Abstract
INTRODUCTION
Demonstrating the “clinical meaningfulness” of slowing early cognitive decline in clinically normal (CN) older adults with elevated amyloid-β (Aβ+) is critical for Alzheimer’s disease (AD) secondary prevention trials and for understanding early cognitive progression.
METHODS
Cox regression analyses were used to determine whether 3-year slopes on the Preclinical Alzheimer’s Cognitive Composite (PACC) predicted MCI diagnosis and Global CDR>0 in 267 Aβ+ CN participating in HABS, AIBL, and ADNI.
RESULTS
Steeper PACC decline over 3 years was associated with increased risk for MCI diagnosis and Global CDR>0 in the following years across all cohorts. Hazard ratios using meta-analytic estimates were 5.47 (95%CI: 3.25–9.18) for MCI diagnosis and 4.49 (95%CI: 2.84–7.09) for CDR>0 in those with subtle decline (>−.14 to −.26 standard deviations/year) on longitudinal cognitive testing.
DISCUSSION
Early “subtle cognitive decline” among Aβ+ CN on a sensitive cognitive composite demonstrably increases risk for imminent clinical disease progression and functional impairment.
Keywords: Alzheimer’s disease, clinical meaningfulness, outcome research, clinical trials methodology, amyloid, secondary prevention, preclinical
1. INTRODUCTION
The AD continuum involves a protracted asymptomatic phase starting with the accumulation of amyloid β (Aβ) plaques and neurofibrillary tangles, followed by subtle yet increasingly persistent cognitive decline, functional impairment, and ultimately the dementia syndrome[1]. At the symptomatic stages of disease, cognitive and functional decline tend to occur in unison [2] and regulators have historically required co-primary outcomes of cognition and function to ensure the clinical meaningfulness of the cognitive effect on functional progression. Advances in our ability to visualize AD neuropathology in vivo have provided the opportunity for early detection during the preclinical phase and have spurred secondary prevention trials to minimize cognitive decline in asymptomatic but at-risk individuals. However, co-primary outcomes are presumed to be challenging for secondary prevention trials because participants lack cognitive or functional impairment at enrollment and are also unlikely to develop significant functional impairment in the timeframes over which such trials are conducted (i.e. 3–5 years)[3, 4]. Recent regulatory guidance for clinical trials in early Alzheimer’s disease (AD) emphasized the importance of establishing the meaningfulness of clinical outcomes[3].
In the absence of functional impairment, one means of inferring clinical meaningfulness at the preclinical stage may be to determine whether subtle longitudinal cognitive decline predicts clinical progression beyond the duration of a trial. The recent National Institute of Aging and Alzheimer’s Association (NIA-AA) research framework describes a transitional stage (e.g., “Stage 2”) along the AD trajectory, in which individuals may exhibit “subtle cognitive decline” on longitudinal cognitive testing as they move from asymptomatic to mildly symptomatic[5]. Quantification of “subtle cognitive decline” remains to be determined. In the same vein, FDA draft guidance offers the possibility of conducting studies long enough to follow individuals over the course of stage 2 until they show functional impairment [3]. However, disease progression is protracted, with a recent study showing that only 20% of Stage 1/ 2 participants progress to MCI/dementia diagnosis after 8 years [6]. Thus, an alternative, more efficient approach is to determine the magnitude of cognitive decline on longitudinal testing that may serve as a proxy for future functional impairment. If a treatment slows this cognitive decline and reduces the likelihood of clinical progression, this would provide evidence for a clinically meaningful therapeutic response. Multiple observational studies have shown that, at the group level, abnormal levels of Aβ (measured with molecular neuroimaging or cerebrospinal fluid) are independently associated with cognitive decline and functional progression [7–9] However, no studies to date have directly quantified the extent of subtle decline measured on longitudinal cognitive testing that is representative of clinically meaningful outcomes (e.g., MCI diagnosis) biomarker-confirmed asymptomatic AD[5].
Here, we determine the extent of cognitive decline on longitudinal testing among Aβ+ CN older adults that predicts risk of subsequent diagnosis of MCI or AD dementia, and separately, progression to a Global Clinical Dementia Rating (CDR) Score greater than 0 after 3 years. To increase the generalizability of risk estimates in relation to cognitive slopes, data was aggregated from participants enrolled in three independent observational studies. To increase the applicability of results, we used the Preclinical Alzheimer’s Cognitive Composite (PACC-5), an outcome currently being used in both pharmacological/non-pharmacological secondary prevention trials. We also assessed whether more subtle functional changes on the CDR Sum of Boxes were associated with concurrent cognitive decline prior to a diagnosis of MCI. Additional analyses in which we further queried these models were conducted within HABS (e.g., reducing the time window of cognitive decline and examining individual measures).
2. METHODS
2.1. Sample characteristics
Participants included individuals from the Harvard Aging Brain Study (HABS), the Australian Imaging, Biomarker and Lifestyle Study (AIBL), and the AD Neuroimaging Initiative (ADNI)[10–12]. All participants were classified as CN at baseline using previously reported study-specific criteria [10–12]. Participants were restricted to those with at least two follow-up neuropsychological assessments post baseline (anchored to year of first Aβ PET scan). Primary analyses focused on a subset of participants classified as having high Aβ (Table 1; n=267). Rates of cognitive and functional decline in the Aβ negative participants (Aβ−) were computed as a comparison to the Aβ positive group (Aβ+) (Supplementary Table 1; n=641).
Table 1.
Baseline Demographic Characteristics of Aβ+ Clinically Normal Participants by Cohort
| Variable | HABS | AIBL | ADNI | Significance Testing (F, χ2) | p |
|---|---|---|---|---|---|
| n | 73 | 84 | 110 | ||
| Age, mean, sd | 74.80 (6.09) | 74.96 (6.92) | 76.16 (6.15) | 2.78 | .065 |
| Female sex, % | 61 | 45* | 64 | 6.93 | .031 |
| Education, mean, sd | 16.15 (2.93) | 13.51 (2.42)* | 16.05 (1.10) | 14.11 | <.0001 |
| MMSE, median, IQR | 29 (28–30) | 29 (27–30) | 29 (28–30) | 0.08 | .924 |
| PACC, mean, sd | 0.02 (0.68) | −0.14 (0.64) | −0.09 (0.60) | 0.48 | .620 |
| Overall Follow-Up, mean, sd, range | 4.35 (1.55) [1.0–6.71] | 4.89(1.28)* [2.77–6.98] | 3.96(1.01)* [1.96–5.24] | 12.94 | <0.0001 |
| Progressors to MCI at year 3+, % | 20 (12/58) | 26 (12/45) | 32 (19/59) | 1.16 | .560 |
| Progressors to CDR>0 at year 3+, % | 23 (10/44) | 31 (14/45) | 39 (23/59) | 1.62 | .444 |
NOTE. HABS: Means and Standard Deviations reported unless otherwise noted. Harvard Aging Brain Study, AIBL: Australian Biomarker and Lifestyle Study, ADNI: Alzheimer’s Disease Neuroimaging Initiative, MMSE: Mini-Mental State Exam, PACC: Preclinical Alzheimer’s Cognitive Composite (5-component), MCI: Mild Cognitive Impairment, sd: standard deviation, CDR: Clinical Dementia Rating, IQR: inter-quartile range
Indicates the cohort which was significantly different from the others using Tukey post-hoc comparisons.
2.2. Cognitive Outcome: The PACC
Use of both the Preclinical Alzheimer’s Cognitive Composite (PACC) [13, 14] and the PACC5 (PACC + semantic fluency), has previously been described in detail in each of these cohorts [15]. The more sensitive PACC5 is used here but referred to throughout as PACC for clarity [13]. In HABS, the PACC includes: Logical Memory Delayed Recall (LMDR), the Free and Cued Selective Reminding Test (FCSRT), the Mini Mental Status Examination (MMSE), the Digit Symbol Substitution Test (DSST), and Category Fluency to animals, vegetables, and fruits (CAT). The PACC similarly includes the MMSE and LMDR for AIBL and ADNI. However, differences in cognitive test batteries across cohorts required substitution with measures assessing the same cognitive process. The PACC has exhibited relative concordance of baseline and slopes among these cohorts[15] despite differences in measures. The PACC was computed separately in each cohort by averaging the z-transformed scores for each measure derived from cohort-specific sample means and standard deviations. ADNI and HABS participants completed the PACC annually compared with 18-month intervals in AIBL.
2.3. Clinical Progression Outcomes: Diagnosis of MCI or AD dementia and Clinical Dementia Rating (CDR)
Measures of clinical disease progression included a diagnosis of MCI or AD dementia as well as a Clinical Dementia Rating Global Score and Sum of Boxes. The CDR was included as a disease progression outcome to ensure that the predictive relationship between PACC decline and MCI diagnosis was not driven by overlap in cognitive measures used both in the PACC and in making a study diagnosis of MCI.
In HABS, the CDR is completed by neuropsychologists and psychiatrists and rated independently from other cognitive testing results. All CDR raters are blinded to participant biomarker status. Quarterly consensus meetings are conducted with 6 or more clinicians as part of a multidisciplinary team. Participants are brought to consensus if they have a global CDR of 0.5 and/or performance falls 1.5 standard deviations below the sample mean on any individual domain-specific composite score [16]. Diagnoses are determined by clinical consensus after reviewing the CDR, cognitive data, and relevant medications/medical history.
In AIBL, the CDR is completed by neuropsychologists and is blinded from the other cognitive testing results. Participants are classified as normal or MCI at each visit by consensus of geriatric psychiatrists, behavioral neurologists and neuropsychologists blinded to Aβ status [17]. MCI subjects met Petersen criteria [18] including subjective and objective cognitive difficulties in the absence of significant functional impairment.
In ADNI, the CDR rater is ideally not involved with any other cognitive or functional assessments. Rating is not limited to MD/PhD level raters. Participants are diagnosed with MCI on the basis of the presence of a memory complaint, an MMSE of 24–30 and a global CDR of 0.5 with a mandatory box score of 0.5 in the memory domain [19]. Diagnosis is made by the site Principal Investigator or designee and includes review of the larger cognitive test battery, functional measures, and medical issues.
2.4. PET data acquisition and analysis
Both HABS and AIBL use the 11C-Pittsburgh Compound-B (PiB) Aβ-PET tracer, while ADNI uses the 18F-AV45 (Florbetapir or FBP) Aβ-PET tracer. The PET acquisition parameters for each study have been published previously [11, 12, 20–22]. In brief, ADNI and AIBL’s PET acquisition time was 50–70 minutes after injection (http://adni.loni.usc.edu/), whereas for HABS, PiB-PET data were collected 40–60 minutes after injection. Cerebellar grey matter was used as the reference region across studies. HABS used a distribution value ratio (DVR) whereas ADNI and AIBL used standardized uptake value ratios (SUVr). We used previously published study-specific regional summary measures and cut-offs to classify individuals as Aβ+. Cut-offs included: HABS>1.2 DVR [22], AIBL>1.40 SUVr [12], ADNI>1.11 SUVr[21].
2.5. Statistical Analyses
Statistical analyses were completed using R version 3.5.0 (packages: survival, ggsurvfit and lme4, pROC, metafor,). Differences in demographics across cohorts and Aβ+/− groups within cohort were examined using a series of one-way ANOVAs for continuous variables and χ2 tests for dichotomous variables.
Ordinary least-squares regression was used to derive individual PACC slopes and intercepts for each participant by cohort over a three-year period (Figure 1). Computation of slopes was restricted to the first 3 years following Aβ PET scans to correspond with the average length of a clinical trial. For studies with annual follow-up (HABS/ADNI), 4 time points were used in contrast with 3 time points in AIBL (18-month follow-up period). To determine the extent to which PACC declined over 3 years in the Aβ+ individuals, a linear mixed effects model controlling for age (centered at 75), sex (female), and education (centered at 16) was utilized for each cohort. Using HABS as an example, we also computed 1 and 2-year slopes to determine whether cognitive decline over a shorter duration could predict functional progression.
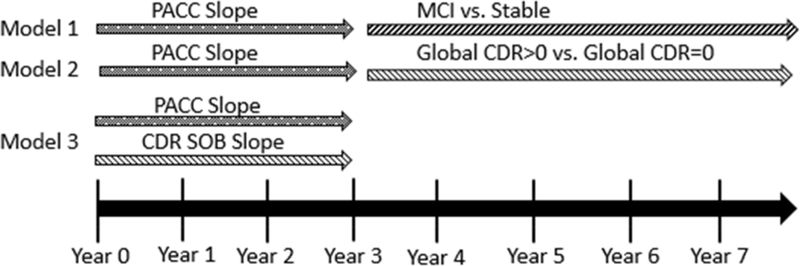
Figure 1.
Schematic of Study Analyses
NOTE. Models 1 and 2 examine the predictive relationship between subtle decline measured on longitudinal cognitive testing (PACC Slope) among normal older adults and subsequent clinical disease progression to either a diagnosis of MCI (Model 1) or a Global CDR>0 (Model 2). Model 3 examines the relationship between concurrent subtle cognitive decline and clinical disease progression (slope of CDR SOB- Sum of Boxes Score). PACC: Preclinical Alzheimer’s Cognitive Composite (5-component).
Given our interest in simulating AD secondary prevention trials, some of which are specifically recruiting older adults with elevated Aβ [23], primary analyses were completed in only those individuals with elevated Aβ. Cox proportional hazards models were used to separately estimate the effect of PACC slope from baseline to year 3 on the risk for clinical progression to MCI/AD dementia at or after year 3. Analyses were controlled for baseline PACC performance, age, sex, and education to account for demographic differences both within and between cohorts (Model 1; Figure 1). The equivalent analysis was completed substituting diagnosis with Global CDR>0 (Model 2). In Models 1 and 2, we restricted our dataset to those who progressed at or after year 3 such that the event of interest (i.e., MCI diagnosis or CDR>0) did not precede the measurement of cognitive slope. A summary meta-analysis estimate was calculated for Models 1 and 2 using the rma function to fit a meta-analytic fixed-effect model from cohort model estimates and confidence intervals. Receiver operating curve (ROC) analysis was used to identify the sensitivity and specificity of PACC slope cutpoints to MCI diagnosis. Finally, we were interested in whether subtle cognitive decline was simultaneously associated with an increase in subtle functional changes. To answer this question, we examined whether PACC slope was associated with evidence for concurrent subtle functional changes prior to MCI by examining the correlation of PACC slope with slope of CDR Sum of Boxes over 3 years (Model 3).
Using HABS as an example we explored whether individual PACC tests were significant predictors of MCI using Cox proportional hazards models in line with Models 1 and 2 above.
All analyses were two-sided and significance was set at p<.05.
3. RESULTS
3.1. Demographic Characteristics
Among the Aβ+ participants, there were no differences across cohorts for age or baseline cognition (Table 1). AIBL participants had a lower proportion of females and lower education compared with HABS and ADNI. Among Aβ+ subjects, mean follow-up in AIBL was longer compared with HABS (p=.022) and ADNI (p<.001). These cohort-differences were comparable when including Aβ− participants (Supplementary Table 1).
3.2. Cognitive Decline by Aβ+ Status and Cohort
Over a 3-year period, Aβ+ participants declined on the PACC in HABS (p<.0001), ADNI (p=.0001) and AIBL (p=.008) (Supplementary Table 2). A different pattern was observed among the Aβ− group, which showed improved performance (practice effect) over the same period in HABS (p=.002) and stability in PACC performance for AIBL and ADNI (Supplementary Table 2; <1% in HABS, 3.7% in AIBL, and 4.2% in ADNI).
3.3. Clinical Progression by Aβ+ Status and Cohort
The proportion of Aβ+ participants who progressed to MCI at year 3 and thereafter was 20% in HABS, 26% in AIBL, and 32% in ADNI (Table 2), which was systematically higher than MCI progression rates observed in Aβ− (Supplementary Table 1).
Table 2.
Model 1: Cox Regression Analyses Showing Progression to MCI Amongst Aβ+
| Progressors to MCI/ Stable | HR (95% CI) | Estimate (se) | p | |
|---|---|---|---|---|
| HABS | ||||
| n=58, events=12 | PACC Slope* | 0.009 (0.001–0.682) | −2.13 (2.22) | .033 |
| PACC Intercept | 0.867 (0.265–2.836) | −0.24 (0.60) | .814 | |
| Age | 1.036 (0.898–1.194) | 0.48 (0.07) | .631 | |
| Sex | 0.076 (0.009–0.652) | −2.35 (1.10) | .019 | |
| Education | 0.917 (0.679–1.240) | −0.56 (0.15) | .575 | |
| AIBL | ||||
| n=45, events=12 | PACC Slope* | 0.000 (0.000–0.021) | −3.71 (2.20) | .000 |
| PACC Intercept* | 0.034 (0.004–0.288) | −3.11 (1.08) | .001 | |
| Age | 0.850 (0.727–0.994) | −2.03 (0.08) | .042 | |
| Sex | 0.857 (0.102–7.241) | −0.141 (1.08) | .887 | |
| Education | 0.680 (0.483–1.081) | −1.628 (0.24) | .103 | |
| ADNI | ||||
| n=59, events=19 | PACC Slope* | 0.143 (0.022–0.911) | −2.06 (0.94) | .039 |
| PACC Intercept | 0.498 (0.246–1.007) | −1.94 (0.35) | .052 | |
| Age | 1.073 (0.984–1.170) | 1.60 (0.04) | .110 | |
| Sex | 0.753 (0.229–2.476) | −0.47 (0.61) | .641 | |
| Education | 0.957 (0.773–1.185) | −0.40 (0.10) | .687 |
NOTE. HABS: Harvard Aging Brain Study, AIBL: Australian Biomarker and Lifestyle Study, ADNI: Alzheimer’s Disease Neuroimaging Initiative, PACC: Preclinical Alzheimer Cognitive Composite-5, HR: Hazard’s Ratio, se: standard error, MCI: Mild Cognitive Impairment, CI: confidence interval
3.4. Cognitive Decline and Subsequent Clinical Disease Progression
Progression to MCI/Dementia (Model 1)
Four participants in HABS (6%), 3 in AIBL (4%), and 12 in ADNI (7%) were excluded from the Cox regression analysis because they progressed to MCI prior to study year 3. Mean time to a diagnosis of MCI/Dementia in Aβ+ CN including those who progressed prior to year 3 was 3.82(1.85) years (HABS), 4.25(1.90) years (AIBL), and 2.89 (1.62) years (ADNI). Follow-up time did not differ between those who progressed to MCI versus those who remained stable in HABS (p=.799) or AIBL (.891), however, stable participants exhibited longer follow-up compared with MCI progressors in ADNI (p<.01).
Results showed that steeper PACC decline was a significant predictor of MCI diagnosis across all 3 cohorts (Table 2). This remained true when controlling for baseline PACC performance, which was also a significant predictor of disease progression in AIBL with a trend on the bounds of significance in ADNI (Table 2). Additional predictors of MCI in Aβ+ CN were female sex (HABS) and age (AIBL).
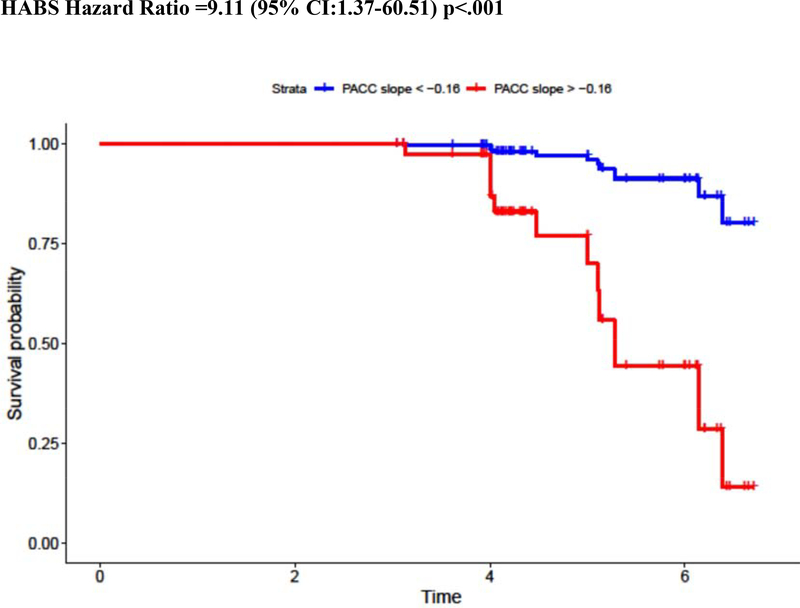
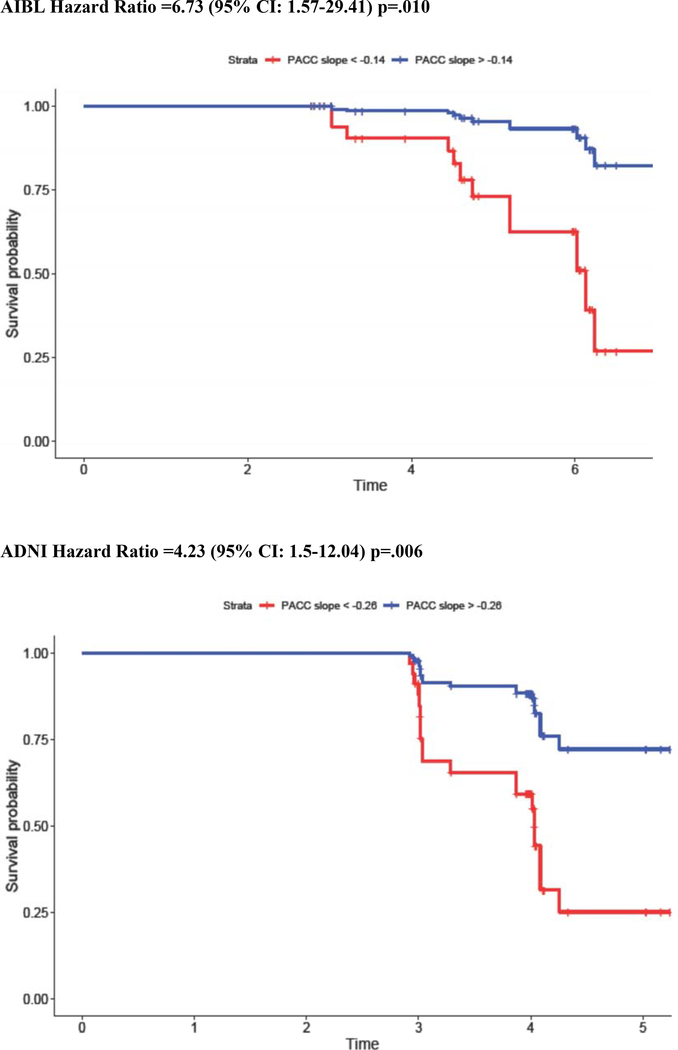
To better visualize and interpret the risk of MCI diagnosis for a given cognitive slope, Cox regression analyses were re-computed using PACC dichotomized into “decliner” versus “stable” groups using the sample-specific Aβ+ slope. For those whose slope was in the lowest tertile (−0.16 in HABS, −0.14 in AIBL, and −0.26 in ADNI), hazard for MCI diagnosis increased by a factor of 9.11 in HABS, 6.73 in AIBL, and 4.23 in ADNI (Figure 2). Combining these estimates across cohorts using meta-analytic techniques showed that overall hazard for MCI diagnosis was 5.47 (95%CI: 3.25–9.18). Sensitivity and specificity of different PACC slope cut points to MCI diagnosis are provided in Supplementary Table 4. As an example, a PACC Slope < −0.16 in HABS is associated with 99.80% sensitivity and 58.80% specificity to MCI diagnosis, a PACC Slope < −0.14 in AIBL is associated with 99.79% sensitivity and 75.00% specificity, and a PACC Slope < −0.26 in ADNI is associated with 99.83% sensitivity and 68.40% specificity to MCI diagnosis.
Figure 2.
Hazard Ratio for MCI Diagnosis in PACC Decliners: Visualization of Model Results
NOTE. Kaplan Meier curves showing the relative risk of MCI diagnosis among initially clinically normal but Aβ+ older adults with steeper (red) versus more stable (blue) PACC slopes in the preceding 3 years. PACC slope is dichotomized into steep versus stable groups using the bottom tertile.
Progression to CDR>0 (Model 2)
Mean time to a CDR>0 in Aβ+ CN, including those who progressed prior to year 3, was 2.84 (1.53) years (HABS), 4.13 (1.9) years (AIBL), and 2.59 (1.51) years (ADNI). Recapitulating results observed in Model 1, steeper PACC decline was a significant predictor of CDR>0 across all 3 cohorts (Table 3). Using the same groupings for PACC “decliner” versus “stable” groups as above, hazard for CDR>0 was 7.13 (95% CI:1.07–47.20, p=0.041) in HABS, 5.08 (95%CI: 1.43–18.18, p=0.011) in AIBL, and 3.78 (95% CI:1.53–9.35, p=0.004) in ADNI. Combining these estimates across cohorts using meta-analytic techniques showed that overall hazard for CDR>0 was 4.49 (95%CI: (2.84–7.09).
Table 3.
Model 2: Cox Regression Analyses Showing Progression to Global CDR>0 Amongst Aβ+
| CDR Progression; Global CDR>0 vs. Global CDR=0 | HR (95% CI) | Estimate (se) | p | |
|---|---|---|---|---|
| HABS | ||||
| n=44, events=10 | PACC5 Slope* | 0.003 (0.000–0.454) | −5.62 (2.47) | 0.023 |
| PACC5 Intercept | 0.797 (0.241–2.833) | −0.23(0.65) | 0.726 | |
| Age | 0.926 (0.802–1.069) | −0.08 (0.07) | 0.296 | |
| Sex | 0.289 (0.053–1.589) | −1.24 (0.87) | 0.154 | |
| Education | 0.973 (0.716–1.321) | −0.03 (0.16) | 0.859 | |
| AIBL | ||||
| n=45, events=14 | PACC5 Slope* | 0.000 (0.000–0.014) | −8.45 (2.14) | 0.000 |
| PACC5 Intercept* | 0.020 (0.002–0.175) | −3.88 (1.09) | 0.000 | |
| Age | 0.855 (0.740–0.989) | −0.16 (0.07) | 0.035 | |
| Sex | 0.268 (0.043–1.666) | −1.32 (0.93) | 0.158 | |
| Education | 0.753 (0.525–1.081) | −0.28 (0.18) | 0.125 | |
| ADNI | ||||
| n=59, events=23 | PACC5 Slope* | 0.064 (0.011–0.386) | −2.75 (0.92) | 0.003 |
| PACC5 Intercept* | 0.360 (0.181–0.717) | −1.02 (0.35) | 0.004 | |
| Age | 0.976 (0.894–1.066) | −0.02 (0.04) | 0.592 | |
| Sex | 0.454 (0.156–1.325) | −0.79 (0.55) | 0.149 | |
| Education | 1.070 (0.862–1.329) | 0.07 (0.11) | 0.539 |
NOTE. HABS: Harvard Aging Brain Study, AIBL: Australian Biomarker and Lifestyle Study, ADNI: Alzheimer’s Disease Neuroimaging Initiative, PACC: Preclinical Alzheimer Cognitive Composite-5, HR: Hazard’s Ratio, se: standard error, MCI: Mild Cognitive Impairment, CI: confidence interval
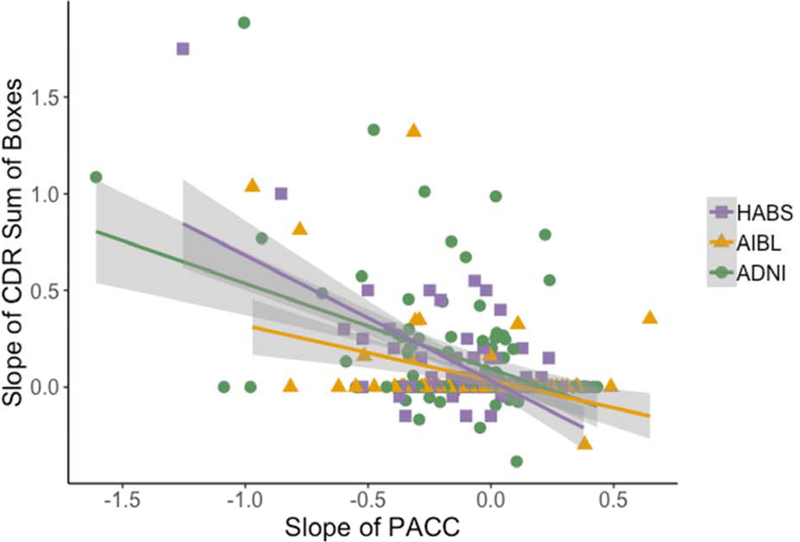
Concurrent Cognitive Decline and Functional Progression (Model 3)
We also examined whether subtle cognitive decline was associated with a concurrent increase in functional symptoms prior to an MCI diagnosis. Across all cohorts, steeper PACC slope was associated with increased Sum of Boxes scores on the CDR (HABS: r= −.612, p<.01; AIBL: r= −.439, p<.01; ADNI: r= −.374, p<.01) over the same 3-year time period (Figure 3). However, 65% of individuals showed no change (Slope=0) on CDR-SOB over 3 years.
Figure 3.
Concurrent subtle cognitive decline and increasing functional impairment over 3 years in Aβ+ CN
NOTE. Correlation between 3-year cognitive slopes and 3-year CDR Sum of Boxes among initially clinically normal but Aβ+ older adults. The correlation between PACC and CDR slope is r= −0.612 (p<.001), r=−0.439, (p<.001), and r=−.374, (p<.001) in HABS, AIBL, and ADNI, respectively.
3.5. Further Analysis of the Association between Cognition and MCI Diagnosis in HABS
Testing the limits of Model 1 within HABS, PACC slope was not a significant predictor of MCI when restricted to either two (p=.399) or one-year (p=.906) follow-up (Supplementary Table 3). Returning to 3-year slopes, the slope of each PACC component (including MMSE, DSST, FCSRT, LMDR and CAT) was a significant predictor of MCI diagnosis at or after 3 years when examined independently (Supplementary Table 3).
4. DISCUSSION
Results across three large observational cohorts indicate that rates of disease progression among initially normal older adults are systematically higher in Aβ+ compared with Aβ− and range from 20 to 32% of Aβ+ progressing to MCI or 23 to 39% progressing to Global CDR>0. Subtle cognitive decline (between −0.14 and −0.26 standard deviations per year on a multi-domain cognitive composite) among these Aβ+ older adults is associated with an approximately 5-fold greater risk of subsequent clinical disease progression (i.e., MCI diagnosis or Global CDR>0). These findings provide strong evidence for the meaningfulness of subtle cognitive decline in the context of biomarker-defined preclinical AD.
Our results provide general parameters for the expected degree of subtle decline measured on longitudinal cognitive testing that is representative of “transitional cognitive decline” in Stage 2 of the revised NIA-AA criteria [24]. We corroborate findings from multiple reports showing that among initially CN older adults, abnormal Aβ is associated with both cognitive decline [9, 25] and functional progression [26]. In contrast with previous work, we examined the predictive utility of longitudinal cognition for imminent clinical disease progression. Furthermore, we focused on CN individuals with biomarker-defined AD (Aβ+).
Criteria for cognitive impairment in MCI is defined as 1.5 standard deviations below normative data [18]and previous studies have shown that the correlation between cognition and function is strongest as the disease progresses [27]. However, we show the extent to which quite subtle cognitive decline (as small as −0.14 to −0.26 standard deviations annually) amongst initially asymptomatic Aβ+ individuals is associated with imminent clinical disease progression, that is, a 5-fold increase in hazard for MCI diagnosis. These findings support the notion that AD treatment effectiveness in secondary prevention may be inferred by examining subtle decline measured on longitudinal cognitive testing alone. Recent FDA draft guidance for industry similarly raises this possibility suggesting it will “consider strongly justified arguments that a persuasive effect on sensitive measures of neuropsychological performance may provide adequate support for a marketing approval” [3]. The persuasiveness of the clinical meaningfulness of cognitive performance would likewise be enhanced with evidence for a large magnitude of effect and a large breadth of effect. Although the magnitude of cognitive decline was relatively subtle, its predictive utility was robust, evident on two separate markers of disease progression (i.e., CDR>0 and MCI diagnosis) and persisting across three cohorts despite differences in methodology (including differences in PACC tests, follow-up duration, and diagnostic procedures) and relatively small sample sizes of Aβ+ individuals with extended follow-up.
Interestingly, baseline PACC performance was not a significant predictor of either MCI or CDR 0.5 in HABS and while baseline cognition did contribute some explanatory variance in ADNI and AIBL, subtle decline measured on longitudinal cognitive testing remained the best predictor of clinical disease progression. This suggests that risk for imminent clinical progression is not solely driven by those further along the trajectory at study initiation as evidenced by lower cognition at study outset, but by those who are subtly declining over time. The scope of subtle cognitive decline’s pervasive relationship with clinical disease progression was further revealed by HABS results showing that decline on each individual task predicted MCI diagnosis independently. Furthermore, there was also evidence that a more subtle increase in functional symptoms (i.e., slope of CDR-SOB) was moderately correlated with concurrent PACC decline, but this was driven by a subset (only 35% showed change on the CDR-SOB). This last finding raises the possibility that traditional coprimary outcomes of cognition and function may be appropriate when targeting those in the latest stages of preclinical AD.
Finally, the reported link between an individual’s own cognitive concerns (rather than those of an informant) and AD biomarkers in asymptomatic individuals [28] suggests that there may be additive utility in examining trajectories of cognitive complaints alongside cognitive decline to predict risk for clinical progression[29]. This may also be extended to measures of mild neurobehavioral changes [30–32]as well as potentially novel measures of health outcomes developed in coordination with patient and caregivers to better identify what is of value from an individual’s perspective (e.g., driving, perceived competence, etc).
4.1. Limitations
Although we pooled data across 3 large observational cohorts, our sample is insufficient to set standards for predicting risk of clinical progression at the individual level for a given slope, age, sex, or genetic profile. Additionally, there may be some circularity in using cognitive slopes to predict MCI diagnosis, which in most cases involves a review of cognitive performance to make this diagnosis. However, our identical finding of 3-year PACC decline on subsequent Global CDR Progression (which is rated independently of cognitive testing) allays concerns regarding circularity and reinforces the robustness of the pattern. Finally, disappointing results from clinical trials testing anti-Aβ therapies at the symptomatic stages of AD certainly raise the question of the relevance of Aβ accumulation to tau spreading, neurodegeneration and cognitive decline. Our results remain agnostic as to whether Aβ is a relevant target for intervention or whether both anti-Aβ and anti-tau therapies in addition to mitigation of other contributing factors may be required at even earlier stages of disease to fully prevent cognitive decline.
4.2. Conclusions
Neuropsychological measures, on face value, do not reflect the everyday cognitive skills needed to function independently; rarely are people faced with matching digits and symbols in daily life or learning unrelated lists of words. However, subtle decline measured on longitudinal cognitive testing was predictive of subsequent MCI diagnosis, which is certainly a meaningful outcome. We may infer that subtle decline measured on longitudinal cognitive testing alone, particularly in the setting of biological markers for a neurodegenerative disease, may serve as a proxy for movement along the AD disease trajectory in future secondary prevention trials.
Supplementary Material
Research in Context.
1). Systematic Review
The extant literature was reviewed using traditional methods. Multiple observational studies have shown that abnormal Aβ among asymptomatic older adults is associated with 1) cognitive decline and 2) functional progression longitudinally. However, the predictive relationship between very subtle cognitive decline and imminent clinical disease progression (i.e., diagnosis of MCI, CDR>0) in asymptomatic Aβ+ individuals is unclear.
2). Interpretation
Results across three large observational cohorts of asymptomatic Aβ+ older adults indicate that subtle 3-year cognitive decline (>−0.14 to −0.25 standard deviations) on the Preclinical Alzheimer’s Cognitive Composite (PACC-5) was associated with a 5.47 increase in hazards for MCI diagnosis and a 4.49 increase in hazard for CDR>0.
3). Future Directions
These findings have important implications for the design and interpretation of results of secondary prevention trials and for interpreting the meaningfulness of subtle cognitive decline in an Aβ+ unimpaired older adult to their risk for AD disease progression.
ACKNOWLEDGMENTS
Funding/Support:
HABS: The Harvard Aging Brain Study is funded by the National Institute on Aging (P01AG036694) with additional support from several philanthropic organizations. REA and KVP (1K23AG053422-01) are supported by K23 awards from NIA and awards from the Alzheimer’s Association. ECM is supported by a K01 award from NIA. RFB is funded with the NHMRC Dementia Research Fellowship (APP1105576).
We would like to thank Michael Properzi for his assistance in preparing the data and Samantha Burnham for input on the statistical plan. We would also like to acknowledge our study personnel, in particular Dylan Kirn, and research coordinators Martha Muniz, Emily Kilpatrick, Paige Sparks, Aubryn Samaroo, Lyssa Manning, and Hannah Klein. Finally, we would like to thank our committed HABS participants.
ADNI: Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
AIBL: Funding for the AIBL study was provided in part by the study partners [Australian Commonwealth Scientific Industrial and research Organization (CSIRO), Edith Cowan University (ECU), Mental Health Research Institute (MHRI), Alzheimer’s Australia (AA), National Ageing Research Institute (NARI), Austin Health, CogState Ltd., Hollywood Private Hospital, Sir Charles Gardner Hospital]. The study also received support from the National Health and Medical Research Council (NHMRC) and the Dementia Collaborative Research Centres program (DCRC2), as well as ongoing funding from the Science and Industry Endowment Fund (SIEF). The authors acknowledge the financial support of the CRC for Mental Health. The Cooperative Research Centre (CRC) program is an Australian Government Initiative.
DISCLOSURES
K Papp has served as a consultant for Biogen Idec.
R Buckley has no disclosures.
E Mormino has served as a consultant for Janssen, Eli Lilly, and Biogen Idec.
P Maruff is a full-time employee of Cogstate Ltd.
V Villemagne has served as a consultant for Bayer Pharma; and received research support from a NEDO grant from Japan.
C Masters is an advisor to Prana Biotechnology Ltd and a consultant to Eli Lilly.
K Johnson has served as paid consultant for Bayer, Biogen Idec, Bristol-Myers Squibb, GE Healthcare, Isis Pharmaceuticals Inc, Janssen Alzheimer’s Immunotherapy, Piramal, Siemens Medical Solutions, Novartis, Roche and Genzyme. He is a site principal investigator co-investigator for Lilly/Avid, Biogen Idec, Bristol-Myers Squibb, Eisai, Pfizer, Janssen Alzheimer Immunotherapy, Merck, and Navidea clinical trials. He has spoken at symposia sponsored by Janssen Alzheimer’s Immunotherapy, GEHC, Lundbeck, and Pfizer. These relationships are not related to the content in the manuscript.
D Rentz has served as a consultant for Eli Lilly, Biogen Idec, Lundbeck Pharmaceuticals, and serves as a member of the Scientific Advisory Board for Neurotrack.
R Sperling has served as a paid consultant for Abbvie, Biogen, Bracket, Genentech, Lundbeck, Merck, Otsuka, Roche, and Sanofi. She has research support from Eli Lilly, Avid, and Janssen. She has spoken at symposia sponsored by Eli Lilly, Biogen, and Janssen Alzheimer Immunotherapy. These relationships are not related to the content in the manuscript.
R Amariglio has served as a consultant for Biogen Idec.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Jack CR, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang Y, Haaksma ML, Ramakers I, et al. Cognitive and functional progression of dementia in two longitudinal studies. Int J Geriatr Psychiatry. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Research FDACfDE. Early Alzheimer’s Disease: Developing Drugs for Treatment: Guidance for Industry. In: FDA; Maryland; 2018. [Google Scholar]
- [4].Graf A, Risson V, Gustavsson A, et al. Assessment of Clinical Meaningfulness of Endpoints in the Generation Program by the Insights to Model Alzheimer’s Progression in Real Life (iMAP) Study. J Prev Alzheimers Dis. 2019;6(2):85–89. [DOI] [PubMed] [Google Scholar]
- [5].Jack C, Bennett D, Blennow K, Carrillo M, Dunn B, Elliott C. NIA-AA research framework: towards a biological definition of Alzheimer’s disease. Alzheimer’s & Dementia. 2018;14(4):535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dang C, Harrington KD, Lim YY, et al. Relationship Between Amyloid-β Positivity and Progression to Mild Cognitive Impairment or Dementia over 8 Years in Cognitively Normal Older Adults. Journal of Alzheimer’s Disease. 2018;65(4):1313–1325. [DOI] [PubMed] [Google Scholar]
- [7].Vos SJ, Xiong C, Visser PJ, et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12(10):957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mormino EC, Betensky RA, Hedden T, et al. Synergistic effect of beta-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol. 2014;71(11):1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lim YY, Maruff P, Pietrzak RH, et al. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain. 2014;137(Pt 1):221–231. [DOI] [PubMed] [Google Scholar]
- [10].Aisen PS, Petersen RC, Donohue MC, et al. Clinical Core of the Alzheimer’s Disease Neuroimaging Initiative: progress and plans. Alzheimers Dement. 2010;6(3):239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dagley A, LaPoint M, Huijbers W, et al. Harvard Aging Brain Study: Dataset and accessibility. NeuroImage. 2017;144(Part B):255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rowe CC, Ellis K, Rimajova M, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31(8):1275–1283. [DOI] [PubMed] [Google Scholar]
- [13].Donohue MC, Sperling RA, Salmon DP, et al. The Preclinical Alzheimer Cognitive Composite: Measuring Amyloid-Related Decline. JAMA Neurol. 2014;71(8):961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Papp KV, Rentz DM, Orlovsky I, Sperling RA, Mormino EC. Optimizing the preclinical Alzheimer’s cognitive composite with semantic processing: The PACC5. Alzheimers Dement. 2017;3(4):668–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Buckley RF, Mormino EC, Amariglio RE, et al. Sex, amyloid, and APOE ε4 and risk of cognitive decline in preclinical Alzheimer’s disease: Findings from three well-characterized cohorts. Alzheimers Dement. 2018;14(9):1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Orlovsky I, Huijbers W, Hanseeuw BJ, et al. The relationship between recall of recently versus remotely encoded famous faces and amyloidosis in clinically normal older adults. Alzheimers Dement. 2017;10:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Villemagne VL, Pike KE, Chételat G, et al. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69(1):181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. [DOI] [PubMed] [Google Scholar]
- [19].Petersen RC, Aisen P, Beckett L, et al. Alzheimer’s disease Neuroimaging Initiative (ADNI) clinical characterization. Neurology. 2010;74(3):201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Landau SM, Mintun MA, Joshi AD, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72(4):578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Landau SM, Breault C, Joshi AD, et al. Amyloid-beta imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J Nucl Med. 2013;54(1):70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mormino E, Betensky RA, Hedden T, et al. Amyloid and APOE e4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology. 2014;82:1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sperling RA, Rentz DM, Johnson KA, et al. The A4 Study: Stopping AD Before Symptoms Begin? Sci Transl Med. 2014;19(6):228fs213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jack C, Bennett D, Blennow K, et al. NIA-AA research framework: towards a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vos SJ, Xiong C, Visser PJ, et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. The Lancet Neurology. 2013;12(10):957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Morris JC, Roe CM, Grant EA, et al. Pittsburgh Compound B Imaging and Prediction of Progression From Cognitive Normality to Symptomatic Alzheimer Disease. Archives of Neurology. 2018;66(12):1469–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu-Seifert H, Siemers E, Selzler K, et al. Correlation between Cognition and Function across the Spectrum of Alzheimer’s Disease. J Prev Alzheimers Dis. 2016;3(3):138–144. [DOI] [PubMed] [Google Scholar]
- [28].Amariglio RE, Becker JA, Carmasin J, et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50(12):2880–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].van Harten AC, Mielke MM, Swenson-Dravis DM, et al. Subjective cognitive decline and risk of MCI: The Mayo Clinic Study of Aging. Neurology. 2018;91(4):e300–e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kuhn E, Moulinet I, Perrotin A, et al. Cross-sectional and longitudinal characterization of SCD patients recruited from the community versus from a memory clinic: subjective cognitive decline, psychoaffective factors, cognitive performances, and atrophy progression over time. Alzheimers Res Ther. 2019;11(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Burhanullah MH, Tschanz JT, Peters ME, et al. Neuropsychiatric Symptoms as Risk Factors for Cognitive Decline in Clinically Normal Older Adults: The Cache County Study. Am J Geriatr Psychiatry. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Caselli RJ, Langlais BT, Dueck AC, et al. Personality Changes During the Transition from Cognitive Health to Mild Cognitive Impairment. J Am Geriatr Soc. 2018;66(4):671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.