Abstract
Objective:
This secondary analysis examined the relationships between Patient Activation Measure (PAM) scores, use of health services, and HgA1C.
Design:
A feasibility study was conducted for a community-based intervention for high-risk adults with uncontrolled diabetes. Data were collected at baseline and monthly, including PAM and modified Diabetes Self-Management Assessment Report Tool.
Intervention:
Participants (n=48) were randomized to a 3-month nurse (RN) telephone management or community health worker (CHW) in-home intervention, focusing on medication adherence, timely follow-up, diabetes self-management coaching, and linkage to resources.
Results:
Sample was mostly female (73%), African-American (90%), low-income (75%), high school education or less (80%) and mean age 59 years. A positive association between PAM score and self-reported diabetes care recommendations was found (r=.356, p=.014) and significant correlation between baseline PAM score and HgA1C levels (r= − .306, p=.029). A paired samples t-test showed statistically significant increases in PAM scores in the CHW intervention group [mean increase +8.5, CI (+2.49 –+14.65)]; baseline (M=60.31, SD=13.3) to end of study [(M=68.89,SD=16.39), t(22)=2.924, p=.008 (two-tailed)].
Conclusion:
A community-based approach to diabetes management demonstrated a positive effect on patient activation. Although disparities in healthcare access among rural, low-income populations exist, community-based interventions show potential for improving patient engagement in diabetes management and recommended health services.
Keywords: Patient engagement, Patient activation, Community Health Worker, Diabetes Mellitus-Type 2, Poverty, Rural population, Health Promotion, Self-Management, Patient Activation Measure
Background
The prevalence of diabetes is substantially higher in rural populations compared to urban populations (Towne et al., 2017). Diabetes is also more common among African-Americans compared to non-Hispanic whites (Centers for Disease Control & Prevention [CDC], 2019). Furthermore, diabetes-related mortality rates are much higher in rural, non-metropolitan areas compared to urban, metropolitan areas and persist among rural African-Americans compared to rural whites at estimated rates of 42.8 per 100,000 versus 33.2 per 100,000, respectively (Callaghan, Towne, Bolin, & Ferdinand, 2017, 2019). Glycemic control is typically worse in younger adults, non-Hispanic blacks, Hispanics, non-married individuals, and uninsured populations than their respective counterparts (Ali, McKeever Bullard, Imperatore, Barker, & Gregg, 2012). For example, approximately 17% non-Hispanic Blacks have higher rates of uncontrolled diabetes, or HgA1C levels greater than 9.0%, compared to 9% in Whites. Diabetes-related complications, such as stroke and kidney disease, disproportionately affect rural and minority populations as well (CDC, 2017; Garcia et al., 2017). Such disparities must be urgently addressed to improve health outcomes in these communities.
Health disparities among rural and minority populations have been well established. These disparities can be partially explained by a lack of access and participation in routine preventive care services (Spleen, Lengerich, Camacho, & Vanderpool, 2014). Rural and minority populations continually face challenges related to diabetes care. African-Americans have a 1.5 higher risk of forgoing medical care due to cost compared to whites, despite recent Medicaid expansion and health insurance reform efforts (Towne et al., 2017). Rural residents are also more likely than urban residents to delay seeking health care and to receive diabetes preventive health services [i.e. diagnostic tests (glucose, urinalysis, A1C, and blood pressure) or patient education (diet/nutrition, exercise, and stress management)], largely due to cost (Hale, Bennett, & Probst, 2010; Towne et al., 2017). Lack of engagement in diabetes preventive services may result in poor health outcomes (Hibbard & Greene, 2013).
Other barriers, such as transportation and competing family priorities, further constrain access to diabetes-related preventive health services. Limited access to specialists, such as eye care professionals, can reduce rural residents’ likelihood of obtaining diabetes-related preventive services (Chou et al., 2012, 2016; Lee et al., 2014). Younger adults, especially those between 18 and 39 years old, are lagging behind their older counterparts in diabetes preventive services, such as dilated eye exams, cholesterol checks, blood pressure screenings, and urine albumin testing (CDC, 2017; Villaroel, Vahratian, & Ward, 2015; Ziller, Lenardson, Paluso, & Janis, 2019). Higher uninsured rates, lower educational attainment, reduced access and lower perceived need for services among younger, rural and minority residents may partially explain lower receipt of preventive services (Ziller et al., 2019).
Conversely, participation in health services may lead to better diabetes health outcomes (Greene & Hibbard, 2012). Individuals who are actively involved in their health are more likely to have lower overall health care costs, obtain recommended diabetes preventive services, and exhibit better health outcomes (Aung, Coll, Williams, & Doi, 2016; Rask et al., 2009; Rogvi, Tapager, Almdal, Schiotz, & Willaing, 2012; Sacks, Greene, Hibbard, Overton, & Parrotta, 2017). Engagement, defined as the active partnership between patients and health care professionals, is necessary for improved health outcomes (Carman et al., 2013). However, scant research has validated the relationship between the level of engagement in diabetes care and health outcomes, especially among rural and minority populations (Schoenberg, Ciciurkaite, & Greenwood, 2017).
Community-based interventions have been known to improve diabetes outcomes, with most evidence for minority, under-resourced, and urban populations; however, increasing evidence is available regarding rural communities (Lepard et al., 2015; Palmas et al., 2015). A recent study of rural-dwelling, African-American women found no significant improvements in glycemic control or blood pressure in the community health worker (CHW) intervention group compared to the control group, but significant weight loss (−1.35 +/− 6.22 kg) was reported (Lutes, Cummings, Littlewood, Dinatale, & Hambridge, 2017). Greater reductions in HgA1C have resulted from exposure to more contact hours from CHW’s or peer advisors, otherwise known as the “dosage effect” (Lepard et al., 2015; Palmas, 2014; Samuel-Hodge et al., 2009; Tang et al., 2014).
The purpose of this secondary data analysis was to examine the association between the level of engagement and utilization of diabetes preventive health services in a rural, Southeastern U.S. community of predominantly African-American adults with type 2 diabetes. The analysis also illustrates the construct of engagement, which Hibbard, Stockard, Mahoney, and Tusler (2004) operationalized as patient activation. Findings from this study can help confirm the potential of community-based interventions to enhance patient engagement and, more importantly, to lessen health disparities among rural, minority adults with diabetes.
In the context of this study, the chosen community is classified as ‘rural’ based on the 2013 National Center for Health Statistics (NCHS) Urban-Rural Classification Scheme for Counties and the estimated county population being 14,275 (U.S. Census Bureau, 2018). According to NCHS, a rural population is generally defined as one that meets the criteria for a non-metropolitan statistical area (non-MSA), or core urban area less than 50,000 population.
Conceptual Framework
The concept of engagement has evolved with patient-centered health care delivery models, specifically the Chronic Care Model (CCM) (Wagner, Austin, & Von Korff, 1996). This model transformed the conventional health care system, allowing for a more collaborative, patient-focused approach (Emanuel & Emanuel, 1992; Wagner et al., 1996). The CCM emphasizes patients as active participants and informed consumers of health care while gaining skills to promote disease self-management (Wagner et al., 1996). The concept of engagement has also been framed as patient activation, namely “[one’s] knowledge, skill and confidence for managing his/her own health and health care” (Hibbard, Mahoney, Stockard, & Tusler, 2005, p. 1919). In this case, patients learn how to participate as effective members of the health care team.
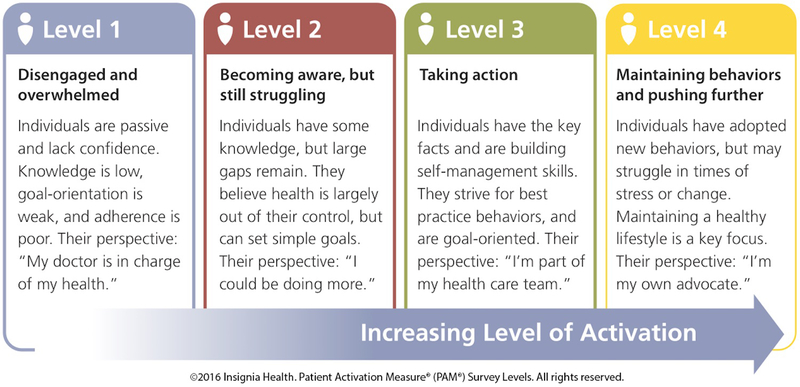
Patient activation comprises a broad definition and continuum of health behaviors, as opposed to the similar construct of self-efficacy that relates to a specific skill or health behavior (Hibbard et al., 2004). Patient activation is a developmental, cognitive process that ultimately promotes adoption of health behaviors (see Figure 1). As patients gain knowledge about their health condition(s) and develop confidence to take corresponding action, patient activation develops. This process is comprised of four levels and starts with acknowledging the importance of patients’ roles in their health care. The next level encompasses various patient-based health behaviors, such as making lifestyle changes, talking to health care providers about their health care, and knowing when to seek help (Harvey, Fowles, Xi, & Terry, 2012). The third level of patient activation consists of gaining more independence in self-management of health care, following providers’ recommendations, and continuing to make necessary lifestyle changes to prevent potential chronic health complications. The final level involves maintaining lifestyle changes and necessary medical treatments, even during times of stress (Graffigna & Barello, 2018). Throughout this continuum, patients maintain active roles in their health care and have the opportunity to become increasingly more confident in self-managing health problems with minimal interference in everyday life activities (Hibbard et al., 2004; Rask et al., 2009; Rijken, Heijmans, Jansen, & Rademakers, 2014).
Figure 1:
Conceptual Model - Levels of Patient Activation (PAM). Copyright 2018 by Insignia. Reprinted with permission.
The Patient Activation Measure (PAM) has been applied to predict health outcomes in numerous populations (Hibbard & Greene, 2013). Higher patient activation has been shown to be positively related to a variety of diabetes outcomes (e.g., control of blood glucose, cholesterol, and blood pressure) (Rogvi et al., 2012). Moreover, higher patient activation levels are associated with a greater likelihood of reported medication adherence, regular physical activity, and diabetes self-management behaviors (Frosch, Rincon, Ochoa, & Mangion, 2010; Mosen et al., 2007; Parchman, Zeber, & Palmer, 2010; Wolever & Dreusicke, 2016). Thus, the results of this secondary data analysis will validate the relationship between level of engagement, as measured by Patient Activation Measure scores, and preventive health behaviors.
The results will also add to the gap in literature concerning the relationship between patient engagement and enhanced health outcomes among rural, minority populations. The potential to enhance patient engagement and improve diabetes outcomes has been demonstrated in several interventional studies with various populations. Examples include African-Americans and Hispanics as well as patients with higher baseline HgA1C levels, low levels of medication adherence, and poor diabetes self-management (Bolen et al., 2014; D’Eramo Melkus et al., 2010; Lorig, Ritter, Ory, & Whitelaw, 2013; Moskowitz, Thom, Hessler, Ghorob, & Bodenheimer, 2013; Philis-Tsimikas, Fortmann, Lleva-Ocana, Walker, & Gallo, 2011; Wolever et al., 2010).
Methods
Design and sample.
Data for this secondary analysis was obtained from a pilot study (Authors, 2016) which examined the feasibility of primary care medical homes using community health workers as care extenders among high-risk persons with type 2 diabetes. High-risk persons targeted were those with uncontrolled diabetes or HgA1C greater than 8%, uncontrolled hypertension, recent hospitalization or urgent care/emergency room visit for diabetes-related diagnosis, reported problems with obtaining medications, or reported need for diabetes management from the primary care provider. The pilot study recruited persons with diabetes from primary care offices (60%), urgent care clinics (16%), hospital inpatient admissions (14%), and emergency departments (10%) within the identified small, rural county. The county’s population characteristics where the study was conducted were comparable to the demographics of the study sample: African-American (60%), high school education or less (79%), and median 2017 income $32,330 (U.S. Census Bureau, 2018). The mean age of the sample was 59 ± 11.6 years and participants were predominantly female (73%) and African-American (90%). More than 75% (n = 33) were low-income (earning less than $25,000 annually) and had a high school education or less (n = 39). The sample included larger proportions of unemployed (34%) or retired (36%) adults compared with working adults (26%).
In the pilot study, participants (n = 58) were randomly assigned to one of three groups: a 3-month intervention group, receiving either nurse (RN) management via telephone (n = 27); a face-to-face management from a community health worker (CHW; n = 26); and a usual care/control group (n = 5). Interventions in the RN telephone management and CHW groups each focused on medication adherence, timely follow-up, diabetes self-management coaching, and referrals to community resources. Baseline and monthly data were collected using eight measures, including PAM scores. Patient activation levels assessed the readiness to change in increasing diabetes self-management behaviors. Several other health behaviors were assessed, such as patients’ eating patterns, tobacco and alcohol use, medication adherence, and diabetes self-efficacy.
The secondary data analysis focused on two variables of interest: PAM scores and the number of diabetes recommendations met. The purpose of this analysis was to examine the relationship between patients’ level of engagement, as quantified by PAM scores, and use of diabetes preventive health services in a rural community of predominantly African-American adults with diabetes. The proposed secondary data analysis was reviewed and approved by the Institutional Review Board of the University of Missouri‐Columbia.
Research Questions
Is there a relationship between PAM score and number of diabetes recommendations met?
Is there a relationship between PAM score and HgA1C level?
Is there a significant change in PAM score following a 3-month RN telephone intervention or CHW intervention?
Measures
Patient Activation Measure.
PAM was developed as a measure of patient engagement (Hibbard et al., 2004). The instrument is well-validated and has demonstrated good reliability (Rasch: 0.81); possible scores range from 0 to 100, corresponding to patient activation levels 1, 2, 3, and 4 (Hibbard et al., 2005). The measure has demonstrated strong associations with constructs such as preventative, self-management, and consumeristic behaviors (Hibbard et al., 2005). The short-form PAM-10, containing 10 items, was used for analysis (Hibbard et al., 2005).
Diabetes Educators’ Self-Management Assessment Reporting Tool (D-SMART).
In the pilot study, the number of self-reported diabetes recommendations met (# DM REC met) were extracted from the Diabetes Educators’ Self-Management Assessment Reporting Tool (D-SMART) used by the RN and CHW interventionists. The American Diabetes Association of Diabetes Educators’ (AADE) identification of seven diabetes self-management behaviors, otherwise known as AADE-7, was based on preliminary D-SMART development (Peyrot et al., 2007). Evaluation of behavioral outcomes includes seven areas of diabetes self-management: being active, healthy eating, taking medication, monitoring, problem-solving, reducing risks, and healthy coping (Peyrot et al., 2007).
Thirteen possible diabetes recommendations were coded as “met” if patients reported the following health care behaviors: HgA1C tested within the past 6 months; a doctor visit, foot exam from a health professional, blood pressure check, cholesterol check, or urine check for protein within the past 12 months; dilated eye exam, dental visit, or flu shot within the past 2 years; and any history of seeing a dietician, attending a diabetes education class, or receiving a pneumonia or hepatitis B vaccine.
Analytic Strategy
PAM scores and D-SMART data from the pilot study (Authors, 2016) were exported into SPSS data files for secondary analysis. Self-reported adverse events were extracted from the D-SMART at baseline and at 3 months. Adverse events included the total number of emergency department visits for high or low blood sugar within the past 3 months, number of hospital admissions within the past 3 months, and number of days missed from school or work. The total number of self-reported adverse events were collapsed into a binary variable, coded as “0” for no adverse events and “1” for one or more adverse events.
Other analyzed covariates included HgA1C level (extracted from each patient’s electronic medical record) and demographic information collected at baseline, including gender, marital status, education, employment status, years of diagnosed diabetes, health insurance status, and literacy level.
The data analysis was based on 48 participants who completed the pilot study, drawn from the RN telephone intervention group (n = 20) and CHW intervention group (n = 24). The usual-care or control group (n = 5) was excluded from analysis due to an insufficient sample size. PAM scores and # DM REC met were analyzed at baseline and 3 months. Data collection for PAM occurred at baseline and at 1-, 2-, and 3-month intervals; however, only baseline and 3-month scores were incorporated for analysis. PAM scores were analyzed for descriptive statistics, independent samples t-test (RN vs. CHW), and a paired samples t-test (mean change baseline to 3 months). SPSS v.24 software was used for analysis.
Results
The independent samples t-test revealed no significant differences in PAM score means between the intervention groups. Comparisons of median PAM scores using the non-parametric Mann-Whitney test of independent samples also demonstrated non-significant differences between the RN telephone intervention and CHW intervention groups (baseline median PAM score: 56.0 [RN], 57.65 [CHW]; 3-month median PAM score: 59.3 [RN], 62.6 [CHW]). At baseline, mean PAM scores were 61.98 (SD = 15.55) for the RN telephone intervention group and 60.48 (SD = 12.88) for the CHW intervention group. At 3 months, the mean PAM scores were 63.85 (SD = 17.85) for the RN telephone intervention group and 68.82 (SD = 16.08) for the CHW intervention group (see Table 1).
Table 1.
Statistical Results for CHW and RN Telephone Management Intervention Groups
| Baseline PAM score (mean) | 60.48 (SD = 12.9) | 61.98 (SD = 15.5) |
| 3-month PAM score (mean) | 68.82 (SD = 16.1) | 63.85 (SD = 17.8) |
| Mean change | + 8.57 ** | + 1.32 (ns) |
| Baseline PAM score (median) | 57.65 | 56.0 |
| 3-month PAM score (median) | 62.6 | 59.3 |
| Mann-Whitney test | ns | ns |
(** = p < .01; ns = not significant)
A paired samples t-test was conducted to evaluate the impact of the intervention on PAM scores. For the RN telephone management group, the mean increase in PAM scores from baseline to month 3 was +1.32, but the difference was not statistically significant (p = .73). A statistically significant increase in PAM scores was observed from baseline to month 3 in the CHW intervention group (M = 60.31, SD = 13.3 [baseline]; M = 68.89, SD = 16.39 [month 3]), t(22) = −2.924, p = .008. The mean increase in PAM score was +8.57 at a 95% confidence interval [2.49, 14.65]. The CHW intervention exhibited an estimated medium effect size (d = 0.58).
Pearson correlations were performed with PAM scores, # DM REC met, and HgA1C levels at baseline and 3 months. At baseline, the mean # DM REC met was 8.9 (SD = 1.89); at 3 months, the mean rose to 9.5 (SD = 1.73) of 13 possible recommendations. A positive association emerged between PAM scores and self-reported #DM REC met (r = .356, p = .014) at the 3-month point but was not significant at baseline. A significant correlation was observed between PAM scores and HgA1C levels (r = −.306, p = .029) at baseline only. The mean HgA1C at baseline was 9.6 (SD = 2.20) compared to 8.59 (SD = 1.71) at 3 months.
Pearson correlations with PAM scores were tested using the following covariates: randomization group, gender, employment status, years of diagnosed diabetes, health insurance status, and literacy level. No significant correlations were found among PAM scores and covariates. Next, a simple linear regression was performed for PAM scores and # DM REC met; PAM scores increased by 3.6 points on average for every additional diabetes recommendation met. Pearson correlations were also determined between PAM scores and the number of adverse events. No significant associations emerged between PAM scores and total adverse events or between PAM scores and individual adverse events.
Poisson regression models were assessed using baseline and 3-month PAM scores as the predictor variable and # DM REC met as the dependent count variable. The model did not result in a significant difference in log expected counts, nor did PAM scores predict # DM REC met.
Discussion
This secondary data analysis focused on a group of rural-dwelling adults living with type 2 diabetes. Results revealed a positive association between patient activation and reported use of diabetes preventive health services. Consistent with prior research, this study found PAM scores to be strong predictors of preventive health behaviors, such as following American Diabetes Association (ADA) recommendations for diabetes preventive services (Frosch et al., 2010; Hibbard & Greene, 2013). On average, PAM scores increased by 3.6 points for every additional diabetes recommendation met. These findings indicated that higher patient activation levels were associated with engagement in preventive health behaviors and medical care.
The preliminary findings of the pilot study, especially the face-to-face CHW intervention, demonstrated potential for a positive impact on patient activation, which could also facilitate patient engagement in recommended diabetes preventive health services. Diabetes self-management can be onerous; the ADA recommends more than 13 preventive activities (American Diabetes Association, 2018). Individuals with diabetes are initially responsible for seeking relevant preventive services; however, collaboration between the health care system and individuals with type 2 diabetes is crucial to promoting engagement in such services (Smith, Berman, Hiratsuka, & Frazier, 2015). Health care providers must consistently monitor patients for diabetes-related complications in addition to patients actively seeking preventive services. Thus, the burden of responsibility in adhering to ADA recommendations falls on both parties.
In the general population, the rate of participation in diabetes preventive health services is higher among women, adults older than 65, and those with higher education levels (CDC, 2018; Hale et al., 2010; Villaroel et al., 2015). Our study sample, which was predominantly older, female, and African-American, was consistent with similar populations in being relatively adherent to recommended diabetes preventive services. On average, our sample of rural-dwelling adults self-reported meeting approximately 9 of 13 diabetes recommendations (i.e., complying with 70% of recommendations). Although the sample had low educational attainment, participants’ level of engagement in preventive services was fairly adequate.
Participants tended to have lower educational status, and approximately 50% reported having attended at least one diabetes class. According to the CDC (2018), attendance at diabetes education remains relatively low (54% on average) for persons with diagnosed diabetes. Rural populations are also less likely to participate in diabetes education, partly due to lack of access to diabetes self-management education (DSME) providers or programs. More than 60% of rural counties in the U.S. lack a DSME program (Rutledge, Masalovich, Blacher & Saunders, 2017). Several community-based DSME programs have been developed and show promise for improving diabetes self-management behavioral outcomes in various populations, including rural communities and minority groups (Lorig et al., 2010; Philis-Tsimikas et al., 2012; Samuel-Hodge et al., 2009; Towne, Smith, Ahn, & Ory, 2014). DSME programs have also demonstrated positive effects on patient activation (Flode, Iversen, Aarflot, & Haltbakk, 2017; Frosch et al., 2010; Ledford, Ledford, & Childress, 2013; Lorig, Ritter, Villa, & Armas, 2009, 2010). However, little is known about how DSME can facilitate patient activation and engagement in diabetes preventive health services. Some studies using CHWs and peer coaches to deliver DSME interventions have found improvements in patient activation levels but did not identify clinically significant improvements in HgA1C levels (Lawson et al., 2013; Lorig et al., 2009; Safford et al., 2015). Although DSME can promote positive health outcomes, education alone may not be sufficient for sustained behavior change (Lepard, Joseph, Agne, & Cherrington, 2015; Norris, Lau, Smith, Schmid, & Engelgau, 2002).
The results of this secondary data analysis support prior evidence that the level of patient activation is associated with use of diabetes preventive health services as well as glycemic control. A retrospective study demonstrated that a 1-point increase in PAM score was associated with a 1.8% increased likelihood of reducing HgA1C less than 8% (Remmers et al., 2009). The high rate of poorly controlled diabetes in the present study’s predominantly low socioeconomic, African-American sample of rural-dwelling adults reflects disparities in U.S adults with diagnosed diabetes. The baseline average HgA1C in this sample was 9.6%, exceeding the ADA recommendation of 7% or less (ADA, 2018). Moreover, the study population had other risk factors for uncontrolled diabetes, including a high poverty rate and median income of $32,300. Living in poverty or in disadvantaged neighborhoods is another risk factor for uncontrolled diabetes (Tabaei et al., 2017).
Although improvements in HgA1C were not investigated in this analysis, a positive relationship between higher patient activation levels and glycemic control was found. Based on previous research, higher baseline HgA1C levels have been associated with larger improvements in glycemic control in patient activation interventions for adults with type 2 diabetes (Bolen et al., 2014). Consequently, individuals with poorly controlled diabetes or with lower patient activation might benefit from CHW or RN telephone-assisted diabetes self-management interventions. The impact of diabetes interventions on long-term diabetes outcomes remains unknown, but improvements in glycemic control could help to prevent diabetes-related complications; a reduction of 1 absolute percentage point in HgA1C among adults with type 2 diabetes has been associated with a 21% reduction in mortality (Stratton et al., 2000).
The CHW intervention group in this study displayed significant improvements in diabetes outcomes (e.g., patient activation) compared to the RN telephone management group. A face-to-face intervention with CHW’s could offer advantages for rural populations compared to telephone support modalities. In several studies, diabetes self-management interventions used a combination of in-person and telephone visits in accordance with patient preferences (Allen et al., 2011; Kangovi et al., 2017; Palmas et al., 2015; Tang et al., 2014). The convenience of either in-home or telephone visits also improved retention compared to travel required for DSME interventions (Lepard et al., 2015). Overall, regardless of the intervention modality, positive clinical outcomes have followed from CHW- and RN-assisted interventions (Rosal et al., 2014; Rothschild et al., 2014; Safford et al., 2015; Wolever et al., 2010). The effect of CHW interventions has demonstrated a more significant reduction of HgA1C levels in older adults, namely over 55 years, than younger counterparts (Campos et al., 2018). However, there remains insufficient evidence to determine whether in-person visits are superior to telephone visits (Ciemins, Coon, Peck, Holloway, & Min, 2011; Lepard et al., 2015; Rosal et al., 2014). Overall, adding collaborative goal setting and motivational support to CHW or peer support appears to offer an advantage over DSME alone (Lepard et al., 2015).
The relationship between PAM and health utilization, namely adverse events, remains unclear. Additionally, the effects of diabetes self-management interventions on lowering hospital admissions and emergency department visits have been inconsistent (Begum, Ozolins, & Dower, 2011; Lorig, 2012; Sacks et al., 2017). A recent randomized controlled trial using a CHW intervention demonstrated significant decreases in hospital admissions but non-significant improvements in PAM scores (Kangovi et al., 2017).
Limitations of our study include higher mean baseline PAM scores compared to similar intervention studies in rural areas (Safford et al., 2015; Schoenberg et al., 2017). Furthermore, more than 75% of the sample was at PAM Level 3 or higher at baseline. The sensitivity of PAM is lower for higher activation levels (Level 4), and ceiling effects have been identified (Hung et al., 2013). Overall, participants with higher baseline HgA1C levels experience greater improvements in clinical outcomes (Bolen et al., 2014). More research is therefore needed to clarify whether participants with lower baseline PAM scores would experience greater improvements in clinical outcomes compared to those with higher baseline scores.
To date, no systematic review or meta-analysis has evaluated the effects of diabetes-focused interventions on patient activation. Bolen et al. (2014) synthesized 138 randomized controlled trials of patient activation interventions for adults with type 2 diabetes; however, PAM levels were not assessed as a diabetes outcome. Patient activation can be a useful measure of diabetes self-management behaviors but has not been widely studied as a primary diabetes outcome (Brewster, Tarrant, & Armstrong, 2015). A comparative effectiveness trial using PAM as a primary outcome was recently designed to evaluate the impacts of diabetes self-management and social support (Page-Reeves et al., 2017). Patient activation may function as a mediator; however, the mechanism of the relationship between patient activation and diabetes outcomes requires further substantiation (Parchman et al., 2010; Williams et al., 2005). More randomized controlled trials, especially among under-resourced, rural, and minority populations, are needed to fully evaluate the usefulness of PAM in health research.
Interventions focused on facilitating engagement in diabetes preventive health services could exert strong influences on diabetes-related long-term morbidity and mortality. The past decade has witnessed an increase in the use of diabetes preventive services along with a reduction in the rate of diabetes-related complications (CDC, 2018; Gregg et al., 2014). A recent 3-year longitudinal study showed that higher patient activation levels resulted in better diabetes outcomes and lower odds of developing diabetes in persons with pre-diabetes (Sacks et al., 2017). More longitudinal studies are needed to evaluate the effect of patient engagement in preventive health behaviors on long-term diabetes outcomes, but current evidence appears promising (Hibbard, Greene, Shi, Mittler, & Scanlon, 2015). A recent 3-year longitudinal study showed that higher patient activation levels resulted in better diabetes outcomes and lower odds of developing diabetes in persons with pre-diabetes (Sacks et al., 2017).
Conclusion
Results of secondary data analysis highlight the need to promote engagement in diabetes preventive services and minimize the risk of long-term complications associated with diabetes. More research is needed to determine whether facilitating engagement in diabetes preventive services could lead to a decrease in health disparities among rural, minority populations. Furthermore, lower rates of engagement in diabetes preventive services found in younger adult populations, specifically those 18 to 39 years, warrant attention. Subsequent studies are needed to determine whether community-based interventions could benefit younger adult populations with diabetes, particularly in enhancing their engagement in relevant preventive services.
Funding:
The primary feasibility study was supported by the NIH National Center for Advancing Translational Sciences (NCATS) through Grant Number UL1 TR001450
Contributor Information
Lynn E. Glenn, University of Missouri Sinclair School of Nursing, Columbia, MO.
Michelle Nichols, Medical University of South Carolina, Charleston, SC.
Maithe Enriquez, University of Missouri Sinclair School of Nursing, Columbia, MO.
Carolyn Jenkins, Medical University of South Carolina, Charleston, SC.
References
- Ali MK, McKeever Bullard K, Imperatore G, Barker L, & Gregg EW (2012)., Characteristics associated with poor glycemic control among adults with self-reported, diagnosed diabetes—National Health and Nutrition Examination Survey, United States, 2007–2010. MMWR Morbidity and Mortality Weekly Reports, 61(2), 32–37. [PubMed] [Google Scholar]
- Allen JK, Dennison-Himmelfarb CR, Szanton SL, Bone L, Hill MN, Levine DM, … Anderson K (2011). Community Outreach and Cardiovascular Health (COACH) Trial: a randomized, controlled trial of nurse practitioner/community health worker cardiovascular disease risk reduction in urban community health centers. Circulation: Cardiovascular Quality and Outcomes, 4(6), 595–602. doi: 10.1161/CIRCOUTCOMES.111.961573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association (2018). Standards of Medical Care in Diabetes—2018. Diabetes Care, 41(Supplement 1). doi: 10.2337/dc18-Sint01 [DOI] [Google Scholar]
- Aung E, Donald M, Coll JR, Williams GM, & Doi SA (2016). Association between patient activation and patient-assessed quality of care in type 2 diabetes: results of a longitudinal study. Health Expectations, 19(2), 356–366. doi: 10.1111/hex.12359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum N, Donald M, Ozolins IZ, & Dower J (2011). Hospital admissions, emergency department utilisation and patient activation for self-management among people with diabetes. Diabetes Research and Clinical Practice, 93(2), 260–267. doi: 10.1016/j.diabres.2011.05.031 [DOI] [PubMed] [Google Scholar]
- Bolen SD, Chandar A, Falck-Ytter C, Tyler C, Perzynski AT, Gertz AM, … Windish DM (2014). Effectiveness and safety of patient activation interventions for adults with type 2 diabetes: systematic review, meta-analysis, and meta-regression. Journal of General Internal Medicine, 29(8), 1166–1176. doi: 10.1007/s11606-014-2855-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster L, Tarrant C, & Armstrong N (2015). ‘Patient activation’ as an outcome measure for primary care?. Family Practice, 32(5), 481–482. doi: 10.1093/fampra/cmv054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Authors (2016, September). Feasibility Pilot: Transforming Patient-Centered Medical Homes into Communities for Underserved Rural Patients Poster session presented at the 22nd Annual Fall Diabetes Symposium for Primary Care Health Professionals, Charleston, SC. [Google Scholar]
- Callaghan TH, Towne SD, Bolin J, Ferdinand A (2017). Diabetes Mortality in Rural America: 1999–2015. Policy Brief. Southwest Rural Health Research Center. Retrieved from: https://www.ruralhealthresearch.org/centers/southwest [Google Scholar]
- Callaghan T, Ferdinand AO, Akinlotan MA, Towne SD Jr., & Bolin J (2019). The Changing Landscape of Diabetes Mortality in the United States Across Region and Rurality, 1999–2016. The Journal of Rural Health, 0(0). doi: 10.1111/jrh.12354 [DOI] [PubMed] [Google Scholar]
- Campos BM, Kieffer EC, Sinco B, Palmisano G, Spencer MS, & Piatt GA (2018). Effectiveness of a Community Health Worker-Led Diabetes Intervention among Older and Younger Latino Participants: Results from a Randomized Controlled Trial. Journal of Geriatrics, 3(3), 47. doi: 10.3390/geriatrics3030047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman KL, Dardess P, Maurer M, Sofaer S, Adams K, Bechtel C, & Sweeney J (2013). Patient and family engagement: a framework for understanding the elements and developing interventions and policies. Health Affairs, 32(2), 223–231. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control (2019). Diabetes Quick Facts. Retrieved August 1, 2019 from: https://www.cdc.gov/diabetes/basics/quick-facts.html [Google Scholar]
- Centers for Disease Control and Prevention (2018). Division of Diabetes Translation. US Diabetes Surveillance System. Retrieved December 1, 2018 from: https://www.cdc.gov/diabetes/data [Google Scholar]
- Centers for Disease Control and Prevention (2017). Stroke Facts. Retrieved December 1, 2018 from: https://www.cdc.gov/stroke/facts.htm [Google Scholar]
- Chou CF, Zhang X, Crews JE, Barker LE, Lee PP, & Saaddine JB (2012). Impact of geographic density of eye care professionals on eye care among adults with diabetes. Ophthalmic Epidemiology, 19(6), 340–349. doi: 10.3109/09286586.2012.722244 [DOI] [PubMed] [Google Scholar]
- Chou CF, Beckles GL, Cheng YJ, & Saaddine JB (2016). Association Between County-Level Characteristics and Eye Care Use by US Adults in 22 States After Accounting for Individual-Level Characteristics Using a Conceptual Framework. JAMA Ophthalmology, 134(10), 1158–1167. doi: 10.1001/jamaophthalmol.2016.3007 [DOI] [PubMed] [Google Scholar]
- Ciemins E, Coon P, Peck R, Holloway B, & Min S-J (2011). Using Telehealth to Provide Diabetes Care to Patients in Rural Montana: Findings from the Promoting Realistic Individual Self-Management Program. Telemedicine Journal and e-Health, 17(8), 596–602. doi: 10.1089/tmj.2011.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Eramo Melkus G, Chyun D, Vorderstrasse A, Newlin K, Jefferson V, & Langerman S (2010). The effect of a diabetes education, coping skills training, and care intervention on physiological and psychosocial outcomes in black women with type 2 diabetes. Biological Research for Nursing, 12(1), 7–19. doi: 10.1177/1099800410369825 [DOI] [PubMed] [Google Scholar]
- Emanuel EJ, & Emanuel LL (1992). Four models of the physician-patient relationship. JAMA, 267(16), 2221–2226. [PubMed] [Google Scholar]
- Fløde M, Iversen MM, Aarflot M, & Haltbakk J (2017). Lasting impact of an implemented self-management programme for people with type 2 diabetes referred from primary care: a one-group, before–after design. Scandinavian Journal of Caring Sciences, 31(4), 789–795. doi: 10.1111/scs.12398 [DOI] [PubMed] [Google Scholar]
- Frosch DL, Rincon D, Ochoa S, & Mangione CM (2010). Activating seniors to improve chronic disease care: results from a pilot intervention study. Journal of American Geriatric Society, 58(8), 1496–1503. doi: 10.1111/j.1532-5415.2010.02980.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MC, Faul M, Massetti G, et al. Reducing Potentially Excess Deaths from the Five Leading Causes of Death in the Rural United States. MMWR Surveillance Summary 2017, 66(No. SS-2):1–7. doi: 10.15585/mmwr.ss6602a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin DJ, Thorpe RJ Jr., McGinty EE, Bower K, Rohde C, Young JH, … Dubay L (2014). Disparities in diabetes: the nexus of race, poverty, and place. American Journal of Public Health, 104(11), 2147–2155. doi: 10.2105/AJPH.2013.301420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graffigna G, & Barello S (2018). Spotlight on the Patient Health Engagement model (PHE model): a psychosocial theory to understand people’s meaningful engagement in their own health care. Patient Preference and Adherence, 12, 1261–1271. doi: 10.2147/PPA.S145646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J, & Hibbard JH (2012). Why does patient activation matter? An examination of the relationships between patient activation and health-related outcomes. Journal of General Internal Medicine, 27(5), 520–526. doi: 10.1007/s11606-011-1931-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg EW, Li Y, Wang J, Rios Burrows N, Ali MK, Rolka D, … Geiss L (2014). Changes in Diabetes-Related Complications in the United States, 1990–2010. New England Journal of Medicine, 370(16), 1514–1523. doi: 10.1056/NEJMoa1310799 [DOI] [PubMed] [Google Scholar]
- Hale NL, Bennett KJ, & Probst JC (2010). Diabetes care and outcomes: disparities across rural America. Journal of Community Health, 35(4), 365–374. doi: 10.1007/s10900-010-9259-0 [DOI] [PubMed] [Google Scholar]
- Harvey L, Fowles JB, Xi M, & Terry P (2012). When activation changes, what else changes? the relationship between change in patient activation measure (PAM) and employees’ health status and health behaviors. Patient Education and Counseling, 88(2), 338–343. doi: 10.1016/j.pec.2012.02.005 [DOI] [PubMed] [Google Scholar]
- Hibbard JH, Stockard J, Mahoney ER, & Tusler M (2004). Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Services Research, 39(4 Pt 1), 1005–1026. doi: 10.1111/j.1475-6773.2004.00269.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbard JH, Mahoney ER, Stockard J, & Tusler M (2005). Development and Testing of a Short Form of the Patient Activation Measure. Health Services Research, 40(6 Pt 1), 1918–1930. doi: 10.1111/j.1475-6773.2005.00438.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbard JH, & Greene J (2013). What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Affairs (Millwood,) 32(2), 207–214. doi: 10.1377/hlthaff.2012.1061 [DOI] [PubMed] [Google Scholar]
- Hibbard JH, Greene J, Shi Y, Mittler J, & Scanlon D (2015). Taking the Long View:How Well Do Patient Activation Scores Predict Outcomes Four Years Later? Medical Care Research and Review, 72(3), 324–337. doi: 10.1177/1077558715573871 [DOI] [PubMed] [Google Scholar]
- Hung M, Carter M, Hayden C, Dzierzon R, Morales J, Snow L, … Samore M (2013). Psychometric assessment of the patient activation measure short form (PAM-13) in rural settings. Quality of Life Research, 22(3), 521–529. doi: 10.1007/s11136-012-0168-9 [DOI] [PubMed] [Google Scholar]
- James CV, Moonesinghe R, Wilson-Frederick SM, Hall JE, Penman-Aguilar A, Bouye K (2017). Racial/Ethnic Health Disparities Among Rural Adults — United States, 2012–2015. MMWR Surveillance Summaries, 66(No. SS-23). doi: 10.15585/mmwr.ss6623a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangovi S, Mitra N, Grande D, Huo H, Smith RA, & Long JA (2017). Community Health Worker Support for Disadvantaged Patients With Multiple Chronic Diseases: A Randomized Clinical Trial. American Journal of Public Health, 107(10), 1660–1667. doi: 10.2105/ajph.2017.303985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson KL, Jonk Y, O’Connor H, Riise KS, Eisenberg DM, & Kreitzer MJ (2013). The impact of Telephonic Health Coaching on Health Outcomes in a High-risk Population. Global Advances in Health and Medicine, 2(3), 40–47. doi: 10.7453/gahmj.2013.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford CJW, Ledford CC, & Childress MA (2013). Extending Physician ReACH: Influencing patient activation and behavior through multichannel physician communication. Patient Education and Counseling, 91(1), 72–78. doi: 10.1016/j.pec.2012.11.011 [DOI] [PubMed] [Google Scholar]
- Lee DJ, Kumar N, Feuer WJ, Chou CF, Rosa PR, Schiffman JC, … Lam BL (2014). Dilated eye examination screening guideline compliance among patients with diabetes without a diabetic retinopathy diagnosis: the role of geographic access. BMJ Open Diabetes Research and Care, 2(1), e000031. doi: 10.1136/bmjdrc-2014-000031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepard MG, Joseph AL, Agne AA, & Cherrington AL (2015). Diabetes self-management interventions for adults with type 2 diabetes living in rural areas: a systematic literature review. Current Diabetes Reports, 15(6), 608. doi: 10.1007/s11892-015-0608-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorig K, Ritter PL, Villa FJ, & Armas J (2009). Community-based peer-led diabetes self-management: a randomized trial. Diabetes Educator, 35(4), 641–651. doi: 10.1177/0145721709335006 [DOI] [PubMed] [Google Scholar]
- Lorig K, Ritter PL, Laurent DD, Plant K, Green M, Jernigan VB, & Case S (2010). Online diabetes self-management program: a randomized study. Diabetes Care, 33(6), 1275–1281. doi: 10.2337/dc09-2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorig K, Ritter PL, Ory MG, & Whitelaw N (2013). Effectiveness of a Generic Chronic Disease Self-Management Program for People With Type 2 Diabetes. Diabetes Educator, 39(5), 655–663. doi: 10.1177/0145721713492567 [DOI] [PubMed] [Google Scholar]
- Lutes L, Cummings D, Littlewood K, Dinatale E, & Hambridge B (2017). A Community Health Worker–Delivered Intervention in African American Women with Type 2 Diabetes: A 12‐Month Randomized Trial. Obesity, 25(8), 1329–1335. doi: 10.1002/oby.21883 [DOI] [PubMed] [Google Scholar]
- Mosen DM, Schmittdiel J, Hibbard J, Sobel D, Remmers C, & Bellows J (2007). Is patient activation associated with outcomes of care for adults with chronic conditions? Journal of Ambulatory Care Management, 30(1), 21–29. [DOI] [PubMed] [Google Scholar]
- Moskowitz D, Thom DH, Hessler D, Ghorob A, & Bodenheimer T (2013). Peer coaching to improve diabetes self-management: which patients benefit most? Journal of General Internal Medicine, 28(7), 938–942. doi: 10.1007/s11606-013-2367-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris SL, Lau J, Smith SJ, Schmid CH, & Engelgau MM (2002). Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care, 25(7), 1159–1171. [DOI] [PubMed] [Google Scholar]
- Page-Reeves J, Regino L, Murray-Krezan C, Bleecker M, Erhardt E, Burge M, … Mishra S (2017). A comparative effectiveness study of two culturally competent models of diabetes self-management programming for Latinos from low-income households. BMC Endocrine Disorders, 17, 46. doi: 10.1186/s12902-017-0192-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmas W, March D, Darakjy S, Findley SE, Teresi J, Carrasquillo O, & Luchsinger JA (2015). Community Health Worker Interventions to Improve Glycemic Control in People with Diabetes: A Systematic Review and Meta-Analysis. Journal of General Internal Medicine, 30(7), 1004–1012. doi: 10.1007/s11606-015-3247-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parchman ML, Zeber JE, Palmer RF (2010). Participatory Decision-Making, Patient Activation, Medication Adherence, and Intermediate Clinical Outcomes in Type 2 Diabetes: A STARNet Study. Annals of Family Medicine, 8, 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrot M, Peeples M, Tomky D, Charron-Prochownik D, Weaver T, AADE Outcomes Project & AADE/UMPC Diabetes Education Outcomes Project (2007). Development of the American Association of Diabetes Educators’ Diabetes Self-management Assessment Report Tool. Diabetes Educator, 33(5), 818–826. doi: 10.1177/0145721707307614 [DOI] [PubMed] [Google Scholar]
- Philis-Tsimikas A, Fortmann A, Lleva-Ocana L, Walker C, & Gallo LC (2011). Peer-led diabetes education programs in high-risk Mexican Americans improve glycemic control compared with standard approaches: a Project Dulce promotora randomized trial. Diabetes Care, 34(9), 1926–1931. doi: 10.2337/dc10-2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask KJ, Ziemer DC, Kohler SA, Hawley JN, Arinde FJ, & Barnes CS (2009). Patient activation is associated with healthy behaviors and ease in managing diabetes in an indigent population. Diabetes Educator, 35(4), 622–630. doi: 10.1177/0145721709335004 [DOI] [PubMed] [Google Scholar]
- Remmers C, Hibbard J, Mosen DM, Wagenfield M, Hoye RE, & Jones C (2009). Is patient activation associated with future health outcomes and healthcare utilization among patients with diabetes? Journal of Ambulatory Care Management, 32(4), 320–327. doi: 10.1097/JAC.0b013e3181ba6e77 [DOI] [PubMed] [Google Scholar]
- Rijken M, Heijmans M, Jansen D, & Rademakers J (2014). Developments in patient activation of people with chronic illness and the impact of changes in self-reported health: results of a nationwide longitudinal study in The Netherlands. Patient Education and Counseling, 97(3), 383–390. [DOI] [PubMed] [Google Scholar]
- Rogvi S, Tapager I, Almdal TP, Schiotz ML, & Willaing I (2012). Patient factors and glycaemic control--associations and explanatory power. Diabetic Medicine, 29(10), e382–389. doi: 10.1111/j.1464-5491.2012.03703.x [DOI] [PubMed] [Google Scholar]
- Rosal MC, Heyden R, Mejilla R, Capelson R, Chalmers KA, Rizzo DePaoli M, … Wiecha JM (2014). A Virtual World Versus Face-to-Face Intervention Format to Promote Diabetes Self-Management Among African American Women: A Pilot Randomized Clinical Trial. JMIR Research Protocol, 3(4). doi: 10.2196/resprot.3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild SK, Martin MA, Swider SM, Tumialán Lynas CM, Janssen I, Avery EF, & Powell LH (2014). Mexican American trial of community health workers: a randomized controlled trial of a community health worker intervention for Mexican Americans with type 2 diabetes mellitus. American Journal of Public Health, 104(8), 1540–1548. doi: 10.2105/AJPH.2013.301439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge SA, Masalovich S, Blacher RJ, Saunders MM (2017). Diabetes Self-Management Education Programs in Nonmetropolitan Counties — United States, 2016. MMWR Surveillance Summaries, 66 (No. SS-10):1–6. doi: 10.15585/mmwr.ss6610a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks RM, Greene J, Hibbard J, Overton V, & Parrotta CD (2017). Does patient activation predict the course of type 2 diabetes? A longitudinal study. Patient Education and Counseling. doi: 10.1016/j.pec.2017.01.014 [DOI] [PubMed] [Google Scholar]
- Safford MM, Andreae S, Cherrington AL, Martin MY, Halanych J, Lewis M, … Richman JS (2015). Peer Coaches to Improve Diabetes Outcomes in Rural Alabama: A Cluster Randomized Trial. Annals in Family Medicine, 13 Suppl 1, S18–26. doi: 10.1370/afm.1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel-Hodge CD, Keyserling TC, Park S, Johnston LF, Gizlice Z, & Bangdiwala SI (2009). A Randomized Trial of a Church-Based Diabetes Self-management Program for African Americans With Type 2 Diabetes. Diabetes Educator, 35(3), 439–454. doi: 10.1177/0145721709333270 [DOI] [PubMed] [Google Scholar]
- Schoenberg NE, Ciciurkaite G, & Greenwood MK (2017). Community to clinic navigation to improve diabetes outcomes. Preventive Medicine Reports, 5, 75–81. doi: 10.1016/j.pmedr.2016.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JJ, Berman MD, Hiratsuka VY, & Frazier RR (2015). The effect of regular primary care utilization on long-term glycemic and blood pressure control in adults with diabetes. Journal of American Board of Family Medicine, 28(1), 28–37. doi: 10.3122/jabfm.2015.01.130329 [DOI] [PubMed] [Google Scholar]
- Spleen AM, Lengerich EJ, Camacho FT, & Vanderpool RC (2014). Health Care Avoidance Among Rural Populations: Results From a Nationally Representative Survey. Journal of Rural Health, 30(1). doi: 10.1111/jrh.12032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton IM, Adler AI, Neil HAW, Matthews DR, Manley SE, Cull CA, … Holman RR (2000). Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ, 321(7258), 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabaei BP, Rundle AG, Wu WY, Horowitz CR, Mayer V, Sheehan DM, & Chamany S (2017). Residential Socioeconomic, Food and Built Environments and Glycemic Control in Individuals With Diabetes in New York City 2007–2013. American Journal of Epidemiology. doi: 10.1093/aje/kwx300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TS, Funnell M, Sinco B, Piatt G, Palmisano G, Spencer MS, … Heisler M (2014). Comparative effectiveness of peer leaders and community health workers in diabetes self-management support: results of a randomized controlled trial. Diabetes Care, 37(6), 1525–1534. doi: 10.2337/dc13-2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towne SD Jr., Smith ML, Ahn S, & Ory MG (2014). The reach of chronic-disease self-management education programs to rural populations. Frontiers in Public Health, 2, 172. doi: 10.3389/fpubh.2014.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towne SD, Bolin J, Ferdinand A, Nicklett EJ, Smith ML, & Ory MG (2017). Assessing Diabetes and Factors Associated with Foregoing Medical Care among Persons with Diabetes: Disparities Facing American Indian/Alaska Native, Black, Hispanic, Low Income, and Southern Adults in the U.S. (2011–2015). International Journal of Environmental Research and Public Health, 14(5), 464. doi: 10.3390/ijerph14050464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau (2018). Quick Facts. Retrieved from: https://www.census.gov/quickfacts/fact/table/bambergcountysouthcarolina,US/PST045218 [Google Scholar]
- Villarroel MA, Vahratian A, Ward BW (2015). Health care utilization among U.S. adults with diagnosed diabetes, 2013 NCHS data brief, no 183 Hyattsville, MD: National Center for Health Statistics; [PubMed] [Google Scholar]
- Wagner EH, Austin BT, & Von Korff M (1996). Organizing care for patients with chronic illness. Milbank Quarterly, 74(4), 511–544. [PubMed] [Google Scholar]
- Williams GC, McGregor H, Zeldman A, Freedman ZR, Deci EL, & Elder D (2005). Promoting glycemic control through diabetes self-management: evaluating a patient activation intervention. Patient Education and Counseling, 56(1), 28–34. doi: 10.1016/j.pec.2003.11.008 [DOI] [PubMed] [Google Scholar]
- Wolever RQ, Dreusicke M, Fikkan J, Hawkins TV, Yeung S, Wakefield J, … Skinner E (2010). Integrative health coaching for patients with type 2 diabetes: a randomized clinical trial. Diabetes Educator, 36(4), 629–639. doi: 10.1177/0145721710371523 [DOI] [PubMed] [Google Scholar]
- Wolever RQ, & Dreusicke MH (2016). Integrative health coaching: a behavior skills approach that improves HbA1c and pharmacy claims-derived medication adherence. BMJ Open Diabetes Research and Care, 4(1), e000201. doi: 10.1136/bmjdrc-2016-000201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziller E, Lenardson J, Paluso N, Janis J. (2019). Preventive Health Service Use among Rural Women Policy Brief-73. Maine Rural Health Research Center; Retrieved from: https://www.ruralhealthresearch.org/publications/1249 [Google Scholar]