Abstract
Background
Whether reprogramming of cardiac resynchronization therapy (CRT) to increase electrical synchrony translates into echocardiographic improvement remains unclear. SyncAV is an algorithm that allows fusion of intrinsic conduction with biventricular pacing. We aimed to assess whether reprogramming chronically implanted CRT devices with SyncAV is associated with improved echocardiographic parameters.
Methods
Patients at a quaternary center with previously implanted CRT devices with a programmable SyncAV algorithm underwent routine electrocardiography-based SyncAV optimization during regular device clinic visits. This analysis included only patients who could be programmed to the SyncAV algorithm (i.e., in sinus rhythm with intrinsic atrioventricular conduction). Echocardiography was performed before and 6 months after CRT optimization.
Results
Of 64 consecutive, potentially eligible patients who underwent assessment, 34 who were able to undergo SyncAV programming were included. Their mean age was 74 ± 9 years, 41% were female, and 59% had ischemic cardiomyopathy. The mean time from CRT implant to SyncAV optimization was 17.8 ± 8.5 months. At 6-month follow-up, SyncAV optimization was associated with a significant increase in left ventricular ejection fraction (LVEF) (mean LVEF 36.5% ± 13.3% vs 30.9% ± 13.3%; P < 0.001) and a reduction in left ventricular end-systolic volume (LVESV) (mean LVESV 110.5 ± 57.5 mL vs 89.6 ± 52.4 mL; P < 0.001) compared with baseline existing CRT programming.
Conclusion
CRT reprogramming to maximize biventricular fusion pacing significantly increased LVEF and reduced LVESV in patients with chronic CRT devices. Further studies are needed to assess if a continuous fusion pacing algorithm improves long-term clinical outcomes and to identify which patients are most likely to derive benefit.
Résumé
Contexte
On ignore si la reprogrammation du dispositif de resynchronisation cardiaque (DRC) afin d’améliorer la synchronisation électrique se traduit réellement par une amélioration échocardiographique. L’algorithme SyncAV permet de fusionner la conduction intrinsèque et la stimulation biventriculaire. Nous avons tenté de déterminer si la reprogrammation à l’aide de l’algorithme SyncAV d’un DRC implanté de façon permanente permet d’améliorer les paramètres échocardiographiques.
Méthodologie
Les patients d’un centre de soins quaternaires porteurs d’un DRC doté d’un algorithme SyncAV programmable ont subi une optimisation électrocardiographique de routine de cet algorithme à l’occasion d’une consultation de suivi. L’analyse ne portait que sur les patients dont le dispositif pouvait être programmé au moyen de l’algorithme SyncAV (c.-à-d. en rythme sinusal avec conduction auriculoventriculaire intrinsèque). Une échocardiographie a été réalisée avant l’optimisation du DRC, puis 6 mois après.
Résultats
Sur les 64 patients consécutifs potentiellement admissibles qui ont fait l’objet d’une évaluation, 34 sujets dont le DRC pouvait être programmé à l’aide de l’algorithme SyncAV ont été retenus. Les sujets avaient en moyenne 74 ± 9 ans; 41 % d’entre eux étaient des femmes, et 59 % présentaient une cardiomyopathie ischémique. Le temps écoulé entre l’implantation du DRC et l’optimisation au moyen de l’algorithme SyncAV était en moyenne de 17,8 ± 8,5 mois. Au moment du suivi à 6 mois, l’optimisation au moyen de l’algorithme SyncAV a été associée à une augmentation significative de la fraction d’éjection ventriculaire gauche (FEVG) (FEVG moyenne de 36,5 % ± 13,3 % vs 30,9 % ± 13,3 %; p < 0,001) et à une réduction du volume télésystolique ventriculaire gauche (VTSVG) (VTSVG moyen de 110,5 ± 57,5 mL vs 89,6 ± 52,4 mL; p < 0,001) comparativement à la programmation initiale du DRC.
Conclusion
La reprogrammation du DRC afin de maximiser la stimulation biventriculaire par fusion a considérablement augmenté la FEVG et réduit le VTSVG chez les patients porteurs d’un DRC permanent. D’autres études sont nécessaires pour déterminer si un algorithme de stimulation par fusion en continu permet d’améliorer les résultats cliniques à long terme et pour établir le profil des patients les plus susceptibles de bénéficier d’une telle intervention.
Cardiac resynchronization therapy (CRT) decreases cardiovascular mortality and symptoms in patients with heart failure.1 However, a lack of response to CRT remains its greatest challenge.2 The ideal method to optimize CRT postimplantation is controversial. Echocardiography has been used for CRT optimization.3 However, routine CRT settings are used for the majority of CRT implants because of the complex and time-consuming nature of echocardiographic optimization.4 In an international survey, 58% of electrophysiologists did not optimize atrioventricular (AV) and ventriculo-ventricular delays.5 Therefore, using the electrocardiogram (ECG) would be an inexpensive and practical process for CRT optimization.4 Narrowing of the QRS complex with biventricular pacing (the paced QRS duration [QRSd]) has been shown to correlate with clinical and echocardiographic improvement,6,7 as well as long-term mortality.8 One study has also reported that ECG-based optimization using the measurement of the narrowest QRS is comparable to echocardiography-based optimization with regard to left ventricle (LV) reverse remodeling.9
SyncAV is a device-based algorithm that is available in some CRT devices manufactured by Abbott (Chicago, IL). The algorithm alters the AV delay to allow biventricular pacing synchronized with intrinsic AV conduction.10 To achieve fusion between intrinsic conduction and biventricular pacing, the device continuously adjusts the AV delay by a set duration (programmable offset between 10 and 120 ms) relative to the measured intrinsic AV conduction interval. This process is dynamic and adjusts according to variations in device-measured intrinsic conduction time, thereby resulting in continuously adapting fusion pacing. Fusion pacing can also be achieved by fusion of LV pacing and intrinsic conduction.11 The concept of fusion optimized interval was previously described by Arbelo et al.,12 who demonstrated a reduction in QRS duration and an acute improvement in hemodynamics compared with nominal CRT programing. The same finding was also described by Varma et al.10 during a de novo implant prospective study using the specific SyncAV algorithm described earlier. Recent published data reported a significant QRS narrowing with programming of SyncAV in existing CRT devices as determined acutely by 12-lead ECG, but did not report longer-term outcomes.13,14 The pacing configuration that achieved the narrowest QRS with SyncAV was biventricular pacing with SyncAV and an optimized offset.10,14
Whether reprogramming of CRT to increase electrical synchrony translates into echocardiographic and functional status improvement remains unclear. We aimed to assess whether reprogramming with SyncAV is associated with an increase in left ventricular ejection fraction (LVEF) and a decrease in left ventricular end-systolic volume (LVESV) compared with routine CRT programming in patients with chronic CRT devices.
Methods
Study population
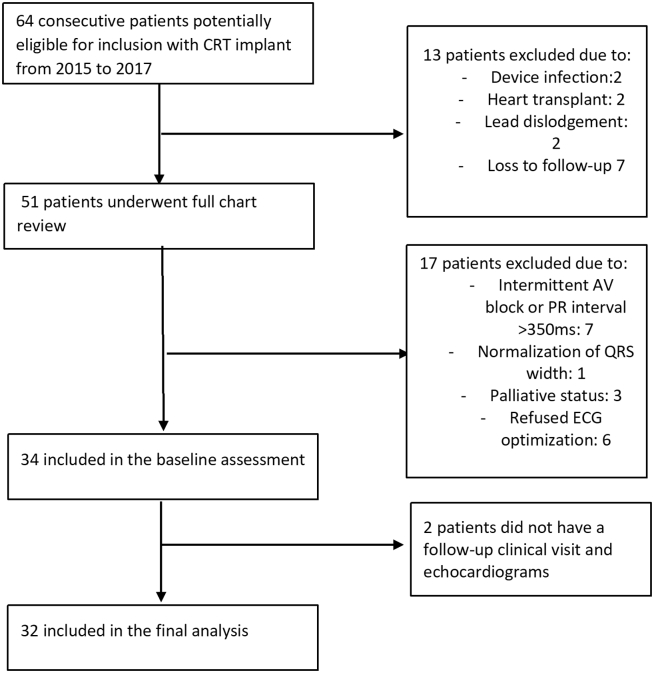
Patients at a single quaternary cardiac center (McGill University Health Center, Montreal, Canada) with a CRT defibrillator or a CRT pacemaker with a programmable SyncAV algorithm (St. Jude Unify Assura and Quadra Assura, or St. Jude Allure Quadra RF; St. Jude Medical, Saint Paul, MN) implanted between January 2014 and November 2017 were evaluated for SyncAV optimization starting in May 2018. The flow diagram for patient selection is shown in Figure 1. Of the 64 potentially eligible consecutive CRT implants, we excluded patients with device explant, lead dislodgement, loss to follow-up, loss of required AV conduction (preventing use of SyncAV algorithm), transition to palliation, or refusal of optimization. The remaining 34 patients had ECG-based optimization performed and were included in this analysis. This study was approved by the McGill University Health Center Institutional Review Board, and patients included in the study fulfilled criteria for CRT implantation as per Canadian Cardiovascular Society guideline recommendations.15
Figure 1.
Flow diagram for patient selection. Modified from AlTurki et al.14 with permission from Elsevier.
Device programming
Details for ECG-based SyncAV optimization were as previously described.14 In brief, devices in all patients at our center were programmed according to operator preference (without use of SyncAV) until December 2017 when ECG-based CRT optimization became the standard of care for newly implanted devices. Routine in-clinic CRT optimization was performed starting May 2018 for patients with chronically implanted devices according to our protocol, including sequential ECGs.14 When programming with SyncAV, the optimal offset achieving the narrowest QRS was used.
Standard programming before the SyncAV optimization involved programming as set by the treating physician according to his/her standard clinical practice; there was no mandated programming protocol. This programming may have been nominal settings or settings selected by the treating physician (considering baseline ECG and postoperative paced ECG to guide programming). None of the devices in the patients were previously programmed using the SyncAV algorithm.
The SyncAV algorithm has been described.13,14 In brief, the SyncAV algorithm periodically extends the AV delay. When intrinsic ventricular events are sensed, the device reprograms the AV delay to a programmed shorter offset (default offset −50 ms) than the measured intrinsic AV interval. The offset can be programmed over a range of values to find the ideal offset that achieves electrical synchrony for each patient. The device was then programmed at that “ideal” offset for each patient.
Electrocardiographic measurements
Standard 12-lead electrocardiography was performed at a paper speed of 25 mm/s and a scale of 10 mm/mV, and QRS duration was measured automatically by the ECG machine (GE MAC 5500 HD Resting ECG System, Boston, MA) as previously described.14 The ECG machine is programmed to measure the earliest onset of the QRS and the latest offset; this translates into the duration from the pacemaker spike until the end of the QRS. QRS duration was subsequently validated manually by a single investigator who was blinded to the clinical data and pacing programming.
Echocardiographic and clinical outcomes
At the baseline visit during which SyncAV programming was activated, all patients had a clinical assessment, including determination of New York Heart Association (NYHA) functional class and a transthoracic echocardiogram. All patients were scheduled for a clinical follow-up and a transthoracic echocardiogram 6 months postoptimization. NYHA functional class, LVEF, LVESV, and mitral regurgitation (MR) severity as assessed on a grade scale (0 = none or trivial, 1 = mild, 2 = moderate, 3 = moderate to severe, and 4 = severe)16 were recorded. Other echocardiographic measurements included left ventricular end-diastolic volume (LVEDV) and pulmonary artery systolic pressure (PASP). LVESV and LVEDV were measured in the apical 4-chamber and apical 2-chamber views and then averaged; LVEF was calculated using Simpson’s biplane method. The echocardiograms were read by level III trained echocardiographers who were unaware of device programming.
We defined a positive LVEF response to CRT as an absolute increase in LVEF ≥ 10%; we find this value to be of clinical relevance. Previous studies have used cutoffs ranging from 5% to 15%.17 LVEF response was assessed ≥ 6 months after the initial implant procedure (compared with LVEF before CRT implant), and LVEF response was subsequently reassessed 6 months after SyncAV ECG optimization (compared with LVEF immediately before SyncAV ECG optimization). In addition, response as measured by a ≥ 15% decrease in LVESV was also assessed 6 months after SyncAV ECG optimization compared with LVESV before SyncAV ECG optimization; the LVESV response after initial CRT could not be assessed because of the absence of data regarding LVESV before initial CRT.
Statistical analysis
All data are presented as mean ± standard deviation for continuous variables and as proportions for categorical variables. A paired t test was used to compare outcomes before and 6 months after SyncAV optimization. A P value of < 0.05 was considered statistically significant. Statistical analysis was performed using StatsDirect 3 (StatsDirect Ltd., 2013, Birkenhead, England).
Results
Patient characteristics
Patient characteristics at the time of SyncAV optimization are summarized in Table 1. At 6 months of follow-up, 94% of patients had complete clinical and echocardiographic data. Their mean age was 74 ± 9 years, 41% were female, and 59% had ischemic cardiomyopathy. The mean time from CRT implant to SyncAV optimization was 17.8 ± 8.5 months. At the time of SyncAV optimization, the mean intrinsic conduction QRSd was 163 ± 24 ms, the mean existing CRT pacing QRSd was 152 ± 25 ms, and the SyncAV optimized mean QRSd was 138 ± 23 ms.
Table 1.
Baseline patient characteristics
| Characteristic | All patients N = 34 |
Initial CRT responders N = 15 |
Initial CRT nonresponders N = 19 |
|---|---|---|---|
| Male, n (%) | 19 (56) | 7 (50) | 12 (63) |
| Age, y (range) | 74 (60-93) | 74 (60-89) | 75 (63-93) |
| Time since implant in mo, mean (range) | 17.8 ± 8.5 | 16.5 ± 9.3 | 17.8 ± 7.2 |
| Ischemic cardiomyopathy, n (%) | 21 (62) | 6 (40) | 15 (79) |
| Hypertension | 28 (82) | 14 (93) | 14 (78) |
| Diabetes mellitus | 8 (24) | 4 (27) | 4 (21) |
| Paroxysmal atrial fibrillation | 10 (29) | 3 (20) | 7 (37) |
| Left bundle branch block | 31 (91)∗ | 15 (100) | 16 (84) |
| CRT defibrillator | 22 (65) | 10 (67) | 12 (63) |
| NYHA, n (%)† | |||
| I | 4 (11.8) | 1 (7) | 3 (16) |
| II | 24 (70.6) | 14 (93) | 10 (53) |
| III | 6 (17.6) | 0 (0) | 6 (31) |
| QRSd (ms) | 163.5 ± 24.3 | 168.1 ± 17.3 | 158.9 ± 29.1 |
| Intrinsic PR interval (ms) | 187.2 ± 36.6 | 184.9 ± 21.1 | 187.3 ± 46.5 |
| LVEF (%) before initial CRT | 24.1 ± 10.1 | 24.9 ± 9.4 | 23.7 ± 10.5 |
| LVEF (%) before SyncAV | 30.9 ± 13.3 | 41.1 ± 9.6 | 23.4 ± 10.6 |
| LVEDV (mL)† | 157.5 ± 56.6 | 133.4 ± 43.5 | 174.1 ± 59.7 |
| LVESV (mL)† | 110.5 ± 57.5 | 75.6 ± 31.8 | 134.5 ± 59.7 |
| Left atrial diameter (cm) | 43.3 ± 7.0 | 42.5 ± 6.6 | 43.9 ± 7.5 |
| Medical therapy for heart failure | |||
| ACEI/ARB | 28 (82) | 12 (80) | 16 (84) |
| β-Blocker | 28 (82) | 12 (80) | 16 (84) |
| MRA | 5 (15) | 2 (13) | 3 (16) |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CRT, cardiac resynchronization therapy; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; MRA, magnetic resonance angiography; NYHA, New York Heart Association; QRSd, QRS duration.
Modified from AlTurki et al.14 with permission from Elsevier.
The remaining 3 were bifascicular block (right bundle branch block and left anterior fascicular block or left posterior fascicular block).
Before SyncAV ECG optimization.
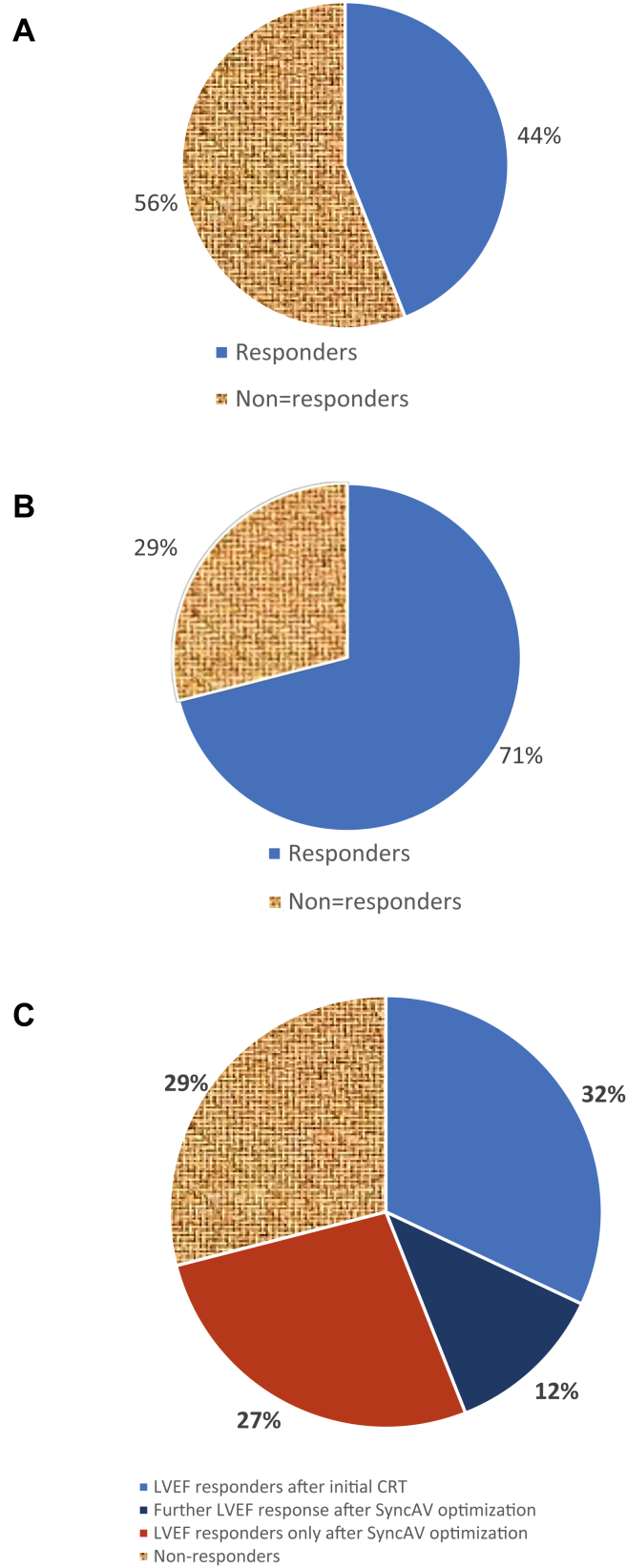
In terms of response to CRT, the mean LVEF was 24.1 ± 10.1 before initial CRT implantation, and with standard CRT programming, 44% of patients had had a significant improvement in LVEF (LVEF responders ≥ 10%), whereas the remaining 56% had not improved their LVEF ≥ 10% and were deemed CRT LVEF nonresponders.
Left ventricular ejection fraction
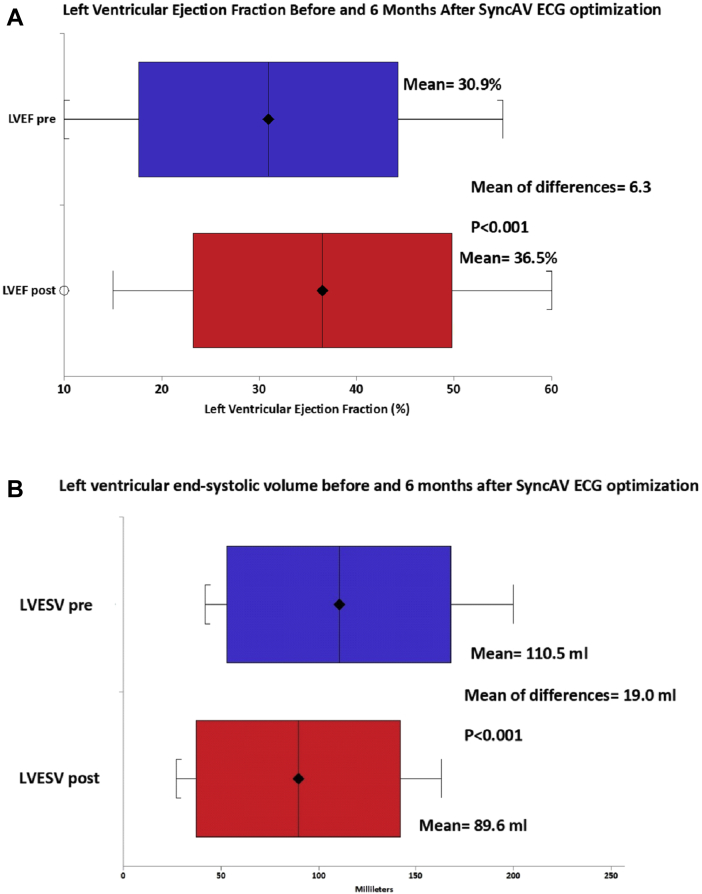
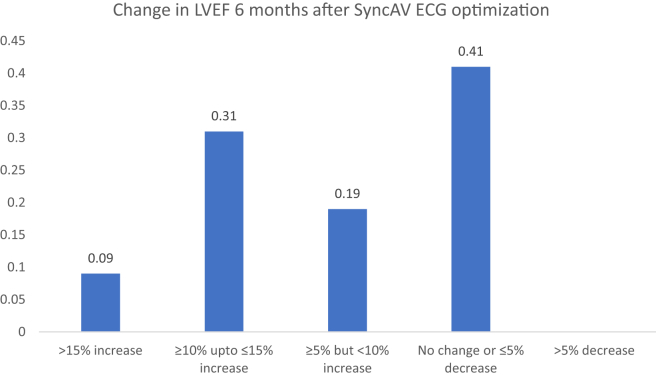
The mean LVEF before SyncAV optimization was 30.9% ± 13.3% (median, 27.5%; interquartile range, 20%-40%) and after 6 months increased to 36.5% ± 13.3% (median, 40%; interquartile range, 25%-50%). The mean difference in LVEF was 6.3%, 95% confidence interval (CI), 3.1%-9.5%, P < 0.001 (Fig. 2A). Of the 32 patients with follow-up LVEF, 40% had an increase ≥ 10%, including 9% who had an increase > 15%. In addition, a further 19% of patients had an increase of ≥ 5% but not reaching 10%, and the remaining (41%) had no significant change in LVEF (Fig. 3). Of those who had not responded to initial CRT (19, 56%), 9 patients (47%) had a significant improvement in LVEF (LVEF responders, ≥10%), and 10 patients (53%) remained nonresponders after SyncAV optimization (Fig. 4). This increased the proportion of total LVEF responders to 71%. In contrast, 33% of those who had already responded to initial CRT had a significant further improvement in LVEF (≥10%). No patient had a significant reduction in LVEF (>5%).
Figure 2.
Change in (A) left ventricular ejection fraction (LVEF) and (B) left ventricular end-systolic volume (LVESV) before and 6 months after SyncAV electrocardiogram (ECG) optimization.
Figure 3.
Patients stratified by the change in LVEF at 6-month follow-up compared with baseline.
Figure 4.
Proportion of patients who responded to cardiac resynchronization therapy (CRT) as defined by an LVEF increase of ≥ 10. (A) After initial CRT. (B) After SyncAV. (C) After SyncAV stratified by initial response to CRT.
In patients who responded to initial CRT, mean LVEF increased from 41.1% ± 9.6% to 45.4% ± 8.2% (P = 0.01) after SyncAV ECG optimization. In patients who did not respond to initial CRT, mean LVEF increased from 23.4% ± 10.6% to 31.4% ± 13.3% (P < 0.001) after SyncAV ECG optimization (Supplemental Fig. S1). There was no difference in change in LVEF after SyncAV optimization between those who had initially responded and those who had not (P = 0.24).
Left ventricular end-systolic volume
Mean LVESV before SyncAV optimization was 110.5 ± 57.5 mL and after 6 months decreased to 89.6 ± 52.4 mL; the mean difference in LVESV was −19.0 mL, 95% CI, −8.3 to −29.6, P < 0.001 (Fig. 2B). After SyncAV ECG optimization, 17 patients (53%) had a significant decrease ≥ 15% in LVESV. Of these patients, 7 (41%) were already LVEF responders after initial CRT and 10 (59%) did not have an LVEF response after initial CRT.
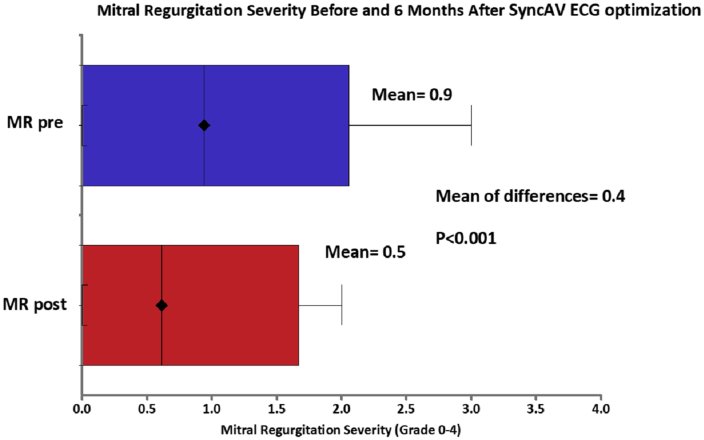
Mitral regurgitation
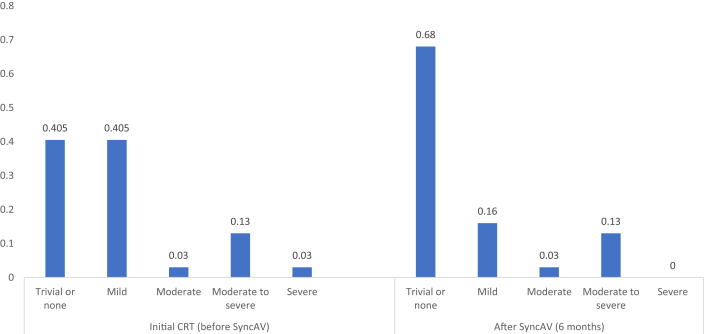
After optimization with SyncAV, there was a significant reduction in the severity of MR (mean MR grade 0.9 ± 1.0 before SyncAV vs 0.5 ± 1.0 after SyncAv optimization; P < 0.001) (Fig. 5). Before SyncAV optimization, 41% of patients had no or trivial MR, 41% had mild MR, 16% had moderate to severe MR, and 3% had severe MR. At 6 months of follow-up after SyncAV optimization, 68% had no or trivial MR, 16% had mild MR, 16% had moderate to severe MR, and none had severe MR. The distribution of MR severity is shown in Figure 6.
Figure 5.
Change in mitral regurgitation (MR) severity before and 6 months after SyncAV ECG optimization.
Figure 6.
Proportion of patients with various MR severity grades after initial CRT (before SyncAV optimization) and after SyncAV optimization.
Other echocardiographic measurements
Mean LVEDV before SyncAV optimization was 157.5 ± 56.6 mL and after 6 months decreased to 141.3 ± 55.7 mL; the mean difference in LVEDV was −14.1 mL, 95% CI, −3.1. to −25.2, P = 0.007. PASP also decreased after SyncAV ECG optimization. Mean PASP was 37.5 ± 14.7 mm Hg before SyncAV optimization and decreased to 32.9 mm Hg ± 10.3 at 6 months after optimization (mean difference −4.2 mm Hg, 95% CI, −0.3 to −8.1, P = 0.04).
NYHA and medication use
No significant difference in NYHA functional class was observed after SyncAV optimization (mean NYHA 2.1 ± 0.5 before SyncAV vs 2.0 ± 0.5 after SyncAV optimization; P = 0.16). The distribution of NYHA functional class is summarized in Supplemental Figure S2. There was no significant difference in the use of heart failure medication (Supplemental Table S1).
Discussion
The main finding of this analysis is that in patients with chronically implanted CRT devices, optimization using a biventricular fusion-pacing algorithm to achieve further reduction in QRSd was associated with a significant increase in LVEF at 6 months after optimization. To the best of our knowledge, this is the first study to demonstrate that an increase in electrical synchrony is associated with echocardiographic improvement in patients chronically implanted with CRTs, irrespective of previous responder status, using an easy, quick, and reproducible ECG-based optimization that can be performed during a regular device clinic follow-up visit. Echocardiographic response to CRT has been assessed using cutoffs of an increase in LVEF ≥ 5% and a decrease in LVESV ≥ 10%.18,19 A combination of an LVEF improvement ≥ 5% and LVESV reduction ≥ 10% was shown to be the best predictor for improved survival.20 In this analysis, stricter cutoffs of an increase in LVEF ≥ 10% and a decrease in LVESV ≥ 15% were used to provide a more specific indicator of CRT response.
Another important finding is the high proportion of patients (44%) classified as nonresponders after initial CRT therapy who subsequently had a significant improvement in LVEF (at least 10% absolute LVEF increase) after optimization using SyncAV. In addition to conversion of nonresponders to responders, QRS narrowing and a further increase in LVEF (at least 10%) were also seen in 1 in every 3 patients who already responded to initial CRT therapy, demonstrating a further improvement in electrical and mechanical synchrony. None of the patients had QRS widening or worsening in the LVEF or MR at 6 months after continuous CRT optimization using the SyncAV algorithm. Trucco et al.21 showed that baseline manual optimization of the AV and ventriculo-ventricular delays, to achieve biventricular fusion pacing, immediately postimplantation leads to a greater proportion of patients achieving both electrical synchrony and LV reverse remodelling at 12 months. Our study validates the long-term effect of an automated continuously optimized biventricular fusion-pacing algorithm.
Unfortunately, CRT device optimization is not routinely performed as revealed in the international survey by Gras et al.5 Approximately 58% of electrophysiologists do not optimize CRT postimplantation and just used the nominal settings.4 Part of this issue is probably related to the time-consuming and complex nature of echocardiographic and intrinsic electrogram-based optimization.4 In addition, multiple studies have shown a lack of benefit of these approaches compared with routine out-of-the-box settings.22 Even in studies that used an ECG-based optimization, the ECG analyses were performed with a paper speed between 50 and 300 mm/s, used computerized recording systems, and required experienced observers for QRS width measurement.12,21 In contrast, we used the standard 12-lead surface ECG at a regular speed of 25 mm/s with automated measurements, which are faster, accurate, and easily reproducible.
Our study was unable to demonstrate a significant improvement in NYHA functional class status, although no patient had a worsening of functional status. The QRS narrowing observed during our analysis (152 ± 25 ms during the baseline evaluation to 138 ± 23 ms after optimization) was similar to that observed in other studies that assessed fusion pacing.4,7 Such a reduction in QRSd has been shown to correlate with clinical outcomes. In a meta-analysis, Korantzopoulos et al.7 showed that QRS narrowing is a strong predictor of clinical and echocardiographic response (or super response) to CRT. LV fusion pacing has been tested using the AdaptiveCRT algorithm, which periodically assesses intrinsic conduction; during normal AV conduction, only LV pacing is provided while biventricular pacing with adjustments of the ventriculo-ventricular timing occurs during prolonged AV conduction.23,24 Adaptive CRT has been shown to be noninferior to nominal CRT with suggestion of improvements in clinical status, echocardiographic parameters, and clinical outcomes, and a reduction in the incidence of atrial fibrillation particularly in patients with normal AV conduction.23, 24, 26, 25 A large prospective, randomized, controlled, multicentre, clinical trial is under way to assess the impact of AdaptiveCRT on cardiovascular outcomes.27
Identifying predictors of nonresponse to CRT remains a great challenge. Despite important advances to improve patient selection based on clinical characteristics, QRS duration, and QRS morphology, the frequency of nonresponse to CRT continues to be a major issue.2,28 Our results suggest that in a considerable proportion of these patients, if sinus rhythm with intrinsic AV conduction is present, a fusion pacing algorithm can improve electrical and mechanical synchrony.
Limitations
This is a single-center study with a limited sample size and 6 months of follow-up after SyncAV optimization. However, significant improvements in LVEF were demonstrable and correlated with QRS narrowing. It is noteworthy that clinical improvement after CRT usually coincides with electrical synchrony and LV reverse remodelling and an increase in LVEF.7,29,30 Furthermore, previous data indicate that patients who respond to CRT in the first 6 months are likely to have further improvement in LVEF at the 1- and 2-year marks.31 Although the trajectory for LVEF after initial CRT cannot be definitively ascertained, most studies assess CRT response at 6 months or 12 months, and the majority of responders usually show improvement at 6 months.18,19,32 In this study, the mean time from implantation of CRT to initial programming of SyncAV was 17.8 ± 8.5 months, and the results of this study assess echocardiographic parameters before and 6 months after SyncAV optimization. In addition, we used stricter cutoffs for LVEF and LVESV to increase the robustness of our results. The study is well powered for the detection of changes in QRSd and LVEF but not for clinical outcomes. Whether the improvement in LVEF translates into better clinical outcomes will require larger randomized studies with longer-term follow-up. A randomized trial of approximately 200 patients is currently under way and will provide the needed insight (NCT03961399). In addition, larger studies will be needed to identify predictors of response to SyncAV optimized pacing. Finally, the determination of response to CRT was based on LVEF and not LVESV because of lack of LVESV data before initial CRT. However, although LVESV is a sensitive marker for LV reverse remodelling, an LVEF increase ≥ 10% is likely to reflect a more clinically meaningful echocardiographic improvement and if anything may underestimate the response to SyncAV. In addition, we have provided the LVESV data before and after SyncAV optimization. The time from initial CRT implant to pre-SyncAV echo varied in each patient, but the time before and after SyncAV was similar at approximately 6 months.
Conclusion
ECG-based CRT optimization using an algorithm to achieve biventricular pacing fused with intrinsic conduction significantly improved electrical synchrony and LVEF in chronically CRT-paced patients. Improved ventricular function at 6 months after CRT optimization was independent of prior response to conventional CRT. This clinic-based method was a simple, safe, and effective means to optimize previously implanted CRT devices. Larger randomized studies are required to compare long-term clinical outcomes between dynamically optimized biventricular fusion pacing and traditional biventricular CRT pacing to inform whether chronic CRT devices with this algorithm in these patients should be reprogrammed.
Funding Sources
Dr Essebag is the recipient of a Clinical Research Scholar Award from the Fonds de recherche du Québec-Santé.
Disclosures
Dr Essebag has received honoraria from Abbott, Biosense Medical, Boston Scientific, and Medtronic.
Footnotes
Ethics Statement: This study was approved by the McGill University Health Center Institutional Review Board.
See page 69 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2019.12.005.
Supplementary Material
References
- 1.Cleland J.G., Abraham W.T., Linde C. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J. 2013;34:3547–3556. doi: 10.1093/eurheartj/eht290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daubert C., Behar N., Martins R.P. Avoiding non-responders to cardiac resynchronization therapy: a practical guide. Eur Heart J. 2017;38:1463–1472. doi: 10.1093/eurheartj/ehw270. [DOI] [PubMed] [Google Scholar]
- 3.Naqvi T.Z. Echocardiography-guided biventricular pacemaker optimization. JACC Cardiovasc Imaging. 2010;3:1168–1180. doi: 10.1016/j.jcmg.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Pujol-Lopez M., San Antonio R., Mont L. Electrocardiographic optimization techniques in resynchronization therapy. Europace. 2019;21:1286–1296. doi: 10.1093/europace/euz126. [DOI] [PubMed] [Google Scholar]
- 5.Gras D., Gupta M.S., Boulogne E. Optimization of AV and VV delays in the real-world CRT patient population: an international survey on current clinical practice. Pacing Clin Electrophysiol. 2009;32(Suppl 1):S236–S239. doi: 10.1111/j.1540-8159.2008.02294.x. [DOI] [PubMed] [Google Scholar]
- 6.Hadjis A., AlTurki A., Proietti R. Predicting response to cardiac resynchronization therapy: use of strict left bundle branch block criteria. Pacing Clin Electrophysiol. 2019;42:431–438. doi: 10.1111/pace.13638. [DOI] [PubMed] [Google Scholar]
- 7.Korantzopoulos P., Zhang Z., Li G. Meta-analysis of the usefulness of change in QRS width to predict response to cardiac resynchronization therapy. Am J Cardiol. 2016;118:1368–1373. doi: 10.1016/j.amjcard.2016.07.070. [DOI] [PubMed] [Google Scholar]
- 8.Jastrzębski M., Baranchuk A., Fijorek K. Cardiac resynchronization therapy-induced acute shortening of QRS duration predicts long-term mortality only in patients with left bundle branch block. Europace. 2018;21:281–289. doi: 10.1093/europace/euy254. [DOI] [PubMed] [Google Scholar]
- 9.Tamborero D., Vidal B., Tolosana J.M. Electrocardiographic versus echocardiographic optimization of the interventricular pacing delay in patients undergoing cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2011;22:1129–1134. doi: 10.1111/j.1540-8167.2011.02085.x. [DOI] [PubMed] [Google Scholar]
- 10.Varma N., O'Donnell D., Bassiouny M. Programming cardiac resynchronization therapy for electrical synchrony: reaching beyond left bundle branch block and left ventricular activation delay. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birnie D., Lemke B., Aonuma K. Clinical outcomes with synchronized left ventricular pacing: analysis of the adaptive CRT trial. Heart Rhythm. 2013;10:1368–1374. doi: 10.1016/j.hrthm.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Arbelo E., Tolosana J.M., Trucco E. Fusion-optimized intervals (FOI): a new method to achieve the narrowest QRS for optimization of the AV and VV intervals in patients undergoing cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2014;25:283–292. doi: 10.1111/jce.12322. [DOI] [PubMed] [Google Scholar]
- 13.Thibault B., Ritter P., Bode K. Dynamic programming of atrioventricular delay improves electrical synchrony in a multicenter cardiac resynchronization therapy study. Heart Rhythm. 2019;16:1047–1056. doi: 10.1016/j.hrthm.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 14.AlTurki A., Lima P.Y., Garcia D. Cardiac resynchronization therapy reprogramming to improve electrical synchrony in patients with existing devices. J Electrocardiol. 2019;56:94–99. doi: 10.1016/j.jelectrocard.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Parkash R., Philippon F., Shanks M. Canadian Cardiovascular Society Guidelines on the Use of cardiac resynchronization therapy: implementation. Can J Cardiol. 2013;29:1346–1360. doi: 10.1016/j.cjca.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Rokey R., Sterling L.L., Zoghbi W.A. Determination of regurgitant fraction in isolated mitral or aortic regurgitation by pulsed Doppler two-dimensional echocardiography. J Am Coll Cardiol. 1986;7:1273–1278. doi: 10.1016/s0735-1097(86)80146-2. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka H., Hara H., Saba S. Prediction of response to cardiac resynchronization therapy by speckle tracking echocardiography using different software approaches. J Am Soc Echocardiogr. 2009;22:677–684. doi: 10.1016/j.echo.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Chung E.S., Leon A.R., Tavazzi L. Results of the Predictors of Response to CRT (PROSPECT) Trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 19.Friedman D.J., Upadhyay G.A., Rajabali A. Progressive ventricular dysfunction among nonresponders to cardiac resynchronization therapy: baseline predictors and associated clinical outcomes. Heart Rhythm. 2014;11:1991–1998. doi: 10.1016/j.hrthm.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Rickard J., Baranowski B., Wilson Tang W.H. Echocardiographic predictors of long-term survival in patients undergoing cardiac resynchronization therapy: what is the optimal metric? J Cardiovasc Electrophysiol. 2017;28:410–415. doi: 10.1111/jce.13175. [DOI] [PubMed] [Google Scholar]
- 21.Trucco E., Tolosana J.M., Arbelo E. Improvement of reverse remodeling using electrocardiogram fusion-optimized intervals in cardiac resynchronization therapy: a randomized study. JACC Clin Electrophysiol. 2018;4:181–189. doi: 10.1016/j.jacep.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Auger D., Hoke U., Bax J.J. Effect of atrioventricular and ventriculoventricular delay optimization on clinical and echocardiographic outcomes of patients treated with cardiac resynchronization therapy: a meta-analysis. Am Heart J. 2013;166:20–29. doi: 10.1016/j.ahj.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Birnie D., Hudnall H., Lemke B. Continuous optimization of cardiac resynchronization therapy reduces atrial fibrillation in heart failure patients: results of the Adaptive Cardiac Resynchronization Therapy Trial. Heart Rhythm. 2017;14:1820–1825. doi: 10.1016/j.hrthm.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Krum H., Lemke B., Birnie D. A novel algorithm for individualized cardiac resynchronization therapy: rationale and design of the adaptive cardiac resynchronization therapy trial. Am Heart J. 2012;163:747–52 e741. doi: 10.1016/j.ahj.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Gasparini M., Birnie D., Lemke B. Adaptive cardiac resynchronization therapy reduces atrial fibrillation incidence in heart failure patients with prolonged AV conduction. Circ Arrhythm Electrophysiol. 2019;12:e007260. doi: 10.1161/CIRCEP.119.007260. [DOI] [PubMed] [Google Scholar]
- 26.Singh J.P., Abraham W.T., Chung E.S. Clinical response with adaptive CRT algorithm compared with CRT with echocardiography-optimized atrioventricular delay: a retrospective analysis of multicentre trials. Europace. 2013;15:1622–1628. doi: 10.1093/europace/eut107. [DOI] [PubMed] [Google Scholar]
- 27.Filippatos G., Birnie D., Gold M.R. Rationale and design of the AdaptResponse trial: a prospective randomized study of cardiac resynchronization therapy with preferential adaptive left ventricular-only pacing. Eur J Heart Fail. 2017;19:950–957. doi: 10.1002/ejhf.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birnie D.H., Tang A.S. The problem of non-response to cardiac resynchronization therapy. Curr Opin Cardiol. 2006;21:20–26. doi: 10.1097/01.hco.0000198983.93755.99. [DOI] [PubMed] [Google Scholar]
- 29.Foley P.W.X., Leyva F., Frenneaux M.P. What is treatment success in cardiac resynchronization therapy? Europace. 2009;11(Suppl 5):v58–v65. doi: 10.1093/europace/eup308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ypenburg C., van Bommel R.J., Borleffs C.J.W. Long-term prognosis after cardiac resynchronization therapy is related to the extent of left ventricular reverse remodeling at midterm follow-up. J Am Coll Cardiol. 2009;53:483–490. doi: 10.1016/j.jacc.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 31.Burns K.V., Gage R.M., Curtin A.E. Long-term echocardiographic response to cardiac resynchronization therapy in initial nonresponders. JACC Heart Fail. 2015;3:990–997. doi: 10.1016/j.jchf.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Varma N., Boehmer J., Bhargava K. Evaluation, management, and outcomes of patients poorly responsive to cardiac resynchronization device therapy. J Am Coll Cardiol. 2019;74:2588–2603. doi: 10.1016/j.jacc.2019.09.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.