Abstract
Objective:
Treatment of patients with thymic malignancies metastatic to the pleura or pericardium is challenging, and benefits of aggressive treatment are unclear. We sought to characterize long-term outcomes in this population.
Methods:
We retrospectively identified patients who underwent resection for de novo thymic malignancies metastatic to the pleura between May 1997 and December 2017. Patients with pleural recurrence after prior thymectomy were excluded. Patient demographics, perioperative treatments, pathologic findings, and postoperative outcomes were collected. Descriptive statistics and Kaplan-Meier analyses were performed.
Results:
Seventy-two patients were included (median age, 51 [range 25-80]; 36/72 women [50%]). Pathologic diagnosis was thymoma in 57 patients (79%) and thymic carcinoma in 15 (21%). Most patients (67/72; 93%) received chemotherapy, radiation, or both. Forty-eight patients underwent thymectomy with pleurectomy, 7 underwent extrapleural pneumonectomy, 10 underwent thymectomy alone, and 7 were unresectable. Macroscopic complete resection was achieved in 52 patients (73%). Five-, 10-, and 15-year overall survivals were 73%, 51%, and 18%, and median overall survival was 11 years (median follow-up, 5.9 years). Forty-six patients (64%) had disease progression (median time to progression, 2.2 years). Repeat episodes of progression and treatment were common (median, 3 episodes/patient). The longest disease-free interval was 12.4 years. Thirteen patients (18%) remain disease-free; 7 (10%) were disease-free for >5 years. The longest ongoing survival without progression or reintervention is 9.9 years.
Conclusion:
Prolonged survival and, in some cases, cure can be achieved in patients with thymic malignancies metastatic to the pleura or pericardium. Aggressive multimodality therapy may be appropriate for select patients.
Thymic malignancies are rare cancers that generally have indolent disease courses, even at advanced stages.1, 3 Patients with advanced stage disease with pleural or pericardial metastases (M1a, TNM 8th edition; Masaoka stage IVa) at presentation have a 5-year overall survival of 50%-84%.1–5 Owing to the rarity of these malignancies and the long follow-up required to study these patients, studies focusing on outcomes after treatment for M1a thymoma are few, and almost all are retrospective.
Management of patients with pleural or pericardial disease is particularly challenging. Surgery is the mainstay of treatment for thymoma, but the utility of resection is less well-defined for patients who have disseminated disease (Figure 1). That metastases tend to be confined to the thoracic cavity raises the theoretical potential for complete surgical eradication and the possibility of rendering the patient disease-free. Despite the presence of multiple lesions, surgery with curative intent is often still performed in such patients. Depending on the tumor burden, aggressive surgery including extended resection and even extrapleural pneumonectomy (EPP)6–9 is sometimes offered.
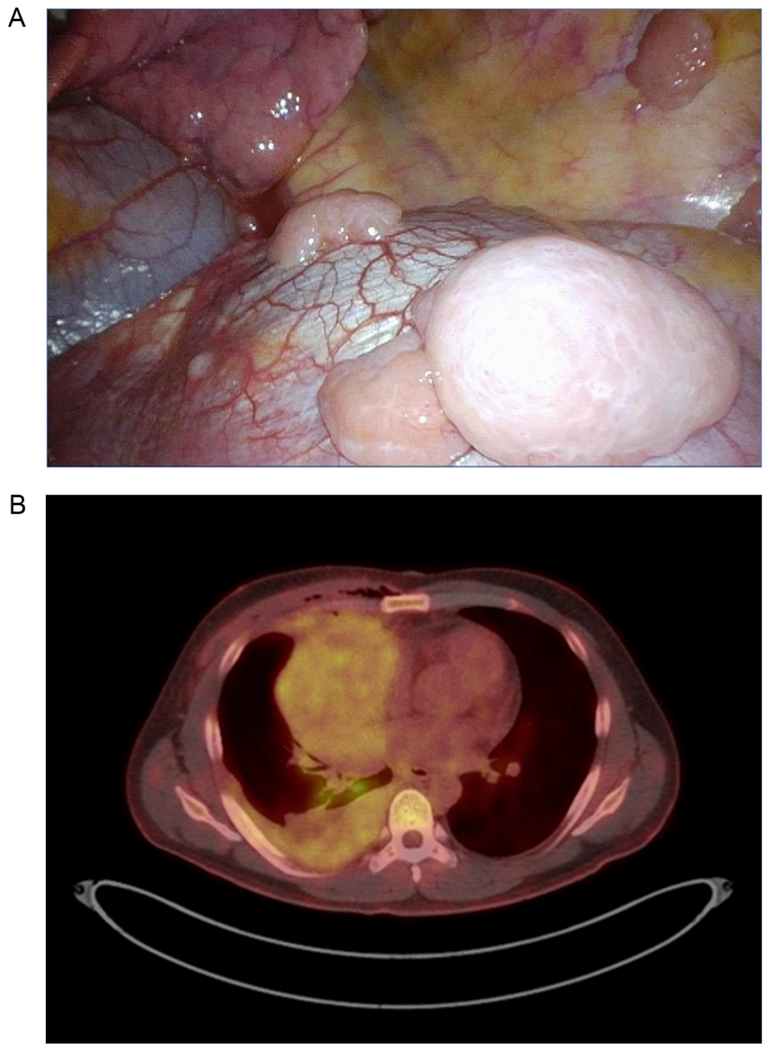
Figure 1.
A, Intraoperative photo of pleural metastases. B, PET/CT image of a patient with thymoma and extensive pleural involvement.
Although it may be possible to render a patient grossly disease-free, whether surgery leads to better long-term disease control or improved outcomes is unclear. Recurrence rates are high and early relapses are common in these patients.10 It remains unclear whether cure is truly achievable. Disease status and freedom from recurrence have been advocated as more clinically relevant endpoints for these patients, rather than survival,11 but accurately identifying the exact timing of a relapse is difficult, as radiographic findings can be ambiguous at first and become apparent only in retrospect over time. Furthermore, if the progression is indolent and the burden of disease is small, clinical evidence of disease progression during surveillance may not necessarily mandate immediate treatment. Observation during these episodes may be reasonable if the patient is otherwise asymptomatic, allowing the patient to extend the time between interventions.
Detailed information on the natural history of the disease and on long-term outcomes is lacking for these patients. Here, we sought to characterize long-term disease-specific outcomes, including survival, rates of progression, and frequency of treatment intervention, as well as disease-free intervals, in this population.
METHODS
Patient Selection
We conducted a retrospective review of a prospectively maintained surgical database to identify all patients with thymic tumors with de novo metastasis to the pleura and/or pericardium at the time of initial presentation who underwent surgical treatment at Memorial Sloan Kettering Cancer Center (MSK) between May 1997 and December 2017. This retrospective study was approved by the MSK institutional review board. Patients who developed pleural recurrences after prior resection of a thymic tumor confined to the mediastinum were excluded. Patients with de novo pleural or pericardial metastasis and resectable pulmonary or nodal disease (previously considered Masaoka stage IVb) were included if they did not have distant hematogenous metastasis.
Data Collection
Patient records were reviewed for demographic information, tumor characteristics, treatment details, and survival outcomes. Demographic information included age, sex, and race. Clinical characteristics included autoimmune conditions and perioperative treatments, including induction and adjuvant therapies. Pathologic data, including histologic diagnosis and margin status, were abstracted from the medical records. Surgical resections were categorized as thymectomy with EPP, thymectomy with pleurectomy, thymectomy with pericardial resection, or unresectable disease. Resection status was determined from the operative notes and pathologic reports and dichotomized as either macroscopically complete resection (R1) or macroscopically incomplete resection (R2), following the convention with respect to pleural resections..
Statistical Analyses and Definitions
Descriptive statistics were generated, and survival analysis was conducted using the Kaplan-Meier method. Survival was calculated from the date of initial surgery to the date of death or the date the patient was last known to be alive. Analyses were conducted using SPSS (version 24). Patients generally underwent serial computed tomographic scans of the chest for follow-up on an annual basis. Patients were considered to have no evidence of disease (NED) at follow-up if they had a macroscopically complete resection and no visible evidence of disease on follow-up imaging.
The endpoints of interest were death and progression. For simplicity, the term “progression” was used to capture all events in patients with a recurrence who had previously been considered NED, as well in patients with known residual disease with evidence of disease progression. As radiographic changes can often be subtle and gradual in these patients, a progression event was defined clinically in the judgment of the treating physician. These clinical diagnoses of progression were associated with a decision to administer treatment such as reoperation, chemotherapy, or radiotherapy or, in some cases, with an explicit decision to continue observation. Progression-free survival was calculated from the date of initial surgery to the date of first progression or last follow-up.
RESULTS
Patient Characteristics
In total, 72 patients met the eligibility criteria. Patient characteristics are shown in Table 1. Median age was 51 years (range, 25-80 years). There were equal numbers of men and women. The majority of patients were white. Four patients (6%) had myasthenia gravis at diagnosis, and 7 patients (10%) developed myasthenia postoperatively. For patients who developed new-onset myasthenia gravis postoperatively, the median time from surgery to diagnosis was 5 years. Two patients each had red cell aplasia and systemic lupus erythematous, 1 of whom also had rheumatoid arthritis.
Table 1.
Patient characteristics (N = 72)
| Characteristic | Median (Range) or No. (%) |
|---|---|
| Age, years | 51 (25-80) |
| Female sex | 36 (50) |
| Race | |
| White | 54 (75) |
| Black | 5 (7) |
| Asian | 8 (11) |
| Other | 5 (7) |
| Autoimmune disorder | |
| Myasthenia gravis | 11 (15) |
| At diagnosis | 4 |
| Delayed presentation | 7 |
| Red cell aplasiaa | 2 (3) |
| Hypogammaglobulinemia | 1 (1) |
| Systemic lupus erythematous alone | 1 (1) |
| Lupus anticoagulant syndrome | 1 (1) |
| Hashimoto’s thyroiditis | 1 (2) |
| Rheumatoid arthritis alone | 2 (3) |
| None | 53 (74) |
Both patients also had systemic lupus erythematous; 1 patient also had rheumatoid arthritis.
Perioperative Treatment
Perioperative treatments are shown in Table 2. Most patients (67; 93%) ultimately received some chemotherapy, radiation, or both in addition to surgery. Sixty-one patients (85%) received induction chemotherapy; 1 of these patients also received concurrent radiation. The majority of patients who underwent induction therapy (n = 36 [50%]) received cisplatin, doxorubicin, and cyclophosphamide. Eight patients received carboplatin and paclitaxel, 6 patients received cisplatin and etoposide, and 2 patients received docetaxel and carboplatin (Supplementary Table 1). Eleven patients went directly to surgery, undergoing no preoperative treatment. In most of these patients (n = 7 [70%]), pleural disease was discovered unexpectedly at the time of surgery.
Table 2.
Treatment received by patients (N = 72)
| Treatment | No. (%) |
|---|---|
| Preoperative chemotherapy | 60 (83) |
| CAP | 36 (60) |
| Carboplatin + paclitaxel | 8 (13) |
| Cisplatin + etoposide | 6 (10) |
| Docetaxel + carboplatin | 2 (3) |
| Other | 8 (13) |
| Chemoradiation (Taxol and 6200 cGy) | 1 (1) |
| None | 11 (15) |
| Surgical procedure | |
| Thymectomy + EPP | 7 (10) |
| Thymectomy + pleurectomy | 48 (67) |
| Thymectomy only | 10 (14) |
| Noncurative resection | 7 (10) |
| Postoperative treatment | |
| Radiation | 31 (43) |
| Brachytherapya | 2 (3) |
| Chemotherapy | 8 (11) |
| Chemoradiation | 5 (7) |
| None | 23 (32) |
| Unknown | 3 (4) |
CAP, cisplatin, doxorubicin, and cyclophosphamide; EPP, extrapleural pneumonectomy.
One patient also had chemotherapy.
Forty-six patients received postoperative therapy consisting of either radiation alone (n = 31 [43%)], chemotherapy alone (n = 8 [11%]), chemoradiotherapy (n = 5 [7%]), brachytherapy alone (n = 1 [1%]), or brachytherapy with chemotherapy (n = 1 [1%]). Postoperative radiation fields included the mediastinum alone in 24 patients (69%) and the mediastinum combined with pleural fields in 9 patients. Ten patients (29%) received radiation to the hemithorax or pleural space without inclusion of the mediastinum. One patient received radiation to a solitary chest wall focus alone, and 1 patient received radiation to the posterior diaphragm only. Twenty-three patients did not undergo postoperative treatment. Information on postoperative therapy was not available for 3 patients.
Surgical Management and Tumor Characteristics
Seven patients underwent thymectomy with EPP, 48 underwent thymectomy with pleurectomy, and 10 underwent thymectomy alone with or without pericardial resection and en bloc resection of involved structures. Owing to extensive disease, 7 patients were deemed to be unresectable and underwent debulking or biopsy only. Tumor characteristics are shown in Table 3. Histologic diagnosis was thymoma in 79% of patients. The majority of thymoma tumors had a histologic subtype of B2 (n = 20 [35%]) or B3 (n = 15 [26%]) (Supplementary Table 2). Among the 15 patients with thymic carcinoma, squamous cell carcinoma was the most common histologic diagnosis (n = 9 [60%]). Fifty-two patients (72%) had a macroscopically complete resection (R1), and 20 (28%) had macroscopic residual disease (R2). Eleven patients (15%) were pathologically downstaged—6 from clinical Masaoka IVa to pathologic III and 5 from clinical Masaoka IVb to pathologic IVa. Seven patients (10%) were upstaged from clinical IVa to pathologic IVb.
Table 3.
Tumor characteristics following resection (N = 72)
| Characteristic | No. (%) |
|---|---|
| Diagnosis | |
| Thymoma | 57 (79) |
| Thymic carcinoma | 15 (21) |
| Resection (R) status | |
| Macroscopic complete resection (R1) | 52 (72) |
| Macroscopic residual disease (R2) | 20 (28) |
| Pathologic stage | |
| III | 6 (8) |
| IVa | 54 (75) |
| IVb | 12 (17) |
Long-Term Outcomes
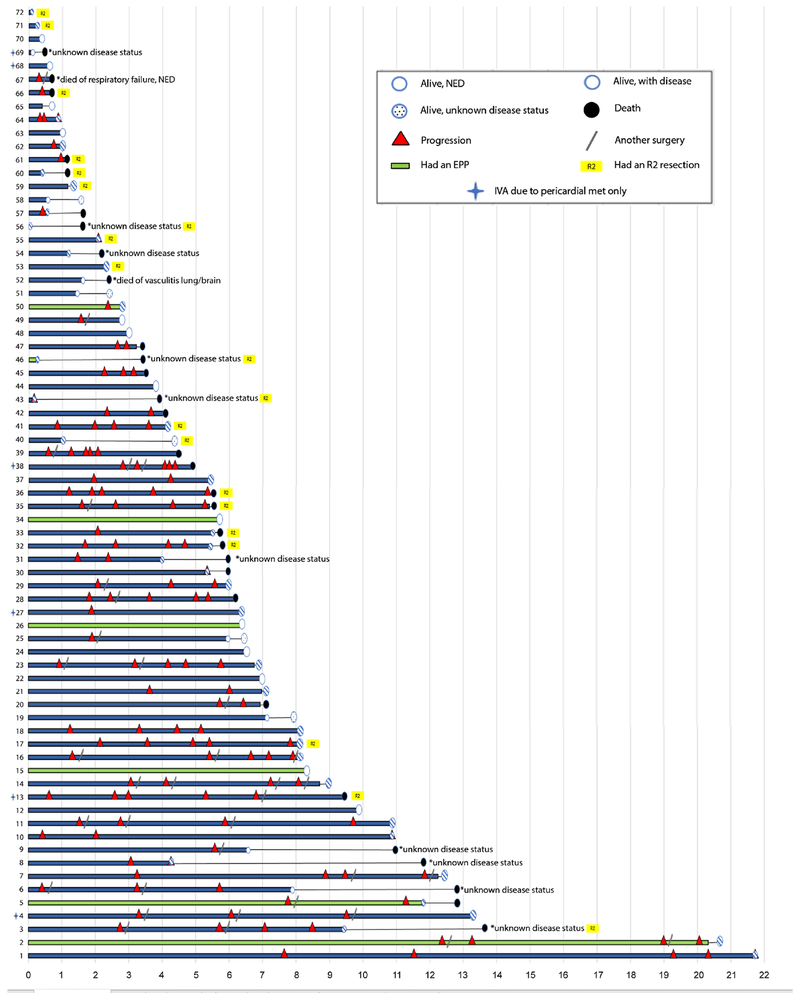
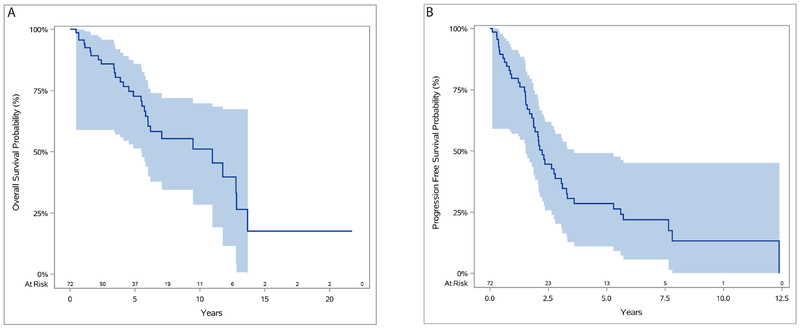
Individual long-term outcomes are shown in Figure 2. At the last follow-up, 42 patients were alive, 13 patients were NED, 24 were alive with disease (AWD), and 5 were alive with unknown disease status. Thirty patients had died. Median follow-up was 5.9 years. The longest survival observed was 21.7 years, during which the patient experienced 5 episodes of disease progression; this patient is currently AWD. The longest progression-free survival was 12.4 years, which was followed by 4 additional episodes of progression, in a patient with an ongoing survival of 20.6 years. Seven (10%) of the patients who are currently NED have been disease-free for >5 years. Five-, 10-, and 15-year overall survivals were 73%, 51%, and 18%, and the median overall survival was 11 years. The longest ongoing survival without any progression or reintervention to date is 9.9 years in a patient who remains NED.
Figure 2.
Plot of all patients and outcomes.
Overall, 26 patients have had no evidence of clinical progression since surgery; median survival for these patients is 7.2 years. Among the 46 patients with evidence of progression, the median number of events is 3 (range, 1-5 events). In the majority of these events, the site of relapse was the pleura (n = 32 [70%]). Other locations of progression include the spine, lungs, and retroperitoneum. Nearly half of patients with clinical progression (n = 21 [46%]) underwent at least 1 additional surgical resection. The 5-year progression-free survival was 29% (Figure 3). and median progression-free survival was 2.2 years.
Figure 3.
Overall survival (A) and progression-free survival (B) for all patients.
Outcomes by Subgroup
Five of the 7 patients who underwent EPP were alive at last contact, and 3 of these patients remain NED, with a median disease-free survival of 6.4 years. Two patients died of unknown causes. The longest disease-free survival observed after EPP was 8.3 years in a patient who is currently NED.
When patients were stratified by R status, those with R2 resection had worse overall survival (median survival, 11.8 [R0/R1] vs 5.5 [R2] years; P = .005) and progression-free survival (median progression-free survival, 2.8 [R0/R1] vs. 1.7 [R2] years; P = .005). Despite the presence of gross residual disease, 5-year overall survival was 63% for patients with R2 resection. R2 resections occurred in cases deemed to be unresectable because of either involvement of critical structures or extensive bulky pleural disease inseparable from the chest wall.
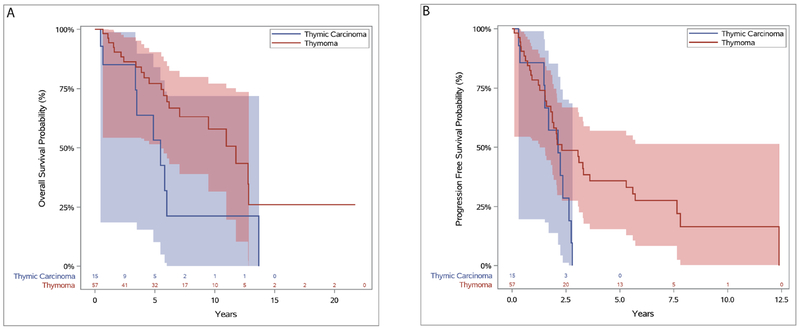
The median survival for the 15 patients with thymic carcinoma was 5.5 years. Six of these patients were alive at last follow-up (NED = 2, AWD = 4), and 9 patients had died. The median survival for the 57 patients with thymoma was 11.8 years (Figure 4).
Figure 4.
Overall survival (A) and progression-free survival (B) for patients with thymoma and thymic carcinoma.
DISCUSSION
To our knowledge, this study presents the largest series of patients with thymic malignancies with de novo pleural or pericardial involvement with long-term disease-specific outcomes (Video 1). Our analysis of these unique patients identified several noteworthy findings. Despite presenting with advanced stage disease, these patients experienced prolonged survival, even in the face of persistent disease. Obtaining a complete resection remains difficult, and most patients developed clinical evidence of recurrence or progression of known residual disease at some point in their lives, often on multiple occasions. These patients generally received multiple episodes of treatment over the course of their lives, but in some cases, in the absence of symptoms, these interventions could be deferred for prolonged periods in favor of observation. Some patients, however, were rendered disease-free, with durable “surgical” remission after completing their initial treatment, even after presenting with bulky pleural disease. These findings on long-term outcomes may help clinicians to counsel patients concerning the treatment and management of these challenging conditions.
Five-year overall survival in the present study (73%) is consistent with that in our previous analysis (78%).9 In the previous analysis, only 1 patient had a follow-up of 10 years. With the additional cases and more-mature data in the present study, we observed that more than half of patients survived at least 10 years, and some are still living >20 years after their initial surgery.
Our results are consistent with those of larger population-based studies: 5-year overall survival was 67% for stage IV patients from the Surveillance, Epidemiology, and End Results database.2 A nationwide study from Japan, comprising 136 patients with pleural dissemination, observed even better outcomes, with a 5-year overall survival of 84%.4
The majority of patients in our cohort (64%) experienced progression after their initial surgery. Even when all gross disease was removed, median progression-free survival was 2.8 years. Our data show that progression of disease is not only likely but is probable to occur multiple times. However, many patients also enjoyed prolonged progression-free intervals of >2 years after an intervention including surgery (19% of all progression events observed). Moreover, and perhaps more importantly from the patient perspective, some patients—in particular, those without symptoms—tolerated prolonged periods of observation with freedom from intervention, even when disease progression was documented. Fortunately, progression of disease often occurs in a manner that allows additional attempts at resection, and patients who can be managed surgically appear to have more favorable outcomes than those undergoing nonsurgical management.10
However, the prolonged survival of these patients in the setting of high rates of progression prompts the question: Would these patients have the same outcomes without surgery? While it is widely accepted that surgery is feasible for these patients, with acceptable levels of morbidity and mortality, whether surgery actually alters the natural history of the disease is unclear. It is possible that the observed outcomes simply reflect the indolence of the disease. If so, observation alone could be a reasonable alternative approach for these patients. Stable disease, especially for a prolonged duration, may be a reasonable goal. However, some patients, knowing that they have active disease, may not be willing to simply be observed, and surgeons may be reluctant to withhold surgery if the disease is resectable.
Although it is likely impossible to directly attribute prolonged survival to surgery in our cohort, it does appear that surgical resection did render some patients disease-free. Of note, among the relatively small number of patients who underwent EPP, 3 of 7 remained with NED, and these patients had a much longer median progression-free survival (6.4 years) than the overall group (2.2 years). Considering that these patients had a higher disease burden in the first place (which necessitated the use of EPP), the improved outcomes seem noteworthy. These results could be attributable to the radicality of the surgery, the ability to radiate the entire hemithorax without having the lung in situ at risk, a particularly indolent disease biology, or some combination of the above. Others have made similar observations about EPP for thymic malignancies,6–9 and such outcomes may present the closest approximation to cure. However, it is important to qualify this statement and note that EPP is an extensive procedure that has been associated with high levels of morbidity and mortality in a large series of patients undergoing EPP for thymic tumors.6
In the present study, 15 patients had thymic carcinoma, a disease known to have a more aggressive course than thymoma.12–14 In line with the data that suggest resection of all gross disease or even debulking may improve outcomes,13 all patients with thymic carcinoma underwent resection with curative intent—in cases of R2 resections, disease was left only on critical structures that were unresectable. R2 patients had a median overall survival of 5.5 years, which is roughly half that for the entire group (11.8 years). Only a third of these patients remained alive at 5 years, and no patients followed up past 3 years were free of disease. Considering that, in an international collection comprising >1000 patients, median overall survival for patients with thymic carcinoma across all stages was 6.6 years,14 it is less clear that aggressive surgical intervention benefits patients with stage IV thymic carcinoma.
Not surprisingly, patients who had a grossly incomplete resections (R2) fared worse, with median overall and progression-free survivals roughly half those for patients with R0/R1 resections (overall survival, 5.5 vs 11.8 years; progression-free survival, 1.7 vs 2.8 years). Still, the relatively long survival associated with thymic malignancies, compared with those for other types of cancers, even in the setting of gross residual disease, again illustrates the indolent course of these malignancies.
The strengths of this study include the relatively large number of patients with de novo pleural or pericardial disease, the availability of detailed and granular clinical data, and the prolonged follow-up including information on disease status. However, our findings must be considered in the context of several limitations. As a retrospective single-institution study, it is subject to selection bias and reflects only the experience of a single specialized institution; therefore, our findings may not be generalizable to all settings. Given that this is a surgical series, there is no comparison to patients who may have either refused or were not offered surgery. We, as an institution, at least attempt resection on all comers, unless there is a clear contraindication to surgery, but we do not have the ability to compare outcomes against those who did not undergo surgery. The lack of a control group precludes any comparative analysis to isolate the effect of surgical intervention, and randomization is impossible given the rarity of this orphan disease. Although the length of follow-up allowed for the assessment of long-term outcomes, it is possible that changes in medical care and practice during the study could have unmeasurable effects on outcomes. Finally, there was no standardized treatment protocol, which is to be expected in this heterogeneous group of patients.
Overall, our findings highlight some unique challenges involved in the treatment of patients with stage IV thymic malignancies. As thymic carcinoma has been shown to have an indolent disease process but is also persistent and stubbornly difficult to eradicate, a thoughtful approach to the overall goals of management should be considered. Cure may prove to be difficult in many cases, and when counselling patients, a discussion including the possibility of local control or palliation and freedom from treatment as acceptable outcomes may be worth considering.11 This may be a challenging concept for some patients to accept, and an explicit discussion backed by data will make the counselling process easier. However, with radical removal of all gross disease, prolonged disease-free survival can be achieved. Philosophically, while we may not be able to promise a “cure,” we may be able to offer, in effect, a “surgical remission.” Finally, because of the indolent disease course, postoperative follow-up and surveillance imaging should likely be done life-long, but no more frequently than annually in patients without symptoms.
Looking to the future, we need to consider how to better describe this heterogeneous group of patients to allow improved prognostication and patient selection for aggressive surgery. A standardized system to quantify and characterize pleural disease will be a step forward. Although we were able to demonstrate that some patients could be rendered disease-free for prolonged periods, and even potentially cured, the lack of a control group prevents us from proving the benefits of surgery. However, given the rare and indolent nature of this disease, it is unlikely that large-scale prospective or randomized studies will be feasible anytime soon. Leveraging the advantages of international focus groups such as the International Thymic Malignancy Interest Group and pooling of data may help us overcome these challenges.15
Supplementary Material
Video 1. Giye Choe, MD, discussing the study.
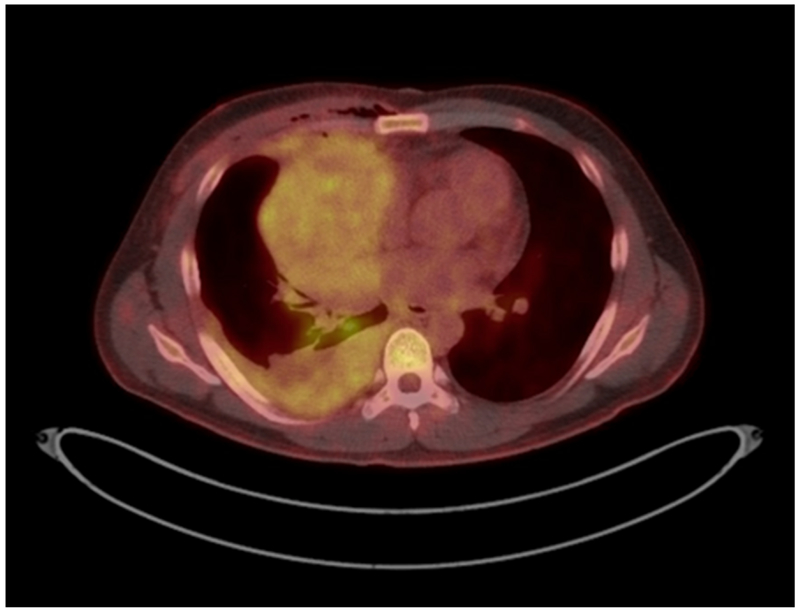
Central Picture:
PET/CT image of patient with stage IVa thymoma, demonstrating pleural involvement.
Central Message:
Surgical resection of stage IVa thymic malignancies can be done with prolonged survival and, in well-selected patients, durable surgical remissions.
Perspective Statement:
We describe the long-term outcomes after surgery for M1a thymic malignancy in granular detail with regard to disease recurrence and survival. Surgery up to extrapleural pneumonectomy can be done with excellent long-term survival, but local progression is frequent, and a tailored surgical strategy should be offered to patients.
Acknowledgments
The authors acknowledge David B. Sewell, Justin Tepe, and Joseph Dycoco for their assistance editing the manuscript.
Funding: This research was funded in part by the NIH/NCI (Cancer Center Support Grant P30 CA008748).
Conflicts of interest: Dr. Valerie Rusch reports grants from Genentech. Dr. Andreas Rimner reports grants from Varian Medical Systems, Boehringer Ingelheim, AstraZeneca, Pfizer, and Merck and personal fees from Varian Medical Systems, Merck, AstraZeneca, Cybrexa, Research to Practice, and More Health. All other authors report no potential conflicts of interest with this study.
Glossary of Abbreviations:
- AWD
alive with disease
- EPP
extrapleural pneumonectomy
- NED
no evidence of disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
IRB Approval: IRB no. 16-1280, approved 8-5-2018.
References
- 1.Ruffini E, Guerrera F, Brunelli A, Passani S, Pellicano D, Thomas P, et al. Report from the European Society of Thoracic Surgeons prospective thymic database 2017: a powerful resource for a collaborative global effort to manage thymic tumours. Eur J Cardiothorac Surg. 2019;55(4):601–9. [DOI] [PubMed] [Google Scholar]
- 2.Hamaji M, Burt BM. Long-term outcomes of surgical and nonsurgical management of stage IV thymoma: a population-based analysis of 282 patients. Semin Thorac Cardiovasc Surg. 2015;27(1):1–3. [DOI] [PubMed] [Google Scholar]
- 3.Detterbeck FC, Stratton K, Giroux D, Asamura H, Crowley J, Falkson C, et al. The IASLC/ITMIG Thymic Epithelial Tumor Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM Classification of Malignant Tumors. J Thorac Onc. 2014;9 Suppl 2:S65–72. [DOI] [PubMed] [Google Scholar]
- 4.Okuda K, Yano M, Yoshino I, Okumura M, Higashiyama M, Suzuki K, et al. Thymoma patients with pleural dissemination: nationwide retrospective study of 136 cases in Japan. Ann Thorac Surg. 2014;97(5):1743–8. [DOI] [PubMed] [Google Scholar]
- 5.Hamanaka K, Koyama T, Matsuoka S, Takeda T, Miura K, Yamada K, et al. Analysis of surgical treatment of Masaoka stage III-IV thymic epithelial tumors. Gen Thorac Cardiovasc Surg. 2018;66(12):731–5. [DOI] [PubMed] [Google Scholar]
- 6.Fabre D, Fadel E, Mussot S, Mercier O, Petkova B, Besse B, et al. Long-term outcome of pleuropneumonectomy for Masaoka stage IVa thymoma. Eur J Cardio-Thoracic Surg. 2011; 39(5):c133–8. [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa Y, Matsuguma H, Nakahara R, Suzuki H, Ui A, Kondo T, et al. Multimodality therapy for patients with invasive thymoma disseminated into the pleural cavity: the potential role of extrapleural pneumonectomy. Ann Thorac Surg. 2009;88(3):952–7. [DOI] [PubMed] [Google Scholar]
- 8.Wright CD. Pleuropneumonectomy for the treatment of Masaoka stage IVa thymoma. Ann Thorac Surg. 2006;82(4):1234–9. [DOI] [PubMed] [Google Scholar]
- 9.Huang J, Rizk NP, Travis WD, Seshan VE, Bains MS, Dycoco J, et al. Feasibility of multimodality therapy including extended resections in stage IVA thymoma. J Thorac Cardiovasc Surg. 2007;134(6):1477–83. [DOI] [PubMed] [Google Scholar]
- 10.Bott MJ, Wang H, Travis W, Riely GJ, Bains M, Downey R, et al. Management and outcomes of relapse after treatment for thymoma and thymic carcinoma. Ann Thorac Surg. 2011;92(6):1984–91. [DOI] [PubMed] [Google Scholar]
- 11.Huang J, Detterbeck FC, Wang Z, Loehrer PJ Sr. Standard outcome measures for thymic malignancies. J Thorac Oncol. 2010;5(12):2017–23. [DOI] [PubMed] [Google Scholar]
- 12.Lin JY, Wei-Shu W, Yen CC, Liu JH, Chen PM, Chiou TJ. Stage IV thymic carcinoma: a study of 20 patients. An J Med Sci. 2005;330(4):172–5. [DOI] [PubMed] [Google Scholar]
- 13.Hishida T, Nomura S, Yano M, Asamura H, Yamashita M, Ohde Y, et al. Long-term outcome and prognostic factors of surgically treated thymic carcinoma: results of 306 cases from a Japanese nationwide database study. Eur J Cardiothorac Surg. 2016;49(3):835–41. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad U, Yao X, Detterbeck F, Huang J, Antonicelli A, Filosso PL, et al. Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg. 2015;149(1):95–100. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Ahmad U, Antonicelli A, Catlin AC, Fang W, Gomez D, et al. Development of the international thymic malignancy interest group international database: an unprecedented resource for the study of a rare group of tumors. J Thorac Oncol. 2014;9(10):1573–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Giye Choe, MD, discussing the study.