Abstract
Background and Objectives
The objective of this study was to evaluate the long-term clinical outcomes and the incidence of permanent pacemaker implantation after catheter ablation in patients with of atrial fibrillation (AF) and sinus node dysfunction (SND).
Methods
Among 3,068 total consecutive patients who underwent AF catheter ablation (AFCA), this study included 222 (9.5%; men 53.2%, 63.7±9.2 years of age, 81.5% paroxysmal AF) with underlying SND and a regular rhythm follow-up. We analyzed the rhythm outcomes, changes in the mean heart rate or heart rate variability, and permanent pacemaker implantation rate.
Results
During 47.5±28.8 months of follow-up, 25 (11.3%) patients received pacemaker implantations due to symptomatic SND. More than half (56.0%, 14/25) underwent a pacemaker implantation within 3 months of the AFCA, and the annual pacemaker implantation rate was 2.0% afterwards. Both the early (68.0% vs. 31.0%, p<0.001) and clinical AF recurrence (68.0% vs. 32.5%, p=0.001) rates and continuous antiarrhythmic drug use after 3 months (44.0% vs. 24.4%, p=0.036) were significantly higher in patients requiring pacemaker implantations than those that did not. An anterior linear ablation (odds ratio [OR], 9.37 [3.03–28.9]; p<0.001) and the E/Em (OR, 1.15 [1.02–1.28]; p=0.018) were independently associated with permanent pacemaker implantations after AFCA in patients with AF and SND.
Conclusions
After AFCA in patients with AF and SND, 1 of 9 patients needed a pacemaker implantation and half needed implantations within 3 months. The AF recurrence rate was significantly higher in those who required pacemaker implantations after the AFCA.
Keywords: Sinus node dysfunction; Atrial fibrillation; Catheter ablation; Pacemaker, artificial
INTRODUCTION
Patients with sinus node dysfunction (SND) are more susceptible to develop atrial fibrillation (AF) and vice versa.1) AF develops in up to half of patients with SND,2) and AF may further exacerbate SND due to a high atrial rate and electrical or structural remodeling of the sinus node.3) Prolonged sinus pauses after AF termination can be commonly observed in patients with SND, which is also known as tachycardia-bradycardia syndrome.
Several studies have reported that successful catheter ablation of AF can induce reverse remodeling of the sinus node function and diminish prolonged sinus pauses upon AF termination.4) Chen et al.5) demonstrated that AF catheter ablation (AFCA) was effective in treating paroxysmal AF-related tachycardia-bradycardia syndrome in terms of restoring sinus rhythm without the need for permanent pacing. Thus recent guidelines have recommended that AFCA can be considered as a strategy before a pacemaker implantation in patients with AF-associated bradycardia as a class IIa indication.6) However, there is limited information on the long-term rhythm outcomes among these populations in a large number of patients. To address this issue, we evaluated the long-term clinical outcomes and incidence of a permanent pacemaker implantation after AFCA in patients with SND. The purpose of this study was to evaluate the rate and timing of a permanent pacemaker implantation and the recurrence rate of AF according to a pacemaker implantation during the long-term follow-up after AFCA in patients with AF and SND.
METHODS
Study population
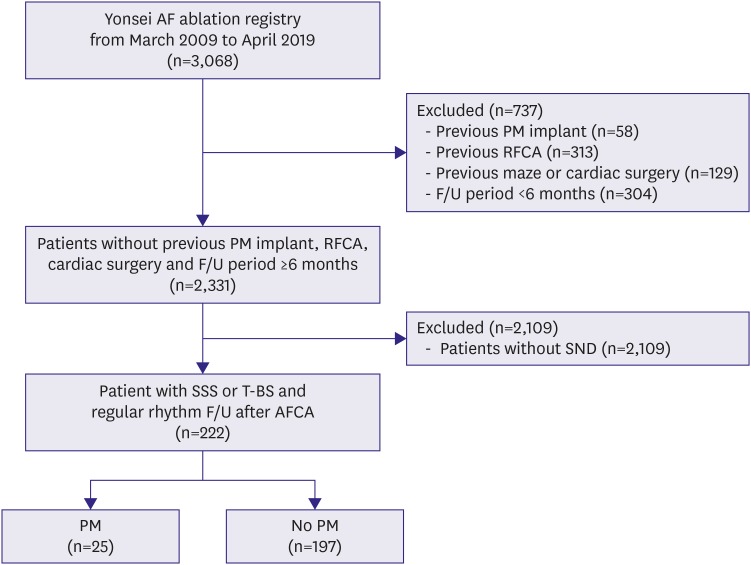
In this study, we defined sick sinus node syndrome or tachycardia-bradycardia syndrome as the inclusion criteria among a total of 3,068 patients out of the Yonsei AF ablation cohort who underwent catheter ablation due to AF from March 2009 to April 2019. After exclusion of 732 patients with 1) valvular AF, 2) previous permanent pacemaker implantation, 3) previous AFCA, 4) previous cardiac surgery or maze procedure, and 5) a post-AFCA follow-up duration of <6 months, 222 patients out of 2,331 with SND were included in this study (Figure 1). SND was defined as symptomatic sinus bradycardia with a sinus rate under 50 beats per minute or sinus pauses of longer than 3 seconds with or without low dose antiarrhythmic drugs (AADs) to maintain sinus rhythm.7) If the documented electrocardiogram (ECG) showed symptomatic SND immediately after AF termination, we classified it as tachycardia-bradycardia syndrome. We compared the patients who underwent a permanent pacemaker implantation with those who did not after the AFCA.
Figure 1. Flowchart of the patient analyses.
AFCA = atrial fibrillation catheter ablation; F/U = follow-up; PM = pacemaker; RFCA = radiofrequency catheter ablation; SND = sinus node dysfunction; SSS = sick sinus syndrome; TBS = tachycardia-bradycardia syndrome.
Electroanatomical mapping and radiofrequency catheter ablation
Three-dimensional (3D) electroanatomical mapping (NavX; Abbott Inc., Minnetonka, MN, USA) was generated using a circumferential pulmonary vein (PV)-mapping catheter (Lasso; Biosense Webster Inc., Diamond Bar, CA, USA) through a long sheath (Schwartz left 1; Abbott Inc.). The 3D geometry of both the left atrium and PVs was generated using the NavX system and then merged with 3D spiral computed tomography (CT) images. Blinded to the patient information, a technician analyzed the color-coded CT-merged NavX voltage maps using custom software (Image Pro; Media Cybernetics Inc., Rockville, MD, USA). An open-irrigated tip catheter (Celsius, Johnson & Johnson Inc., Diamond Bar, CA, USA; NaviStar ThermoCool, Biosense Webster Inc.; ThermoCool SF, Biosense Webster Inc.; ThermoCool SmartTouch, Biosense Webster Inc.; Coolflex, Abbott Inc.; 30–35 W; 47°C.; FlexAbility, Abbott Inc.; ThermoCool SmartTouch, Biosense Webster Inc.; and TactiCath, Abbott Inc.) was used for the AFCA. All patients underwent a de novo procedure with a circumferential PV isolation (CPVI). The majority of the patients underwent the creation of cavotricuspid isthmus (CTI) block during the de novo procedure unless there was AV conduction disease. As an extra-PV left atrial ablation, we conducted additional linear ablation including a roof line, posterior inferior line (posterior box lesion), and anterior line, particularly in patients with persistent AF. A left lateral isthmus ablation, right atrial ablation, and complex fractionated electrogram (CFAE) ablation were performed in the minority of the patients at the operator's discretion. We defined an extra-PV left atrial ablation as additional linear ablation with or without a CFAE ablation following the CPVI. The de novo procedure ended when there was no immediate recurrence of AF within 10 minutes after cardioversion with an isoproterenol infusion (5–10 μg/min).
Post-ablation management and follow-up
Patients visited the outpatient clinic at 1, 3, 6, and 12 months and every 6 months thereafter or whenever symptoms developed after the AFCA. ECG was performed at every visit. Twenty-four-hour Holter monitoring was performed at 3, 6, and 12 months and then every 6 months according to the 2012 Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia Society expert consensus statement guidelines.8) Patients who suffered from symptoms of palpitations underwent Holter/event-monitor examinations to investigate the possibility of an arrhythmia recurrence. We defined an AF recurrence as any episode of atrial tachycardia or AF lasting for 30 seconds or more. Any electrocardiography documentation of an AF recurrence after the 3-month blanking period was classified as a clinical recurrence.
Statistical analysis
Continuous variables are expressed as the mean±standard deviation. Categorical variables are reported as the count (percentage). To compare the baseline characteristics and clinical outcomes between the 2 groups, we used the Student's t-test or Mann-Whitney test for continuous variables and the χ2 test for categorical variables. A Kaplan-Meier analysis with a log-rank test was used to compare the clinical recurrence rates according to the presence or absence of SND. The incidence of a pacemaker implantation and the rhythm outcomes after the catheter ablation were analyzed. All statistical analyses were performed using SPSS (Statistical Package for Social Sciences, Chicago, IL, USA) software for Windows (version 25.0).
RESULTS
Patient characteristics and permanent pacemaker implantation rates
Among 2,331 patients who underwent an AFCA, 222 had clinically significant symptomatic SND without a permanent pacemaker before the AFCA (Table 1). Among the 222 patients with SND, 41.9% (n=93) had sick sinus node syndrome, 55.4% (n=123) tachycardia-bradycardia syndrome, and 2.7% (n=6) both. The patients with SND were older (p<0.001) and included proportionally more women (p<0.001), paroxysmal AF (p<0.001), hypertension (p=0.002), a prior history of a stroke (p<0.001), higher CHA2DS2-VASc score (p<0.001), the ratio of the early diastolic peak mitral inflow velocity and early diastolic mitral annular velocity (E/Em) on echocardiography (p<0.001), and a lower body mass index (p=0.014) or lower prescription rates of β-blockers (p<0.001) or AAD (p<0.001) than those without SND.
Table 1. Baseline clinical characteristics in the patients with SND and those without.
| Variables | Total (n=2,331) | SND (n=222) | No SND (n=2,109) | p value | |
|---|---|---|---|---|---|
| Age (years) | 58.2±10.8 | 63.7±9.2 | 57.6±10.8 | <0.001 | |
| Male | 1,730 (74.2) | 118 (53.2) | 1,612 (76.4) | <0.001 | |
| Paroxysmal AF | 1,635 (70.2) | 181 (81.5) | 1,454 (69.0) | <0.001 | |
| AF duration (months) | 38.7±47.1 | 31.2±32.9 | 39.5±48.4 | 0.296 | |
| BMI (kg/m2) | 25.0±3.1 | 24.5±3.0 | 25.1±3.1 | 0.014 | |
| Comorbidities | |||||
| Heart failure | 215 (9.2) | 19 (8.6) | 196 (9.3) | 0.719 | |
| Hypertension | 1,080 (46.3) | 125 (56.3) | 955 (45.3) | 0.002 | |
| Diabetes | 348 (14.9) | 33 (14.9) | 315 (14.9) | 0.975 | |
| Stroke/TIA | 269 (11.5) | 43 (19.4) | 226 (10.7) | <0.001 | |
| Vascular disease | 259 (11.1) | 29 (13.1) | 230 (10.9) | 0.331 | |
| CHA2DS2-VASc score | 1.7±1.5 | 2.4±1.7 | 1.6±1.5 | <0.001 | |
| Echocardiographic measures | |||||
| LA dimension (mm) | 41.1±6.0 | 41.3±6.2 | 41.1±6.0 | 0.602 | |
| LV ejection fraction (%) | 63.6±16.9 | 64.2±8.0 | 63.5±17.6 | 0.085 | |
| E/Em | 10.0±4.1 | 11.6±4.5 | 9.8±4.0 | <0.001 | |
| Medications | |||||
| ACEi/ARB | 787 (33.8) | 87 (39.2) | 700 (33.3) | 0.076 | |
| β-blocker | 813 (35.0) | 53 (23.9) | 760 (36.1) | <0.001 | |
| Statin | 683 (29.4) | 82 (36.9) | 601 (28.6) | 0.009 | |
| AAD | 431 (18.5) | 16 (7.2) | 415 (19.7) | <0.001 | |
Values are presented as mean±standard deviation or number (%).
AAD = antiarrhythmic drug; ACEi = angiotensin-converting enzyme inhibitor; AF = atrial fibrillation; ARB = angiotensin receptor blocker; BMI = body mass index; E/Em = mitral inflow velocity/mitral annulus tissue velocity; LA = left atrium; LV = left ventricle; SND = sinus node dysfunction; TIA = transient ischemia attack.
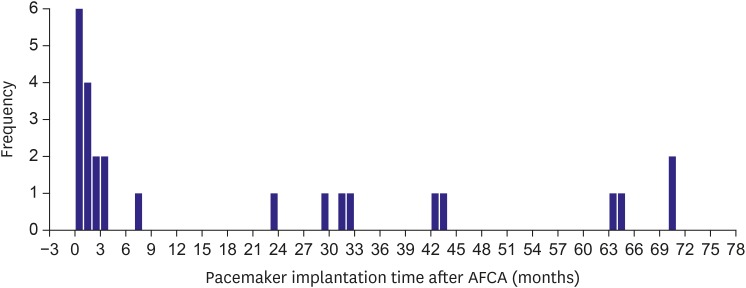
During the 47.5±28.8-month follow-up period of the 222 SND patients after the AFCA, 25 (11.3%) patients received a pacemaker implantation due to symptomatic SND. Table 2 compares the patients who underwent a permanent pacemaker implantation after the AFCA and those without. Of the 222 patients with SND, pacemaker implantation was performed in 14 (15.1%) of the 93 patients without tachycardia-bradycardia syndrome, and in 11 (8.5%) of the 129 patients with tachycardia-bradycardia syndrome (odds ratio [OR], 0.53; p=0.138). There was no significant difference in the clinical characteristics and medications between the patients with a pacemaker implantation and those without. Among the 25 patients who underwent a permanent pacemaker implant after the AFCA, more than half (56.0%) underwent pacemaker procedures within 3 months after the AFCA and the annual pacemaker implantation rate was 2.0% thereafter (mean follow-up duration 54.4±25.4 months, Figure 2).
Table 2. Baseline clinical characteristics in the patients who required a pacemaker and those that did not after the AFCA.
| Variables | Total (n=222) | PM (n=25) | No PM (n=197) | p value | |
|---|---|---|---|---|---|
| Age (years) | 63.7±9.2 | 64.3±8.9 | 63.6±9.2 | 0.735 | |
| Male | 118 (53.2) | 12 (48.0) | 106 (53.8) | 0.584 | |
| Paroxysmal AF | 181 (81.5) | 20 (80.0) | 161 (81.7) | 0.834 | |
| AF duration (months) | 31.2±32.9 | 33.7±41.1 | 30.9±32.2 | 0.524 | |
| BMI (kg/m2) | 24.5±3.0 | 24.2±2.8 | 24.6±3.0 | 0.826 | |
| Sick sinus node syndrome | 93 (41.9) | 14 (56.0) | 79 (40.1) | 0.129 | |
| Tachy-bradycardia syndrome | 123 (55.4) | 10 (40.0) | 113 (57.4) | 0.100 | |
| Mixed type | 6 (2.7) | 1 (4.0) | 5 (2.5) | 0.516 | |
| Comorbidities | |||||
| Heart failure | 19 (8.6) | 1 (4.0) | 18 (9.1) | 0.703 | |
| Hypertension | 125 (56.3) | 16 (64.0) | 109 (55.3) | 0.410 | |
| Diabetes | 33 (14.9) | 4 (16.0) | 29 (14.7) | 0.772 | |
| Stroke/TIA | 43 (19.4) | 38 (19.3) | 0.933 | ||
| Vascular disease | 29 (13.1) | 6 (24.0) | 23 (11.7) | 0.085 | |
| CHA2DS2-VASc score | 2.4±1.7 | 2.7±1.8 | 2.4±1.6 | 0.548 | |
| Echocardiographic measures | |||||
| LA dimension, mm | 41.3±6.2 | 41.2±7.1 | 41.3±6.1 | 0.739 | |
| LV ejection fraction (%) | 64.2±8.0 | 66.6±7.5 | 63.9±8.1 | 0.172 | |
| E/Em | 11.6±4.5 | 13.6±6.2 | 11.3±4.2 | 0.104 | |
| Medications | |||||
| ACEi/ARB | 87 (39.2) | 14 (56.0) | 73 (37.1) | 0.068 | |
| β-blocker | 53 (23.9) | 7 (28.0) | 46 (23.4) | 0.607 | |
| Statin | 82 (36.9) | 9 (36.0) | 73 (37.1) | 0.918 | |
| AAD | 16 (7.2) | 4 (16.0) | 12 (6.1) | 0.089 | |
Values are presented as mean±standard deviation or number (%).
AAD = antiarrhythmic drug; ACEi = angiotensin-converting enzyme inhibitor; AFCA = atrial fibrillation catheter ablation; AF = atrial fibrillation; ARB = angiotensin receptor blocker; BMI = body mass index; E/Em = mitral inflow velocity/mitral annulus tissue velocity; LA = left atrium; LV = left ventricle; PM = pacemaker; TIA = transient ischemia attack.
Figure 2. Pacemaker implantation timing after the catheter ablation procedure.
AFCA = atrial fibrillation catheter ablation.
Atrial fibrillation catheter ablation and atrial fibrillation recurrence rates
The procedure-related factors and ablation outcomes between the pacemaker and no pacemaker groups are presented in Table 3. The mean procedure time (p=0.009) and ablation time (p=0.044) were significantly longer in the patients who underwent pacemaker implantations than in those who did not. Extra-PV left atrial linear ablation, such as a roof line (p=0.008), posterior inferior line (p=0.008), or anterior line (p<0.001) was more commonly conducted in the pacemaker group than no pacemaker group. The procedure-related major complication rates did not differ between the 2 groups. Although the AAD prescription rate at discharge did not significantly differ between the 2 groups (16.0% vs. 6.1%, p=0.089), it was significantly higher at 3 months after the AFCA (44.0% vs. 24.4%, p=0.036) and at the timing of the last follow-up (72.0% vs. 24.9%, p<0.001) in the pacemaker group than the no pacemaker group.
Table 3. Procedure related characteristics in the patients with SND.
| Procedure outcomes | Overall (n=222) | PM (n=25) | No PM (n=197) | p value | |
|---|---|---|---|---|---|
| Procedure time (minutes) | 181.0±54.9 | 214.5±74.8 | 176.7±50.5 | 0.009 | |
| Ablation time (seconds) | 4,673.6±1,473.1 | 5,225.4±1,601.2 | 4,603.6±1,445.4 | 0.044 | |
| Fluoroscopy time (minutes) | 38.6±15.3 | 44.2±19.6 | 37.9±14.6 | 0.093 | |
| Complications* | 9 (4.1) | 1† (4.0) | 8 (4.1) | 1.000 | |
| Major complications | 5 (2.3) | 0 | 5 (2.5) | 1.000 | |
| Tamponade | 4 (1.8) | 0 | 4 (2.0) | 1.000 | |
| Arteriovenous fistula | 1 (0.4) | 0 | 1 (0.5) | 1.000 | |
| Mean LA voltage (mV) | 1.27±0.60 | 1.03±0.46 | 1.29±0.61 | 0.105 | |
| Extra-PV LA Ablation (%) | |||||
| Roof line | 64 (29.4) | 13 (52.0) | 51 (26.4) | 0.008 | |
| Posterior inferior line | 51 (23.0) | 11 (44.0) | 40 (20.3) | 0.008 | |
| Anterior line | 47 (21.2) | 14 (56.0) | 33 (16.8) | <0.001 | |
| Left lateral isthmus line | 8 (3.6) | 1 (4.0) | 7 (3.6) | 1.000 | |
| Cavotricuspid isthmus line | 207 (93.2) | 25 (100) | 182 (92.4) | 0.229 | |
| SVC-septal line | 142 (64.0) | 15 (60.0) | 127 (64.5) | 0.661 | |
| Extra-PV triggers (IRAF, %) | 22 (13.6) | 1 (6.3) | 21 (14.4) | 0.699 | |
| Follow-up (months) | 47.5±28.8 | 54.4±25.4 | 46.6±29.2 | 0.158 | |
| Early recurrence | 78 (35.1) | 17 (68.0) | 61 (31.0) | <0.001 | |
| Recurrence as AT | 28 (36.4) | 7 (41.2) | 21 (35.0) | 0.640 | |
| Clinical recurrence | 81 (36.5) | 17 (68.0) | 64 (32.5) | 0.001 | |
| Recurrence as AT | 26 (31.7) | 8 (47.1) | 18 (27.7) | 0.127 | |
| AADs at discharge | 16 (7.2) | 4 (16.0) | 12 (6.1) | 0.089 | |
| AADs 3 months after RFCA | 59 (26.6) | 11 (44.0) | 48 (24.4) | 0.036 | |
| AADs at recurrence | 71 (32.0) | 12 (48.0) | 59 (29.9) | 0.068 | |
| AADs at final follow-up | 67 (30.2) | 18 (72.0) | 49 (24.9) | <0.001 | |
| CV for recurrence | 27 (12.2) | 5 (20.0) | 22 (11.2) | 0.203 | |
| Final rhythm in sinus | 197 (88.7) | 21 (84.0) | 176 (89.3) | 0.497 | |
Values are presented as mean±standard deviation or number (%).
AAD = antiarrhythmic drug; AT = atrial tachycardia; CV = cardioversion; IRAF = immediate reinitiation of atrial fibrillation; LA = left atrium; PM = pacemaker; PV = pulmonary vein; RFCA = radiofrequency catheter ablation; SND = sinus node dysfunction; SVC = superior vena cava.
*Complications: pericarditis, cardiac tamponade, mild jugular hematoma, femoral arteriovenous fistula; †Jugular hematoma: self-resolved; Major complications: tamponade which needed pericardiocentesis; Femoral arteriovenous fistula which needed a fistulectomy.
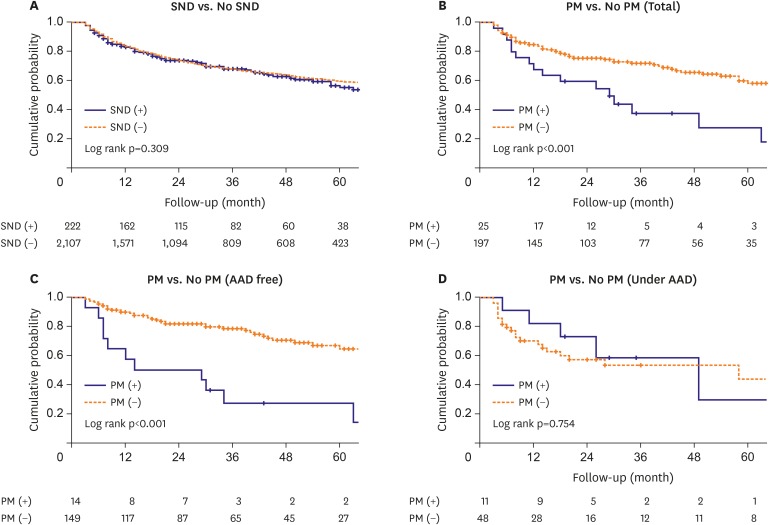
During the 47.5±28.8-month follow-up, both the early (68.0% vs 31.0%, p<0.001) and clinical AF recurrence (68.0% vs. 32.5%, p=0.001) rates were significantly higher in the patients who required a pacemaker implantation than in those who did not. Kaplan-Meier analyses showed there was no significant difference in the AF recurrence-free survival between the patients with underlying pre-ablation SND and those without (log rank p=0.309, Figure 3A), but the clinical recurrence rate was significantly higher in the patients who required a pacemaker implantation than in those who did not (Log rank p<0.001, Figure 3B). This trend was consistent in AAD-free patient groups (log rank p<0.001, Figure 3C), but not in the patients under the AAD effects (log rank p=0.754, Figure 3D).
Figure 3. Kaplan-Meier analysis with a log-rank test for the AF recurrence after the AFCA. (A) Patients with SND vs. without SND. (B) Patients who required a pacemaker implantation versus no pacemaker implantation. (C) AAD-free recurrence rates in the pacemaker group versus no pacemaker group. (D) AF recurrence under AADs in the pacemaker group versus no pacemaker group.
AAD = anti-arrhythmic drug; AF = atrial fibrillation; AFCA = atrial fibrillation catheter ablation; SND = sinus node dysfunction; PM = pacemaker.
Associated factors with a pacemaker implantation after the atrial fibrillation catheter ablation
We compared the pre-AFCA and post-AFCA 3-month heart rate variability between the pacemaker group and no pacemaker group (Supplementary Table 1). The minimum heart rate (p<0.001) and mean heart rate (p<0.001) were significantly increased in the no pacemaker group, but not in the pacemaker group (before the pacemaker implantation) at 3 months after the AFCA (minimum heart rate [p=0.100] and mean heart rate [p=0.099], respectively). The reduction in the root mean square of the successive differences (p=0.001), high frequency (p=0.001), or low frequency (p<0.001) was also significant in the no pacemaker group, but not in the pacemaker group (before the pacemaker implantation).
In the multivariate logistic regression analysis, the E/Em (OR, 1.15 [1.02–1.28]; p=0.018) and anterior linear ablation (OR, 9.37 [3.03–28.95]; p<0.001) were independently associated with a permanent pacemaker requirement after the AFCA in the patients associated with SND (Table 4).
Table 4. Logistic regression analyses for a pacemaker implantation after the AFCA in patients with underlying SND.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age* | 1.009 (0.964–1.056) | 0.705 | ||
| Male sex* | 0.792 (0.344–1.823) | 0.584 | ||
| Paroxysmal AF* | 0.894 (0.315–2.542) | 0.834 | ||
| BMI | 0.961 (0.834–1.108) | 0.586 | ||
| Heart failure | 0.414 (0.053–3.245) | 0.401 | ||
| Hypertension | 1.435 (0.605–3.404) | 0.412 | ||
| Diabetes mellitus | 1.103 (0.353–3.449) | 0.866 | ||
| Stroke or TIA | 1.046 (0.369–2.965) | 0.933 | ||
| Vascular disease | 2.389 (0.865–6.596) | 0.093 | ||
| CHA2DS2-VASc* | 1.116 (0.875–1.422) | 0.378 | ||
| LA size | 0.999 (0.934–1.068) | 0.969 | ||
| LV ejection fraction* | 1.049 (0.988–1.113) | 0.116 | ||
| E/Em* | 1.103 (1.014–1.199) | 0.022 | 1.146 (1.023–1.284) | 0.018 |
| Mean LA voltage (mV)* | 0.415 (0.152–1.134) | 0.086 | ||
| PreAFCA mean heart rate | 1.001 (0.963–1.041) | 0.962 | ||
| Roof line* | 3.016 (1.293–7.038) | 0.011 | ||
| Posterior inferior line* | 3.084 (1.302–7.307) | 0.011 | ||
| Anterior line* | 6.325 (2.640–15.154) | <0.001 | 9.371 (3.034–28.948) | <0.001 |
| SVC-septal line | 0.827 (0.353–1.938) | 0.662 | ||
AFCA = atrial fibrillation catheter ablation; AF = atrial fibrillation; BMI = body mass index; CI = confidence interval; E/Em = mitral inflow velocity/mitral annulus tissue velocity; LA = left atrium; LV = left ventricle; LVEF = left ventricular ejection fraction; OR = odds ratio; SND = sinus node dysfunction; SVC = superior vena cava; TIA = transient ischemia attack.
*The variables used in the multivariate analysis are as follows: age, sex, paroxysmal AF, CHA2DS2-VASc score, LVEF, E/Em, mean LA voltage (mV), PreAFCA mean heart rate (ms), roof line, posterior inferior line, and anterior line.
DISCUSSION
The main findings of this study were that 11.3% of the AF patients with SND eventually underwent a permanent pacemaker implantation after the AFCA during a mean follow-up period of 47.5±28.8-months, and 56% of them had permanent pacemakers within 3 months of the AFCA. The annual pacemaker implantation rate was 2.0% thereafter. Although the AF/AT recurrence rate after the AFCA between the patients with SND and those without exhibited no significant difference, the early and clinical recurrence rates and continuous AAD use were significantly higher in the permanent pacemaker implantation group than in the no pacemaker group among the patients with SND. We found that an anterior linear ablation during the de novo procedure and the E/Em were independently associated with a permanent pacemaker implantation after the AFCA in the patients with AF and SND.
Although SND is caused by functional or histological deterioration of the sinus node located in the right atrium, the primary mechanisms of AF triggers and the maintenance are known to be driven by the left atrium and PVs. Nevertheless, both AF and SND have much in common with each other. Recently, PITX2, the top 1st common genetic loci of AF, has been implicated not only in the PV development9) but has also been linked to the multiple genes (TBX5, Gja1, or SCN5a) involved in the development of the sinus node and right and left atrial asymmetry.10) Atrial arrhythmias associated with SND are known to be present in 40% to as much as 70% of those patients.11) Many variables including structural and electrical remodeling, genetic mutations, a cholinergic effect, and reverse remodeling after AF rhythm control explain the SND accompanied by AF.
In patients with AF, remodeling and significant atrial fibrosis near the sinus node area are associated with clinically significant SND.3) In previous studies, SND could be induced under conditions of pacing-induced chronic AF, a prolonged intra-atrial conduction time, and a decreased atrial refractoriness under sustained AF.12) A prolonged sinus node recovery time and slower intrinsic heart rates were gradually reversed after termination of AF. Down-regulation of the sinoatrial node ion channel (HCN, hyperpolarization-activated cyclic nucleotide-gated) caused by atrial tachycardia may result in a decreased expression of the ion channels in the sinus node, which operate as a pacemaker and may cause SND.13)
Catheter ablation has been used to treat AF patients for several decades. Previous studies have shown that paroxysmal AF with SND can be treated by AFCA,5) although the association and causality between AFCA in SND has not been fully elucidated. Sparks et al.14) demonstrated that both paroxysmal and chronic atrial flutter exhibited an improvement in the sinus node recovery time 3 weeks after atrial flutter catheter ablation. Catheter ablation in patients with AF-induced bradycardia may reduce the need for AADs and a pacemaker implantation.5) Thus, a successful reduction in the AF burden by AFCA leads to electrical reverse remodeling and reduces the permanent pacemaker requirement by reducing the use of drugs that suppress the SN function. However, SND associated with irreversible structural remodeling and replacement fibrosis may persist despite AF rhythm control,15) eventually leading to a permanent pacemaker implantation. Although atrial scar burden might be related to the pacemaker requirement, LA voltage did not differ between pacemaker group and no pacemaker group with statistical significance and right atrial voltage data were not available in this study. Previously we reported post-AF ablation high sinus heart rate in patients with significant vagal modulation and its association with favorable rhythm outcome after catheter ablation.16) In the present study, the mean heart rate was significantly increased in no pacemaker group, but not in the pacemaker group. There is one possibility that the increase in heart rate after AFCA ameliorated the pacemaker requirement in patients with significant vagal modulation.
However, since AFCA itself is a destructive surgery, iatrogenic aggravation of the SND due to atrial tissue damage or vascular injury to the sinus nodal artery cannot be excluded. A higher risk of post-operative SND after a bi-atrial maze procedure compared to a left atrial maze procedure was reported in patients who underwent mitral valve surgery.17) In this study, the AFCA procedure time and ablation time were significantly longer in the pacemaker implantation group than in those without, and extra-PV ablation including an anterior line was independently associated with a permanent pacemaker implantation after the AFCA. Therefore, the cause and effect relationship between the higher AF recurrence rate and the eventual permanent pacemaker implantation group is not clear.
In a previous study that compared patients with SND and those without, the LA dimension in the SND patients was larger than that in those without SND.18) Several studies have shown that factors including atrial remodeling and congestive heart failure that suggest stretched atria have an effect on the sinus node function.19) In patients who underwent mitral valve surgery and concomitant maze procedures, post-operative SND that required a pacemaker was more commonly observed in patients with moderate to severe tricuspid regurgitation than in their counterparts.17) This suggests that tricuspid regurgitation might contribute to right atrial and SN remodeling. In the present study, the E/Em, which indicates the diastolic function of the left ventricle, was independently associated with a permanent pacemaker implantation after the AFCA. We previously reported that a high E/Em and elevated LA pressure were significantly associated with atrial structural remodeling reflected by the LA volume and LA voltage and a higher recurrence of AF after the AFCA.20)
The striking finding of this study was that an anterior linear ablation21) was an independent risk factor for a permanent pacemaker implantation after the AFCA in patients with underlying SND. An anterior line is a linear lesion that connects the anterior mitral ring to the roof line. Pak et al.21) reported that the bidirectional block rate and procedural success rate were significantly better after an anterior linear ablation than after a left lateral isthmus ablation in patients with persistent AF. We occasionally experienced post-ablation SND after the anterior linear ablation, but most SND recovered within 24 hours after the procedure. This may be due to the anterior line passing through the area of the sinus nodal artery, which exists parallel inside of Bachmann's bundle. However, we confirmed that the anterior line was the significant independent risk factor of a permanent pacemaker implantation after the AFCA in patients with AF and SND.
This study had several limitations. First, it is a retrospective study, conducted in a single center, and only included patients with concurrent AF and SND. Therefore, there could have been a selection bias. Second, elderly patients with severely symptomatic SND generally preferred AAD treatment after a pacemaker implantation rather than AFCA. Therefore, this study had a selection bias for AFCA, and the outcome of this study cannot be generalized to all patients with SND and AF. Third, although 24-hour Holter monitoring was performed at 3, 6, and 12 months and then every 6 months in both groups, the rhythm monitoring could have been more aggressive, and the detection sensitivity for AF recurrence was higher in the pacemaker group when the atrial high rate episodes were detected by the device than in no pacemaker group. Nonetheless, this study had implications for evaluating the long-term prognosis in patients with evidence-based indications for an AFCA prior to a pacemaker implantation.
After AFCA in patients with AF and SND, 1 out of 9 patients needed a pacemaker implantation and half underwent a placement within 3 months. The AF recurrence rate was significantly higher in the patients who required a pacemaker implantation after the AFCA. The causal-result relationship between the higher AF recurrence rate and the eventual permanent pacemaker implantation group is not clear.
ACKNOWLEDGMENTS
We thank Mr. John Martin for his linguistic assistance.
Footnotes
Funding: This work was supported by a grant (HI18C0070) and (HI19C0114) from the Ministry of Health and Welfare and a grant (NRF-2017R1A2B4003983) from the Basic Science Research Program run by the National Research Foundation of Korea (NRF), which is funded by the Ministry of Science, ICT & Future Planning (MSIP).
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Yu HT, Pak HN.
- Data curation: Kim TH, Uhm JS, Kim JY, Joung B, Lee MH.
- Writing - original draft: Hwang TH, Yu HT.
- Writing - review & editing: Pak HN.
SUPPLEMENTARY MATERIAL
The heart rate variability according to the presence of a pacemaker implantation
References
- 1.Nielsen JC, Thomsen PE, Højberg S, et al. A comparison of single-lead atrial pacing with dual-chamber pacing in sick sinus syndrome. Eur Heart J. 2011;32:686–696. doi: 10.1093/eurheartj/ehr022. [DOI] [PubMed] [Google Scholar]
- 2.Lamas GA, Lee K, Sweeney M, et al. The mode selection trial (MOST) in sinus node dysfunction: design, rationale, and baseline characteristics of the first 1000 patients. Am Heart J. 2000;140:541–551. doi: 10.1067/mhj.2000.109652. [DOI] [PubMed] [Google Scholar]
- 3.Chang HY, Lin YJ, Lo LW, et al. Sinus node dysfunction in atrial fibrillation patients: the evidence of regional atrial substrate remodelling. Europace. 2013;15:205–211. doi: 10.1093/europace/eus219. [DOI] [PubMed] [Google Scholar]
- 4.Hocini M, Sanders P, Deisenhofer I, et al. Reverse remodeling of sinus node function after catheter ablation of atrial fibrillation in patients with prolonged sinus pauses. Circulation. 2003;108:1172–1175. doi: 10.1161/01.CIR.0000090685.13169.07. [DOI] [PubMed] [Google Scholar]
- 5.Chen YW, Bai R, Lin T, et al. Pacing or ablation: which is better for paroxysmal atrial fibrillation-related tachycardia-bradycardia syndrome? Pacing Clin Electrophysiol. 2014;37:403–411. doi: 10.1111/pace.12340. [DOI] [PubMed] [Google Scholar]
- 6.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 7.Kusumoto FM, Schoenfeld MH, Barrett C, et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;140:e382–482. doi: 10.1161/CIR.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 8.Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9:632–696.e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Lee JY, Kim TH, Yang PS, et al. Korean atrial fibrillation network genome-wide association study for early-onset atrial fibrillation identifies novel susceptibility loci. Eur Heart J. 2017;38:2586–2594. doi: 10.1093/eurheartj/ehx213. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Klysik E, Sood S, Johnson RL, Wehrens XH, Martin JF. Pitx2 prevents susceptibility to atrial arrhythmias by inhibiting left-sided pacemaker specification. Proc Natl Acad Sci U S A. 2010;107:9753–9758. doi: 10.1073/pnas.0912585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamas GA, Lee KL, Sweeney MO, et al. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med. 2002;346:1854–1862. doi: 10.1056/NEJMoa013040. [DOI] [PubMed] [Google Scholar]
- 12.Elvan A, Wylie K, Zipes DP. Pacing-induced chronic atrial fibrillation impairs sinus node function in dogs. Electrophysiological remodeling. Circulation. 1996;94:2953–2960. doi: 10.1161/01.cir.94.11.2953. [DOI] [PubMed] [Google Scholar]
- 13.Yeh YH, Burstein B, Qi XY, et al. Funny current downregulation and sinus node dysfunction associated with atrial tachyarrhythmia: a molecular basis for tachycardia-bradycardia syndrome. Circulation. 2009;119:1576–1585. doi: 10.1161/CIRCULATIONAHA.108.789677. [DOI] [PubMed] [Google Scholar]
- 14.Sparks PB, Jayaprakash S, Vohra JK, Kalman JM. Electrical remodeling of the atria associated with paroxysmal and chronic atrial flutter. Circulation. 2000;102:1807–1813. doi: 10.1161/01.cir.102.15.1807. [DOI] [PubMed] [Google Scholar]
- 15.Cha TJ, Ehrlich JR, Zhang L, et al. Dissociation between ionic remodeling and ability to sustain atrial fibrillation during recovery from experimental congestive heart failure. Circulation. 2004;109:412–418. doi: 10.1161/01.CIR.0000109501.47603.0C. [DOI] [PubMed] [Google Scholar]
- 16.Yu HT, Kim TH, Uhm JS, et al. Prognosis of high sinus heart rate after catheter ablation for atrial fibrillation. Europace. 2017;19:1132–1139. doi: 10.1093/europace/euw142. [DOI] [PubMed] [Google Scholar]
- 17.Cho MS, Heo R, Jin X, et al. Sick sinus syndrome after the maze procedure performed concomitantly with mitral valve surgery. J Am Heart Assoc. 2018;7:e009629. doi: 10.1161/JAHA.118.009629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi K, Fukunaga M, Yamaji K, et al. Impact of catheter ablation for paroxysmal atrial fibrillation in patients with sick sinus syndrome - important role of non-pulmonary vein foci - Circ J. 2016;80:887–894. doi: 10.1253/circj.CJ-15-1384. [DOI] [PubMed] [Google Scholar]
- 19.Morton JB, Sanders P, Vohra JK, et al. Effect of chronic right atrial stretch on atrial electrical remodeling in patients with an atrial septal defect. Circulation. 2003;107:1775–1782. doi: 10.1161/01.CIR.0000058164.68127.F2. [DOI] [PubMed] [Google Scholar]
- 20.Park J, Joung B, Uhm JS, et al. High left atrial pressures are associated with advanced electroanatomical remodeling of left atrium and independent predictors for clinical recurrence of atrial fibrillation after catheter ablation. Heart Rhythm. 2014;11:953–960. doi: 10.1016/j.hrthm.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Pak HN, Oh YS, Lim HE, Kim YH, Hwang C. Comparison of voltage map-guided left atrial anterior wall ablation versus left lateral mitral isthmus ablation in patients with persistent atrial fibrillation. Heart Rhythm. 2011;8:199–206. doi: 10.1016/j.hrthm.2010.10.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The heart rate variability according to the presence of a pacemaker implantation