Abstract
Background
The intensity of inflammatory response triggered by cardiopulmonary bypass (CPB) during cardiac surgery has been associated with adverse outcomes. Neutrophils might contribute to organ injury through the liberation of DNA histone-based structures named “neutrophil extracellular traps” (NETs). Our objective was to assess circulating NETs levels before and after cardiac surgery in low-risk and high-risk patients.
Methods
This prospective cohort study included 2 groups of patients undergoing elective cardiac surgery with the use of CPB. The first group consisted of low-risk patients (European System for Cardiac Operative Risk Evaluation II ≤ 1%), and the second group included high-risk patients (European System for Cardiac Operative Risk Evaluation II ≥ 5%). Blood samples were drawn pre-CPB and 3 hours post-CPB separation. Measurements of circulating NETs, interleukin-6, C-reactive protein, myeloperoxidase, citrullinated histone 3, and pentraxin-related protein 3 levels were performed at each time point.
Results
Twenty-four patients, 12 high-risk and 12 low-risk patients, were included. Circulating NETs measurements changed from a median of 0.054 before CPB to 0.084 at 3 hours post-CPB separation, with a median increase of 0.037 (P < 0.001) per patient. No difference was noted between the high-risk and low-risk groups. A linear relationship was found between the circulating NETs measurements 3 hours post-CPB and CPB duration (ß = 0.047; confidence interval, 0.012-0.081; P = 0.01 R2 = 0.27). A correlation was found between the change in NETs with citrullinated histone 3 and myeloperoxidase levels, but not between NETs and other inflammatory biomarkers.
Conclusions
Circulating NETs measurements increases during cardiac surgery with postsurgical levels proportional to CPB duration. The clinical significance of NETs production during cardiac surgery should be further investigated.
Résumé
Contexte
L’intensité de la réponse inflammatoire déclenchée par la circulation extracorporelle en cours de chirurgie cardiaque a été associée à des résultats défavorables. Les neutrophiles pourraient contribuer à des lésions organiques par la libération de structures d’ADN lié à des histones appelées « pièges extracellulaires des neutrophiles » (ou NETs, de l’anglais neutrophil extracellular traps). Nous nous sommes donné pour objectif d’évaluer les taux de ces pièges extracellulaires dans la circulation avant et après la chirurgie cardiaque chez des patients exposés à des risques faibles et à des risques élevés.
Méthodologie
Cette étude prospective de cohortes a été menée chez deux groupes de patients subissant une chirurgie cardiaque non urgente au cours de laquelle nous avons eu recours à la circulation extracorporelle. Le premier groupe était composé de patients exposés à un faible risque (EuroSCORE II [European System for Cardiac Operative Risk Evaluation II] ≤ 1 %), et le second, de patients exposés à un risque élevé (EuroSCORE II ≥ 5 %). Des échantillons sanguins étaient prélevés avant la mise sous circulation extracorporelle, puis 3 heures après le sevrage de la circulation extracorporelle. Les taux de NETs circulants, d’interleukine-6, de protéine C réactive, de myéloperoxydase, d’histones 3 citrullinées et de protéines apparentées à la pentraxine 3 ont été mesurés à chaque point d’évaluation.
Résultats
Les données obtenues chez vingt-quatre patients, 12 dans le groupe à risque élevé et 12 dans le groupe à faible risque, ont été incluses dans l’analyse. Le taux médian de NETs circulants est passé de 0,054 avant la circulation extracorporelle à 0,084 trois heures après le sevrage, avec une augmentation médiane de 0,037 (p < 0,001) par patient. Aucune différence n’a été notée entre les deux groupes. Une relation linéaire a été observée entre la mesure du taux de NETs circulants 3 heures après le sevrage et la durée de la circulation extracorporelle (ß = 0,047; intervalle de confiance : 0,012-0,081; p = 0,01; R2= 0,27). Une corrélation a été notée entre la variation du taux de NETs comportant des histones 3 citrullinés et du taux de myéloperoxydase, mais pas entre le taux de NETs et ceux des autres biomarqueurs de l’inflammation.
Conclusions
Le taux de NETs circulants augmente pendant une chirurgie cardiaque, le taux après la chirurgie étant proportionnel à la durée de la circulation extracorporelle. L’importance clinique de la production de NETs pendant la chirurgie cardiaque doit faire l’objet d’études plus approfondies.
Surgery involving cardiopulmonary bypass (CPB) results in a general inflammatory response of varying intensity.1 The innate immune system is the main culprit of the adverse effects seen with unabated general inflammation response in pathological settings. Neutrophils, which are well known as the principal cells involved in host defense against microbial pathogen infections in the innate immune system,2,3 are also playing a key role in the inflammatory response to injury.3,4
In addition to phagocytosis and degranulation, the production of neutrophil extracellular traps (NETs) was identified as another distinct neutrophil function.5,6 NETs are web-like structures composed of decondensed chromatin and antimicrobial proteins. Although NETs contribute to defense against infection, they have also been implicated in the pathophysiology of multiple diseases, such as vasculitis,7,8 transfusion-associated lung injury,9,10 thrombotic microangiopathy,11 preeclampsia,12 cancer metastasis,13 acute respiratory distress syndrome,14 sepsis,15,16 and acute kidney injury (AKI).17, 18, 19 Local liberation of histones, such as citrullinated histone 3 (H3Cit), and myeloperoxidase (MPO) are thought to cause endothelial injury and exacerbate inflammation-induced organ injury. Recent evidence also implicates a potential role of NETs in linking sterile inflammation with thrombosis.20,21 The formation and deposition of NETs could impair perfusion at the microcirculation level because it has been demonstrated in some models of organ dysfunction in which microcirculatory compromise is well described, such as the myocardial no-reflow phenomenon and septic AKI.22, 23, 24
In the context of cardiac surgery, production of NETs could have pathophysiological implications. Patients with vascular disease and other comorbidities such as diabetes may have chronic inflammation, resulting in a priming of circulating neutrophils before surgery. The general inflammatory reaction triggered by CPB may consequently result in a burst of NETs production in the perioperative period. Occlusion and injury of the pulmonary and systemic microcirculation by an intense NETs formation triggered by CPB may be implicated in the pathogenesis of adverse patient outcomes by inducing organ injury in multiple systems.
The objective of this study was to perform circulating NETs quantification in patients undergoing cardiac surgery. We hypothesized that circulating NETs levels are increased 3 hours after surgery compared with baseline measurements before CPB. Furthermore, we hypothesized that this increase might be more pronounced in high-risk diabetic patients. We also investigated whether NETs production may be associated with the duration of CPB and the release of other markers known to be associated with NETosis (MPO, H3Cit), neutrophil activation (pentraxin-related protein 3 [PTX3]), and inflammatory mediators such as interleukin-6 (IL-6) and C-reactive protein (CRP).
Material and Methods
Participants
This prospective cohort study (NET1; ICM#2016-2060) included patients undergoing elective cardiac surgery with the use of CPB. Adult participants (aged ≥ 18 years) able to provide informed consent were admissible. Patients were screened and approached the day before scheduled surgery. Two groups of equal size were formed on the basis of preoperative risk assessment. The low-risk group included patients with a European System for Cardiac Operative Risk Evaluation (euroSCORE) II25 ≤ 1% without diabetes, and the high-risk group included participants with a euroSCORE II ≥ 5% and with known diabetes mellitus. We excluded patients undergoing urgent surgery or unable to provide consent, and patients with active infection, malignancy, autoimmune diseases (arthritis, vasculitis, inflammatory bowel disease), acute ischemic event in the last 14 days, or severe chronic kidney disease (estimated glomerular filtration rate < 15 mL/min/1.73 m2 based on the Modified Diet in Renal Disease equation).26 The project was approved by the local ethics board. Written informed consent was obtained for all participants. Patient recruitment was performed from November 2016 to July 2017. Serums were also collected from 12 healthy volunteers who were enrolled in the NEUTRO study (6) (ICM #2012-1374) and the SAUNA FMD study (6) (ICM #2017-2179).
Patient assessment
Before surgery, demographic information and medical history were recorded from the participants’ records, including prior diagnosis of chronic hypertension, tobacco use, and peripheral artery disease. Other information included functional status using the New York Heart Association (NYHA) classification27 left ventricular ejection fraction based on preoperative transthoracic echocardiography, planned procedure(s), baseline renal function based on the most recent available serum creatinine measurement, and recent diagnosis of myocardial infarct (< 90 days). The euroSCORE II was calculated from the gathered information using an online calculator.25 Finally, the following laboratory parameters were also collected the day before surgery: leucocyte, neutrophil, and lymphocyte count.
During the perioperative period, two 5 mL blood samples were collected in the operating room after the induction of general anaesthesia but before CPB initiation. A second series of samples were collected in the intensive care unit after surgery precisely 3 hours after CPB separation.
The following information was gathered during the perioperative period: duration of the procedure, intraoperative fluid balance, complications during or shortly after CPB separation, duration of intensive care unit and hospital stay after surgery, complications after surgery including AKI, stroke, pneumonia, arrhythmias, delirium, major bleeding, and urgent reinterventions for any indications. Hemodynamic parameters were also recorded at CPB separation. A difficult CPB separation was defined as the need for at least 1 vasopressor (norepinephrine, vasopressin) and 1 inotropic medication (epinephrine, dobutamine, intravenous milrinone) during CPB separation or the use of mechanical circulatory support after the first CPB weaning attempt.28 AKI was defined as an increase of > 26 μmol/L or > 50% in serum creatinine as per the Kidney Diseases: Improving Global Outcome group criteria.
Quantification of NETs and biomarkers
NETs-associated MPO-DNA complexes were quantified as previously described.7,29 Briefly, 5 μg/mL of mouse anti-human MPO antibody (Bio-Rad Antibodies, Hercules, CA) was coated to 96-well microtiter plates. After a 1-hour blocking with 1% bovine serum albumin, serum samples were added together with a peroxidase-labeled anti-DNA monoclonal antibody (component 2 of the Cell Death Detection ELISA PLUS kit, Millipore Sigma/Roche, Oakville, ON). After a 2-hour incubation on the shaker at 320 rpm, the peroxidase substrate was added according to the manufacturer's instructions. The optical absorbance was measured at 405 nm. CRP in serum samples was quantified by nephelometry at the Biochemistry Laboratory at the Montreal Heart Institute. IL-6, MPO, and PTX3 were measured using a Luminex Assay (R&D Systems, Minneapolis, MN). H3Cit was measured using the Citrullinated Histone H3 (Clone 11D3) ELISA Kit (Cayman Chemical, Ann Arbor, MI).
Statistical analysis
For the sample size calculation, it was determined that a total sample size of 24 patients gives a 90% power to detect an effect size (Cohen’s d) of 0.69 for an increase in the NETs measurements during the perioperative period in a 2-sided paired Student t test with a 95% confidence level. Additionally, 2 groups of 12 patients give 90% power to detect an effect size (Cohen’s d) of 1.39 in the difference of NETs measurements between the high-risk and low-risk groups.
Data are presented as frequency (percentages) and mean ± standard deviation or median (interquartile range [IQR], quartile 1; quartile 3) where appropriate. The normality of the data for continuous variables was assessed graphically using histograms and the Shapiro–Wilk test. The difference between NETs measurements before and after CPB was assessed using the Student t test for paired samples or Wilcoxon rank-sum test for paired sample depending on the distribution of the data. The difference between parameters in the low-risk and high-risk groups was assessed using the Student t test for independent samples or the Mann–Whitney U test for independent samples depending on the distribution for continuous variables, and chi-square test for dichotomous variables. The difference among 3 or more groups was assessed using 1-way analysis of variance or Kruskal–Wallis test depending on the distribution. When a P < 0.05 was observed, the difference between individual groups was further assessed using multiple comparisons with Bonferroni correction.
The relationship between the NETs measurements and the duration of CPB was assessed using linear regression, and the results are presented as ß coefficient with 95% confidence intervals and R2 for model fit. Outliers were defined as a data point more than 1.5 × IQR lower than the first quartile or higher than the third quartile (Tukey’s rule)30 and were excluded from the linear regression analysis. A multivariable linear regression analysis was used to determine if the association remained significant after adjustment for baseline NETs measurements. The relationship between NETs measurements and other inflammatory biomarkers was assessed using Spearman correlation, and results are presented as correlation coefficients (r). Significance level for all tests was set at 95%.
Results
From November 2016 to July 2017, 24 patients were included in the study. The characteristics of the studied subjects are presented in Table 1. Patients in the high-risk group had a lower left ventricular ejection fraction (38%; IQR, 33; 50%) vs 60% (IQR, 55; 65%) P < 0.001), worse functional class (P < 0.001), greater proportion of peripheral artery disease (1 vs 0 patients, P = 0.03), and chronic kidney disease (7 patients [58.3%] vs 1 patient [8.3%], P = 0.009). The duration of the procedure was significantly longer in high-risk patients (213 [IQR, 175-239] minutes vs 136 [IQR, 113-175] minutes P = 0.01). The mean age of all patients was 62.2 ± 14.9 years and statistically not different from the mean age of healthy volunteers used for comparison (63.9 ± 6.3 years; 6 men and 6 women).
Table 1.
Patient characteristics
| All patients (n = 24) | Low-risk (n = 12) | High-risk (n = 12) | P value | |
|---|---|---|---|---|
| Preoperative characteristics | ||||
| Age (y) | 62.2 ± 14.9 | 57.4 ± 14.6 | 66.9 ± 14.2 | 0.12 |
| BMI (kg/m2) | 31.0 ± 7.3 | 29.7 ± 5.0 | 32.3 ± 9.1 | 0.40 |
| euroSCORE II (%) | 4.0 ± 3.5 | 0.8 ± 0.2 | 7.2 ± 1.9 | < 0.001 |
| Left ventricular ejection fraction (%) | 55 (38-60) | 60 (55-65) | 38 (33-50) | < 0.001 |
| Functional class (NYHA) | < 0.001 | |||
| Class I | 5 (20.8%) | 5 (41.7%) | 0 (0%) | |
| Class II | 7 (29.2%) | 5 (41.7%) | 2 (16.7%) | |
| Class III | 12 (50.0%) | 2 (16.7%) | 10 (83.3%) | |
| Class IV | 0 (0.0%) | 0 (0%) | 0 (0%) | |
| Hypertension | 16 (66.7%) | 7 (58.3%) | 9 (75%) | 0.39 |
| Smoker | 5 (20.8%) | 1 (8.3%) | 4 (33.3%) | 0.13 |
| Peripheral artery disease | 1 (4.2%) | 0 (0%) | 1 (8.3%) | 0.03 |
| Recent myocardial infarct (< 90 d) | 2 (8.3%) | 1 (8.3%) | 1 (8.3%) | 1.0 |
| Chronic kidney disease (eGFR < 60 mL/m/1.73 m2) | 8 (33.3%) | 1 (8.3%) | 7 (58.3%) | 0.009 |
| Type of surgery | 0.07 | |||
| - Isolated coronary artery | 7 (29.2%) | 4 (33.3%) | 3 (25.0%) | |
| - Bypass | 10 (41.7%) | 7 (58.3%) | 3 (25.0%) | |
| - Isolated valvular procedure | 7 (29.2%) | 1 (8.3%) | 6 (50.0%) | |
| Leukocyte count before surgery | 8.1 ± 2.1 | 7.7 ± 1.2 | 8.5 ± 2.8 | 0.38 |
| Neutrophil count before surgery | 5.2 ± 1.4 | 4.9 ± 1.1 | 5.5 ± 1.7 | 0.34 |
| Lymphocyte count before surgery | 1.9 ± 0.8 | 1.8 ± 0.7 | 1.9 ± 1.0 | 0.73 |
| Intraoperative characteristics | ||||
| Duration of surgery (min) | 175 (136-223) | 136 (113-175) | 213 (175-239) | 0.01 |
| Duration of CPB (min) | 89 (50-113) | 61 (46-103) | 93 (80-121) | 0.13 |
| Duration of aortic clamping (min) | 63 (40-77) | 49 (28-69) | 72 (62-80) | 0.07 |
| Intraoperative fluid balance (L) | 1.3 (0.8-1.9) | 1.3 (0.7-1.9) | 1.3 (0.8-2.0) | 1.00 |
Data are presented as mean ± standard deviation or median (IQR) where appropriate.
BMI, body mass index; CPB, cardiopulmonary bypass; eGFR, estimated glomerular filtration rate; euroSCORE, European System for Cardiac Operative Risk Evaluation; NYHA, New York Heart Association.
Before the initiation of CPB, circulating NETs measurements were higher in the patients compared with healthy controls (0.054 [IQR, 0.039-0.073] vs 0.026 [IQR, 0.021-0.048], P = 0.022). However, no significant difference was noted between the pre-CPB NETs measurements between the high-risk and low-risk groups 0.046 (IQR, 0.039-0.069) vs 0.064 (IQR, 0.039-0.091), P = 0.56, as shown in Table 2.
Table 2.
Measurements of circulating NETs
| All patients (n = 24) | Low-risk (n = 12) | High-risk (n = 12) | P value | |
|---|---|---|---|---|
| NETs measurements before CPB | 0.054 (0.039-0.073) | 0.064 (0.039-0.091) | 0.046 (0.039-0.069) | 0.56 |
| NETs measurements 3 h after CPB | 0.084 (0.066-0.144) | 0.113 (0.061-0.193) | 0.081 (0.067-0.101) | 0.40 |
| Median change in NETs measurements | 0.037 (0.019-0.063) | 0.043 (0.026-0.103) | 0.027 (0.014-0.045) | 0.19 |
| Median fold-change in NETs measurements | 1.60 (1.34-2.37) | 1.83 (1.35-2.48) | 1.51 (1.28-2.30) | 0.55 |
For comparative purpose, the median NETs measurement in 12 healthy volunteers was 0.026 (IQR, 0.021-0.048). Results are presented as median (IQR). CPB, cardiopulmonary bypass; NETs, neutrophil extracellular traps.
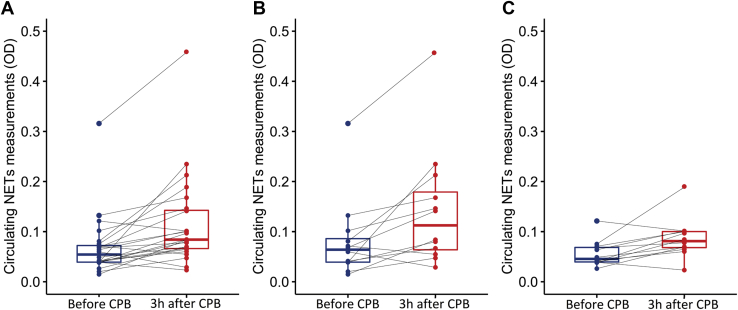
A significant increase in circulating NETs measurements was observed 3 hours after CPB (median change: +0.037 [0.019-0.063], P < 0.0001), as shown in Figure 1A. This increase was observed in both the low-risk and high-risk groups as shown in Figure 1B and C (median change of 0.043 [IQR, 0.026-0.103] P = 0.010 and 0.027 [IQR, 0.014-0.045] P = 0.008, respectively). However, there was no difference between the high-risk and low-risk groups in regard to the median change or NETs measurement 3 hours after CPB (P = 0.19) (Table 2).
Figure 1.
Circulating neutrophil extracellular traps (NETs) in the perioperative period. (A) Measurements in all patients (n = 24), (B) in low-risk patients (n = 12), and (C) in high-risk patients (n = 12). Significant differences were present between measurement before cardiopulmonary bypass (CPB) and after CPB (Wilcoxon signed-rank test, P < 0.05). OD, optical density.
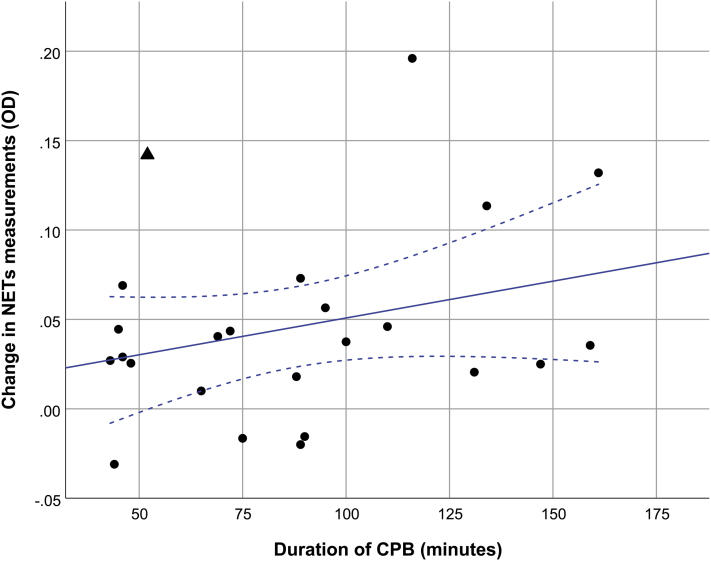
One patient in the low-risk group was identified as an outlier because he had elevated NETs measurements before CPB (outlier: 0.316, median in the group: 0.054 [IQR, 0.039-0.073]) and 3 hours after CPB (outlier: 0.458, median in the group: 0.084 [IQR, 0.066-0.144]). A brief clinical description of this outlier is presented in Supplemental Appendix S1. After exclusion of this outlier, a positive linear association was observed between the duration of CPB and the NETs measurement 3 hours after CPB (ß = 0.047 [CI, 0.012-0.081] P = 0.01 R2 = 0.27) (Fig. 2). This association remained significant after adjustment for baseline NETs measurements (ß = 0.039 [CI, 0.004-0.074] P = 0.03) (Table 3).
Figure 2.
Relationship between circulating NETs measurement 3 hours after surgery and the duration of CPB. Linear regression line is shown with 95% confidence intervals (CIs) (blue line) (ß = 0.047; CI, 0.012-0.081; P = 0.01, R2 = 0.27). The triangle marker denotes the outlier that was excluded for the analysis. CPB, cardiopulmonary bypass; NETs, neutrophil extracellular traps; OD, optical density.
Table 3.
Multivariable linear regression models to determine the association between the duration of CPB and NETs measurements after adjustment for baseline NETs measurements
| Univariable |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| ß | CI | P value | ß | CI | P value | |
| Duration of CPB | 0.047 | 0.012-0.081 | 0.011 | 0.039 | 0.004-0.074 | 0.032 |
| NETs measurements before CPB | 0.811 | 0.042-1.581 | 0.040 | 0.570 | −0.166 to 1.307 | 0.12 |
CI, confidence interval; CPB, cardiopulmonary bypass; NETs, neutrophil extracellular traps.
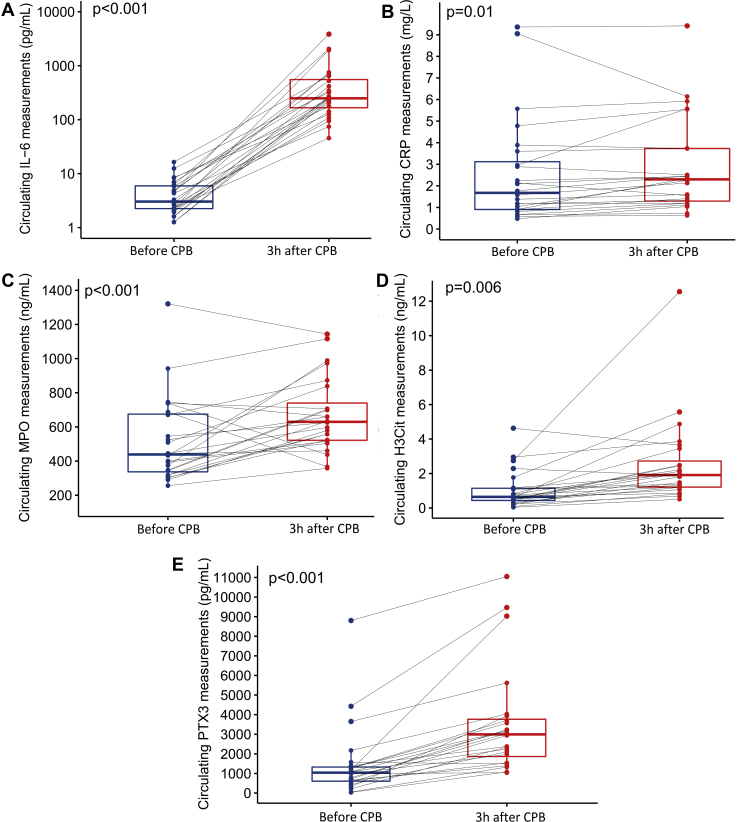
Baseline measurements of IL-6 and CRP were higher in the studied patients compared with healthy controls (3.05 pg/mL [2.21-6.02] vs 1.26 pg/mL [1.17-2.39] P = 0.002; and 1.68 mg/L [0.91-3.28] vs 0.66 mg/L [0.43-1.13] P = 0.002, respectively), whereas no significant baseline differences were found in MPO, H3Cit, and PTX3. Patients in the high-risk group had higher IL-6 measurement at baseline compared with patients in the low-risk group (5.38 pg/mL [3.82-7.77] vs 2.30 pg/mL [1.78-2.47] P = 0.004) (Supplemental Table S1), but there was no difference in baseline CRP measurements as shown in Supplemental Table S1. No significant difference was observed in MPO, H3Cit, and PTX3 levels.
During the perioperative period, a significant elevation in IL-6, CRP, MPO, H3Cit, and PTX3 was observed in the studied patients (median change: 245 pg/mL [IQR, 153-577] P < 0.001 and 0.28 mg/L [IQR, 0.04-0.55], P = 0.011) (Fig. 3). No differences between the changes or the postoperative levels of all biomarkers were observed between the low-risk and high-risk groups (Supplemental Tables S2-S6). Significant correlations were found between the change in NETs measurements and the change in MPO, H3Cit during the perioperative period (r = 0.58, P = 0.003 and r = 0.67, P < 0.001, respectively), and the absolute measurements of these markers 3 hours after CPB (r = 0.62, P = 0.001 and r = 0.52, P = 0.01, respectively). The baseline measurement of NETs before CPB was also correlated with MPO (r = 0.44, P = 0.03) but not with H3Cit. No correlation was found between NETs measurements and IL-6, CRP, or PTX3 at any time point. Furthermore, no correlation was present between the change in IL-6, CRP, or PTX3 and the change in NETs during the perioperative period (Supplemental Table S7).
Figure 3.
Measurement of (A) interleukin-6 (IL-6; pg/mL), (B) C-reactive protein (CRP; mg/L), (C) myeloperoxidase (MPO; ng/mL), (D) citrullinated histone 3 (H3Cit; ng/mL), and (E) pentraxin-related protein (PTX3; pg/mL) during the perioperative period. Significant differences were present between measurement before CPB for all biomarkers (Wilcoxon signed-rank test, P < 0.05). CPB, cardiopulmonary bypass.
After cardiac surgery, 50% of patients developed complications, including difficult separation for CPB, AKI, stroke, delirium, pneumonia, arrhythmia (atrial fibrillation, atrioventricular block), haemorrhage, and urgent reoperation (Table 4). Patients in the high-risk group had a longer hospital stay (8.5 [5.5-9.5] days vs 5.0 [4.5-5.5] days P = 0.020). Circulating NETs measurements before CPB and 3 hours after CPB, and the difference between baseline and 3 hours after CPB were not associated with individual complications or with a composite end point of major complications (Supplemental Table S8). There was no significant difference in NETs measurements in participants who received blood transfusions during surgery compared with those who did not (Supplemental Table S9).
Table 4.
Length of stay and complications after cardiac surgery
| All patients (n = 24) | Low-risk patients (n = 12) | High-risk patients (n = 12) | P value | |
|---|---|---|---|---|
| Duration of ICU stay (d) | 2.0 (1.0-2.0) | 1.5 (1.0-2.0) | 2.0 (1.0-3.0) | 0.242 |
| Duration of hospital stay (d) | 5.5 (5.0-8.5) | 5.0 (4.5-5.5) | 8.5 (5.5-9.5) | 0.020 |
| Duration of mechanical ventilation (h) | 5.0 (3.0-16.5) | 2.5 (2.5-36.0) | 6.0 (2.0-11.5) | 0.932 |
| Difficult separation from bypass | 3 (12.5%) | 2 (16.7%) | 1 (8.3%) | 1.00 |
| AKI | 6 (25.0%) | 1 (8.3%) | 5 (41.7%) | 0.155 |
| Stroke | 1 (4.2%) | 0 (0.0%) | 1 (8.3%) | 1.00 |
| Haemorrhage | 3 (12.5%) | 1 (8.3%) | 2 (16.7%) | 1.00 |
| Urgent reoperation | 2 (8.3%) | 1 (8.3%) | 1 (8.3%) | 1.00 |
| Arrhythmia | 5 (20.8%) | 3 (25.0%) | 2 (16.7%) | 1.00 |
| Delirium | 3 (12.5%) | 0 (0.0%) | 3 (25.0%) | 0.217 |
| Pneumonia | 1 (4.2%) | 0 (0.0%) | 1 (8.3%) | 1.00 |
| Death | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | - |
| Any complication | 12 (50.0%) | 6 (50.0%) | 6 (50.0%) | 1.00 |
AKI, acute kidney injury; ICU, intensive care unit.
Discussion
In this study, we observed an increase in circulating NETs measurements in patients undergoing on-pump cardiac surgery. Before surgery, the subjects had a median NETs measurement 2-fold higher than healthy controls, which increased significantly after surgery, attaining a median level more than 3-fold higher than in healthy controls. No difference between circulating NETs measurements was observed between the low-risk and high-risk patients. The post-CPB NETs measurement was proportional to the duration of CPB. We observed a correlation between NETs quantification and biomarkers known to be associated with NETs production, such as MPO and H3Cit. However, no association was observed with the other inflammatory biomarkers studied or with clinical complications after surgery.
We are the first to demonstrate that cardiac surgery with the use of CPB is associated with release of NETs. Paunel-Gorgulu et al.31 demonstrated an increase in cell-free DNA in the perioperative period, with prolonged CPB being associated with greater elevations. However, cell-free DNA may be produced by apoptosis, necrosis, and cell damage induced by the contact of blood to the extracorporeal circuit, and cell-free DNA measurements may not be proportional to NETs release as it was demonstrated in patients undergoing liver transplantation.32 Therefore, cell-free DNA is not a direct evidence of NETs production. We used an assay to detect MPO-associated DNA to specifically assess cell-free DNA originating from neutrophils, therefore providing evidence of circulating NETs increase during the CPB procedure. Furthermore, we supported these results by demonstrating a parallel increase in circulating MPO and H3Cit, which are known to be associated with NETs release.33,34
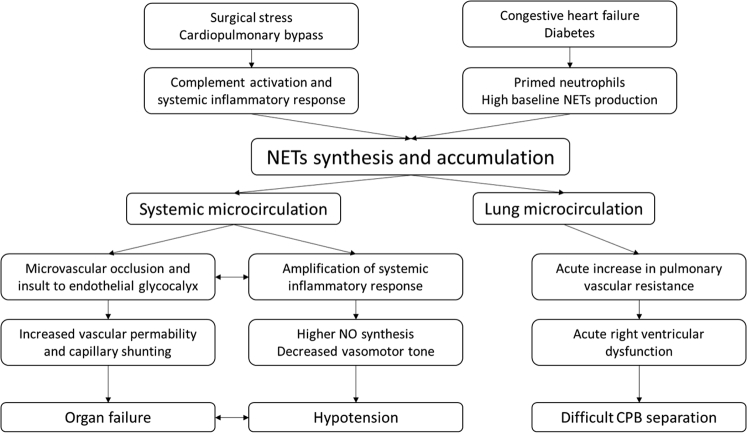
The triggers leading to NETs formation can be of multiple origins, including in response to microorganisms,15 from the contact with activated platelets15 or activated endothelial cells, and during the general inflammation response via proinflammatory cytokines.35 In the specific context of “on-pump” cardiac surgery, contact with the CPB circuit is known to cause activation of the coagulation cascade and complement system activation resulting in production of C3a and C5a, which are potent proinflammatory chemokines. Ischemia–reperfusion injury and operative trauma promote endothelial cell activation and expression of adhesion molecules, which promote migration of neutrophils to the site of injury.36 Unabated NETs production may be a major pathway leading to injury in critically ill patients (Fig. 4). Recent evidence has shown that NETs formation by neutrophils collected from a cohort of critically ill patients independently predicts mortality and disseminated intravascular coagulation.37 Consequently, inhibiting NETs formation by the use of peptidyl arginine deiminase inhibitors38 or inducing degradation of circulating NETs by using exogenous DNase I may represent promising therapeutic avenues.39
Figure 4.
Potential clinical implications of NETs after CPB. CPB, cardiopulmonary bypass; NETs, neutrophil extracellular traps; NO, nitric oxide.
Our original hypothesis was that NETs formation would be correlated with production of IL-6, an early inflammation marker contributing to neutrophil activation. However, we found that measurement of NETs post-CPB was positively associated with CPB duration but not with IL-6 production. Consequently, it is possible that NETs formation in the perioperative period is triggered by direct contact with the extracorporeal circuit or with other pathways, such as complement activation, platelet activation, and endothelial cell activation. On the basis of our in vitro study,40 in which the peak of NETs synthesis was observed within 3 hours poststimulation with proinflammatory mediators, we elected to measure circulating NETs 3 hours postprocedure. Additionally, it is possible that measuring NETs at this time point after CPB separation does not allow enough time for the triggering of NETs formation by the general inflammatory response associated with CPB. In support of this, CRP increase was minimal in the studied period.41 CRP production by the hepatocytes is stimulated by IL-6, but the increase in serum is delayed, usually peaking 24 hours after the initiation of the inflammatory response.41 Therefore, this marker is associated with the late inflammatory response of CPB and thus may contribute to NETs formation later than the immediate postoperative period. In support of this, Qin et al.42 reported that IL-6 levels increase early after cardiac surgery, but other mediators of inflammation, such as mitochondrial DNA, have a more delayed response. However, they found that the peak concentration of mitochondrial DNA correlates with the intensity of early IL-6 release.42 Another possibility is that NETs measured in the circulation could be an underestimation of the total amount of NETs being produced because NETs could be deposited in the capillary bed or bound to primed neutrophils sequestered in systemic and pulmonary microvascular beds.43
PTX3 is an acute-phase biomarker that increases dramatically in the context of sepsis or other inflammatory disorders. It is hypothesized that PTX3 may act to protect against the adverse consequences of NETs by binding circulating histone, thereby preventing endothelial injury and acting as an anti-inflammatory mediator in patients undergoing cardiac surgery.44,45 We observed that the PTX3 level increased during the perioperative period and reached abnormal levels (> 2000 pg/mL) after CPB, but did not reach high levels commonly seen in sepsis (> 14,000 pg/mL).46,47 Additionally, we found no correlations between PTX3 and NETs measurements during the perioperative period.
Study limitations
This study has several limitations. First, the small sample size was not sufficient to assess the clinical implications of circulating NETs measurements. Furthermore, what represents a clinically significant increase in circulating NETs measurements in the perioperative period of a major surgery is unknown. In addition, only 2 time points were studied, which preclude us to precisely determine when the increase in NETs formation occurs and if NETs production continues beyond 3 hours after cardiac surgery. Regarding the outlier patient, we were not able to explain the observed outlier NETs value, considering that all the other biomarkers values were within the normal range observed for the other patients before CPB. Finally, all patients underwent cardiac surgery with the use of CPB. A control group undergoing off-pump cardiac surgery would be needed to confirm that exposure to CPB is the primary factor leading to production of NETs.
Conclusions
In this exploratory study, we demonstrate for the first time that circulating NETs increase during cardiac surgery. The exposure of blood to the extracorporeal circuit during CPB may trigger production of NETs. Whether the phenomenon may predispose patients to complications after cardiac surgery should be further investigated to determine if circulating NETs represent a potential treatment target.
Acknowledgements
The authors thank the volunteers (patients and healthy donors) for providing us with blood samples and Dr Jan-Alexis Tremblay for his contribution to Figure 4 of the article.
Funding Sources
This work was supported by the Richard I. Kaufman Endowment Fund in Anesthesia and Critical Care, Montreal, Quebec (A.Y.D.), and the Montreal Heart Institute Foundation, Montreal, Quebec (M.G.S.). W.B.-S. was the recipient of a studentship from the Fonds de Recherche du Québec en Santé, Montreal, Quebec. The funding sources had no involvement in this article.
Disclosures
Cayman Chemical provided free H3Cit ELISA kits. A.Y.D. is a Speaker for CAE Healthcare and Masimo. The other authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The research reported has adhered to the relevant ethical guidelines.
See page 47 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2019.12.001.
Supplementary Material
References
- 1.Warren O.J., Smith A.J., Alexiou C. The inflammatory response to cardiopulmonary bypass: part 1--mechanisms of pathogenesis. J Cardiothorac Vasc Anesth. 2009;23:223–231. doi: 10.1053/j.jvca.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Rigby K.M., DeLeo F.R. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol. 2012;34:237–259. doi: 10.1007/s00281-011-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinnula V.L., Soini Y., Kvist-Makela K., Savolainen E.R., Koistinen P. Antioxidant defense mechanisms in human neutrophils. Antioxid Redox Signal. 2002;4:27–34. doi: 10.1089/152308602753625825. [DOI] [PubMed] [Google Scholar]
- 4.Mayadas T.N., Tsokos G.C., Tsuboi N. Mechanisms of immune complex-mediated neutrophil recruitment and tissue injury. Circulation. 2009;120:2012–2024. doi: 10.1161/CIRCULATIONAHA.108.771170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinkmann V., Reichard U., Goosmann C. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 6.Urban C.F., Reichard U., Brinkmann V., Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 7.Kessenbrock K., Krumbholz M., Schonermarck U. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida M., Sasaki M., Sugisaki K., Yamaguchi Y., Yamada M. Neutrophil extracellular trap components in fibrinoid necrosis of the kidney with myeloperoxidase-ANCA-associated vasculitis. Clin Kidney J. 2013;6:308–312. doi: 10.1093/ckj/sft048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas G.M., Carbo C., Curtis B.R. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood. 2012;119:6335–6343. doi: 10.1182/blood-2012-01-405183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caudrillier A., Kessenbrock K., Gilliss B.M. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122:2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arai Y., Yamashita K., Mizugishi K. Serum neutrophil extracellular trap levels predict thrombotic microangiopathy after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:1683–1689. doi: 10.1016/j.bbmt.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Gupta A.K., Hasler P., Holzgreve W., Gebhardt S., Hahn S. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol. 2005;66:1146–1154. doi: 10.1016/j.humimm.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Cools-Lartigue J., Spicer J., McDonald B. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013 Jul 1 doi: 10.1172/JCI67484. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bendib I., de Chaisemartin L., Granger V. Neutrophil extracellular traps are elevated in patients with pneumonia-related acute respiratory distress syndrome. Anesthesiology. 2019;130:581–591. doi: 10.1097/ALN.0000000000002619. [DOI] [PubMed] [Google Scholar]
- 15.Clark S.R., Ma A.C., Tavener S.A. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 16.Gao X., Hao S., Yan H. Neutrophil extracellular traps contribute to the intestine damage in endotoxemic rats. J Surg Res. 2015;195:211–218. doi: 10.1016/j.jss.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 17.Jansen M.P., Emal D., Teske G.J. Release of extracellular DNA influences renal ischemia reperfusion injury by platelet activation and formation of neutrophil extracellular traps. Kidney Int. 2017;91:352–364. doi: 10.1016/j.kint.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Nakazawa D., Kumar S.V., Marschner J. Histones and neutrophil extracellular traps enhance tubular necrosis and remote organ injury in ischemic AKI. J Am Soc Nephrol. 2017;28:1753–1768. doi: 10.1681/ASN.2016080925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raup-Konsavage W.M., Wang Y., Wang W.W. Neutrophil peptidyl arginine deiminase-4 has a pivotal role in ischemia/reperfusion-induced acute kidney injury. Kidney Int. 2018;93:365–374. doi: 10.1016/j.kint.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brill A., Fuchs T.A., Savchenko A.S. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10:136–144. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinod K., Wagner D.D. Thrombosis: tangled up in NETs. Blood. 2014;123:2768–2776. doi: 10.1182/blood-2013-10-463646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boneschansker L., Inoue Y., Oklu R., Irimia D. Capillary plexuses are vulnerable to neutrophil extracellular traps. Integr Biol (Camb) 2016;8:149–155. doi: 10.1039/c5ib00265f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ge L., Zhou X., Ji W.J. Neutrophil extracellular traps in ischemia-reperfusion injury-induced myocardial no-reflow: therapeutic potential of DNase-based reperfusion strategy. Am J Physiol Heart Circ Physiol. 2015;308:H500–H509. doi: 10.1152/ajpheart.00381.2014. [DOI] [PubMed] [Google Scholar]
- 24.Prowle J.R., Bellomo R. Sepsis-associated acute kidney injury: macrohemodynamic and microhemodynamic alterations in the renal circulation. Semin Nephrol. 2015;35:64–74. doi: 10.1016/j.semnephrol.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Nashef S.A., Roques F., Sharples L.D. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41:734–745. doi: 10.1093/ejcts/ezs043. [DOI] [PubMed] [Google Scholar]
- 26.Levey A.S., Bosch J.P., Lewis J.B. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 27.The Criteria Committee of the New York Heart Association . 9th ed. Little, Brown & Co; Boston, MA: 1994. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. [Google Scholar]
- 28.Denault A.Y., Tardif J.C., Mazer C.D., Lambert J., BART Investigators Difficult and complex separation from cardiopulmonary bypass in high-risk cardiac surgical patients: a multicenter study. J Cardiothorac Vasc Anesth. 2012;26:608–616. doi: 10.1053/j.jvca.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 29.Wang H., Sha L.L., Ma T.T. Circulating level of neutrophil extracellular traps is not a useful biomarker for assessing disease activity in antineutrophil cytoplasmic antibody-associated vasculitis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoaglin D.C., John W. Tukey and data analysis. Stat Sci. 2003:311–318. [Google Scholar]
- 31.Paunel-Gorgulu A., Wacker M., El Aita M. cfDNA correlates with endothelial damage after cardiac surgery with prolonged cardiopulmonary bypass and amplifies NETosis in an intracellular TLR9-independent manner. Sci Rep. 2017;7:17421. doi: 10.1038/s41598-017-17561-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Meijenfeldt F.A., Burlage L.C., Bos S. Elevated plasma levels of cell-free DNA during liver transplantation are associated with activation of coagulation. Liver Transplant. 2018;24:1716–1725. doi: 10.1002/lt.25329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mauracher L.M., Posch F., Martinod K. Citrullinated histone H3, a biomarker of neutrophil extracellular trap formation, predicts the risk of venous thromboembolism in cancer patients. J Thromb Haemost. 2018;16:508–518. doi: 10.1111/jth.13951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schechter M.C., Buac K., Adekambi T. Neutrophil extracellular trap (NET) levels in human plasma are associated with active TB. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182587. e0182587-e0182587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oehmcke S., Morgelin M., Herwald H. Activation of the human contact system on neutrophil extracellular traps. J Innate Immun. 2009;1:225–230. doi: 10.1159/000203700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Day J.R., Taylor K.M. The systemic inflammatory response syndrome and cardiopulmonary bypass. Int J Surg. 2005;3:129–140. doi: 10.1016/j.ijsu.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Abrams S.T., Morton B., Alhamdi Y. A novel assay for neutrophil extracellular trap formation independently predicts disseminated intravascular coagulation and mortality in critically ill patients. Am J Respir Crit Care Med. 2019;200:869–880. doi: 10.1164/rccm.201811-2111OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aliko A., Kamińska M., Falkowski K. Discovery of novel potential reversible peptidyl arginine deiminase inhibitor. Int J Mol Sci. 2019;20:2174. doi: 10.3390/ijms20092174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang S., Xie T., Sun S. DNase-1 treatment exerts protective effects in a rat model of intestinal ischemia-reperfusion injury. Sci Rep. 2018;8:17788. doi: 10.1038/s41598-018-36198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavoie S.S., Dumas E., Vulesevic B. Synthesis of human neutrophil extracellular traps contributes to angiopoietin-mediated in vitro proinflammatory and proangiogenic activities. J Immunol. 2018;200:3801–3813. doi: 10.4049/jimmunol.1701203. [DOI] [PubMed] [Google Scholar]
- 41.Noly P.E., Labbe P., Thorin-Trescases N. Reduction of plasma angiopoietin-like 2 after cardiac surgery is related to tissue inflammation and senescence status of patients. J Thorac Cardiovasc Surg. 2019;158:792–802.e5. doi: 10.1016/j.jtcvs.2018.12.047. [DOI] [PubMed] [Google Scholar]
- 42.Qin C., Liu R., Gu J. Variation of perioperative plasma mitochondrial DNA correlate with peak inflammatory cytokines caused by cardiac surgery with cardiopulmonary bypass. J Cardiothorac Surg. 2015;10:85. doi: 10.1186/s13019-015-0298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Summers C., Singh N.R., White J.F. Pulmonary retention of primed neutrophils: a novel protective host response, which is impaired in the acute respiratory distress syndrome. Thorax. 2014;69:623–629. doi: 10.1136/thoraxjnl-2013-204742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daigo K., Takamatsu Y., Hamakubo T. The protective effect against extracellular histones afforded by long-pentraxin PTX3 as a regulator of NETs. Front Immunol. 2016;7:344. doi: 10.3389/fimmu.2016.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kunes P., Mandak J., Holubcova Z., Kolackova M., Krejsek J. The long pentraxin PTX3: a candidate anti-inflammatory mediator in cardiac surgery. Perfusion. 2013;28:377–389. doi: 10.1177/0267659113483799. [DOI] [PubMed] [Google Scholar]
- 46.Kunes P., Lonsky V., Mandak J. The long pentraxin 3 in cardiac surgery: distinct responses in “on-pump” and “off-pump” patients. Scand Cardiovasc J. 2007;41:171–179. doi: 10.1080/14017430701324262. [DOI] [PubMed] [Google Scholar]
- 47.Uusitalo-Seppälä R., Huttunen R., Aittoniemi J. Pentraxin 3 (PTX3) is associated with severe sepsis and fatal disease in emergency room patients with suspected infection: a prospective cohort study. PloS One. 2013;8 doi: 10.1371/journal.pone.0053661. e53661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.