Abstract
Electron cryo-tomography allows for high-resolution imaging of stereocilia in their native state. Because their actin filaments have a higher degree of order, we imaged stereocilia from mice lacking the actin crosslinker plastin 1 (PLS1). We found that while stereocilia actin filaments run 13 nm apart in parallel for long distances, there were gaps of significant size that were stochastically distributed throughout the actin core. Actin crosslinkers were distributed through the stereocilium, but did not occupy all possible binding sites. At stereocilia tips, protein density extended beyond actin filaments, especially on the side of the tip where a tip link is expected to anchor. Along the shaft, repeating density was observed that corresponds to actin-to-membrane connectors. In the taper region, most actin filaments terminated near the plasma membrane. The remaining filaments twisted together to make a tighter bundle than was present in the shaft region; the spacing between them decreased from 13 nm to 9 nm, and the apparent filament diameter decreased from 6.4 to 4.8 nm. Our models illustrate detailed features of distinct structural domains that are present within the stereocilium.
Keywords: Hair cell, stereocilia, cryo-electron microscopy, actin, volumetric model
Introduction
Our senses of hearing and balance depend on the mechanosensitive hair bundles of the inner ear’s sensory cells, the hair cells. A bundle protrudes from a hair cell’s apical surface and consists of rows of actin-filled stereocilia, which are arranged in a staircase (Roberts et al., 1988; Gillespie and Müller, 2009; Fettiplace and Kim, 2014). Each stereocilium consists of a distal tip, a shaft, and a proximal taper region, with the plasma membrane enclosing a highly-crosslinked actin filament core. About 400 actin filaments are found in each mouse utricle stereocilium (Krey et al., 2016); they are thought to run uninterrupted (Corwin and Warchol, 1991) from the tip along the shaft, parallel to the stereocilium longitudinal axis, before they end near the plasma membrane in the taper region (Tilney et al., 1986). The most central filaments condense into a rootlet structure, which penetrates deep into the actin meshwork of the cuticular plate and provides a pivot point that anchors the stereocilia (Tilney et al., 1980). In conventional transmission electron microscopy images, the core of the taper region and the initial insertion of the rootlet shows very high contrast when stained with osmium tetroxide, indicating either a very high protein density, an unusually high affinity for osmium, or both.
Actin filaments are heavily cross-linked by PLS1 (plastin 1; also known as fimbrin) (Sobin and Flock, 1983; Tilney et al., 1989; Daudet and Lebart, 2002), ESPN (espin) (Zheng et al., 2000; Sekerkova et al., 2004; Sekerkova et al., 2006), and FSCN2 (fascin 2) (Shin et al., 2010; Chou et al., 2011; Hwang et al., 2015). Early transmission electron microscopy studies suggested that stereocilia cores had a paracrystalline crosslinker pattern with full crosslinker occupancy (DeRosier et al., 1980; Jacobs and Hudspeth, 1990; Hackney et al., 1993). Mice lacking PLS1 display an actin filament core that is better ordered when compared to wild type mouse stereocilia (Krey et al., 2016), and strongly resembles the hexagonally packed actin core of chick cochlea (Tilney et al., 1983) and chick utricle (Shin et al., 2013).
Quantitative mass spectrometry analysis in combination with electron tomography of high-pressure frozen, freeze-substituted, and resin-embedded tissue samples provided an estimated inventory of stereocilia proteins in chick utricle and showed that actin crosslinkers were not as abundant and regularly spaced as expected for a paracrystalline array (Shin et al., 2013).
While hair cells with damaged hair bundles show some capacity for repair or replacement (Robertson et al., 1980; Liberman and Dodds, 1987; Gale et al., 2002), the stereocilia actin core is generally thought to be very robust. How the filamentous core is maintained is not entirely clear, however. Fluorescence studies of transfected mammalian hair cells in culture indicated that tagged actin or tagged ESPN incorporate into stereocilia tips and spread down the stereocilium (Rzadzinska et al., 2004). Those findings suggested a continuous treadmilling mechanism, where actin is polymerized at the stereocilia tip and depolymerized near the taper region membrane, thus resulting in a net movement of all actin filaments and their crosslinkers towards the base of a stereocilium (Rzadzinska et al., 2004). This model was challenged by a study that included multi-isotope imaging mass spectrometry, differential temporal expression of labeled actin proteins in vivo, and fluorescence recovery after photobleaching; these experiments showed that while protein turned over rapidly at stereocilia tips, most protein remained stationary for weeks in the shaft, arguing against treadmilling (Zhang et al., 2012). Moreover, McDermott and colleagues used fluorescence imaging of genetically encoded in intact zebrafish larvae ear and showed dynamic turnover of both fascin 2b and actin β throughout the hair bundle (Hwang et al., 2015), further questioning the treadmilling model of actin turnover in stereocilia.
In the taper region, peripherally located actin filaments end in close proximity to the plasma membrane whereas the central actin filaments of the actin core extend through the rootlet region into the cuticular plate (Itoh and Nakashima, 1980; Tilney et al., 1980; Itoh, 1982; Slepecky and Chamberlain, 1982; Tilney and DeRosier, 1986). In resin-embedded, osmium-stained samples, the rootlets appear as dark structures, which prevents discrimination of internal features.
The actin core is connected to its surrounding plasma membrane via RDX near the taper region (Kitajiri et al., 2004; Pataky et al., 2004; Zhao et al., 2012). In addition, unconventional myosins also serve as actin-to-membrane connectors throughout the stereocilium (Hasson et al., 1997). For example, MYO6 is found at stereocilia bases (Hasson et al., 1997), MYO7A along the entire shaft (Morgan et al., 2016), and MYO3A, MYO3B, and MYO15A at stereocilia tips (Belyantseva et al., 2003; Schneider et al., 2006; Merritt et al., 2012). The location of MYO1C is controversial; it is either concentrated at the upper tip link insertion side (Garcia et al., 1998; Steyger et al., 1998), where adaptation is thought to occur, or is found throughout the stereocilia membrane (Belyantseva et al., 2005). In mammalian vestibular hair cells, MYO1H is the predominant myosin-I isoform (Krey et al., 2015).
Electron cryo-tomography (cryo-ET) has emerged as a powerful method for examining macromolecular structures in cells in general (Baker et al., 2017; Oikonomou and Jensen, 2017; Hutchings and Zanetti, 2018) and the cytoskeleton in particular (Jasnin et al., 2013; Turgay et al., 2017; McIntosh et al., 2018; Sun et al., 2018). We have previously reported cryo-ET studies at ~3–4 nm resolution of frozen-hydrated individual stereocilia, which were isolated by blotting them away from the sensory epithelia onto a poly-lysine-coated grid (Metlagel et al., 2019). Here we describe a simplified volumetric model of the actin core in the tip, shaft and taper regions of stereocilia harvested from utricular sensory epithelia of murine Pls1−/− mice. We present here an ultrastructural 3D analysis of unstained, frozen-hydrated samples in an unstained near-native state. Although actin filaments adopt a highly ordered 3D organization in the shaft region, we also found significant longitudinal gaps along the actin filaments, indicating that actin filaments do not run uninterrupted from taper to tip. Examining the actin-actin crosslinker distribution, we found that only a fraction of possible crosslinking sites were occupied. In the tapered region, actin filaments adopt a complex 3D organization; the central core of the actin-filament bundle twists along the filament axis, which leads to a compacted, dense rootlet structure. Our results reveal new structural features of the stereocilia actin core and provide near-native dimensions for these features.
Results
While we collected and reconstructed 26 tomograms of vestibular stereocilia from Pls1−/− mutant mice, our in-depth analysis was focused on four tomograms, two for the tip and shaft region and two for the taper region. Our quantitative results presented here stem from one tomogram each of the shaft and taper regions and the conclusions derived from these data were confirmed by the other two tomograms. All Pls1−/− stereocilia reconstructed and examined displayed a higher degree of order for the actin core when compared to wild-type samples (Krey et al., 2016; Metlagel et al., 2019), with Fourier analysis yielding an estimated 4.3 nm resolution for the Pls1−/− density maps examined. This limited resolution prevented us from docking atomic models into the density maps, but allowed us to build simplified volumetric ball-and-stick models into the density map, and to determine filament and cross-link numbers, dimensions, curvature, distances, and spacings.
Manual model building followed by refinement
We obtained cryo-tomograms of structurally intact stereocilia that were blotted from utricles of Pls1−/− mice. Examination of a single ~1 nm cryo-tomogram slice central overview of the tip and shaft region (Fig. 1A), as well as a 10 nm cryo-tomogram slab close-up of the shaft region (Fig. 1B), revealed regularly spaced filamentous densities, which become more easily visible when tilting the 10 nm slab by ~75 degrees out of plane (Fig. 1C). Tilting of the density maps along the stereocilia axis made it much easier to detect the actin-filament axis and place a ball-and-stick starting model onto the map. Rather than placing individual models one-by-one for each filament into the density maps, we simultaneously placed up to 17 parallel strands with an original center-to-center spacing of 12.5 nm (Fig. 1D). Once we obtained by visual inspection an acceptable global fit for an individual actin model layer to the ~10 nm density slab (Fig. 1D), we locally adjusted each of the filament models manually to be at the center of the observed density maps (Fig. 1E). Length adjustments of the models in the tip region (Fig. 1F) resulted in a first model for each actin filament layer (Fig. 1G). After such longitudinal-orientation model building, we validated our model by replacing each cross-sectional slice (Fig. 1H) by a slice that represents an average density over 30 nm of slices (Fig. 1I–J) at the respective position, which greatly improved the signal-to-noise ratio. We refined the position of each filament model for each 30 nm-cross-averaged slice (Fig. 1K), resulting in a hexagonally close-packing 3D bundle (Figure 1L–M). Using our semi-automated filament tracing approach (Sazzed et al., 2018), we validated the manually-built model. In addition, the semi-automated approach allowed us to fill in positions between the manually placed position along each filament, optimizing the fit locally.
Figure 1. Volumetric model building of actin filaments into tip and shaft, or shaft and taper region of a Pls1−/− stereocilium.
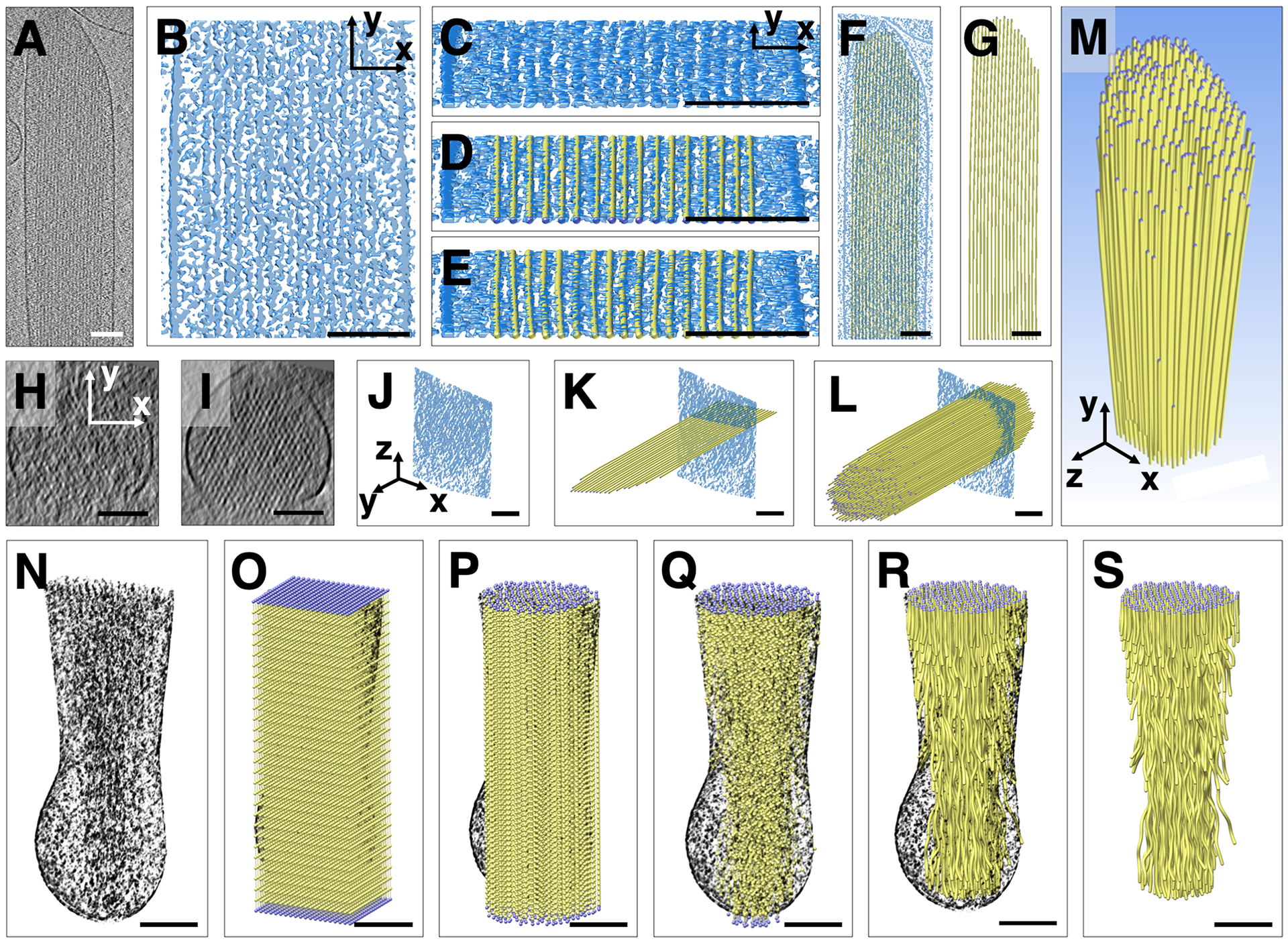
(A) Cryo-tomographic grayscale map of a Pls1−/− stereocilium; slice of ~1 nm thickness in longitudinal (XY) orientation. (B) Solid rendering (using the UCSF Chimera Surface labeling function) of a central portion of the shaft region, depicting a complex scenery of parallel actin filament and cross-linker densities. (C) Same shaft region as depicted in B, but rotated around the X-axis by ~80°, which allows to view almost precisely along the filament axis, making the filamentous nature of the densities more obvious. (D) Manual fitting of one layer of a simplified balls-and-sticks-model of parallel, tube-like actin filaments. (E) After global fitting for an individual model layer of parallel actin-filament tubes, the position of each ball was adjusted to achieve a local fit of each of the actin-filament tubes to their respective density. (F, G) Single layer model shown with (F) or without (G) a central slab density map. (H) Single slice of density map in cross-sectional (XZ) orientation. Note that the map is very noisy and periodicity is difficult to see. (I, J) A 30 nm Y-axis-averaged cross-sectional (XZ) slice showing clear periodicity of hexagonal packing of actin filaments in orthographic view. The model is rotated in panel J so the square slab is visible from the side. (K) Longitudinal single actin layer model superimposed onto single slice of a 30 nm-averaged cross-sectional map. Note that the model can be very accurately positioned into the density map shown in cross-sectional orientation. (L) The entire actin bundle model superimposed onto single slice of 30 nm-averaged cross-sectional map. (M) Entire model of actin filaments of the stereocilia actin core shown in perspective view. (N) Partial cutaway of tomographic volume in taper region with all density outside of the membrane (ice) removed. The filament model was combined with this volume in O-R. (O) Rectangular prism containing 20 × 20 × 30 balls, with 20 × 20 balls spaced ~12 nm apart in the cross-sectional plane of the taper region/rootlet and spaced 20 nm apart in the longitudinal stereocilia axis. (P, R) Corresponding balls along the filament axis were connected by sticks. Only balls inside a cylinder with a radius of the stereocilia membrane in the shaft region (top) were retained. (Q) Only balls corresponding to features of the density map inside the confines of the stereocilia membrane were retained. (S) Final coarse model of actin filament core, including the shaft, taper, and rootlet regions. Scale bars = 100 nm.
For the tapered region of the stereocilia (Fig. 1N), we took a different approach, reflecting the fact that individual actin filaments in the bundle undergo a more complex path in the rootlet portion of density map. We started out by placing an array of 20 × 20 × 30 balls onto the corresponding density map (Fig. 1O). Corresponding balls were then connected along the filament axis. All model balls that fell outside the actin core density map were eliminated (Fig. 1P–Q). For each of the 30 cross-sectional layers spaced 20 nm apart we adjusted the position of balls using a 20-slice average at each of the positions (Fig. 1R).
Global bending of the actin filament core
Actin filaments in the stereocilia were parallel to one another throughout the shaft and tip region, and in the shaft region did not deviate from the stereocilia main axis (Fig. 2a). However, near the tip, all actin filaments deviated from the main axis towards the side of the lower stereocilium by 5 degrees on the side adjacent to a shorter neighboring stereocilium, and up to 8 degrees on the tall neighbor’s side (Fig. 2B). All filaments were bent in the same direction, which was obvious when viewing the volumetric ball-and-stick model head on (Fig. 2C). This curvature resulted in a displacement of the filament tips by ~10–15 nm, which is hardly noticeable in longitudinal views (Fig. 2A), but becomes more obvious when viewed at an angle along the filament axis (Fig. 2B), or head-on (Fig. 2C). Note that all filaments underwent the same curvature and thus remained parallel to one another.
Figure 2. Actin core curvature and gaps in the Pls1−/− stereocilium model.
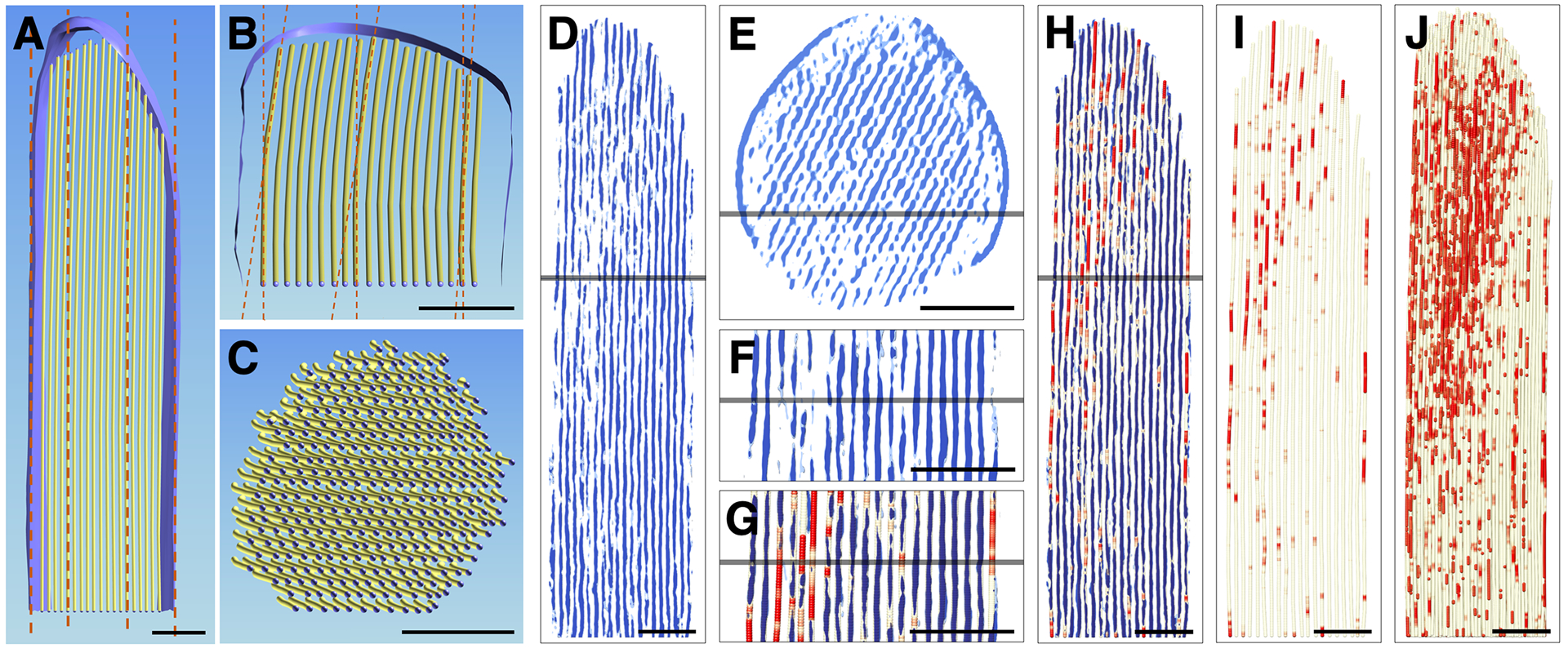
(A-C) Three views of a single central layer of actin in the model. (A) Longitudinal view of a single central layer of actin in the stereocilia model. Dotted line indicates main axis of actin filaments and stereocilia. (B) Same actin layer rotated ~75° along the X-axis. Foreshortening emphasizes the small deviations of 5–8° near the tip of stereocilia. Plasma membrane surface rendering is shown in A and B. (C) Cross-section view revealed that all actin filaments curve near the tip into the same direction, i.e., away from lower tip link insertion site. (D) Slab of 10 nm through 3D volume where each pixel inside the stereocilia membrane along the Y-axis has been replaced by a 30 nm Y-axis average to increase the signal-to-noise ratio in the direction of the filament. Note that significant gaps exist in the 30 nm averaged map. (E) Cross-sectional view of 30 nm averaged map rotated around X-axis. Note the periodicity of the density map and several gaps in the periodic pattern. (F) Close-up view of D. (G, H) Model of actin filament superimposed onto 30 nm averaged map. Care was taken to refine position of the model to reflect that 30 nm average map. Three map thresholds were chosen; we used cutoffs of ~20% above, exactly at, and ~20% below the average of the density map at the filaments and at the space between the actin filaments. Red color-coding reflects actual density value of the ball position with red being well below the average density, and thus a gap in the density map averaged for 30 nm along the actin filament axis. (I, J) Gap model (red) of actin filament core in single 2D layer (I) and the 3D bundle (J). Scale bars = 100 nm.
Actin filament gaps
A thorough analysis of a map where each XZ slice was replaced by its 30 nm-slab average (Fig. 2D–H) revealed holes in the density map, both in longitudinal (Fig. 2D, 2F–H) and in cross-sectional (Fig. 2E) orientations. We overlaid our actin filament model onto the 30 nm averaged map and color-coded the model red in the gap regions where the density was missing (Fig. 2G). Gaps typically ranged in length from ~20 to ~75 nm, and are shown in Fig. 2I for an individual model layer or in Fig 2J for the entire actin filament core. There was no obvious pattern to the distribution of the gaps, and they were found throughout the stereocilium; there did appear to be more gaps near the tip of stereocilium, however (Fig. 2J). We also examined three tomograms of stereocilia isolated from wild-type mouse utricle. While we did not develop a model for a wild-type stereocilium, we noted that gaps were also present in the shaft region of these stereocilia.
To ensure that the gaps in the actin filaments were not an artifact of 30 nm slab averaging of actin filaments that were hypothetically locally displaced, we examined maps that had been denoised but were not averaged. We visualized ~1 nm thin cross-sectional slices of a non-averaged but non-linear anisotropic diffusion denoised map (Fig. S1A–C), slices from a 10 nm slab-averaged map (Fig. S1D–F), and slices from a 30 nm slab-averaged map (Fig. S1G–I). False-color visualization emphasized the location of the high and low-density peaks. We then probed the grayscale cross-sectional images using a 5 × 7 pixel window and measured the integrated density over this window both for the high density peaks, its neighboring low-density areas, as well as the position and its immediate surrounding of the actin-filament gap (Fig. S1J–M). The integrated density value at the position of the actin gap and its immediate surrounding matched well the background densities, which differed significantly from the high-density values at the peaks (Fig. S1N). These results suggest that in those gap regions, there is no actin filament density.
Actin-to-membrane connectors in tip and shaft regions
To better understand the interaction of actin filaments with macromolecules in the stereocilia tip region, we divided the density in our structure that lies between the top of the actin filaments (yellow lines) and the tip membrane (blue) into two regions, which we color-coded golden and maroon. Maroon-colored structures correspond to density within 10 nm of the distal end of the actin filaments, and thus constitutes the density map for proteins that may bind directly to actin filaments (Fig. 3A). In Fig. 3A, a 10-nm slab of the density that corresponds to a single actin model layer is shown. Fig. 3B shows the density as a 3D object in longitudinal orientation; the actin paracrystal was omitted in this view. When rotated 90° around the X-axis, one obtains an en-face view of the maroon-colored density that resides in close proximity to the actin filament ends. Note that there are a number of maroon-colored shapes of similar dimensions that are located near the actin filaments, whose ends are indicated by small dots (Fig. 3C, D). In Fig. 3D, the balls have a diameter of 6 nm, in accordance with the dimensions of actin filaments.
Figure 3. Actin-membrane crosslinkers in the tip and shaft regions of the Pls1−/− stereocilium model.
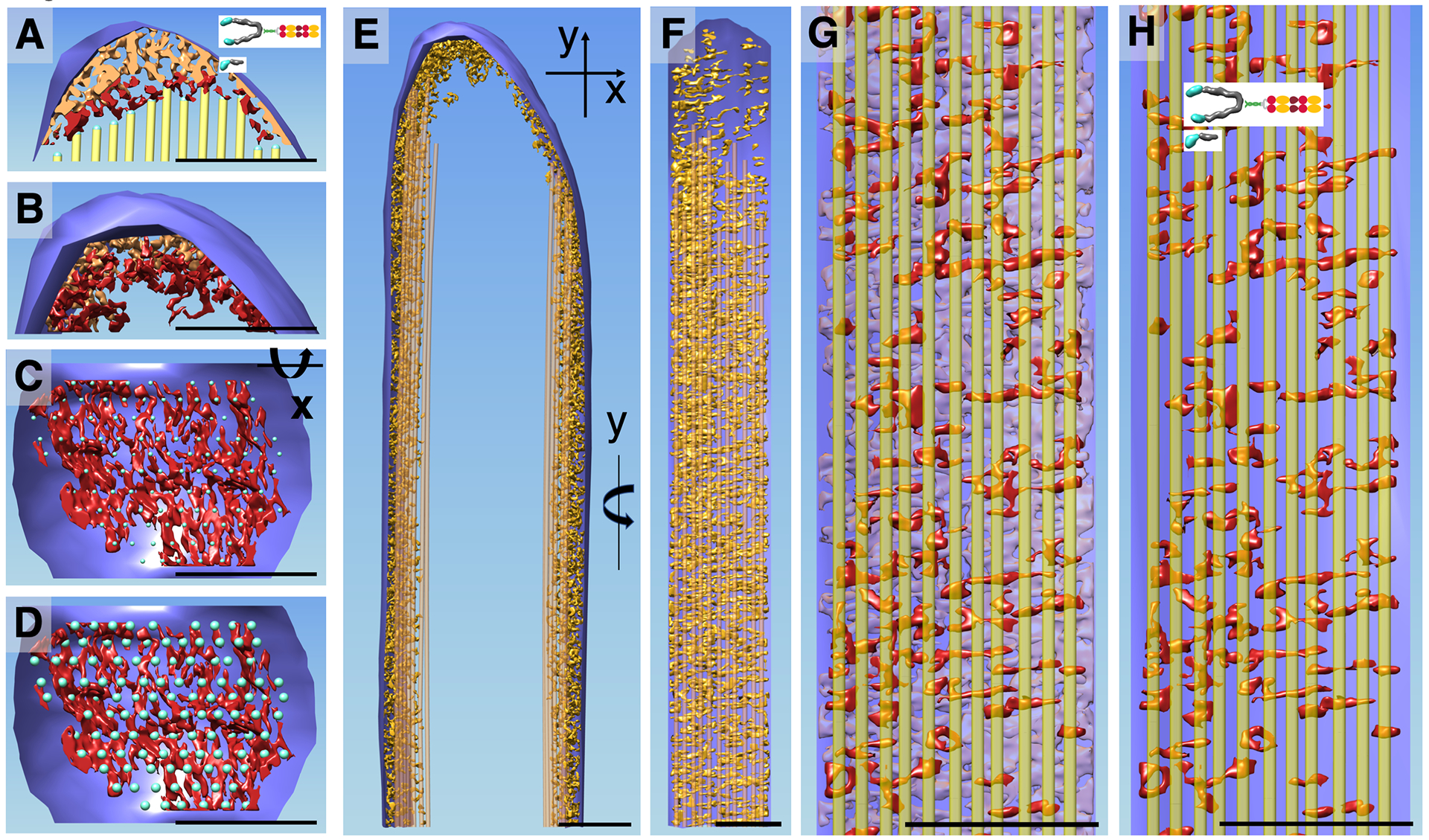
(A) Central density slab of the tip region with the most distal portion of the corresponding central actin-filament model layer. Note complex density near the lower tip link insertion site (left third of stereocilia tip), in stark contrast to the close proximity of the actin filament on the opposite stereocilia tip side. Red colored density rendering depicts map density within 10 nm proximity to the end of the actin filament. (B) 3D density corresponding to A. (C) En-face view onto the density map in 10 nm proximity to the actin filaments, showing a number of density lobes of similar size and what appears to be a non-random distribution. Small balls depict center location of each actin filament. (D) Same view as C but with blue balls representing the approximate actin filament diameter. Note that most but not all actin filaments show a corresponding red-colored density map. (E) Stereocilia in longitudinal orientation with the outermost layer of the actin filament of the corresponding circumferential membrane stretch of the entire stereocilia (õne third). Density between outermost actin layer and membrane is depicted in golden color. (F) Corresponding en face view of the shaft region by rotating the right portion of E by 90 degrees around the Y-axis. (G, H) Close up views of F with red density corresponding to those in close proximity (10 nm) to the outermost actin filament layer and transparent purple density corresponding to density between the red density and the membrane plane. Note that the membrane is depicted as a single plane. (H) Outermost actin layer (yellow) and red density map within 10 nm proximity to the actin filaments. Note that the red densities are similar and size, shape and orientation with the densities seen in C and D and are consistent with models of unconventional myosins. Scale bars = 100 nm
In addition to connectors at stereocilia tips, we also studied the space between the actin filament core (yellow lines) and the plasma membrane (blue) in the shaft region (Fig. 3E–F). Our analysis was restricted to about one-third of the entire stereocilia membrane due to the missing wedge of data collection and the resulting data anisotropy. Fig. 3E shows the densities in the space between the plasma membrane and the most outer layer of actin filament in profile. Rotation around the Y-axis by 90° allows an en-face view of the density, which appears to be very complex. In order to simplify the scenery, we color-coded the density map within 10 nm proximity to the most outer actin filament layer (Fig. 3G–H). For a section of 115 nm × 400 nm, we counted ~120 structures that are both close to the actin filaments and to the membrane (Fig. 3H), which extrapolates to ~9000 actin-to-membrane connectors for a stereocilium of 5 μm. While the resolution is insufficient to determine molecular identity, many of the densities seen in Fig. 3H show similarity in size and shape to myosins visualized at similar resolution (Whittaker et al., 1995; Jontes and Milligan, 1997a; Jontes and Milligan, 1997b).
Actin crosslinkers
To determine the 3D organization of the actin core, we created a simplified ball-and-stick model that represents actin filaments and crosslinkers. For quantitative analysis we focused on the layer direction in this hexagonally packed actin core, which was the least affected by the missing wedge-related data anisotropy. We examined the density map for three different stereocilia regions at a slab thickness of 10 nm, containing one layer of actin filaments (Fig. 4A–D). Superimposition of six adjacent slabs suggests the crosslinkers are randomly distributed (Fig. 4E). We adjusted the actin-filament model to best fit locally the density map. While we represented the actin filament as a round uniform cylinder, this is a gross simplification; the native actin filament is a helically-wound polymer, and at any point along the filament it has an elliptical cross-section, with periodically thicker and thinner densities.
Figure 4. Actin-actin crosslinker model building.
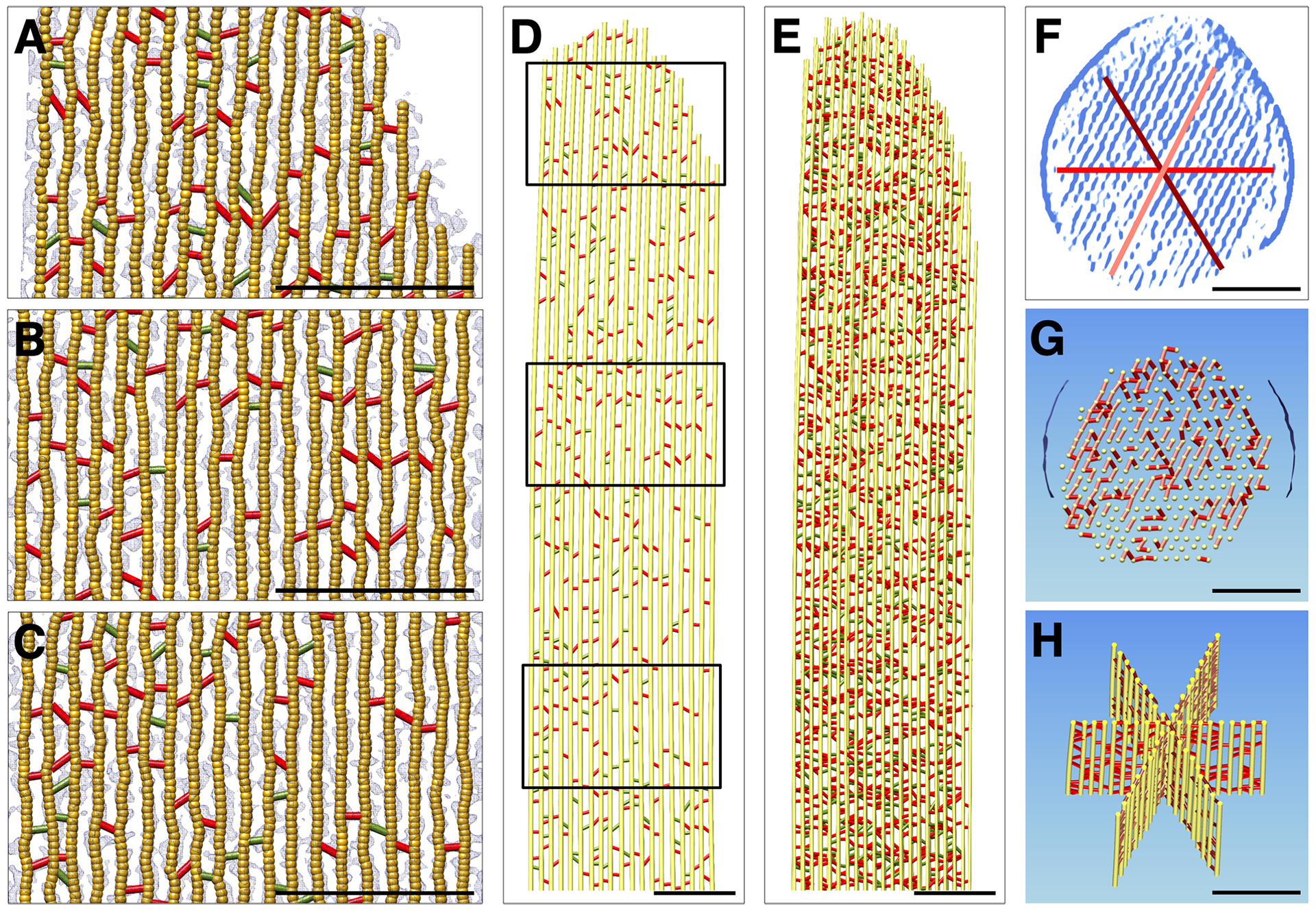
Models showing a single actin filament plane in longitudinal orientation from the Pls1−/− stereocilium model. (A-C) Overlay of crosslinker models with density map corresponding with boxed areas in panel D (A, top; B, middle; C, bottom). Red crosslinkers are located at 36 nm intervals and are likely FSCN2 and ESPN. Green crosslinkers are at non-36 nm intervals and are likely other actin-binding proteins. (D) Crosslinker model of entire plane corresponding to red line in panel F. (E) Crosslinker model of six superimposed actin filament planes (the model actin diameter was reduced to 4 nm to better show the crosslinkers). (F) Location of three central planes on cross-section of the model. (G) Crosslinkers in a cross-sectional slab of 15 nm. (H) Three central planes overlaid in a 200 nm slab showing crosslinkers. Scale bars = 100 nm.
We counted crosslinkers from six well oriented slabs of the model that were perpendicular to the electron beam, each of which contained 19 actin filaments of 1080 nm length. We found 293 ± 7 (mean ± SD) actin-actin crosslinkers that were at intervals of 37 nm (red in Fig. 4), as well as 100 ± 14 links that did not fit this pattern (green in Fig. 4). Because of the elongation of the density map along the direction of the electron beam (data anisotropy), counting cross-linkers in the other two principle directions was less reliable and led to overestimates of the number of crosslinkers. Using the above crosslinker density and a total of 340 actin filaments (321 filament pairs), the extrapolated total number of crosslinkers is 65,200 ± 1500 for the 4.5 μm shaft of a prototypical stereocilium of 5 μm length. The 340 actin filaments, averaging 4.75 μm (including the taper, which does not contain crosslinkers and has progressively shorter filaments towards the actin core periphery), will comprise ~585,000 actin monomers, yielding a crosslinker/actin monomer ratio of 0.111. The theoretical maximum is 0.23 crosslinkers/monomer (DeRosier and Tilney, 1982), which suggests a crosslinker occupancy of 48%. Adding in the crosslinkers that do not appear at 37 nm intervals suggests a crosslinker occupancy of 64%, but some of these structures may not be true actin-actin crosslinkers.
Fig. 4F shows a 30 nm-averaged cross-sectional central slab through the stereocilium, which makes the hexagonal pattern of the actin core very obvious and allows the three main axes to be easily determined. In Fig. 4G, a corresponding model of a 10 nm central slab is shown with actin-filaments shown head on as yellow circles; red, firebrick red, and salmon colored crosslinkers signify actin-actin crosslinkers for each of the main three axes. Three central planes representing the three principal directions of hexagonal packing are shown in Fig. 4H.
Actin 3D organization in the shaft regions
Filaments remained straight and therefore parallel to one another along the main stereocilia axis. We measured the actin-actin spacing at different locations of the actin core in the shaft region (Figs. 5A–D). The average spacing was 12.6 ± 1.2 nm (mean ± SD; n=2803), and did not differ at various positions along the stereocilium shaft (Fig. 5E). Distributions were fit well with a single Gaussian model, which indicates uniformity of crosslinking through the stereocilium.
Figure 5. Changes in actin-actin spacing and 3D organization from shaft region to taper/rootlet region.
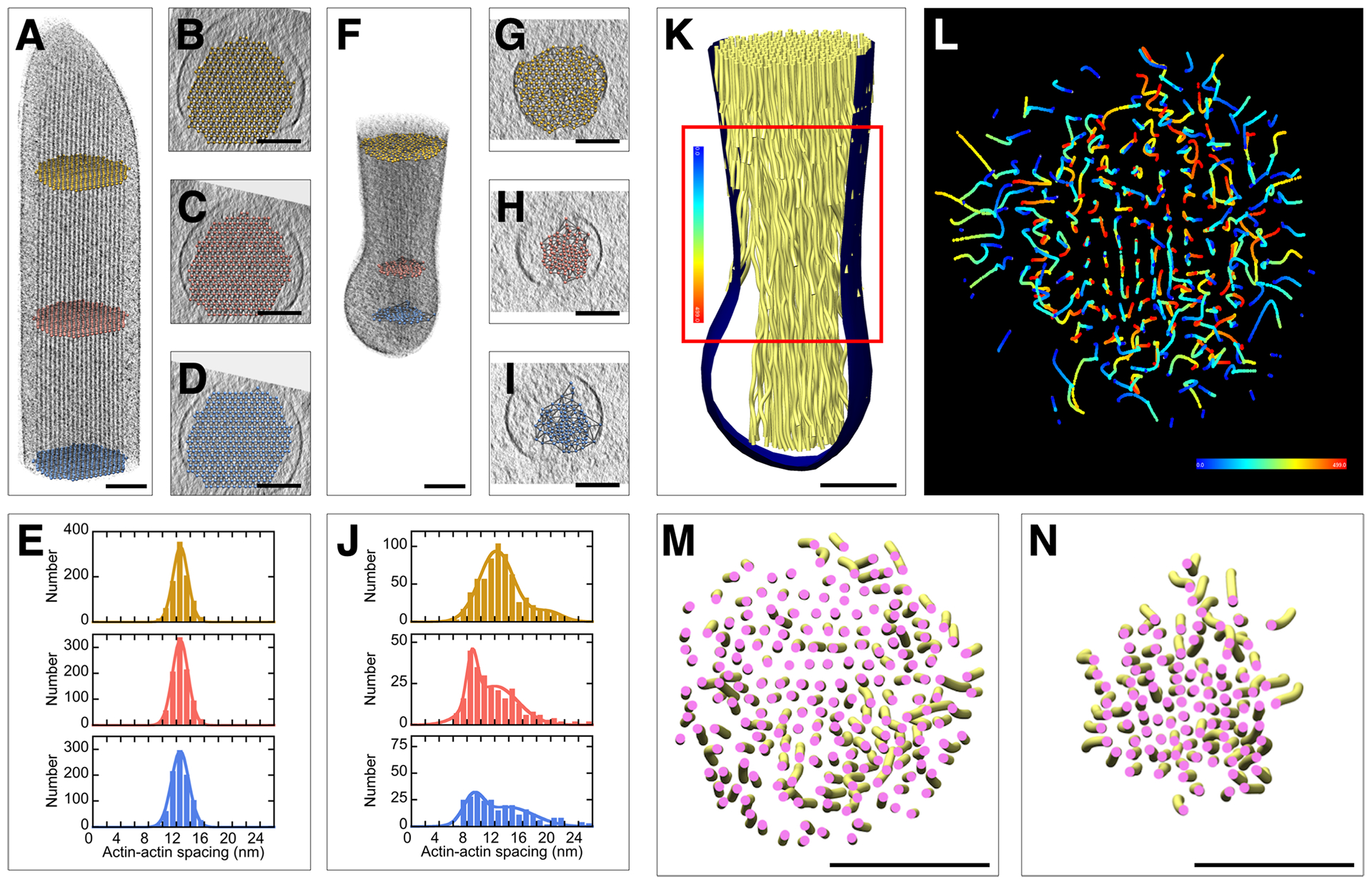
(A-E) Stereocilia actin filament spacing in shaft region of the Pls1−/− stereocilium model. (A) Longitudinal orientation with balls in yellow, red, or blue at different locations of the shaft region. (B-D) Corresponding cross-sectional views with ball-model superimposed, allowing quantification of actin-actin spacing. (E) Histograms of actin-actin distance in shaft region. Single-Gaussian fits with peaks at 13.1 nm (yellow), 13.1 nm (red), and 13.1 nm (blue). (F-I) Stereocilia actin filament spacing in taper region. (F) Longitudinal orientation with balls in yellow, red, or blue at different locations of the taper/rootlet region. (G-I) Corresponding cross-sectional views with ball model superimposed, allowing quantification of actin-actin spacing. (J) Histogram of actin-actin distance in taper/rootlet region. Note the change of spacing between shaft and rootlet region. Double-Gaussian fits with peaks at 12.8 and 20.0 nm (yellow); 9.2 and 12.5 nm (red); and 9.5 and 14.3 nm (blue). (K-L) Traces of actin filaments are they travel from the tapered region (blue) through the rootlet region (red). (M) Actin tube model in taper region. (N) Actin tube model in rootlet region. Note compaction of actin filaments in rootlet region and rotational twisting of actin filaments particularly around the core region. Scale bars = 100 nm.
Actin 3D organization in the taper and rootlet regions
Fig. 1N suggests that our model contains not only the stereocilia taper, where the number of actin filaments is reduced before the stereocilium meets the hair cell soma, but also part of the rootlet, which normally extends into the soma and cuticular plate, anchoring the stereocilium to the cell (Furness et al., 2008). Examination of Fig. 1N shows that the membrane remains in close proximity to the actin core most of the distance to the stereocilium base, but abruptly disconnects from the cytoskeleton and forms a bubble around the last part. We suggest that the membrane is anchored to the stereocilium in the taper region (Tilney et al., 1986) but does not bind to the rootlet proper, which is fully intracellular and has no exposure to the plasma membrane (Furness et al., 2008).
Actin filaments in the taper region (Figs. 5F–I) adopted a more complex 3D organization than they did in the shaft. The spacing of actin filaments decreased from ~13 nm near the shaft region to majority spacing of ~9 nm in the rootlet (Fig. 5J). Many peripheral filaments end near the taper region membrane (Fig. 5K), whereas the inner actin core continues, with many but not all filaments adopting a curved or twisted trajectory (Figs. 5L–N), most apparent in a color-coded path-tracing of each actin filament (Fig. 5L). As can be seen in Fig. 5M–N, actin filaments transition from a loose organization in the taper region just above the rootlet (Fig. 5M) to a tightly packed arrangement in the rootlet region (Fig. 5N). The rootlet is osmiophilic, as is the central core of the stereocilium actin through the taper and up into the stereocilium shaft (Furness et al., 2008). However, our density maps did not reveal any structural correlates of this central osmiophilic core beyond the compacting of actin filaments.
We examined the structure of the rootlet region in more detail, comparing it to the shaft (Fig. 6). Shaft filaments were regularly spaced as described (Fig. 6A). Periodicities were not obvious in the 10 nm slab but fast Fourier transform (FFT) spatial analysis showed not only a 12.3 nm row line that corresponds to actin-actin spacing, but also 35 and 6 nm periodicities (Fig. 6D). These repeats correspond to the actin half-turn and the helical twist (DeRosier and Tilney, 1982).
Figure 6. Altered filament structure in the rootlet region.
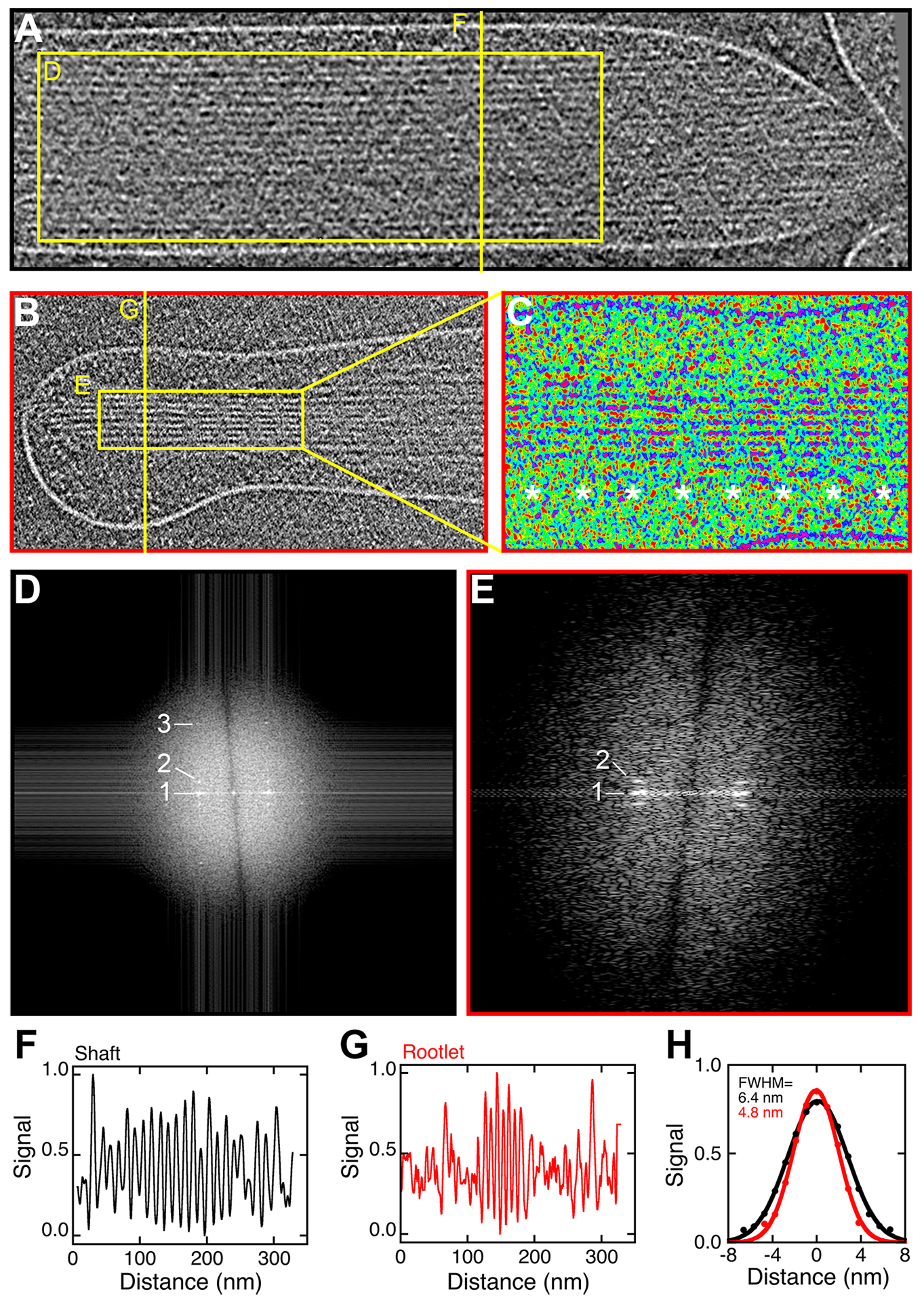
(A) Stereocilia shaft of the Pls1−/− stereocilium model. Box used for FFT analysis and line used for profile plot are indicated. (B) Rootlet region. (C) Image of rootlet region from panel B in pseudocolor to highlight repeats at ~36 nm (asterisks). (D) FFT analysis of boxed region in panel A (rotated 90°). Row line labeled 1 corresponds to 12.3 ± 0.2 (mean ± range; n=2). Layer line labeled 2 corresponds to 35 ± 6 nm (mean ± SD; n=4). Layer line 3 corresponds to 6.1 ± 0.1 nm (mean ± SD; n=4). (E) FFT analysis of boxed region in panel B (rotated 90°). Row line labeled 1 corresponds to 8.22 ± 0.1 (mean ± range; n=2). Layer line labeled 2 corresponds to 37 ± 5 nm (mean ± SD; n=4). (F-G) Profiles corresponding to line scans of images in A and B. (H) Average of six cycles each of the periodic structures in F and G. Fit is single Gaussian with full width at half maximum (FWHM) indicated in the figure.
Filaments appeared more closely packed in the rootlet region, and additional repeating structures were apparent (Fig. 6B). We used pseudocoloring to highlight these periodicities in the rootlet region; there was an obvious banding pattern seen every ~36 nm (Fig. 6C, asterisks). The row lines in the FFT analysis confirmed the tight spacing of actin filaments (8.22 ± 0.01 nm), and confirmed the presence of a repeat at 35 ± 6 nm (Fig. 6E).
We used line scans to demonstrate the filament periodicity but also to examine the filaments’ diameters. Interestingly, the apparent diameter of the filaments in the rootlet region (4.8 nm) was less than that in the shaft region (6.4 nm), although filaments appeared to be continuous from shaft to rootlet (Fig. 6F–H). Tight packing of actin filaments and an apparent decrease in actin filament diameter was also seen when mixtures of TRIOBP and actin were compared to mixtures of espin and TRIOBP (Kitajiri et al., 2010), which is consistent with the suggestion that the rootlet region highlighted in Fig. 6 corresponds to actin filaments bundled by TRIOBP.
Discussion
We focused here on the 3D structure of Pls1−/− mutant mouse utricle stereocilia. A preliminary comparison of wild-type and knockout tomographic data sets revealed the higher order of the actin core in Pls1−/− knockout stereocilia (Metlagel et al., 2019), and so by using the mutant tomograms, our model of the 3D organization of the actin core was significantly improved. The resolution in our tomograms was limited to 4.3 nm, however, which did not allow us to directly reveal the molecular identity of features visible in the density maps. Nevertheless, we were able to build simplified geometrical (volumetric) models, such as ball-and-stick models, into the density maps, which allowed us to determine the 3D organization and quantify the number of the actin filaments, actin-actin crosslinkers, and actin-membrane connectors.
Volumetric model building, first by manual fitting and then further refined by semi-automated fitting (Sazzed et al., 2018), resulted in actin models that ran parallel along the full length of the shaft and into the tip region. No abrupt changes in the actin filament orientation was observed. Accordingly, we averaged the map along 30 nm each of the actin filament models and thus replaced each voxel in the density map with a 30 nm average value. The resulting averaging not only increased the signal-to-noise ratio of the map and thus made the refinement of the actin model position much easier, but it also smoothed over fluctuations in map density caused by noise. A gap still visible in an averaged map therefore corresponded to a lack of density that extended over a significant length span, and so these gaps must be due to discontinuity of the underlying actin filaments. Actin filaments throughout the shaft therefore run parallel to one another with a defined separation, yet do not extend the length of a stereocilium. These results suggest either that actin filaments are not formed continuously from taper to tip, or that once the actin core has formed, it undergoes depolymerization of significant stretches. The gaps we encountered support the recent finding that actin turnover occurs throughout the stereocilium actin core (Hwang et al., 2015), and are not consistent with the treadmilling model for stereocilia actin turnover (Rzadzinska et al., 2004).
Gaps in the actin-filament structure should expose binding sites for barbed-end capping proteins, such as the heterodimeric capping protein and twinfilins TWF1 and TWF2. While antibodies against CAPZB and TWF2 strongly label row 2 tips in mature cochlear hair cells, labeling is not exclusively at stereocilia tips (Avenarius et al., 2017) and this broader stereocilia labeling could reflect capping in the gap regions.
Distinct structural features at stereocilia tips
We noticed several interesting features at stereocilia tips. For example, near tips, actin filaments deviated significantly from the main stereocilia axis in the direction opposite that of the tip link insertion site. While tip links were not present in the stereocilia model, the insertion site for the tip link can be readily inferred by the prolate shape of the tip, seen in all rows of stereocilia except for the tallest (Rzadzinska et al., 2004). This region of the stereocilium is subject to dynamic actin remodeling, even in adult animals (Zhang et al., 2012, Perrin et al., 2013, Narayanan et al., 2015), and actin’s structure at the tips is under the control of Ca2+ entering through transduction channels (Vélez-Ortega et al., 2017). While not conclusive, our results raise the possibility that transduction both stimulates local actin polymerization but also leads to deflection of filaments away from the site of local Ca2+ entry.
Density corresponding to proteins fills the gap between the end of the actin core and the plasma membrane; this distance is small opposite the putative tip link insertion, but can reach 40–50 nm where the tip link is presumed to insert. This protein density likely corresponds to the osmiophilic structure found underneath the tip link insertion, capping the actin core (Furness and Hackney, 1985), which is known as the lower tip link density (LTLD). When under tension, the stereocilia membrane can be pulled away from the LTLD by ~15 nm (Assad et al., 1991), which was not seen in our model. Whether the density we observe includes tethers for the transduction channels (Powers et al., 2012), which could correspond to the gating spring (Corey and Hudspeth, 1983), is not known at present.
MYO3A, MYO3B, and MYO15A localize to stereocilia tips (Belyantseva et al., 2003; Schneider et al., 2006; Merritt et al., 2012). MYO15A in particular is thought to be deposited at high levels at the ends of the actin core (Belyantseva et al., 2003); in the shorter stereocilia rows, isoform 1 of MYO15A (MYO15A-L, the long form) is the only isoform present and is found just underneath the tip link insertions (Fang et al., 2015). In the density maps corresponding to stereocilia tips, we observed structures that may correspond to unconventional myosins. Moreover, protein density at stereocilia tips could include the large N-terminal extension of MYO15A-L. Higher resolution is needed, however, to reveal the macromolecular identity of these structures.
Actin-to-membrane connectors
We estimated that a 5 μm mouse utricle stereocilium has ~9000 actin-to-membrane connectors, which could be RDX, myosins, or other proteins. While fewer connectors were estimates for chick stereocilia, 5800–7300 per 5 μm (Shin et al., 2013), chick stereocilia are more narrow and hence have a smaller membrane circumference. In our model, many of these connectors were tadpole-shaped, similar to how myosins appear at a similar resolution (Whittaker et al., 1995; Jontes and Milligan, 1997a; Jontes and Milligan, 1997b). Future high-resolution cryo-tomograms should provide the opportunity for docking high-resolution structures of the myosin motor domain on to the actin-to-membrane connectors seen along the stereocilia shafts. The connector count corresponded well with that estimated by quantitative mass spectrometry, which found ~7200 members of the ERM (ezrin, radixin, moesin) family, 260 MYO1H, 60 MYO3A and 3B, 1300 MYO6, and 230 MYO7A in P23 utricle stereocilia (Krey et al., 2015), for a total of 9100 potential actin-to-membrane connectors. This correlation suggests that most actin-to-membrane connectors could be members of the ERM or myosin families.
Actin-to-membrane connectors are likely essential for maintenance of membrane tension in the stereocilia, which is important for controlling stereocilia shape (Prost et al., 2007) and transduction-channel gating (Powers et al., 2012; Powers et al., 2014; Peng et al., 2016). In most cells, the actin cytoskeleton and connectors that bridge it to the membrane are necessary to establish and control membrane tension (Pontes et al., 2017). Many membrane-to-actin connectors, including RDX and the myosin I family, bind strongly to PIP2, which is also essential for maintaining adhesion of the membrane to the cytoskeleton (Raucher et al., 2000). Notably, PIP2 is prominent in stereocilia (Hirono et al., 2004; Effertz et al., 2017). Actin-to-membrane connectors occupy far fewer than the theoretical maximum number sites on the periphery of the actin core, which may allow for efficient transport of proteins along the shaft. Too many connectors could form a physical barrier that impedes the movement of molecules, especially myosin-based transport along the periphery of the actin core. Our view of the connectors is necessarily static; however, the dynamics not only of myosin movement but also association and dissociation kinetics of the connectors will substantially impact the effect of the connectors on transport.
Actin-actin crosslinkers
We also studied extensively the number and distribution of actin-actin crosslinkers, which were similar to estimates previously made from our correlative study incorporating quantitative mass spectrometry and electron tomography of chick utricle stereocilia (Shin et al., 2013). In our model mouse utricle Pls1−/− stereocilium, which is 5 μm in length, we estimated the presence of ~65,000 crosslinkers for 585,000 stereocilia actin monomers in 340 actin filaments. These values compare to 60,000–90,000 crosslinkers and 400,000 stereocilia actin monomers in a chick stereocilium of the same length, albeit with 210 actin filaments (Shin et al., 2013). Targeted proteomics of CD-1 mouse stereocilia suggested the presence of 30,500 PLS1, 16,100 FSCN2, and 14,800 ESPN in a stereocilium of 400,000 actin monomers (Krey et al., 2016). Removing the PLS1 molecules (because we are modeling Pls1−/− stereocilia) and extrapolating to the larger number of actin monomers in our model stereocilium, those mass spectrometry experiments predict a total of 45,000 non-PLS1 actin-actin crosslinkers per stereocilium, reasonably close to the 65,000 we counted. Several actin crosslinkers increased in abundance, although not significantly, in targeted proteomics measurements comparing wild-type and Pls1−/− stereocilia (Krey et al., 2016). Upregulation of crosslinker numbers or increased stereocilia targeting could in part account for the apparent discrepancy.
The uniformity of actin-actin spacing throughout the stereocilium suggests either that a single type of crosslinker controls that spacing or that multiple crosslinkers have similar properties. Rigidity of actin-espin crosslinks and flexibility of actin-fascin crosslinks suggests that the uniform actin-actin spacing is set by ESPN rather than FSCN2 (Shin et al., 2009), although they should be present at similar abundance. We noticed that individual actin layers showed little sign of clustering of actin-actin crosslinkers. Together with the results from gap analysis, this results argues that the actin core’s 3D organization is more gel-like than paracrystalline, consistent with the dynamic nature of actin exchange within the core (Hwang et al., 2015).
As we noted in our preliminary study (Metlagel et al., 2019), the actin-actin spacing measured here (12–13 nm) is considerably larger than the ~8 nm measured previously for Pls1−/− mutants (Krey et al., 2016). One of the major advantages of the electron cryo-tomography approach we used here is that we prepare samples by rapid freezing, with no fixation, dehydration, and staining steps that could distort stereocilia dimensions. For this reason, we believe that the 12.6 nm actin-actin spacing is an accurate estimate for actin filaments crosslinked by a combination of FSCN2 and ESPN, and suggests that diffusion of small proteins could readily occur within the stereocilia actin core. Parenthetically, this comparison highlights the large tissue distortion that conventional electron microscopy techniques introduce.
Filaments in the taper and rootlet regions
As has been shown previously (Tilney et al., 1980), peripheral actin filaments terminated in the taper region at the plasma membrane. Several membrane or membrane-associated proteins, including PTPRQ, RDX, and CLIC5 (Goodyear et al., 2003; Pataky et al., 2004; Gagnon et al., 2006; Zhao et al., 2012; Salles et al., 2014), are associated with the taper region at the membrane. TPRN (taperin) also localizes to this area (Rehman et al., 2010), but is largely found in the stereocilium core (Zhao et al., 2016). TPRN is localized to the region of the insertion by GRXCR2, which also concentrates at stereocilia tapers as well (Liu et al., 2018). How the pointed ends of actin filaments are anchored at the membrane remains unknown, although the RDX-CLIC5-TPRN-MYO6 complex (Salles et al., 2014) could be responsible.
The central actin filaments in the taper region adopted a twisting path that resulted in the compaction of the filaments in the rootlet. The compression we observed of the actin core corresponds to the absence of actin-actin crosslinkers previously reported for tapers of mouse utricle stereocilia (Krey et al., 2016). In the region of the taper and rootlet, the central filaments are brought together into a tight bundle, which has been previously shown with transmission electron microscopy (Itoh, 1982; Slepecky and Chamberlain, 1982). The spacing between the filaments that we measured (~8 nm) in the rootlet region is considerably smaller than that seen through the stereocilia shaft (12–13 nm), and the filaments appear to be of a narrower diameter; these observations raise the possibility that the rootlet could be composed of a filamentous protein distinct from actin. We note, however, that mixing actin and TRIOBP in vitro creates tightly packed filament bundles with actin filaments that appear to be of reduced diameter compared to those crosslinked with espin (Kitajiri et al., 2010). Moreover, experiments using the S1 (actin-binding) fragment of myosin II or actin antibodies show that rootlet filaments are indeed actin (Tilney et al., 1980; Slepecky and Chamberlain, 1985). Our data are thus consistent with the proposal that rootlets are formed of actin and that TRIOBP wraps around these rootlet filaments to create a tight bundle, presumably for structural integrity (Kitajiri et al., 2010; Katsuno et al., 2019).
In the experiments that generated the isolated stereocilia, where stereocilia adhered to poly-lysine-coated glass and the tissue was lifted away, the rootlet structure was fractured near the stereocilia insertion point. While the reduced order of the stereocilia actin filaments could be in part due to this force-dependent fracturing, conventional transmission electron microscope imaging of this region shows considerable structural changes from the main shaft region (Tilney et al., 1980, Tilney and DeRosier, 1986, Furness et al., 2008).
Future ultrastructural imaging should help define better the region at the stereocilia insertion where actin filaments in the stereocilia taper transition into the TRIOBP-mediated bundle, which then extends into the cuticular plate as the rootlet proper. In particular, imaging the stereocilia insertion in an intact cell, where the stereocilia retain their connection to the cell, will be important for demonstration of the native rootlet structure. Note that the osmiophilic density seen in electron micrographs usually extends from below the stereocilia insertion to well into a stereocilium; recent experiments show that the splice form TRIOBP-4 is responsible for actin-filament bundling for the portion of the rootlet within the stereocilia, while TRIOBP-5 locates exclusively to the rootlet portion within the cuticular plate (Katsuno et al., 2019). Other proteins presumably mediate the structural transition from the taper to the rootlet.
Implications
Use of the Pls1−/− mutant mouse line allowed for a more direct comparison to our previous work on chick utricle stereocilia, which have relatively low levels of PLS1 compared to FSCN2 (Shin et al., 2013). Moreover, the high order of the Pls1−/− cytoskeleton (Krey et al., 2016) improved our ability to accurately develop the stereocilium model. This model improved substantially on our previous model for chick stereocilia, which used resin-embedding processing for transmission electron microscopy (Shin et al., 2013). The dominant actin-actin crosslinker distance in chick utricle stereocilia was ~8 nm, which corresponds well to the actin-actin spacing in mouse utricle stereocilia prepared from Pls1−/− mutants using conventional processing but is much smaller than the ~13 nm measured here. Electron cryo-tomography using rapidly frozen samples, as we used here, provides the ability to see the cytoskeleton in a near-native state with little or no distortion, and thus the measurement values reported here are likely to reflect those of native stereocilia.
Our results also have implications for the mechanical properties of stereocilia. Estimates of the stiffness of stereocilia shafts assume isotropic behavior, i.e., long filaments and uniform crosslinking (Howard and Ashmore, 1986, Duncan and Grant, 1997). Gaps introduce potential points of mechanical weakness in the stereocilium shaft. Nevertheless, it is likely that the compliance of the rootlet region is far less than that of the shaft (Howard and Ashmore, 1986), even with gaps in the actin filament structure.
The native structure of the rootlet region revealed with cryo-ET is also consistent with previous predictions about rootlet mechanics. There is no evidence for actin crosslinkers in the rootlet, and the actin filaments are bundled very tightly together, perhaps by TRIOBP. Comparison of the estimated stereocilia rotational stiffness to theoretical predictions for rootlet mechanics suggested that the actin filaments should be free to slide, allowing for significant compliance at the rootlet (Howard and Ashmore, 1986, Pacentine et al., 2020). Our structural results are consistent with this model.
Development of a model for a Pls1−/− stereocilium has thus enabled new insight into the structure and function of mouse utricle stereocilia. Future modeling of stereocilia structure will include those of wild-type mouse utricle, as well as stereocilia from inner and outer hair cells of the mouse cochlea. Moreover, as methods for identifying specific molecules within electron cryo-tomograms improve, we will be able better define the functional role of those molecules. Our stereocilium model is an important step in that direction.
Materials and Methods
Blotting of stereocilia onto microscope grids, vitrification, cryo-tomographic data collection and 3D reconstruction have all been described in detail previously (Metlagel et al., 2019). In short, the sensory epithelium was blotted onto the lacey carbon support film of an EM grid, transferring intact stereocilia to the grid. Samples were vitrified using ultra-rapid plunge-freezing. Single axis cryo-tomograms were collected on a Krios transmission electron microscope (Thermo Fisher) operated at 300 kV with a nominal defocus of 3.5–4.5 μm using a Falcon 2 camera in integration mode at 0.47 to 0.59 nm pixel size. The typical dose for single axis data collection was 80–100 electrons/Å2). Tomogram 3D volumes were reconstructed using IMOD (Kremer et al., 1996), using either weighted back-projection or the SIRT method (Agulleiro and Fernandez, 2011). To improve contrast, we filtered tomograms with recursive median or bilateral filtering in Priism (Chen et al., 1992) or IMOD for initial inspection.
Pixel values of the 16-bit original cryo-tomograms were contrast-inverted and inspected at varying rotation angles using the slicer window of the IMOD viewer 3dmod. Once a specific XYZ rotation angle was identified that resulted in actin filaments being located in a single plane, the tomogram was rotated by using IMOD command rotatevol to align the actin filament orientation with the tomographic coordinate system. The resulting tomogram was then cropped by using IMOD command trimvol to only contain the relevant stereocilium and its immediate surroundings. The tomogram was then filtered with nonlinear anisotropic diffusion via IMOD.
We used UCSF–Chimera software (Pettersen et al., 2004) for tomographic 3D volume visualization, model building and quantitative analysis. In order to obtain 10 nm and 30 nm averaged maps, we first aligned the tomograms so that the actin filament axes were parallel to Y-axis. We then replaced each XZ cross-sectional plane with an average of 10 nm slab by averaging 5 or 16 slices of ~0.9 nm thickness, respectively on both sides of the XY cross-sectional plane. Using a modified version of the UCSF Chimera script actinlattice.py (https://www.cgl.ucsf.edu/chimera/data/actin-fit-may2017/actinlattice.py), we roughly placed volumetric models of a group of actin filaments, one layer at a time. To accommodate curvature in actin filaments, the filament models were further divided into smaller segments using the UCSF Chimera script dividelink.py (http://plato.cgl.ucsf.edu/trac/chimera/attachment/wiki/Scripts/dividelinks.py). Each segment was then manually adjusted to reflect the curvature of the actin core. Volumetric models placed manually were validated and further refined using a semi-automated computational actin-tracing algorithm (Sazzed et al., 2018)
The above-described manual protocol was used for the shaft region of the stereocilia, but was not suitable for modeling the taper and rootlet region because of the complex path of the filaments through that region. Instead of modeling one XY plane at a time for the taper, we placed a model of an entire actin filament bundle into the density map. Each filament of the bundle model was divided along the filament axes into 20 nm segments. We then compared the position of each 20 nm segment with an averaged slab of 20 cross-sectional XZ planes, and adjusted the location of each segment. Like in the shaft region, we further refined and validated the filament tracing using the semi-automated computational actin-tracing algorithm (Sazzed et al., 2018).
In order to visualize the locations of filament gaps, we divided the actin bundle model into small segments using dividelink.py script; we evaluated the density map of these very small segments using the UCSF Chimera function value at atom position, which reads voxel value of the tomogram at each set of marker coordinates. These values were then rendered as red color at the desired threshold using Render by Attribute tool.
The stereocilia membrane model was created by tracing the stereocilium membrane every ten XY-planes using Volume Tracer tool, which allows manual tracing and model placement on tomogram. After tracing the clearly visible portion of membrane, the Surface function within the Volume Tracer was used to create the surface model. The space between the membrane and the actin core was visualized using the mask command in UCSF Chimera, which enables selectively cropping of a 3D volume that is a specified distance from a surface. The Hide Dust function was used to remove densities below a specified size threshold value.
Actin-actin crosslinkers were modeled as cylinders using Volume Tracer. Inter-actin distance measurements were performed by examining placed markers at three different XY cross-sections using Volume Tracer. The findclash command was used to automatically identify and to connect all nearby markers within specified distance, resulting in a so-called pseudobond model. To ensure connection between all adjacent markers, the search distance was larger than the previously established actin-actin spacing. This resulted in some markers being connected despite not being the nearest markers. Excess connectors were removed by the UCSF Chimera script removecross.py (http://plato.cgl.ucsf.edu/trac/chimera/attachment/wiki/Scripts/removecross.py). The pseudobond model lengths were determined using UCSF Chimera script pblengths.py (http://plato.cgl.ucsf.edu/trac/chimera/attachment/wiki/Scripts/pblengths.py).
For visualization purposes and figure creation, we frequently displayed only a portion of the model or the density map using the per-model clipping UCSF Chimera command mclip. Per-model clipping allows settings related to model clipping, such as slab thickness and slab direction, to be set individually. Color-coded visualization of the filament paths in the taper and rootlet region along the z-direction was done using the plugin TrackMate (Tinevez et al., 2017) for Fiji (https://fiji.sc/).
Where mentioned in the Results section, we created a map where each stereocilia cross-section density map slice was replaced with a 30 nm average along that axis, which simplified manual and semiautomated tracing and trace refinement (Sazzed et al., 2018).
Tomograms for two stereocilia shaft regions and two taper/rootlet regions are available at: https://figshare.com/s/6b85b44f2c10bba77d95
Supplementary Material
Figure S1. Actin gaps do not result from an averaging effect. (A) Single cross-sectional plane of original raw tomogram, approximately 1nm thick. (B) Same plane as A, with false color to better enhance lack of density. (C) Close up of red rectangle of B, where asterisk indicates the location of the gap. (D) Single cross-sectional plane of 10 nm averaged tomogram, representing approximately 10 nm thick slab≥ (E) Same plane as D, with false color to better enhance lack of density. (F) Close up of red rectangle on Supplemental figure E, where asterisk indicates the location of the gap. (G) Single cross-sectional plane of 30 nm averaged tomogram, representing approximately 30 nm thick slab. (H) Same plane as G, with false color to better enhance lack of density. (I) Close up of red rectangle on Supplemental figure H, where asterisk indicates the location of the gap. (J) Example locations for sample window taken at actin filaments. (K) Example locations for inter-filament locations. (L) Example locations in the gap region (but not centered at the gap). (M) Gap location. Scale bars: A-B, D-E, G-H, J-M, 100 nm; C, F, I, 50 nm. (N) Plot of intensities measured at locations of actin filaments, locations between filaments (inter-filament), locations surrounding the gap, and the gap itself. Inter-filament and gap region measurements were significantly different from measurements at actin filaments (***, p<0.0001).
Supplemental Video 1 (Song_Map01_NoAverage). Scroll-through video of XZ-cross-sectional slices of nonlinear anisotropic diffusion of denoised un-averaged density map, displayed in false-color representation to emphasize high density regions (pink and red) as well as low density regions (blue).
Supplemental Video 2 (Song_Map01_10nm_Average). Scroll-through video of XZ-cross-sectional slices of density map where each slice along the Y-axis has been replaced by an 10 nm average slab of the density map. Map is displayed in false-color representation to emphasize high density regions (pink and red) as well as low density regions (blue).
Supplemental Video 3 (Song_Map01_30nm_Average). Scroll-through video of XZ-cross-sectional slices of density map where each slice along the Y-axis has been replaced by an 30 nm average slab of the density map. Map is displayed in false-color representation to emphasize high density regions (pink and red) as well as low density regions (blue).
Electron cryo-tomography allows visualization of native stereocilia structure
Imaging mouse utricle stereocilia lacking PLS1 allows for increased structural order
Actin filaments are spaced ~13 nm apart but have significant gaps
Stereocilia have ~9000 membrane connectors and 65,000 actin crosslinkers
Actin filaments in the taper region become more tightly packed to form the rootlet
Acknowledgements
PGBG was supported by National Institutes of Health grant R01 DC011034. MA was supported by National Institutes of Health grant P01 GM051487.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Agulleiro JI, Fernandez JJ, 2011. Fast tomographic reconstruction on multicore computers. Bioinformatics 27, 582–583. [DOI] [PubMed] [Google Scholar]
- Assad JA, Shepherd GMG, Corey DP, 1991. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron 7, 985–994. [DOI] [PubMed] [Google Scholar]
- Baker LA, Grange M, Grünewald K, 2017. Electron cryo-tomography captures macromolecular complexes in native environments. Curr Opin Struct Biol 46, 149–156. [DOI] [PubMed] [Google Scholar]
- Belyantseva IA, Boger ET, Friedman TB, 2003. Myosin XVa localizes to the tips of inner ear sensory cell stereocilia and is essential for staircase formation of the hair bundle. Proc Natl Acad Sci USA 100, 13958–13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyantseva IA, Boger ET, Naz S, Frolenkov GI, Sellers JR, Ahmed ZM, Griffith AJ, Friedman TB, 2005. Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat Cell Biol 7, 148–156. [DOI] [PubMed] [Google Scholar]
- Chen H, Clyborne WK, Sedat JW, Agard DA, 1992. Priism: an integrated system for display and analysis of 3-D microscope images. Proceedings of SPIE 1660, 784–790. [Google Scholar]
- Chou SW, Hwang P, Gomez G, Fernando CA, West MC, Pollock LM, Lin-Jones J, Burnside B, McDermott BM, 2011. Fascin 2b is a component of stereocilia that lengthens actin-based protrusions. PLoS One 6, e14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey DP, Hudspeth AJ, 1983. Kinetics of the receptor current in bullfrog saccular hair cells. J Neurosci 3, 962–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin JT, Warchol ME, 1991. Auditory hair cells: structure, function, development, and regeneration. Annu Rev Neurosci 14, 301–333. [DOI] [PubMed] [Google Scholar]
- Daudet N, Lebart MC, 2002. Transient expression of the t-isoform of plastins/fimbrin in the stereocilia of developing auditory hair cells. Cell Motil Cytoskeleton 53, 326–336. [DOI] [PubMed] [Google Scholar]
- DeRosier DJ, Tilney LG, 1982. How actin filaments pack into bundles. Cold Spring Harb Symp Quant Biol 46 Pt 2, 525–540. [DOI] [PubMed] [Google Scholar]
- DeRosier DJ, Tilney LG, Egelman E, 1980. Actin in the inner ear: the remarkable structure of the stereocilium. Nature 287, 291–296. [DOI] [PubMed] [Google Scholar]
- Effertz T, Becker L, Peng AW, Ricci AJ, 2017. Phosphoinositol-4,5-bisphosphate regulates auditory hair cell mechanotransduction channel pore properties and fast adaptation. J Neurosci pii: 1351–17. doi: 10.1523/JNEUROSCI.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Q, Indzhykulian AA, Mustapha M, Riordan GP, Dolan DF, Friedman TB, Belyantseva IA, Frolenkov GI, Camper SA, Bird JE, 2015. The 133-kDa N-terminal domain enables myosin 15 to maintain mechanotransducing stereocilia and is essential for hearing. eLife 4, e08627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace R, Kim KX, 2014. The physiology of mechanoelectrical transduction channels in hearing. Physiol Rev 94, 951–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness DN, Hackney CM, 1985. Cross-links between stereocilia in the guinea pig cochlea. Hearing Res 18, 177–188. [DOI] [PubMed] [Google Scholar]
- Furness DN, Mahendrasingam S, Ohashi M, Fettiplace R, Hackney CM, 2008. The dimensions and composition of stereociliary rootlets in mammalian cochlear hair cells: comparison between high- and low-frequency cells and evidence for a connection to the lateral membrane. J Neurosci 28, 6342–6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon LH, Longo-Guess CM, Berryman M, Shin JB, Saylor KW, Yu H, Gillespie PG, Johnson KR, 2006. The chloride intracellular channel protein CLIC5 is expressed at high levels in hair cell stereocilia and is essential for normal inner ear function. J Neurosci 26, 10188–10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale JE, Meyers JR, Periasamy A, Corwin JT, 2002. Survival of bundleless hair cells and subsequent bundle replacement in the bullfrog’s saccule. J Neurobiol 50, 81–92. [DOI] [PubMed] [Google Scholar]
- Garcia JA, Yee AG, Gillespie PG, Corey DP, 1998. Localization of myosin-Iβ near both ends of tip links in frog saccular hair cells. J Neurosci 18, 8637–8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie PG, Müller U, 2009. Mechanotransduction by hair cells: models, molecules, and mechanisms. Cell 139, 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear RJ, Legan PK, Wright MB, Marcotti W, Oganesian A, Coats SA, Booth CJ, Kros CJ, Seifert RA, Bowen-Pope DF, Richardson GP, 2003. A receptor-like inositol lipid phosphatase is required for the maturation of developing cochlear hair bundles. J Neurosci 23, 9208–9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney CM, Fettiplace R, Furness DN, 1993. The functional morphology of stereociliary bundles on turtle cochlear hair cells. Hear Res 69, 163–175. [DOI] [PubMed] [Google Scholar]
- Hasson T, Gillespie PG, Garcia JA, MacDonald RB, Zhao Y, Yee AG, Mooseker MS, Corey DP, 1997. Unconventional myosins in inner-ear sensory epithelia. J Cell Biol 137, 1287–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono M, Denis CS, Richardson GP, Gillespie PG, 2004. Hair cells require phosphatidylinositol 4,5-bisphosphate for mechanical transduction and adaptation. Neuron 44, 309–320. [DOI] [PubMed] [Google Scholar]
- Hutchings J, Zanetti G, 2018. Fine details in complex environments: the power of cryo-electron tomography. Biochem Soc Trans [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang P, Chou SW, Chen Z, McDermott BM, 2015. The stereociliary paracrystal Is a dynamic cytoskeletal scaffold in vivo. Cell Rep 13, 1287–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, 1982. Preservation and visualization of actin-containing filaments in the apical zone of cochlear sensory cells. Hear Res 6, 277–289. [DOI] [PubMed] [Google Scholar]
- Itoh M, Nakashima T, 1980. Structure of the hair rootlets on cochlear sensory cells by tannic acid fixation. Acta Otolaryngol 90, 385–390. [DOI] [PubMed] [Google Scholar]
- Jacobs RA, Hudspeth AJ, 1990. Ultrastructural correlates of mechanoelectrical transduction in hair cells of the bullfrog’s internal ear. Cold Spring Harb Symp Quant Biol 55, 547–561. [DOI] [PubMed] [Google Scholar]
- Jasnin M, Asano S, Gouin E, Hegerl R, Plitzko JM, Villa E, Cossart P, Baumeister W, 2013. Three-dimensional architecture of actin filaments in Listeria monocytogenes comet tails. Proc Natl Acad Sci USA 110, 20521–20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jontes JD, Milligan RA, 1997a. Three-dimensional structure of Brush Border Myosin-I at approximately 20 A resolution by electron microscopy and image analysis. J Mol Biol 266, 331–342. [DOI] [PubMed] [Google Scholar]
- Jontes JD, Milligan RA, 1997b. Brush border myosin-I structure and ADP-dependent conformational changes revealed by cryoelectron microscopy and image analysis. J Cell Biol 139, 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuno T, Belyantseva IA, Cartagena-Rivera AX, Ohta K, Crump SM, Petralia RS, Ono K, Tona R, Imtiaz A, Rehman A, Kiyonari H, Kaneko M, Wang YX, Abe T, Ikeya M, Fenollar-Ferrer C, Riordan GP, Wilson EA, Fitzgerald TS, Segawa K, Omori K, Ito J, Frolenkov GI, Friedman TB, Kitajiri SI, 2019. TRIOBP-5 sculpts stereocilia rootlets and stiffens supporting cells enabling hearing. JCI Insight 4, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajiri S, Fukumoto K, Hata M, Sasaki H, Katsuno T, Nakagawa T, Ito J, Tsukita S, Tsukita S, 2004. Radixin deficiency causes deafness associated with progressive degeneration of cochlear stereocilia. J Cell Biol 166, 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajiri S, Sakamoto T, Belyantseva IA, Goodyear RJ, Stepanyan R, Fujiwara I, Bird JE, Riazuddin S, Riazuddin S, Ahmed ZM, Hinshaw JE, Sellers J, Bartles JR, Hammer JA, Richardson GP, Griffith AJ, Frolenkov GI, Friedman TB, 2010. Actin-bundling protein TRIOBP forms resilient rootlets of hair cell stereocilia essential for hearing. Cell 141, 786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, McIntosh JR, 1996. Computer visualization of three-dimensional image data using IMOD. J Struct Biol 116, 71–76. [DOI] [PubMed] [Google Scholar]
- Krey JF, Krystofiak ES, Dumont RA, Vijayakumar S, Choi D, Rivero F, Kachar B, Jones SM, Barr-Gillespie PG, 2016. Plastin 1 widens stereocilia by transforming actin filament packing from hexagonal to liquid. J Cell Biol 215, 467–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krey JF, Sherman NE, Jeffery ED, Choi D, Barr-Gillespie PG, 2015. The proteome of mouse vestibular hair bundles over development. Sci Data 2, 150047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW, 1987. Acute ultrastructural changes in acoustic trauma: serial-section reconstruction of stereocilia and cuticular plates. Hear Res 26, 45–64. [DOI] [PubMed] [Google Scholar]
- Liu C, Luo N, Tung CY, Perrin BJ, Zhao B, 2018. GRXCR2 regulates taperin localization critical for stereocilia morphology and hearing. Cell Rep 25, 1268–1280.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh JR, O’Toole E, Morgan G, Austin J, Ulyanov E, Ataullakhanov F, Gudimchuk N, 2018. Microtubules grow by the addition of bent guanosine triphosphate tubulin to the tips of curved protofilaments. J Cell Biol 217, 2691–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt RC, Manor U, Salles FT, Grati M, Dose AC, Unrath WC, Quintero OA, Yengo CM, Kachar B, 2012. Myosin IIIB uses an actin-binding motif in its espin-1 cargo to reach the tips of actin protrusions. Curr Biol 22, 320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlagel Z, Krey JF, Song J, Swift MF, Tivol WJ, Dumont RA, Thai J, Chang A, Seifikar H, Volkmann N, Hanein D, Barr-Gillespie PG, Auer M, 2019. Electron cryo-tomography of vestibular hair-cell stereocilia. J Struct Biol 206, 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CP, Krey JF, Grati M, Zhao B, Fallen S, Kannan-Sundhari A, Liu XZ, Choi D, Müller U, Barr-Gillespie PG, 2016. PDZD7-MYO7A complex identified in enriched stereocilia membranes. eLife 5, e18312. doi: 10.7554/eLife.18312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan P, Chatterton P, Ikeda A, Ikeda S, Corey DP, Ervasti JM, Perrin BJ, 2015. Length regulation of mechanosensitive stereocilia depends on very slow actin dynamics and filament-severing proteins. Nat Commun 6, 6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikonomou CM, Jensen GJ, 2017. Cellular Electron Cryotomography: Toward Structural Biology In Situ. Annu Rev Biochem 86, 873–896. [DOI] [PubMed] [Google Scholar]
- Pataky F, Pironkova R, Hudspeth AJ, 2004. Radixin is a constituent of stereocilia in hair cells. Proc Natl Acad Sci USA 101, 2601–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng AW, Gnanasambandam R, Sachs F, Ricci AJ, 2016. Adaptation Independent Modulation of Auditory Hair Cell Mechanotransduction Channel Open Probability Implicates a Role for the Lipid Bilayer. J Neurosci 36, 2945–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin BJ, Strandjord DM, Narayanan P, Henderson DM, Johnson KR, Ervasti JM, 2013. β-actin and fascin-2 cooperate to maintain stereocilia length. J Neurosci 33, 8114–8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE, 2004. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 25, 1605–1612. [DOI] [PubMed] [Google Scholar]
- Pontes B, Monzo P, Gauthier NC, 2017. Membrane tension: A challenging but universal physical parameter in cell biology. Semin Cell Dev Biol 71, 30–41. [DOI] [PubMed] [Google Scholar]
- Powers RJ, Kulason S, Atilgan E, Brownell WE, Sun SX, Barr-Gillespie PG, Spector AA, 2014. The local forces acting on the mechanotransduction channel in hair cell stereocilia. Biophys J 106, 2519–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RJ, Roy S, Atilgan E, Brownell WE, Sun SX, Gillespie PG, Spector AA, 2012. Stereocilia membrane deformation: implications for the gating spring and mechanotransduction channel. Biophys J 102, 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prost J, Barbetta C, Joanny JF, 2007. Dynamical control of the shape and size of stereocilia and microvilli. Biophys J 93, 1124–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucher D, Stauffer T, Chen W, Shen K, Guo S, York JD, Sheetz MP, Meyer T, 2000. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell 100, 221–228. [DOI] [PubMed] [Google Scholar]
- Rehman AU, Morell RJ, Belyantseva IA, Khan SY, Boger ET, Shahzad M, Ahmed ZM, Riazuddin S, Khan SN, Riazuddin S, Friedman TB, 2010. Targeted capture and next-generation sequencing identifies C9orf75, encoding taperin, as the mutated gene in nonsyndromic deafness DFNB79. Am J Hum Genet 86, 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WM, Howard J, Hudspeth AJ, 1988. Hair cells: transduction, tuning, and transmission in the inner ear. Annu Rev Cell Biol 4, 63–92. [DOI] [PubMed] [Google Scholar]
- Robertson D, Johnstone BM, McGill TJ, 1980. Effects of loud tones on the inner ear: a combined electrophysiological and ultrastructural study. Hear Res 2, 39–43. [DOI] [PubMed] [Google Scholar]
- Rzadzinska AK, Schneider ME, Davies C, Riordan GP, Kachar B, 2004. An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal. J Cell Biol 164, 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles FT, Andrade LR, Tanda S, Grati M, Plona KL, Gagnon LH, Johnson KR, Kachar B, Berryman MA, 2014. CLIC5 stabilizes membrane-actin filament linkages at the base of hair cell stereocilia in a molecular complex with radixin, taperin, and myosin VI. Cytoskeleton (Hoboken) 71, 61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazzed S, Song J, Kovacs JA, Wriggers W, Auer M, He J, 2018. Tracing Actin Filament Bundles in Three-Dimensional Electron Tomography Density Maps of Hair Cell Stereocilia. Molecules 23, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ME, Dose AC, Salles FT, Chang W, Erickson FL, Burnside B, Kachar B, 2006. A new compartment at stereocilia tips defined by spatial and temporal patterns of myosin IIIa expression. J Neurosci 26, 10243–10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekerkova G, Zheng L, Loomis PA, Changyaleket B, Whitlon DS, Mugnaini E, Bartles JR, 2004. Espins are multifunctional actin cytoskeletal regulatory proteins in the microvilli of chemosensory and mechanosensory cells. J Neurosci 24, 5445–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekerkova G, Zheng L, Mugnaini E, Bartles JR, 2006. Differential expression of espin isoforms during epithelial morphogenesis, stereociliogenesis and postnatal maturation in the developing inner ear. Dev Biol 291, 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Purdy Drew KR, Bartles JR, Wong GC, Grason GM, 2009. Cooperativity and frustration in protein-mediated parallel actin bundles. Phys Rev Lett 103, 238102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JB, Krey JF, Hassan A, Metlagel Z, Tauscher AN, Pagana JM, Sherman NE, Jeffery ED, Spinelli KJ, Zhao H, Wilmarth PA, Choi D, David LL, Auer M, Barr-Gillespie PG, 2013. Molecular architecture of the chick vestibular hair bundle. Nat Neurosci 16, 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JB, Longo-Guess CM, Gagnon LH, Saylor KW, Dumont RA, Spinelli KJ, Pagana JM, Wilmarth PA, David LL, Gillespie PG, Johnson KR, 2010. The R109H variant of fascin-2, a developmentally regulated actin crosslinker in hair-cell stereocilia, underlies early-onset hearing loss of DBA/2J mice. J Neurosci 30, 9683–9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepecky N, Chamberlain SC, 1982. Distribution and polarity of actin in the sensory hair cells of the chinchilla cochlea. Cell Tissue Res 224, 15–24. [DOI] [PubMed] [Google Scholar]
- Slepecky N, Chamberlain SC, 1985. Immunoelectron microscopic and immunofluorescent localization of cytoskeletal and muscle-like contractile proteins in inner ear sensory hair cells. Hear Res 20, 245–260. [DOI] [PubMed] [Google Scholar]
- Sobin A, Flock A, 1983. Immunohistochemical identification and localization of actin and fimbrin in vestibular hair cells in the normal guinea pig and in a strain of the waltzing guinea pig. Acta Otolaryngol 96, 407–412. [DOI] [PubMed] [Google Scholar]
- Steyger PS, Gillespie PG, Baird RA, 1998. Myosin Iβ is located at tip link anchors in vestibular hair bundles. J Neurosci 18, 4603–4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SY, Kaelber JT, Chen M, Dong X, Nematbakhsh Y, Shi J, Dougherty M, Lim CT, Schmid MF, Chiu W, He CY, 2018. Flagellum couples cell shape to motility in Trypanosoma brucei. Proc Natl Acad Sci U S A 115, E5916–E5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, DeRosier DJ, Mulroy MJ, 1980. The organization of actin filaments in the stereocilia of cochlear hair cells. J Cell Biol 86, 244–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS, Saunders JC, DeRosier DJ, 1986. Actin filaments, stereocilia, and hair cells of the bird cochlea. III. The development and differentiation of hair cells and stereocilia. Devel Biol 116, 100–118. [DOI] [PubMed] [Google Scholar]
- Tilney LG, DeRosier DJ, 1986. Actin filaments, stereocilia, and hair cells of the bird cochlea. IV. How the actin filaments become organized in developing stereocilia and in the cuticular plate. Dev Biol 116, 119–129. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Egelman EH, DeRosier DJ, Saunders JC, 1983. Actin filaments, stereocilia, and hair cells of the bird cochlea. II. Packing of actin filaments in the stereocilia and in the cuticular plate and what happens to the organization when the stereocilia are bent. J Cell Biol 96, 822–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney MS, Tilney LG, Stephens RE, Merte C, Drenckhahn D, Cotanche DA, Bretscher A, 1989. Preliminary biochemical characterization of the stereocilia and cuticular plate of hair cells of the chick cochlea. J Cell Biol 109, 1711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinevez JY, Perry N, Schindelin J, Hoopes GM, Reynolds GD, Laplantine E, Bednarek SY, Shorte SL, Eliceiri KW, 2017. TrackMate: An open and extensible platform for single-particle tracking. Methods 115, 80–90. [DOI] [PubMed] [Google Scholar]
- Turgay Y, Eibauer M, Goldman AE, Shimi T, Khayat M, Ben-Harush K, Dubrovsky-Gaupp A, Sapra KT, Goldman RD, Medalia O, 2017. The molecular architecture of lamins in somatic cells. Nature 543, 261–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélez-Ortega AC, Freeman MJ, Indzhykulian AA, Grossheim JM, Frolenkov GI, 2017. Mechanotransduction current is essential for stability of the transducing stereocilia in mammalian auditory hair cells. eLife 6, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker M, Wilson-Kubalek EM, Smith JE, Faust L, Milligan RA, Sweeney HL, 1995. A 35-A movement of smooth muscle myosin on ADP release [see comments]. Nature 378, 748–751. [DOI] [PubMed] [Google Scholar]
- Zhang DS, Piazza V, Perrin BJ, Rzadzinska AK, Poczatek JC, Wang M, Prosser HM, Ervasti JM, Corey DP, Lechene CP, 2012. Multi-isotope imaging mass spectrometry reveals slow protein turnover in hair-cell stereocilia. Nature 481, 520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wu Z, Müller U, 2016. Murine Fam65b forms ring-like structures at the base of stereocilia critical for mechanosensory hair cell function. Elife 5, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Williams DE, Shin JB, Brugger B, Gillespie PG, 2012. Large membrane domains in hair bundles specify spatially constricted radixin activation. J Neurosci 32, 4600–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Sekerkova G, Vranich K, Tilney LG, Mugnaini E, Bartles JR, 2000. The deaf jerker mouse has a mutation in the gene encoding the espin actin-bundling proteins of hair cell stereocilia and lacks espins. Cell 102, 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Actin gaps do not result from an averaging effect. (A) Single cross-sectional plane of original raw tomogram, approximately 1nm thick. (B) Same plane as A, with false color to better enhance lack of density. (C) Close up of red rectangle of B, where asterisk indicates the location of the gap. (D) Single cross-sectional plane of 10 nm averaged tomogram, representing approximately 10 nm thick slab≥ (E) Same plane as D, with false color to better enhance lack of density. (F) Close up of red rectangle on Supplemental figure E, where asterisk indicates the location of the gap. (G) Single cross-sectional plane of 30 nm averaged tomogram, representing approximately 30 nm thick slab. (H) Same plane as G, with false color to better enhance lack of density. (I) Close up of red rectangle on Supplemental figure H, where asterisk indicates the location of the gap. (J) Example locations for sample window taken at actin filaments. (K) Example locations for inter-filament locations. (L) Example locations in the gap region (but not centered at the gap). (M) Gap location. Scale bars: A-B, D-E, G-H, J-M, 100 nm; C, F, I, 50 nm. (N) Plot of intensities measured at locations of actin filaments, locations between filaments (inter-filament), locations surrounding the gap, and the gap itself. Inter-filament and gap region measurements were significantly different from measurements at actin filaments (***, p<0.0001).
Supplemental Video 1 (Song_Map01_NoAverage). Scroll-through video of XZ-cross-sectional slices of nonlinear anisotropic diffusion of denoised un-averaged density map, displayed in false-color representation to emphasize high density regions (pink and red) as well as low density regions (blue).
Supplemental Video 2 (Song_Map01_10nm_Average). Scroll-through video of XZ-cross-sectional slices of density map where each slice along the Y-axis has been replaced by an 10 nm average slab of the density map. Map is displayed in false-color representation to emphasize high density regions (pink and red) as well as low density regions (blue).
Supplemental Video 3 (Song_Map01_30nm_Average). Scroll-through video of XZ-cross-sectional slices of density map where each slice along the Y-axis has been replaced by an 30 nm average slab of the density map. Map is displayed in false-color representation to emphasize high density regions (pink and red) as well as low density regions (blue).


