESULTS
C. difficile upregulates the putative Zn transporter ZupT during host-mediated metal limitation.
Calprotectin is essential to the immune response to CDI and calprotectin-mediated metal limitation is antimicrobial to C. difficile (3, 7). An RNA sequencing study by our group showed that the putative Zn transporter ZupT is one of the most highly upregulated genes in the presence of calprotectin in vitro (19). To begin to assess the contribution of ZupT to the response to host-mediated metal starvation, the transcriptional induction of zupT during treatment with recombinant calprotectin was validated using reverse transcription-quantitative PCR (qRT-PCR). In the presence of 0.35 mg/ml calprotectin, a >200-fold transcriptional increase of zupT was observed compared to the untreated controls (Fig. 1A). To confirm that this response was specific to metal limitation, C. difficile was treated with a chemical chelator N,N,N’,N’-tetrakis (2-pyridinylmethyl)-1,2-ethanediamine (TPEN; 50 μM). Exposure to TPEN-mediated metal starvation revealed a 500-fold increase in zupT transcripts compared to the untreated control (Fig. 1B). These results demonstrate that transcription of zupT is a robust response by C. difficile to metal limitation.
Fig. 1.

zupT is upregulated during nutrient metal limitation. C. difficile was grown in the presence of 0.35 mg/ml calprotectin (A) or 50 μM TPEN (B). zupT transcripts were measured via qRT-PCR and are shown as fold change from untreated cells (n = 3) with standard deviation. CP, calprotectin. Statistical significance was determined by t test (*, P < 0.05).
Inactivation of zupT decreases resistance to metal limitation.
Based on the increased expression of zupT in the presence of calprotectin, we hypothesized that the encoded protein may play a central role in Zn acquisition during metal limitation by the host. To test this, a strain of C. difficile inactivated for zupT (zupT::CT) was generated using the ClosTron system (20). The lack of zupT rendered the bacteria more sensitive to calprotectin-mediated metal starvation, as growth was delayed over time in the presence of 0.35 mg/ml calprotectin and overall percent growth was reduced by 80% compared to the wild-type (WT) strain at 6 h (Fig. 2A and B). Similar results were observed when the growth of the bacteria was assessed in 50 μM TPEN, which led to a 40% reduction in growth of the mutant compared to the WT strain (Fig. 2C and D). The growth of zupT::CT in either condition is recovered by expressing zupT in trans under the control of the predicted promoter-containing region upstream of zupT (ZupT::CT pJS116_zupT; Fig. 2A to D). Taken together, these data suggest that ZupT functions to obtain metals, such as Zn, in the face of host nutritional immunity and may play an important role in reducing the effect on C. difficile of nutrient metal starvation.
FIG 2.

ZupT is required for growth during metal limitation. Growth curves of WT C. difficile, mutant zupT::CT, and complemented zupT::CT pJS116_zupT grown in the presence of 0.35 mg/ml calprotectin (A) or 50 μM TPEN (C). Percent growth based off maximum OD at 6 h is shown for calprotectin (B) and TPEN (D). Statistical significance was determined by t test (*, P < 0.05).
ZupT imports Zn during metal limitation.
ZupT is highly expressed during competition with calprotectin and zinc uptake is essential to C. difficile survival during calprotectin-mediated metal starvation; however, it remains unknown if this putative metal transporter imports Zn into the cell (7, 19). To investigate the ability of ZupT to take up Zn, cell cultures were grown in Zn-limited media and pulsed with a minor Zn stable isotope (70Zn) prior to analysis of intracellular 70Zn levels by inductively coupled plasma mass spectrometry (ICP-MS). The zupT::CT strain displayed a 10-fold reduction in intracellular 70Zn compared to the WT strain in these experiments (Fig. 3). The uptake of 70Zn was restored in the zupT::CT pJS116_pzupT-zupT complemented strain. These data demonstrate the direct contribution of ZupT to increasing intracellular Zn concentrations as a strategy to combat Zn-limiting conditions (Fig. 3), and strongly support the assignment of ZupT as a Zn transporter.
FIG 3.
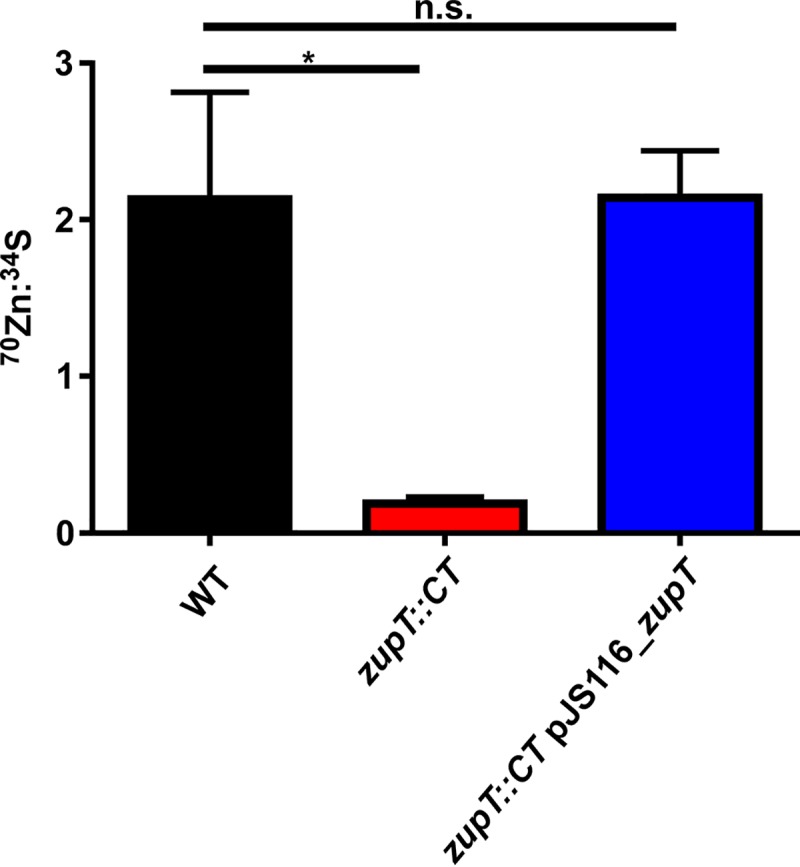
ZupT imports Zn in response to metal limitation. 70Zn uptake measured by ICP-MS. WT C. difficile, mutant zupT::CT, and complemented zupT::CT pJS116_zupT were pregrown in the presence of 50 μM TPEN and then supplemented with 70Zn. Following incubation, 70Zn uptake was measured. Statistical significance was determined by t test (*, P < 0.05; n.s., not significant).
ZupT mutants show a colonization defect in the gastrointestinal tract during infection.
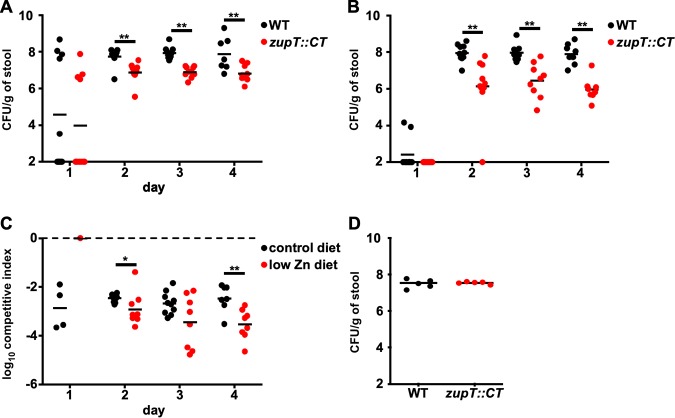
The reduced ability of zupT::CT to resist Zn starvation in the presence of the innate immune protein calprotectin suggests that ZupT may be required in a mouse model of CDI. To test this, mice were placed on a custom formulated diet that is Zn-free (7). This Zn-free diet was supplemented with standard levels of Zn (control diet: 29 mg/kg) and animals were fed the diet for 1 week prior to infection (7). Mice were then coinfected with WT and zupT::CT spores of C. difficile at a 1:1 ratio and disease was monitored for 4 days. Following treatment with the control diet, the WT strain colonized to ∼108 CFU per gram of stool (Fig. 4A). The zupT::CT strain displayed a 1 log reduction in colonization compared to the WT strain (Fig. 4A). Next, to examine the role of ZupT during conditions of Zn deficiency, we coinfected mice that were fed a custom formulated diet lacking Zn supplementation (low-Zn diet: 0 mg/kg). Following infection, we observed a 2 to 3 log defect in the zupT::CT strain under these extreme Zn-limited conditions (Fig. 4B). In both dietary conditions, the zupT::CT mutant showed a significant reduction in competitive index compared to WT and this reduction was increased in the low Zn diet (Fig. 4C). To ensure the zupT::CT strain does not contain an innate colonization defect, mice fed the control diet were mono-infected with either WT or zupT::CT strains. There were no signification differences in colonization between the WT or zupT::CT strain throughout the infection (Fig. 4D). Together, these data suggest that ZupT is required for C. difficile to overcome nutrient Zn limitation imposed by the host during CDI.
FIG 4.
Strains of C. difficile inactivated for zupT exhibit a defect in a competitive murine model of C. difficile infection. WT C. difficile and zupT::CT spores were orally gavaged at a 1:1 ratio into antibiotic pretreated mice. Mice were fed a low-Zn diet (A) or a control diet (B) prior to infection and CFU were measured for each strain using differential media. Competitive index (zupT::CT/WT) was measured for each dietary condition (C). Each individual point is representative of an individual mouse and the bar represents the mean. CFU were enumerated during a 4-day time course of infection of mice on the control diet infected with WT or zupT::CT spores (D). Statistical significance was determined by t test (*, P < 0.05; **, P < 0.01).
DISCUSSION
Transition metals are essential micronutrients for all living organisms, and invading pathogens must acquire these metals in the host environment to colonize and establish infection (2). This is complicated even further in the gastrointestinal tract, as enteric pathogens must also compete with resident microbiota for transition metals. Calprotectin-dependent nutritional immunity is essential to defend against CDI (7). However, recent studies have demonstrated that increased fecal calprotectin is a hallmark of severe CDI, suggesting that during severe infection, C. difficile must employ strategies to persist and thrive in the gastrointestinal tract despite nutritional immunity (7, 13–15). In this study, we explored a potential mechanism by which C. difficile competes with the host for transition metals during infection. Previously, our group demonstrated that calprotectin is an essential component of the innate immune response to CDI and calprotectin-mediated transition metal limitation is inhibitory to C. difficile growth (7). Furthermore, we showed that subinhibitory concentrations of calprotectin dramatically alter the transcriptional profile and metabolism of C. difficile in vitro (19).
Here, we explored the role of one of the most highly upregulated genes in the presence of calprotectin, zupT. Previous studies have shown that zupT is upregulated under metal stress, but this putative metal transporter has not been studied experimentally in C. difficile (19, 21, 22). We demonstrated that zupT expression was robustly induced in the presence of calprotectin or a chemical chelator, and that inactivation of zupT decreased the ability of C. difficile to grow in the presence of subinhibitory concentrations of calprotectin. Using ICP-MS, we further demonstrated that zupT mutant cells have a defect in heavy Zn uptake, suggesting that ZupT is indeed a bona fide Zn transporter in C. difficile. In mice fed standard Zn diets, as well as Zn-free diets, strains of C. difficile lacking functional ZupT showed decreased fitness compared to wild type. Taken together, this suggests that C. difficile employs strategies to compete with the host and the resident microbiota for metals during infection.
ZupT has been reported in several studies to be one of the most highly upregulated genes in C. difficile during metal stress, suggesting this importer plays a key role in metal homeostasis for this important pathogen (19, 21, 22). However, despite this strong transcriptional response, inactivation of zupT does not completely inhibit the ability of C. difficile to colonize the host or grow in the presence of lower concentrations of calprotectin (Fig. 2 and 4). This suggests that C. difficile harbors additional metal import systems that may play similar roles in acquisition of Zn during. In support of this hypothesis, multiple putative Fe (CDR20291_1545-1548, CDR20291_1326-1328, CDR20291_2771-2774) and cation (CDR20291_0516-0157) transporters are transcriptionally increased in the presence of calprotectin (19). The phenomenon of functional redundancy in metal transport is shared among numerous enteric pathogens, including Enterococcus faecalis, E. coli, and multiple strains of Salmonella (16, 23–25). This has been specifically reported for ZupT homologs in several organisms. For example, the loss of ZupT in combination with the ZnuABC transporter in uropathogenic E. coli leads to a cumulative effect on fitness during urinary tract infections, whereas there was no decrease observed in a strain lacking only ZupT (24). In Salmonella enterica infection, strains lacking ZupT are attenuated in natural resistance-associated macrophage protein 1 (Nramp1)+/+ mice, but strains lacking both ZnuABC and ZupT exhibit markedly pronounced attenuation during infection (16). Future studies that simultaneously interrogate multiple Zn-transport systems are needed to define the mechanisms of Zn import in C. difficile during host nutritional immunity.
Taken together, these data demonstrate that metal import in C. difficile is an important response to host-mediated nutritional immunity during infection. Future work is needed to characterize the response of C. difficile to transition metal limitation in the gut and to define the role for functional redundancy in metal transporters, as these pathways represent an intriguing target for therapeutic development.
MATERIALS AND METHODS
Ethics statement.
All animal experiments under protocol M1700053 were reviewed and approved by the Institutional Animal Care and Use Committee of Vanderbilt University. Procedures were performed according to the institutional policies, NIH guidelines, Animal Welfare Act, and American Veterinary Medical Association guidelines on euthanasia.
Bacterial strains, growth conditions, and plasmids.
Strains used in this study are listed in Table 1. C. difficile strains were grown at 37°C in an anaerobic chamber (85% nitrogen, 10% hydrogen, 5% carbon dioxide, Coy Lab Products) in brain heart infusion broth (BD Life Sciences) supplemented with 0.5% yeast extract (BD Life Sciences) and 0.1% cysteine (Sigma-Aldrich) (BHIS) or in C. difficile minimal media (CDMM) as described previously (26, 27). Escherichia coli strains were grown in lysogeny broth (LB) or agar (LBA), supplemented with 50 μg/ml kanamycin when necessary. Bacillus subtilis strains were grown on LBA or in BHI broth supplemented with 5 μg/ml tetracycline or 2.5 μg/ml chloramphenicol (27). All antibiotics were purchased from Sigma-Aldrich.
TABLE 1.
Bacterial strains and plasmids used in this study.
| Bacterial strain or plasmid | Relevant feature or genotype | Reference |
|---|---|---|
| Clostridioides difficile R20291 | (29) | |
| Clostridioides difficile zupT::CT | Intron inserted into zupT | This study |
| Bacillus subtilis JH BS2 | Carries Tn196 | (28) |
| Escherichia coli DH5α | (30) | |
| Escherichia coli MG1655 | RecA+ | (31) |
| pJS107 | ClosTron plasmid | (28) |
| pJS107_zupT | ClosTron plasmid with intron targeted to zupT | This study |
| pJS116 | Stable C. difficile plasmid | (28) |
| pJS116_pzupT-zupT | zupT::CT complementation plasmid | This study |
zupT::CT strain generation. Gene inactivation was achieved using the ClosTron system as described previously (20, 27). Briefly, gBlocks containing specific modifications for insertion into the genome were generated using the TargeTronics algorithm (http://www.targetrons.com) and synthesized by Integrated DNA Technologies. The gBlocks were cloned into the pCR-Blunt vector using the Zero Blunt PCR cloning kit (Thermo Fisher Scientific) followed by restriction digest with BsrGI and HindIII (NEB) and ligation (NEB T4 ligase) into pJS107. Plasmids were transformed into the recA+ E. coli MG1655 through a standard heat shock protocol followed by transformation into B. subtilis JH2 using an established method (28). B. subtilis strains containing the pJS107_zupT plasmids were mated with C. difficile R20291 overnight at 37°C by plating and mixing together 100 μl of each strain onto a BHIS plate in the anaerobic chamber. Plates were scraped and transferred into 2 ml of BHIS prior to plating 200 μl onto BHIS plates containing 20 μg/ml thiamphenicol and 50 μg/ml kanamycin (BHISthiamp20kan50). Colonies from these plates were patched onto new BHISthiamp20kan50 and BHIS plates containing 5 μg/ml tetracycline (BHIStet5). Patched colonies that were tetracycline sensitive were patched again onto new BHISthiamp20kan50 and BHIStet5 plates. Colonies that remained tetracycline sensitive were streaked onto BHIS plates containing 20 μg/ml lincomycin (BHISlinc20). Inactivation of the zupT gene was confirmed by performing PCR to identify a 1.5-kbp shift in gene size using gDNA extracted as previously described from colonies that were lincomycin resistant (27, 28).
Complementation plasmids. Complementation plasmids (Table 1) were created by amplifying the zupT gene and intergenic region from C. difficile strain R20291. The amplicon was then cloned into the pJS116 plasmid backbone. C. difficile strains were transformed as described above with the removal of the lincomycin selection and were maintained on BHISthiamp20 to ensure plasmid retention (28).
Growth assays. Calprotectin was produced as described previously (6–9). Freshly streaked bacterial colonies were used to inoculate 5 ml of BHIS or BHISthiamp20 and grown for 16 h at 37°C. Cultures were subcultured 1:50 into fresh BHIS or BHISthiamp20 and grown for 6 h at 37°C prior to 1:50 inoculation into a 1:1 mixture of calprotectin buffer (20 mM Tris-HCl, 100 mM NaCl, 5 mM β-mercaptoethanol, 3 mM CaCl2) with BHIS or BHISthiamp20 containing calprotectin or into BHIS or BHISthiamp20 containing N,N,N’,N’,tetrakis (2-pyridinylmethyl)-1,2-ethanediamine (TPEN, Sigma) at the indicated concentrations. All growth assays were performed in a 96-well plate in 200 μl of media. Optical density at 600 nm (OD600) served as a measurement of growth and was measured every 30 min for the indicated total time in an EpochII microplate reader (BioTek).
RNA extraction and quantitative qPCR.
C. difficile cultures were grown anaerobically in triplicate in a 1:1 mixture of calprotectin buffer and BHIS with calprotectin or TPEN added in indicated concentrations at 37°C to an OD600 of 0.3. After growth, a 1:1 solution of acetone:ethanol was added to an equal volume of the culture. Samples were stored at –80°C until used for RNA extraction. Samples were thawed on ice, pelleted, and resuspended in 750 μl of LETS buffer (1 M LiCl, 0.5 M EDTA, 1 M Tris pH 7.4). Cells were transferred to tubes containing lysing matrix B beads (MP Biomedicals) and lysed by a FastPrep-24 (MP Biomedicals) bead beater for 45 s at 6 m/s. Lysed samples were heated for 5 min at 55°C and pelleted by centrifugation for 10 min. The supernatant was transferred to a fresh tube and 1 ml TRIzol (Thermo Fisher Scientific) was added. Chloroform (200 μl) was added to each sample and vortexed prior to separation of the aqueous and organic layers by centrifugation for 15 min. The aqueous (upper) layer was transferred to a fresh tube and the RNA was precipitated through the addition of 1 ml isopropyl alcohol. Samples were incubated for 10 min and RNA was pelleted by centrifugation for 10 min. Supernatant was removed and the RNA pellet was washed with 200 μl of 70% ethanol. Samples were air dried for 1 min, then resuspended in 100 μl RNase free water. DNA contamination was removed through the addition of 8 μl RQ1 DNase, 12 μl 10× RQ1 buffer, and 2 μl RNase inhibitor (Promega) to the purified RNA. Samples were DNase treated for 2 h and purified using the RNeasy miniprep RNA cleanup kit (Qiagen). RNA concentration was determined using the Synergy 2 with Gen 5 software (BioTek) and 2 μg was reverse transcribed by M-MLV reverse transcriptase (Thermo Fisher Scientific) in the presence of RNase inhibitor (Promega) and random hexamers (Promega). Reaction mixtures lacking the reverse transcriptase were used to control for DNA contamination. Newly created cDNA was diluted 1:100 and was used in qRT-PCR using iQ SYBR green supermix (BIO-RAD) utilizing the primer pairs in Table 2. Amplification was achieved using a 3-step melt cure program on a CFX96 qPCR cycler (BIO-RAD). Transcript abundance was calculated using the ΔΔCT method normalized by the rpoB gene.
TABLE 2.
Oligonucleotides used in this study
| Name | Sequence (5′ to 3′) | Description |
|---|---|---|
| qRT_zupT_F | ccagtttattatgccacaggag | qRT-PCR forward primer for zupT |
| qRT_zupT_R | tcgctcctaaaggctcagac | qRT-PCR reverse primer for zupT |
| qRT_rpoB_F | tgctgttgaaatggttcctg | qRT-PCR housekeeping gene forward primer |
| qRT_rpoB_R | cggttggcatcatcattttc | qRT-PCR housekeeping gene reverse primer |
| R20291_zupT _F | agcttaggattttctgctggtgt | Forward primer to check for intron insertion into zupT |
| R20291_zupT_R | tcgctcctaaaggctcagac | Reverse primer to check for intron insertion into zupT |
Animals and experimental models of Clostridioides difficile infection.
All animal experiments were approved by the Animal Care and Use Committee of Vanderbilt University (protocol M1700053). C57BL/6 male mice at 6 to 8 weeks old were purchased from Jackson Laboratories and given one week to equilibrate their microbiota prior to experimentation. Mice were housed with autoclaved water, food, and bedding and all experimental manipulations were performed in a biosafety level 2 laminar flow hood. Mice were housed in individual cages under the same conditions during the experiment and all mice were culture negative for C. difficile prior to infection. One week prior to infection, mice were placed on diets containing altered metal levels as previously described (7). For the C. difficile infection model, mice were given cefoperazone at 0.5 mg/ml in drinking water ad libitum for 5 days followed by a 2-day recovery period and subsequent infection. Mice were infected via oral gavage with a 1:1 mixture of 1 × 105 spores of C. difficile (NAP1/BI/027 strain R20291) and zupT::CT or 1 × 105 spores of each individual strain resuspended in phosphate-buffered saline (PBS). Mice were monitored for survival or were euthanized after reaching a terminal endpoint of appearing moribund or experiencing weight loss of >20% from baseline. C. difficile CFU were quantified daily from fecal samples. Samples were diluted and homogenized in PBS and serial plated onto taurocholate cycloserine cefoxitin fructose agar (TCCFA) with or without lincomycin for enumeration as CFU per gram of feces. Competitive index (zupT::CT/WT) was measured for each dietary condition.
Uptake of 70Zn isotope.
C. difficile strains were grown anaerobically in BHIS or BHISthiamp20 containing 50 μM TPEN to an OD600 of 0.5. 70ZnO (Cambridge Isotope Laboratories) was added to each sample at a final concentration of 25 μM and samples were incubated at 37°C for 1 h. A 1:1 mixture of acetone:ethanol was added and the cells were pelleted by centrifugation (4000 × g for 10 min). Cells were subsequently washed twice and resuspended in 1× PBS. Samples were transferred to metal-free 15 ml conical tubes (VWR), digested overnight in 50% Optima-grade nitric acid (Thermo Fisher Scientific) and 12.5% hydrogen peroxide at 50°C prior to 1:10 dilution in Millipore water for inductively coupled plasma mass spectrometry analysis.
Inductively coupled plasma mass spectrometry.
Elemental quantification on acid-digested liquid samples was performed using an Agilent 7700 inductively coupled plasma mass spectrometer (Agilent, Santa Clara, CA) attached to a Teledyne CETAC Technologies ASX-560 autosampler (Teledyne CETAC Technologies, Omaha, NE). The following settings were fixed for the analysis: Cell Entrance = −40 V; Cell Exit = −60 V; Plate Bias = −60 V; OctP Bias = −18 V; and collision cell Helium Flow = 4.5 ml/min. Optimal voltages for Extract 2, Omega Bias, Omega Lens, OctP RF, and Deflect were determined empirically before each sample set was analyzed. Element calibration curves were generated using ARISTAR ICP Standard Mix (VWR, Radnor, PA). Samples were introduced by peristaltic pump with 0.5 mm internal diameter tubing through a MicroMist borosilicate glass nebulizer (Agilent). Samples were initially taken up at 0.5 rps for 30 s followed by 30 s at 0.1 rps to stabilize the signal. Samples were analyzed in Spectrum mode at 0.1 rps, collecting three points across each peak and performing three replicates of 100 sweeps for each element analyzed. The sampling probe and tubing were rinsed for 20 s at 0.5 rps with 2% nitric acid between every sample. Data were acquired and analyzed using the Agilent Mass Hunter Workstation Software version A.01.02. Data of the investigated metal ion were normalized to the 34S abundance of each sample.
Statistical analyses.
Statistical analyses were performed using GraphPad Prism version 8. Specific statistical tests, replicate numbers, calculated errors, and other information for each experiment are reported in the figure legends.
ACKNOWLEDGMENTS
We thank Joseph A. Sorg for providing plasmids and strains utilized in this study and for advice and critical feedback related to this work.
This work was supported by the National Institutes of Health (NIH) grant R01 AI101171 and the Vanderbilt Digestive Diseases Research Center NIH grant P30DK058404. J.P.Z. was supported by F32AI120553 and K22AI7220. R.J.K. was supported by the National Institute of General Medical Sciences training grant T32GM065086 and by the National Institute of Allergy and Infectious Diseases training grant T32AI007281.
REFERENCES
- 1.Hood MI, Skaar EP. 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer LD, Skaar EP. 2016. Transition metals and virulence in bacteria. Annu Rev Genet 50:67–91. doi: 10.1146/annurev-genet-120215-035146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zackular JP, Chazin WJ, Skaar EP. 2015. Nutritional immunity: S100 proteins at the host-pathogen interface. J Biol Chem 290:18991–18998. doi: 10.1074/jbc.R115.645085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinberg ED. 1975. Nutritional immunity. Host’s attempt to withold iron from microbial invaders. JAMA 231:39–41. doi: 10.1001/jama.231.1.39. [DOI] [PubMed] [Google Scholar]
- 5.Zygiel EM, Nolan EM. 2018. Transition metal sequestration by the host-defense protein calprotectin. Annu Rev Biochem 87:621–643. doi: 10.1146/annurev-biochem-062917-012312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kehl-Fie TE, Chitayat S, Hood MI, Damo S, Restrepo N, Garcia C, Munro KA, Chazin WJ, Skaar EP. 2011. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe 10:158–164. doi: 10.1016/j.chom.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zackular JP, Moore JL, Jordan AT, Juttukonda LJ, Noto MJ, Nicholson MR, Crews JD, Semler MW, Zhang Y, Ware LB, Washington MK, Chazin WJ, Caprioli RM, Skaar EP. 2016. Dietary zinc alters the microbiota and decreases resistance to Clostridium difficile infection. Nat Med 22:1330–1334. doi: 10.1038/nm.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaddy JA, Radin JN, Loh JT, Piazuelo MB, Kehl-Fie TE, Delgado AG, Ilca FT, Peek RM, Cover TL, Chazin WJ, Skaar EP, Scott Algood HM. 2014. The host protein calprotectin modulates the Helicobacter pylori cag type IV secretion system via zinc sequestration. PLoS Pathog 10:e1004450. doi: 10.1371/journal.ppat.1004450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hood MI, Mortensen BL, Moore JL, Zhang Y, Kehl-Fie TE, Sugitani N, Chazin WJ, Caprioli RM, Skaar EP. 2012. Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathog 8:e1003068. doi: 10.1371/journal.ppat.1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lessa FC, Winston LG, McDonald LC, Team EIPCdS. 2015. Burden of Clostridium difficile infection in the United States. N Engl J Med 372:2369–2370. doi: 10.1056/NEJMc1505190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abt MC, McKenney PT, Pamer EG. 2016. Clostridium difficile colitis: pathogenesis and host defence. Nat Rev Microbiol 14:609–620. doi: 10.1038/nrmicro.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sammons JS, Toltzis P, Zaoutis TE. 2013. Clostridium difficile infection in children. JAMA Pediatr 167:567–573. doi: 10.1001/jamapediatrics.2013.441. [DOI] [PubMed] [Google Scholar]
- 13.Zackular JP, Skaar EP. 2018. The role of zinc and nutritional immunity in Clostridium difficile infection. Gut Microbes 9:469–476. doi: 10.1080/19490976.2018.1448354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao K, Santhosh K, Mogle JA, Higgins PD, Young VB. 2016. Elevated fecal calprotectin associates with adverse outcomes from Clostridium difficile infection in older adults. Infect Dis (Lond) 48:663–669. doi: 10.1080/23744235.2016.1186832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Kim H, Oh HJ, Kim HS, Hwang YJ, Yong D, Jeong SH, Lee K. 2017. Fecal calprotectin level reflects the severity of Clostridium difficile infection. Ann Lab Med 37:53–57. doi: 10.3343/alm.2017.37.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerasi M, Liu JZ, Ammendola S, Poe AJ, Petrarca P, Pesciaroli M, Pasquali P, Raffatellu M, Battistoni A. 2014. The ZupT transporter plays an important role in zinc homeostasis and contributes to Salmonella enterica virulence. Metallomics 6:845–853. doi: 10.1039/c3mt00352c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grass G, Wong MD, Rosen BP, Smith RL, Rensing C. 2002. ZupT is a Zn(II) uptake system in Escherichia coli. J Bacteriol 184:864–866. doi: 10.1128/jb.184.3.864-866.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lonergan ZR, Skaar EP. 2019. Nutrient zinc at the host-pathogen interface. Trends Biochem Sci 44:1041–1056. doi: 10.1016/j.tibs.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez CA, Beavers WN, Weiss A, Knippel RJ, Zackular JP, Chazin W, Skaar EP. 2019. The immune protein calprotectin impacts Clostridioides difficile metabolism through zinc limitation. mBio 10:e02289-19. doi: 10.1128/mBio.02289-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heap JT, Kuehne SA, Ehsaan M, Cartman ST, Cooksley CM, Scott JC, Minton NP. 2010. The ClosTron: mutagenesis in Clostridium refined and streamlined. J Microbiol Methods 80:49–55. doi: 10.1016/j.mimet.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Hastie JL, Hanna PC, Carlson PE. 2018. Transcriptional response of Clostridium difficile to low iron conditions. Pathog Dis 76. doi: 10.1093/femspd/fty009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho TD, Ellermeier CD. 2015. Ferric uptake regulator fur control of putative iron acquisition systems in Clostridium difficile. J Bacteriol 197:2930–2940. doi: 10.1128/JB.00098-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colomer-Winter C, Flores-Mireles AL, Baker SP, Frank KL, Lynch AJL, Hultgren SJ, Kitten T, Lemos JA. 2018. Manganese acquisition is essential for virulence of Enterococcus faecalis. PLoS Pathog 14:e1007102. doi: 10.1371/journal.ppat.1007102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabri M, Houle S, Dozois CM. 2009. Roles of the extraintestinal pathogenic Escherichia coli ZnuACB and ZupT zinc transporters during urinary tract infection. Infect Immun 77:1155–1164. doi: 10.1128/IAI.01082-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diaz-Ochoa VE, Lam D, Lee CS, Klaus S, Behnsen J, Liu JZ, Chim N, Nuccio SP, Rathi SG, Mastroianni JR, Edwards RA, Jacobo CM, Cerasi M, Battistoni A, Ouellette AJ, Goulding CW, Chazin WJ, Skaar EP, Raffatellu M. 2016. Salmonella mitigates oxidative stress and thrives in the inflamed gut by evading calprotectin-mediated manganese sequestration. Cell Host Microbe 19:814–825. doi: 10.1016/j.chom.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cartman ST, Minton NP. 2010. A mariner-based transposon system for in vivo random mutagenesis of Clostridium difficile. Appl Environ Microbiol 76:1103–1109. doi: 10.1128/AEM.02525-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knippel RJ, Zackular JP, Moore JL, Celis AI, Weiss A, Washington MK, DuBois JL, Caprioli RM, Skaar EP. 2018. Heme sensing and detoxification by HatRT contributes to pathogenesis during Clostridium difficile infection. PLoS Pathog 14:e1007486. doi: 10.1371/journal.ppat.1007486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francis MB, Allen CA, Shrestha R, Sorg JA. 2013. Bile acid recognition by the Clostridium difficile germinant receptor, CspC, is important for establishing infection. PLoS Pathog 9:e1003356. doi: 10.1371/journal.ppat.1003356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stabler RA, He M, Dawson L, Martin M, Valiente E, Corton C, Lawley TD, Sebaihia M, Quail MA, Rose G, Gerding DN, Gibert M, Popoff MR, Parkhill J, Dougan G, Wren BW. 2009. Comparative genome and phenotypic analysis of Clostridium difficile 027 strains provides insight into the evolution of a hypervirulent bacterium. Genome Biol 10:R102. doi: 10.1186/gb-2009-10-9-r102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 31.Blattner FR, Plunkett G, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]