Abstract
Recognition of invading pathogens and execution of defensive responses are crucial steps in successfully combating infectious diseases. Inflammasomes are a group of diverse, signal-transducing complexes with critical roles in both processes. While the responses mediated by inflammasomes are vital to host defense, disruption of inflammasome regulation or activity can lead to the development of autoimmune and sterile inflammatory diseases, including cancer. The field of inflammasome research has rapidly expanded to identify novel regulatory pathways, new inflammasome components, and the mechanistic activation of these complexes. In this review, we discuss recent insights into the regulation of inflammasomes by interferon regulatory factor proteins, newly discovered mechanisms of activation for the NLRP1B and NLRP6 inflammasomes, and recent studies exploring the viability of inflammasome-modulating immunotherapies.
Keywords: inflammasome, caspase-1, gasdermin, infection, inflammation, pyroptosis, interleukin
INTRODUCTION
Inflammasomes are critical mediators of the innate immune response to infection [1]. Upon activation, these multimeric death complexes assemble to function as activation platforms for caspase-1 (CASP1) autoproteolysis [1]. The most well-established inflammasomes are NLRP1 (nucleotide-binding domain leucine-rich repeat containing [NLR] family, pyrin domain [PYD]containing 1), NLRP3 (NLR family, PYD-containing 3), NLRC4 (NLR family, caspase activation and recruitment domain [CARD]-containing 4), AIM2 (absent in melanoma 2), and pyrin. Evidence suggests other NLR family receptors, including NLRP6 and NLRP9B may also form functional inflammasomes [2]. Activation of inflammasomes triggers a cascade of responses, including release of interleukins 1β (IL-1β) and 18 (IL-18) and the induction of pyroptotic, or inflammatory, cell death through cleavage of gasdermin D (GSDMD) [3, 4]. The responses governed by inflammasome signal transduction lead to protection from infectious diseases. However, dysregulation in inflammasome signaling can lead to a hyperinflammatory state, culminating in the development of autoinflammatory disorders, neurodegenerative diseases, and cancer progression [5–9]. To protect against aberrant inflammation, numerous pathways in the cell regulate inflammasome activation [10]. Here, we highlight recent studies examining novel regulation and mechanisms of inflammasome activation and the growing interest in inflammasome-targeting therapies.
INFLAMMASOME SIGNAL TRANSDUCTION
Infection or injury leads to the release of immunostimulatory molecules known as pathogen-associated or damage-associated molecular patterns (PAMPs or DAMPs, respectively) and recognition of these molecules is vital to mounting a defensive response [11, 12]. Pattern recognition sensors, including Toll-like and NOD-like receptors (TLRs and NLRs, respectively), initiate several innate immune responses, including activation of inflammasomes [13].
As would be expected for such a critical process, inflammasome activation is tightly regulated [2]. Type I interferon signaling and interferon regulatory factor proteins (IRFs) have emerged as key regulators of optimal inflammasome activation [14–19]. IRF1 regulates expression of components necessary for NLRP3 and AIM2 activation after infection, including z-DNA binding protein 1 (ZBP1) and guanylate binding proteins (GBPs) [14, 18, 19]. Kayagaki, et al. found that IRF2 regulates expression of the pyroptotic executioner GSDMD and that pyroptosis and IL-1β release could be abolished by mutation of an IRF2 binding site within the GSDMD promoter sequence [17**]. Karki, et al. identified IRF8 as a novel regulator of the NLR family apoptosis inhibitory protein (NAIP)-NLRC4 inflammasome [16*]. Depletion of IRF8 impaired transcription of ligand-sensing NAIPs, resulting in an inadequate response to NLRC4-activating bacteria [16*].
Inflammasome assembly subsequently induces pyroptosis. Mechanistically different from apoptosis and necroptosis, pyroptosis leads to formation of pores in the cellular membrane and release of proinflammatory cytokines [20]. The execution of pyroptosis is mediated by GSDMD, a bipartite protein comprised of an N-terminal effector and C-terminal regulatory domain [4]. After activation by the inflammasome, CASP1 cleaves GSDMD, liberating the effector domain. The N-terminal region of GSDMD then self-oligomerizes and creates a pore-forming complex, allowing for cytokine release and effectively executing pyroptotic cell death. While the role of other gasdermins in pyroptosis remains less clear, most exhibit pore-forming capabilities [21], and recent studies have established a role for Gasdermin E in mediating cell death [22–24].
To further complicate our understanding of the regulation of inflammasomes and pyroptosis, several molecules participate in cell death pathways previously considered to be autonomous. In the absence of GSDMD, cells treated with pyroptotic stimuli have been shown to undergo a caspase-8 (CASP8)-dependent form of cell death [23]. Another recent study described the involvement of transforming growth factor-β activated kinase 1 (TAK1) and receptor-interaction serine/threonine-protein kinase 1 (RIPK1), proteins involved in apoptosis and necroptosis, in NLRP3 inflammasome activation [25]. Gurung et al. described the roles of CASP8 and FADD (Fas-associated protein with death domain), proteins normally associated with apoptotic cell death, in inflammasome regulation [26]. As evidence of crosstalk between apoptosis, necroptosis, and pyroptosis has become increasingly apparent, study of the intricate connections and complex interplay between death signaling is swiftly becoming an area of major research interest.
MECHANISMS OF INFLAMMASOME ACTIVATION AND ASSEMBLY
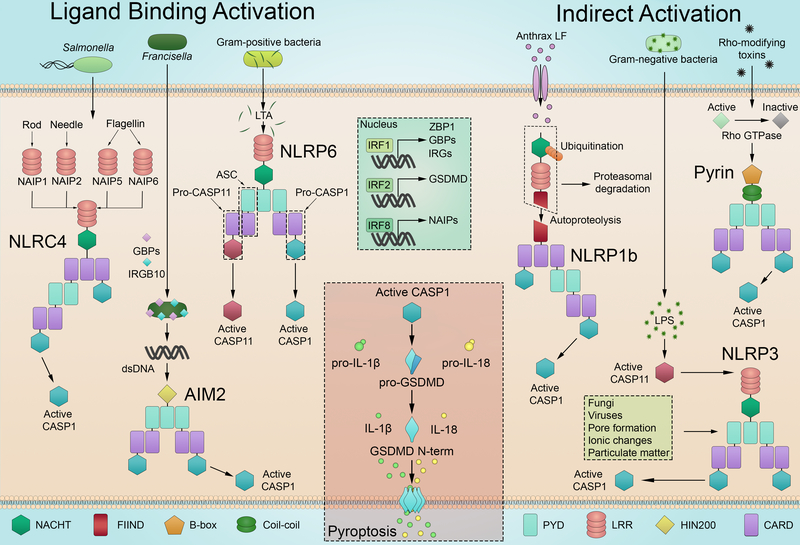
Though the inflammasomes all contribute to host defense, each is distinct in terms of ligand recognition, complex composition, and mechanism of activation (Figure 1). Activation can be achieved through direct inflammasome-ligand binding (AIM2, NAIP-NLRC4) or through indirect sensing of damage or infection (NLRP1, NLRP3, pyrin).
Figure 1:
Inflammasomes can be activated through direct ligand binding or indirect mechanisms of activation. Ligand-binding activation: NAIP proteins recognize ligands from Salmonella and other bacteria to activate the NLRC4 inflammasome. AIM2 binds to cytosolic DNA freed by GBP and IRG proteins to assemble the AIM2 inflammasome and initiate pyroptosis. The NLRP6 inflammasome is activated by cytosolic LTA from Gram-positive bacteria, including L. monocytogenes, to cleave CASP11 and CASP1, though CASP1 does not appear to cleave GSDMD when activated by NLRP6. Indirect activation: The FIIND domain of NLRP1B undergoes autoproteolysis but stays associated with the C-terminus of the protein. Anthrax protective antigen and lethal factor (PA/LF) lead to N-terminal NLRP1B cleavage, targeting it for ubiquitination and degradation and allowing the C-terminus to activate the inflammasome. The pyrin inflammasome becomes active after Rho GTPase inactivation by toxins. NLRP3 is activated through a variety of mechanisms. dsDNA, double-stranded DNA; LRR, leucine-rich repeat; nGSDMD, N-terminal region of gasdermin D; NOD, nucleotide oligomerization domain.
While our understanding of inflammasome composition is still developing, the basic structure of an inflammasome complex includes a sensor, adaptor molecule(s), and a binding platform for CASP1. Complex assembly is mediated through homotypic interactions between the domains of the component proteins (Figure 1). Inflammasomes can interact directly with CASP1 through CARDs or by utilizing the adaptor apoptosis-associated speck-like (ASC) protein to mediate interaction between PYD-containing sensors and CARD-containing CASP1.
NLRP1
NLRP1 was the first inflammasome discovered, but its mechanism of activation has remained elusive. Recently, a mechanism of ‘functional degradation’ controlling murine NLRP1B activation has been described [27**, 28**, 29]. Cleavage of NLRP1B within a function-to-find (FIIND) domain and proteasome activity are both required for inflammasome activation [30, 31]. After cleavage, the N-terminal fragment of NLRP1B continues to interact with the remainder of the protein. Sandstrom et al. and Chui et al. now demonstrated that activators of the NLRP1B inflammasome, including anthrax lethal toxin, cleave the N-terminal fragment of NLRP1B, targeting it for ubiquitination and degradation [27**, 28**]. This frees the C-terminal CARD of the protein to form the inflammasome complex. Strikingly, the authors showed that N-terminal region’s sequence is immaterial for NLRP1B activation, dispelling notions of a sequence-specific autoinhibitory role of the N-terminus [28**]. These findings present intriguing possibilities for the activation of other FIIND-domain containing proteins activation, such as CARD8.
NLRP3
A clear mechanism of activation for this inflammasome is currently unknown, though many have been proposed. The NLRP3 inflammasome assembles in response to several stressors, including destabilization of phagosomes due to particulate matter, changes in ionic flux, and ATP [32], and is implicated in a number of autoinflammatory diseases [7, 8, 33, 34]. Mitochondrial DNA (mtDNA) synthesis has recently been found to contribute to NLRP3 activation. Zhong et al. showed that loss of mtDNA results in decreased activation of the NLRP3 inflammasome, with no effects on AIM2 inflammasome activity [35]. The authors further interrogated the mechanisms of TLR4- and IRF1-mediated mtDNA synthesis after LPS stimulation [35]. Additionally, NEK7, a mitotic kinase, is implicated in NLRP3 activation [36, 37]. The results of a recent, exciting structural examination of the NLRP3 and NEK7 complex by cryo-electron microscopy indicate that NEK7 may function as a bridge between NLRP3 subunits to facilitate assembly [38**].
Additionally, the NLRP3 inflammasome can be activated through non-canonical means by human caspases-4 and −5 or murine caspase-11 (CASP11). Studies have demonstrated that CASP11 responds to intracellular LPS to cleave GSDMD [3, 4]. Activation of CASP11 and induction of pyroptosis lead to the activation of NLRP3.
NAIP/NLRC4
The NLRC4 inflammasome is the only known inflammasome to utilize NLR family apoptosis inhibiting proteins (NAIPs) to sense pathogens. In response to flagellin and type III secretion system (T3SS) proteins, NAIPs recruit NLRC4 into an inflammasome complex, where the NLRC4 protein interacts directly with CASP1 through CARD-CARD interactions [39–41]. Though mice have multiple NAIPs, humans have only one. Murine NAIPs 1 and 2 recognize T3SS rod and needle proteins, respectively, while NAIPs 5 and 6 recognize flagellin [39, 40]. The presence of multiple murine NAIPs with no known ligand indicates that other currently unknown signals may activate the NLRC4 inflammasome. While the NLRC4 inflammasome protects against infections such as Salmonella, mutations resulting in hyperactivation of this inflammasome can also lead to autoinflammation and disease [42, 43].
AIM2
The AIM2 inflammasome recognizes and directly binds to cytosolic DNA, resulting in activation [44–46]. After infection of murine macrophages with Francisella novicida, interferon signaling leads to the upregulation of interferon responsive genes. Several resulting proteins, including guanylate binding proteins (GBPs) and IRGB10, localize to the invading bacteria and release the foreign DNA into the cytosol [47]. After binding to the DNA, AIM2 recruits ASC and CASP1, triggering pyroptosis and providing protection from Francisella infection [48].
Pyrin
Like NLRP1B, the pyrin inflammasome does not directly sense a bacterial ligand. Rather, activation is dependent upon modifications of host proteins by pathogenic factors. Under normal conditions, pyrin is kept inactive through phosphorylation. Bacterial effectors, including TcdA/B, inactivate host RhoA, resulting in the loss of pyrin phosphorylation and its subsequent activation [49]. Mutations within the gene encoding for pyrin are linked to the development of the autoinflammatory disease familial Mediterranean fever [5, 49], and it was recently determined that GSDMD is crucial in the pathogenesis of an experimental model of this disease [50].
NLRP6
In addition to the well-established inflammasomes, further details are emerging regarding other, lesser-studied inflammasomes. Several recent studies have focused on NLRP6 (NLR family, PYD-containing 6), an intestinal NLR with diverse inflammatory roles [51]. Hara et al. identified lipoteichoic acid (LTA) from Listeria monocytogenes as the activator of a functional, non-canonical inflammasome by NLRP6 [52*]. A subsequent structural study supported this finding, as the PYD of NLRP6 could achieve filamentous self-assembly and recruitment of ASC, hallmarks of an active inflammasome [53]. CASP11 is recruited by NLRP6 in an ASC-dependent manner, leading to CASP-1 maturation and subsequent pro-IL-1β and pro-IL-18 cleavage. Utilizing immunoprecipitation and bio-layer interferometry, this study demonstrated a direct interaction between NLRP6 and cytosolic LTA. Furthermore, mice deficient in NLRP6 or CASP11 were protected from L. monocytogenes-induced death and IL-18 treatment restored susceptibility [52*]. Interestingly, the authors note no cleavage of GSDMD in response to LTA or Listeria infection, though CASP1 cleavage was observed.
THERAPEUTIC TARGETTING OF INFLAMMATION
The relationship between host defense and deadly, unregulated inflammation is complex and nuanced, and interest in inflammation-modulating therapeutics, specifically those targeting members of the IL-1 family, is growing [54]. Both IL-1α and IL-1β signal through the receptor IL-1R; however, these proinflammatory cytokines are not redundant. Rather, Lukens, et al. showed that osteomyelitis development depends on IL-1β but not IL-1α, while murine inflammatory disease resembling human neutrophilic dermatosis is dependent on IL-1α, not IL-1β [55, 56]. This divergence in interleukin signaling pathways indicates that tailored therapeutics, targeting either inflammasome-dependent IL-1β or inflammasome-independent IL-1α will be more effective than total IL-1R blockade due to the minimization of off-target effects.
Recent studies have begun investigating the therapeutic effects of modulating inflammasome activation and IL-1β signaling. Segovia et al. recently provided evidence that inflammasome activation in the context of immune checkpoint inhibitor (ICI) therapy can have synergistic, protective effects [57**]. The authors identified a negative regulator of NLRP3 inflammasome activity, the transmembrane protein TMEM176B, which is responsible for regulating internal Ca2+ levels. Loss of TMEM176B led to increased NLRP3 activity, the protective effects of ICI therapy [57**]. However, as previously noted, chronic inflammation can lead to the development of disease. Therefore, therapies blocking IL-1β signaling have begun to gain prominence. In 2011, the Canakinumab Anti-Inflammatory Thrombosis Outcome Study (CANTOS) was initiated [58]. This large, phase 3 clinical trial aimed to examine the consequences of canakinumab-mediated IL-1β neutralization and to test the long-standing hypothesis that hyperinflammation contributes to cardiovascular disease. CANTOS demonstrated that neutralization of IL-1β reduced incidences of cardiovascular disease. Protection occurred independently of lipid content in patient plasma, directly implicating inflammation in disease pathogenesis. As many current treatments focus on reducing lipids within the cardiovascular system, this study opens up potential avenues for the development of novel therapeutics [58]. Further analysis of the CANTOS data revealed a decrease in lung cancer incidence in those treated with canakinumab, suggesting IL-1β blockade may be a potential preventative treatment for lung cancer [59]. Taken together, these studies and others suggest that the role of IL-1 in promoting or preventing disease is complex and context specific. As maturation of IL-1β, but not IL-1α, is directly dependent on inflammasome activation, the complexes themselves also present intriguing therapeutic targets.
CONCLUSIONS
The delicate balance between defense and disease is one that has evolved into a fine-tuned network of signaling pathways and molecules. Infection triggers cellular responses, such as IRF-dependent transcriptional activity [16*, 17**], that contribute to inflammasome activation. Inflammasomes can be activated through direct binding of pathogenic ligands, such as the NLRP6 response to LTA [52*], or through indirect mechanisms, such as the functional degradation of NLRP1B [28**, 29]. Activation of inflammasomes triggers a cleavage cascade, resulting in pyroptotic cell death. This signaling network coordinates a robust and sturdy defense against damage and pathogenic invaders, but aberrations in this response promote the development of inflammatory disease. Modulation of inflammasome activation is being explored in the treatment of various diseases, including cancer immunotherapy [57**, 58]. In summary, numerous advances have been made in the recent years in our understanding of inflammasome regulation, as well as the therapeutic potential of inflammasome-mediated processes, with more exciting discoveries to come as the lines between defense and disease become more refined.
HIGHLIGHTS.
IRF proteins regulate critical components of inflammasome activation
The NLRP1B inflammasome is activated through ‘functional degradation’.
NLRP6 senses lipoteichoic acid to assemble into an inflammasome.
Increased inflammasome activity enhances immune checkpoint therapy.
IL-1β neutralization reduces cardiovascular event and lung cancer incidences.
ACKNOWLEDGEMENTS
The authors acknowledge many investigators in the field whose primary data could not be cited in this review because of space limitations. The authors would like to thank members of the Kanneganti lab for helpful feedback during the editing of this review. Scientific editing and writing support were provided by Rebecca Tweedell, PhD. This work was supported by the National Institutes of Health grants CA163507, AR056296, AI124346, and AI101935 and by ALSAC to T-DK. The authors have no financial conflicts of interest to disclose.
REFERENCES
- 1.Martinon F, Burns K, and Tschopp J, The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell, 10 (2002), pp. 417–426. [DOI] [PubMed] [Google Scholar]
- 2.Man SM and Kanneganti TD, Regulation of inflammasome activation. Immunol Rev, 265 (2015), pp. 6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kayagaki N, et al. , Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature, 526 (2015), pp. 666–671. [DOI] [PubMed] [Google Scholar]
- 4.Shi J, et al. , Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature, 526 (2015), pp. 660–665. [DOI] [PubMed] [Google Scholar]
- 5.French FMFC, A candidate gene for familial Mediterranean fever. Nat Genet, 17 (1997), pp. 25–31. [DOI] [PubMed] [Google Scholar]
- 6.Karki R and Kanneganti TD, Diverging inflammasome signals in tumorigenesis and potential targeting. Nat Rev Cancer, 19 (2019), pp. 197–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDermott MF and Aksentijevich I, The autoinflammatory syndromes. Curr Opin Allergy Clin Immunol, 2 (2002), pp. 511–516. [DOI] [PubMed] [Google Scholar]
- 8.Savic S, et al. , Autoinflammatory syndromes and cellular responses to stress: pathophysiology, diagnosis and new treatment perspectives. Best Pract Res Clin Rheumatol, 26 (2012), pp. 505–533. [DOI] [PubMed] [Google Scholar]
- 9.Voet S, et al. , Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol Med, (2019), p. e10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broz P and Dixit VM, Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol, 16 (2016), pp. 407–420. [DOI] [PubMed] [Google Scholar]
- 11.Akira S and Hemmi H, Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett, 85 (2003), pp. 85–95. [DOI] [PubMed] [Google Scholar]
- 12.Schaefer L, Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem, 289 (2014), pp. 35237–35245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawai T and Akira S, The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol, 11 (2010), pp. 373–384. [DOI] [PubMed] [Google Scholar]
- 14.Briard B, et al. , Fungal ligands released by innate immune effectors promote inflammasome activation during Aspergillus fumigatus infection. Nat Microbiol, 4 (2019), pp. 316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry T, et al. , Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J Exp Med, 204 (2007), pp. 987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karki R, et al. , IRF8 Regulates Transcription of Naips for NLRC4 Inflammasome Activation. Cell, 173 (2018), pp. 920–933 e913.* Here, authors identified IRF8 as a crucial regulator of NAIPs, the ligand sensors of the NAIP/NLRC4 inflammasome. Loss of IRF8 decreased expression of NAIPs 1, 2, 5, and 6 and abolished NLRC4 activation without impacting the activation of other inflammasomes. IRF8-deficient mice were found to be more susceptible to bacterial infections recognized by the NLRC4 inflammasome.
- 17.Kayagaki N, et al. , IRF2 transcriptionally induces GSDMD expression for pyroptosis. Sci Signal, 12 (2019), p. eaax4917.** This recent study from Kayagaki, et al. found that IRF2 regulates the expression of the executioner of pyroptosis, GSDMD. Deficiency in IRF2 significantly reduced both mRNA and protein levels of GSDMD, decreasing IL-1β release and pyroptotic cell death. The authors further identified an uncharacterized IRF2 binding site within the promoter of GSDMD and found that disruption of this binding motif was sufficeint to impair GSDMD expression.These findings suggest IRF2 may be a viable target in autoinflammatory diseases.
- 18.Kuriakose T, et al. , IRF1 Is a Transcriptional Regulator of ZBP1 Promoting NLRP3 Inflammasome Activation and Cell Death during Influenza Virus Infection. J Immunol, 200 (2018), pp. 1489–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Man SM, et al. , The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat Immunol, 16 (2015), pp. 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergsbaken T, Fink SL, and Cookson BT, Pyroptosis: host cell death and inflammation. Nat Rev Microbiol, 7 (2009), pp. 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding J, et al. , Pore-forming activity and structural autoinhibition of the gasdermin family. Nature, 535 (2016), pp. 111–116. [DOI] [PubMed] [Google Scholar]
- 22.Lu H, et al. , Molecular Targeted Therapies Elicit Concurrent Apoptotic and GSDME-Dependent Pyroptotic Tumor Cell Death. Clin Cancer Res, 24 (2018), pp. 6066–6077. [DOI] [PubMed] [Google Scholar]
- 23.Rogers C, et al. , Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat Commun, 10 (2019), p. 1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, et al. , GSDME mediates caspase-3-dependent pyroptosis in gastric cancer. Biochem Biophys Res Commun, 495 (2018), pp. 1418–1425. [DOI] [PubMed] [Google Scholar]
- 25.Malireddi RKS, et al. , TAK1 restricts spontaneous NLRP3 activation and cell death to control myeloid proliferation. J Exp Med, 215 (2018), pp. 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurung P, et al. , FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol, 192 (2014), pp. 1835–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandstrom A, et al. , Functional degradation: A mechanism of NLRP1 inflammasome activation by diverse pathogen enzymes. Science, 364 (2019), p. eaau1330.** In a back-to-back articles with Chui, et al., Sandstrom et al. found that lethal factor from B. anthracis targets the N-terminus of NLRP1B for degradation. As the N-terminus of the protein is cleaved from the C-terminal domain due to FIIND autoprocessing, degradation frees the CARD of the C-terminal domain to oligomerize into the active inflammasome. This work, along with the work from Chui, et al. links the previously reported requirements of N-terminal NLRP1B cleavage, FIIND cleavage, and proteasomal activity into a unified mechanism of NLRP1B inflammasome activation.
- 28.Chui AJ, et al. , N-terminal degradation activates the NLRP1B inflammasome. Science, 364 (2019), pp. 82–85.** In back-to-back articles with Sandstrom, et al., Chui and co-authors described the mechanism of activation of the NLRP1B inflammasome. Using a genome-wide CRISPR-Cas9 screening approach, the authors found that proteins involved in the N-end rule proteasomal degradation are required for NLRP1B activation.
- 29.Xu H, et al. , The N-end rule ubiquitin ligase UBR2 mediates NLRP1B inflammasome activation by anthrax lethal toxin. EMBO J, (2019), p. e101996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okondo MC, et al. , Inhibition of Dpp8/9 Activates the Nlrp1b Inflammasome. Cell Chem Biol, 25 (2018), pp. 262–267 e265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frew BC, Joag VR, and Mogridge J, Proteolytic processing of Nlrp1b is required for inflammasome activity. PLoS Pathog, 8 (2012), p. e1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He Y, Hara H, and Nunez G, Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem Sci, 41 (2016), pp. 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffman HM and Broderick L, The role of the inflammasome in patients with autoinflammatory diseases. J Allergy Clin Immunol, 138 (2016), pp. 3–14. [DOI] [PubMed] [Google Scholar]
- 34.Lamkanfi M and Dixit VM, Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol, 28 (2012), pp. 137–161. [DOI] [PubMed] [Google Scholar]
- 35.Zhong Z, et al. , New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature, 560 (2018), pp. 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He Y, et al. , NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature, 530 (2016), pp. 354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi H, et al. , NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol, 17 (2016), pp. 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharif H, et al. , Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature, (2019).** Sharif, et al. reported the cryo-electron microscopy structure of human NLRP3 in complex with NEK7. This structure revealed the binding site of NEK7 on NLRP3, with three distinct points of contact, mediated through electrostatic interactions. Their results suggest a scaffolding role for NEK7 in facilitating active NLRP3 oligomerization.
- 39.Yang J, et al. , Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proc Natl Acad Sci U S A, 110 (2013), pp. 14408–14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Y, et al. , The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature, 477 (2011), pp. 596–600. [DOI] [PubMed] [Google Scholar]
- 41.Zhao Y and Shao F, The NAIP-NLRC4 inflammasome in innate immune detection of bacterial flagellin and type III secretion apparatus. Immunol Rev, 265 (2015), pp. 85–102. [DOI] [PubMed] [Google Scholar]
- 42.Canna SW, et al. , An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet, 46 (2014), pp. 1140–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romberg N, et al. , Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat Genet, 46 (2014), pp. 1135–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burckstummer T, et al. , An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol, 10 (2009), pp. 266–272. [DOI] [PubMed] [Google Scholar]
- 45.Hornung V, et al. , AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature, 458 (2009), pp. 514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernandes-Alnemri T, et al. , AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature, 458 (2009), pp. 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Man SM, et al. , IRGB10 Liberates Bacterial Ligands for Sensing by the AIM2 and Caspase-11-NLRP3 Inflammasomes. Cell, 167 (2016), pp. 382–396 e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rathinam VA, et al. , The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol, 11 (2010), pp. 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park YH, et al. , Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol, 17 (2016), pp. 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanneganti A, et al. , GSDMD is critical for autoinflammatory pathology in a mouse model of Familial Mediterranean Fever. J Exp Med, 215 (2018), pp. 1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levy M, et al. , NLRP6: A Multifaceted Innate Immune Sensor. Trends Immunol, 38 (2017). pp. 248–260. [DOI] [PubMed] [Google Scholar]
- 52.Hara H, et al. , The NLRP6 Inflammasome Recognizes Lipoteichoic Acid and Regulates Gram-Positive Pathogen Infection. Cell, 175 (2018), pp. 1651–1664 e1614.* Hara, et al. identified Gram-positive LTA as a ligand for the NLRP6 inflammasome. The authors demonstrated that NLRP6-mediated activation of caspase-11 leads to the activation of caspase-1 and subseqent protection from Gram-positive bacterial infections, including Listeria monocytogenes.
- 53.Shen C, et al. , Molecular mechanism for NLRP6 inflammasome assembly and activation. Proc Natl Acad Sci U S A, 116 (2019), pp. 2052–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dinarello CA, Simon A, and van der Meer JW, Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov, 11 (2012), pp. 633–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lukens JR, et al. , Critical role for inflammasome-independent IL-1beta production in osteomyelitis. Proc Natl Acad Sci U S A, 111 (2014), pp. 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lukens JR, et al. , RIP1-driven autoinflammation targets IL-1alpha independently of inflammasomes and RIP3. Nature, 498 (2013), pp. 224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Segovia M, et al. , Targeting TMEM176B Enhances Antitumor Immunity and Augments the Efficacy of Immune Checkpoint Blockers by Unleashing Inflammasome Activation. Cancer Cell, 35 (2019), pp. 767–781 e766.** Segovia and co-authors determined that the transmembrane protein TMEM176B is a novel regulator of NLRP3 inflammasome activity. The authors further provided evidence of inflammasome activation enhancing ICI therapies, raising exciting therapeutic possibilities.
- 58.Ridker PM, et al. , Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J, 162 (2011), pp. 597–605. [DOI] [PubMed] [Google Scholar]
- 59.Ridker PM, et al. , Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet, 390 (2017), pp. 1833–1842. [DOI] [PubMed] [Google Scholar]