Abstract
Macrophages are found in large numbers in the adipose tissue where they closely associate with the adipocytes and the vasculature. Adipose tissue macrophages are a heterogenous population of cells with ‘hard wired’ diversity brought upon by distinct developmental lineages. The purpose of this review is to provide a brief history of macrophages in control of adipose tissue metabolism with the emphasis on the importance of macrophage ontogeny.
Keywords: Macrophages, adipocytes, adipose issue, insulin resistance, Csf1r, Pdgf
Introduction
Adipose tissue and organismal metabolism
Daily and seasonal variations in caloric intake dictate a need for cycles of energy storage and expenditurê). To accommodate these changes in nutritional availability, all eukaryotes from yeast to man have evolved mechanisms to store calories in the form of lipid droplets(2). Amongst all eukaryotes only vertebrates including all mammals, birds, reptiles, amphibians and many fish have cells that are readily identifiable as adipocytes, although the anatomical location of these cells varies greatly between species(2, 3). At the molecular level, orthologous lipid storage genes performing similar functions can be found in worms, flies, and mammals(4). Adipocytes store large amounts of energy in the form of esterified lipids in a manner that is not toxic to the cell or the organism as a whole, a function that is indispensable to the survival of the organism(1, 5, 6). Adipocytes release fatty acids into the circulation when glucose is limiting. These fatty acids are generated by breaking down triacylglycerols that contain more energy per unit mass than do carbohydrates and can essentially be stored anhydrously(1, 5–7).
In addition to the primary function of adipocytes and the adipose tissue in energy storage, there seem to exist adipose depot-specific functions. Transplantation studies have demonstrated that transferring subcutaneous fat to the visceral compartment leads to reduced adiposity and improvement in glucose homeostasis in the recipient tissue(8). Additionally, many diseases that affect adipose tissue show depot-specific outcomes. For example, glucocorticoid excess is associated with redistribution of fat only to visceral stores with relative wasting of subcutaneous fat. A similar pattern is seen in the acquired lipodystrophy associated with certain HIV treatment regimens(9). Congenital lipodystrophy can also preferentially affect specific depots, with different patterns of fat loss associated with distinct genetic lesions (For a comprehensive review see 9).
Macrophages in organismal and adipose tissue metabolism
Macrophages were first hypothesized to play a role in energy metabolism via their function in inflammation, a phenomenon that is associated with insulin resistance, hyperglycemia, and altered fatty acid synthesis(10–12). However, it was not until the early 1980s that it was experimentally demonstrated that macrophage-derived factors, when added to human adipocyte cells, could decrease insulin response(10), reduce lipoprotein lipase activity(11), and decrease fatty acid synthesis(12). In the following decades, It was shown that obesity is associated with a heightened inflammatory condition(13, 14) that involves activation and recruitment of bone-marrow derived monocyte/macrophages into the adipose tissue of obese animals(15, 16), a phenomenon that causes inflammation, insulin resistance, and type 2 diabetes(10, 13–15, 17–28). It is now known that all the adipose tissue depots of healthy or obese animals contain a large population of innate and adaptive immune cells, numerically dominated by macrophages that surround the adipocytes and vasculature(19, 20, 29).
Macrophage heterogeneity in the adipose tissue
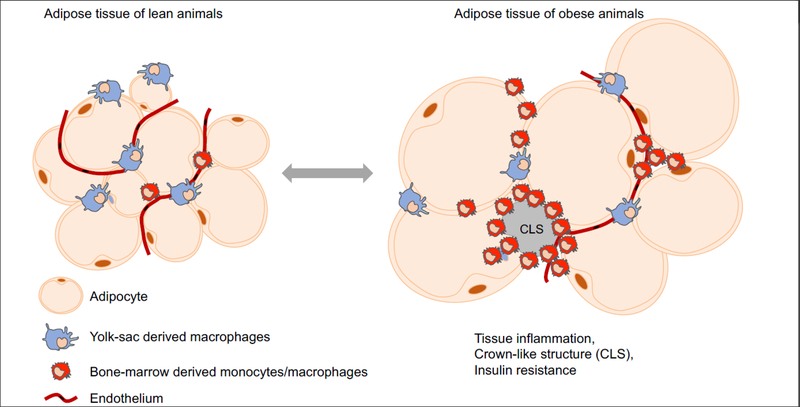
Adipose tissue macrophages are a diverse population of cells that express distinct surface markers and possess unique anatomical locations(29–33). This diversity in macrophages is in part proposed to result from reprogramming of one macrophage subset into another(34–41). In this model, adipose tissue macrophages alter their transcriptional programs to ‘polarize’ from an homeostatic state into an inflammatory state or vice versa depending on the environmental cues. For example, macrophages are proposed to ‘polarize’ into an inflammatory state in the adipose tissue of obese animals and result in obesity associated complications such as insulin resistance(7, 18, 42–47). However, recent studies(34–41) indicate that there exist layers of ‘hard wired’ macrophage diversity that result from developmental processes that take place in the embryo and the adult bone marrow to define myeloid cell function in vivo. For example, erythro-myeloid progenitors(EMP) that reside in the yolk-sac differentiate during embryogenesis to give rise to long lived, self-maintaining, tissue resident macrophages such as microglia and Kupffer cells(19, 21). In contrast, bone-marrow hematopoietic stem cells (HSC) differentiate at steady state along several lineages to generate a number of distinct cells types, including several monocyte subsets that infiltrate tissues to give rise to macrophage-like cells with short lifespans that are continuously renewed from the bone-marrow HSCs(19, 21, 33). There now exists experimental evidence indicating the presence of such ‘hard wired’ developmental heterogeneity in the adipose tissue macrophages. In amphibians, the adipose tissue contains both self-renewing macrophages that populate the adipose tissue before the establishment of bone marrow hematopoiesis and macrophages that originate from the bone-marrow(33). Similarly in mice, there exist a population of adipose tissue macrophages that are long lived(29), self-renewing(48), and molecularly distinct from the other macrophage subsets(32) suggesting a potential developmental heterogeneity in the adipose tissue macrophages of mice. Indeed, recent studies on the heterogeneity of macrophages using fate mapping models indicate that in the adipose tissue, yolk-sac derived tissue resident macrophages and bone-marrow derived monocytes/macrophages coexist (33)(manuscript under review)(Figure 1). Of note, a population of adipose tissue macrophages that express markers reminiscent of yolk-sac derived macrophages in mice are also found in humans, suggesting a potential developmental heterogeneity comparable to mice and amphibians(30).
Figure 1). Macrophages of distinct developmental origin coexist in the adipose tissue of lean and obese animals.
Upon high fat diet feeding, adipocytes and by extension the adipose tissue undergo dramatic expansion. Adipocyte hypertrophy is associated with endoplasmic reticulum stress which results in cell death and recruitment of monocytes into the adipose tissue. The recruited cells are primarily found in association with dead/dying adipocytes in “crown-like structures”. The present body of data indicate that macrophages dynamically adapt their population sizes (the proportion and the absolute number of different macrophage subsets) to accommodate changes associated with dramatic alterations in the size of the adipocytes and the adipose tissue.
The presence of developmentally distinct macrophage populations in the adipose tissue suggests that ‘reprogramming’ of macrophages proposed in context of obesity and inflammation could reflect a change in the proportion and the absolute number of different macrophage subsets resulting from recruitment of bone-marrow derived monocytes into the adipose tissue of obese animals(Figure 1).
Homeostatic roles of macrophages in the adipose tissue
Macrophages regulate fat storage in the adipose tissue
Macrophages are present in the adipose tissue of lean as well as obese animals(7, 18, 42–47, 49–55) (Figure 1). The function of these macrophages is well characterized in the context of obesity and metabolic syndrome(described below), however, their function at steady state and their contribution to lipid storage in the adipose tissue remains somewhat enigmatic. Depletion of all macrophages in mice using CSF1 blocking antibodies postnatally provided perhaps the first glimpse into the function of macrophages. These experiments revealed that in the absence of all macrophages adipocytes are smaller in size(51). Later studies revealed that macrophages are necessary for the formation of adipocytes in tissue engineering chambers(50, 56). However, it was not until the generation of Csf1r mutant rats that it became apparent that macrophages are required for proper development of the adipose tissue(57). Interestingly, the defective adipose tissue formation does not seem to require the absence of all macrophages as experiments in Trib1 deficient mice that lack specifically mannose receptor+ macrophages in the adipose tissue can recapture the same phenotype(32). This unique subset of macrophages is found directly associated with the adipocytes and is long lived, self-maintaining, and most likely of embryonic origin(29, 32) suggesting a role for yolk-sac derived macrophages in proper development of the adipose tissue. Our studies in mice that specifically lack tissue resident macrophages but still maintain HSC-derived monocytes/macrophages demonstrate that yolk-sac derived macrophages are required for expansion of adipocytes postnatally and adipocyte hypertrophy upon high fat diet feeding(manuscript under review). This function is in part mediated through the production of PDGFcc, a PDGF/VEGF family growth factor, by yolk-sac derived macrophages(manuscript under review). It is noteworthy that other macrophage-derived factors such TNF have also been implicated in adipocyte differentiation as well as modulation of adipocyte size(53, 58). These observations on the role of macrophages in control of adipocyte expansion is of particular interest as too much fat(obesity) and too little fat(lipodystrophy) are both associated with metabolic complications such as insulin resistance and hyperglycemia(1).
Metabolic sensors in macrophages
In response to a lipid rich diet, adipose tissue macrophages of embryonic origin increase PDGFcc transcription to promote adipocyte hypertrophy(manuscript under review). This observation requires that macrophages act as metabolic sensors in the adipose tissue. In agreement with this hypothesis, macrophages respond to dietary changes in Drosophila and mice(59, 60) and in particular, some adipose tissue macrophages are observed in close proximity to the vasculature where they may sample the circulation(29). This sampling of the blood would allow for the sensing of circulating metabolites in addition to removal of potentially noxious material.
What could then be the nutritional sensor in macrophages that monitors changes in circulating metabolites? The nuclear receptors (NRs) are promising candidates for this function. NRs are a large family of ligand-activated transcription factors that regulate several important processes, including development, reproduction, and metabolism(61, 62). NRs respond to lipophilic hormones and vitamins, but more importantly dietary lipids(63). NRs include members such as the Peroxisome proliferator activated receptors(PPAR) and liver X receptors(LXR)(61–63).
Several studies have shown that the administration of PPARy agonists inhibits the development of atherosclerosis in low-density lipoprotein(LDL) receptor–deficient(LDLR−/−)(64, 65) and apolipoprotein E-deficient(apoE−/−) mice(66). It is proposed that these effects of PPARy agonists are in part mediated through PPARy activation in macrophages therefore leading to upregulation of scavenger receptor CD36(75) and genes responsible for cholesterol efflux(67). These transcriptional changes promote removal of lipids and fatty acids from the environment and stimulate efflux and loading of such molecules into lipoproteins for transport to the liver where they are processed(67), thus shifting the balance from lipid loading to lipid efflux in the atherosclerotic lesions. PPARy may also exert anti-inflammatory effects in macrophages directly(68) or indirectly through LXR(69–73). Disruption of PPARy in myeloid cells impairs insulin response, glucose homeostasis, and downregulates the expression of genes involved in oxidative phosphorylation and mitochondrial homeostasis in the skeletal muscle and liver(74). This leads to decreased insulin sensitivity in these tissues(74). PPARy reduces monocyte CCR2 expression(76, 77) therefore contributing to altered recruitment of monocytes into different tissues including adipose tissue of obese animals. Other members of the peroxisome proliferator activated receptors are also implicated in macrophage biology. For example, macrophages sense very-low-density lipoprotein (VLDL) via the activation of the nuclear receptor PPAR5 (78) to upregulate genes involved in lipid handling such as Perilipin. Altogether, these studies indicate that PPAR and LXR activity in macrophages may control lipid metabolism, glucose metabolism, and monocyte recruitment into the adipose tissue of obese animals therefore providing a link between circulating fatty acids, lipid sensing in macrophages, inflammation in the adipose tissue, and complications associated with metabolic syndrome.
Pathogenic roles of macrophages in obesity
Macrophages in adipose tissue inflammation
Obesity promotes adipocyte hypertrophy and endoplasmic reticulum stress(82) which may be the initial signals to trigger inflammation(31, 83) in the adipose tissue. Upon adipose tissue inflammation in obese animals, monocytes are recruited to the adipose tissue under the influence of secreted proteins such as MCP-1(CCL2)(19, 20). These recruited monocytes/macrophages are often found surrounding the dead/dying adipocytes in so-called ‘crown-like structures’ where they scavenge cell debris and free lipid droplets(84)(Figure 1). It is proposed that adipose tissue inflammation and monocyte recruitment are responsible for insulin resistance. Accordingly, targeted ablation of either MCP-1 or its receptor (CCR2) reduces monocyte infiltration of the adipose tissue and improves insulin sensitivity(21). Interestingly, these mice do not present with changes in body weight, suggesting that bone-marrow derived monocytes/macrophages may have little to no role in adipocyte hypertrophy or weight gain in animals on lipid rich diet(21). Overexpression of MCP-1 in the adipose tissue causes monocyte recruitment and insulin resistance(27, 28). Decrease in monocyte infiltration into the adipose tissue is also associated with a reduction in crown-like structures, TNF, and iNOS in the adipose tissue of obese animals(19, 21, 24), further emphasizing the function of bone-marrow derived monocytes/macrophages in the obesity associated inflammation and the subsequent metabolic syndrome.
Of note, cultured adipocytes in vitro made hypertrophic with oleic acid display insulin resistance without triggering an inflammatory response(85) suggesting monocyte infiltration may not be the only contributing factor to induction of insulin resistance in the adipose tissue.
Macrophages in ectopic fat deposition
Excess energy intake in lieu of limiting adipocyte hypertrophy results in the overflow of lipids that need to be stored in cells not specialized for long term fat storage in a process termed ectopic fat deposition(86). Ectopic deposition of lipids is often accompanied with inflammation in the affected organs such as the liver and skeletal muscle(15, 16, 21, 24–28). Interestingly, the development of insulin resistance in tissues such as the liver and skeletal muscle are often preceded by local deposition of lipids, suggesting that this process may directly or indirectly contribute to insulin resistance(87). Studies in mice deficient for MCP-1 or its receptor (CCR2) suggest that ectopic fat deposition requires the infiltration of monocytes into the affected organs(27)(manuscript under review). It is not clear how bone marrow-derived monocytes/macrophages promote ectopic fat deposition. There are indications that induction of inflammation in the liver may be sufficient to induce fat deposition in hepatocytes, however, the role of monocytes was not formally ruled out in these experiments(88). The proposed role of bone marrow-derived monocytes/macrophages in ectopic fat deposition demonstrates a functional dichotomy between these cells and EMP-derived macrophages that are required for fat storage in specialized fat storing cells i.e adipocytes. This further emphasizes the necessity to consider the developmental origin of macrophages when studying their functions in the adipose tissue and organismal metabolism.
Development of therapeutic targeting macrophages for treatment of obesity and accompanied morbidities
Elegant studies on the origin and function of macrophages in the adipose tissue of lean and obese animals have provided new and exciting opportunities for development of therapies that may improve the life of individuals suffering from morbid obesity. Targeted ablation of either MCP-1 or its receptor(CCR2) improves insulin sensitivity(19, 21, 24, 26, 89). Similarly, Ccr2 silencing or antagonism in the adipose tissue of wildtype mice or those suffering from genetic causes of obesity, such as leptin receptor deficient mice, results in reduced ectopic fat deposition and inflammation leading to improved glucose and insulin resistance(19, 21,24, 26, 89). These results suggest that targeting the CCR2/CCL2 axis may prove a viable therapeutic option in obese individuals suffering from metabolic syndrome. Indeed, a clinical trial has indicated the beneficial effects of the CCR2 antagonist CCX140-B on glycemic parameters in human subjects(90). However, it is important to note that targeted ablation of CCR2/CCL2 axis does not affect body weight(21). An approach that may prove successful in correcting the weight gain due to over feeding is through targeting of PDGF-receptor signaling. These receptors play complex roles in control of adipocyte size and therefore lipid storage(91–95). Our study on the role of adipose tissue macrophages in lean and obese mice demonstrate that blockade of macrophage-derived PDGFcc may be beneficial in preventing further weight gain in obese individuals(91–95)(manuscript under review). This treatment, however, does not prevent complications associated with obesity and therefore may be best used in combination with CCR2 antagonists.
An alternative approach is the use of CSF1R inhibitors or blocking antibodies, the benefit of such an approach is that it depletes all macrophages including yolk-sac derived macrophages that control adipocyte hypertrophy(96)(manuscript under review) and bone-marrow derived monocytes/macrophages that contribute to insulin resistance and ectopic fat deposition(15, 16, 21,24–28). However, systemic complications of macrophages depletion by CSF1R inhibitors and antibodies may preclude such dramatic measures.
Summary and outlook
In the last few decades, many studies using new genetic mouse models have provided insights into the roles of macrophages in the adipose tissue. Albeit many questions are still open, it is now demonstrated that yolk-sac derived macrophages promote the postnatal expansion of adipocytes during early development and control adipocyte hypertrophy in obese mice. In contrast, bone-marrow derived monocytes/macrophages are primarily involved in adipose tissue inflammation, ectopic fat deposition, and insulin resistance. Many challenges lie in identification of mechanisms by which macrophages sense the nutritional status of the organism and then inform adipocytes of the ever-changing environment. Mounting evidence suggests a role for yolk-sac derived macrophages in formation and expansion of adipocytes in adult mice, but it not clear if these cells may be involved in adipose tissue wasting or lipodystrophy. In regard to bone-marrow derived monocytes/macrophages, it is not apparent how and why macrophages promote ectopic lipid uptake by cells not specialized for fat storage such as hepatocytes. It is also not clear for what purpose bone-marrow derived monocytes/macrophage may be recruited to the adipose tissue of obese animals. One proposed reason is to act as buffers for the dramatic increase in lipid flux(97). Future work investigating the respective contributions of lineage-specific functions of macrophages will greatly benefit our understanding of adipose tissue biology and lipid storage in specialized and non-specialized fat storing cells.
Highlights.
Adipose tissue is populated with developmentally distinct populations of macrophages
Yolk-sac derived adipose tissue macrophages control adipose tissue development and adipocyte hypertrophy
Bone-marrow derived monocytes/macrophages contribute to insulin resistance and lipid storage in cells not specialized for long term fat storage such as hepatocytes
Acknowledgements
This work was supported by NIH/NCI P30CA008748 to MSKCC, NIH/NIAID 1R01AI130345, NIH/NHLBI R01HL138090, Ludwig institute for Cancer research basic immunology grant and Cycle For Survival grants to FG. This work was also supported by Leducq transatlantic network of excellence to FG, NIH/NCI F32CA225036 to NC. The authors acknowledge Maria Pokrovskii, James Muller, and all members of the Geissmann lab for helpful suggestions and editing the manuscript.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444(7121):847–53. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ottaviani E, Malagoli D, Franceschi C. The evolution of the adipose tissue: a neglected enigma. Gen Comp Endocrinol. 2011;174(1):1–4. doi: 10.1016/j.ygcen.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Pond CM. The Fats of Life. Cambridg: Cambridge Univ. Press; 1998. [Google Scholar]
- 4.Young SG, Zechner R. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes & development. 2013;27(5):459–84. doi: 10.1101/gad.209296.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135(2):240–9. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 6.Wise A Adipocyte number and size in hypothalamic obesity induced in weanling mice by gold thioglucose and bipiperidyl mustard. Nutr Metab. 1975;19(5–6):291–8. [DOI] [PubMed] [Google Scholar]
- 7.Hotamisligil GS. Foundations of Immunometabolism and Implications for Metabolic Health and Disease. Immunity. 2017;47(3):406–20. doi: 10.1016/j.immuni.2017.08.009.* An elegant and comprehensive review on the role of immune cells and macrophages in metabolism with an emphasis on the history of this field.
- 8.Tran TT, Kahn CR. Transplantation of adipose tissue and stem cells: role in metabolism and disease. Nat Rev Endocrinol. 2010;6(4):195–213. doi: 10.1038/nrendo.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg A Clinical review#: Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96(11):3313–25. doi: 10.1210/jc.2011-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pekala P, Kawakami M, Vine W, Lane MD, Cerami A. Studies of insulin resistance in adipocytes induced by macrophage mediator. The Journal of experimental medicine. 1983;157(4):1360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawakami M, Pekala PH, Lane MD, Cerami A. Lipoprotein lipase suppression in 3T3-L1 cells by an endotoxin-induced mediator from exudate cells. Proceedings of the National Academy of Sciences of the United States of America. 1982;79(3):912–6. doi: 10.1073/pnas.79.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pekala PH, Kawakami M, Angus CW, Lane MD, Cerami A. Selective inhibition of synthesis of enzymes for de novo fatty acid biosynthesis by an endotoxin-induced mediator from exudate cells. Proceedings of the National Academy of Sciences of the United States of America. 1983;80(9):2743–7. doi: 10.1073/pnas.80.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. [DOI] [PubMed] [Google Scholar]
- 14.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. The Journal of clinical investigation. 1995;95(5):2409–15. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito A, Suganami T, Yamauchi A, Degawa-Yamauchi M, Tanaka M, Kouyama R, Kobayashi Y, Nitta N, Yasuda K, Hirata Y, Kuziel WA, Takeya M, Kanegasaki S, Kamei Y, Ogawa Y. Role of CC chemokine receptor 2 in bone marrow cells in the recruitment of macrophages into obese adipose tissue. The Journal of biological chemistry. 2008;283(51):35715–23. doi: 10.1074/jbc.M804220200. [DOI] [PubMed] [Google Scholar]
- 16.Parker R, Weston CJ, Miao Z, Corbett C, Armstrong MJ, Ertl L, Ebsworth K, Walters MJ, Baumart T, Newland D, McMahon J, Zhang P, Singh R, Campbell J, Newsome PN, Charo I, Schall TJ, Adams DH. CC chemokine receptor 2 promotes recruitment of myeloid cells associated with insulin resistance in nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2018;314(4):G483–G93. doi: 10.1152/ajpgi.00213.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirsch J, Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metab. 1976;5(2):299–311. [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin T, Ackerman SE, Shen L, Engleman E. Role of innate and adaptive immunity in obesity-associated metabolic disease. The Journal of clinical investigation. 2017;127(1):5–13. doi: 10.1172/JCI88876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr. Obesity is associated with macrophage accumulation in adipose tissue. The Journal of clinical investigation. 2003;112(12):1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of clinical investigation. 2003;112(12):1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW Jr., CCR2 modulates inflammatory and metabolic effects of high-fat feeding. The Journal of clinical investigation. 2006;116(1):115–24. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suganami T, Ogawa Y. Adipose tissue macrophages: their role in adipose tissue remodeling. Journal of leukocyte biology. 2010;88(1):33–9. doi: 10.1189/jlb.0210072. [DOI] [PubMed] [Google Scholar]
- 23.Surmi BK, Hasty AH. Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Future Lipidol. 2008;3(5):545–56. doi: 10.2217/17460875.3.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J, Chung K, Choi C, Beloor J, Ullah I, Kim N, Lee KY, Lee SK, Kumar P. Silencing CCR2 in Macrophages Alleviates Adipose Tissue Inflammation and the Associated Metabolic Syndrome in Dietary Obese Mice. Mol Ther Nucleic Acids. 2016;5:e280. doi: 10.1038/mtna.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan TJ, Miao Z, Zhao BN, Ertl LS, Wang Y, Krasinski A, Walters MJ, Powers JP, Dairaghi DJ, Baumgart T, Seitz LC, Berahovich RD, Schall TJ, Jaen JC. Experimental evidence for the use of CCR2 antagonists in the treatment of type 2 diabetes. Metabolism. 2013;62(11):1623–32. doi: 10.1016/j.metabol.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Obstfeld AE, Sugaru E, Thearle M, Francisco AM, Gayet C, Ginsberg HN, Ables EV, Ferrante AW Jr., C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes. 2010;59(4):916–25. doi: 10.2337/db09-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. The Journal of clinical investigation. 2006;116(6):1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. The Journal of biological chemistry. 2006;281(36):26602–14. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 29.Silva HM, Bafica A, Rodrigues-Luiz GF, Chi J, Santos PDA, Reis BS, Hoytema van Konijnenburg DP, Crane A, Arifa RDN, Martin P, Mendes D, Mansur DS, Torres VJ, Cadwell K, Cohen P, Mucida D, Lafaille JJ. Vasculature-associated fat macrophages readily adapt to inflammatory and metabolic challenges. The Journal of experimental medicine. 2019;216(4):786–806. doi: 10.1084/jem.20181049.** This study demonstrates the presence of long-lived macrophages in the adipose tissue and show that distinct adipose tissue macrophages alter their absolute numbers to adapt to metabolic stress and inflammation.
- 30.Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G, Zlabinger GJ, Stulnig TM. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond). 2007;31(9):1420–8. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 31.Huber J, Kiefer FW, Zeyda M, Ludvik B, Silberhumer GR, Prager G, Zlabinger GJ, Stulnig TM. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J Clin Endocrinol Metab. 2008;93(8):3215–21. doi: 10.1210/jc.2007-2630. [DOI] [PubMed] [Google Scholar]
- 32.Satoh T, Kidoya H, Naito H, Yamamoto M, Takemura N, Nakagawa K, Yoshioka Y, Morii E, Takakura N, Takeuchi O, Akira S. Critical role of Trib1 in differentiation of tissue-resident M2-like macrophages. Nature. 2013;495(7442):524–8. doi: 10.1038/nature11930. [DOI] [PubMed] [Google Scholar]
- 33.Hassnain Waqas SF, Noble A, Hoang AC, Ampem G, Popp M, Strauss S, Guille M, Roszer T. Adipose tissue macrophages develop from bone marrow-independent progenitors in Xenopus laevis and mouse. Journal of leukocyte biology. 2017;102(3):845–55. doi: 10.1189/jlb.1A0317-082RR. PubMed PMID: 28642277;** This elegant study provides the first evidence for a prenatal origin of adipose tissue macrophages.
- 34.Gomez Perdiguero E, Geissmann F. Myb-Independent Macrophages: A Family of Cells That Develops with Their Tissue of Residence and Is Involved in Its Homeostasis. Cold Spring Harbor symposia on quantitative biology. 2013. Epub 2013/10/15. doi: 10.1101/sqb.2013.78.020032. [DOI] [PubMed] [Google Scholar]
- 35.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518(7540):547–51. Epub 2014/12/04. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nature immunology. 2015;17(1):2–8. doi: 10.1038/ni.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geissmann F, Mass E. A stratified myeloid system, the challenge of understanding macrophage diversity. Seminars in immunology. 2015;27(6):353–6. doi: 10.1016/j.smim.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perdiguero EG, Klapproth K, Schulz C, Busch K, de Bruijn M, Rodewald HR, Geissmann F. The Origin of Tissue-Resident Macrophages: When an Erythro-myeloid Progenitor Is an Erythro-myeloid Progenitor. Immunity. 2015;43(6):1023–4. doi: 10.1016/j.immuni.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 39.Mass E, Ballesteros I, Farlik M, Halbritter F, Gunther P, Crozet L, Jacome-Galarza CE, Handler K, Klughammer J, Kobayashi Y, Gomez-Perdiguero E, Schultze JL, Beyer M, Bock C, Geissmann F. Specification of tissue-resident macrophages during organogenesis. Science. 2016;353(6304). doi: 10.1126/science.aaf4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nature immunology. 2016;17(1):2–8. doi: 10.1038/ni.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacome-Galarza CE, Percin GI, Muller JT, Mass E, Lazarov T, Eitler J, Rauner M, Yadav VK, Crozet L, Bohm M, Loyher P, Karsenty G, Waskow C, Geissmann F. Developmental origin, functional maintenance and genetic rescue of osteoclasts Nature. 2019. doi: 10.1038/s41586-019-1105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hume DA. The Many Alternative Faces of Macrophage Activation. Frontiers in immunology. 2015;6:370. doi: 10.3389/fimmu.2015.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pollard JW. Trophic macrophages in development and disease. Nature reviews Immunology. 2009;9(4):259–70. Epub 2009/03/14. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation. 2007;117(1):175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56(1):16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. The Journal of biological chemistry. 2007;282(48):35279–92. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 47.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 Activation of Kupffer Cells by PPARÖ Ameliorates Obesity-Induced Insulin Resistance. Cell metabolism. 2008;7(6):496–507. doi: 10.1016/i.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, Shen Y, Czech MP, Aouadi M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell metabolism. 2014;19(1):162–71. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han J, Lee JE, Jin J, Lim JS, Oh N, Kim K, Chang SI, Shibuya M, Kim H, Koh GY. The spatiotemporal development of adipose tissue. Development. 2011;138(22):5027–37. doi: 10.1242/dev.067686. [DOI] [PubMed] [Google Scholar]
- 50.Debels H, Galea L, Han XL, Palmer J, van Rooijen N, Morrison W, Abberton K. Macrophages play a key role in angiogenesis and adipogenesis in a mouse tissue engineering model. Tissue Eng Part A. 2013;19(23–24):2615–25. doi: 10.1089/ten.TEA.2013.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei S, Lightwood D, Ladyman H, Cross S, Neale H, Griffiths M, Adams R, Marshall D, Lawson A, McKnight AJ, Stanley ER. Modulation of CSF-1-regulated post-natal development with anti-CSF-1 antibody. Immunobiology. 2005;210(2–4):109–19. doi: 10.1016/j.imbio.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Lee YH, Petkova AP, Granneman JG. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell metabolism. 2013;18(3):355–67. doi: 10.1016/j.cmet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, Scherer PE. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell metabolism. 2014;20(1):103–18. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biswas SK, Mantovani A. Orchestration of metabolism by macrophages. Cell metabolism. 2012;15(4):432–7. Epub 2012/04/10. doi: 10.1016/j.cmet.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 55.Pang C, Gao Z, Yin J, Zhang J, Jia W, Ye J. Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. American journal of physiology Endocrinology and metabolism. 2008;295(2):E313–22. doi: 10.1152/ajpendo.90296.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hemmrich K, Thomas GP, Abberton KM, Thompson EW, Rophael JA, Penington AJ, Morrison WA. Monocyte chemoattractant protein-1 and nitric oxide promote adipogenesis in a model that mimics obesity. Obesity (Silver Spring). 2007;15(12):2951–7. doi: 10.1038/oby.2007.352. [DOI] [PubMed] [Google Scholar]
- 57.Pridans C, Raper A, Davis GM, Alves J, Sauter KA, Lefevre L, Regan T, Meek S, Sutherland L, Thomson AJ, Clohisey S, Bush SJ, Rojo R, Lisowski ZM, Wallace R, Grabert K, Upton KR, Tsai YT, Brown D, Smith LB, Summers KM, Mabbott NA, Piccardo P, Cheeseman MT, Burdon T, Hume DA. Pleiotropic Impacts of Macrophage and Microglial Deficiency on Development in Rats with Targeted Mutation of the Csf1r Locus. J Immunol. 2018;201(9):2683–99. doi: 10.4049/jimmunol.1701783.** A descriptive analysis of rats deficient in Csf1r reveals loss of visceral adipose tissue in these mice.
- 58.Cawthorn WP, Heyd F, Hegyi K, Sethi JK. Tumour necrosis factor-alpha inhibits adipogenesis via a beta-catenin/TCF4(TCF7L2)-dependent pathway. Cell death and differentiation. 2007;14(7):1361–73. doi: 10.1038/sj.cdd.4402127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woodcock KJ, Kierdorf K, Pouchelon CA, Vivancos V, Dionne MS, Geissmann F. Macrophage-derived upd3 cytokine causes impaired glucose homeostasis and reduced lifespan in Drosophila fed a lipid-rich diet. Immunity. 2015;42(1):133–44. doi: 10.1016/j.immuni.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Remmerie A, Scott CL. Macrophages and lipid metabolism. Cellular immunology. 2018;330:27–42. doi: 10.1016/j.cellimm.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chambon P How I became one of the fathers of a superfamily. Nature medicine. 2004;10(10):1027–31. doi: 10.1038/nm1004-1027. [DOI] [PubMed] [Google Scholar]
- 62.Evans R A transcriptional basis for physiology. Nature medicine. 2004;10(10):1022–6. doi: 10.1038/nm1004-1022. [DOI] [PubMed] [Google Scholar]
- 63.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294(5548):1866–70. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 64.Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. The Journal of clinical investigation. 2000;106(4):523–31. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Collins AR, Meehan WP, Kintscher U, Jackson S, Wakino S, Noh G, Palinski W, Hsueh WA, Law RE. Troglitazone inhibits formation of early atherosclerotic lesions in diabetic and nondiabetic low density lipoprotein receptor-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2001;21(3):365–71. [DOI] [PubMed] [Google Scholar]
- 66.Chen Z, Ishibashi S, Perrey S, Osuga J, Gotoda T, Kitamine T, Tamura Y, Okazaki H, Yahagi N, Iizuka Y, Shionoiri F, Ohashi K, Harada K, Shimano H, Nagai R, Yamada N. Troglitazone inhibits atherosclerosis in apolipoprotein E-knockout mice: pleiotropic effects on CD36 expression and HDL. Arteriosclerosis, thrombosis, and vascular biology. 2001;21(3):372–7. [DOI] [PubMed] [Google Scholar]
- 67.Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, Liao D, Nagy L, Edwards PA, Curtiss LK, Evans RM, Tontonoz P. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Molecular cell. 2001;7(1):161–71. [DOI] [PubMed] [Google Scholar]
- 68.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 69.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nature medicine. 2003;9(2):213–9. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 70.Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA, Tontonoz P. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(22):12097–102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Costet P, Luo Y, Wang N, Tall AR. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. The Journal of biological chemistry. 2000;275(36):28240–5. doi: 10.1074/jbc.M003337200. [DOI] [PubMed] [Google Scholar]
- 72.Schwartz K, Lawn RM, Wade DP. ABC1 gene expression and ApoA-I-mediated cholesterol efflux are regulated by LXR. Biochemical and biophysical research communications. 2000;274(3):794–802. doi: 10.1006/bbrc.2000.3243. [DOI] [PubMed] [Google Scholar]
- 73.Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289(5484):1524–9. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- 74.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–20. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moore KJ, Rosen ED, Fitzgerald ML, Randow F, Andersson LP, Altshuler D, Milstone DS, Mortensen RM, Spiegelman BM, Freeman MW. The role of PPAR-gamma in macrophage differentiation and cholesterol uptake. Nature medicine. 2001;7(1):41–7. doi: 10.1038/83328. [DOI] [PubMed] [Google Scholar]
- 76.Han KH, Chang MK, Boullier A, Green SR, Li A, Glass CK, Quehenberger O. Oxidized LDL reduces monocyte CCR2 expression through pathways involving peroxisome proliferator-activated receptor gamma. The Journal of clinical investigation. 2000;106(6):793–802. doi: 10.1172/JCI10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Babaev VR, Yancey PG, Ryzhov SV, Kon V, Breyer MD, Magnuson MA, Fazio S, Linton MF. Conditional knockout of macrophage PPARgamma increases atherosclerosis in C57BL/6 and low-density lipoprotein receptor-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2005;25(8):1647–53. doi: 10.1161/01.ATV.0000173413.31789.1a. [DOI] [PubMed] [Google Scholar]
- 78.Chawla A, Lee CH, Barak Y, He W, Rosenfeld J, Liao D, Han J, Kang H, Evans RM. PPARdelta is a very low-density lipoprotein sensor in macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(3):1268–73. doi: 10.1073/pnas.0337331100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Byles V, Covarrubias AJ, Ben-Sahra I, Lamming DW, Sabatini DM, Manning BD, Horng T. The TSC-mTOR pathway regulates macrophage polarization. Nature communications. 2013;4:2834. doi: 10.1038/ncomms3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Covarrubias AJ, Aksoylar HI, Horng T. Control of macrophage metabolism and activation by mTOR and Akt signaling. Seminars in immunology. 2015;27(4):286–96. doi: 10.1016/j.smim.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hallowell RW, Collins SL, Craig JM, Zhang Y, Oh M, Illei PB, Chan-Li Y, Vigeland CL, Mitzner W, Scott AL, Powell JD, Horton MR. mTORC2 signalling regulates M2 macrophage differentiation in response to helminth infection and adaptive thermogenesis. Nature communications. 2017;8:14208. doi: 10.1038/ncomms14208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 83.Rebuffe-Scrive M, Surwit R, Feinglos M, Kuhn C, Rodin J. Regional fat distribution and metabolism in a new mouse model (C57BL/6J) of non-insulin-dependent diabetes mellitus. Metabolism. 1993;42(11):1405–9. [DOI] [PubMed] [Google Scholar]
- 84.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 85.Kim JI, Huh JY, Sohn JH, Choe SS, Lee YS, Lim CY, Jo A, Park SB, Han W, Kim JB. Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Molecular and cellular biology. 2015;35(10):1686–99. doi: 10.1128/MCB.01321-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lettner A, Roden M. Ectopic fat and insulin resistance. Curr Diab Rep. 2008;8(3):185–91. [DOI] [PubMed] [Google Scholar]
- 87.Kraegen EW, Clark PW, Jenkins AB, Daley EA, Chisholm DJ, Storlien LH. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes. 1991;40(11):1397–403. doi: 10.2337/diab.40.11.1397. [DOI] [PubMed] [Google Scholar]
- 88.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nature medicine. 2005;11(2):183–90. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kang YS, Lee MH, Song HK, Ko GJ, Kwon OS, Lim TK, Kim SH, Han SY, Han KH, Lee JE, Han JY, Kim HK, Cha DR. CCR2 antagonism improves insulin resistance, lipid metabolism, and diabetic nephropathy in type 2 diabetic mice. Kidney international. 2010;78(9):883–94. doi: 10.1038/ki.2010.263. [DOI] [PubMed] [Google Scholar]
- 90.de Zeeuw D, Bekker P, Henkel E, Hasslacher C, Gouni-Berthold I, Mehling H, Potarca A, Tesar V, Heerspink HJ, Schall TJ, Group CBDNS. The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: a randomised trial. Lancet Diabetes Endocrinol. 2015;3(9):687–96. doi: 10.1016/S2213-8587(15)00261-2. [DOI] [PubMed] [Google Scholar]
- 91.Onogi Y, Wada T, Kamiya C, Inata K, Matsuzawa T, Inaba Y, Kimura K, Inoue H, Yamamoto S, Ishii Y, Koya D, Tsuneki H, Sasahara M, Sasaoka T. PDGFRbeta Regulates Adipose Tissue Expansion and Glucose Metabolism via Vascular Remodeling in Diet-Induced Obesity. Diabetes. 2017;66(4):1008–21. doi: 10.2337/db16-0881.* This study demonstrates the role of PDGFRp singling in adipose tissue size and adipocyte hypertrophy upon high fat diet feeding.
- 92.Gao Z, Daquinag aC, Su F, Snyder B, Kolonin MG. PDGFRalpha/PDGFRbeta signaling balance modulates progenitor cell differentiation into white and beige adipocytes. Development. 2018;145(1). doi: 10.1242/dev.155861.** This study demonstrates PDGF receptor ligands affect adipocyte size. PDGFaa decreases adipocyte size whereas PDGFdd promotes adipocyte size.
- 93.Sun C, Berry WL, Olson LE. PDGFRalpha controls the balance of stromal and adipogenic cells during adipose tissue organogenesis. Development. 2017;144(1):83–94. doi: 10.1242/dev.135962.* Constitute PDGFRa singling results in small adipose tissue.
- 94.Haider N, Dusseault J, Larose L. Nck1 Deficiency Impairs Adipogenesis by Activation of PDGFRalpha in Preadipocytes. iScience. 2018;6:22–37. doi: 10.1016/j.isci.2018.07.010.* Deletion of NCK1, a Src homology containing adaptor, downstream of PDGFRa results in smaller adipocytes in the subcutaneous adipose tissue depots.
- 95.Vaziri C, Faller DV. Down-regulation of platelet-derived growth factor receptor expression during terminal differentiation of 3T3-L1 pre-adipocyte fibroblasts. The Journal of biological chemistry. 1996;271(23):13642–8. [DOI] [PubMed] [Google Scholar]
- 96.Valdearcos M, Douglass JD, Robblee MM, Dorfman MD, Stifler DR, Bennett ML, Gerritse I, Fasnacht R, Barres BA, Thaler JP, Koliwad SK. Microglial Inflammatory Signaling Orchestrates the Hypothalamic Immune Response to Dietary Excess and Mediates Obesity Susceptibility. Cell metabolism. 2017;26(1):185–97 e3. doi: 10.1016/j.cmet.2017.05.015.* This study demonstrates the efficacy of CSF1R inhibition in preventing weigh gain in mice on high fat diet.
- 97.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW Jr., Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. The Journal of clinical investigation. 2010;120(10):3466–79. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]