Abstract
INTRODUCTION:
Some Alzheimer disease biomarker studies found amyloid changes 20 years or more in advance of expected symptoms while cognitive changes lagged for over a decade, but this apparent lag might reflect the sensitivities of the biomarker and cognitive assays used. How far in advance of incident amnestic mild cognitive impairment (MCI) does cognition begin to decline?
METHODS:
Longitudinal neuropsychological study of an apolipoprotein E e4 enriched cohort of cognitively normal individuals at entry. Linear mixed models for MCI converters (n=65) and nonconverters (n=719) fitted for each neuropsychological measure; annual changes compared between groups before and after linear model intersections (inflection points).
RESULTS:
34 of 35 cognitive measures and 9 of 18 behavioral measures declined faster post-inflection in the MCI converters; the earliest cognitive inflection point was nearly 20 years in advance of MCI diagnosis.
DISCUSSION:
The preclinical duration of cognitive and behavioral changes approaches the earliest reported biomarker changes.
Keywords: Pre-MCI, Preclinical Alzheimer’s disease, Preclinical Memory Decline, Cognitive Aging, Age-Related Cognitive Decline
1.0. Background
The transition from normal to pathological cognitive aging is riddled with many questions and controversies. For clinicians, is a modestly suboptimal performance on a mental status test age appropriate or pathological? A related research concern, especially pressing with the rise of preclinical therapeutic trials for Alzheimer’s disease (AD), is determining how long before symptoms develop does pathology begin? Some biomarker studies in autosomal dominant AD (ADAD) kindreds1,2 and in late onset AD (LOAD) cohorts3 found changes related to amyloid 20 years or more in advance of expected symptoms while cognitive changes lagged for over a decade. But might this apparent lag in cognition reflect the relative sensitivity of the various biomarker and cognitive assays used in such studies?
The first evidence of preclinical AD derived from clinical-pathological correlations of cognitively normal elders whose brains harbored cortical amyloid4,5. Similar traces of AD pathology have been identified in younger apolipoprotein E (APOE) e4 carriers6. More recently, biomarker studies in living patients have shown cerebral amyloid burden becomes more prevalent and pronounced with increasing age even in the absence of symptomatic memory loss7,8. Mild cognitive impairment (MCI) represents the earliest symptomatic stage typically of a neurodegenerative dementia and most often AD9,10. MCI was originally anchored to memory but has since been expanded to include essentially any cognitive profile that is thought to represent pathology beyond that of normal aging while still admitting that non-neuropathological causes including normal aging and depression for example are possible in a nontrivial number of cases9.
Whether due to pure AD, another degenerative disease, or a mix of degenerative and vascular pathologies, as a clinical syndrome, MCI is important to recognize as it portends further decline in the majority of patients for whom it is hoped earlier intervention might slow or prevent disease progression to the stage of dementia and ultimately death. Herein we present evidence that cognition begins to decline roughly 20 years before the diagnosis of incident MCI, much earlier than previous studies have shown.
2.0. Methods
2.1. Subjects.
From January 1, 1994 through June 30, 2016, cognitively normal residents of Maricopa County age 21 years and older were recruited through local media ads, underwent APOE genotyping and longitudinal neuropsychological assessment every two years. APOE genotype was determined using Taqman Single Nucleotide Polymorphism assays. All APOE e4 homozygotes were matched by age, sex, and education to one ε4 heterozygote (HTZ; all with the ε3/4 genotype) and two ε4 non-carriers. Additional heterozygous persons and non-carriers who were otherwise eligible for enrollment were also recruited so that roughly half of the entire cohort represents matched quartos while remaining members were unmatched but otherwise fulfilled entry criteria. Screening tests included a medical history (including review of medications in all cases and medical records when provided), neurological examination, the Folstein Mini-Mental Status Exam (MMSE), Hamilton Depression (Ham-D) Rating Scale, Functional Activities Questionnaire (FAQ), Instrumental Activities of Daily Living (IADL), and Structured Psychiatric Interview for DSM-III-R. We excluded anyone with potentially confounding medical, neurologic, or psychiatric problems (essentially any condition that might adversely affect cognitive abilities such as end stage organ disease, stroke, or active major depression). Anyone with a history, self-reported or documented, of a psychotic disorder or psychiatric hospitalization was excluded. As expected most participants expressed a personal interest in AD and were introspective about whether their episodes of forgetting meant anything. Such concern was not exclusionary, but subjective memory complaints severe enough to motivate clinical evaluation were exclusionary even if objective testing revealed no impairment. No one met published criteria for MCI9, AD11, other forms of dementia or major depressive disorder12. Entry criteria included scores of at least 27 on the MMSE (with at least 1 of 3 on the recall subtest), 10 or less on the Ham-D, and perfect scores on the FAQ and IADL.
Data were reviewed at each visit and MCI suspected by consensus of two behavioral neurologists (RJC and BKW) and a neuropsychologist (DECL) in those individuals who endorsed symptoms of memory loss (corroborated by a close informant), and exhibited objective decline from previous performance that consistently affected multiple neuropsychological tests within the same cognitive domain13,14. MCI suspects were then invited to complete a clinical neurological evaluation that included standard laboratory and radiological assessments following which a formal diagnosis was made and provided to the patient. The time between completion of neuropsychological testing and ultimate MCI diagnosis varied but was typically between one and two months.
All individuals gave their written, informed consent to participate in the study and have the results of the APOE test withheld from them which was approved by the Mayo Clinic Institutional Review Board.
2.2. Neuropsychological Assessment.
A comprehensive neuropsychological battery was administered every one to two years, and is summarized in the Table 1. Our initial 1994 battery was expanded in 1998. The National Alzheimer’s Coordinating Committee issued the Uniform Data Set (UDS) in 2004 and so UDS tests were added then. The neuropsychological tests (cognitive and behavioral) used within our battery are detailed in reference 15, and encompass four broadly defined cognitive domains (memory, executive skills, language, and visuospatial skills), as well as behavioral measures. Regarding subjective cognition, participants and their informants completed the paired Multidimensional Assessment of Neurodegenerative Symptoms Questionnaire including both self (MANS-Self) and Observer (MANS-Observer) versions16. The MANS are paired self and informant based questionnaires comprised of 87 questions that assess changes over the preceding year in daily habits, personality, and motor functioning. It employs a quantitative scale for rating the frequency of a symptom from 0 (never) to 4 (routinely), with intermediate values of 1 (once), 2 (occasionally), and 3 (more than monthly); scores can range from 0-344 with higher scores indicating more frequent and severe symptoms.
Table 1.
Cognitive and Behavioral Measures
| Test | Score | Major Domain | Year Added |
|---|---|---|---|
| Folstein Minimental State Exam | 0-30 | General | 1994 |
| Mattis Dementia Rating Scale | 0-144 | General | 1998 |
| WAIS-R Information | Age Scaled Score | General | 1994 |
| WAIS-R Vocabulary | Age Scaled Score | Language | 1994 |
| WAIS-R Similarities | Age Scaled Score | Language | 1994 |
| Boston Naming Test | 0-60 | Language | 1994 |
| Token Test | 0-44 | Language | 1994 |
| Judgement of Line Orientation | 0-30 | Visuospatial | 1994 |
| Facial Recognition Test | 0-50 | Visuospatial | 1994 |
| WAIS-R Block Design | Age Scaled Score | Visuospatial | 1994 |
| Complex Figure Test-copy | 0-36 | Visuospatial | 1994 |
| Digit span forward length (from WAIS-R) | Forward Digit Span (absolute number) | Executive | 2004 (UDS) |
| Digit Span backward length (from WAIS-R) | Backward Digit Span (absolute number) | Executive | 2004 (UDS) |
| WAIS-R Mental Arithmetic | Age Scaled Score | Executive | 1994 |
| Paced Auditory Serial Attention Task-3 second | 0-50 | Executive | 1998 |
| Paced Auditory Serial Attention Task-2 second | 0-50 | Executive | 1998 |
| WAIS-R Digit Symbol Substitution | Age Scaled Score | Executive | 1994 |
| Trail Making Test Part A | Seconds to Complete | Executive | 2004 (UDS) |
| Trail Making Test Part B | Seconds to Complete | Executive | 2004 (UDS) |
| Controlled Oral Word Association Test | Total Number of Words | Executive | 1994 |
| Animals Fluency | Total Number of Animals Named | Executive | 2004 (UDS) |
| Vegetable Fluency | Total Number of Vegetables Named | Executive | 2004 (UDS) |
| Wisconsin Card Sorting Test-Total Errors | Total Errors | Executive | 1998 |
| Wisconsin Card Sorting Test-Categories Completed | Categories Completed | Executive | 1998 |
| Wisconsin Card Sorting Test-Perseverative Errors | Number of Perseverative Errors | Executive | 1998 |
| Complex Figure Test-recall | 0-36 | Memory | 1994 |
| Auditory Verbal Learning Test-Total Learning | Number Words Over 5 Trials | Memory | 1994 |
| Auditory Verbal Learning Test-% Recall | % Words Recalled at 30 Minutes | Memory | 1994 |
| Auditory Verbal Learning Test-Long Term Memory | Number of Words Recalled at 30 Minutes | Memory | 1994 |
| Visual Retention Test | Total Correct | Memory | 1994 |
| Selective Reminding Test-Immediate Free Recall | Total Correct Without Delay | Memory | 1998 |
| Selective Reminding Test-Delayed Free Recall | Total Correct After Delay | Memory | 1998 |
| Wechsler Memory Scale-R Logical Memory-Immediate Recall | Absolute Number Story Items | Memory | 2004 (UDS) |
| Wechsler Memory Scale-R Logical Memory-Delayed Recall | Absolute Number Story Items | Memory | 2004 (UDS) |
| Subjective Cognition and Social Standing | |||
| Multidimensional Assessment of Neurodegenerative Symptoms-Informant | 0-348 | Subjective Cognitive Change | 1996 |
| Multidimensional Assessment of Neurodegenerative Symptoms-Self | 0-348 | Subjective Cognitive Change | 1996 |
| Behavioral Measures | |||
| Geriatric Depression ScaleTotal | 0-30 | Depression | 2004 (UDS) |
| Hamilton Depression Scale | 0-49 | Depression | 1994 |
| Beck Depression Inventory | 0-67 | Depression | 1994 |
| Personality Assessment Inventory | |||
| PAI_CSc_Somatization | T score | Somatization | 1998 |
| -PAI_SOM_Conversion | T score | Somatization | 1998 |
| -PAI_SOM_Hypocondriasis | T score | Somatization | 1998 |
| -PAI_SOM_Somatization | T score | Somatization | 1998 |
| PAI_CSc_Anxiety | T score | Anxiety | 1998 |
| -PAI_ANX_A | T score | Anxiety | 1998 |
| -PAI_ANX_C | T score | Anxiety | 1998 |
| -PAI_ANX_P | T score | Anxiety | 1998 |
| PAI_CSc_Anxiety Related Disorders | T score | Anxiety Related Disorders | 1998 |
| -PAI_ARD_Obessive | T score | Anxiety Related Disorders | 1998 |
| -PAI ARD Phobia | T score | Anxiety Related Disorders | 1998 |
| -PAI_ARD_Trauma | T score | Anxiety Related Disorders | 1998 |
| PAI_CSc_Depression | T score | Depression | 1998 |
| -PAI_DEP_Affective | T score | Depression | 1998 |
| -PAI_DEP_Cognitive | T score | Depression | 1998 |
| -PAI_DEP_Physiological | T score | Depression | 1998 |
| PAI_CSc_Mania | T score | Mania | 1998 |
| -PAI_MAN_Affective | T score | Mania | 1998 |
| -PAI_MAN_Grandiosity | T score | Mania | 1998 |
| -PAI_MAN_Irritability | T score | Mania | 1998 |
| PAI_CSc_Paranoia | T score | Paranoia | 1998 |
| -PAI_PAR_Hypervigilance | T score | Paranoia | 1998 |
| -PAI_PAR_Persecution | T score | Paranoia | 1998 |
| -PAI_PAR_Resentment | T score | Paranoia | 1998 |
| PAI_CSc_Schizophrenia | T score | Schizophrenia | 1998 |
| -PAI_SCZ_Psychotic Experiences | T score | Schizophrenia | 1998 |
| -PAI_SCZ_Social Detachment | T score | Schizophrenia | 1998 |
| -PAI_SCZ_Thought Disorder | T score | Schizophrenia | 1998 |
| PAI_TSc_Aggression | T score | Aggression | 1998 |
| -PAI_AGG_Attitude | T score | Aggression | 1998 |
| -PAI_AGG_Physical | T score | Aggression | 1998 |
| -PAI_AGG_Vverbal | T score | Aggression | 1998 |
| PAI_CSc_Antisocial | T score | Antisocial | 1998 |
| -PAI_ANT_Antisocial Behaviors | T score | Antisocial | 1998 |
| -PAI_ANT_Egocentricity | T score | Antisocial | 1998 |
| -PAI_ANT_Stimulus Seeking | T score | Antisocial | 1998 |
| PAI_CSc_Borderline | T score | Borderline | 1998 |
| -PAI_BOR_Affective Instability | T score | Borderline | 1998 |
| -PAI_BOR_Identity Problems | T score | Borderline | 1998 |
| -PAI_BOR_Negative Relationships | T score | Borderline | 1998 |
| -PAI_BOR_Self Harm | T score | Borderline | 1998 |
| PAI_CSc_Drugs | T score | Drugs | 1998 |
| PAI_CSc_Alcohol | T score | Alcohol | 1998 |
| PAI_TSc_Nonsupport | T score | Nonsupport | 1998 |
| PAI_TSc_Treatment Rejection | T score | Treatment Rejection | 1998 |
| PAI_TSc_Traumatic Stress | T score | Stress | 1998 |
| PAI_TSc_Suicidal ideation | T score | Suicide | 1998 |
| PAI_ISc_Dominance | T score | Dominance | 1998 |
| PAI_ISc_Warmth | T score | Warmth | 1998 |
WAIS-R=Wechsler Adult Intelligence Scale-Revised; CSc=Clinical Scale; TSc=Treatment Scale; ISc=Interpersonal Scale
2.3. Statistical Analysis
Analyses were performed by BTL, ACD, YC, and YS. In order to determine how far in advance of incident MCI cognitive decline begins, we assessed the neuropsychological trajectories between individuals diagnosed with incident amnestic MCI during the study period (converters) and those remaining cognitively normal (nonconverters). For each of the neuropsychological measures, we constructed a linear mixed model accounting for the cross-sectional and longitudinal nature of the data to compare the longitudinal trajectories between converters and nonconverters. Quadratic terms were initially considered, however their addition did not outperform first-order linear modeling. Within the fitted linear model, the age at which the two neuropsychological measure trajectories intersect was considered an ‘inflection point’ whereby converter and nonconverter paths diverge. A confidence interval for the inflection point was generated using 500 bootstrapped samples. The inflection point was then used as a marker to form a piecewise linear mixed effects regression model used to assess pre- and post-inflection effects (before and after the converter and nonconverter trajectories diverge) and allow for comparison of annual change (slopes) between groups. Regression contrast analysis within the piecewise linear mixed model framework was used to compare the annual change in neuropsychological measure scores between converters and nonconverters, both before the inflection point and after the inflection point, separately. Additionally, we performed cross sectional comparisons at five and ten years prior to the age of MCI conversion. The average conversion age of the 65 converters was 73, so for the matched set of comparable nonconverters we used those who were within two years of ages 68 and 63 respectively.
Study characteristics are presented using means and standard deviations (SD), frequencies, and relative risks (RR). Mean values and frequency distributions were compared between converters and nonconverters using t-tests and chi-squared tests, respectively. Kaplan-Meier (KM) method was used to estimate time to MCI diagnosis within groups. Logrank tests and Cox proportional hazards regression were used to compare time to MCI diagnosis between groups. Statistical modeling was conducted using the statistical software SAS version 9.4 (SAS Institute Inc., Cary, NC).
3.0. Results
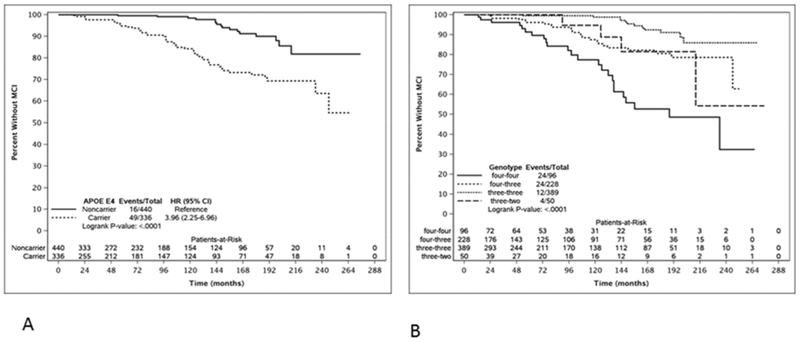
Over a mean duration of 9.5 years (range 1.1-20.5 years) 65 individuals were diagnosed with incident amnestic MCI at a mean age of 73 (S.D. 6.8) years. (An additional patient who was an APOE e4 homozygote developed a nonamnestic dysexecutive form of MCI that was later found to reflect an unsuspected progranulin mutation with autopsy confirmed frontotemporal lobar degeneration in addition to AD, and was excluded from this analysis). 719 remained clinically normal over a mean observational period of 7.2 (S.D. 6.5) years. Demographic data are summarized in table 2. Figures 1a and 1b shows the Kaplan-Meier curves by APOE e4 carrier status and genotype respectively. APOE e4 carriers had roughly four times the risk of MCI diagnosis than non-carriers (HR 3.96; 95%CI: 2.25, 6.96). Men had over twice the risk of MCI diagnosis than women (HR 2.25; 95%CI: 1.42, 3.58). Sixteen experienced clinically significant decline in additional cognitive domains (as well as memory) including executive (n=10), visuospatial (n=7), and/or language (n=5). Of these 16, 12 were e4 carriers. 151 members of the entire cohort had an amyloid PET scan. Of those with MCI, it was positive in 6 of 12, and of the nonconverters it was positive in 24 of 139.
Table 2.
Entry Demographics
| Converters (N=65) | Nonconverters (N=719) | p | |
|---|---|---|---|
| Age at entry (years) – mean (SD) | 63.6 (7.4) | 56.1 (11.6) | <0.001 |
| Education (years) – mean (SD) | 15.8 (2.8) | 15.8 (2.5) | 0.94 |
| Time to diagnosis/duration followup (years) – mean (SD) | 9.5 (4.5) | 7.2 (6.5) | 0.006 |
| Women – n (%) | 35 (53.8%) | 533 (74.1%) | <0.001 |
| APOE e4 carriers – n (%) | 49 (75.4%) | 287 (40.4%) | <0.001 |
| Folstein Minimental State Exam – mean (SD) | 29.3 (0.9) | 29.7 (0.7) | <0.001 |
| Hamilton Depression Scale – mean (SD) | 2.5 (3.0) | 2.5 (3.3) | 0.87 |
Figure 1.
Kaplan-Meier curves of time to mild cognitive impairment contrasting a) APOE e4 carriers and noncarriers; and b) by APOE genotype.
48 have had subsequent followup from their MCI diagnosis ranging from one to 12.5 years and all have shown further decline. Four developed symptoms suggesting dementia with Lewy bodies: dream enactment behavior in three and mild parkinsonism in one. All four were APOE e4 carriers (three were e4 homozygotes). Two others had a stroke between eight and 16 years after MCI diagnosis, and both died. Ten in all have since died an average of 8.5 years after MCI diagnosis, and seven came to autopsy with AD confirmed in all but one case in who cerebral rarefaction was the major finding nearly six years after MCI was diagnosed. Two had additional findings of synucleinopathy, neither of who exhibited clinical parkinsonism (one was an APOE e4 carrier). Among the MCI nonconverters six developed Parkinson’s disease, three of who were e4 carriers.
Supplementary Table 1 shows the neuropsychological and behavioral data at entry for the originally well matched APOE e4 carrier and noncarrier groups as well as the inflection point baseline data for the MCI converter and nonconverter groups at which point differences in age and memory performance have become evident. Of 56 tested neuropsychological measures, the inflection point and subsequent piecewise linear mixed models could be estimated for 53 outcomes. Table 3 summarizes the results for these 35 cognitive and 18 behavioral measures (Supplementary Table 2 is an expanded version with the complete data set). 34 of 35 cognitive neuropsychological scores including both subjective cognition measures, all three general intellect, all four language, all four visuospatial, all nine memory, and 12 of 13 executive measures showed a significantly steeper slope of decline post-inflection in the MCI converters relative to the nonconverters with the sole exception being a reverse digit span test although this may reflect the significantly greater rate of decline pre-inflection in the MCI converters. Figure 2 summarizes the inflection point ages for each cognitive measure. The earliest cognitive inflection point was nearly 20 years in advance of MCI diagnosis. Multiple declarative memory tests declined early, and generally had the narrowest confidence intervals. Inflection point ages of delayed recall scores were as follows: Auditory Verbal Learning Test (AVLT) Long Term Memory (LTM) 53.4 years, AVLT % recall 54 years, Selective Reminding Test (SRT) delayed free recall 54.8 years, Complex Figure Test (CFT) recall 55 years, Wechsler Memory Scale-Revised (WMS-R) logical memory delayed recall 58.6 years. Immediate recall (and related measures) inflection points were as follows: AVLT Total Learning 55.2 years, SRT immediate free recall 55.4 years, Visual Retention Test 55.9 years, WMS-R logical memory immediate recall 58.6 years. The inflection age of self-reported subjective cognition nearly overlapped logical memory delayed recall while informant-based change lagged self-observation by another two years. The earliest inflection point, however, was for the CFT-copy, age 53.3 years while the next earliest visuospatial test, the Judgement of Line Orientation (JLO) was two years later.
Table 3.
Trajectories (Slopes) of Cognitive and Behavioral Measures Before and After Inflection Points of MCI Converters and Nonconverters
| Trajectory Slope Overall | Trajectory Slope Before AOI | Trajectory Slope After AOI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age of Inflection | MCI Nonconverters | MCI Converters | p | MCI Nonconverters | MCI Converters | p | MCI Nonconverters | MCI Converters | p | |
| General Intellect | ||||||||||
| Folstein MMSE | 57.2 | −0.005 | −0.106 | <0.001 | 0.0042 | −0.017 | 0.59 | −0.008 | −0.113 | <0.001 |
| Dementia Rating Scale | 59.4 | −0.02 | −0.55 | <0.001 | 0.0564 | −0.08 | 0.34 | −0.059 | −0.594 | <0.001 |
| WAIS Information | 54.7 | 0.062 | −0.038 | <0.001 | 0.0562 | 0.1097 | 0.60 | 0.0631 | −0.043 | <0.001 |
| Language | ||||||||||
| WAIS Vocabulary | 58.8 | 0.0501 | −0.031 | <0.001 | 0.0798 | −0.038 | 0.05 | 0.0377 | −0.031 | <0.001 |
| WAIS Similar | 53.5 | 0.0962 | 0.0278 | <0.001 | 0.1041 | 0.1673 | 0.74 | 0.0948 | 0.0248 | <0.001 |
| Bostn Naming Test | 58.1 | −0.018 | −0.411 | <0.001 | 0.0862 | −0.199 | 0.02 | −0.055 | −0.431 | <0.001 |
| Token Test | 63.0 | −0.008 | −0.071 | 0.002 | 0.0034 | −0.022 | 0.63 | −0.018 | −0.084 | 0.008 |
| Visuospatial | ||||||||||
| Judgement of Line Orientation | 55.3 | −0.029 | −0.246 | <0.001 | 0.0208 | 0.3924 | 0.15 | −0.042 | −0.264 | <0.001 |
| Facial Recognition Test | 56.7 | −0.071 | −0.267 | <0.001 | −0.015 | 0.0192 | 0.89 | −0.089 | −0.28 | <0.001 |
| WAIS Block Design | 57.2 | 0.0442 | −0.104 | <0.001 | 0.1043 | 0.1087 | 0.96 | 0.026 | −0.12 | <0.001 |
| Complex Figure Test-Copy | 53.3 | −0.02 | −0.251 | <0.001 | 0.0015 | 0.0803 | 0.80 | −0.024 | −0.258 | <0.001 |
| Executive | ||||||||||
| WAIS Digit Span Backward | 64.8 | −0.007 | −0.05 | 0.045 | 0.0051 | −0.197 | <0.001 | −0.018 | −0.025 | 0.77 |
| WAIS Arithmetic | 57.5 | −0.01 | −0.14 | <0.001 | 0.0227 | −0.012 | 0.65 | −0.021 | −0.151 | <0.001 |
| PASAT 3 Second | 59.5 | 0.0716 | −0.9 | <0.001 | 0.3661 | 0.422 | 0.88 | −0.085 | −1.058 | <0.001 |
| PASAT 2 Second | 58.3 | 0.2999 | −0.4 | <0.001 | 0.6485 | 0.6922 | 0.93 | 0.1438 | −0.494 | <0.001 |
| WAIS Digit Symbol Items | 55.5 | −0.474 | −1.245 | <0.001 | −0.175 | −11.58 | 0.001 | −0.528 | −1.196 | <0.001 |
| TMT-A (seconds) | 55.1 | 0.1415 | 0.7659 | 0.001 | −0.083 | 0.8082 | 0.91 | 0.193 | 0.7655 | 0.003 |
| TMT-B (seconds) | 58.1 | 0.8977 | 4.6749 | <0.001 | 0.3074 | 1.6676 | 0.81 | 1.0913 | 4.7389 | <0.001 |
| COWAT | 63.7 | 0.1998 | −0.315 | <0.001 | 0.3982 | 0.0375 | 0.02 | 0.0051 | −0.456 | <0.001 |
| Animal Fluency (UDS) | 61.5 | −0.115 | −0.647 | <0.001 | 0.0113 | −0.663 | 0.07 | −0.182 | −0.644 | <0.001 |
| Vegetable Fluency (UDS) | 55.2 | −0.098 | −0.368 | <0.001 | 0.0351 | 0.8062 | 0.75 | −0.126 | −0.372 | <0.001 |
| WCST Categories | 61.8 | −0.069 | −0.179 | <0.001 | −0.026 | −0.106 | 0.11 | −0.103 | −0.194 | <0.001 |
| WCST Errors | 60.8 | 0.714 | 1.9292 | <0.001 | 0.3292 | 1.6364 | 0.03 | 0.9706 | 1.9737 | <0.001 |
| WCST Persev Errors | 59.8 | 0.3414 | 1.0579 | <0.001 | 0.106 | 0.7815 | 0.09 | 0.4775 | 1.0892 | <0.001 |
| Memory | ||||||||||
| Complex Figure Test-Recall | 55.0 | 0.159 | −0.491 | <0.001 | 0.2803 | 0.8931 | 0.10 | 0.1323 | −0.546 | <0.001 |
| AVLT Total Learning | 55.2 | −0.087 | −1.046 | <0.001 | 0.1763 | 0.3401 | 0.73 | −0.145 | −1.105 | <0.001 |
| AVLT Recall | 54.0 | −0.043 | −2.668 | <0.001 | 0.1821 | −2.867 | 0.08 | −0.088 | −2.663 | <0.001 |
| AVLT Long Term Memory | 53.4 | −0.003 | −0.388 | <0.001 | 0.0688 | −0.302 | 0.19 | −0.015 | −0.39 | <0.001 |
| Visual Retention Test | 55.9 | −0.032 | −0.193 | <0.001 | 0.0113 | 0.102 | 0.34 | −0.044 | −0.209 | <0.001 |
| SRT Immediate Free Recall | 55.4 | 0.2512 | −1.452 | <0.001 | 0.4728 | 1.6022 | 0.13 | 0.193 | −1.551 | <0.001 |
| SRT Delayed Free Recall | 54.8 | 0.0509 | −0.213 | <0.001 | 0.0843 | 0.5869 | 0.06 | 0.0416 | −0.234 | <0.001 |
| Logical Memory Immediate Recall (UDS) | 58.6 | −0.003 | −0.467 | <0.001 | 0.0442 | −0.683 | 0.14 | −0.019 | −0.461 | <0.001 |
| Logical Memory Delayed Recall (UDS) | 58.4 | 0.013 | −0.554 | <0.001 | 0.057 | −0.558 | 0.26 | −0.001 | −0.553 | <0.001 |
| Subjective Cognition | ||||||||||
| MANS Informant | 60.7 | 0.0605 | 2.6147 | <0.001 | −0.435 | 1.6059 | 0.02 | 0.3557 | 2.756 | <0.001 |
| MANS Self | 58.6 | 0.0466 | 2.2717 | <0.001 | −0.468 | 0.1588 | 0.59 | 0.2474 | 2.4463 | <0.001 |
| Depression | ||||||||||
| Geriatric Depression Scale (UDS) | 58.1 | −0.018 | 0.2008 | <0.001 | −0.117 | 0.2547 | 0.49 | 0.0106 | 0.1994 | 0.002 |
| Hamilton Depression Scale | 56.3 | −0.026 | 0.0615 | <0.001 | −0.011 | −0.061 | 0.74 | −0.03 | 0.0685 | <0.001 |
| Beck Depression Inventory | 65.6 | 0.0271 | 0.1623 | 0.001 | −0.016 | 0.1469 | 0.04 | 0.0912 | 0.1703 | 0.17 |
| PAI-CSC-DEP | 60.3 | 0.0827 | 0.4877 | <0.001 | −0.043 | 0.3706 | 0.06 | 0.1621 | 0.5065 | <0.001 |
| Personality Assessment Inventory | ||||||||||
| PAI-CSC-DEP | 60.3 | 0.0827 | 0.4877 | <0.001 | −0.043 | 0.3706 | 0.06 | 0.1621 | 0.5065 | <0.001 |
| PAI-CSC-ANT | 56.2 | −0.105 | −0.036 | 0.15 | −0.236 | 0.0487 | 0.31 | −0.064 | −0.04 | 0.64 |
| PAI-CSC-ANX | 59.2 | −0.038 | 0.3315 | <0.001 | −0.106 | 0.0407 | 0.47 | −0.004 | 0.3655 | <0.001 |
| PAI-CSC-ARD | 59.9 | −0.015 | 0.1766 | 0.003 | −0.139 | 0.2391 | 0.08 | 0.0572 | 0.1677 | 0.13 |
| PAI-CSC-BOR | 58.7 | −0.115 | 0.1028 | <0.001 | −0.299 | −0.174 | 0.55 | −0.028 | 0.1311 | 0.006 |
| PAI-CSC-DRG | 59.0 | −0.085 | 0.0331 | 0.11 | −0.165 | 0.2274 | 0.15 | −0.041 | 0.0121 | 0.51 |
| PAI-CSC-PAR | 63.6 | −0.061 | 0.102 | 0.005 | −0.133 | −0.068 | 0.61 | 0.0219 | 0.1667 | 0.05 |
| PAI-CSC-SCZ | 61.6 | −0.061 | 0.2334 | <0.001 | −0.141 | −0.071 | 0.65 | 0.0027 | 0.3041 | <0.001 |
| PAI-CSC-SOM | 62.1 | 0.15 | 0.4068 | <0.001 | 0.0955 | 0.3173 | 0.14 | 0.1967 | 0.4302 | 0.001 |
| PAI-ISC-DOM | 57.3 | −0.125 | −0.239 | 0.06 | −0.066 | −0.005 | 0.84 | −0.147 | −0.255 | 0.10 |
| PAI-ISC-WRM | 68.6 | −0.008 | −0.171 | 0.01 | 0.0425 | −0.05 | 0.34 | −0.161 | −0.318 | 0.17 |
| PAI-TSC-AGG | 54.6 | −0.078 | 0.0591 | 0.01 | −0.203 | −0.01 | 0.68 | −0.047 | 0.0611 | 0.06 |
| PAI-TSC-NON | 68.2 | 0.0584 | 0.1958 | 0.07 | −0.012 | 0.0089 | 0.85 | 0.2624 | 0.4015 | 0.26 |
| PAI-TSC-STR | 62.4 | −0.089 | 0.126 | 0.007 | −0.187 | −0.009 | 0.36 | 0.0055 | 0.1639 | 0.10 |
| PAI-TSC-SUI | 68.5 | 0.0203 | 0.1837 | 0.008 | −0.008 | 0.1602 | 0.06 | 0.1066 | 0.2114 | 0.32 |
Figure 2.
Cognitive Test Score Ages of Inflection. The ages at which overall linear trajectories intersect for each cognitive measure between those who developed MCI and those who remained cognitively normal. Error bars represent 95% confidence intervals based on 500 bootstrapped samples.
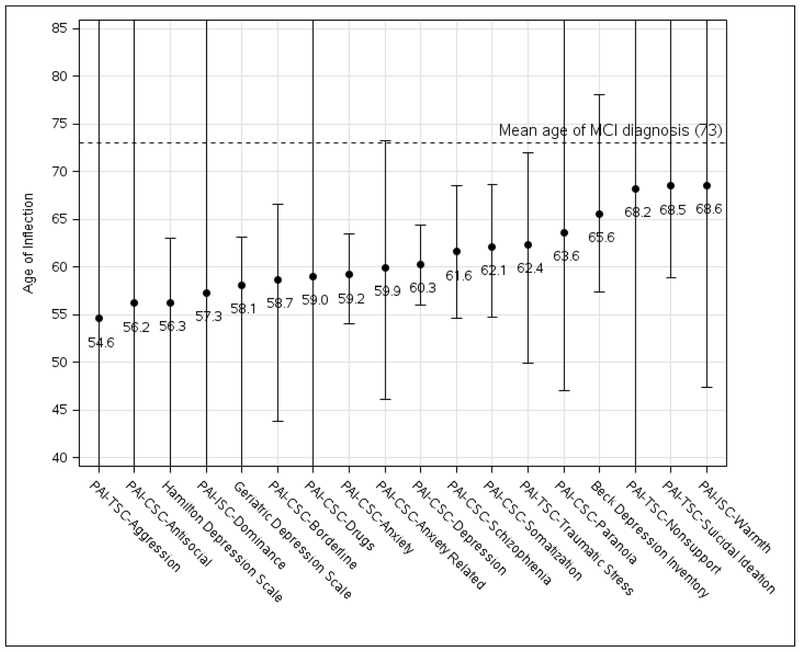
Figure 3 summarizes the inflection point ages for each behavioral measure. Confidence intervals were wide for all. Three of four depression measures declined more steeply post-inflection in the MCI converters, one of which was the Personality Assessment Inventory (PAI) Clinical Scale for Depression. Another four PAI Clinical Scale scores also statistically significantly worsened post-inflection in the MCI converters including Somatization, Anxiety, Borderline, and Schizopheniform symptoms. The earliest inflection point (PAI Treatment Scale for Aggression [PAI-TSc-AGG]; p=0.06) nearly rivaled the earliest cognitive change 18 years prior to MCI diagnosis, though the confidence interval was very wide.
Figure 3.
Behavioral Test Score Ages of Inflection. The ages at which overall linear trajectories intersect for each behavioral measure showed a statistically significant change in slope between those who developed MCI and those who remained cognitively normal. Error bars represent 95% confidence intervals based on 500 bootstrapped samples.
Supplementary Table 3 shows the cross sectional comparisons five and ten years prior to MCI conversion. At ten years prior to conversion there were too few who had completed the Uniform Data Set tests and so these are omitted (including, for example Logical Memory). Otherwise, both comparisons found that all memory measures were lower in the eventual MCI converters, although the difference was greater at five than at ten years prior to diagnosis. In addition, at five years prior to diagnosis several non-memory measure differences reached statistical significance.
4.0. Discussion
The cognitive and behavioral ages of inflection identified in this longitudinal cohort study represent the earliest reported. This was likely facilitated by our young lower age limit of enrollment, the long duration of our observational period, and our analysis of incident diagnoses based upon within-subject decline. Our findings are consistent with and extend the findings of previous studies that have shown weaker memory performances at the preclinical stage of AD that predict subsequent dementia17–21 with differences identified as much as 10 years before dementia diagnosis19, as well as with a meta-analysis of 47 studies showing preclinical decline in multiple cognitive domains20. Multiple studies have found memory performance to be a sensitive predictor of MCI conversion to dementia22–25. Our findings are also consistent with our previous reports of accelerated memory decline in healthy APOE e4 carriers26,27 and extend them to a true “pre-MCI” stage. Our finding of a fourfold increase in amnestic MCI risk attributed to APOE e4 is consistent with previous studies28, and our finding that male sex more than doubled MCI risk is consistent with the Olmsted County data29,30. Regarding e2, despite its known protective effect against AD, carriers unexpectedly had a higher conversion rate than e4 heterozygotes, but the number of e2 converters was too small to draw further conclusions.
Nine memory measures from all five memory tests administered declined prior to diagnosis. Word list tests including the AVLT and SRT declined earlier than the logical memory test which is based on recalling a story, a finding shared by previous studies in both preclinical17,19 and MCI based22–25,31 cohorts. All memory measures were significantly lower as well in cross sectional comparisons five and ten years prior to MCI conversion, illustrating the sensitivity of word list memory paradigms that could be useful in preclinical AD therapeutic trials. Over the pre-MCI course, tests that were sensitive to other cognitive domains also declined, even though memory was the main clinical complaint. To what degree a specific test changed may reflect its “within-domain” sensitivity (for example, how sensitive a visuospatial test is to visuospatial impairment) or in some cases might instead reflect “cross-sensitivity” with memory. For example, the MMSE inflection point was roughly two years sooner than the Dementia Rating Scale (DRS). If we consider orientation, learning, and memory to all reflect memory, then memory makes up a higher proportion of the MMSE than the DRS. Tests can be considered as generally reflective of a given domain but in reality may reflect multiple domains to different degrees. Sixteen patients in this study developed clinically evident non-amnestic features (in addition to memory decline). A recent analysis of four large cohorts of AD dementia patients (that were not limited to incident cases) found that non-amnestic features were more likely in APOE e4 non-carriers32. Non-AD pathologies may partially account for our findings, though we found that APOE e4 carriers were heavily over-represented in our atypical group. Ten developed clinical synucleinopathies and another two were subclinical found at autopsy. Of these twelve, eight were e4 carriers, consistent with a recent large study of Lewy body related disease33.
Behavioral measures also changed during the pre-MCI period despite the absence of any clinically identified behavioral problems, though the confidence intervals were wide in all cases. The source of these behavioral changes is not certain but the median raphe34 and locus ceruleus35 are sites of early pathology, and the neurochemistry of agitation in AD dementia reflects an alteration of multiple monoaminergic neurochemical projection systems36. Additionally, in patients experiencing visual hallucinations in the setting of dementia with Lewy bodies37 as well as Parkinson’s disease38 cortical GABA-ergic interneuron signaling is impaired in the absence of visual cortex synucleinopathy. Regarding depression, for which we found three of four tests declining more rapidly in MCI converters relative to nonconverters post-inflection, we have previously shown in a normal aging cohort that there was no differential acceleration in depression scores among e4 carriers39. During the transition from normal aging to MCI, however, there is a change in personality and behavioral measures including depression scores40. This temporal pattern suggests that depression, rather than being a risk factor, is more likely an early manifestation of neurodegeneration. Further observation will be needed to confirm whether these subclinical changes during the pre-MCI stage are predictive of subsequent behavioral disorders.
AD has been thought to be the single most common cause of MCI9,10, but neuropathological studies of patients dying with MCI are few. Petersen et al found mild AD-like changes in 10 of 15 patients dying with amnestic MCI at a mean age of 88.9 years41. In a series of 34 subjects with amnestic MCI (aMCI; mean age at death 88 years) and 15 with nonamnestic MCI (mean age at death (mean age at death 83 years), Dugger et al reported that 53% in each group met neuropathological criteria for AD though the aMCI group had denser temporal lobe neurofibrillary tangle pathology42. In an autopsy series of 1337 patients derived from 4 different Alzheimer Disease Centers, 343 had MCI that remained stable until death an average of 4 years later (mean age at death 89.5 years) and of these, 72.3% had Braak stage III or higher, the majority of which had additional pathologies43. Similarly, in a large autopsy series among patients dying at a mean age of 90 years, 95% had at least one age-related pathology, nearly 60% had three or more, and the rate of mixed pathology was higher in those with a clinical diagnosis of AD44. Whether the extent of mixed pathology at these very advanced ages is representative of pathology at the incident MCI age of our subjects is unclear.
There are several limitations of our study. First, MCI is pathologically heterogeneous and while AD likely accounts for the majority of cases in our APOE e4 enriched cohort, patients who have come to autopsy often have had a mixed neuropathological picture and occasionally a primarily non-AD cause42,43,45. The latter is uncommon, however, among amnestic presentations, and given that the age at death of autopsied MCI patients has been in the late 80s it is far from clear whether the neuropathology of MCI at the younger ages in our study would be the same. Second, the general lack of amyloid and tau biomarkers precludes our ability to determine the relative timing of cognitive and biomarker changes in these subjects. However, unlike most previous studies, we found the timing of cognitive decline rivaled the earliest reported biomarker changes and should prompt reconsideration of the relative sensitivities of the methods used in other studies. This is consistent with findings from the Alzheimer’s Disease Neuroimaging Initiative Cohort in a study modeling neurodegenerative disease progression. Of seven included variables including cerebrospinal fluid amyloid and tau, hippocampal volumes, and several cognitive measures, it was the AVLT LTM score that changed before all others46. Third, as shown in Supplementary Table 1, although age at entry for APOE e4 carriers and noncarriers was the same at entry, once subjects were reclassified by clinical outcome, older age at entry was associated with greater risk for incident MCI resulting (which is not surprising) in fewer time points of data preceding the inflection point that separated converters and nonconverters. This actually raises the possibility that the age of inflection may be even earlier on some tests, and underscores the importance of enrolling younger individuals in preclinical studies. Fourth, our findings reflect group rather than individual analyses, so we cannot account for individual differences that would likely impact a patient’s subsequent course. This would include the possibility that some of those in the nonconverter group will eventually become converters. Judging from the width of the confidence intervals memory decline, as expected, remains not only an early but also the most consistent finding. Finally, the changes on behavioral measures were of no current clinical significance, but appeared to vary greatly between individuals, so their implications for potential behavioral disturbances will require further observation.
In summary, we found cognitive and behavioral changes begin nearly 20 years before incident MCI, a duration that approaches the earliest reported biomarker changes.
Supplementary Material
Research In Context.
Systematic Review:
In addition to evaluating studies related to preclinical Alzheimer’s disease (AD) through traditional sources we reviewed the history of current thinking from the discoveries leading to the amyloid hypothesis to the phylogeny of amyloid precursor protein (APP), and asked how to resolve the evident discrepancies between this highly conserved gene/protein, amyloid targeted treatment failures, and the incontrovertible genetic evidence linking APP alteration to the cause of AD.
Interpretation:
In contrast to previously reported and widely assumed findings of an extreme time lag between the earliest reported amyloid biomarker changes and cognitive decline, we found the earliest cognitive changes occur 20 years before incident mild cognitive impairment, rivaling the earliest reported biomarker changes challenging current disease models.
Future Directions:
Alternate pathophysiological models exploring a loss of a critical APP-related function (rather than a gain of toxicity) better fit this chronology and should be further explored to identify alternate therapeutic targets.
Acknowledgments
This work was supported by the National Institute on Aging (P30AG019610, R01AG031581), and the Arizona Alzheimer’s Research Consortium (E.M. Reiman, PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bateman RJ, Xiong C, Benzinger TLS, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. New Engl J Med 2012; 367: 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDade E, Wang G, Gordon BA, et al. Longitudinal cognitive and biomarker changes in dominantly inherited Alzheimer disease. Neurology 2018; 91: e1295–e1306. Doi: 10.1212/WNL.0000000000006277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid B deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol 2013; 12(4): 357–367. [DOI] [PubMed] [Google Scholar]
- 4.Crystal H, Dickson D, Fuld P, et al. Clinico-pathological studies in dementia: nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology 1988; 38(11): 1682–1687. [DOI] [PubMed] [Google Scholar]
- 5.Dickson DW, Crystal HA, Mattiace LA, et al. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging 1992; 13(1): 179–189. [DOI] [PubMed] [Google Scholar]
- 6.Kok E, Haikonen S, Luoto T, Huhtala H, Goebeler S, Haapasalo H, Karhunen PJ. Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann Neurol 2009; 65: 650–657. [DOI] [PubMed] [Google Scholar]
- 7.Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia. A meta-analysis. JAMA 2015; 313(19): 1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donohue MC, Sperling RA, Petersen R, Sun CK, Weiner MW, Aisen PS for ADNI. Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA 2017; 317(22): 2305–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen RC, Smith GE, Ivnik RJ, et al. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. JAMA 1995; 273(16): 1274–1278. [PubMed] [Google Scholar]
- 11.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Washington D.C., American Psychiatric Association, 1994. [Google Scholar]
- 13.Caselli RJ, Reiman EM, Locke EC, et al. Cognitive domain decline in healthy apolipoprotein E e4 homozygotes before the diagnosis of mild cognitive impairment. Arch Neurol 2007; 64(9): 1306–1311. [DOI] [PubMed] [Google Scholar]
- 14.Caselli RJ, Chen K, Lee W, Alexander GE, Reiman EM. Correlating cerebral hypometabolism with future memory decline in subsequent converters to amnestic pre-mild cognitive impairment. Arch Neurol 2008; 65(9): 1231–1236. [DOI] [PubMed] [Google Scholar]
- 15.Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 4th ed. New York: Oxford University Press; 2004. [Google Scholar]
- 16.Locke DE, Dassel KB, Hall G, Baxter LC, Woodruff BK, Hoffman Snyder C, Miller BL, Caselli RJ. Assessment of patient and caregiver experiences of dementia-related symptoms: development of the Multidimensional Assessment of Neurodegenerative Symptoms questionnaire. Dement Geriatr Cogn Disord. 2009; 27(3):260–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grober E, Kawas C. Learning and retention in preclinical and early Alzheimer’s disease. Psychol Aging 1997; 12(1): 183–188. [DOI] [PubMed] [Google Scholar]
- 18.Bondi MW, Salmon DP, Galasko D, Thomas RG, Thal LJ. Neuropsychological function and apolipoprotein E genotype in the preclinical detection of Alzheimer’s disease. Psychol Aging 1999; 14(2): 295–303. [DOI] [PubMed] [Google Scholar]
- 19.Tierney MC, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology 2005; 64: 1853–1859. [DOI] [PubMed] [Google Scholar]
- 20.Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer’s disease: a meta-analysis. Neuropsychology 2005; 19(4): 520–531. [DOI] [PubMed] [Google Scholar]
- 21.Jedynak BM, Lang A, Liu B, et al. for ADNI. A computational neurodegenerative disease progression score: method and results with the Alzheimer’s disease neuroimaging initiative cohort. Neuroimage 2012; 63(3): 1478–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devanand DP, Liu X, Tabert MH, et al. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer’s disease. Biol Psychiatry 2008; 64(10): 871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomar JJ, Bobes-Bascaran MT, Conejero-Goldberg C, Davies P, Goldberg TE for ADNI. Utility of combinations of biomarkers, cognitive markers, and risk factors to predict conversion from mild cognitive impairment to Alzheimer disease in patients in the Alzheimer’s Disease Neuroimaging Initiative. Arch Gen Psychiatry 2011; 68(9): 961–969. [DOI] [PubMed] [Google Scholar]
- 24.Heister D, Brewer JB, Magda S, Blennow K, McEvoy LK, for ADNI. Predicting MCI outcome with clinically available MRI and CSF biomarkers. Neurology 2011; 77: 1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landau SM, Harvey D, Madison CM, et al. for ADNI. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology 2010; 75: 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, Baxter LC, Rapcsak SZ, Shi J, Woodruff BK, Locke DE, Snyder CH, Alexander GE, Rademakers R, Reiman EM. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009. July 16; 361(3):255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caselli RJ, Locke DE, Dueck AC, Knopman DS, Woodruff BK, Hoffman-Snyder C, Rademakers R, Fleisher AS, Reiman EM. The neuropsychology of normal aging and preclinical Alzheimer’s disease. Alzheimers Dement. 2014. January; 10(1):84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farrer LA, Cupples A, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. JAMA 1997; 278: 1349–1356. [PubMed] [Google Scholar]
- 29.Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology 2010; 75(10): 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts RO, Geda YE, Knopman DS, et al. The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology 2012; 78(5): 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabin LA, Pare N, Saykin AJ, Brown MJ, Wishart HA, Flashman LA, Santulli RB. Differential memory test sensitivity for diagnosing amnestic mild cognitive impairment and predicting conversion to Alzheimer’s disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2009; 16(3): 357–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheltens NME, Tijms BM, Koene T, et al. Cognitive subtypes of probable Alzheimer’s disease robustly identified in four cohorts. Alz Dem 2017; 13(11): 1226–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickson DW, Heckman MG, Murray ME, et al. APOE e4 is associated with severity of Lewy body pathology independent of Alzheimer pathology. Neurology 2018; 91: e1182–e1195. Doi: 10.1212/WNL.0000000000006212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mann DMA, Yates PO, Serotonin nerve cells in Alzheimer’s disease. J Neurol Neurosurg, Psychiatry 1982; 43: 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bondareff W, Mountjoy CQ, Roth M. Loss of neurons of origin of the adrenergic projection to cerebral cortex (nucleus locus ceruleus) in senile dementia. Neurology 1982; 32: 164–168. [DOI] [PubMed] [Google Scholar]
- 36.Liu KY, Stringer AE, Reeves SJ, Howard RJ. The neurochemistry of agitation in Alzheimer’s disease: a systematic review. Aging Res Rev 2018; 43: 99–107. [DOI] [PubMed] [Google Scholar]
- 37.Khundakar AA, Hanson PS, Erskine D, et al. Analysis of primary visual cortex in dementia with Lewy bodies indicates GABAergic involvement associated with recurrent visual hallucinations. Acta Neuropathol Commun 2016; 4: 66 Doi 10.1186/s40478-016-0334-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Firbank MJ, Parikh J, Murphy N, et al. Reduced occipital GABA in Parkinson disease with visual hallucinations. Neurology 2018; 91:e675–e685. Doi: 10.1212/WNL.0000000000006007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Locke DE, Dueck AC, Stonnington CM, Knopman DS, Geda YE, Caselli RJ. Depressive symptoms in healthy apolipoprotein E epsilon4 carriers and noncarriers: a longitudinal study. J Clin Psychiatry. 2013; 74: (12)1256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caselli RJ, Langlais BT, Dueck AC, Henslin BR, Johnson TA, Woodruff BK, Hoffman-Snyder C, Locke DEC. Personality changes during the transition from cognitive health to mild cognitive impairment. J Am Geriatr Soc. 2018; 66 (4):671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol 2006; 63: 665–672. [DOI] [PubMed] [Google Scholar]
- 42.Dugger BN, Davis K, Malek-Ahmadi M, et al. Neuropathological comparisons of amnestic and nonamnestic mild cognitive impairment. BMC Neurol 2015; 15: 146 Doi: 10.1186/s12883-015-0403-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abner EL, Kryscio RJ, Schmitt FA, et al. Outcomes after diagnosis of mild cognitive impairment in a large autopsy series. Ann Neurol 2017; 81: 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyle PA, Yu L, Leurgans SE, Wilson RS, Brookmeyer R, Schneider JA, Bennett DA. Attributable risk of Alzheimer’s dementia attributed to age-related neuropathologies. Ann Neurol 2019; 85(1): 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology 2006; 67: 467–473. [DOI] [PubMed] [Google Scholar]
- 46.Jedynak BM, Lang A, Liu B, et al. for ADNI. A computational neurodegenerative disease progression score: method and results with the Alzheimer’s disease neuroimaging initiative cohort. Neuroimage 2012; 63(3): 1478–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.