Abstract
Background:
Dysphagia following stroke is prevalent, however dysphagia treatment is often applied haphazardly and outcomes unclear. Neuromuscular Electrical stimulation (NMES) has received increased attention as a treatment for post stroke dysphagia; but application data remain conflicted.
Objective:
This study investigated effectiveness and safety of an exercised-based swallowing therapy (McNeill Dysphagia Therapy: MDTP) +NMES for dysphagia rehabilitation following stroke.
Methods:
Stroke patients (n=53, age: 66 [13.2], 47.2% male) with dysphagia admitted to sub-acute rehabilitation hospital were randomized to MDTP+NMES [NMES], MDTP+ sham NMES [MDTP] or usual care [UC] swallowing therapy groups. Patients were treated for one hour per day for 3 weeks and monitored to 3-months by a blinded evaluator. Outcomes included clinical swallowing ability, oral intake, weight, patient perception of swallow, and occurrence of dysphagia–related complications.
Results:
Post treatment dysphagia severity and treatment response were significantly different between groups (p ≤0.0001). MDTP demonstrated greater positive change than either NMES or UC arms, including increase in oral intake (χ2=5, p ≤ 0.022) and improved functional outcome by 3-months post stroke (RR = 1.72, 1.04–2.84). Exploratory Cox regression revealed the MDTP group conferred the greatest benefit in time to “return to pre-stroke diet” of 4.317 [95% CI: 1.08–17.2, p< .03].
Conclusion:
Greater benefit (e.g. reduction in dysphagia severity, improved oral intake, and earlier return to pre-stroke diet) resulted from a program of MDTP alone vs. NMES or UC.
Keywords: Swallowing dysfunction, Stroke, swallowing therapy, exercise, NMES randomized controlled trial
Introduction:
Dysphagia following stroke is prevalent, [1, 2] and associated with increased morbidities [3], and poorer outcome [4]. The effectiveness of common dysphagia treatments remain uncertain as treatment studies have often been plagued by variable methodology and results [5].
Neuromuscular Electrical stimulation (NMES) is a popular modality for dysphagia treatment, with many speech pathologists choosing this treatment type as the primary form of intervention [6]. NMES is a form of electrotherapy used to strengthen muscular contractions during swallowing. It is commonly delivered as a transcutaneous approach using a dual channel system (VitalStim® Model 5900, Chattanooga Group, Hixson TN) Some authors suggest that NMES paired with behavioral swallowing therapy best facilitates improvement [7]. However, studies evaluating NMES in dysphagia treatment post stroke have also demonstrated conflicting results [8]. Weak design, lack of controls, variable timing of treatment, and variable applications of technique have resulted in studies susceptible to systematic bias. As a result of these efforts equipoise continues. Within a double blind randomized controlled trial this study investigated NMES as an adjunctive modality in the treatment of post stroke dysphagia. We hypothesized that the addition of NMES to a peer reviewed and published program of exercise-based swallowing therapy, the McNeill Dysphagia Therapy program (MDTP) would enhance functional outcome post stroke in comparison to usual care swallowing therapy.
Methods:
Setting:
This trial evaluated the effectiveness of McNeill Dysphagia Therapy Program (MDTP) with adjunctive NMES in sub-acute stroke patients with dysphagia. Fifty-three stroke patients ( age: 66 [13.2], 47.2% male) admitted to a rehabilitation facility were screened for dysphagia and randomized to receive 3 weeks (15 sessions) of daily MDTP +NMES (NMES), MDTP + Sham NMES (MDTP) or usual care (UC) swallowing treatment.
The local Institutional Review Board approved the study protocol.
Study design
Randomized, double -blind, placebo controlled clinical trial.
Participants:
All patients presenting to the sub-acute rehabilitation hospital over a two -year period were screened for inclusion into the study. Patients were initially identified by the treating stroke neurologist and subsequently approached by a study speech pathologist that reviewed study procedures and obtained informed consent. Screening procedures included a detailed medical history, physical and neurological examination, and speech/swallowing examination.
Patients were eligible if stroke was confirmed by the attending neurologist according to the WHO definition, demonstrated dysphagia on admission to rehabilitation defined by a score <178 on the Mann Assessment of Swallowing Ability (MASA) [9] and had no previous history of swallowing disability, or surgery of the head or neck. Patients had to be able to adhere to behavioral treatment regimens, provide written informed consent to participate, and be followed-up for the next 3-months.
Background demographics included co-morbidity, pre-morbid function, clinical stroke syndrome, stroke pathology, and etiological stroke subtype. Stroke assessment included the National Institutes of Health Stroke Scale (NIHSS) [10, 11], Modified Rankin Score (mRS) [12], Modified Barthel Index (mBI) [13], Western Aphasia Battery [14] and Mini mental state exam [15] to screen global cognitive function for therapy. All patients received a baseline and post treatment swallowing assessments which included MASA, modified barium swallow study (MBS), and Functional Oral Intake scale (FOIS) [16]. Results were made available to treating clinicians from each intervention arm to assist the direction of each treatment.
Randomization
Recruited patients were randomly assigned to treatment arms using computer assisted random permuted blocks (fixed block size =6, one to one allocation ratio across treatment groups). The randomization schedule was secured remotely from the study environment at a study coordinating center. The remote study coordinator provided assignment by phone to the onsite coordinator and treating therapist in each group.
Interventions
All treatments were provided by speech language pathologists working within the rehabilitation setting. All therapists were experienced clinicians with > 5 years active dysphagia rehabilitative experience.
NMES Application:
NMES was provided using the VitalStim® system. Both the active NMES and sham protocols used a single electrode placement [All electrodes are placed vertically along the midline from above the hyoid bone to immediately superior to the cricoid cartilage]. This placement was reported to be the most commonly applied setting used for swallowing rehabilitation [22]. Following electrode placement stimulation was introduced via ascending amplitude strategy until “motor” level amplitude was achieved. This procedure followed previously published methodology [17].
McNeill Dysphagia Therapy Program [MDTP]:
MDTP is an exercise-based dysphagia intervention with good reported outcomes in severe cases of adult dysphagia [17–21]. MDTP focuses on swallowing as an exercise and incorporates specific criteria for initial oral bolus materials for therapy and how to advance or regress oral bolus materials during therapy. Progression during treatment follows an 11 step “food hierarchy”. Simple swallowing instructions are provided to the patient and clinicians monitor and modify each swallow attempt.
Intervention groups
[UC]. “Usual care” treatment (control):
Behavioral swallowing therapy comprising combinations of treatment strategies / maneuvers chosen from a standard hierarchy. Techniques were derived from reported common techniques in a published case-based survey of speech pathologists treating dysphagic stroke patients [6]. All listed techniques were common behavioral dysphagia treatment methods applied to post stroke dysphagia. A single dedicated experienced UC therapist provided all UC treatment. This therapist had no previous training in the application of NMES or MDTP. Based upon patient evaluation data, the UC therapist designed and directed the treatment intensity and applied the UC treatment daily for one-hour over a consecutive 3-week period (anticipated total of 15 hours or until discharge if earlier than 3-weeks). The UC therapist was blind to the other arms of the study.
[NMES]: MDTP +NMES Active treatment:
A standardized behavioral swallowing intervention, MDTP [17–21], combined with NMES. A single dedicated NMES therapist provided all treatment. This NMES therapist was trained and experienced in MDTP and NMES. Treatment was applied daily for one-hour over a consecutive 3-week period (anticipated total of 15 hours or until discharge if earlier than 3-weeks).
[MDTP] MDTP +Sham NMES treatment:
Behavioral swallowing therapy [MDTP] identical to that provided to the NMES treatment group, but with sham NMES stimulation. Sham stimulation consisted of a replica VitalStim device that delivered 3 minutes of active stimulation at onset, followed by 20% incremental declinations of stimulation over a further 3-minute period. After this ramp down period, the electrodes remained non-stimulating, until the unit was switched off for > 5 minutes. At all times the unit displayed the highest stimulation setting established and held for > 60 seconds within the first 3 minutes of activation (i.e., stimulation threshold). Therapists and patients in both NMES and MDTP groups were instructed that some patients might not feel the stimulation due to sensory accommodation to the signal. A single dedicated therapist provided all MDTP treatment. This MDTP therapist was trained and experienced in both MDTP and NMES procedures. Both the MDTP therapist and patient were blind to the sham application of current through the electrodes. The MDTP therapist applied the treatment daily for one-hour over a consecutive 3-week period (anticipated total of 15 hours or until discharge if earlier than 3-weeks).
All patients were treated in the general wards of the sub-acute rehabilitation hospital. Treatment details, progress, and outcome were monitored by treating therapists. Patients were reassessed post treatment and 3-months after therapy using the baseline measures. Patients not able to attend the 3-month appointment were reviewed by phone.
Masking / Blinding
Research personnel involved in the study were unaware of treatment allocation. Patients allocated to UC, and the speech pathologist treating patients assigned to UC conducted treatment as usual and were unaware of the other study applications. All subjects were referred /evaluated as per usual management procedure and all swallow imaging performed by a blinded clinician. Patients were designated to the treatment arms via computer randomized number from the remote study center to the onsite coordinator and therapists. Patients and speech pathologists assigned to the two intervention arms [MDTP+NMES and MDTP+SHAM] were unaware of the provision of active stimulation or sham stimulation. Active and Sham units were provided via a concealed number allocation that was not disclosed to the therapists. No communication between treating therapists regarding the study content was permitted or occurred.
Neurological assessments were conducted by physicians blinded prior to randomization. Outcomes were assessed by a blinded independent evaluator (IE).
Outcome Events
Endpoints were assessed by the IE at baseline, post treatment, and 3-months. Following treatment patients completed a diary card to record any change in health, swallow, or diet status between the post treatment and 3-month review. Primary outcome included improvement in clinical swallowing ability (MASA score), and oral intake level (FOIS). Secondary outcomes included modified barium swallow outcomes (dysphagia and aspiration), patient self-perception of swallowing ability; body weight, time to recover pre-stroke diet, and the occurrence of dysphagia–related health complications.
Definitions:
Full Response was defined a-priori as improvement ≥10 points in MASA scores from baseline and improvement of ≥2 scale points on the FOIS, without significant weight loss or dysphagia-related complication. The response criteria for MASA score change was based upon previous analysis of reliable clinical change (responsiveness) from a published RCT [23] in stroke patients which demonstrated, RCI=10.52 (SEM 3.13).
Partial response was defined as a 5–10 point increase with or without a 2-point change in FOIS score, with or without weight loss or complications.
Non-response was defined as experiencing < 5-point increment in MASA scores compared to baseline performance with or without weight loss or complications.
Relapse: was defined as >5 point decrement in the MASA score from the post treatment endpoint with or without significant weight loss or dysphagia related complication.
Dependency was defined as a Modified Rankin Scale score of ≥3, or a Barthel index score ≤15.
Chest Infection was defined by presentation of at least three symptoms: fever >38 degrees Celsius, productive cough, abnormal respiratory exam [tachypnea (>22/min)], tachycardia, inspiratory crackles, bronchial breathing], arterial hypoxemia (PO2<70 mmHg), culture of a relevant pathogen, and /or positive chest radiograph in a patient with suspected chest infection.
Clinically significant weight change was defined as +/− 3% or more weight change over the study period.
Abnormal diet was defined as dietary intake (oral or non-oral) requiring a restricted consistency or special preparation before it can be consumed safely.
Functional swallowing recovery was defined as return to pre-stroke diet without swallowing complication.
Statistical analysis
Sample size calculations were based on published data of MDTP+ NMES treatment [17] reporting a mean MASA score (primary outcome) increase post treatment from 160.5 (17.4) to181.75 (8.5) [Cohen’s d effect size =1.54]. Using these data, a sample size of N=51 would yield a power of 80% to detect a difference of 22% (proportion dysphagic) between NMES vs. UC with alpha = 0.05. Calculations were performed using G-Power [24] and Power and Precision 2.0 [25]. Primary comparison was between treatment arms MDTP +NMES/ MDTP+SHAM vs UC.
Parametric statistics were undertaken for normally distributed variables and non-parametric Cochran Q tests, for skewed variables. Relative risk ratios and their 95% confidence intervals were derived for outcomes. Continuous endpoints were reviewed using repeated measures ANOVA, Friedman’s ANOVA, or Kruskal-Wallis test. Chi-square was employed for discrete counts of adverse and dietary events. Exploratory Cox proportional hazards regression analysis, adjusted for age, stroke severity, and swallowing disability was performed to estimate hazard ratios with corresponding 95% CI’s for time to return to pre-stroke diet. The Kaplan-Meier method was used to estimate survival curves. The log-rank test was used to compare survival curves. Median Ratio was used as a descriptive time-to-event measure to quantify the magnitude of benefit from interventions (i.e. median ratio = placebo median time/treatment median time)
Results:
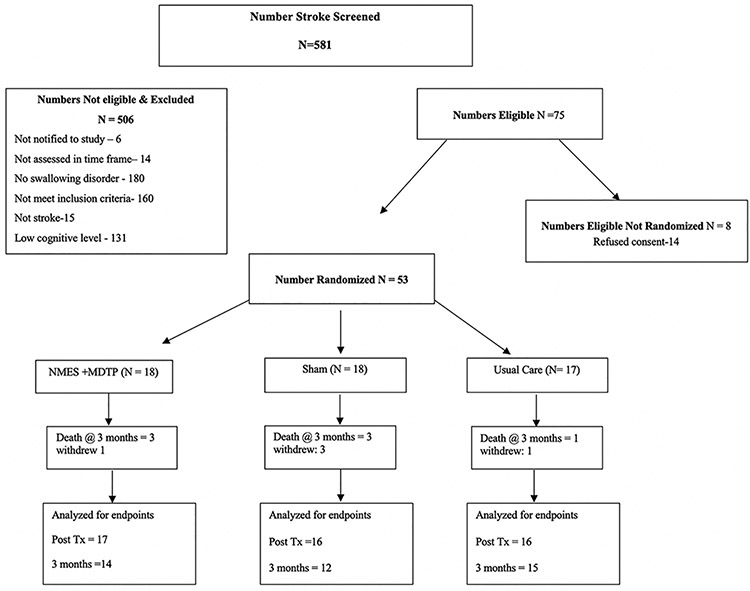
581 suspected strokes were referred to the study team between Jan 2009 and December 2010. From these, 75 (13%) were eligible for inclusion in the study (Figure 1). Written informed consent was obtained from 53 (71 %) eligible patients, who were randomized to UC (n=17), MDTP+NMES (n=18) and MDTP+SHAM (n=18) treatment arms (Table 1). The trial closed once recruitment numbers were met. Ineligible patients did not differ significantly from enrolled patients in stroke type, severity or location. Reasons for non-enrollment are provided in Figure 1. The proportion of baseline factors was similar across study groups (Table 1). Mean time post stroke was 7.67(SD: 5.4) days, and mean time to randomization into the study was 0.35 (SD: 0.68) days after admission. Time post stroke was not significantly different among groups. The mean duration of inpatient stay was 12.4 (SD: 5.7) days. Length of inpatient stay was not different among groups. Over the study period five patients withdrew, and seven were lost to follow up, 5 patients died from complications associated with their primary diagnosis and another 2 died from recurrent stroke. No adverse events were related to the study interventions. Data were complete to 3-months for 41 survivors. Analysis was conducted by intent to treat.
Figure 1.
Trial Profile
Table 1.
Sample Demographics
| Demographic | +MDTP NMES (active NMES) | MDTP+SHAM (MDTP alone) | Usual Care |
|---|---|---|---|
| N=18 | N=18 | N=17 | |
| Age (years), (mean, SD) | 62.7 (12.2) | 70.6 (11.8) | 64.3 (14.7) |
| Gender (male) | 10 | 8 | 7 |
| Race (count) | |||
| -Caucasian | 15 | 16 | 16 |
| -African American | 3 | 2 | 1 |
| Clinical Syndrome (count) | |||
| -TACI | 3 | 2 | 5 |
| -PACI | 8 | 6 | 7 |
| -LACI | 1 | 7 | 1 |
| -POCI | 6 | 1 | 3 |
| Pathology (count) | |||
| -Cerebral infarction | 16 | 17 | 16 |
| -Cerebral hemorrhage | 2 | 1 | 1 |
| Stroke severity (NIHSS) (mean, SD) | 8.8 (4.1) | 9.3 (4.1) | 8.2 (3.7) |
| Stroke Handicap (mean, SD) | |||
| -Modified Rankin | 4.5 (0.61) | 4.46 (0.51) | 4.56 (0.51) |
| Stroke disability (mean, SD) | |||
| -Modified Barthel | 5.27(3.4) | 5.53 (2.8) | 5.56 (2.6) |
| Mini Mental State Exam (mean, SD) | 23.3 (5.4) | 20.12 (7.2) | 23.6 (6.48) |
| Weight (mean, SD) | 177.2 (39.4) | 162 (47.4) | 188.7 (42) |
| BMI (mean, SD) | 29.18 (6.87) | 25.4 (6.39) | 30.57 (7.7) |
| Stroke status (count) | |||
| -First ever | 11 | 13 | 10 |
| -Multiple | 7 | 5 | 7 |
| Days post stroke (mean, SD) | 7.83 (3.9) | 8.47 (7.17) | 6.7 (5.1) |
| Days to randomization (mean, SD) | 0.22 (0.43) | 0.5 (0.78) | 0.35 (0.78) |
| MASA score (mean, SD) | 157.8 (16.5) | 154.62 (18.87) | 158.4 (15.82) |
| FOIS score (mean, SD) | 3.72 (1.44) | 3.25 (1.61) | 4.35 (1.8) |
| G-Tube presence (count) | 6 | 5 | 2 |
| Patient perception (mean, SD) | 7(2.3) | 7.7(2.3) | 6.6 (2.6) |
| Aspiration on VFE (count) | 10/18 (55%) | 7/17 (41%) | 10/17(59%) |
Intervention
Time from stroke onset and sub-acute rehabilitation admission until initiation of swallowing treatment was not different between groups (Table 1). Number of swallowing therapy sessions and duration of sessions for patients assigned to each intervention group did not differ significantly (Table 2).
Table 2.
Post treatment outcomes
| Post Treatment | MDTP +NMES (active NMES) | MDTP +SHAM † (MDTP alone) | Usual Care |
|---|---|---|---|
| N=17 | N=16 | N=16 | |
| #Treatment sessions (mean, SD) | 7.5 (3.5) | 8.93 (3) | 9.2 (3.5) |
| Days admitted- (mean, SD) | 12.16 (5.4) | 12.12 (4.2) | 13 (7.2) |
| NMES Stimulation level (mean, SD) | 6.63 (3.2) | 5.16 (1.4)§ | ‡NA |
| Primary Outcomes | |||
| MASA score (mean, SD) | 172.7 (16.7) | 172 (12.3) | 171.7 (17.3) |
| MASA Change (mean, SD) | 15.2 (12.5) | 17.6 (11.4) | 13.3 (13.7) |
| FOIS change (mean) | 1.2(.97) | 2.1(1.4) | 0.53 (.83) |
| Secondary Outcomes | |||
| G-Tube remaining (count) | 3 | 0 | 2 |
| Tube change-post Tx | 3 | 4 | 0 |
| Patient perception (mean, SD) | 8.76 (1.5) | 8.87 (1.5) | 8.09 (1.8) |
| Dysphagia resolved (count - MASA>178) | 7 | 5 | 3 |
| Aspiration on VFE (%) | 6/17 (35%) | 4/15 (27%) | 5/16 (31%) |
| Weight loss (mean, SD) | 4.3 (2.0) | 4 (4) | 8.2 (10.7) |
| Treatment response | |||
| Responder (full) % | 4/17 (23%) | 9/15 (60%) | 1/16 (6%) |
| Responder (partial) % | 8/17 (47%) | 4/15 (26%) | 4/16 (25%) |
| Total death (count) | 3 | 3 | 1 |
Sham/Faux stimulation only,
N/A = not appropriate
Compliance
Evaluation of Blinding
To confirm adequacy of blinding, study clinicians were asked to estimate group allocation (post treatment) using a secured vote system. Kappa analysis of clinician estimated treatment allocation at the end of the study (k= 0.26), confirmed adequate concealment to prevent bias.
Treatment Compliance
Compliance with prescribed treatments was defined as completing a minimum of two thirds of the program within the available length of the hospital stay. Compliance in all three groups was approximately 70%. No difference was identified between groups in the compliance levels to treatments offered.
Outcomes:
Clinical Swallowing Ability:
The proportion of patients demonstrating dysphagia post-treatment (MASA score ≤ 178) did not differ among the study arms. However, change in MASA severity rating post treatment for the groups was significantly different (χ2= 24.8, p ≤0.0001), the MDTP alone arm demonstrating greater overall positive change than either MDTP+NMES or UC arms, [effect size = 1.37; 95% CI: 0.68–2.07] (Table 2).
Modified Barium Swallow Study Outcomes:
The proportion of patients demonstrating MBS identified dysphagia post-treatment was 41.6 %. Proportions differed significantly among the study arms (χ2=4.083, p≤0.043) with fewer of the MDTP alone arm continuing to display dysphagia. Similarly, resolution of aspiration occurred in 25%. Aspiration events identified post treatment for the groups was also significantly different (χ2=7.73, p ≤0.021), the MDTP alone arm demonstrating greater overall positive change than either MDTP+NMES or UC arms [effect size =1.26; 95% CI: 0.60–2.57].
Oral Intake:
The MDTP+SHAM arm demonstrated the greatest increase in oral intake level (FOIS scores) post treatment (F (2, 45) = 11.38, p≤0.0001), [effect size d= 1.7; 95% CI 0.88-.017]. The MDTP+NMES arm also demonstrated significant change in comparison to the usual care group (t =−3.19, p ≤0.003). (Table 2)
Weight Change:
No significant difference in mean weight change post treatment and at 3-months was identified among treatment arms. However, the categorical cut point for significant weight change (+/− 3% from baseline) demonstrated a descriptive increase in the number of patients demonstrating a ≥3% weight loss within the UC group at the post treatment time point (Table 2)
Treatment Response:
No difference in therapeutic intensity (number of therapy hours applied) was found between groups (Table 2). In total 73% of all patients demonstrated a treatment response to therapy including 86% of MDTP+SHAM group, 70% MDTP+NMES, and 33% UC. Full response was identified in 35% of patients at the post-treatment time point. The rate of full responders’ post-treatment was significantly higher in the MDTP alone group (χ2=9.6, p ≤0.001) compared to the UC [RR = 9 (95% CI: 1.29 −62.5), NNT = 1.87, effect size d=1.56; but not compared to MDTP+NMES [RR =3.5 (95%CI: 0.44–28.2)], NNT = 5.9, effect size d=0.73. The rate of partial responders was not significantly different between groups.
Self-Perception of Swallowing Ability
Mean changes in patient self-perception scores were not different among groups. A significant linear within group’s effect was identified for time post treatment (F (2, 70) =18.7, p≤0.0001) but no between groups difference. Swallowing self-perception scores demonstrated improvement across time in all groups.
Time–to-Event Analysis
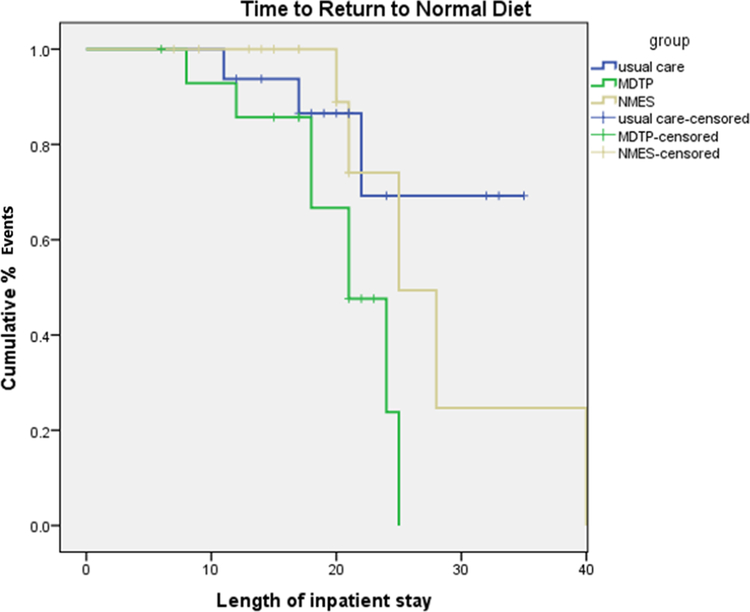
Return to Pre-stroke Diet (Figure 2):
Figure 2.
Kaplan-Meier Survival curve for risk adjusted time to return to pre-stroke diet by intervention group
Person-time for each participant was calculated from the date of treatment initiation to discharge from the sub-acute rehabilitation hospital. Each participant thus contributed only one end point. At the post-treatment time point, 53% of MDTP+SHAM cases reached pre-stroke diet levels compared to 23% of MDTP+NMES (RR: 2.27, 0.85–6.03) and 17.6% UC (RR = 3.0, 1.01–9.36). By three months post-treatment 68% of survivors returned to pre-stroke diet levels including 91% of MDTP+SHAM, 64% MDTP+NMES and 53% UC. (RR = 1.72, 1.04–2.84; NNT = 2.6), and (RR = 1.21, 0.65–2.23, NNT = 9.1).
Exploratory Cox regression analyses (adjusted for age, NIHSS, stroke classification/type, Rankin and MASA baseline score) revealed a significant association (log rank test: χ2=6.39, P<0.04) of reduced time to return to pre-stroke diet by MDTP treatment group, independent of other prognostic factors [Tables 3–4]. Compared with the reference group (UC), the significant risk-adjusted hazard ratio identified for MDTP alone) was 4.317 [95% CI: 1.08–17.2, p< .03]. A hazard ratio > 3 corresponding to a >75% chance of a patient returning to a pre-stroke diet earlier if participating in the MDTP alone group. Further, chi square difference test (x2=7.29, P<.026) suggested an acceptable model fit [Table 4]. The median ratio (MR) indicating the median time at which half the cases are resolved (or magnitude of benefit) for the MDTP alone group in “return to pre-stroke diet” compared to MDTP+NMES was 1.2, and to UC was 1.5.
Table 3.
Actuarial risk for return to normal diet by group
| Number at risk (% non-normal diet) | Length of inpatient stay | |||
|---|---|---|---|---|
| Treatment Group | 0 | 10 | 20 | 30 |
| UC | 16 (100%) | 15 (93) | 9 (85) | 4 (72) |
| MDTP alone | 15 (100%) | 12 (86) | 6 (31) | 0 (0) |
| MDTP +NMES | 16 (100%) | 15 (94) | 9 (71) | 1 (24) |
Log Rank test: x2=6.397, p<.041.
Table 4.
Cox regression analysis (final model): Time to return to normal diet at 3-months. (n=53): Overall χ2 <.026
| Covariate | Coeff (b) | SE (b) | Significance (p) | Hazard ratio for unit change | 95% CI |
|---|---|---|---|---|---|
| Group: | |||||
| MDTP alone | 1.462 | 0.706 | 0.038 | 4.317 | 1.081–17.23 |
| MDTP +NMES | 0.441 | 0.766 | 0.564 | 1.555 | 0.347–6.97 |
Coeff (b) denotes regression coefficient; SE, standard error.
Dysphagia Related Complications
Too few patients experienced chest infection or other dysphagia–related complications to permit statistical analysis. A single MDTP+NMES patient experienced a chest infection within the 3-month follow up period. An additional MDTP+NMES patient reported dehydration during the post treatment period.
Follow up:
Follow up was completed by phone in 58% (24/41) of patients and 41% (17 /41) attended an in-person follow up appointment permitting the MASA to be re-administered. A statistically significant difference was found among groups in MASA score at 3 -months [H=6.3, 2 df, p =0.043]. The MDTP+SHAM group demonstrated higher mean swallowing scores overall [ = 195.2, SD: 5.5]. Within MDTP+NMES and UC groups, nine patients (22%) continued to demonstrate lower swallowing function scores (MASA and or FOIS) and two remained G-tube dependent.
Relapse rates:
At the 3-month reassessment three patients (two UC, one MDTP+NMES) demonstrated relapse with a mean reduction of 6.3 (SD: 1.5) points in MASA score, additional diet modification and >3% weight loss post active treatment. Among patients reporting outcome via phone review, two patients (MDTP+NMES group) reported relapse including complications of pneumonia and dehydration since discharge (noted above).
Discussion
This study identified significant benefit in post-stroke swallowing outcome from the application of an exercise-based swallowing therapy protocol (MDTP) without active NMES. Thus, the primary hypothesis that MDTP+NMES would result in superior outcomes was not upheld. However, exercised based swallowing therapy (MDTP) with and without NMES, resulted in greater benefit (i.e. improved oral intake, reduced dysphagia severity level, and more rapid return to pre-stroke diet) than usual care therapy.
Application of behavioral swallowing therapy approaches to post-stroke dysphagia rehabilitation is an accepted practice, [6] however the addition of adjunctive NMES has been strongly debated. The addition of electrical stimulation to traditional swallowing treatment following stroke may complement voluntary swallowing exercise, shortening rehabilitation time and enhancing outcome [26–28]. However, data supporting this contention is ambiguous. To date five published randomized controlled trials (n = 161) and three quasi-randomized clinical trials (n = 247) have examined the efficacy of NMES in post-stroke dysphagia with varying outcomes. Although six of these studies report positive outcomes, all are limited by poor treatment design, use of non-validated measures and systematic investigator bias [29]. Further, recent published meta-analyses disagree with each other, one identifying no significant difference in post-stroke dysphagia resolution from four of these trials (n=175) [8]. The other reporting a positive effect of NMES paired with traditional swallowing therapy from 8 studies [7]. Unfortunately, this last meta-analysis pooled studies with dramatically different formulations of “traditional swallowing therapy” with significant heterogeneity scores (I2 =85%) suggesting inadequate statistical pooling.
In contrast, the current study identified attenuated benefit from the addition of NMES to standardized exercise-based therapy (MDTP). Reasons for this apparent negative effect of NMES are speculative. Addition of NMES to MDTP may facilitate less efficient muscle training by way of preferential recruitment of fast fibers or the “reverse recruitment order” principle. In our study the preferential recruitment of type II fast fibers from NMES may have opposed muscle fiber recruitment from voluntary practice incorporated in the MDTP protocol. This opposition may have attenuated the “efficiency “of muscle response, i.e. less time spent in normal voluntary contraction practice. This reduced specificity of training may account for the apparent negative impact of NMES. Support for this theory remains contentious [30], and resolution of the debate surrounding reverse recruitment by electrical stimulation is beyond the scope of this study.
Another postulated reason for the reduction in benefit noted within the NMES arm is the impact of age related muscle plasticity and a selective response to electrical stimulation by age. Recent work has identified a selective response by older patients to sensory levels (low amplitude) of stimulation compared to tetanic (motor level) stimulation [31]. Differences in muscle plasticity with increasing age are noted as muscle fibers are replaced by connective and fat tissue, and muscle conditioning by NMES becomes more difficult. Consequently the training response to NMES is likely slower as the connective tissue and fat does not participate directly in contraction. Moreover, return to baseline levels (de-training) after the cessation of electrical stimulation is quicker in the elderly. Training of elderly skeletal muscle might better involve lower stimulation levels and longer training protocols with more prolonged periods of rest between contractions [32]. Our study employed a motor level NMES protocol and thus cannot explore the role of sensory level stimulation for post-stroke dysphagia rehabilitation.
Finally, given the short period of training employed in this study (up to 3-weeks) it is possible that the different responses between MDTP alone and MDTP+NMES intervention arms relates directly to an improved motor control elicited from the more voluntary exercise-based therapy [MDTP] early in rehabilitation. Prior research has identified that NMES does not improve coordination between muscle groups and does not facilitate motor learning and the coordination of complex movements [33]. Consequently, benefit identified in our MDTP group may be largely from early capitalization on motor learning.
Superior outcomes noted in MDTP+NMES and MDTP+SHAM groups compared to the UC group may also have resulted from the therapy formulation applied. The MDTP protocol provides a standardized exercise-based therapy platform while UC is often capricious. We chose not to match the UC, and MDTP/ NMES groups for specific behavioral techniques or number of techniques trialed for several reasons. First, session treatment time is prescribed by health care requirements of a rehabilitation center and also the NMES protocol, therefore making the groups equitable at this level. Second, our goal was to simulate (as close as possible) current clinical practice for dysphagia rehabilitation. Prior research has demonstrated that applied dysphagia practice typically consists of differing intensities of a variable range of behavioral treatment techniques [6]. In light of this and to reduce potential variability, we included a hierarchy of common treatment choices for clinicians to select from. The treating UC clinician formulated and chose each treatment specific to individual patients dysphagic issues from the prescribed hierarchy of techniques (+/− a bolus), using information from clinical and MBS assessment. We believe this approach reflected UC intervention consistent with real world application.
The strength of this investigation rests on the fact that the study employed a true double blind placebo controlled randomized design. Although somewhat small, the study sample was well characterized, baseline data were complete, and follow-up was prospective, with minimal loss to 3-months post stroke. Moreover, the study employed significant control for bias and confounding, the maintenance of blinding was strong, and an independent evaluator assessed all patients. Consequently, despite the capitated sample size, the clinical effect sizes recorded were moderate to large suggesting true benefit from intervention.
We believe that these data have several implications for clinical practice. Our analyses of time to return to pre-stroke diet during the study time period revealed that diet recovery improved over time in all groups. Most importantly however, the time for diet recovery in patients within the MDTP alone arm was significantly quicker than the other comparators suggesting that 3-weeks of exercise-based swallowing intervention may reduce dependence on alternate or modified diets in stroke rehabilitation patients.
Conclusion
This double-blind placebo controlled RCT identified greatest benefit from the application of exercise-based behavioral swallowing therapy (MDTP) paired with sham electrical stimulation over that of the same therapy paired with active NMES and a usual care comparator. Importantly, benefit from MDTP + active NMES while less than MDTP alone, conferred greater benefit than usual care practice. These results add to the preliminary data on the effectiveness of intensive exercise-based behavioral swallowing interventions for patients following stroke. These data support the inclusion of shorter-term intense behavioral intervention for swallowing to enable efficient allocation of resources in post- acute stroke rehabilitation
Acknowledgements:
This study was supported a grant from NIH/ NCMRR: NIH-R21HD054752. The authors wish to thank Ms. Diana Miller and her Speech Pathology staff for their contributions.
Footnotes
ClinicalTrials.gov Identifier: NCT01279824- full trial accessible from https://clinicaltrials.gov/
Contributor Information
Giselle D. Carnaby, School of Communication Sciences & Disorders, University of Central Florida.
Lisa LaGorio, Department of Communication Disorders and Sciences, Rush University.
Scott Silliman, Department of Neurology, University of Florida, Jacksonville.
Michael Crary, School of Communication Sciences & Disorders, University of Central Florida.
References:
- 1.Mann G, Hankey GJ, Cameron D. Swallowing disorders following acute stroke: prevalence and diagnostic accuracy. Cerebrovasc Dis. 2000; 10:380–6. [DOI] [PubMed] [Google Scholar]
- 2.Mann G, Hankey GJ, Cameron D. Swallowing function after stroke: prognosis and prognostic factors at 6months. Stroke. 1999; 30:744–8. [DOI] [PubMed] [Google Scholar]
- 3.Martino R, Foley N, Bhogal S, Diamant N, Speechly M, Teasell R. Dysphagia after stroke: Incidence diagnosis and pulmonary complications. Stroke. 2005; 36: 2756–2763 [DOI] [PubMed] [Google Scholar]
- 4.Sharma JC, Fletcher S, Vassallo M, Ross I. What influences outcome of stroke- pyrexia or dysphagia. Int JClin Pract. 2001; 55: 17–20. [PubMed] [Google Scholar]
- 5.Carnaby G, Madhavan A A Systematic Review of Randomized Controlled Trials in the field of Dysphagia Rehabilitation. Current Phys Med Reports. 2013; December (1) 4:197–215. [Google Scholar]
- 6.Carnaby G, Harenberg L. What is “Usual Care” in Dysphagia Rehabilitation: A Survey of U.S.A Dysphagia Practice Patterns? Dysphagia. 2013; 28 (4): 567–74. [DOI] [PubMed] [Google Scholar]
- 7.Chen YW, Chang KH, Chen HC, Liang WM, Wang YH, Lin YN. The effects of surface electrical stimulation on post-stroke dysphagia: a systemic review and meta-analysis. Clin Rehabil. 2016; 30(1): 24–35. [DOI] [PubMed] [Google Scholar]
- 8.Tan C, Liu Y, Li W, Liu J, and Chen L. “Transcutaneous neuromuscular electrical stimulation can improve swallowing function in patients with dysphagia caused by non‐stroke diseases: a meta‐analysis.” Journal of oral rehabilitation. 2013; 40 (6): 472–480. [DOI] [PubMed] [Google Scholar]
- 9.Mann GD The Mann assessment of swallowing ability. Clifton Park: Singular Thompson Learning; 2002. [Google Scholar]
- 10.Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R,Hertzberg V, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke.1989;20:864–70 [DOI] [PubMed] [Google Scholar]
- 11.Goldstein LR, Samsa GP. Reliability of the National Institutes of Health Stroke Scale. Extension to nonneurologists in the context of a clinical trial. Stroke. 1997; 28:307–10. [DOI] [PubMed] [Google Scholar]
- 12.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S,Donnan G, Schneider D, Diez-Tejedor E, Trouillas P. Randomised double-blind placebo-controlled trialof thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998; 352:1245–51. [DOI] [PubMed] [Google Scholar]
- 13.Collin C, Wade DT, Davis S, Horne V. The Barthel ADL index: a reliability study. International Disability Studies. 1988; 10:61–3. [DOI] [PubMed] [Google Scholar]
- 14.Kertesz A (1982). The Western Aphasia Battery. New York: Grune & Stratton. [Google Scholar]
- 15.Folstein MF, Folstein SE, & McHugh PR Mini-Mental State. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 16.Crary M,Carnaby-Mann G, Groher G Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil 2005; 86:1516–1520. [DOI] [PubMed] [Google Scholar]
- 17.Carnaby-Mann G, Crary M Adjunctive Neuromuscular Electrical Stimulation for Treatment-refractory Dysphagia Ann Otol Rhinol Laryngol 2007; 117: 279–287 [DOI] [PubMed] [Google Scholar]
- 18.Carnaby-Mann G, Crary M (2010) McNeill Dysphagia Therapy Program (MDTP): A case control study. Arch Phys Med Rehabil. 2010; 91(5): 743–9 [DOI] [PubMed] [Google Scholar]
- 19.Crary MA, Carnaby-Mann GD, LaGorio L, Carvajal P. Functional and physiological outcomes from an exercise-based dysphagia intervention program: MDTP. Arch Phys Med Rehabil. 2012; 93(7):1173–8. [DOI] [PubMed] [Google Scholar]
- 20.Lan Y, Ohkubo M, Berretin-Felix G, Sia I, Carnaby-Mann GD, Crary MA. Normalization of temporal aspects of swallowing physiology following the McNeill Dysphagia Therapy program (MDTP). Annals of Oto-Rhino-Laryngology 2012; 121(8):525–32. [DOI] [PubMed] [Google Scholar]
- 21.Sia I, Carvajal P, Lacy A, Crary MA, Carnaby-Mann G. Hyoid and Laryngeal Excursion Kinematics - Magnitude, Duration and Velocity- Changes Following An Exercise-Based Dysphagia Treatment: MDTP. Dysphagia. 2014; December 8 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.[VitalStim FDA submission] FDA 2001, http://www.fda.gov/cdrh/510k/sumjun01.html - accessed on January 26, 2015.
- 23.Carnaby G, Hankey GJ, Pizzi J. Behavioral intervention for dysphagia in acute stroke: a randomized controlled trial. Lancet Neurol. 2006; 5(1):31–7. [DOI] [PubMed] [Google Scholar]
- 24.Erdfelder E, Faul F, & Buchner A G POWER: A general power analysis program. Behavior Research Methods, Instruments, & Computers. 1996; 28: 1–11. [Google Scholar]
- 25.Bornstein M Rothstein H, Cohen J. Power and Precision: A Computer Program for Statistical Power Analysis and Confidence Intervals. Englewood, NJ: Biostat; 2000. [Google Scholar]
- 26.Freed ML, Freed L, Chatburn RL, Christian M Electrical stimulation for swallowing disorders caused by stroke. Respir Care 2001; 46:466–474. [PubMed] [Google Scholar]
- 27.Lim KB, Lee HJ, Lim SS, Choi YI. Neuromuscular electrical and thermal-tactile stimulation for dysphagia caused by stroke: a randomized controlled trial. J Rehabil Med 2009; 41(3):174–178. [DOI] [PubMed] [Google Scholar]
- 28.Permsirivanich W, Tipchatyotin S, Wongchai M, et al. Comparing the effects of rehabilitation swallowing therapy vs. neuromuscular electrical stimulation therapy among stroke patients with persistent pharyngeal dysphagia: a randomized controlled study. J Med Assoc Thai. 2009; 92(2):259–265. [PubMed] [Google Scholar]
- 29.Humbert I, Michou E,MacRae P, Cruijdo L. Electrical Stimulation and swallowing: How much do we know? Semin Speech Lang. 2012; 33(3): 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gregory C, Bickel S. Recruitment patterns in human skeletal muscle during electrical stimulation. Phys Ther.2005; 85:358–364. [PubMed] [Google Scholar]
- 31.Berretin-Felix G, Sia I, Barikroo A, Carnaby GD, Crary MA. Immediate effects of transcutaneous electrical stimulation on physiological swallowing effort in older versus young adults. Gerodontology. 2014; November 12 [E-pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 32.Chekanov VS, Karakozov P, Rieder M, Zander G. Age related skeletal muscle response to electrical stimulation. ASAIO J. 2000; 46(4): 474–481 [DOI] [PubMed] [Google Scholar]
- 33.Paillard T Combined application of neuromuscular electrical stimulation and voluntary muscular contractions. Sports Med. 2008; 38(2): 161–77. [DOI] [PubMed] [Google Scholar]