Abstract
Targeting early lesion in breast cancer is more therapeutically effective. We have previously identified an oncoprotein GT198 (PSMC3IP) in human breast cancer. Here we investigated GT198 in MMTV-PyMT mouse mammary gland tumors and found that GT198 is a shared early lesion in both species. Similar to human breast cancer even before a tumor appears, cytoplasmic GT198 is overexpressed in mouse tumor stroma including pericyte stem cells, descendent adipocytes, fibroblasts, and myoepithelial cells. Using recombinant GT198 protein as an antigen, we vaccinated MMTV-PyMT mice and found that the GT198 vaccine delayed mouse tumor growth and reduced lung metastasis. The antitumor effects were linearly correlated with vaccinated mouse serum titers of GT198 antibody, which recognized cell surface GT198 protein on viable tumor cells confirmed by FACS. Furthermore, GT198+ tumor cells isolated from MMTV-PyMT tumor induced faster tumor growths than GT198− cells when re-implanted into normal FVB/N mice. Together, this first study of GT198 vaccine in mouse showed its effectiveness in antitumor and anti-metastasis. The finding supports GT198 as a potential target in human immunotherapy since GT198 defect is shared in both human and mouse.
Keywords: Breast cancer, tumor-associated antigen, cancer vaccine, pericytes, tumor stroma, immunotherapy
1. Introduction
In 1953, the mouse polyomavirus was isolated as one of the first DNA viruses with its potential role in tumorigenesis [1, 2]. Polyomavirus was found to induce tumors when inoculated into a variety of mouse tissues. Subsequently, it was established that polyomavirus expresses several tumor antigens, including the middle T antigen, which is necessary and sufficient for polyomavirus-induced tumor transformation [3–5].
The polyomavirus middle T antigen is a membrane-bound protein containing 421 amino acids with multiple phosphorylation sites. It does not have kinase activity itself but serves as a scaffold to interact and activate a panel of kinases and phosphatases, including but not limited to, Src family tyrosine kinases, protein phosphatase 2A, PI3 kinase, Shc and Ras, which in turn stimulate cellular signal transduction pathways [2, 4]. In particular, the Ras pathway with downstream mitogen-activated protein (MAP) kinase activation has been extensively studied for tumorigenesis in molecular or cellular contexts, and in mouse tumor models [3, 4, 6]. Because complex nuclear protein factors are targeted by virus-induced kinase signaling, the nuclear mechanism responsible for tumor initiation remains undetermined.
The effects of middle T antigen in mouse mammary glands were previously investigated in transgenic mice carrying mouse mammary tumor virus long terminal repeat (MMTV-PyMT), where the middle T antigen can be specifically expressed in mammary glands under the MMTV promoter. This resulted in widespread, multifocal, and spontaneous mammary tumor formation with onset as early as 3–4 weeks of age [7]. Similarly, mammary gland-specific expression of v-Ha-ras oncogene in MMTV-Ras transgenic mice also showed widespread and spontaneous mammary gland tumors [8]. Tumor metastasis is often found in mouse lung and lymph nodes.
Despite substantial evidence of tumor formation in mouse models, the obvious difference may exist between mouse and human tumor initiation, which become an increasing concern [9–11]. Since the goal of cancer research is the development of therapies for human with drug target identification, it was a challenge to reconcile, for example, human breast cancer versus mouse mammary gland tumor, in their physiology, pathology, and molecular signaling.
We have previously identified a human breast cancer initiating oncogene called GT198 (gene symbol PSMC3IP) [12]. In this study, we investigate oncoprotein GT198 expression in MMTV-PyMT and MMTV-Ras mice and carry out vaccination using GT198 protein as an antigen. We found that the differences between human and mouse can be reconciled. Specifically, distinct tumor initiating stimuli in human and mouse resulted in a common GT198-mediated pathway of tumor development in both species.
GT198 is a DNA-binding protein dimer, containing 217 amino acids in its monomer [13]. GT198 binds to single- and double-stranded DNAs and the binding is not sequence-specific. GT198 is a transcriptional coactivator stimulating nuclear receptor-mediated gene activation [13, 14]. GT198 protein is MAP kinase phosphorylation regulated [12, 13]. GT198 is also known as Hop2/TBPIP, acts as a crucial DNA repair factor by participating in homologous DNA recombination and regulating meiosis [15–17].
The human GT198 gene is located at chromosome 17q21, 470 Kb proximal to BRCA1, a hot cancer locus previously linked to breast and ovarian cancer predisposition. Germline mutations in GT198 have been identified in familial and early-onset human breast and ovarian cancer [18, 19], as well as in familial ovarian disease [20]. Somatic mutations in GT198 are prevalent and recurrent in sporadic breast, ovarian, prostate, uterine, and fallopian tube cancers, where mutant GT198 leads to constitutive activation in transcription [18, 21].
GT198 regulates mouse stem cells at the embryoid body stage [21]. In sporadic human breast and ovarian cancers, the GT198 gene is mutated in the tumor microenvironment. In breast cancer, the mutant cells are pericyte stem cells and the descendent vascular smooth muscle cell lineage, including myoepithelial cells, fibroblasts, and adipocytes [12]. GT198 affects stromal cells in common solid tumors [22], including hormone-producing luteinized theca cells in ovarian cancer [23], pericytes in skin and brain tumors [24], and myofibroblasts in prostate and bladder cancers [25].
Importantly, GT198 protein is an anticancer drug target. Clinically effective oncology drugs such as paclitaxel, doxorubicin analogs, and etoposide, are found to directly bind and inhibit GT198 protein [22, 26]. The finding is not surprising since GT198 shares protein sequence homology with both DNA topoisomerase I and II [22], which are previously thought as targets of doxorubicin and etoposide. GT198 is also inhibited by certain herbal medicines known to be clinically successful. As a drug target, GT198 protein may also have potential to serve as an antigen in therapeutic cancer vaccine.
An ideal cancer vaccine antigen should have high tumor specificity as well as high immunogenicity [27]. GT198 fulfills the criteria to be an ideal antigen. First, the overall expression pattern of GT198 resembles a class of proteins called cancer-testis antigens with high levels of expression in cancer, embryo, testis, but low levels in most adult normal tissues [24]. This will minimize the toxicity of the vaccine in normal cells. Secondly, targeting to GT198+ tumor stromal cells and their progenitors is expected to remove the stimuli driving tumor growth. The phenotype of stromal cells is more stable than that of tumor cells, reducing the chance of immune escape. This is an advantage over many other tumor-associated antigens found at the late stages of tumor. Thirdly, GT198 normally is a nuclear protein but deregulated GT198 is overexpressed in cell cytoplasm and membrane [24], adding another layer of tumor specificity in therapy. Finally, GT198 is a small protein at 217 amino acids enriched with alpha-helices based on the secondary structure prediction. Polyclonal GT198 antibody had high antibody titer indicating a likely high immunogenicity. We therefore tested GT198 protein as a cancer vaccine antigen in this study.
In this report, we found that GT198 is overexpressed in tumor stromal cells including pericytes, adipocytes, and myoepithelial cells in MMTV-PyMT and MMTV-Ras mouse mammary glands. We carried out vaccination using recombinant GT198 protein as antigen in MMTV-PyMT mice and confirmed that GT198 vaccine delayed mouse tumor growth. Since this is the first time to test GT198 vaccination in mice, we performed a pilot study but thoroughly evaluated each aspect in tumor development, including serum antibody production, tumor growth, lung metastasis, FACS analysis of immune cells in mouse organs, and FVB/N mouse re-implantation using GT198 positive tumor cells derived from the MMTV-PyMT tumor.
We suggest that GT198 vaccination has significant promise to warrant further investigation for human immunotherapy targeting GT198. We conclude that even though tumor initiating stimuli are distinct in human and mouse, oncoprotein GT198 is a shared target in both species resulting in a common pathway of tumor development. The oncoprotein in human breast cancer finally provides an explanation of polyomavirus-induced mammary tumor initiation in mice.
2. Materials and methods
2.1. Immunohistochemistry
Polyclonal rabbit antibody against GT198 was affinity purified and previously described [12, 13]. FFPE sections of mouse tumors and lungs from MMTV-PyMT (FVB/N-Tg(MMTV-PyVT) 634Mul/J) [7], MMTV-Ras (FVB.Cg-Tg(MMTV-vHa-ras)SH1Led/J) [8], and MMTV-Neu (FVB/N-Tg(MMTVneu)202Mul/J) [28], mice were deparaffinized and dehydrated through xylene and ethanol series, followed by antigen retrieval in 10 mM sodium citrate buffer, pH 6.0, containing 0.05% Triton at 90°C for 20 min. Anti-GT198 (1:200) was incubated at 4°C overnight. Antibody binding was detected using a biotinylated secondary antibody followed by detecting reagents (Abcam, Cambridge, MA). Sections were counterstained with hematoxylin.
2.2. His-GT198 protein purification
N-terminal His-tagged recombinant human GT198 protein (aa 1–217) was expressed in E. coli BL21(DE3)pLysS and purified through Ni-NTA-agarose (Qiagen, #30210) as previously described [21]. Proteins were eluted by 200 mM imidazole, desalted and concentrated using Amicon YM-10 spin columns before used in GT198 antibody detection.
2.3. GT198 inclusion body as antigen in vaccination
The glutathione S-transferase (GST) fusion human GT198 protein was expressed in E. coli BL21(DE3)pLysS and insoluble inclusion body was collected as antigen in mouse vaccination since insoluble antigen has greater efficacy in vaccination. Briefly, the isolated inclusion body containing 95% pure GT198 protein (Supplementary Figure S1a) was repeatedly washed by sonication in PBS and sterilized by 70% ethanol. Incomplete Freund’s adjuvant (IFA) was mixed with PBS at 1:1 ratio together with GT198 protein pellet, and was sonicated using a sterilized probe to produce GT198 antigen at 1 mg/ml for each subcutaneous injection at 100 μg in 100 μl. GST was too soluble to yield inclusion body so that soluble GST protein served as control.
2.4. GT198 vaccination in mouse tumor models
An institutional animal care and use committee (IACUC) approval was obtained (#2014–0625). In MMTV-PyMT mice, GT198 vaccinations (n=3, an additional one was censored) together with controls (n=4) were carried out in 4-week-old female mice after confirmed by genotyping. Because GT198 expression in tumor stroma can be found in as early as three weeks (Fig. 1a), vaccination is designed to be as early as possible. Mice were subcutaneously injected at 100μg of GT198 inclusion body in 100μl IFA solution, in every other week starting from week four until week 16 (Fig. 2b). Controls were GST in IFA. Mouse tail blood was collected at each vaccine time point to produce serum (5–10 μl). The antibody titers were measured at the end of experiment using His-tagged GT198-coated 96-well white plate (100 ng GT198 and 5 μg BSA/well), which was incubated with 200 μl of 1:1000 or 1:3000 diluted mouse sera in duplicate wells, and detected by HRP-conjugated anti-mouse antibody with ECL detection reagents. Antibody titers were counted by a Dynex luminometer. Tumor volumes were also measured at each vaccine time point from week 4 to week 16. The volume calculations were obtained using the formula V = (W(2) × L)/2 with caliper measurements. Tumor volume and GT198 antibody binding correlation were further statistically analyzed.
Fig. 1. Cytoplasmic GT198 expression in mouse mammary gland tumors of MMTV-PyMT and MMTV-Ras transgenic mice.
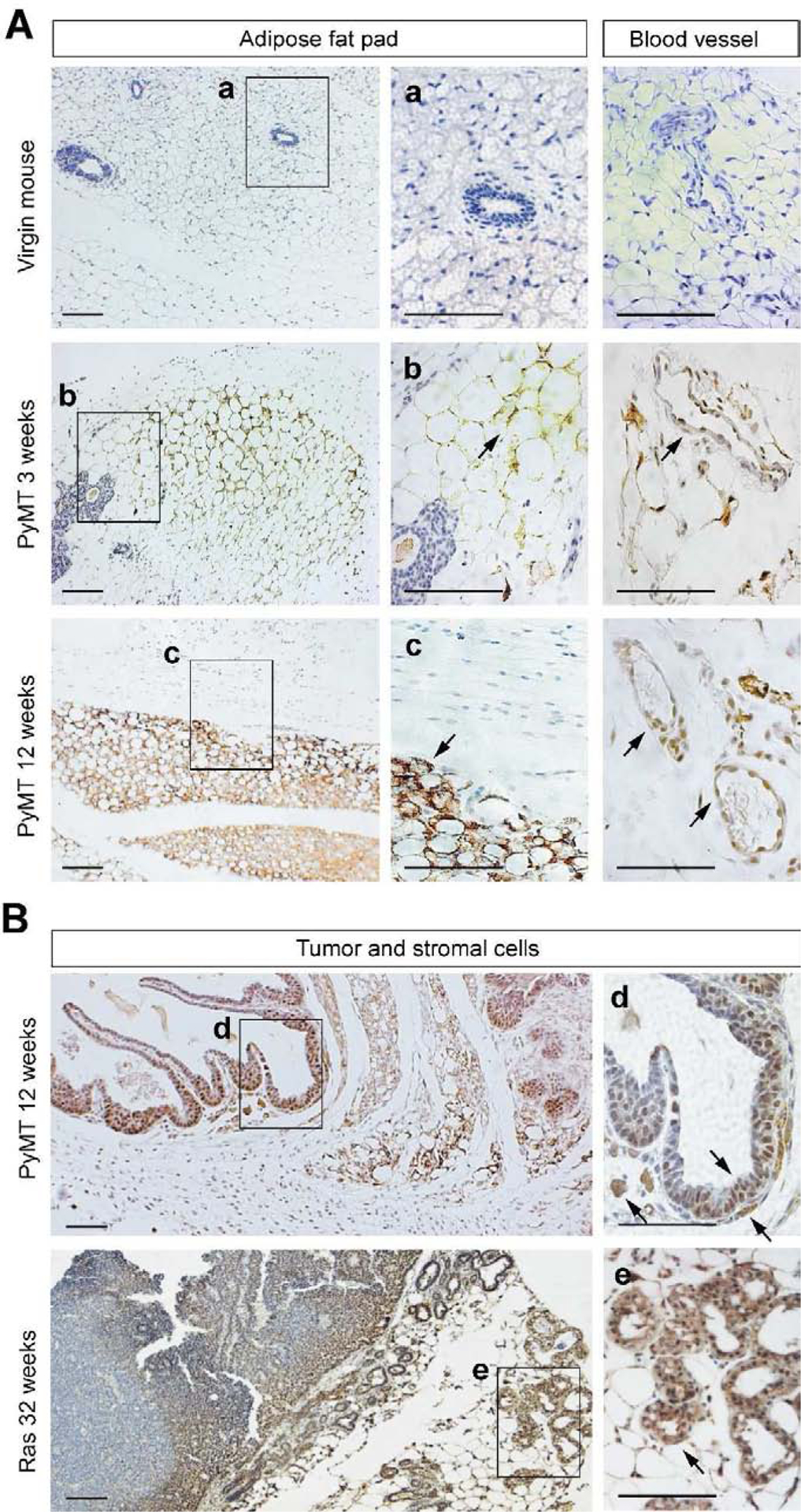
A. GT198 expression in fat pads and blood vessels of mouse mammary gland tumor microenvironment. Virgin non-transgenic mouse mammary gland serves as a negative control. At three weeks of age, before tumor appears in PyMT mice, GT198 is expressed in adipocytes of the fat pad and in pericytes of the capillary. At 12 weeks when the tumor appears, cytoplasmic GT198 is highly expressed in the fat pad. B. GT198 expression in mammary gland tumor of MMTV-PyMT mouse at 12 weeks of age, and in MMTV-Ras mouse at 32 weeks (8 months). Strong GT198 expression is in adipocytes, myoepithelial cells, and stromal fibroblasts. Nuclear expression is found in epithelial tumor cells at early stages of tumor. Arrows indicate GT198 positive cells. Boxed areas are enlarged at the right with corresponding labels. Sections were counter-stained with hematoxylin. Scale bars = 100 μm.
Fig. 2. GT198 vaccination delays tumor growth in MMTV-PyMT transgenic mice.
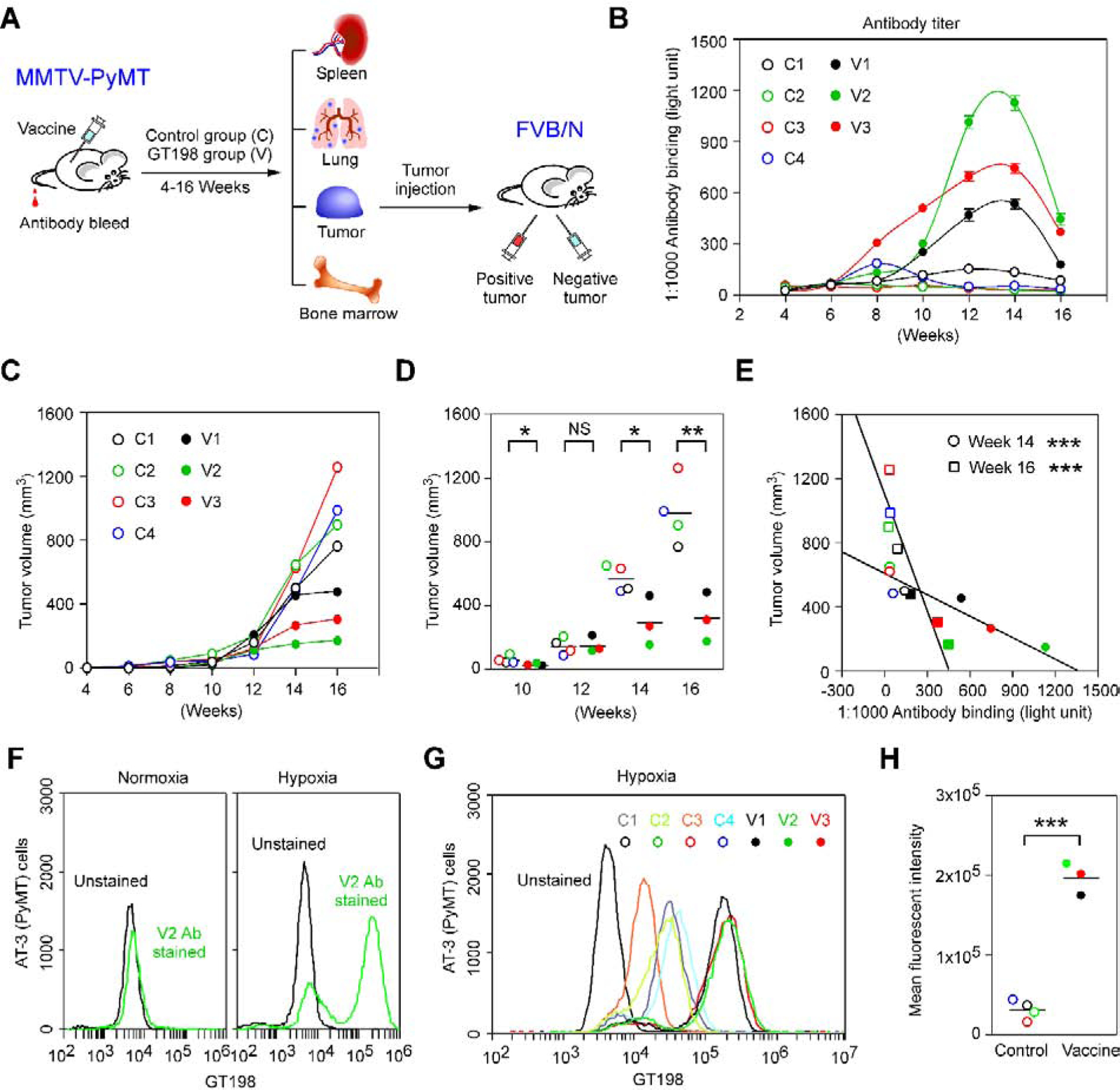
A. A model of the study design. Human recombinant GST-GT198 inclusion body as antigen (100μg) was subcutaneously injected in MMTV-PyMT mice, with tumor volume measured, and tail blood collected during 4–16 weeks of age. Lung metastasis and mouse organs were analyzed at week 16. The GT198 positive and negative tumor cells were further re-implanted in pairs into each side of FVB/N mice (n=6) for tumor growth analysis. B. Using his-tagged GT198 coated 96-well plates, GT198 antibody titer in diluted mouse tail blood sera (1:1000, n=2) was measured in each mouse. C-D. Tumor volumes were measured and analyzed in the vaccine group (n=3) and control group (n=4). P * < 0.05; P ** < 0.01; NS, not significant. E. Reciprocal linear correlation of tumor volumes with serum anti-GT198 concentrations at weeks 14 and 16. F. Vaccine-induced anti-GT198 antibody (V2, week 14) increased the binding to cultured viable AT-3 (PyMT) tumor cells with PyMT origin under hypoxia condition. G. GT198 antibody binding to hypoxic AT-3 cells was compared among sera from vaccine and control mice. H. Same data are presented by scattergram. The vaccine group has a significant increase of anti-GT198 recognition to AT-3 tumor cells. P *** < 0.001.
In FVB/N mice, tumor re-implantation was carried out in six female mice at 6-week-old of age. GT198+ tumor cells were obtained from MMTV-PyMT tumor with smaller tumor size and with tumor stromal contents. GT198− tumor cells were obtained from MMTV-PyMT tumor with larger tumor size and at the center of the tumor. The tumor cells at the center of a larger tumor are generally more GT198 negative (Fig. 1b), in contrast to GT198 positive in tumor stroma. This was later confirmed by immunohistochemistry staining of GT198. Each mouse (n=6) was implanted with GT198− cells at the left, and GT198+ cells at the right sides of the mammary glands (106 cells in 100 μl), and followed for additional seven weeks with tumor volume measurements.
In B6 mice for vaccine and adjuvant evaluations (n=4), GT198 vaccination using the inclusion body was similarly carried out by comparison of three adjuvants. Subcutaneous injections of Incomplete Freund’s adjuvant (IFA, 1:1) or TiterMax (1:1), or intramuscular injection of Anti-CD40 and Poly IC (50 μg each) were used with 100 μg GT198 in 100 μl volume.
2.5. Lung metastasis analysis
Both sides of the lung in MMTV-PyMT mice were prepared in FFPE sections, immunohistochemically stained with anti-GT198 (1:150), and immunofluorescent stained with anti-GT198 and anti-Ki67 as a proliferation marker. Foci numbers were counted from both sides of the lung in the analysis.
2.6. FACS analysis
FACS analysis and data acquisition were performed on a flow cytometer (Accuri C6, BD Biosciences). Immune cells were isolated from spleen, lung, tumor, and bone marrow from the vaccine or control MMTV-PyMT mice. FACS analysis was carried out for myeloid (CD45+, CD11b+), M1 macrophage (CD45+, CD86+, CD11b+), and B cells (CD45+, CD19+). Analysis for lymphocyte (CD45+), M2 macrophage (CD45+, CD206+, CD11b+), MDSC (CD45+, Gr1+, CD11b+), T helper cells (CD45+, CD4+), and T cytotoxic cells (CD45, CD8+) were also carried out but are not shown. Hypoxia AT-3 cells which is a PyMT mouse tumor cell line were generated in DMEM with 10% fetal bovine serum, in 1% oxygen and 5% CO2 at 37°C overnight. Living normoxia and hypoxia AT-3 cells were stained in PBS with 1% BSA on ice using anti-GT198 and anti-rabbit Alexa 448. A minimum of 10,000 cells within the gated region were analyzed.
2.7. GT198 antibody titer analysis
The antibody titers were measured using collected mouse sera. Briefly, mouse tail blood (20 μl) was left on ice for 30 min and centrifuged to collect sera (10 μl). White MicroLite™ 2+ 96-well plates (Thermo Scientific, #7572) were coated to dry overnight at 37°C with 400 ng/well of recombinant His-tagged GT198 proteins together with 5 μg/well of purified BSA (NEB) in a volume of 50 μl. BSA alone was included as a control for background. Duplicate wells were used for each experimental point (n = 2). The GT198-coated plates were blocked with 5% BSA in PBS with 0.1% Triton X-100 (TPBS) for 1 h. The antibody binding was carried out in 200 μl of 1:1000 or 1:3000 diluted mouse sera in duplicate wells, and detected by HRP-conjugated anti-mouse antibody with ECL detection reagents. Antibody titers were counted by a Dynex luminometer.
2.8. Immunofluorescence
Paraffin-embedded mouse tumor tissue sections were deparaffinized through xylene and ethanol series, followed by antigen retrieval in 10 mM sodium citrate buffer, pH 6.0, containing 0.05% Triton at 90°C for 20 min. Immunofluorescence double staining was carried out in 1% horse serum using rabbit anti-GT198 (1:150) and mouse anti-Ki67 (1:200, DAKO). Secondary antibodies were anti-mouse or anti-rabbit Alexa Fluor-conjugated antibodies (Invitrogen, Carlsbad, CA). Slides were counterstained with DAPI before visualization by fluorescence microscopy.
2.9. Statistical analysis
Statistical analyses were carried out using GraphPad Prism software. Scattergrams with means are presented using tumor volume scores, metastasis foci numbers, or fluorescent intensity. In tumor volume and antibody binding correlation assay, P values were determined by linear regression. In antibody titer measurements, data represent mean ± s.e.m of duplicate experiments (n = 2). Duplicates rather than triplicates were used in each experimental data point due to high consistency. P values in scattergrams were calculated using unpaired two-tailed t-test. * P<0.05, ** P<0.01, *** P <0.001; NS, not significant. A P value of less than 0.05 is considered statistically significant.
3. Results
3.1. High levels of GT198 expression in mouse mammary gland tumor stroma
We have previously identified GT198 overexpression in human reactive breast tumor stroma due to GT198 somatic mutations [12]. Here we performed immunohistochemical staining of GT198 in mouse mammary glands of MMTV-PyMT and MMTV-Ras transgenic mice [7, 8]. We found robust and widespread expression of GT198 in mammary glands of transgenic but not virgin mice (Fig. 1). The positive cells include pericytes in blood vessels, adipocytes, fibroblasts, myoepithelial cells, as well as epithelial cells. In 3-week-old MMTV-PyMT mouse mammary gland before a tumor can be detected, GT198 is already expressed in adipocytes and pericytes (Fig. 1A). The same GT198 expression pattern is present in MMTV-Ras mice (Fig. 1B). The expression is mostly cytoplasmic, which is a characteristic of GT198 activation with splice variant expression [21]. However, tumor cells have nuclear GT198 expression which is reduced at advanced stages of the tumor growth (Fig. 1B).
GT198 expression pattern in the mouse is similar to that observed in the human breast cancer. However, no somatic mutation of the mouse GT198 gene was detectable in mouse mammary glands (sequence data not shown). GT198 was originally cloned through activating Sos-Ras pathway [13], and GT198 variant can be stimulated by MAP kinase activity [12]. Thus, the overexpression of GT198 variant in transgenic mice is possibly kinase-induced and has bypassed the requirement of somatic mutations in human, which induce GT198 variant activation [21], with cytoplasmic overexpression in human breast cancers [12].
3.2. GT198 vaccination in MMTV-PyMT mice
Oncoprotein GT198 activation is an early event in tumor development and occurs in pericytes and stromal microenvironment before tumor cells appear. We therefore tested GT198 vaccination to determine whether serum anti-GT198 antibody in mouse has a protective effect on tumor growth.
The MMTV-PyMT transgenic mice were subcutaneously vaccinated using insoluble sonicated recombinant human GST-GT198 inclusion bodies (n=3) or GST control (n=4) during 4–16 weeks of age every two weeks (Fig. 2A–B and Supplementary Fig. S1). The adjuvant used was incomplete Freund adjuvant (IFA) due to its higher efficacy when compared to other adjuvants tested (Supplementary Fig. S1B–C). Blood sera were collected for GT198 antibody titer analysis with tumor volumes concurrently measured at each vaccination time point. Mouse serum anti-GT198 antibody was increased in vaccinated mice than the controls, although serum antibody appeared to be consumed by GT198 positive tumors and decreased at the late stages of tumor development (Fig. 2B). There was a significant delay of tumor growths in the vaccinated group than the control mice (Fig. 2C–D). Importantly, tumor volumes showed a reciprocal linear correlation to serum antibody titers in all mice (Fig. 2E). The serum anti-GT198 antibody in each mouse was further evaluated by FACS analysis, and the results suggested that vaccine-induced antibodies can recognize cell surface GT198 protein in viable cultured hypoxic AT-3 tumor cells (Fig. 2F–G), and the increasing recognition by GT198 antibody is significant in vaccinated mice than the controls (Fig. 2H). The antibody binding was also detectable using viable MMTV-PyMT tumor cells or PY8119 (PyMT) tumor cell line (not shown), consistent with our previous report that surface GT198 is expressed on hypoxic tumor cells [24]. These data together confirmed that serum GT198 antibody would target tumor cells in vivo in MMTV-PyMT transgenic mice.
3.3. GT198 vaccination decreases lung metastasis
At the end of the vaccination, mouse organs including lung, spleen, bone marrow, and tumor were collected for further analyses (Fig. 2A). The numbers of metastatic foci in both side of the lung were counted after immunohistochemical staining of GT198 (Fig. 3A). The lung loci numbers were decreased in vaccinated mice compared to the controls (Fig. 3B–C). Metastatic foci contain GT198 positive tumor cells, located mostly at the edge of foci (Fig. 3D), and are more proliferative (Fig. 3E).
Fig. 3. GT198 vaccination decreases tumor metastasis in the lungs of MMTV-PyMT mice.
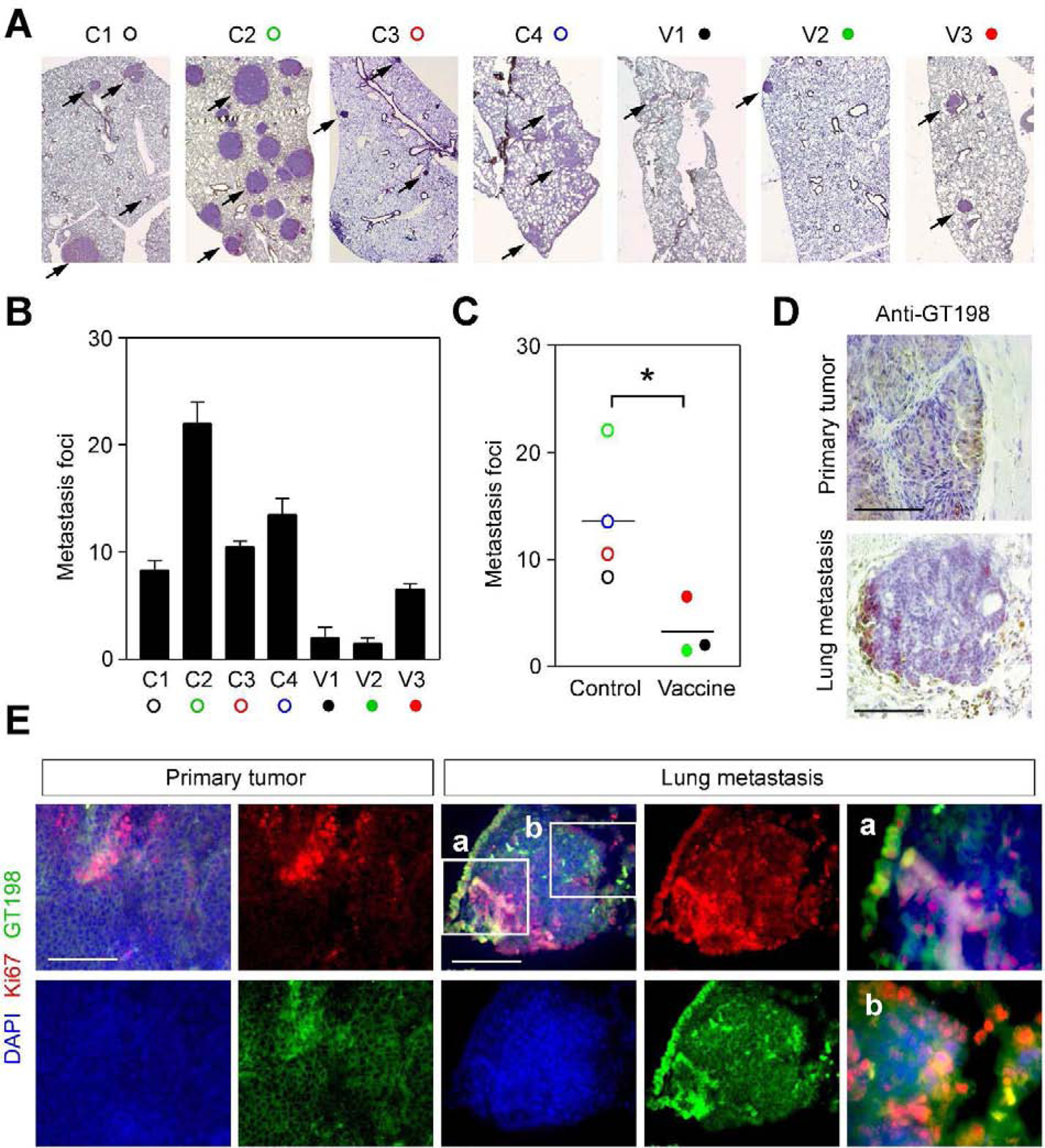
A. Immunohistochemical staining of GT198 for lung metastasis in vaccine and control groups of mice. B. Numbers of lung metastasis foci in each mouse. Both sides of the lung (n=2) were FFPE blocked and stained to count numbers of foci. C. The averages of foci numbers are presented by scattergram. P * < 0.05. D. Immunohistochemical staining of GT198 to compare a primary tumor in the mammary gland and a metastatic tumor in the lung. E. Immunofluorescent staining of the proliferation marker Ki67 (red), showing GT198 positive cells (green) are proliferative in both primary and lung metastatic tumors. Slides were counterstained with DAPI.
3.4. Immune cell population in vaccinated mice
We further analyzed the immune cell populations from mouse organs in representative vaccinated (V1, V2) and control mice (C1, C2). We analyzed myeloid, M1 macrophage, B cell populations (Fig. 4). We also analyzed total lymphocyte, M2 macrophage, MDSC, T helper cells, and T cytotoxic cells (not shown). We found that there were no significant changes among vaccinated and control groups. However, there was a potential increase of myeloid, M1 macrophage, B cell populations only in tumors of vaccinated mice (Fig. 4), although statistically insignificant. These data suggest that the mouse cellular immune response remained comparable between GT198 vaccinated mice and the control group.
Fig. 4. FACS analyses of immune cell populations in GT198-vaccinated and control MMTV-PyMT mice.
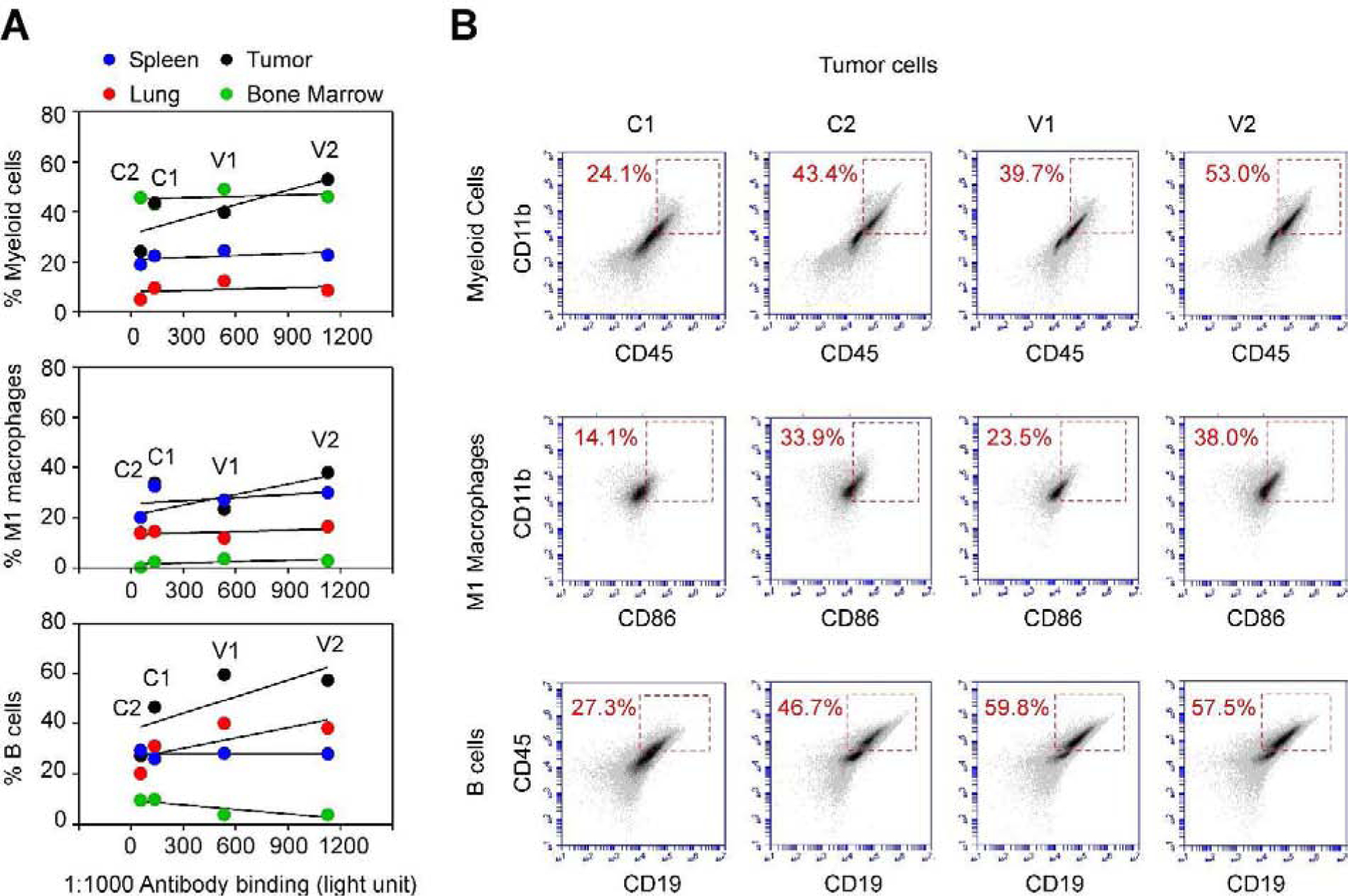
A. At week 16, FACS analyses of myeloid, M1 macrophage, and B cell populations were carried out using organs derived from the vaccine (V1, V2) and control (C1, C2) mice. Positive cell populations in the spleen (blue), lung (red), bone marrow (green), and mammary gland tumor (black) were graphed correlating to GT198 antibody titers at week 14 in each mouse. Three immune cell populations were potentially increased in tumors of mice with elevated anti-GT198 antibody, although not statistically significant. P > 0.05. B. Flow cytometry quadrant dot plots with immune cell markers as indicated on the x-axis and y-axis. The numbers at each corner indicate the percentage of positive cells in tumors.
However, these analyses were carried out at the end of the vaccination experiment when mouse organs can be collected, but when antibody titers already dropped in the vaccinated group (Fig. 2B). It may be critical for future studies to evaluate the immune response during the course of vaccination. Since there is a possibility that GT198 vaccine stimulates in vivo cellular immune response, in addition to detectable serum response.
3.5. GT198 positive cells from MMTV-PyMT tumor promote tumor growth when re-implanted into non-transgenic FVB/N mice
GT198+ tumor stromal cells are observed before the tumor starts and GT198 vaccination delayed the tumor growth. These observations suggest that GT198+ cells may drive tumor development. To further test this hypothesis, we compared GT198+ and GT198− cells by re-implanting them into non-transgenic FVB/N mice (n=6) with a background similar to the MMTV-PyMT mice (Fig. 5A). We implanted both cells in each side of the same mouse to achieve better control.
Fig. 5. GT198-positive tumor cells promote tumor growth when re-implanted.
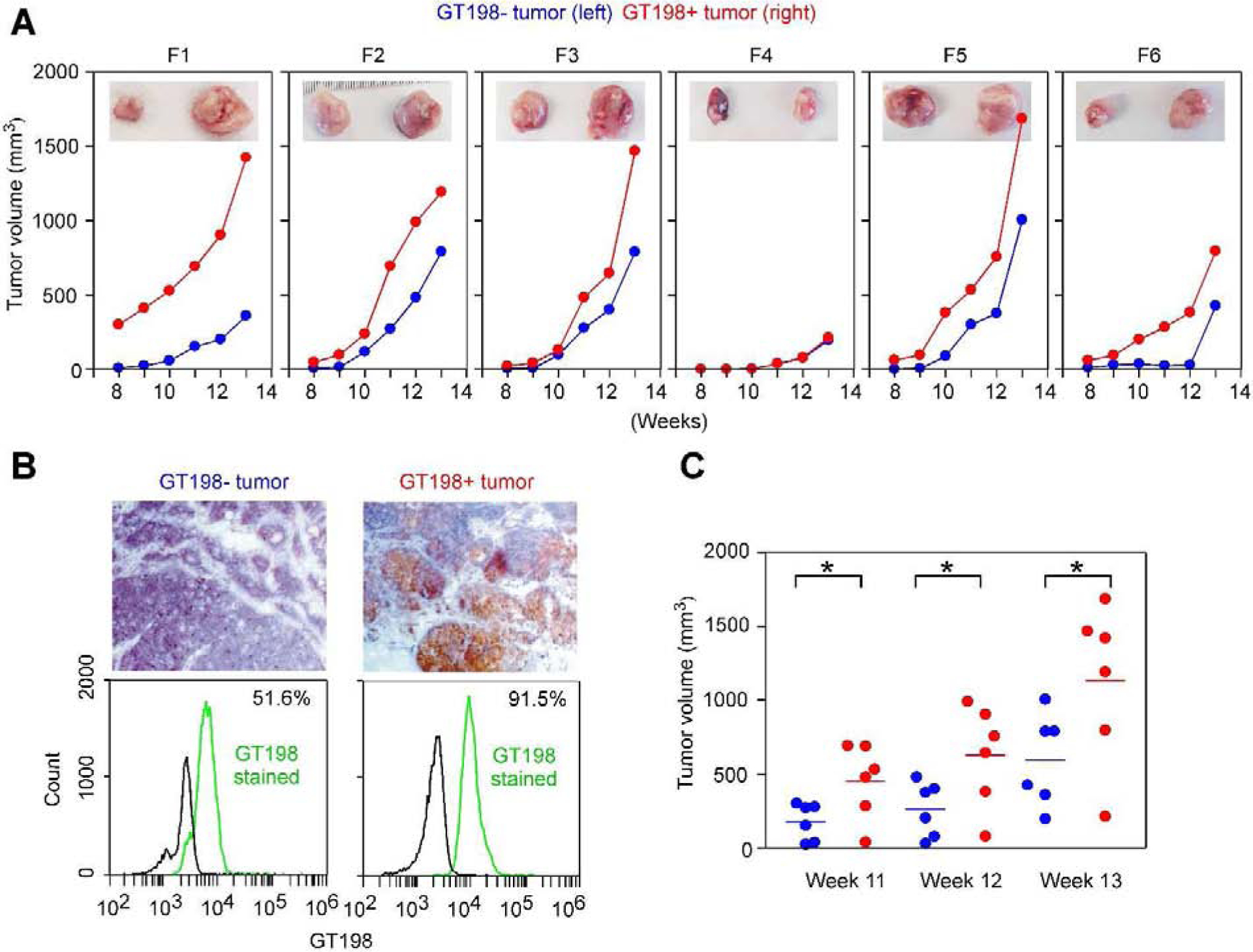
A. GT198+ and GT198− tumor cells (106 cells in 100 μl) derived from MMTV-PyMT mouse were further re-implanted into six FVB/N mice (F1-F6) with GT198− cells at the left and GT198+ cells at the right sides of mammary glands in each mouse. The mice were followed with tumor volume measurement for additional seven weeks. The tumors at week 13 were photographed and are shown at the top (GT198−, left; GT198+, right). B. Immunohistochemical staining of GT198 in MMTV-PyMT tumors used for re-implantation (top panels). FACS analysis of GT198 expression in the tumor cells. 91.5% cells from the GT198+ tumor are positive, although 51.6% of cells from GT198− tumor are also weakly positive (bottom panels). C. Tumor volumes in weeks 8–10 from (A) were analyzed by Prism scattergram showing that GT198+ tumor cells significantly promote tumor growth than GT198− tumor cells. P * < 0.05.
GT198+ cells were derived from a smaller sized MMTV-PyMT tumor, reddish in color, which contained more stromal cells confirmed by microscopic examination. GT198− cells were derived from more massive sized tumor center, white in color, which contained mostly negative tumor cells evaluated by immunohistochemistry (Fig. 5B). These cells weakly expressed GT198 as evaluated by FACS analysis (Fig. 5B). After an equal number of cells (106 cells) were injected subcutaneously at week 6 of age in FVB/N mice, tumor volumes were measured weekly for additional 7 weeks. The tumors were collected at the end of experiments at week 13. The data showed that GT198+ cells significantly promoted tumor growth than GT198− cells (Fig. 5A–C). Our studies collectively suggest that GT198+ cells promote mouse tumor development, and GT198 vaccination decreases tumor growth in MMTV-PyMT mice.
4. Discussion
The polyomavirus middle T antigen-induced spontaneous mouse tumor is one of the most extensively studied mouse tumor models [4]. It was predicted that the middle T antigen targets protein factors relevant to human tumorigenesis, and this is an evolutionarily conserved function utilized by the virus to propel host cell growth [2]. This targeted factor has now emerged as GT198.
As a transcriptional coactivator, stem cell regulator, and crucial DNA repair factor, GT198 is in position to serve as such an oncoprotein. Deregulated GT198 activity in mutant GT198 or splice variant overexpression leads to potent apoptosis [21]. We consider GT198 as a p53-like molecule in their similar dimeric structures, functions in DNA repair and cell cycle, splice variants always retaining DNA-binding domains [29], as well as unusual abundant somatic mutations [21]. GT198 is also directly inhibited by paclitaxel which affects cell mitosis [26].
However, the mechanism of tumorigenesis is not merely promoting cell growth as previously thought, but is activating pericyte stem cells on blood vessels and producing stromal microenvironment responsible for epithelium growth [12]. This is an important point since cytotoxic drugs are not necessarily effective antitumor drugs in human. In part because that mouse tumor cells are all fast growing and human cancer cells are slow developing in years [30].
When human cancers and rodent tumor models are compared, the GT198+ vessel pericytes are found as a common feature (Fig. 6). In human breast cancer, the GT198 gene carries somatic mutations, which activate GT198+ vessel pericytes in tumor microenvironment [12]. In human glioblastoma xenografts of rat brain, human GT198+ vessel pericytes induce rat brain tumor through angiogenesis [24]. In this study, the spontaneous mouse tumors carrying MMTV-PyMT or MMTV-Ras transgenes contain GT198+ pericytes. Similar GT198+ pericytes were found in MMTV-Neu transgenic mouse mammary tumors (Supplementary Fig. S2), whereas non-transgenic mouse mammary glands are GT198− (Supplementary Fig. S3). Consistently, even though distinct cancer-inducing stimuli are present in various systems, a common GT198-mediated pathway is shared by all of them (Fig. 6). In particular, GT198 positive angiogenic pericyte stem cells are a common feature leading to activated tumor microenvironment to propel epithelial tumor cells.
Fig. 6. GT198+ pericyte stem cells are a common feature in human and mouse tumor development.
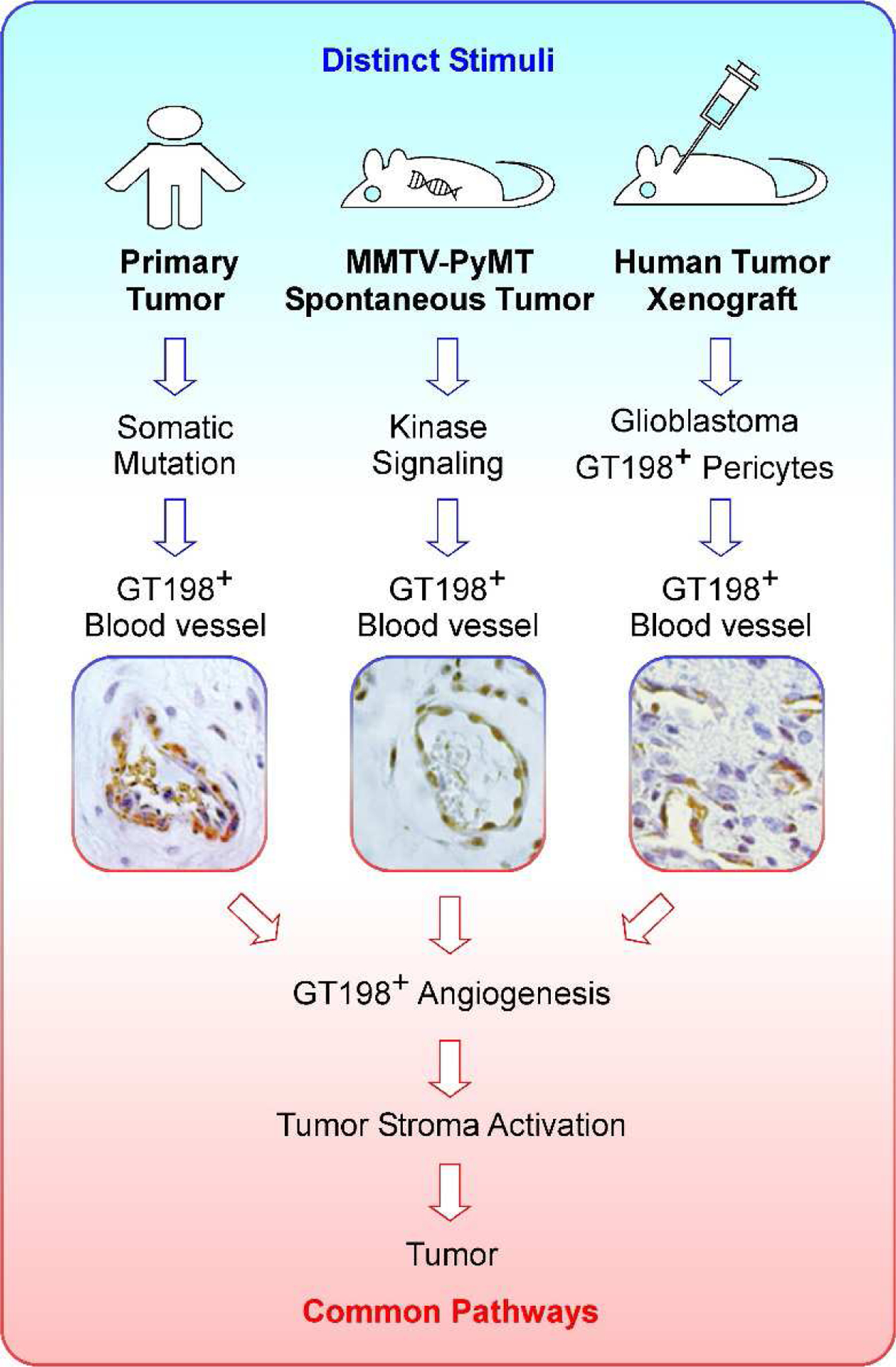
GT198-mediated tumor development in human and mouse have a shared common pathway, in which pericyte stem cells in the blood vessel are activated and overexpress GT198. Activated GT198+ pericytes further induce tumor angiogenesis in the tumor microenvironment, and in turn, stimulate tumor growth. In human cancers (left panel), the GT198 gene carries somatic mutations, and GT198+ vessel pericytes are present in multiple common solid tumors [12, 22–24]. A human breast cancer blood vessel is shown. In spontaneous mouse tumors carrying transgenes (middle panel), GT198+ pericytes are present in the blood vessel (photo from Fig. 1a, PyMT 12 weeks). In human tumor xenografts (right panel), GT198+ vessel pericytes are activated and induce tumor angiogenesis. Human glioblastoma in rat brain is shown [24]. Therefore, GT198+ pericyte stem cells are a shared feature in a common pathway in tumor development in both human and mouse. This model suggests that cancer initiation is distinct, but tumor development pathway is the same in both human and rodent.
The key to this mechanism is that pericytes are stem cells, and GT198 is a stem cell regulator. We have previously shown that GT198 splice variant is down-regulated during normal stem cell differentiation [21], so that variant activation with GT198 cytoplasmic expression in pericytes is mimicking the stem cell status or preventing them from normal differentiation. This creates a differentiation-blocked abnormal mammary gland stroma containing over activated adipocytes, fibroblasts, and myoepithelial cells; whose primary jobs are to promote the growth of epithelial cells. In addition, PyMT antigen induces MAP kinase pathway, which is known to stimulate variant GT198 activity [12], thereby leading to GT198 variant overexpression (Fig. 1). A potential positive feedback loop may exist between MMTV-driven PyMT and kinase-driven GT198 variant, which also stimulates the MMTV promoter [21]. During mammary gland differentiation, the required downregulation of GT198 variant can be blocked by this positive feedback loop, overly activating stromal cells for tumor development. GT198+ pericytes are also present in the tumor stroma of multiple human solid tumors [24]. Therefore, cancer initiation stimuli are distinct, but GT198-mediated tumor development pathway is consistent in both human and rodent (Fig. 6).
GT198-positive cells are a driving force of tumor that could be targeted in anticancer therapy. This idea is already supported by the fact that paclitaxel and doxorubicin directly inhibit GT198 [22, 26]. The current study provides further support that GT198 can be a potential cancer vaccine. One reason for GT198 antigen being effective is that GT198 protein has cytoplasmic as well as membrane translocation when deregulated or overexpressed. A well-observed phenomenon is that many otherwise nuclear oncoproteins such as p53 [31], Rb [32], BRCA1 [33], EWS [34], are cytoplasmic or membrane translocated when mutated or altered in cancer. For GT198, although immunohistochemical studies may only show cytoplasmic staining, we previously found that GT198 protein can be detected by its antibody on living cell surfaces by FACS analysis [24], and GT198 surface level increased during hypoxia condition. In this study, surface GT198 can also be detected by FACS analysis using viable AT-3 cells (Fig. 2F–G) and tumor cells (Fig. 5B). In addition, we tested a number of cultured mouse cancer cell lines and conformed that GT198 is present on the cell surfaces of viable cancer cells (Supplementary Fig. S4). Thus, the membrane translocation of nuclear tumor antigens is a critical advantage facilitating the development of cancer vaccine.
To achieve an ideal vaccination efficacy, a hormonal response is essential. Our data showed that all three vaccinated mice had robust hormonal responses but decreased their serum anti-GT198 antibody at the advanced stages of the tumor (Fig. 2b). It appears that the serum antibodies in mice were used up due to increasing GT198+ tumors. Besides, we initially designed four mice in the vaccine group, but one was censored. This sick mouse had a normal amount of T cells comparable to controls but had a 50-fold decrease of B cells without production of anti-GT198, and had tumor growth outside mammary gland (not shown).
Nonetheless, it implies that GT198 vaccination may have a protective effect against tumor only when the individual is healthy with a normal hormonal response. In human, if older patients have weak immune systems, GT198 protein vaccine would be less effective, whereas monoclonal GT198 antibody therapy could be a better option. This first study of GT198 vaccination in mice aims to pave the way for the future investigations of immunotherapy targeting GT198.
In summary, we find that GT198 is overexpressed in mammary glands of MMTV-PyMT and MMTV-Ras mice. GT198 expression in mouse pericyte stem cells and tumor stromal cells are consistent with that in human reactive breast cancer stroma. GT198 vaccination in MMTV-PyMT mice delayed mouse tumor growth and reduced lung metastasis. Our study indicates that even though distinct cancer-initiation stimuli exist between mouse and human, the defective oncoprotein GT198 is a shared mechanism of tumor development in both species. Therefore, GT198 can be targeted in human cancer therapy.
Supplementary Material
Highlights:
GT198 expression is an early lesion in MMTV-PyMT mouse tumor.
GT198 vaccine delayed MMTV-PyMT mouse tumor growth.
Human breast cancer and mouse mammary gland tumor share a common GT198 pathway.
Acknowledgements
We thank Dr. Yukai He for discussion of immunotherapy. We thank Dr. Juan Wu for testing vaccine adjuvants. We thank Dr. Jianming Xu for the study of GT198 expression in mice.
Funding
This work was supported in part by the Georgia Cancer Coalition Distinguished Cancer Scholar Award to L.K., the National Institutes of Health grant CA160216 and CA172048 to A.S.A; CA062130 to N.F.M.; and American Cancer Society grant IRG-14-193-01 grant to B.R.A.
Abbreviations:
- GT198
gene name with gene symbol PSMC3IP, also called TBPIP and Hop2
- MMTV
mouse mammary tumor virus long terminal repeat
- PyMT
polyomavirus middle T antigen
- IFA
Incomplete Freund’s adjuvant
- MDSC
myeloid-derived suppressor cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
LK is the inventor of GT198 patents. The other authors declare no conflict of interest.
References
- [1].Gross L, A filterable agent, recovered from Ak leukemic extracts, causing salivary gland carcinomas in C3H mice, Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine, 83 (1953) 414–421. [DOI] [PubMed] [Google Scholar]
- [2].Dilworth SM, Polyoma virus middle T antigen and its role in identifying cancer-related molecules, Nat Rev Cancer, 2 (2002) 951–956. [DOI] [PubMed] [Google Scholar]
- [3].Fluck MM, Schaffhausen BS, Lessons in signaling and tumorigenesis from polyomavirus middle T antigen, Microbiology and molecular biology reviews : MMBR, 73 (2009) 542–563, Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gottlieb KA, Villarreal LP, Natural biology of polyomavirus middle T antigen, Microbiology and molecular biology reviews : MMBR, 65 (2001) 288–318 ; second and third pages, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].van der Meijden E, Feltkamp M, The Human Polyomavirus Middle and Alternative T-Antigens; Thoughts on Roles and Relevance to Cancer, Frontiers in microbiology, 9 (2018) 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schaffhausen BS, Roberts TM, Lessons from polyoma middle T antigen on signaling and transformation: A DNA tumor virus contribution to the war on cancer, Virology, 384 (2009) 304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Guy CT, Cardiff RD, Muller WJ, Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease, Mol Cell Biol, 12 (1992) 954–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sinn E, Muller W, Pattengale P, Tepler I, Wallace R, Leder P, Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo, Cell, 49 (1987) 465–475. [DOI] [PubMed] [Google Scholar]
- [9].Fantozzi A, Christofori G, Mouse models of breast cancer metastasis, Breast Cancer Res, 8 (2006) 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG, Inflammation LSCRP Host Response to Injury, Genomic responses in mouse models poorly mimic human inflammatory diseases, Proc Natl Acad Sci U S A, 110 (2013) 3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Eruslanov EB, Singhal S, Albelda SM, Mouse versus Human Neutrophils in Cancer: A Major Knowledge Gap, Trends in cancer, 3 (2017) 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang Z, Peng M, Cheng L, Jones K, Maihle NJ, Mivechi NF, Ko L, GT198 Expression Defines Mutant Tumor Stroma in Human Breast Cancer, Am J Pathol, 186 (2016) 1340–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ko L, Cardona GR, Henrion-Caude A, Chin WW, Identification and characterization of a tissue-specific coactivator, GT198, that interacts with the DNA-binding domains of nuclear receptors, Mol Cell Biol, 22 (2002) 357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Satoh T, Ishizuka T, Tomaru T, Yoshino S, Nakajima Y, Hashimoto K, Shibusawa N, Monden T, Yamada M, Mori M, Tat-binding protein-1 (TBP-1), an ATPase of 19S regulatory particles of the 26S proteasome, enhances androgen receptor function in cooperation with TBP-1-interacting protein/Hop2, Endocrinology, 150 (2009) 3283–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cho NW, Dilley RL, Lampson MA, Greenberg RA, Interchromosomal homology searches drive directional ALT telomere movement and synapsis, Cell, 159 (2014) 108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Enomoto R, Kinebuchi T, Sato M, Yagi H, Shibata T, Kurumizaka H, Yokoyama S, Positive role of the mammalian TBPIP/HOP2 protein in DMC1-mediated homologous pairing, J Biol Chem, 279 (2004) 35263–35272. [DOI] [PubMed] [Google Scholar]
- [17].Petukhova GV, Pezza RJ, Vanevski F, Ploquin M, Masson JY, Camerini-Otero RD, The Hop2 and Mnd1 proteins act in concert with Rad51 and Dmc1 in meiotic recombination, Nat Struct Mol Biol, 12 (2005) 449–453. [DOI] [PubMed] [Google Scholar]
- [18].Peng M, Bakker JL, DiCioccio RA, Gille JJP, Zhao H, Odunsi K, Sucheston L, Jaafar L, Mivechi NF, Waisfisz Q, Ko L, Inactivating mutations in GT198 in familial and early-onset breast and ovarian cancers, Genes Cancer, 4 (2013) 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schubert S, Ripperger T, Rood M, Petkidis A, Hofmann W, Frye-Boukhriss H, Tauscher M, Auber B, Hille-Betz U, Illig T, Schlegelberger B, Steinemann D, GT198 (PSMC3IP) germline variants in early-onset breast cancer patients from hereditary breast and ovarian cancer families, Genes Cancer, 8 (2017) 472–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zangen D, Kaufman Y, Zeligson S, Perlberg S, Fridman H, Kanaan M, Abdulhadi-Atwan M, Abu Libdeh A, Gussow A, Kisslov I, Carmel L, Renbaum P, Levy-Lahad E, XX ovarian dysgenesis is caused by a PSMC3IP/HOP2 mutation that abolishes coactivation of estrogen-driven transcription, Am J Hum Genet, 89 (2011) 572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Peng M, Yang Z, Zhang H, Jaafar L, Wang G, Liu M, Flores-Rozas H, Xu J, Mivechi NF, Ko L, GT198 splice variants display dominant negative activities and are induced by inactivating mutations, Genes Cancer, 4 (2013) 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ko L, Human solid cancer decoded, Zenodo (2019) 10.5281/zenodo.3236836. [DOI] [Google Scholar]
- [23].Peng M, Zhang H, Jaafar L, Risinger JI, Huang S, Mivechi NF, Ko L, Human Ovarian Cancer Stroma Contains Luteinized Theca Cells Harboring Tumor Suppressor Gene GT198 Mutations, J Biol Chem, 288 (2013) 33387–33397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang L, Wang Y, Rashid MH, Liu M, Angara K, Mivechi NF, Maihle NJ, Arbab AS, Ko L, Malignant pericytes expressing GT198 give rise to tumor cells through angiogenesis, Oncotarget, 8 (2017) 51591–51607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang L, Liu Y, Cheng L, Zhao C, Ko L, Mutant GT198 in angiogenesis as a common origin of human prostate and bladder BioRxiv, (2019) 10.1101/726679. [DOI] [Google Scholar]
- [26].Yang Z, Gurvich VJ, Gupta ML, Mivechi NF, Ko L , Oncoprotein GT198 is a direct target of taxol, BioRxiv, (2019) 10.1101/675579 [DOI] [Google Scholar]
- [27].Hollingsworth RE, Jansen K, Turning the corner on therapeutic cancer vaccines, NPJ vaccines, 4 (2019) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ, Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease, Proc Natl Acad Sci U S A, 89 (1992) 10578–10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Khoury MP, Bourdon JC, p53 Isoforms: An Intracellular Microprocessor?, Genes Cancer, 2 (2011) 453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Komlodi-Pasztor E, Sackett DL, Fojo AT, Inhibitors targeting mitosis: tales of how great drugs against a promising target were brought down by a flawed rationale, Clin Cancer Res, 18 (2012) 51–63. [DOI] [PubMed] [Google Scholar]
- [31].Green DR, Kroemer G, Cytoplasmic functions of the tumour suppressor p53, Nature, 458 (2009) 1127–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pickard A, Wong PP, McCance DJ, Acetylation of Rb by PCAF is required for nuclear localization and keratinocyte differentiation, J Cell Sci, 123 (2010) 3718–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wilson CA, Payton MN, Elliott GS, Buaas FW, Cajulis EE, Grosshans D, Ramos L, Reese DM, Slamon DJ, Calzone FJ, Differential subcellular localization, expression and biological toxicity of BRCA1 and the splice variant BRCA1-delta11b, Oncogene, 14 (1997) 1–16. [DOI] [PubMed] [Google Scholar]
- [34].Belyanskaya LL, Gehrig PM, Gehring H, Exposure on cell surface and extensive arginine methylation of ewing sarcoma (EWS) protein, J Biol Chem, 276 (2001) 18681–18687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


