Abstract
The varied list of agonists that activate innate lymphoid cells (ILCs) continues to grow, but whether and how these signals interact is not well defined, especially in vivo. ILC subsets share master transcription factors, chromatin landscapes, and effector cytokines with their corresponding T helper (Th) cell subsets. Here we discuss how studies of these two cell types can inform each other. Specifically, we outline a framework in which ILC agonists are grouped by the transcription factors they activate. Optimal ILC activation requires at least three items from a “menu” of non-redundant signals that collectively replicate the STAT and TCR signaling that drives effector Th cell function. This conceptual model may also apply to TCR-independent “bystander” activation of Th cells.
Keywords: innate lymphoid cell, ILC2, Th2, bystander T cell
Introduction
Innate lymphoid cells (ILCs) are tissue-resident immune cells widely distributed throughout the body. Over the past decade, intensive research into ILC biology has established roles for these cells in the maintenance of tissue homeostasis and orchestration of inflammation and immunity in response to infection or tissue damage. ILCs derive from a common lymphoid progenitor cell, which also gives rise to T cells of the adaptive immune system. ILC subsets are similar to effector T cell subsets in their dependence on specific lineage-defining transcription factors and their production of hallmark cytokines. A consensus nomenclature has thus divided these cells into ILC1, ILC2, and ILC3 subsets to reflect their similarity to Th1, Th2, and Th17 CD4+ T cells, respectively [1]. The existence of a dedicated ILCreg subset to match T regulatory cells remains controversial and will not be discussed further; nor will we focus on natural killer (NK) cells, the innate counterpart for CD8+ T cells.
The development, localization, and biological functions of ILCs have been well-reviewed elsewhere [2-5]. In this Opinion article we instead consider how to make sense of the numerous signals that activate ILCs. We propose a framework for understanding how such signals integrate to regulate ILC function, and perhaps also innate-like responses in effector T cells. Specifically, we note that optimal transcription of most Th and ILC effector cytokines requires a lineage-specific STAT and all three of the transcription factors activated downstream of TCR signaling: NF-κB, AP-1, and NFAT. Using ILC2s and Th2 cells as an example, we discuss evidence that multiple agonists combine to replicate this TCR signal in ILCs and in T effector cells activated in a TCR-independent fashion.
Th and ILC similarity
Given the similarities between ILCs and CD4+ Th cells, past lessons learned from extensive study of adaptive T cells may also prove useful for understanding ILC biology. Indeed, ILCs are strikingly similar to effector Th cells at both the transcriptomic and epigenomic levels in mice [6]. ILC and Th relationships appear to be more variable in humans, although ILC2s and Th2s were not included in the study [7]. Profiling of chromatin accessibility between ILC groups has identified distinct landscapes established early during lineage specification that remain relatively fixed over the life of the cell, consistent with the potential for rapid effector cytokine production [6]. ILC chromatin accessibility is distinct from that of naïve CD4+ T cells, but as T cells differentiate, their chromatin is remodeled to resemble the transcriptional landscape of ILCs. In the context of lung helminth infection, differentiated Th2 cells undergo rapid epigenetic remodeling such that greater than 70% of the newly accessible loci are shared in common with ILC2s residing in the same tissue, resulting in strikingly similar gene expression [6,8]. Together these findings suggest that ILC effector cytokine expression is likely regulated by the same transcriptional pathways that drive effector gene expression in corresponding Th subsets.
T cell versus ILC activation
Naïve CD4+ T cells require cognate peptide/MHC binding to the TCR, co-stimulation through CD28, and a cytokine signal to direct differentiation into effector subsets. Once activated, however, the epigenome of cytokine loci is modified as described above and the requirement for co-stimulation falls away. Effector CD4+ T cells are thus maintained by cytokine-driven STAT signaling and activated through the TCR. The STAT signal varies between CD4+ subsets, with Th1 cells requiring STAT1 and STAT4, Th2 cells requiring STAT5 and STAT6, and Th17 cells requiring STAT3 [9]. TCR signaling, on the other hand, is stereotyped and always leads to activation of NF-κB, AP-1, and NFAT. The upstream regulatory regions of effector cytokine loci often contain binding sites for all of these transcription factors, so that they collectively drive optimal cytokine expression, in some cases through direct cooperation. Such cooperativity is best defined for NFAT and AP-1, which form a complex that binds regulatory elements with higher affinity than is afforded by either factor alone [10,11]. NFAT:AP-1 cooperative binding sites are found in the promoters of Il2, Il4, Il5, Il13, Csf2, Ifng, Tnfa and others [12].
ILCs and CD4+ Th cells share many features in common, including production of cytokines that drive stereotyped immune responses against specific classes of pathogens. A key difference between these cell types, however, is the way in which they are activated by extracellular signals. Whereas CD4+ T cells respond to cognate peptide/MHC complexes via the TCR, ILCs lack TCRs and instead rely on expression of cell surface receptors specific for cytokines, neuropeptides, and eicosanoids. Integration of such signals is hypothesized to attune ILCs to the local microenvironment and allow them to respond rapidly to deviations from homeostasis [13].
Broadly, ILC1s are said to be activated by the cytokines IL-12 and IL-18 to produce IFN-γ and TNF-α; ILC2s are activated by IL-33, IL-25, and TSLP to produce IL-5 and IL-13; and ILC3s are activated by IL-1β and IL-23 to produce IL-22, IL-17, and GM-CSF. While intuitive and often cited, this simple scheme belies the complexity of ILC activation in vivo. For example, at least 9 additional physiologic agonists have been reported to promote cytokine expression in ILC2s [3,14,15]. Further, recent studies have begun to reveal how ILCs are specifically adapted to their microenvironment, such that ILCs of the same group can express different repertoires of activating receptors depending on their tissue localization [14,16]. A current challenge in the field is therefore to identify the array of signals that drive ILC activation in specific tissues and during different disease states, and to map out the redundancy and synergy between these signals.
A framework for understanding ILC activation
In considering ILC activation, we will focus here on ILC2s, as these have been extensively studied and a number of unique activating signals have been identified to date. ILC2s were formally identified in 2010 by three groups [17-19]. In these initial descriptions, the epithelial cytokines IL-33 and IL-25 were noted for their ability to activate murine ILC2s of the lung, small intestine, and adipose tissues to produce the cytokines IL-5, IL-9, and IL-13. An analogous human cell type, also responsive to IL-33 and IL-25, was quickly identified [20]. Soon after these reports, TSLP was shown to activate ILC2s, particularly those residing in the skin [21,22]. Thus, the triad of IL-33, IL-25, and TSLP became established as the key cytokines regulating ILC2 activation and have since been highly cited in any work discussing ILC2 biology.
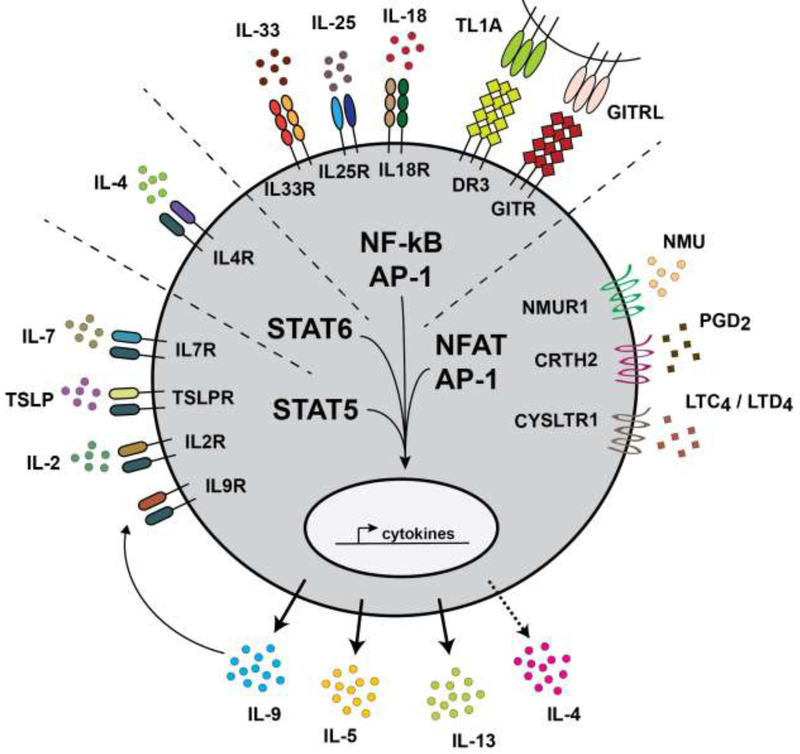
While each of these signals undoubtedly serves important roles, we believe that grouping IL-33, IL-25, and TSLP together is an artifact of history and lacks a biological basis. Instead, we propose a framework for understanding ILC2 activation based on the induction and cooperation of transcription factors that mediate effector gene expression. Given the high overlap of transcriptomes and accessible chromatin loci in ILC2 and Th2 subsets [6], it seems likely that similar transcriptional processes occur in both cell types. Drawing on our understanding of T cell signaling then, we predict that optimal ILC2 proliferation and activation requires STAT5, STAT6, NF-κB, AP-1, and NFAT. In Th2 cells, the TCR alone accounts for NF-κB, AP-1, and NFAT, but we propose that ILC2s must integrate multiple signals to compensate for their lack of a TCR. Therefore, we suggest that it is useful to group ILC2 activating signals by the transcription factors they induce (Figure 1), as others have also begun to do [23].
Figure 1. A Framework for ILC Activation.
The signals that activate ILC effector functions can be organized into four groups based on the transcription factors that they mobilize: (1) STAT5; (2) STAT6; (3) NF-κB/AP-1; and (4) NFAT/AP-1. Under physiologic conditions in vivo where agonist availability is limited, optimal ILC activation likely requires at least one signal from each category. Collectively, these signals replicate the STAT and TCR signaling that regulate adaptive T cell function. This figure groups ILC2-activating signals, but the framework can also be applied to other ILC subsets and to TCR-independent activation of bystander T cells.
ILC2 Activating Signals
STAT Signaling
Several STAT5-coupled receptors have been shown to regulate ILC2s, including those for IL-7, IL-2, TSLP, and IL-9 [3]. Of note, activated ILC2s secrete IL-9 and an autocrine role for this cytokine has been reported [24]. ILC2s also express the STAT6-coupled IL-4/13 receptor, which is required for their proliferation and function during type 2 inflammation [25,26].
NF-κB/AP-1 Signaling
IL-33 and IL-25 were the first ILC2 activating signals identified; both of these epithelial cytokines induce NF-κB and AP-1 downstream of their receptors [23]. It is important to note, however, that relative expression levels of these receptors on ILC2s varies between tissues. For example, the IL-33 receptor is highly expressed by ILC2s in the lung and adipose tissue, whereas the IL-25 receptor is predominantly expressed on ILC2s of the small intestine [14,27,28]. Additional ILC2 activating signals capable of inducing NF-κB and AP-1 include TL1A (and its receptor DR3), IL-18, and GITRL (and its receptor GITR) [14,15,29-31].
NFAT/AP-1 Signaling
More recent work has identified several signals capable of activating NFAT and AP-1 in ILC2s. Airway ILC2s are highly responsive to cysteinyl leukotrienes, inflammatory lipids derived from arachidonic acid [32]. Leukotriene signaling through the receptor CYSLTR1 generates a calcium flux that activates calcineurin, resulting in NFAT translocation from the cytosol to the nucleus and subsequent expression of effector cytokines [33,34]. ILC2s of the small intestine are similarly responsive to leukotriene signaling (McGinty and von Moltke, unpublished). A second arachidonic acid derivative, prostaglandin D2, activates human ILC2s through the receptor CRTH2, which is also expressed on effector Th2 cells. PGD2 is particularly potent as a chemoattractant, but has also been linked to NFAT induction [35,36]. Finally, the neuropeptide neuromedin U was recently shown to activate ILC2s in the small intestine and lung in an NFAT-dependent manner [37-39].
Signal integration in ILC2s
In many cases ILC2 activating signals have been studied in isolation and declared to be the dominant regulator of ILC2s in a given tissue or model. The absence of ILC2 activation in mice deficient for one activating signal does not, however, preclude the existence of additional non-redundant signals. Further, activation of ILC2s by supraphysiological levels of recombinant cytokine, neuropeptide, or lipid does not fully determine their sufficiency. For example, many in vitro experiments incubate ILC2s in 10 ng/ml or more of cytokine for multiple days. Such experiments are important to demonstrate what a cytokine can do, but may not be representative of its function in vivo. Also, when exogenous cytokine is delivered in vivo, it binds to ILC2s that are already homeostatically exposed to IL-33, IL-2, IL-25 and perhaps other signals [40-42]. We propose that in the context of rapid activation and limiting agonist concentrations, the importance of cooperative signaling is amplified. Therefore, under physiologic conditions in vivo, ILC2s likely integrate multiple signals to mobilize the transcription factors known to drive cytokine expression in Th2 cells.
Experimental evidence to support this model of signal integration is accumulating. For example, although caveats about agonist concentration and incubation time apply, in vitro ILC2 stimulation has repeatedly demonstrated the efficacy of combining a STAT5 signal (IL-7, IL-2, or TSLP) with an NF-κB/AP-1 signal (IL-33 or IL-25) [17,18,43]. As predicted by our model, in these assays overlapping signals such as IL-33 and IL-25 were generally redundant. In vitro synergy between IL-4 (STAT6) and IL-33 has also been reported, although at high concentration either signal alone is sufficient to induce measurable cytokine secretion [25]. More recently, leukotrienes, which mobilize NFAT in ILC2s, have received attention and their synergy with IL-33 was a particular focus of these studies [33,34]. Both in vivo and in vitro, sub-optimal doses of IL-33 or leukotriene induced little ILC2 activation, but when given together, effector cytokine production was boosted well above levels predicted for simple additive interaction. Such synergy has also been demonstrated between IL-33 and the NFAT-inducing agonist NMU [44]. Conversely, deletion of either the IL-33 receptor or ALOX5, the enzyme required to synthesize leukotrienes, demonstrated the non-redundant requirement for both signals during type 2 inflammation in the lung. In human ILC2s, effector cytokine production is enhanced in the combined presence of agonists mobilizing STAT5 (IL-2), NF-κB/AP-1 (IL-33 and IL-25), and NFAT (PGD2), whereas inhibition of autocrine PGD2 reduces cytokine production, suggesting signal integration is required here as well [45,46].
Signal integration in ILC1s and ILC3s
How might our framework for ILC2 activation apply to other ILC subsets? The importance of STAT and NF-κB/AP-1-inducing signals in ILC1 and ILC3 proliferation and activation is well documented [3,4], but NFAT-mobilizing signals have not been reported for either cell type, despite expression of NFAT family members at levels equal to or greater than in ILC2s [16]. An NFAT binding site has not been reported upstream of Il22, but the other ILC1 and ILC3 effector cytokines, such as Il17a, Csf2, Ifng, and Tnfa, are all regulated by NFAT [47]. NK cells and some ILC3s express activating NK receptors (NKR) that replicate TCR signaling, including NFAT activation, by using ITAMs to activate SYK kinases. Whether and how NFAT is mobilized in ILC1s and NKR-negative ILC3s remains to be determined, although the latter appear to express the leukotriene receptor CYSLTR1 [16].
TCR-independent T cell activation
Given that the Th2 transcriptome and chromatin landscape converge on an ILC2-like phenotype as Th2 effector cells differentiate, it is worth considering how lessons from ILC studies might inform our understanding of effector T cell regulation and function. For example, Th2 priming in the lymph node induces expression of cytokine receptors found on ILC2s, and the terminal differentiation into Th2 effector cells requires tissue-derived IL-25, IL-33, and TSLP [8]. Whether NFAT activation also contributes to the differentiation of Th2 effectors has not been examined.
There is also accumulating evidence of TCR-independent T cell responses. Such “bystander” responses to heterologous stimuli have been reviewed elsewhere [48], but certain examples are illustrative of the similarities to ILC activation. For example, Th2 cells generated in response to the helminth Ascaris suum are reactivated by the phylogenetically distant helminth Nippostrongylus brasiliensis, even when MHC is blocked [49]. Instead, this response requires the IL-33 receptor, which is up-regulated as part of the Th2 effector program. Similarly, a recent study identified skin-resident commensal-specific T cells that homeostatically produce IL-17A but are poised to produce IL-5 and IL-13 in response to many of the same signals that regulate ILC2s: IL-33, IL-25, and IL-18 [31]. Similar mixed-lineage resident cells expressing IL-13 and ILC2-like receptors have been described in human skin. Tissue T regulatory cells also bear striking resemblance to ILC2s, including expression of GATA3 and receptors for IL-33, IL-2 (high-affinity), and perhaps leukotrienes [50,51]. In the context of autoimmune encephalomyelitis, bystander activation of memory Th17 cells by IL-1β and IL-23 can contribute to pathogenesis [52]. Lastly, just like ILCs, innate-like T cells including γδ cells, MAIT cells, and NKT cells are tissue resident, acquire a poised effector state during development, and can rapidly secrete cytokine in response to tissue-derived signals, such as IL-18, IL-23, NK receptor ligands, and others [53-55].
We propose that the transcription factor framework we outlined for ILC activation, in which several signals cooperate to activate the full complement of required transcription factors, likely also applies to TCR-independent activation of T cells. The studies cited above mostly examined the ability of individual cytokines to induce TCR-independent responses, but additional signals are likely important under physiologic conditions. Indeed, some studies have emphasized the importance of signal integration in the absence of a TCR signal. For example, TCR-independent cytokine production by in vitro differentiated Th cells is optimally induced by combining an NF-κB/AP-1 signal with the appropriate STAT signal for each Th subset [56]. Similarly, IL-1β (NF-κB/AP-1) and IL-23 (STAT3) synergistically induce IL-17 secretion from intestinal γδ T cells [57]. The element missing from all of these studies, however, is a consideration of NFAT activation. As with the NKR+ ILC3s, NK and some γδ T cells express NKRs that can activate NF-κB, AP-1, and NFAT simultaneously, and human effector Th2 cells express the PGD2 receptor CRTH2 and the leukotriene receptor CYSLTR1, both of which could potentially induce the Ca2+ flux needed for NFAT activation [58,59]. Whether and how NFAT is activated in other effector T cells in a TCR-independent manner has, to our knowledge, not been studied.
Caveats
Although this review emphasizes the similarities between ILCs and effector T cells, this comparison should not be taken too far. The TCR remains the predominant regulator of T cell activation and there are important transcriptional differences between T cells and ILCs. For example, Th2 cells readily secrete IL-4 and only begin to make IL-5 when they are fully activated [8]. Conversely, ILC2s secrete IL-5 constitutively, but only make IL-4 when NFAT is activated [32,40], and a recent study identified discordant regulation of IL-5 and IL-13 that occurs in a subset of lung ILC2s but not Th2 cells [44]. We also note that comparisons between ILC and Th cell transcriptomes and chromatin were made using recently generated Th effectors. Similarly, several studies of TCR-independent CD4+ T cell activation used in vitro generated effector cells. More work will therefore be needed to assess relationships between ILCs and memory Th cells, and to test if the rules of TCR-independent activation vary between different memory subsets (e.g. resident memory vs. effector memory). Lastly, this review has focused on activating signals, but the proposed framework can be extended to negative regulators and the various mechanisms they use to impede ILC function.
Summary & Outlook
This review outlined how multiple signals integrate to induce effector cytokine production in ILCs and T cells. In this context, grouping agonists by the transcription factor(s) they activate provides a conceptual framework, with optimal cytokine secretion predicted to occur when at least one agonist from each group is present. From an evolutionary perspective, this mechanism provides increased regulatory control and the opportunity to encode different information with each signal. For example, coincidence detection, in which activation results from temporally close but spatially distributed signals, might ensure that maximal ILC activation occurs only when two or more upstream sensing pathways have been stimulated. At the same time, there is some temporal segregation of signals across homeostasis and inflammation. For example, ILC2s receive an NF-κB/AP-1 signal (e.g. IL-33 or IL-25) both homeostatically and during inflammation, while NFAT-inducing leukotrienes and NMU are acutely produced or released only in response to a defined stimulus [33,40,41]. Recent studies have begun to consider the relative contributions of such signals in different contexts across and even within tissues [14,44], but additional work is needed to fully disentangle the complexities of ILC and TCR-independent T cell activation in vivo.
Acknowledgements
We thank R. Locksley for discussions that shaped our thinking about ILC activation. We thank M. Fontana for helpful discussions and critical reading of the manuscript. JWM is supported by the University of Washington Immunology Training Grant (T32 AI106677). JVM is a Searle Scholar. The laboratory is supported by NIH 1DP2 OD024087 (JVM) and the University of Washington.
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, et al. : Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol 2013, 13:145–149. [DOI] [PubMed] [Google Scholar]
- 2.Eberl G, Colonna M, Santo JPD, McKenzie ANJ: Innate lymphoid cells: A new paradigm in immunology. Science 2015, 348:aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klose CSN, Artis D: Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nature Immunology 2016, 17:765–774. [DOI] [PubMed] [Google Scholar]
- 4.Spits H, Bernink JH, Lanier L: NK cells and type 1 innate lymphoid cells: partners in host defense. Nature Immunology 2016, 17:758–764. [DOI] [PubMed] [Google Scholar]
- 5.Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, et al. : Innate Lymphoid Cells: 10 Years On. Cell 2018, 174:1054–1066. [DOI] [PubMed] [Google Scholar]
- 6.Shih H-Y, Sciumè G, Mikami Y, Guo L, Sun H-W, Brooks SR, Urban JF, Davis FP, Kanno Y, O’Shea JJ: Developmental Acquisition of Regulomes Underlies Innate Lymphoid Cell Functionality. Cell 2016, 165:1120–1133.** This study describes the similarity between transcriptomes and chromatin landscapes of ILCs and effector Th cells.
- 7.OI Koues, Collins PL, Celia M, Robinette ML, Porter SI, Pyfrom SC, Payton JE, Colonna M, Oltz EM: Distinct Gene Regulatory Pathways for Human Innate versus Adaptive Lymphoid Cells. Cell 2016, 165:1134–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Dyken SJ, Nussbaum JC, Lee J, Molofsky AB, Liang H-E, Pollack JL, Gate RE, Haliburton GE, Ye CJ, Marson A, et al. : A tissue checkpoint regulates type 2 immunity. Nature Immunology 2016, 17:1381–1387.** This study highlights the importance of non-TCR signals for terminal differentiation of Th2 cells.
- 9.Zhu J, Yamane H, Paul WE: Differentiation of effector CD4 T cell populations. Annu Rev Immunol 2010, 28:445–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain J, McCafffrey PG, Miner Z, Kerppola TK, Lambert JN, Verdine GL, Curran T, Rao A: The T-cell transcription factor NFAT p is a substrate for calcineurin and interacts with Fos and Jun. Nature 1993, 365:352. [DOI] [PubMed] [Google Scholar]
- 11.Jain J, Miner Z, Rao A: Analysis of the preexisting and nuclear forms of nuclear factor of activated T cells. J Immunol 1993, 151:837–848. [PubMed] [Google Scholar]
- 12.Macián F, López-Rodríguez C, Rao A: Partners in transcription: NFAT and AP-1. Oncogene 2001, 20:2476–2489. [DOI] [PubMed] [Google Scholar]
- 13.von Moltke J, Locksley RM: I-L-C-2 it: type 2 immunity and group 2 innate lymphoid cells in homeostasis. Curr Opin Immunol 2014, 31:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricardo-Gonzalez RR, Van Dyken SJ, Schneider C, Lee J, Nussbaum JC, Liang H-E, Vaka D, Eckalbar WL, Molofsky AB, Erle DJ, et al. : Tissue signals imprint ILC2 identity with anticipatory function. Nat Immunol 2018, 19:1093–1099.** This study provides comprehensive transcriptome analysis to highlight the shared and divergent features of ILC2s in different tissues.
- 15.Nagashima H, Okuyama Y, Fujita T, Takeda T, Motomura Y, Moro K, Hidaka T, Omori K, Sakurai T, Machiyama T, et al. : GITR cosignal in ILC2s controls allergic lung inflammation. J Allergy Clin Immunol 2018, 141:1939–1943.e8. [DOI] [PubMed] [Google Scholar]
- 16.Robinette ML, Fuchs A, Cortez VS, Lee JS, Wang Y, Durum SK, Gilfillan S, Colonna M, the Immunological Genome Consortium, Shaw L, et al. : Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nature Immunology 2015, 16:306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J-I, Ohtani M, Fujii H, Koyasu S: Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 2010, 463:540–544. [DOI] [PubMed] [Google Scholar]
- 18.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA, Bucks C, Kane CM, Fallon PG, Pannell R, et al. : Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 2010, 464:1367–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price AE, Liang H-E, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM: Systemically dispersed innate IL-13–expressing cells in type 2 immunity. Proc Natl Acad Sci U S A 2010, 107:11489–11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H: Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nature Immunology 2011, 12:1055–1062. [DOI] [PubMed] [Google Scholar]
- 21.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, Hepworth MR, Voorhees ASV, Comeau MR, Artis D: TSLP Elicits IL-33–Independent Innate Lymphoid Cell Responses to Promote Skin Inflammation. Science Translational Medicine 2013, 5:170ra16–170ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, Huang L-C, Johnson D, Scanlon ST, McKenzie ANJ, et al. : A role for IL-25 and IL-33–driven type-2 innate lymphoid cells in atopic dermatitis. Journal of Experimental Medicine 2013, 210:2939–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabata H, Moro K, Koyasu S: The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunological Reviews 2018, 286:37–52. [DOI] [PubMed] [Google Scholar]
- 24.Turner J-E, Morrison PJ, Wilhelm C, Wilson M, Ahlfors H, Renauld J-C, Panzer U, Helmby H, Stockinger B: IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J Exp Med 2013, 210:2951–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motomura Y, Morita H, Moro K, Nakae S, Artis D, Endo TA, Kuroki Y, Ohara O, Koyasu S, Kubo M: Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity 2014, 40:758–771. [DOI] [PubMed] [Google Scholar]
- 26.Symowski C, Voehringer D: Th2 cell-derived IL-4/IL-13 promote ILC2 accumulation in the lung by ILC2-intrinsic STAT6 signaling in mice. Eur J Immunol 2019, doi: 10.1002/eji.201948161. [DOI] [PubMed] [Google Scholar]
- 27.Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, Bucks C, Wu X, Kane CM, Neill DR, et al. : IL-33 is more potent than IL-25 in provoking IL-13–producing nuocytes (type 2 innate lymphoid cells) and airway contraction. Journal of Allergy and Clinical Immunology 2013, 132:933–941. [DOI] [PubMed] [Google Scholar]
- 28.Molofsky AB, Nussbaum JC, Liang H-E, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM: Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med 2013, 210:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu X, Pappu R, Ramirez-Carrozzi V, Ota N, Caplazi P, Zhang J, Yan D, Xu M, Lee WP, Grogan JL: TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers. Mucosal Immunol 2014, 7:730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meylan F, Hawley ET, Barron L, Barlow JL, Penumetcha P, Pelletier M, Sciumè G, Richard AC, Hayes ET, Gomez-Rodriguez J, et al. : The TNF-family cytokine TL1A promotes allergic immunopathology through group 2 innate lymphoid cells. Mucosal Immunol 2014, 7:958–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrison OJ, Linehan JL, Shih H-Y, Bouladoux N, Han S-J, Smelkinson M, Sen SK, Byrd AL, Enamorado M, Yao C, et al. : Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science 2019, 363.** This study identifies adaptive T cells in the skin that rapidly secrete cytokines in response to some of the same agonists that regulate ILC2s.
- 32.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH: Lung Type 2 innate lymphoid cells express CysLT1R that regulates Th2 cytokine production. J Allergy Clin Immunol 2013, 132:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Von Moltke J, O’Leary CE, Barrett NA, Kanaoka Y, Austen KF, Locksley RM: Leukotrienes provide an NFAT-dependent signal that synergizes with IL-33 to activate ILC2s. Journal of Experimental Medicine 2017, 214:27–37.* This and the following reference highlight the synergy of IL-33 and leukotrienes in ILC2 activation
- 34.Lund SJ, Portillo A, Cavagnero K, Baum RE, Naji LH, Badrani JH, Mehta A, Croft M, Broide DH, Doherty TA: Leukotriene C4 Potentiates IL-33-Induced Group 2 Innate Lymphoid Cell Activation and Lung Inflammation. J Immunol 2017, 199:1096–1104.** This and the previous reference highlight the synergy of IL-33 and leukotrienes in ILC2 activation
- 35.Xue L, Salimi M, Panse I, Mjösberg JM, McKenzie ANJ, Spits H, Klenerman P, Ogg G: Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol 2014, 133:1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xue L, Gyles SL, Barrow A, Pettipher R: Inhibition of PI3K and calcineurin suppresses chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2)-dependent responses of Th2 lymphocytes to prostaglandin D(2). Biochem Pharmacol 2007, 73:843–853. [DOI] [PubMed] [Google Scholar]
- 37.Klose CSN, Mahlakõiv T, Moeller JB, Rankin LC, Flamar A-L, Kabata H, Monticelli LA, Moriyama S, Putzel GG, Rakhilin N, et al. : The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 2017, 549:282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cardoso V, Chesné J, Ribeiro H, García-Cassani B, Carvalho T, Bouchery T, Shah K, Barbosa-Morais NL, Harris N, Veiga-Fernandes H: Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 2017, 549:277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallrapp A, Riesenfeld SJ, Burkett PR, Abdulnour R-EE, Nyman J, Dionne D, Hofree M, Cuoco MS, Rodman C, Farouq D, et al. : The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 2017, 549:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang H-E, et al. : Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 2013, 502:245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Von Moltke J, Ji M, Liang H-E, Locksley RM: Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 2016, 529:221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, Englezakis A, Barlow JL, Hams E, Scanlon ST, et al. : MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 2014, 41:283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohapatra A, Van Dyken SJ, Schneider C, Nussbaum JC, Liang H-E, Locksley RM: Group 2 innate lymphoid cells utilize the IRF4-IL-9 module to coordinate epithelial cell maintenance of lung homeostasis. Mucosal Immunol 2016, 9:275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagashima H, Mahlakõiv T, Shih H-Y, Davis FP, Meylan F, Huang Y, Harrison OJ, Yao C, Mikami Y, Urban JF, et al. : Neuropeptide CGRP Limits Group 2 Innate Lymphoid Cell Responses and Constrains Type 2 Inflammation. Immunity 2019, 0.* This study higlights the synergy between IL-33 and NMU in ILC2 activation and identifies context-dependent stimulatory and inhibitory effects of the neuropeptide CGRP on ILC2s.
- 45.Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, Wechsler ME, Israel E, Levy BD: Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med 2013, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maric J, Ravindran A, Mazzurana L, Van Acker A, Rao A, Kokkinou E, Ekoff M, Thomas D, Fauland A, Nilsson G, et al. : Cytokine-induced endogenous production of prostaglandin D2 is essential for human group 2 innate lymphoid cell activation. J Allergy Clin Immunol 2019, 143:2202–2214.e5.* This study provides evidence of signal integration in activation of human ILC2s
- 47.Hermann-Kleiter N, Baier G: NFAT pulls the strings during CD4+ T helper cell effector functions. Blood 2010, 115:2989–2997. [DOI] [PubMed] [Google Scholar]
- 48.Whiteside SK, Snook JP, Williams MA, Weis JJ: Bystander T Cells: A Balancing Act of Friends and Foes. Trends Immunol 2018, 39:1021–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo L, Huang Y, Chen X, Hu-Li J, Urban JF, Paul WE: Innate immunological function of TH2 cells in vivo. Nat Immunol 2015, 16:1051–1059.** This study identifies TCR-independent activation of bystander Th2 cells in vivo.
- 50.Zeng Q, Sun X, Xiao L, Xie Z, Bettini M, Deng T: A Unique Population: Adipose-Resident Regulatory T Cells. Front Immunol 2018, 9:2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klicznik MM, Morawski PA, Höllbacher B, Varkhande SR, Motley SJ, Kuri-Cervantes L, Goodwin E, Rosenblum MD, Long SA, Brachtl G, et al. : Human CD4+CD103+ cutaneous resident memory T cells are found in the circulation of healthy individuals. Science Immunology 2019, 4:eaav8995.* This study describes resident Th cells with some ILC-like features in human skin.
- 52.Lee H-G, Lee J-U, Kim D-H, Lim S, Kang I, Choi J-M: Pathogenic function of bystander-activated memory-like CD4+ T cells in autoimmune encephalomyelitis. Nat Commun 2019, 10:709.* This study describes cytokine signals that activate bystander Th17 cells in vivo.
- 53.Garner LC, Klenerman P, Provine NM: Insights Into Mucosal-Associated Invariant T Cell Biology From Studies of Invariant Natural Killer T Cells. Front Immunol 2018, 9:1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vantourout P, Hayday A: Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol 2013, 13:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doisne J-M, Soulard V, Bécourt C, Amniai L, Henrot P, Havenar-Daughton C, Blanchet C, Zitvogel L, Ryffel B, Cavaillon J-M, et al. : Cutting edge: crucial role of IL-1 and IL-23 in the innate IL-17 response of peripheral lymph node NK1.1- invariant NKT cells to bacteria. J Immunol 2011, 186:662–666. [DOI] [PubMed] [Google Scholar]
- 56.Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, Paul W: IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci USA 2009, 106:13463–13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duan J, Chung H, Troy E, Kasper DL: Microbial colonization drives expansion of IL-1 receptor 1-expressing and IL-17-producing gamma/delta T cells. Cell Host Microbe 2010, 7:140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spinozzi F, Russano AM, Piattoni S, Agea E, Bistoni O, de Benedictis D, de Benedictis FM: Biological effects of montelukast, a cysteinyl-leukotriene receptor-antagonist, on T lymphocytes. Clin Exp Allergy 2004, 34:1876–1882. [DOI] [PubMed] [Google Scholar]
- 59.Cosmi L, Annunziato F, Galli MIG null, Maggi RME null, Nagata K, Romagnani S: CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur J Immunol 2000, 30:2972–2979. [DOI] [PubMed] [Google Scholar]