Abstract
Background:
Household endotoxin levels have been variably associated with risk for asthma and atopy.
Methods:
We studied participants from the 2005–2006 National Health and Nutrition Examination Survey (NHANES, n=6963), a large cohort representative of the US population (age 1–84 years). We built logistic regression models to test for associations between house dust endotoxin and sensitization to specific foods (milk, egg, and peanut). To experimentally explore the detected epidemiologic associations, peripheral blood mononuclear cells (PBMCs) were collected from 21 children (age 1–19 years) mono-food allergic (i.e. sensitized and clinically reactive) to milk, egg, or peanut and non-allergic controls for stimulation with endotoxin and secreted cytokine measurement. For each food allergy, linear mixed effects models were built to test the association between endotoxin stimulation and cytokine level.
Results:
Among NHANES subjects, the geometric mean household endotoxin level was 15.5 EU/mg (GSE 0.5). Prevalence of food allergen sensitization (sIgE>0.35 kUA/L) varied by food: milk 5.7%, egg 4.0%, and peanut 7.9%. In models adjusted for potential confounders (age, race, country of birth, total people per household, US region, and history of wheezing in the past year), household endotoxin level was associated with sensitization to milk (OR 1.7, 95%CI 1.2–2.1) and egg (OR 1.4, 95%CI 1.01–1.9), but not peanut (OR 0.98, 95%CI 0.8–1.2). Interferon-γ levels of endotoxin-stimulated PBMCs from children allergic to milk or egg, but not peanut, were significantly lower compared to controls in linear mixed effects models adjusted for repeated measures, experimental variables, age, and inter-individual variability (P-values 0.007, 0.018, and 0.058, respectively,).
Conclusion:
Higher household endotoxin is associated with increased odds of milk and egg sensitization. Altered cytokine responsiveness to endotoxin is also observed in PBMCs from individuals with milk and egg allergy.
Keywords: cytokine, endotoxin, food allergy, food sensitization, house dust
Introduction
The prevalence of food allergy in children has been increasing in recent years and now affects 8% of children.1,2 Environmental factors could be contributing to this increase, including household levels of endotoxin. Endotoxins are lipopolysaccharide (LPS) molecules that form a component in the outer cell walls of Gram-negative bacteria. They are found at varying levels in household dust and ambient air.3 Although the relationship between endotoxin and asthma has been frequently studied3–5, there have been fewer studies on endotoxin and food allergy-related outcomes.6–7
Studies of endotoxin and atopic outcomes to date have yielded heterogeneous results. Higher levels of household endotoxin have been associated with increased asthma prevalence and wheezing, suggesting risk from endotoxin exposure.3 In contrast, exposure to endotoxin in early life, particularly in rural areas, has also been associated with lower asthma prevalence.5 Other studies of endotoxin level and hay fever or eczema outcomes have shown mixed results.4
Within food allergy, the effects reported for endotoxin have also been disparate. In one cohort, higher levels of bedroom endotoxin during the first year of life were found to be protective for the development of overall food allergy and egg allergy.6 On the other hand, a prior study found no significant relationship between endotoxin levels and egg or milk sensitization, with the exception of increased egg sensitization among those 50 years and older with higher household endotoxin levels.7
Advancing our understanding of the relationship between endotoxin exposure and food allergy could contribute to prevention efforts and further our mechanistic understanding of environmental influences on food allergy. Here we present a complementary epidemiologic and experimental study of endotoxin and food allergy outcomes.
We hypothesized that the epidemiologic association between household endotoxin and food allergen sensitization would vary by food allergen. Complementary to this, we also hypothesized that endotoxin stimulation of peripheral blood mononuclear cells from children with food allergy would yield cytokine responses that vary by specific food allergy. We first evaluated the association between house dust endotoxin levels and food allergen sensitization to milk, egg and peanut among 6963 participants of the National Health and Nutrition Examination Survey (NHANES, n=6963), a large cohort representative of the United States (US) population. We then performed complementary experimental work to assess differences in peripheral blood mononuclear cells (PBMC) response to endotoxin based on food allergy status using PBMCs collected from food allergic children.
Methods
Epidemiologic study population
We studied participants from the 2005–2006 National Health and Nutrition Examination Survey (NHANES), a US cross-sectional study conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention. This survey encompassed 10,348 US participants, ages 1 year and older. Sampling was designed to be representative of the US population. The study protocol and procedures were approved by the NCHS Institutional Review Board with informed consent obtained from all subjects.8 Details on study design and methods can be found on the NHANES website (http://www.cdc.gov/nchs/nhanes.htm).9
Because prior studies demonstrated variation in food allergen sensitization by US region as well as level of urbanization among NHANES 2005–2006 participants,10 we obtained access to restricted region-level data for this study by submitting a proposal to the NCHS Research Data Center and then accessing the 2005–2006 NHANES data on-site at the Hyattsville, MD, USA site of the NCHS. NCHS does not make region-level data publicly available to protect the confidentiality of NHANES participants.
Household endotoxin level measurement
Household endotoxin was collected from each participant’s bed and bedroom floor using a standardized protocol and trained technicians.11 A Sanitaire™ Model 3683 vacuum cleaner and Mitest™ Dust Collector (Indoor Biotechnologies, Inc., Charlottesville, VA) were used to collect samples. One-square yard of both the bed and adjacent floors were vacuumed for a total of 2 minutes. Dust specimens were sent to a laboratory at the University of Iowa, stored at −80°C, and dust endotoxin levels were measured using Limulus amebocyte lysate assay. A kinetic chromogenic assay was used to measure dust extracts, and endotoxin from E. coli 0155 was used to establish standard curves.11
House dust endotoxin measurements were available for 6,963 NHANES 2005–2006 participants. Endotoxin level in endotoxin units (EU) per milligram of sieved dust (EU/mg) was log10 transformed for analysis due to its skewed distribution. Summary statistics are reported as the geometric mean per sieved weight of house dust, consistent with many previous studies of endotoxin.4,7 While some studies have examined endotoxin load (i.e. endotoxin mass per area vacuumed12), studies of NHANES and other cohorts4,6,7,13–16 have focused on endotoxin mass concentration, which is endotoxin per mass of dust. Perzanowski et al. demonstrated that the only allergic health effects (eczema, wheezing) significantly associated with endotoxin were observed with concentration of endotoxin and not load.12 The following formula was used to calculate the endotoxin amount per sieved dust weight: (Endotoxin EU/ml)*(1ml/50 mg dust) = EU/mg dust.11
Food allergen sensitization
Serum-specific IgE levels were measured using Thermo Scientific ImmunoCAP Specific IgE.17,18 Serum-specific IgE levels for cow’s milk, hen’s egg white, and peanut were measured for study participants age 1 year and older. A serum-specific IgE level ≥ 0.35 kUA/L was considered to be positive for food allergen sensitization. Milk, egg, and peanut serum IgE levels were dichotomized for analysis, e.g. not milk sensitized (milk serum IgE <0.35 kUA/L) and milk sensitized (milk serum IgE >0.35 kUA/L). Although food allergy sensitization was measured, clinical symptoms of food allergy were not assessed in NHANES 2005–2006 participants.
Statistical analyses of the epidemiologic data
To account for the complex sampling and survey design, survey weights and strata were used in accordance with the NHANES Analytic and Reporting Guidelines.19 Comparisons among the study population were completed using Student’s t-test to compare means, and Pearson’s chi-squared test to compare proportions.
To assess the relationship between log10-transformed endotoxin level and food allergen sensitization (each for milk, egg, and peanut), food allergen sensitization was fitted into logistic regression models as the dependent variable with log10-transformed endotoxin as a continuous, independent variable. Three separate models were created with milk, egg, or peanut sensitization as the dependent variable. Based on prior literature, the following variables (assessed by participant questionnaires) were considered as potential confounders: age4 (as a continuous variable), gender, race, country of birth, total number of people per household (as a continuous variable), time in US, socio-economic status, region of US,10 level of urbanization, and indicators of other atopic disease (history of wheezing in the past year, doctor’s diagnosis of asthma, family history of asthma, hay fever symptoms in the past year, doctor’s diagnosis of hay fever, an itchy rash coming and going for 6 or more months, and doctor’s diagnosis of eczema). Socio-economic status was represented by a poverty-index ratio (PIR), which is the ratio of income to the family’s poverty threshold. A low PIR of less than 1.00 is below the official poverty threshold.20 A family with a PIR of less than 1.85 qualifies for US federal food assistance.4 The following variables were found to be associated with both endotoxin concentration and food allergen sensitization (i.e. confounders), and they were included in the multivariable logistic regression model to further assess the association between endotoxin concentration and specific food allergen sensitization: age, race, country of birth, total people per household, US region, and history of wheezing in the past year.
Analyses were conducted using STATA 11 and 13 (StataCorp, College Station, TX).
Study population for the experimental study
To complement findings from our epidemiologic analysis of NHANES subjects, we collected peripheral blood samples from healthy, non-atopic children and children who were mono-food allergic to either milk, egg, or peanut (i.e. each was singly food allergic to milk only, egg only, or peanut only) for the experimental studies described below. These children were recruited from pediatrics clinics within the Mount Sinai Health System (New York, NY, USA). The study was approved by the Icahn School of Medicine at Mount Sinai Institutional Review Board, with each participant or legal guardian providing written informed consent. Five subjects were recruited for each food-allergic group (milk, egg, and peanut) along with six non-atopic, healthy controls of similar age for a total of 21 subjects. Non-atopic, healthy controls had no clinical history of food allergy, allergic rhinoconjunctivitis, atopic dermatitis, asthma, drug allergy, or venom allergy, and all healthy controls were each within 1 year of age of food-allergic subjects. Subjects with food allergy to milk, egg, or peanut were diagnosed by a Mount Sinai pediatric allergist as determined by positive oral food challenge or history of convincing symptoms with positive skin prick testing and/or elevated serum-specific IgE levels for that food. Participants with multiple food allergies or a concurrent diagnosis of drug allergy, venom allergy, or the following conditions active within the past 12 months were excluded: allergic rhinoconjunctivitis, atopic dermatitis, or asthma. None of the selected participants had symptoms or signs of any environmental allergy (i.e. no reported symptoms or signs of allergic rhinoconjunctivitis in the past 12 months as determined by a pediatric allergist).
Endotoxin stimulation of PBMCs from milk, egg, and peanut allergic subjects
Peripheral blood mononuclear cells (PBMCs) were isolated from each study subject’s blood sample by Ficoll-Paque PLUS (GE Healthcare, Uppsala, Sweden) density centrifugation and stimulated with two concentrations of endotoxin (LPS 10 ng/ml and 100 ng/ml) and media (RPMI/10%FCS), each in duplicate. Supernatants were collected at 24 hours, 48 hours, and 5 days. The following cytokine levels were measured using a multiplex assay (Millipore™ Luminex®, magnetic beads): interferon (IFN)-γ, IL-6, IL-12, IL-4, and IL-5.
Differences in cytokine response were compared between food allergic subjects (each for milk, egg, and peanut) and non-allergic healthy controls. For each food allergy, data were analyzed using linear mixed effects models (xtmixed) to allow for repeated measurements of cytokines for each subject and multiple incubation time periods and endotoxin stimulation concentrations to be included within the same model. Cytokine level was modeled as the dependent variable and the following were included as independent or fixed effect variables: food allergy status, subject age, endotoxin concentration (LPS 0 ng/ml (media only), LPS 10 ng/ml, LPS 100 ng/ml), length of incubation (24 hours, 48 hours, 5 days), and presence of duplicates. To account for inter-individual differences, subject was included as a random-effects parameter.
Results
Study population for the epidemiologic analysis
Baseline characteristics of the NHANES 2005–2006 subjects examined in this study (n=6963) are summarized in Table I. There were approximately equal proportions of males and females, and the majority of subjects were adults (75.4%) and living above the poverty income ratio (67.2%). The subjects were largely non-Hispanic white and non-Hispanic black; most Hispanics identified as Mexican American. The number of people per household ranged from 1 (10.7%) and 2 (25.4%) to 3 (20.2%), 4 (20.1%), and ≥ 5 (23.7%). Subjects lived in all regions of the United States, with 71.6% based in urban areas.
Table I.
Baseline Characteristics of the NHANES 2005–2006 Subjects
| All | Milk | Egg | Peanut | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N=6963 | Sensitized N=350 | Non-Sensitized N=5749 | P-value | Sensitized N=248 | Non-Sensitized N=5923 | P-value | Sensitized N=489 | Non-Sensitized N=5690 | P-value | |
| Age | <0.0001 | <0.0001 | 0.023 | |||||||
| 1–5 years | 480 (7.0%) | 72 (20.6%) | 259 (4.5%) | 47 (19.0%) | 290 (4.9%) | 27 (5.5%) | 313 (5.5%) | |||
| 6–17 years | 1206 (17.6%) | 87 (24.8%) | 943 (16.4%) | 41 (16.7%) | 1007 (17.0%) | 109 (22.2%) | 939 (16.5%) | |||
| ≥18 years | 5166 (75.4%) | 191 (54.6%) | 4547 (79.1%) | 159 (64.3%) | 4632 (78.2%) | 354 (72.3%) | 4444 (78.1%) | |||
| Gender - Female | 3558 (51.1%) | 159 (45.4%) | 2949 (51.3%) | 0.17 | 127 (51.1%) | 3021 (51.0%) | 0.99 | 175 (35.7%) | 2976 (52.3%) | 0.0006 |
| Race | 0.11 | 0.56 | 0.0001 | |||||||
| Non-Hispanic white | 4742 (68.1%) | 215 (61.4%) | 3996 (69.5%) | 177 (71.2%) | 4081 (68.9%) | 270 (55.2%) | 3989 (70.1%) | |||
| Other Hispanic | 251 (3.6%) | 17 (4.8%) | 201 (3.5%) | 7 (2.7%) | 213 (3.6%) | 25 (5.2%) | 193 (3.4%) | |||
| Mexican-American | 655 (9.4%) | 35 (10.0%) | 540 (9.4%) | 19 (7.7%) | 557 (9.4%) | 61 (12.5%) | 518 (9.1%) | |||
| Non-Hispanic Black | 856 (12.3%) | 53 (15.1%) | 673 (11.7%) | 27 (10.8%) | 711 (12.0%) | 99 (20.3%) | 637 (11.2%) | |||
| Other | 453 (6.5%) | 30 (8.7%) | 351 (6.1%) | 19 (7.6%) | 367 (6.2%) | 34 (6.9%) | 353 (6.2%) | |||
| Region | 0.014 | 0.71 | 0.20 | |||||||
| Northeast | 1010 (14.5%) | 40 (12.0%) | 795 (14.8%) | 29 (12.2%) | 807 (14.6%) | 55 (12.1%) | 781 (14.7%) | |||
| Midwest | 1769 (25.4%) | 74 (22.4%) | 1364(25.4%) | 62 (26.3%) | 1376 (24.9%) | 89 (19.5%) | 1350 (25.4%) | |||
| South | 2033 (29.2%) | 138 (41.9%) | 1499 (27.9%) | 80 (34.0%) | 1609 (29.1%) | 153 (33.5%) | 1541 (29.0%) | |||
| West | 2159 (31.0%) | 78 (23.7%) | 1719 (32.0%) | 65 (27.5%) | 1736 (31.4%) | 160 (34.9%) | 1642 (30.9%) | |||
| Urban | 4986 (71.6%) | 194 (63.4%) | 3644 (71.6%) | 0.037 | 141 (68.3%) | 3738 (71.2%) | 0.45 | 342 (77.7%) | 3546 (70.6%) | 0.037 |
| Number of People per Household | 3.4 (1.6) | 3.6 (1.9) | 3.3 (1.6) | 0.028 | 3.3 (1.6) | 3.3 (1.6) | 0.99 | 3.7 (1.8) | 3.3 (1.6) | 0.010 |
| Country of Birth | 0.0003 | 0.0002 | 0.20 | |||||||
| United States | 6119 (87.9%) | 337 (96.3%) | 5018 (87.3%) | 241 (97.2%) | 5177 (87.4%) | 417 (85.2%) | 5007 (88.0%) | |||
| Mexico | 313 (4.5%) | 5 (1.5%) | 276 (4.8%) | 5 (2.0%) | 278 (4.7%) | 31 (6.3%) | 256 (4.5%) | |||
| Elsewhere | 529 (7.6%) | 8 (2.3%) | 454 (7.9%) | 2 (0.81%) | 462 (7.8%) | 42 (8.5%) | 427 (7.5%) | |||
| Time in US ≥ 4 years | 4178 (60.0%) | 138 (39.3%) | 3570 (62.1%) | 0.084 | 151 (61.0%) | 3643 (61.5%) | 0.98 | 235 (48.1%) | 3579 (62.9%) | 0.036 |
| Family PIR <1.85 (Poverty) | 2182 (32.8%) | 135 (38.5%) | 1839 (32.0%) | 0.083 | 79 (31.8%) | 1919 (32.4%) | 0.88 | 182 (37.1%) | 1822 (32.0%) | 0.24 |
| Wheezing in Chest in Past Year | 1128 (16.2%) | 79 (22.5%) | 937 (16.3%) | 0.12 | 63 (25.6%) | 971 (16.4%) | 0.014 | 118 (24.1%) | 916 (16.1%) | 0.001 |
Number (%) or Mean (Standard Deviation)
Sensitized = Serum specific IgE ≥ 0.35 kUA/L; Non-Sensitized = Serum specific IgE <0.35 kUA/L
Means were compared with Student’s t-test, and proportions were compared with Pearson’s Chi-squared test.
PIR = Poverty Income Ratio (the ratio of income to the family’s poverty threshold)16
Household Endotoxin Levels
Among all subjects, the geometric mean endotoxin level was 15.5 EU/mg (geometric standard error (GSE) 0.50). Household endotoxin levels differed by age, with the highest geometric mean endotoxin level among children age 1 to 5 years, followed by older children age 6–17 years, and then adults age ≥ 18 years (P<0.0001) (Table II). Racial and regional differences were observed, with the higher average household endotoxin levels among those of Mexican-American background compared to Non-Hispanic whites (P<0.0001), and among subjects born in Mexico relative to those born in the United States (P=0.001) (Table II). Within the US, the Western region had significantly higher household endotoxin levels compared to the Northeast (P=0.045). Lower socioeconomic status (poverty-income-ratio<1.85) and households with 5 or more people also had higher average endotoxin levels (P<0.0001). Because endotoxin has been previously associated with asthma, we also evaluated for wheeze in the past year among the study subjects, finding that those who had wheezed in the past year had higher household endotoxin levels (geometric mean 17.8 EU/mg, GSE 1.27) than those who had not wheezed in the past year (geometric mean 15.2 EU/mg, GSE 0.53) (P=0.04). There were no differences in household endotoxin level by sex, urbanicity, or time living in the United States (Table II).
Table II:
Household Endotoxin Levels of NHANES 2005–2006 Subjects
| Geometric Mean (GSE) in EU/mg* | |
|---|---|
| Overall | 15.5 (0.50) |
| Age | |
| 1–5 years | 29.6 (1.25) |
| 6–17 years | 23.0 (1.37) |
| ≥18 years | 13.2 (0.41) |
| Gender | |
| Female | 15.9 (0.56) |
| Male | 15.2 (0.61) |
| Race | |
| Non-Hispanic white | 14.8 (0.55) |
| Other Hispanic | 14.2 (2.58) |
| Mexican-American | 23.5 (1.17) |
| Non-Hispanic Black | 15.2 (1.35) |
| Other | 16.3 (1.14) |
| Region | |
| Northeast | 14.5 (1.35) |
| Midwest | 14.8 (1.12) |
| South | 13.8 (0.85) |
| West | 18.2 (1.07) |
| Urbanicity | |
| Rural | 16.3 (0.91) |
| Urban | 15.2 (0.65) |
| Number of People per Household | |
| 1 | 12.9 (0.69) |
| 2 | 12.6 (0.95) |
| 3 | 16.6 (1.20) |
| 4 | 14.5 (0.70) |
| ≥5 | 20.9 (1.52) |
| Country of Birth | |
| United States | 15.5 (0.50) |
| Mexico | 22.4 (1.84) |
| Elsewhere | 13.2 (1.19) |
| Time in US | |
| 1–3 years | 17.8 (2.06) |
| 4+ years | 15.5 (1.67) |
| Family PIR | |
| PIR<1.85 (Poverty) | 20.0 (0.81) |
| PIR≥1.85 | 13.5 (0.53) |
Food Allergen Sensitization
13.5% of subjects were sensitized to at least one food allergen (specific IgE ≥ 0.35 kUA/L) among milk, egg, and peanut, with 5.7% sensitized to milk, 4.0% sensitized to egg, and 7.9% sensitized to peanut. As expected, sensitization to each of these food allergens was significantly associated with age (milk P<0.0001, egg P<0.0001, peanut P=0.023), with sensitization most prevalent among children age 1–5 years (22.0%, 14.2%, and 8.0%) and least prevalent among adults (4.1%, 3.4%, and 7.5%) respectively for milk, egg, and peanut.
Additional baseline characteristics of the subjects stratified by milk, egg, and peanut sensitization are summarized in Table I. In addition to age (P<0.0001), milk sensitization was also associated with birth in the US (P=0.0003), higher number of people per household (P=0.028), and residence in the Southern US (P=0.014) and in a rural area (P=0.037) (Table I). There were no significant differences in milk sensitization by gender, race, time in the US, or poverty level. Similar to milk, egg sensitization was also associated with age (P<0.0001) and birth in the US (P=0.0002), but not with other characteristics. Peanut sensitization was associated with multiple demographic factors, including age (P=0.023), male gender (P=0.0006), higher number of people per household (P=0.010), less time living in the US (P=0.036), and residence in an urban area (P=0.037). Racial categories including “Other Hispanic,” “Mexican American,” and “Non-Hispanic Black” had a higher proportion of peanut-sensitized patients (P=0.0001).
Association between household endotoxin levels and sensitization to milk or egg but not peanut
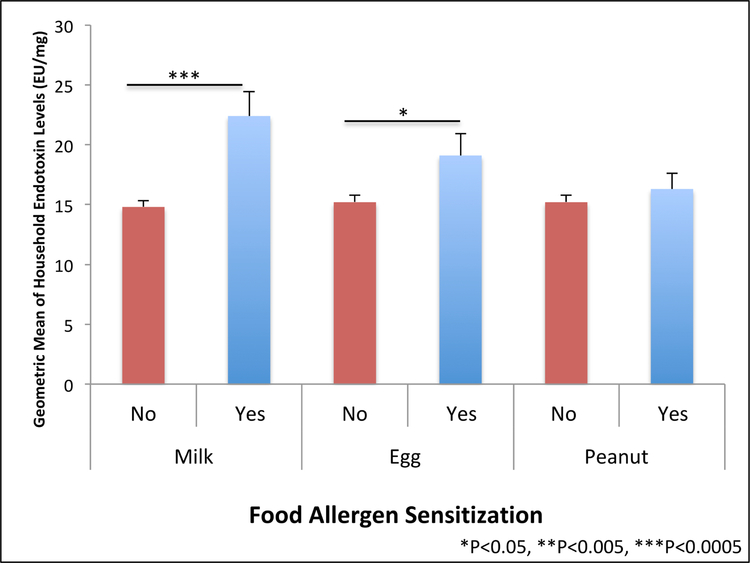
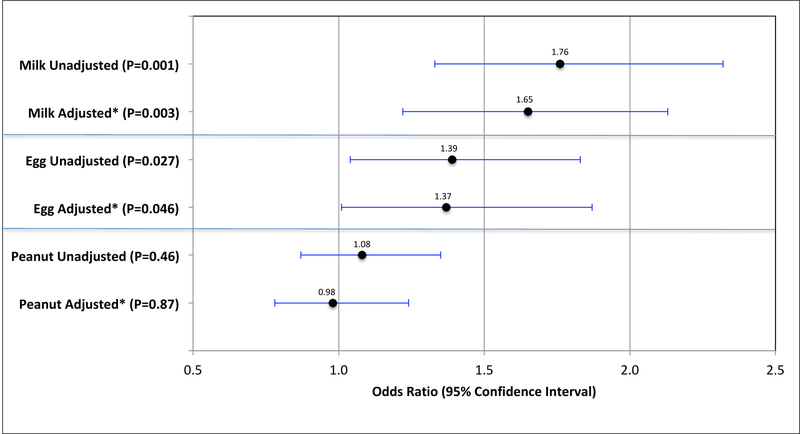
Geometric mean household endotoxin levels were significantly higher among subjects sensitized to milk (22.4 EU/mg, GSE 2.02) versus those without milk sensitization (14.8 EU/mg, GSE 0.55) (t test P<0.0001) (Figure 1). Each 10-fold increment in endotoxin level was associated with 1.8-fold odds (95% CI 1.3–2.3) of sensitization to milk (Figure 2). To account for potential confounders, we built multivariable logistic regression models adjusted for age, race, country of birth, total people per household, US region, and history of wheezing in the past year, finding that each 10-fold increment in endotoxin level was associated with 1.7-fold odds (95% CI 1.2–2.2) of milk sensitization in these adjusted models (Figure 2). Results for the association between endotoxin concentration and food sensitization stratified by age are shown in Supplementary Table E1.
FIG 1.
Geometric mean of household endotoxin levels by food allergen sensitization among NHANES 2005–2006 subjects (n=6963). Food allergen sensitization defined as serum specific IgE >0.35 kUA/L. Error bars depict geometric standard error.
FIG 2.
Odds of food allergen sensitization with each 10-fold increase in household endotoxin level among NHANES 2005–2006 subjects (n=6963). *Adjusted for: age, race, birth country, total people in household, region of US, and wheezing in the past year.
The results for household endotoxin levels among egg-sensitized subjects followed the same direction as for milk sensitization. Geometric mean household endotoxin levels were higher among subjects sensitized to egg (19.1 EU/mg, GSE 1.83) versus those without egg sensitization (15.2 EU/mg, SD 0.56) (t test P=0.027) (Figure 1). Each 10-fold increment in endotoxin level was associated with 1.4-fold odds (95% CI 1.04–1.8) of egg sensitization, and multivariable logistic regression models adjusted for the same potential confounders yield an adjusted OR of 1.4 (95% CI 1.01–1.9) (Figure 2).
Interestingly, household endotoxin levels were not significantly different between those sensitized and not sensitized to peanut (t test P=0.46) (Figure 1), and there were no significant associations between household endotoxin level and peanut sensitization in the unadjusted (OR 1.1, 95% CI 0.9–1.4) or adjusted (aOR 0.98, 95% CI 0.8–1.2) models (Figure 2).
Study population for the experimental study
Inspired by our epidemiologic findings of varying associations between household endotoxin level and specific food allergen sensitization, we next performed complementary, laboratory-based experiments to examine the effects of endotoxin stimulation on peripheral blood mononuclear cells (PBMCs) from 21 children mono-food allergic to milk, egg, or peanut, and healthy controls. Specifically, we obtained peripheral blood samples from these 21 children, isolated PBMCs from these samples, and stimulated them with endotoxin (LPS). The baseline characteristics of these subjects are shown in Table III. Among all 21 subjects, 52% were female and the mean age was 8.0 years (SD 5.4, range 1–19 years). There were no significant differences in age (P=0.93) or gender (P=0.83) between the healthy controls and food allergic subjects.
Table III.
Characteristics of the Experimental Study Population
| Healthy Control (n=6) |
Peanut Allergic (n=5) |
Milk Allergic (n=5) |
Egg Allergic (n=5) |
|
|---|---|---|---|---|
| Female | 4 (67%) | 2 (40%) | 3 (60%) | 2 (40%) |
| Age: years | 7.8 (6.2) | 5.6 (3.0) | 11.6 (6.6) | 7.0 (4.1) |
| sIgE* (kUA/L) | n/a | 14.7 (19.0) | 44.8 (51.9) | 52.2 (48.2) |
| SPT* (wheal mm) | n/a | 11.3 (4.7) | 7.7 (1.5) | 8.8 (2.2) |
|
PBMC yield
(#cells × 106/1 ml) |
1.3 (0.77) | 1.5 (0.79) | 1.1 (0.36) | 1.0 (0.18) |
To respective allergen
Mean (SD) or number (%) indicated
Endotoxin stimulation of PBMCs from milk, egg, and peanut allergic subjects
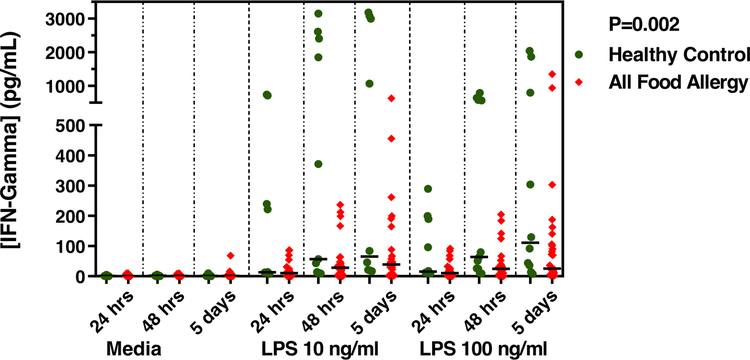
Among all 21 subjects, the median yield of PBMCs was 1.04 × 106 cells/ml (Interquartile range (IQR) 0.94 × 106 to 1.36 × 106) with no significant difference in yield (P= 0.77) in subjects with any food allergy (mean 1.18 × 106 cells/ml, SD 0.52 × 106) vs. healthy control (mean 1.26 × 106 cells/ml, SD 0.77 × 106) (Table III). PBMCs from each subject were stimulated with 0, 10, and 100 ng/ml endotoxin each for 24 hours, 48 hours, and 5 days of incubation, and each in duplicate. The levels of the cytokines in the supernatants under each of these conditions are shown in Supplementary Table E2. Linear mixed effects modeling of these cytokine levels accounting for stimulation with variable endotoxin concentration, multiple lengths of incubation, duplicates, as well as subject age and inter-individual effects within each model revealed that higher levels of endotoxin exposure was associated with lower IFN-γ level production by PBMCs from children with any food allergy (milk, egg, or peanut) relative to non-allergic healthy controls (Coefficient −347.8 pg/mL, 95% CI −572.4 to −123.2 pg/mL, P=0.002) (Figure 3).
FIG 3.
Interferon-γ levels (pg/mL) following stimulation with endotoxin of PBMCs from healthy non-allergic children (n=6) and children with food allergy to milk, egg, or peanut (n=15). Hours/days shown are length of incubation with media or endotoxin (lipopolysaccharide (LPS)) at 10 ng/ml and 100 ng/ml. P-value calculated using linear mixed effects model adjusted for age, length of incubation, stimulus (media, LPS 10 ng/ml, LPS 100 ng/ml), and presence of duplicates. Medians indicated by black lines.
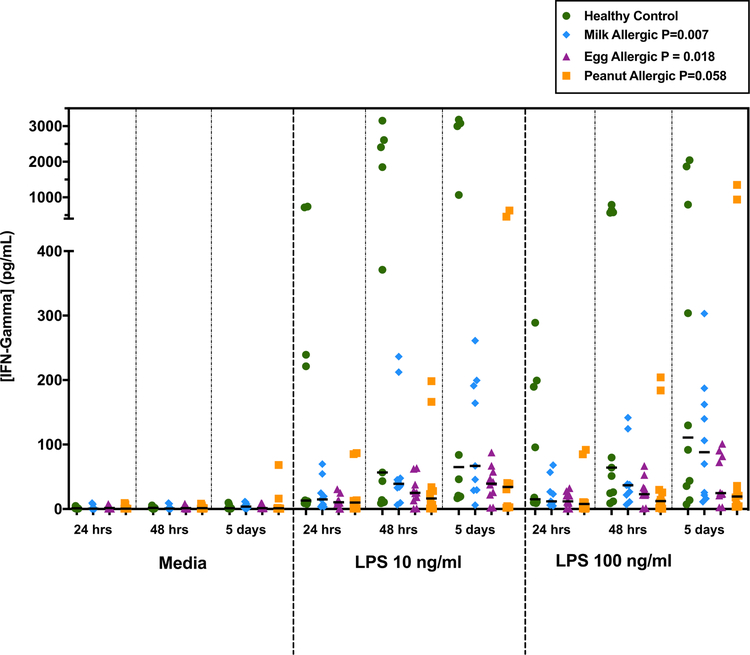
Linear mixed model results for endotoxin-stimulated cytokine production for each individual food allergy showed significant results for IFN-γ among children with milk and egg allergy, but not those with peanut allergy. Specifically, IFN-γ production by PBMCs from milk allergic patients (Coefficient −415.8 pg/mL, 95% CI −720.5 to −111.1 pg/mL, P=0.007) and egg allergic patients (Coefficient −349.7 pg/mL, 95% CI −640.1 to −59.4 pg/mL, P=0.018) were each significantly lower than that by healthy controls (Figure 4). Among peanut allergic subjects, IFN-γ levels trended toward lower levels than healthy controls but were not significant (Coefficient −283.8 pg/mL, 95% CI −577.1 to 9.6 pg/mL, P=0.058) (Figure 4). The other Th1-related cytokines IL6 and IL12, trended toward decreased levels in response to endotoxin across milk, egg, and peanut allergic subjects, but these effects did not reach statistical significance in linear mixed effects models (IL-6: milk P=0.066, egg P=0.23, peanut P=0.42) and IL-12: milk P=0.49, egg P=0.38, peanut P=0.87) (Supplementary Table E2). Conversely, the Th2 cytokines, IL-4 and IL-5, trended toward increased levels in response to endotoxin across milk, egg, and peanut allergic subjects, but these trends were also not statistically significant (IL-4: milk P=0.63, egg P=0.68, peanut P=0.54 and IL-5: milk P=0.63, egg P=0.74, peanut P=0.31) (Supplementary Table E2).
FIG 4.
Interferon-γ levels (pg/mL) following stimulation with endotoxin of PBMCs from healthy non-allergic children (n=6) and children mono-food allergic to milk (blue points, n=5), egg (purple points, n=5), and peanut (orange points, n=5). Hours/days shown are length of incubation with media or endotoxin (lipopolysaccharide (LPS)) at 10 ng/ml and 100 ng/ml. P-value calculated using linear mixed effects model adjusted for age, length of incubation, stimulus (media, LPS 10 ng/ml, LPS 100 ng/ml), and presence of duplicates. Medians indicated by black lines.
Discussion
The results from this complementary epidemiologic and experimental study suggest that the associations between endotoxin, food allergen sensitization, and food allergy can differ by individual food allergen. Among 6963 children and adults from the 2005–2006 NHANES cohort, higher household endotoxin levels were associated with increased odds of sensitization to milk and egg, but not peanut, in both unadjusted models and models adjusted for potential confounders. In parallel to these epidemiologic findings, our experimental study of cytokine production by endotoxin-stimulated PBMCs from 21 milk, egg, and peanut allergic children and healthy controls showed that production of IFN-γ was significantly decreased for milk-allergic and egg-allergic children relative to healthy non-allergic controls. While peanut-allergic children trended towards an attenuated IFN-γ response, this difference was not significant. The results from our epidemiologic study of the NHANES population, along with our experimental results from a targeted study of 21 children mono-food allergic to milk, egg, or peanut and controls, particularly for IFN-γ, prompt consideration that individual food allergen sensitizations and allergies may have differing associations with endotoxin exposure.
Few studies have evaluated the relationship between endotoxin exposure and food allergen sensitization. Our results differ from an earlier study of the NHANES 2005–2006 cohort that reported an association between higher household endotoxin levels and egg sensitization only among adults ages 50 years and older.7 The study targeted allergen sensitization to all allergens (environmental and food), and the data examined were limited to approximately 5200 NHANES participants who were 6 years and older, where participants were stratified into 3 age groups (children, adults, and older adults) that were each analyzed separately.7 In contrast, we included 6963 NHANES subjects age 1 year and older and employed multivariable regression adjusted for age and other confounders. As sensitization and food allergy are more prevalent in younger children,21 it was important to include these younger subjects in a study of food allergen sensitization, and we controlled for age in our models in addition to considering possible effect modification by age. Furthermore, our focus on food allergen sensitization in particular allowed us to incorporate potential confounders specifically relevant to food allergen sensitization.
In contrast, an independent study of 516 children from an inner-city birth cohort suggested that household endotoxin levels have a protective association with the development of food allergies in children.6 Food allergy was defined by positive serum-specific IgE (≥0.35 kUA/L) to milk, egg, or peanut and clinical confirmation, which was met by 51 children out of the 516 children included in the study. Higher bedroom endotoxin levels collected at 3 months of life were significantly associated with decreased odds of overall food allergies (milk, egg or peanut) and egg allergy by 5 years of age. There was no significant association between endotoxin levels and the development of milk or peanut allergy. As NHANES 2005–2006 does not include children younger than age 1 year, our study does not encompass household endotoxin levels during infancy.
The potential reasons for effects for milk and egg sensitization/allergy distinct from peanut are interesting to consider and merit further study. On a rudimentary level, cow’s milk and hen’s egg are animal-based, versus peanut, which is plant-based. Clinically, milk and egg allergy are more likely to be outgrown in comparison to peanut allergy.21 This is the first study to compare responses to endotoxin between milk, egg, and peanut allergic subjects. There are differences in the epidemiology and natural history of these specific food allergies, and our results suggest that endotoxin response may differ as well. Further experimental studies with larger sample sizes will be needed to investigate the mechanistic underpinnings of these findings.
We found positive associations between household endotoxin level and sensitization to milk or egg, suggesting that endotoxin is a risk-conferring factor. Our finding is both consistent and contrasting to previous studies of endotoxin and allergy-related outcomes more broadly. A Canadian study found that bedroom endotoxin levels were protective for asthma in children age 6–12 years.22 This protective effect was not noted in children older than 12 years, however. In a longitudinal birth cohort in the US, increased home endotoxin levels in infancy were associated with decreased eczema by age 1 year, reduced risk of environmental allergen sensitization by school age, and decreased IL-13 production by allergen stimulated PBMCs.23–25 In this same cohort, prolonged exposure to endotoxin was also shown to be protective with reduced odds of asthma in children with repeated measurements of high endotoxin levels in infancy and at school age.26 Another birth cohort study of an inner-city population reported that children with higher endotoxin concentrations in the home were less likely to have eczema at age 1 year, but were more like to have wheezed within the past year at age 2 years.12 In a cross-sectional study of children from rural areas in Austria, Germany, Switzerland, infant exposure to stables and drinking of farm milk (i.e. higher endotoxin levels) was associated with lower frequencies of asthma, hay fever, and atopic sensitization relative to later stable exposure in childhood without infant exposure.5 Two-thirds of the mothers of these infants who had been exposed to the farming environment within the first year of life were also active on the farm during their pregnancies. 5 Thus, endotoxin exposure prenatally and in infancy could be protective, while higher household endotoxin levels later in life could be a risk factor for allergy-related outcomes, including food allergen sensitization, particularly to milk and egg, as we found in this study.
In addition to age of exposure, studies of asthma have shown that differences in the effects of endotoxin can also be attributed to genetic variability.27–28 For example, among subjects with a specific genetic polymorphism in ACAA1 (SNP rs156265) exposure to higher household endotoxin levels confers protection against asthma.29 Therefore, varying effects of endotoxin on atopy-related outcomes could be attributed not only to age of exposure but also gene-by-environment interactions.
Parallel to our epidemiologic results of a positive association between household endotoxin level and milk and egg sensitization among NHANES participants, we further demonstrated that the Th1 cytokine response, IFN-γ, was attenuated in endotoxin-stimulated PBMCs from children with food allergies, particularly those allergic to milk and egg. IFN-γ was the only cytokine for which significant effects were observed. IFN-γ is a key mediator of Th1 response and plays a crucial role in isotype switching and IgE regulation.30 Recent studies evaluating novel therapies for food allergy use IFN-γ as an outcome measure for evaluating response to therapy31 and even as adjuvant therapy for food immunotherapy.32
Several studies have previously shown that allergic children have immature Th1 function in comparison to non-allergic children.33–34 Most recently, Tulic et al. found differences in responsiveness to TLR ligands, including endotoxin, between allergic (eczema, food allergy, asthma, or positive sensitization) and non-allergic children.35 The innate cytokine response (IL-1β, IL-6, TNF-α, and IL-12) was found to be initially hyper-responsive at birth (cord blood) among allergic children in comparison to non-allergic children, but the response to endotoxin and other TLR ligands became attenuated by 5 years of age. IFN-γ responses to allergens and mitogens were also decreased for allergic children during the first 5 years of life, suggesting a lack of appropriate maturation of Th1 function. Even though the inflammatory response to microbes was initially strong in newborns, the Th1 response to allergens became weaker and the Th2 response became predominant with age.36 This increased inflammatory response at birth has also been shown specifically among food allergic children in comparison to children without food allergies.37 Zhang et al. reported that CD14+ monocytes from cord blood exposed to endotoxin (LPS) express higher amounts of inflammatory cytokines, specifically IL-1β, IL-6, and TNF-α in children who later developed confirmed food allergies, demonstrating that these food allergic children had increased monocyte responsiveness at the time of birth. These inflammatory cytokines have been shown to lead to non-classic Th2 differentiation and impaired naive Treg function, a possible mechanism in which endotoxin exposure could affect the development of food allergies. As our population was focused specifically on children with food allergies ages 1 year and older and no cord blood was collected, we also demonstrated an attenuated IFN-γ production, representing a weakened Th1 response to endotoxin among food-allergic children compared to non-allergic controls.
There were some limitations to this study. Data from NHANES 2005–2006 are cross-sectional with household endotoxin level and health outcome variables measured at the same time, rendering it challenging to determine causal associations. However, a strength of studying this population is that it is based on a large sample intended to be representative of the US population. While specific assessment of food allergy within NHANES would have been ideal, and food allergen sensitization does not equate a clinical diagnosis of food allergy, there was no information collected in NHANES 2005–2006 on food-related allergic reactions. To partially address this limitation and assess the degree to which findings from NHANES might be relevant to outcomes in children with clinical food allergy, we undertook experimental studies of endotoxin-stimulated PBMCs from twenty-one children with clinical food allergy based on allergist diagnosis and confirmatory allergy testing. Although the NHANES study population included adults and children and was predominantly adult, and our experimental study population included only children and a more limited sample size, the results from both our epidemiologic and experimental studies consistently highlighted differences in those sensitized or allergic to milk or egg specifically.
In summary, this is a unique study evaluating the relationship between endotoxin and sensitization and allergy to specific food allergens (milk, egg, and peanut) using a combined epidemiologic and experimental approach in two different populations. Among NHANES subjects representative of the US population, elevated household endotoxin levels were significantly associated with increased odds of milk and egg sensitization, but not peanut sensitization. In complementary experimental studies of PBMCs from children with food allergy, IFN-γ production in response to endotoxin stimulation was attenuated among food allergic children relative to healthy non-allergic controls. Consistent with our epidemiologic results, children with allergy to milk or egg produced significantly less IFN-γ in response to endotoxin, while differences were not significant among peanut allergic children. The results from this and prior studies suggest that the relationship between endotoxin and food sensitization/allergy is complex with differing effects not only dependent on age of exposure and genetic variation as suggested by prior work, but also on the type of food allergy.
Supplementary Material
Acknowledgements:
We thank Dieudonne Nahigombeye, MS, statistician for the CDC/National Center for Health Statistics, Research Data Center, for his assistance in the application process to access the restricted NHANES data. We thank Jade Andrade for assistance with subject recruitment. We also thank the children and their parents who participated in this study.
Funding Source: Funding for this project was awarded in part by the Pediatric Scholars Program from the Department of Pediatrics, Icahn School of Medicine at Mount Sinai. AT is supported in part by The Louis and Rachel Rudin Foundation, Inc. SB, AG, GG, and AD are supported in part by NIH R01 AI118833 and U19 AI136053.
Abbreviations:
- CI
confidence interval
- GSE
geometric standard error
- IFN
interferon
- IL
interleukin
- IQR
interquartile range
- LPS
lipopolysaccharide
- NCHS
National Center for Health Statistics
- NHANES
National Health and Nutrition Examination Survey
- OR
odds ratio
- PBMCs
peripheral blood mononuclear cells
- SD
standard deviation
- TLR
toll-like receptor
Footnotes
Conflict of Interest
The authors have no conflicts to disclose.
References
- 1.Sicherer SH, Sampson HA. Food Allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention and management. J Allergy Clin Immunol 2018;141:41–58. [DOI] [PubMed] [Google Scholar]
- 2.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 2011;128:9–17. [DOI] [PubMed] [Google Scholar]
- 3.Thorne PS, Cohn RD, Mav D, Arbes SJ, Zeldin DC. Predictors of endotoxin levels in US housing. Environ Health Perspect 2009;117:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorne PS, Mendy A, Metwali N, Salo P, Co C, Jaramillo R, et al. Endotoxin Exposure: Predictors and Prevalence of Associated Asthma Outcomes in the United States. Am J Respir Crit Care Med 2015;192:1287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riedler J, Braun-Fahrländer C, Eder W, Schreuer M, Waser M, Maisch S, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet 2001;358:1129–33. [DOI] [PubMed] [Google Scholar]
- 6.McGowan EC, Bloomberg GR, Gergen PJ, Visness CM, Jaffee KF, Sandel M, et al. Influence of early-life exposures on food sensitization and food allergy in an inner-city birth cohort. J Allergy Clin Immunol 2015;135:171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Min KB, Min JY. Exposure to household endotoxin and total and allergen-specific IgE in the US population. Environ Pollut 2015;199:148–54. [DOI] [PubMed] [Google Scholar]
- 8.NCHS Research Ethics Review Board (ERB) Approval Hyattsville, MD: National Center for Health Statistics; 2012. Available at https://www.cdc.gov/nchs/nhanes/irba98.htm. Accessed January 1, 2019. [Google Scholar]
- 9.Public data general release file documentation Hyattsville, MD: National Center for Health Statistics; 2005. Available at https://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/general_data_release_doc_05_06.pdf. Accessed January 1, 2019. [Google Scholar]
- 10.Salo PM, Arbes SJ Jr, Jaramillo R, Calatroni A, Weir CH, Sever ML, et al. Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005–2006. J Allergy Clin Immunol 2014;134:350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allergens – Household Dust (ALDUST_D) Hyattsville, MD: National Center for Health Statistics; 2009. Available at https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/ALDUST_D.htm. Accessed January 1, 2019. [Google Scholar]
- 12.Perzanowski MS, Miller RL, Thorne PS, Barr RG, Divjan A, Sheares BJ, et al. Endotoxin in inner-city homes: Associations with wheeze and eczema in early childhood. J Allergy Clin Immunol 2006;117:1082–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connor GT, Lynch SV, Bloomberg GR, Kattan M, Wood RA, Gergen PJ, et al. Early-life home environment and risk of asthma among inner-city children. J Allergy Clin Immunol 2018;141:1468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai PS, Sheehan WJ, Gaffin JM, Petty CR, Coull BA, Gold DR, et al. School Endotoxin Exposure and Asthma Morbidity in Inner-city Children. Chest 2015;148:1251–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnig C, Reboux G, Roussel S, et al. Indoor dust and air concentrations of endotoxin in urban and rural environments. Lett Appl Microbiol 2013;56:161–7. [DOI] [PubMed] [Google Scholar]
- 16.Sheehan WJ, Hoffman EB, Fu C, Baxi SN, Bailey A, King EM, et al. Endotoxin exposure in inner-city schools and homes of children with asthma. Ann Allergy Asthma Immunol 2012;108:418–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laboratory Procedures Manual Elmhurst, IL: 2008. Available at https://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/al_ige_d_met_specific_ige_total_ige.pdf. Accessed January 1, 2019. [Google Scholar]
- 18.Allergen Specific IgE(s) and Total IgE in Serum (AL_IGE_D) Hyattsville, MD: National Center for Health Statistics; 2009. Available at https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/AL_IGE_D.htm. Accessed January 1, 2019. [Google Scholar]
- 19.Analytic and Reporting Guidelines Hyattsville, MD: National Center for Health Statistics; 2005. Available at https://wwwn.cdc.gov/nchs/data/nhanes/2005-2006/nhanes_analytic_guidelines_dec_2005.pdf. Accessed January 1, 2019. [Google Scholar]
- 20.Demographic Variables and Sample Weights (DEMO_D) Hyattsville, MD: National Center for Health Statistics; 2009. Available at https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/DEMO_D.htm. Accessed January 1, 2019. [Google Scholar]
- 21.Savage J, Sicherer S, Wood R. The Natural History of Food Allergy. J Allergy Clin Immunol Pract 2016;4:196–203. [DOI] [PubMed] [Google Scholar]
- 22.Lawson JA, Dosman JA, Rennie DC, Beach JR, Newman SC, Crowe T, et al. Endotoxin as a determinant of asthma and wheeze among rural dwelling children and adolescents: a case-control study. BMC Pulm Med 2012;12:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phipatanakul W, Celedon JC, Raby BA, Litonjua AA, Milton DK, Sredl D, et al. Endotoxin exposure and eczema in the first year of life. Pediatrics 2004;114:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celedon JC, Milton DK, Ramsey CD, Litonjua AA, Ryan L, Platts-Mills TA, et al. Exposure to dust mite allergen and endotoxin in early life and asthma and atopy in childhood. J Allergy Clin Immunol 2007;120:144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abraham JH, Finn PW, Milton DK, Ryan LM, Perkins DL, Gold DR. Infant home endotoxin is associated with reduced allergen-stimulated lymphocyte proliferation and IL-13 production in childhood. J Allergy Clin Immunol 2005;116:431–7. [DOI] [PubMed] [Google Scholar]
- 26.Sordillo JE, Hoffman EB, Celedón JC, Litonjua AA, Milton DK, Gold DR. Multiple microbial exposures in the home may protect against asthma or allergy in childhood. Clin Exp Allergy 2010;40:902–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saçkesen C, Karaaslan C, Keskin O, Tokol N, Tahan F, Civelek E, et al. The effect of polymorphisms at the CD14 promoter and the TLR4 gene on asthma phenotypes in Turkish children with asthma. Allergy 2005; 60:1485–92. [DOI] [PubMed] [Google Scholar]
- 28.Zambelli-Weiner A, Ehrlich E, Stockton ML, Grant AV, Zhang S, Levett PN, et al. Evaluation of the CD14/−260 polymorphism and house dust endotoxin exposure in the Barbados Asthma Genetics Study. J Allergy Clin Immunol 2005;115:1203–9. [DOI] [PubMed] [Google Scholar]
- 29.Sordillo JE, Sharma S, Poon A, Lasky-Su J, Belanger K, Milton DK, et al. Effects of endotoxin exposure on childhood asthma risk are modified by a genetic polymorphism in ACAA1. BMC Med Genet 2011;12:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbas AK, Lichtman AH, Pillai S. Cellular and Molecular Immunology 9th ed. Philadelphia (PA): Elsevier; 2018. [Google Scholar]
- 31.Srivastava KD, Siefert A, Fahmy TM, Caplan MJ, Li XM, Sampson HA. Investigation of peanut oral immunotherapy with CpG/peanut nanoparticles in a murine model of peanut allergy. J Allergy Clin Immunol 2016;138:536–43. [DOI] [PubMed] [Google Scholar]
- 32.Noh G, Jang EH. Dual specific oral tolerance induction using interferon gamma for IgE-mediated anaphylactic food allergy and the dissociation of local skin allergy and systemic oral allergy: tolerance or desensitization? J Investig Allergol Clin Immunol 2014;24:87–97. [PubMed] [Google Scholar]
- 33.Prescott SL, Noakes P, Chow BW, Breckler L, Thornton CA, Hollams EM, et al. Presymptomatic differences in toll-like receptor function in infants who develop allergy. J Allergy Clin Immunol 2008;122:391–9. [DOI] [PubMed] [Google Scholar]
- 34.Schaub B, Liu J, Höppler S, Haug S, Sattler C, Lluis A, et al. Impairment of T-regulatory cells in cord blood of atopic mothers. J Allergy Clin Immunol 2008;121:1491–9. [DOI] [PubMed] [Google Scholar]
- 35.Tulic MK, Hodder M, Forsberg A, McCarthy S, Richman T, D’Vaz N, et al. Differences in innate immune function between allergic and nonallergic children: new insights into immune ontogeny. J Allergy Clin Immunol 2011;127:470–78. [DOI] [PubMed] [Google Scholar]
- 36.Prescott SL, Martino D, Hodder M, Richman T, Tulic MK. Progress in understanding postnatal immune dysregulation in allergic disease. World Allergy Organ J 2010;3:162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Collier F, Naselli G, Saffery R, Tang ML, Allen KJ, et al. Cord blood monocyte-derived inflammatory cytokines suppress IL-2 and induce nonclassic “T(H)2-type” immunity associated with development of food allergy. Sci Transl Med 2016;8:321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.