Abstract
The majority of variation in six traits critical to the growth, survival and reproduction of plant species is thought to be organised along just two dimensions, corresponding to strategies of plant size and resource acquisition. However, it is unknown whether global plant trait relationships extend to climatic extremes, and if these interspecific relationships are confounded by trait variation within species. We test whether trait relationships extend to the cold extremes of life on Earth using the largest database of tundra plant traits yet compiled. We show that tundra plants demonstrate remarkably similar resource economic traits, but not size traits, compared to global distributions, and exhibit the same two dimensions of trait variation. Three quarters of trait variation occurs among species, mirroring global estimates of interspecific trait variation. Plant trait relationships are thus generalizable to the edge of global trait-space, informing prediction of plant community change in a warming world.
Subject terms: Biogeography, Macroecology, Theoretical ecology, Ecology
It is unclear whether plant trait relationships found at the global scale extend to climatic extremes. Here the authors analyse six major aboveground traits to show that known plant trait relationships extend to the tundra biomes and exhibit the same two dimensions of variation detected at the global scale.
Introduction
Despite the vast diversity of life on Earth, vascular plants are limited by trade-offs in leaf1, wood2, seed3 and root4 traits, enabling the characteristics of global plant species to be organised along a few general dimensions5–8. Two dimensions, plant size (large and woody vs. small and non-woody) and resource economics (acquisitive vs. conservative), have been shown to describe the majority of variation in six widely-sampled plant traits, which together represent key differences in plant form and function8. Such trait relationships predict community assembly9,10 and ecosystem functions11,12 across biogeographic gradients13 and in response to environmental change12,14. However, our current understanding of trait relationships has largely been formulated using tropical and temperate data, which comprise over 90% of global trait observations15 (Fig. 1). Although some site-specific studies exist16,17, whole-plant trait relationships have not been widely tested at the environmental extremes of plant life such as the cold tundra biome, where plants could exhibit rare or unique trait relationships resulting from adaptation to extreme environmental conditions18,19.
Fig. 1. Tundra trait data within geographical and climate space.
a Map of trait observation sites for six plant traits, indicating global trait observations in TRY (grey points), tundra species observations in TRY (orange points) and TTT data (purple points). b Location of trait collection sites in climate space for all available plant species (grey) and tundra species (blue). Major biomes are mapped onto climate space (T-Tundra; B-Boreal Forest; TG-Temperate Grassland; TeF-Temperate Deciduous Forest; TeRF-Temperate Rain Forest; TrF-Tropical Deciduous Forest; TrRF-Tropical Rain Forest; Sa-Savanna; D–Desert)146. c Number of trait observations (upper panel) and species (lower panel) for all available plant species (grey) and tundra species (blue), by latitude. Dotted curves indicate global distributions with the inclusion of TTT collected data.
Our current understanding of global trait relationships is also based on the assumption that the majority of trait variation occurs among species20. However, trait variation within communities is ultimately driven by differences among individuals, rather than species21. Large within-species trait variation could thus obscure or alter interspecific trait relationships22–24, restricting their potential for ecological prediction across scales and among biomes. Within-species variation accounts for approximately 25% of trait variation at the global scale21, but has been hypothesised to be greater in extreme environments due to environmental filtering of trait expression25, at local geographical scales where species richness is low26,27, and for species that span large biogeographical gradients21 due to wide niche breadth. The tundra biome thus provides an optimal system to test our current understanding of trait variation within plant communities due to a small species pool28, large species ranges13, and extreme environmental conditions29.
In this study, we test whether our existing understanding of plant trait relationships extends to the tundra biome. We establish the largest database of Arctic and alpine tundra plant traits ever compiled by combining 20,991 records from the TRY database15 with 30,616 records from the Tundra Trait Team (TTT)30, representing 89% of the tundra species pool. We select six globally well-sampled plant traits: adult plant height, leaf area, seed mass, leaf mass per area (LMA), leaf nitrogen, and leaf dry matter content (LDMC) (Supplementary Table 1). These traits underpin two important dimensions of global trait space8, and link to ecosystem functions including primary productivity7, carbon storage31, and nutrient cycling12. We test three hypotheses: (1) Trait expression among tundra species will be constrained relative to global trait space due to extreme environmental conditions, yet will exhibit the same two dimensions of plant form and function. (2) The contribution of within-species trait variation to total trait variation in the tundra will be greater than the global average of 25%21. (3) The contribution of within-species trait variation to total trait variation will be greater at local rather than at larger geographical scales. We show that tundra plants exhibit constrained size traits, but not resource traits, relative to global species. However, tundra plants exhibit the same two dimensions of trait variation, indicating that plant trait relationships are generalizable to cold extremes of life on Earth. We also show that differences among species comprise the majority of trait variation, but that trait variation within species becomes increasingly important at small geographical scales.
Results and discussion
Trait expression is constrained in the tundra
We found that tundra species occupied a constrained subset of global trait space for size-related traits but not resource economic traits (Fig. 2a, c, Supplementary Fig. 1). Many tundra species, such as the prostrate, small-leaved, and wind-dispersed evergreen shrub Cassiope hypnoides, were located at the very edge of global trait space, consistent with adaptation to extreme environmental conditions in the tundra32. Given that tundra plant communities are found above treeline, and therefore by definition exclude tree species, we expected to see reduced plant height among tundra species compared to global species. However, we found that lower plant height corresponded with smaller leaf area and seed mass (Fig. 2a, axis 1, Supplementary Fig. 1), as would be predicted from global trait relationships5. In contrast, traits associated with resource economics occupied almost the full global range (Fig. 2a, axis 2), with both highly acquisitive species such as chickweed (Stellaria media), and highly conservative species such as crowberry (Empetrum nigrum) present at tundra sites. Species with faster resource-related traits and larger size-related traits were associated with warmer environments within the tundra (Fig. 2b, Supplementary Fig. 1), potentially informing the adaptive capacity to climate warming within and among tundra plant communities.
Fig. 2. Global trait relationships are maintained in the tundra biome despite constrained size, but not resource economic, traits among tundra species.
a Global trait-space defined by six plant traits for 1,358 plant species in the global dataset (grey points) and 219 tundra species (blue points). b Distribution of trait space for tundra species only. Note that PCA axes are reversed in tundra data relative to global data. Points are coloured by temperature category, corresponding to the mean annual temperature of trait collection sites for each species (Cold < −1 °C, Mid > −1 °C but <1 °C, Warm >1 °C, Supplementary Fig. 1). Arrows indicate the direction and weighting of trait vectors. We also tested the consistency of the patterns found (c) within global trait space and for (d) tundra trait space using subset of “extreme” tundra species that included only those species found only north of the Arctic circle or at sites with a MAT < 0 °C.
Global trait relationships extend to the tundra biome
We found that plant trait relationships among tundra species were consistent with global patterns (Fig. 2b, d), despite a limited range of trait values and lower species richness in the tundra biome. The two dimensions of global trait space (plant size and resource economics) aligned with trait relationships among tundra species (Supplementary Fig. 2), and together explained 64.5% of trait variation in the tundra. However, the relative importance of the PCA axes was reversed relative to global data (Supplementary Fig. 3), suggesting that tundra plant strategies are primarily differentiated by resource economics. Leaf area was more strongly associated with plant size among tundra species and with resource economics among global species (Supplementary Fig. 4). In contrast, leaf dry matter content (LDMC) was more strongly associated with resource economics in the tundra. LDMC correlated closely with stem density, which was associated with plant size and structure among global plant species8, especially among tree species2. Nevertheless, trait co-variation was maintained in the tundra despite the absence of trees, which comprise half of global trait space8 and have been a focus of many previous studies of plant trait relationships1,2,5,6.
The majority of trait variation occurs among species
We found that differences among species explained the majority of trait variation in the tundra biome, accounting for an average of 76.8% of variation across the six traits examined (Fig. 3a, Fig. 4) and reinforcing one of the key assumptions of trait-based ecology20. Functional group categorisation alone explained an average of 25.6% of trait variation across all six traits, but varied substantially by trait;33 differences among species still accounted for the majority of trait variation even if functional group classifications were removed. The contribution of within-species variation to total trait variation (23.2%) was surprisingly close to the global mean (25%21), despite harsh environmental conditions and large species ranges in the tundra. However, within-species variation differed substantially by trait, accounting for as much as 55% of trait variation for leaf nitrogen, in line with previous studies15,21. Size-related traits demonstrated greater overall variation than resource economic traits, even though variation relative to global trait space was constrained along the size-related axis (Fig. 2a). Overall, our findings support the hypothesis that species-level variation comprises the majority of the global spectrum of plant form and function8,20, underlining the importance of species richness and turnover in determining plant community characteristics, trait diversity, and linkages to ecosystem function.
Fig. 3. Sources of trait variation for six plant traits in the tundra biome.
a Relative proportion of trait variation explained by functional group (deciduous shrubs, evergreen shrubs, graminoids, forbs; yellow), species (red) and within species (blue). b Total trait variation, represented by the coefficient of variation (ratio of the standard deviation to the mean), and component sources of trait variation.
Fig. 4. Sources of trait variation across geographic scales.
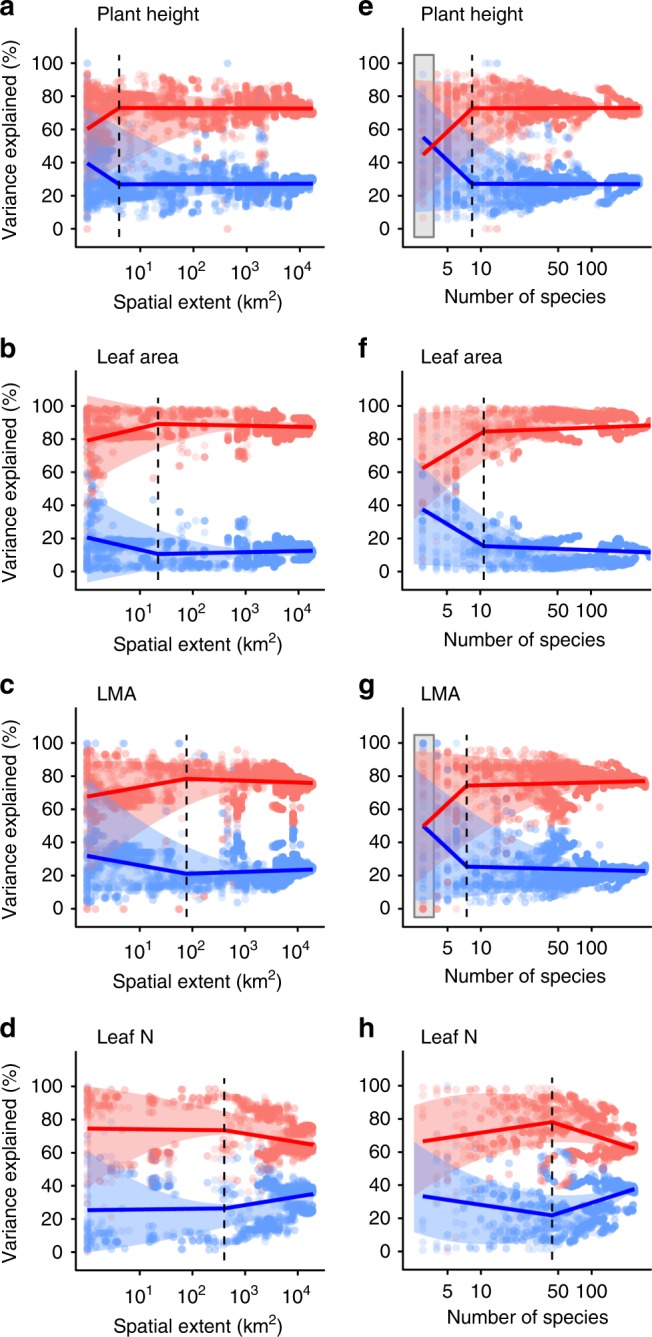
Among-species trait variation (red) accounts for the majority of total trait variation across the tundra, but the importance of within-species trait variation (blue) increases at local scales and at low species richness in most traits. Sources of trait variation across geographical scale (a–d) and species richness (e–h) for plant height, leaf area, LMA and leaf nitrogen (see also Supplementary Figs. 5–7). Coloured lines indicate linear break point model fits with one break point (dashed line). Grey boxes indicate where differences between among-species and within-species variation are not significant (P > 0.05).
Trait variation across geographic scales
We found that the contribution of within-species trait variation was largely consistent across geographic scales (Fig. 4a-d), but comprised a greater proportion of total variation at local scales (<10 km2), approximately the size of current high-resolution cells in gridded climate datasets. Sites with low sampled species richness also exhibited high within-species variation (<10 species; Fig. 4e-h), suggesting that spatial patterns were at least in part driven by a small species pool at local scales. Although both theoretical models27 and empirical studies21 have suggested that the contribution of within-species trait variation should increase at local scales, we demonstrate this relationship from the plot to the biome scale.
Our findings indicate that the relationships between plant traits found at the global scale are generalizable even at the climatic edge of global trait-space. Our results suggest that plants are subjected to globally consistent trade-offs in trait expression7,8,16 despite dramatically different environmental constraints17, growth forms29 and evolutionary history6 across biomes. Our findings reinforce claims that relationships between these widely measured plant traits are indicative of fundamental trade-offs in plant life strategy8,9, including resource acquisition (LMA, LA, LN, LDMC)7, competition (PH, SM, LA, LDMC)34, and reproduction (PH, SM)5. However, plant size and resource economics have yet to be integrated with other key facets of plant life strategy such as phenological35,36, chemical and below-ground traits4,18. These less frequently measured traits need to be incorporated into analyses to more comprehensively capture how extreme biomes such as the tundra occupy global trait space.
Tundra plant species showed remarkable variation in resource economic traits within the tundra biome relative to global trait space8. Given the low vascular plant diversity associated with many tundra environments, this variation in plant leaf resource economics is notably high and suggests that tundra species have developed a wide range of ecological strategies to cope with extreme conditions and limiting resources. In contrast, tundra plant species occupied half the global range of size-related traits, potentially indicating that two of the major axes of global trait variation may be differentially selected by environmental conditions, and could thus respond differently to environmental change. If the spatial patterns observed in our study are indicative of temporal responses13,37, climate change will likely shift trait distributions towards increased plant height, leaf area and seed mass, as has already been observed at some sites38,39, and for plant height at the tundra biome scale13.
Contrary to our expectations, the contribution of within-species trait variation in the tundra was lower than global estimates21. Our findings support previous studies indicating that the relative importance of within-species variation decreases with increasing environmental stress25, leading to wide functional divergence between species, as found for resource economic traits33,40. Lower within-species variation may further indicate that plasticity is lower among tundra species, which are typically slow growing and nutrient limited41. However, rapid and sustained plastic responses to environmental change have been documented at some tundra sites38,39. Indeed, large differences in both trait expression, as demonstrated here, and plasticity have been found to promote coexistence in other resource-limited comminities36. If the majority of trait variation occurs among species, and if phenotypic plasticity is comparatively low, shifts in community-level traits following environmental change may occur more slowly than would be predicted from biogeographic gradients13,37. More substantial trait change would thus require the immigration of new species from warmer sites.
We found that within-species trait variation comprised a large component of trait variation at local scales—the scale at which many critical ecological processes occur11. Despite the importance of trait differences among species in the tundra, we nevertheless found that that within-species variation accounted for approximately one quarter of total trait variation, and thus should not be ignored in trait-based analyses42. High within-species trait variation at local scales has previously been predicted from ecological theory21,27, and may result from low local-scale species richness27 or reveal the influence of local-scale environmental variability (i.e., topography, snow, drainage, etc.)24,43–45. Accounting for multiple sources of trait variation has been shown to constrain trait-based vegetation models7,12 and subsequently to improve prediction of the response of key ecosystem processes to environmental change11,13,46. However, such trait-based modelling approaches are rare, and require precisely geo-referenced trait databases that link trait records to environmental variables. We therefore support calls to collect additional trait data in changing and novel climate conditions13,30, to improve the techniques and technologies used to remotely sense plant trait information28, and to incorporate trait variation into Earth system modelling47.
Overall, our findings demonstrate that relationships and trade-offs among six fundamental plant traits are generalisable across lineages and among biomes to the cold extremes of the planet. It remains to be tested whether such relationships are consistent in other environmental extremes, such as desert ecosystems. Understanding the differences in trait expression across global trait-space offers fundamental insights into the rules that underpin evolution, community assembly, and ecosystem response to environmental change. Quantifying trait expression and variation thus offers a clearer picture of how plant communities and ecosystem functions will respond as climate change alters environmental conditions around the world.
Methods
Tundra biome definition
In line with previous biome-scale assessments of tundra vegetation community change48–50, we defined the tundra biome as the vegetated regions above treeline at high latitude and high altitude. Tundra species were identified as those present in sampling plots from two biome-scale experiments, the International Tundra Experiment (ITEX)51 and associated sites49, and the sUMMITDiv network52, or those present at trait collection sites with a mean annual temperature below 0oC. Tundra plant communities include many widely-distributed and locally common species that are found across large geographical gradients and a variety of environments51. We included trait records of tundra species collected outside of tundra environments in this study because i) we were specifically interested in the maximum potential within-species variation among tundra species, ii) defining tundra environments on a purely climatic basis (excluding biotic community in the definition) is very difficult and would require arbitrary decisions regarding biome boundaries, and iii) many trait records in the TRY database do not contain georeferenced collection coordinates and thus would be impossible to classify based on environment (Fig. 1b).
Trait selection
We selected six plant traits: plant height (PH, maximum measured height), seed mass (SM, dry mass), leaf area per leaf (LA, fresh leaf area), leaf mass per area (LMA, ratio of leaf dry mass to fresh leaf area), leaf dry matter content (LDMC, ratio of leaf dry mass to fresh leaf mass), and leaf nitrogen (LN, nitrogen per unit leaf dry mass). These six traits are considered to represent fundamental dimensions of ecological strategy8,53 and are commonly measured in the tundra30, thus maximising trait coverage.
Trait data collection
We extracted trait data from the TRY15 3.0 database (available at www.try-db.org) for tundra species1,2,17,54–142. We extracted traits of all tundra species from the TRY database regardless of location to maximise the capture of trait variation per species. We supplemented TRY data with additional trait data from the “Tundra Trait Team” (TTT) database30. All species names from ITEX, TRY and TTT were matched to accepted names in The Plant List using the R package Taxonstand (v. 1.8) before merging the datasets143. We assigned species to four traditional functional groups—evergreen shrubs, deciduous shrubs, graminoids, and forbs—based on previous classification of ITEX species49. We excluded stem-specific density (SSD) from all analyses, except for comparisons with Diaz et al.8 (Supplementary Figs. 9–11) since SSD had low collection coverage in the tundra and was available for too few species (n = 52). We also extracted trait data and collection site coordinates from TRY 3.0 for all global species to provide global context in geographical-climate-space and trait-space analyses following previously published approaches in Kattge et al.15 and Diaz et al.8.
Data cleaning-TRY
TRY trait data were subjected to a multi-step cleaning process. Firstly, all values that did not represent individual measurements or species means were excluded. Secondly, we identified overlapping datasets within TRY and removed duplicate observations whenever possible. The following datasets were identified as having partially overlapping observations: GLOPNET–Global Plant Trait Network Database, The LEDA Traitbase, Abisko and Sheffield Database, Tundra Plant Traits Database, and KEW Seed Information Database (SID).
Thirdly, we removed duplicates within each TRY dataset (e.g., if a value is listed once as “mean” and once as “best estimate”) by first calculating the ratio of duplicated values within each dataset, and then removing duplicates from datasets with more than 30% duplicated values. This cut-off was determined by manual evaluation of datasets at a range of thresholds. Datasets with fewer than 30% duplicated values were not cleaned in this way as any internally duplicate values were assumed to be true duplicates (i.e., two different individuals were measured and happened to have the same measurement value).
Finally, we removed all species mean observations from the “Niwot Alpine Plant Traits” database and replaced them with the original individual observations as provided by the trait collector (Marko J. Spasojevic) in order to ensure all trait measurements were for individuals.
Data cleaning–TRY and TTT combined
Both datasets were checked for improbable values, with the goal of excluding likely errors or measurements with incorrect units, but without excluding true extreme values. It was particularly important to avoid artificial reduction in the range of trait values in this study, since we were explicitly interested in trait variation. We followed a series of data-cleaning steps, in each case estimating an error risk for a given observation (x) by calculating the difference between x and the mean (excluding x) of the group in question and then dividing by the standard deviation of the group. We employed a hierarchical data cleaning method, because the standard deviation of a trait value is related to the mean and sample size. First, we checked individual records against the entire distribution of observations of that trait and removed any records with an error risk greater than 8.0 (i.e., a value more than eight standard deviations away from the trait mean). For species that occurred in four or more unique datasets within TRY or TTT (i.e., different data contributors), we estimated a species mean per dataset and removed observations for which the species mean error risk was greater than 3.0 (i.e., the species mean of that dataset was more than three SD’s away from the species mean across all datasets). For species that occurred in fewer than four unique datasets, we estimated a genus mean per dataset and removed observations in datasets for which the error risk based on the genus mean was greater than 3.5. Finally, we compared individual records directly to the distribution of values for that species. For species with fewer than four records, we did not remove any values. For species with more than four records, we excluded values above an error risk y, where y was dependent on the number of records of that species and ranged from an error risk of 2.25 for species with fewer than 10 records to an error risk of 4.0 for species with more than 30 records. This procedure was performed on the complete tundra trait database – including species and traits not presented here. In total 3515 observations (2.8%) were removed. In all cases, we visually checked the excluded values against the distribution of all observations for each species to ensure that our trait cleaning protocol was reasonable.
All trait observations with latitude/longitude information were mapped using the R package ‘mapproj’144 and checked for illogical values (e.g., falling in the ocean). These values were corrected from the original publications or by contacting the data contributor whenever possible. Where locations could not be verified, geo-referenced coordinates were removed and the trait data not included in geographic analyses.
Final trait database
After cleaning out duplicates and suspected mistakes, we retained 51,657 unique trait observations (of which 20,991 were already in TRY and 30,616 were newly contributed by the Tundra Trait Team) across the six traits of interest. Of the 447 identified species in the ITEX dataset, 397 (89%) had trait data available from TRY or TTT for at least one trait (range 60–100% per site). Those species without trait data generally represent rare or uncommon species unique to each site. On average, trait data were available for 97% of total plant cover across all sites (range 39–100% per site; Supplementary Table 1).
Data compiled through the Tundra Trait Team are available in Bjorkman et al.30. The total TTT database submitted to TRY includes traits not considered in this study, as well as tundra species that do not occur in our vegetation survey plots, for a total of 54,210 trait observations on 530 species. For more information on trait data and trait cleaning methods see Bjorkman et al.13 and Bjorkman et al.30.
Climate data
To plot the distribution of tundra trait data within climate space, we used the coordinates of all unique collection sites for both tundra (TRY and TTT) and global (TRY) datasets.
CHELSA climate variables (mean annual temperature–BIO10_1 and mean annual precipitation–BIO10_12, http://chelsa-climate.org/) were extracted for all trait observations with latitude/longitude values recorded (39,573 records in total, 12,434 of which were from TRY and 27,139 from TTT). Because most observations did not include information about elevation, temperature estimates for individual trait observations were not corrected for elevation.
We calculated the ‘temperature class’ of tundra species based on the mean summer temperature of trait collection sites for each species. Mean summer temperature was considered to be the most ecologically meaningful climatic variable since it captures conditions during the growing season for each plant species. We extracted summer temperatures from the CHELSA dataset based on BioClim variable BIO10_1. We assigned species to three temperature classes: Cold tundra = mean summer temperature less than −1 °C, Mid tundra = mean summer temperature greater than −1 °C but less than 1 °C, Warm tundra = summer temperature of coldest site greater than 1 °C.
Analysis of trait relationships
All analyses were conducted in R (v. 3.3.3). Code is available at github.com/hjdthomas/Tundra_trait_variation.
We performed principal component analysis (PCA) on plant traits for all global species, and for tundra species only using the R package ‘prcomp’. As far as possible, we replicated the methods outlined in Diaz et al. (2016)8, though this was not always possible due to the use of gap-filled traits and additional data not included in TRY in Diaz et al. (2016)8. We log transformed trait values to account for log-normal distributions, which is considered appropriate for data with different measurement scales8. To test whether the inclusion of SSD altered results, and for comparison with Diaz et al. (2016)8, we performed supplementary analysis using a conversion from SSD to LDMC based on the correlation between these two traits8,145, since LDMC and SSD individually have fewer trait observations than other traits at the global scale. Although only an approximate conversion, this greatly increases the number of species available for the analyses and does not affect the distribution of trait-space or direction of trait loadings (Supplementary Figs. 8–10). We did not use converted values in the main analysis to avoid introducing additional sources of variation.
To visualise trait-space, we plotted the first two PCA axes and direction and weighting of trait loadings. We performed PCA on the full global dataset (including tundra data) and highlighted tundra species within the overall distribution to identify the location of tundra species within global trait-space. We repeated PCA using tundra species only to compare global trait relationships with tundra trait relationships. We compared all pairwise trait correlations for both global and tundra species (Supplementary Fig. 2) to investigate consistency in trait-trait relationships, and investigated the strength and direction of trait loadings for global and tundra analyses to compare the location and relative importance of PCA axes (Supplementary Fig. 3). Finally, we calculated the contribution of traits loadings to each PCA axis using the ‘fviz_contrib’ function in the R package ‘factoextra’ (Supplementary Fig. 4).
To investigate whether the location of species within tundra trait-space or along the two major axes of variation was influenced by climate, we categorised species according to temperature class (point colour). We plotted the distribution of species along PCA axes for each temperature class to test whether trait variation within multivariate trait-space was influenced by the species’ thermal range (Supplementary Fig. 1).
Variance partitioning
To investigate the sources of trait variation in the tundra, we conducted variance partitioning by fitting a generalised linear mixed-effects model to the variance across nested classification hierarchy (functional group/species) using the R package ‘nlme’. We then conducted a variance component analysis on this model using the ‘varcomp’ function in the R package ‘ape’. Partitioning was performed on a trait-by-trait basis, so does not account for co-variation between traits. We used unexplained variance to represent the within-species variation (including within-individual variation), though some unexplained variation could also arise from measurement error. Variance partitioning was conducted for each site with greater than three observations per trait per species, and at least three sampled species, and summarised using the mean of all sites. To complement variance partitioning, we also calculated the coefficient of variation (CV; the ratio of the standard deviation to the mean) to compare variation among traits. CV was calculated for each trait for all species.
Variance partitioning across geographic scale
To assess how variance explained by differences within-species and between-species varied with geographic scale, we iteratively grouped sites based on geographic proximity. We calculated the geographic distance between all trait sampling sites using the R package ‘geosphere’. We excluded sampling sites with fewer than three species per site, or fewer than two trait observations per species. To test the sensitivity of findings to trait or species availability, we also conducted a sensitivity analysis excluding sites with fewer than five species per site or trait observations per species (Supplementary Figs. 12, 13). For a given trait sampling site, we conducted variance partitioning analysis at the site scale (scale = 0). We then added data from the nearest site (shortest geographical distance) and conducted variance partitioning analysis on this expanded dataset (geographic scale = distance from starting site to most distant site). We added sites iteratively until all sites were included i.e., the biome-scale was reached. We repeated this analysis across all trait sampling sites. To examine whether sources of trait variance were affected by differences in species richness at different geographical scales, we also calculated the species richness (number of unique species for which we have trait measurements, i.e., size of the measured species pool) of the dataset at each sampling step.
To summarise the relationship between variance explained, geographic scale and species richness, we performed a breakpoint analysis using the R package ‘segmented’ with one break point. To calculate errors, we grouped trait observation sites into 5 km or 1 species bins and calculated the 95% intervals of the spread of values. These were plotted as error bounds using a second-order polynomial smooth. We visualised all analyses with geographic scale and species richness presented on a log10 scale to highlight change at local scales. For non-logged figures see Supplementary Figs. 6, 7. To investigate if differences in the contribution of within-species and among-species variation to total trait variation were significant, we additionally grouped data into 10 equal bins, and tested the significance of source of variance using linear models, with a significance threshold of 0.05. We highlighted insignificant bins on figures as grey shaded areas, which indicate scales at which the amount of within-species variation is not significantly different from among-species variation. We examined (1) the mean contribution of within-species and among-species variation to overall trait variation, and (2) proportion of sampling combinations for which within-species variation accounted for at least one third and one half of overall trait variation above and below each break point (Supplementary Fig. 5). Finally, we tested the sensitivity of all analysis to species selection by repeating analyses for only trait sampling sites north of the Arctic Circle (66.5 °N) or from collection locations with a mean annual temperature below 0 °C (Supplementary Figs. 14–20).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
Additional data and contributions were provided by L. Andreu-Hayles, O. Atkin, A. Blach Overgaard, J. Dickie, S. Dullinger, B. Enquist, J. Fang, K. Fleischer, H. Ford, G. Freschet, E. Garnier, R. Halfdan Jørgensen, K. Harper, S. Harrison, M. Harze, J. Hille Ris Lambers, R. Jackson, R. Klady, S. Kuleza, A. Lavalle, F. Louault, B. Medlyn, R. Milla, J. Ordonez, C. Pladevall, H. Poorter, C. Price, P. Semenchuk, F. Schweingruber, B. Shipley, A. Siefert, L. Street, J. Tremblay, E. Weiher, C. Wirth, I. Wright and the Royal Botanic Gardens Kew Seed Information Database (SID). We thank innumerable field technicians, logistics teams, graduate and undergraduate assistants for help with data collection, and parks, wildlife refuges, field stations and the local and indigenous people for the opportunity to conduct research on their land. Thanks to all those that have reviewed this manuscript for their insightful and constructive comments. The project was funded by the UK Natural Environment Research Council (ShrubTundra Project NE/M016323/1 [I.M.-S., H.T., A.B.] and NERC doctoral training partnership grant NE/L002558/1 [H.T.]), and the Synthesis Centre of the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig (DFG FZT 118; sTundra working group). The study has been supported by the TRY initiative on plant traits (http://www.try-db.org). The TRY initiative and database is hosted at the Max Planck Institute for Biogeochemistry, Jena, Germany. TRY is currently supported by DIVERSITAS/Future Earth and the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig. Authors were supported by: Academy of Finland [A.E.], ArcticNet [E.F., E.L., L.H., O.G.], Arctic Research Centre, Aarhus University [J.N.N.], BBSRC David Phillips Fellowship [F.d.V.], Carl Tryggers Stiftelse för Vetenskaplig Forskning and Qatar Petroleum (QUEX-ESC-QP-RD-18/19 [J.M.A.], Carlsberg Foundation [S.N., S.S.N.], Centre d’études nordiques [O.G.], Danish Council for Independent Research-Natural Sciences [S.N., S.S.N.], Danish National Research Foundation [B.E.], Deutsche Forschungsgemeinschaft DFG [N.R., M.D.], Energy Exascale Earth System Model (E3SM) project, funded by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research [B.B.-L.], EU-F7P INTERACT [A.B.], EU-INTERACT [MH], European Research Council Synergy Grant ERC-2013-SyG-610028 IMBALANCE-P [J.P.], Fonds de recherche du Quebec: Nature et technologies [O.G.], Marie Skłodowska Curie Actions [D.B.], MOBILITY PLUS [A.B.], Montagna di Torricchio Nature Reserve [G.C.], National Aeronautics and Space Administration [S.S.], NASA's Arctic Boreal Vulnerability Experiment (ABoVE) [S.J.G., L.T.B.], Natural Environment Research Council (UK) [NE/M019160/1; B.B.], Natural Sciences and Engineering Research Council of Canada [B.S., E.F., E.L., J.J., M.V., P.M., O.G., P.G., T.Z.], National Science Foundation (USA) [K.G., R.H.; Arctic Natural Sciences program S.J.G, L.T.B], Netherlands Organization for Scientific Research [R.S.], The Niwot Ridge LTER (NSF DEB-1637686) [M.J.S, K.N.S.], Northern Scientific Training Program [O.G.], NWO Earth and Life Sciences (NWO-ALW), project ALWPP.2016.008 [MH], Memorial University [L.H.], Organismo Autónomo Parques Nacionales [J.M.N.], Polar Continental Shelf Program [E.F., E.L., O.G.], Research Council of Norway [J.S.], Russian Science Foundation, #16-14-10208 [V.O.], Swedish Research Council [D.B., E.K., R.B.], Swiss National Science Foundation [A.K., E.F.], University of Zurich Research Priority Program on Global Change and Biodiversity [G.S.S.], U.S. Department of Energy, Office of Biological and Environmental Research-Energy Exascale Earth System Model [B.B.L.], U.S. Department of Energy, Office of Biological and Environmental Research-Next-Generation Ecosystem Experiments in the Arctic (NGEE Arctic) [C.I.], University of Zurich Research Priority Program on Global Change and Biodiversity [M.I.G.], and the Villum Foundation [S.S.N.]. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author contributions
H.T., I.M.-S. and A.B. conceived the study. H.T. performed statistical analysis with additional input from I.M.-S. and A.B. H.T. wrote the manuscript with input from I.M.-S. and A.B., and contributions from all authors. A.B. compiled the TTT database with assistance from I.M.-S., H.T. and S.A.B. H.T., A.B., I.M.-S., S.E., J.K., S.D., M.V., D.B., J.C., B.F., G.H., R.H., S.N., J.P., C.R., G.S.S., M.W., S.W., W.C., P.B., D.G., S.G., K.G., N.R., N.S., M.S., J.A., H.A., A.A.R., S.A.B., Mt.B., L.B., R.B., A.B., A.B., M.C., K.C., L.C., E.C., B.E., A.E., E.F., O.G., P.G., M.H., L.H., J.H., J.J., K.H., M.I.G., C.I., F.J.,. E.K., A.K., L.J.L., T.C.L., E.L., C.L., A.M., A.M., J.N.N., S.N., J.N., S.O., J.O., V.O., A.P., S.R., R.S., J.S., K.S., K.T., M.T., A.T., U.T., M.T., S.V., T.V., S.W., P.W., T.Z., M.B., B.B., P.v.B., B.B.L., G.C., B.C., F.S.C. III, J.C., M.D., W.G., S.J., M.K., P.M., U.N., Y.O., W.O., J.P., P.P., P.R., B.S., S.S. and F.d.V. contributed data. I.M.-S., S.E. and A.B. led the sTundra working group. I.M.-S. supervised H.T. and acquired funding for the project.
Data availability
All trait data compiled through the Tundra Trait Team are available at http://github.com/TundraTraitTeam/TraitHub, see Bjorkman et al.30. Additional global trait data are available through the TRY database (www.try-db.org)15.
Code availability
Code is available at http://github.com/hjdthomas/Tundra_trait_variation.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Ignacio Perez-Ramos, Jonathan Storkey, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-15014-4.
References
- 1.Wright IJ, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- 2.Chave J, et al. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009;12:351–366. doi: 10.1111/j.1461-0248.2009.01285.x. [DOI] [PubMed] [Google Scholar]
- 3.Westoby M, Jurado E, Leishman MR. Comparative evolutionary ecology of seed size. Trends Ecol. Evol. 1992;7:368–372. doi: 10.1016/0169-5347(92)90006-W. [DOI] [PubMed] [Google Scholar]
- 4.Iversen CM, et al. The unseen iceberg: plant roots in arctic tundra. N. Phytologist. 2015;205:34–58. doi: 10.1111/nph.13003. [DOI] [PubMed] [Google Scholar]
- 5.Westoby M. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil. 1998;199:213–227. doi: 10.1023/A:1004327224729. [DOI] [Google Scholar]
- 6.Westoby M, Wright IJ. Land-plant ecology on the basis of functional traits. Trends Ecol. Evol. 2006;21:261–268. doi: 10.1016/j.tree.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Reich PB. The world-wide ‘fast-slow’ plant economics spectrum: a traits manifesto. J. Ecol. 2014;102:275–301. doi: 10.1111/1365-2745.12211. [DOI] [Google Scholar]
- 8.Díaz S, et al. The global spectrum of plant form and function. Nature. 2016;529:167–171. doi: 10.1038/nature16489. [DOI] [PubMed] [Google Scholar]
- 9.McGill BJ, Enquist BJ, Weiher E, Westoby M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006;21:178–185. doi: 10.1016/j.tree.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Cornwell WK, Ackerly DD. Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecol. Monogr. 2009;79:109–126. doi: 10.1890/07-1134.1. [DOI] [Google Scholar]
- 11.Suding KN, et al. Scaling environmental change through the community-level: a trait-based response-and-effect framework for plants. Glob. Change Biol. 2008;14:1125–1140. doi: 10.1111/j.1365-2486.2008.01557.x. [DOI] [Google Scholar]
- 12.Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: revisting the Holy Grail. Funct. Ecol. 2002;16:545–556. doi: 10.1046/j.1365-2435.2002.00664.x. [DOI] [Google Scholar]
- 13.Bjorkman AD, et al. Plant functional trait change across a warming tundra biome. Nature. 2018;562:57–62. doi: 10.1038/s41586-018-0563-7. [DOI] [PubMed] [Google Scholar]
- 14.Moran EV, Hartig F, Bell DM. Intraspecific trait variation across scales: Implications for understanding global change responses. Glob. Change Biol. 2016;22:137–150. doi: 10.1111/gcb.13000. [DOI] [PubMed] [Google Scholar]
- 15.Kattge J, et al. TRY-a global database of plant traits. Glob. Change Biol. 2011;17:2905–2935. doi: 10.1111/j.1365-2486.2011.02451.x. [DOI] [Google Scholar]
- 16.Reich PB, Walters MB, Ellsworth DS. From tropics to tundra: Global convergence in plant functioning. Proc. Natl Acad. Sci. USA. 1997;94:13730–13734. doi: 10.1073/pnas.94.25.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freschet GT, Cornelissen JHC, van Logtestijn RSP, Aerts R. Evidence of the ‘plant economics spectrum’ in a subarctic flora. J. Ecol. 2010;98:362–373. doi: 10.1111/j.1365-2745.2009.01615.x. [DOI] [Google Scholar]
- 18.Myers-Smith IH, Thomas HJD, Bjorkman AD. Plant traits inform predictions of tundra responses to global change. N. Phytologist. 2019;221:1742–1748. doi: 10.1111/nph.15592. [DOI] [PubMed] [Google Scholar]
- 19.Wigley BJ, et al. Leaf traits of African woody savanna species across climate and soil fertility gradients: evidence for conservative versus acquisitive resource-use strategies. J. Ecol. 2016;104:1357–1369. doi: 10.1111/1365-2745.12598. [DOI] [Google Scholar]
- 20.Shipley Bill, De Bello Francesco, Cornelissen J. Hans C., Laliberté Etienne, Laughlin Daniel C., Reich Peter B. Reinforcing loose foundation stones in trait-based plant ecology. Oecologia. 2016;180(4):923–931. doi: 10.1007/s00442-016-3549-x. [DOI] [PubMed] [Google Scholar]
- 21.Siefert A, et al. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol. Lett. 2015;18:1406–1419. doi: 10.1111/ele.12508. [DOI] [PubMed] [Google Scholar]
- 22.Anderegg LDL, et al. Within-species patterns challenge our understanding of the leaf economics spectrum. Ecol. Lett. 2018;21:734–744. doi: 10.1111/ele.12945. [DOI] [PubMed] [Google Scholar]
- 23.Laughlin DC, et al. Intraspecific trait variation can weaken interspecific trait correlations when assessing the whole-plant economic spectrum. Ecol. Evol. 2017;7:8936–8949. doi: 10.1002/ece3.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De La Riva EG, Olmo M, Poorter H, Ubera JL, Villar R. Leaf mass per area (LMA) and its relationship with leaf structure and anatomy in 34 mediterranean woody species along a water availability gradient. PLoS ONE. 2016;11:e0148788. doi: 10.1371/journal.pone.0148788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fajardo A, Piper FI. Intraspecific trait variation and covariation in a widespread tree species (Nothofagus pumilio) in southern Chile. N. Phytologist. 2011;189:259–271. doi: 10.1111/j.1469-8137.2010.03468.x. [DOI] [PubMed] [Google Scholar]
- 26.Messier J, McGill BJ, Enquist BJ, Lechowicz MJ. Trait variation and integration across scales: is the leaf economic spectrum present at local scales? Ecography. 2017;40:685–697. doi: 10.1111/ecog.02006. [DOI] [Google Scholar]
- 27.Albert CH, Grassein F, Schurr FM, Vieilledent G, Violle C. When and how should intraspecific variability be considered in trait-based plant ecology? Perspect. Plant Ecol. Evol. Syst. 2011;13:217–225. doi: 10.1016/j.ppees.2011.04.003. [DOI] [Google Scholar]
- 28.Jetz W, et al. Monitoring plant functional diversity from space. Nat. Plants. 2016;2:1–5. doi: 10.1038/nplants.2016.24. [DOI] [PubMed] [Google Scholar]
- 29.Pearson RG, et al. Shifts in Arctic vegetation and associated feedbacks under climate change. Nat. Clim. Change. 2013;3:673–677. doi: 10.1038/nclimate1858. [DOI] [Google Scholar]
- 30.Bjorkman AD, et al. Tundra Trait Team: a database of plant traits spanning the tundra biome. Glob. Ecol. Biogeogr. 2018;27:1402–1411. doi: 10.1111/geb.12821. [DOI] [Google Scholar]
- 31.Manning P, et al. Simple measures of climate, soil properties and plant traits predict national-scale grassland soil carbon stocks. J. Appl. Ecol. 2015;52:1188–1196. doi: 10.1111/1365-2664.12478. [DOI] [Google Scholar]
- 32.Billings WD. Constraints to plant growth, reproduction, and establishment in arctic environments. Arct. Alp. Res. 1987;19:357. doi: 10.2307/1551400. [DOI] [Google Scholar]
- 33.Thomas HJD, et al. Traditional plant functional groups explain variation in economic but not size-related traits across the tundra biome. Glob. Ecol. Biogeogr. 2019;28:78–95. doi: 10.1111/geb.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunstler G, et al. Plant functional traits have globally consistent effects on competition. Nature. 2016;529:1–15. doi: 10.1038/nature16476. [DOI] [PubMed] [Google Scholar]
- 35.Bjorkman AD, Vellend M, Frei ER, Henry GHR. Climate adaptation is not enough: warming does not facilitate success of southern tundra plant populations in the high Arctic. Glob. Change Biol. 2017;23:1540–1551. doi: 10.1111/gcb.13417. [DOI] [PubMed] [Google Scholar]
- 36.Pérez-Ramos, I. M., Matías, L., Gómez-Aparicio, L. & Godoy, Ó. Functional traits and phenotypic plasticity modulate species coexistence across contrasting climatic conditions. Nat. Commun.10, 1–11 (2019). [DOI] [PMC free article] [PubMed]
- 37.Elmendorf SC, et al. Experiment, monitoring, and gradient methods used to infer climate change effects on plant communities yield consistent patterns. Proc. Natl Acad. Sci. USA. 2015;112:448–452. doi: 10.1073/pnas.1410088112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hudson JMG, Henry GHR, Cornwell WK. Taller and larger: shifts in Arctic tundra leaf traits after 16 years of experimental warming. Glob. Change Biol. 2011;17:1013–1021. doi: 10.1111/j.1365-2486.2010.02294.x. [DOI] [Google Scholar]
- 39.Myers‐Smith Isla H., Grabowski Meagan M., Thomas Haydn J. D., Angers‐Blondin Sandra, Daskalova Gergana N., Bjorkman Anne D., Cunliffe Andrew M., Assmann Jakob J., Boyle Joseph S., McLeod Edward, McLeod Samuel, Joe Ricky, Lennie Paden, Arey Deon, Gordon Richard R., Eckert Cameron D. Eighteen years of ecological monitoring reveals multiple lines of evidence for tundra vegetation change. Ecological Monographs. 2019;89(2):e01351. doi: 10.1002/ecm.1351. [DOI] [Google Scholar]
- 40.Hoffmann AA, Merilä J. Heritable variation and evolution under favourable and unfavourable conditions. Trends Ecol. Evol. 1999;14:96–101. doi: 10.1016/S0169-5347(99)01595-5. [DOI] [PubMed] [Google Scholar]
- 41.Baruah G, Molau U, Bai Y, Alatalo JM. Community and species-specific responses of plant traits to 23 years of experimental warming across subarctic tundra plant communities. Sci. Rep. 2017;7:2571. doi: 10.1038/s41598-017-02595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Violle C, et al. The return of the variance: Intraspecific variability in community ecology. Trends Ecol. Evol. 2012;27:244–252. doi: 10.1016/j.tree.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 43.Opedal ØH, Armbruster WS, Graae BJ. Linking small-scale topography with microclimate, plant species diversity and intra-specific trait variation in an alpine landscape. Plant Ecol. Diversity. 2015;8:305–315. doi: 10.1080/17550874.2014.987330. [DOI] [Google Scholar]
- 44.Elberling B. Annual soil CO2 effluxes in the High Arctic: The role of snow thickness and vegetation type. Soil Biol. Biochem. 2007;39:646–654. doi: 10.1016/j.soilbio.2006.09.017. [DOI] [Google Scholar]
- 45.McGraw JB. Experimental ecology of Dryas octopetala ecotypes. III. Environ. Factors Plant Growth Arct. Alp. Res. 1985;17:229–239. [Google Scholar]
- 46.Soudzilovskaia NA, et al. Functional traits predict relationship between plant abundance dynamic and long-term climate warming. Proc. Natl Acad. Sci. USA. 2013;110:18180–18184. doi: 10.1073/pnas.1310700110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wullschleger SD, et al. Plant functional types in Earth system models: past experiences and future directions for application of dynamic vegetation models in high-latitude ecosystems. Ann. Bot. 2014;114:1–16. doi: 10.1093/aob/mcu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Myers-Smith IH, et al. Climate sensitivity of shrub growth across the tundra biome. Nat. Clim. Change. 2015;5:887–891. doi: 10.1038/nclimate2697. [DOI] [Google Scholar]
- 49.Elmendorf SC, et al. Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nat. Clim. Change. 2012;2:453–457. doi: 10.1038/nclimate1465. [DOI] [Google Scholar]
- 50.Bliss, L. C., Heal, O. W. & Moore, J. J. Tundra Ecosystems: A Comparative Analysis. (CUP Archive, 1981).
- 51.Henry GHR, Molau U. Tundra plants and climate change: the International Tundra Experiment (ITEX) Glob. Change Biol. 1997;3:1–9. doi: 10.1111/j.1365-2486.1997.gcb132.x. [DOI] [Google Scholar]
- 52.Steinbauer MJ, et al. Accelerated increase in plant species richness on mountain summits is linked to warming. Nature. 2018;556:231–234. doi: 10.1038/s41586-018-0005-6. [DOI] [PubMed] [Google Scholar]
- 53.Weiher E, et al. Challenging theophrastus: a common core list of plant traits for functional ecology. J. Vegetation Sci. 1999;10:609–620. doi: 10.2307/3237076. [DOI] [Google Scholar]
- 54.Choat B, et al. Global convergence in the vulnerability of forests to drought. Nature. 2012;491:752–755. doi: 10.1038/nature11688. [DOI] [PubMed] [Google Scholar]
- 55.Burrascano S, et al. Wild boar rooting intensity determines shifts in understorey composition and functional traits. Community Ecol. 2015;16:244–253. doi: 10.1556/168.2015.16.2.12. [DOI] [Google Scholar]
- 56.Byun C, de Blois S, Brisson J. Plant functional group identity and diversity determine biotic resistance to invasion by an exotic grass. J. Ecol. 2013;101:128–139. doi: 10.1111/1365-2745.12016. [DOI] [Google Scholar]
- 57.Campbell C, et al. Acclimation of photosynthesis and respiration is asynchronous in response to changes in temperature regardless of plant functional group. N. Phytologist. 2007;176:375–389. doi: 10.1111/j.1469-8137.2007.02183.x. [DOI] [PubMed] [Google Scholar]
- 58.Castro-Díez P, Puyravaud JP, Cornelissen JHC, Villar-Salvador P. Stem anatomy and relative growth rate in seedlings of a wide range of woody plant species and types. Oecologia. 1998;116:57–66. doi: 10.1007/s004420050563. [DOI] [PubMed] [Google Scholar]
- 59.Cerabolini BEL, et al. Can CSR classification be generally applied outside Britain? Plant Ecol. 2010;210:253–261. doi: 10.1007/s11258-010-9753-6. [DOI] [Google Scholar]
- 60.Ciocarlan, V. The illustrated Flora of Romania. Pteridophyta et Spermatopyta. (Editura Ceres, 2009).
- 61.Cornelissen JHC. An experimental comparison of leaf decomposition rates in a wide range of temperate plant species and types. J. Ecol. 1996;84:573. doi: 10.2307/2261479. [DOI] [Google Scholar]
- 62.Cornelissen JHC, et al. Functional traits of woody plants: correspondence of species rankings between field adults and laboratory-grown seedlings? J. Vegetation Sci. 2003;14:311–322. doi: 10.1111/j.1654-1103.2003.tb02157.x. [DOI] [Google Scholar]
- 63.Cornelissen JHC, et al. Leaf digestibility and litter decomposability are related in a wide range of subarctic plant species and types. Funct. Ecol. 2004;18:779–786. doi: 10.1111/j.0269-8463.2004.00900.x. [DOI] [Google Scholar]
- 64.Cornelissen JHC, Diez PC, Hunt R. Seedling growth, allocation and leaf attributes in a wide range of woody plant species and types. J. Ecol. 1996;84:755. doi: 10.2307/2261337. [DOI] [Google Scholar]
- 65.Cornwell WK, et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008;11:1065–1071. doi: 10.1111/j.1461-0248.2008.01219.x. [DOI] [PubMed] [Google Scholar]
- 66.Craine JM, et al. Functional consequences of climate change-induced plant species loss in a tallgrass prairie. Oecologia. 2011;165:1109–1117. doi: 10.1007/s00442-011-1938-8. [DOI] [PubMed] [Google Scholar]
- 67.Craine JM, Towne EG, Ocheltree TW, Nippert JB. Community traitscape of foliar nitrogen isotopes reveals N availability patterns in a tallgrass prairie. Plant Soil. 2012;356:395–403. doi: 10.1007/s11104-012-1141-7. [DOI] [Google Scholar]
- 68.Craine JM, et al. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. N. Phytologist. 2009;183:980–992. doi: 10.1111/j.1469-8137.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 69.Craine JM, Lee WG, Bond WJ, Williams RJ, Johnson LC. Environmental constraints on a global relationship among leaf and root traits of grasses. Ecology. 2005;86:12–19. doi: 10.1890/04-1075. [DOI] [Google Scholar]
- 70.Diaz S, et al. The plant traits that drive ecosystems: evidence from three continents. J. Vegetation Sci. 2004;15:295–304. doi: 10.1111/j.1654-1103.2004.tb02266.x. [DOI] [Google Scholar]
- 71.Dainese M, Bragazza L. Plant traits across different habitats of the Italian Alps: a comparative analysis between native and alien species. Alp. Bot. 2012;122:11–21. doi: 10.1007/s00035-012-0101-4. [DOI] [Google Scholar]
- 72.Everwand G, Fry EL, Eggers T, Manning P. Seasonal variation in the relationship between plant traits and grassland carbon and water fluxes. J. Ecol. 2014;17:1095–1108. [Google Scholar]
- 73.Fitter AH, Peat HJ. The ecological flora database. J. Ecol. 1994;82:415–425. doi: 10.2307/2261309. [DOI] [Google Scholar]
- 74.Atkin OK, Westbeek MHM, Cambridge ML, Lambers H, Pons H. Leaf Respiration in Light and Darkness. Plant Physiol. 1997;113:961–965. doi: 10.1104/pp.113.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fonseca CR, Overton JM, Collins B, Westoby M. Shifts in trait-combinations along rainfall and phosphorus gradients. J. Ecol. 2000;88:964–977. doi: 10.1046/j.1365-2745.2000.00506.x. [DOI] [Google Scholar]
- 76.Fry EL, Power SA, Manning P. Trait-based classification and manipulation of plant functional groups for biodiversity-ecosystem function experiments. J. Vegetation Sci. 2014;25:248–261. doi: 10.1111/jvs.12068. [DOI] [Google Scholar]
- 77.Gallagher RV, Leishman MR. A global analysis of trait variation and evolution in climbing plants. J. Biogeogr. 2012;39:1757–1771. doi: 10.1111/j.1365-2699.2012.02773.x. [DOI] [Google Scholar]
- 78.Garnier E, et al. Assessing the effects of land-use change on plant traits, communities and ecosystem functioning in grasslands: A standardized methodology and lessons from an application to 11 European sites. Ann. Bot. 2007;99:967–985. doi: 10.1093/aob/mcl215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Green, W. USDA PLANTS Compilation, version 1, 09-02-02. NRCS: The PLANTS Database (2009).
- 80.Han W, Fang J, Guo D, Zhang Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. N. Phytologist. 2005;168:377–385. doi: 10.1111/j.1469-8137.2005.01530.x. [DOI] [PubMed] [Google Scholar]
- 81.Guy AL, Mischkolz JM, Lamb EG. Limited effects of simulated acidic deposition on seedling survivorship and root morphology of endemic plant taxa of the Athabasca Sand Dunes in well-watered greenhouse trials. Botany. 2013;91:176–181. doi: 10.1139/cjb-2012-0162. [DOI] [Google Scholar]
- 82.Hickler, T. Plant functional types and community characteristics along environmental gradients on Oland’s Great Alvar (Sweden). (University of Lund, Sweden, 1999).
- 83.Fagúndez J, Izco J. Seed morphology of two distinct european species of Erica L. (Ericaceae) Acta Botanica Malacit. 2008;33:1–9. [Google Scholar]
- 84.Kühn I, Durka W, Klotz S. BiolFlor-A new plant-trait database as a tool for plant invasion ecology. Diversity Distrib. 2004;10:363–365. doi: 10.1111/j.1366-9516.2004.00110.x. [DOI] [Google Scholar]
- 85.Bahn, M. et al. Leaf photosynthesis, nitrogen contents and specific leaf area of grassland species in mountain ecosystems under different land use. in Land use changes in European mountain ecosystems: ECOMONT concepts and results. Blackwell, Vienna, Austria 247–255 (1999).
- 86.Kattge J, Knorr W, Raddatz T, Wirth C. Quantifying photosynthetic capacity and its relationship to leaf nitrogen content for global-scale terrestrial biosphere models. Glob. Change Biol. 2009;15:976–991. doi: 10.1111/j.1365-2486.2008.01744.x. [DOI] [Google Scholar]
- 87.Kazakou E, Vile D, Shipley B, Gallet C, Garnier E. Co-variations in litter decomposition, leaf traits and plant growth in species from a Mediterranean old-field succession. Funct. Ecol. 2006;20:21–30. doi: 10.1111/j.1365-2435.2006.01080.x. [DOI] [Google Scholar]
- 88.Kerkoff A, Fagan WF, James J, Elser, Brian J. Enquist. Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants. Am. Naturalist. 2006;168:E103–E122. doi: 10.1086/507879. [DOI] [PubMed] [Google Scholar]
- 89.Kichenin E, Wardle DA, Peltzer DA, Morse CW, Freschet GT. Contrasting effects of plant inter- and intraspecific variation on community-level trait measures along an environmental gradient. Funct. Ecol. 2013;27:1254–1261. doi: 10.1111/1365-2435.12116. [DOI] [Google Scholar]
- 90.Kleyer M, et al. The LEDA Traitbase: a database of life-history traits of the Northwest European flora. J. Ecol. 2008;96:1266–1274. doi: 10.1111/j.1365-2745.2008.01430.x. [DOI] [Google Scholar]
- 91.Louault F, Pillar VD, Aufrère J, Garnier E, Soussana JF. Plant traits and functional types in response to reduced disturbance in a semi-natural grassland. J. Vegetation Sci. 2005;16:151–160. doi: 10.1111/j.1654-1103.2005.tb02350.x. [DOI] [Google Scholar]
- 92.Loveys BR, et al. Thermal acclimation of leaf and root respiration: an investigation comparing inherently fast- and slow-growing plant species. Glob. Change Biol. 2003;9:895–910. doi: 10.1046/j.1365-2486.2003.00611.x. [DOI] [Google Scholar]
- 93.Moretti M, Legg C. Combining plant and animal traits to assess community functional responses to disturbance. Ecography. 2009;32:299–309. doi: 10.1111/j.1600-0587.2008.05524.x. [DOI] [Google Scholar]
- 94.Medlyn BE, et al. Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis. N. Phytologist. 2001;149:247–264. doi: 10.1046/j.1469-8137.2001.00028.x. [DOI] [PubMed] [Google Scholar]
- 95.Mencuccini M. The ecological significance of long-distance water transport: Short-term regulation, long-term acclimation and the hydraulic costs of stature across plant life forms. Plant Cell Environ. 2003;26:163–182. doi: 10.1046/j.1365-3040.2003.00991.x. [DOI] [Google Scholar]
- 96.Bakker C, Rodenburg J, Van Bodegom PM. Effects of Ca- and Fe-rich seepage on P availability and plant performance in calcareous dune soils. in. Plant Soil. 2005;275:111–122. doi: 10.1007/s11104-005-0438-1. [DOI] [Google Scholar]
- 97.Meziane D, Shipley B. Interacting determinants of specific leaf area in 22 herbaceous species: effects of irradiance and nutrient availability. Plant Cell Environ. 1999;22:447–459. doi: 10.1046/j.1365-3040.1999.00423.x. [DOI] [Google Scholar]
- 98.Milla R, Reich PB. Multi-trait interactions, not phylogeny, fine-tune leaf size reduction with increasing altitude. Ann. Bot. 2011;107:455–465. doi: 10.1093/aob/mcq261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Niinemets U. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology. 2001;82:453–469. doi: 10.1890/0012-9658(2001)082[0453:GSCCOL]2.0.CO;2. [DOI] [Google Scholar]
- 100.Ogaya R, Peñuelas J. Comparative field study of Quercus ilex and Phillyrea latifolia: photosynthetic response to experimental drought conditions. Environ. Exp. Bot. 2003;50:137–148. doi: 10.1016/S0098-8472(03)00019-4. [DOI] [Google Scholar]
- 101.Onoda Y, et al. Global patterns of leaf mechanical properties. Ecol. Lett. 2011;14:301–312. doi: 10.1111/j.1461-0248.2010.01582.x. [DOI] [PubMed] [Google Scholar]
- 102.Ordoñez JC, et al. Plant strategies in relation to resource supply in mesic to wet environments: does theory mirror nature? Am. Naturalist. 2010;175:225–239. doi: 10.1086/649582. [DOI] [PubMed] [Google Scholar]
- 103.Paula S, et al. Fire-related traits for plant species of the Mediterranean Basin. Ecology. 2009;90:46470. doi: 10.1890/08-1309.1. [DOI] [Google Scholar]
- 104.Peco B, De Pablos I, Traba J, Levassor C. The effect of grazing abandonment on species composition and functional traits: the case of dehesa grasslands. Basic Appl. Ecol. 2005;6:175–183. doi: 10.1016/j.baae.2005.01.002. [DOI] [Google Scholar]
- 105.Adler PB, Milchunas DG, Lauenroth WK, Sala OE, Burke IC. Functional traits of graminoids in semi-arid steppes: A test of grazing histories. J. Appl. Ecol. 2004;41:653–663. doi: 10.1111/j.0021-8901.2004.00934.x. [DOI] [Google Scholar]
- 106.Pierce S, Brusa G, Sartori M, Cerabolini BEL. Combined use of leaf size and economics traits allows direct comparison of hydrophyte and terrestrial herbaceous adaptive strategies. Ann. Bot. 2012;109:1047–1053. doi: 10.1093/aob/mcs021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bakker C, Van Bodegom PM, Nelissen HJM, Ernst WHO, Aerts R. Plant responses to rising water tables and nutrient management in calcareous dune slacks. Plant Ecol. 2006;185:19–28. doi: 10.1007/s11258-005-9080-5. [DOI] [Google Scholar]
- 108.Pierce S, Brusa G, Vagge I, Cerabolini BEL. Allocating CSR plant functional types: the use of leaf economics and size traits to classify woody and herbaceous vascular plants. Funct. Ecol. 2013;27:1002–1010. doi: 10.1111/1365-2435.12095. [DOI] [Google Scholar]
- 109.Pierce S, Ceriani RM, DE Andreis R, Luzzaro A, Cerabolini B. The leaf economics spectrum of Poaceae reflects variation in survival strategies. Plant Biosyst. 2007;141:337–343. doi: 10.1080/11263500701627695. [DOI] [Google Scholar]
- 110.Pierce S, Luzzaro A, Caccianiga M, Ceriani RM, Cerabolini B. Disturbance is the principal α-scale filter determining niche differentiation, coexistence and biodiversity in an alpine community. J. Ecol. 2007;95:698–706. doi: 10.1111/j.1365-2745.2007.01242.x. [DOI] [Google Scholar]
- 111.Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. N. Phytologist. 2009;182:565–588. doi: 10.1111/j.1469-8137.2009.02830.x. [DOI] [PubMed] [Google Scholar]
- 112.Poschlod P, Kleyer M, Jackel A-K, Dannemann A, Tackenberg O. BIOPOP—A database of plant traits and internet application for nature conservation. Folia Geobotanica. 2003;38:263–271. doi: 10.1007/BF02803198. [DOI] [Google Scholar]
- 113.Prentice IC, et al. Evidence of a universal scaling relationship for leaf CO2 drawdown along an aridity gradient. N. Phytologist. 2011;190:169–180. doi: 10.1111/j.1469-8137.2010.03579.x. [DOI] [PubMed] [Google Scholar]
- 114.Preston KA, Cornwell WK, DeNoyer JL. Wood density and vessel traits as distinct correlates of ecological strategy in 51 California coast range angiosperms. N. Phytologist. 2006;170:807–818. doi: 10.1111/j.1469-8137.2006.01712.x. [DOI] [PubMed] [Google Scholar]
- 115.Price CA, Enquist BJ. Scaling mass and morphology in leaves: an extension of the wbe model. Ecology. 2007;88:1132–1141. doi: 10.1890/06-1158. [DOI] [PubMed] [Google Scholar]
- 116.Pyankov VI, Kondratchuk AV, Shipley B. Leaf structure and specific leaf mass: the alpine desert plants of the Eastern Pamirs, Tadjikistan. N. Phytologist. 1999;143:131–142. doi: 10.1046/j.1469-8137.1999.00435.x. [DOI] [Google Scholar]
- 117.Quested HM, et al. Decomposition of sub-arctic plants with differing nitrogen economies: a functional role for hemiparasites. Ecology. 2003;84:3209–3221. doi: 10.1890/02-0426. [DOI] [Google Scholar]
- 118.Blonder B, et al. The leaf-area shrinkage effect can bias paleoclimate and ecology research. Am. J. Bot. 2012;99:1756–1763. doi: 10.3732/ajb.1200062. [DOI] [PubMed] [Google Scholar]
- 119.Reich PB, Oleksyn J, Wright IJ. Leaf phosphorus influences the photosynthesis-nitrogen relation: a cross-biome analysis of 314 species. Oecologia. 2009;160:207–212. doi: 10.1007/s00442-009-1291-3. [DOI] [PubMed] [Google Scholar]
- 120.Reich PB, et al. Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecol. Lett. 2008;11:793–801. doi: 10.1111/j.1461-0248.2008.01185.x. [DOI] [PubMed] [Google Scholar]
- 121.Kew, R. B. G. Seed information database (SID). Version 7.1. http://www.kew.org/data/sid(2008).
- 122.Scherer-Lorenzen M, Schulze ED, Don A, Schumacher J, Weller E. Exploring the functional significance of forest diversity: A new long-term experiment with temperate tree species (BIOTREE) Perspect. Plant Ecol. Evol. Syst. 2007;9:53–70. doi: 10.1016/j.ppees.2007.08.002. [DOI] [Google Scholar]
- 123.Schweingruber, F. & Landolt, W. The xylem database. Swiss Federal Research Institute WSLhttp://www.wsl.ch/dendropro/xylemdb/ (2005).
- 124.Sheremet’ev, S. N. Herbs on the soil moisture gradient (water relations and the structural-functional organization). (KMK, Moscow, 2005).
- 125.Shipley B. Trade-offs between net assimilation rate and specific leaf area in determining relative growth rate: relationship with daily irradiance. Funct. Ecol. 2002;16:682–689. doi: 10.1046/j.1365-2435.2002.00672.x. [DOI] [Google Scholar]
- 126.Shipley B. Structured interspecific determinants of SLA in 34 species of herbaceous angiosperms. Funct. Ecol. 1995;9:312–319. doi: 10.2307/2390579. [DOI] [Google Scholar]
- 127.Shipley B, Lechowicz MJ. The functional co-ordination of leaf morphology, nitrogen concentration, and gas exchange in 40 wetland species. Ecoscience. 2000;7:183–194. doi: 10.1080/11956860.2000.11682587. [DOI] [Google Scholar]
- 128.Shipley B, Parent M. Germination responses of 64 wetland species in relation to seed size, minimum Time to reproduction and seedling relative growth rate. Source Funct. Ecol. 1991;5:111–118. doi: 10.2307/2389561. [DOI] [Google Scholar]
- 129.Blonder B, Violle C, Enquist BJ. Assessing the causes and scales of the leaf economics spectrum using venation networks in Populus tremuloides. J. Ecol. 2013;101:981–989. doi: 10.1111/1365-2745.12102. [DOI] [Google Scholar]
- 130.Shipley B, Vu TT. Dry matter content as a measure of dry matter concentration in plants and their parts. N. Phytologist. 2002;153:359–364. doi: 10.1046/j.0028-646X.2001.00320.x. [DOI] [Google Scholar]
- 131.Spasojevic MJ, Suding KN. Inferring community assembly mechanisms from functional diversity patterns: The importance of multiple assembly processes. J. Ecol. 2012;100:652–661. doi: 10.1111/j.1365-2745.2011.01945.x. [DOI] [Google Scholar]
- 132.Tucker SS, Craine JM, Nippert JB. Physiological drought tolerance and the structuring of tallgrass prairie assemblages. Ecosphere. 2011;2:48. doi: 10.1890/ES11-00023.1. [DOI] [Google Scholar]
- 133.Van Bodegom PM, Sorrell BK, Oosthoek A, Bakker C, Aerts R. Separating the effects of partial submergence and soil oxygen demand on plant physiology. Ecology. 2008;89:193–204. doi: 10.1890/07-0390.1. [DOI] [PubMed] [Google Scholar]
- 134.Minden V, Kleyer M. Testing the effect-response framework: key response and effect traits determining above-ground biomass of salt marshes. J. Vegetation Sci. 2011;22:387–401. doi: 10.1111/j.1654-1103.2011.01272.x. [DOI] [Google Scholar]
- 135.Minden V, Andratschke S, Spalke J, Timmermann H, Kleyer M. Plant trait-environment relationships in salt marshes: Deviations from predictions by ecological concepts. Perspect. Plant Ecol. Evolution Syst. 2012;14:183–192. doi: 10.1016/j.ppees.2012.01.002. [DOI] [Google Scholar]
- 136.Vergutz, L., Manzoni, S., Porporato, A., Novais, R. F. & Jackson, R. B. A Global Database of Carbon and Nutrient Concentrations of Green and Senesced Leaves. (2012).
- 137.Vile, D. Significations fonctionnelle et ecologique des traits des especes vegetales: exemple dans une succession post-cultural mediterraneenne et generalisations. (2005).
- 138.Han W, et al. Floral, climatic and soil pH controls on leaf ash content in China’s terrestrial plants. Glob. Ecol. Biogeogr. 2012;21:376–382. doi: 10.1111/j.1466-8238.2011.00677.x. [DOI] [Google Scholar]
- 139.Wirth C, Lichstein J. The imprint of species turnover on old-growth forest carbon balances-Insights from a trait-based model of forest dynamics. Old Growth Forest. SE-5. 2009;207:81–113. [Google Scholar]
- 140.Sandel B, Corbin JD, Krupa M. Using plant functional traits to guide restoration: a case study in California coastal grassland. Ecosphere. 2011;2:1–16. doi: 10.1890/ES10-00175.1. [DOI] [Google Scholar]
- 141.Chen Y, Han W, Tang L, Tang Z, Fang J. Leaf nitrogen and phosphorus concentrations of woody plants differ in responses to climate, soil and plant growth form. Ecography. 2013;36:178–184. doi: 10.1111/j.1600-0587.2011.06833.x. [DOI] [Google Scholar]
- 142.Medlyn BE, et al. Effects of elevated CO2 on photosynthesis in European forest species: a meta-analysis of model parameters. Plant Cell Environ. 1999;22:1475–1495. doi: 10.1046/j.1365-3040.1999.00523.x. [DOI] [Google Scholar]
- 143.Cayuela L, Granzow-de la Cerda Í, Albuquerque FS, Golicher DJ. Taxonstand: an R package for species names standardisation in vegetation databases. Methods Ecol. Evol. 2012;3:1078–1083. doi: 10.1111/j.2041-210X.2012.00232.x. [DOI] [Google Scholar]
- 144.McIlroy, D., Brownrigg, R., Minka, T. P. & Bivand, R. mapproj: Map Projections. 1–2 (2014).
- 145.Büntgen U, Psomas A, Schweingruber FH. Introducing wood anatomical and dendrochronological aspects of herbaceous plants: Applications of the Xylem Database to vegetation science. J. Vegetation Sci. 2014;25:967–977. doi: 10.1111/jvs.12165. [DOI] [Google Scholar]
- 146.Chapin FS, III, Matson PA, Vitousek P. Principles of terrestrial ecosystem ecology. Springe. Sci. Bus. Media. 2011 doi: 10.5860/choice.40-2771. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All trait data compiled through the Tundra Trait Team are available at http://github.com/TundraTraitTeam/TraitHub, see Bjorkman et al.30. Additional global trait data are available through the TRY database (www.try-db.org)15.
Code is available at http://github.com/hjdthomas/Tundra_trait_variation.





