Abstract
Acute kidney injury (AKI) is a serious complication in the intensive care unit (ICU), which may increase the mortality of critically ill patients. The red blood cell distribution width (RDW) has proved useful as a predictor of short-term prognosis in critically ill patients with AKI. However, it remains unknown whether RDW has a prognostic value of long-term all-cause mortality in these patients. The data of 18279 critically ill patients with AKI at first-time hospital admission were extracted from the Medical Information Mart for Intensive Care III (MIMIC-III) database. The tertiles of the RDW values were used to divide subjects into three groups, namely RDW < 13.6% for the low RDW group, 13.6% ≤ RDW < 15.2% for the middle RDW group and RDW ≥ 15.2% for the high RDW group. Demographic data, mortality, 4-year survival time and severity scale scores were compared among groups. The Kaplan-Meier analysis and the Cox regression analysis were performed to assess the impact of RDW on all-cause mortality in AKI patients. The receiver operating characteristic (ROC) curve analysis was done to evaluate the prognostic value of RDW on the long-term outcome of critically ill patients with AKI. The median age of the enrolled subjects was 65.6 years. AKI patients with a higher RDW value had significantly shorter survival time and higher death rate. By the Kaplan-Meier analysis, patients in the higher RDW group presented significantly shorter survival time and higher death rate. The Cox regression model indicated RDW as an independent risk factor of all-cause mortality of AKI patients (HR 1.219, 95% CI, 1.211 to 1.228). By the ROC analysis, RDW appeared more efficient in predicting long-term prognosis as compared with conventional severity scales. The AUC of RDW (95% CI, 0.712 to 0.725) was significantly higher than other severity scale scores. In conclusion, RDW is positively correlated to survival time of 4-year follow-up in critically ill patients with AKI, and RDW is an independent prognostic factor of long-term outcomes of these patients.
Subject terms: Acute kidney injury, Risk factors
Introduction
Acute kidney injury (AKI) is one of the most common complications in critically ill patients, which results in poor prognosis and high risk of death1. Approximately 57% of patients in the intensive care unit (ICU) were complicated with AKI, and up to 27% died consequently during hospitalization2. When AKI occurs concomitantly with other severe organ dysfunctions like myocardial infarction or sepsis, the mortality rate is further increased to 45% to 60%3.
A number of scoring systems are being used to evaluate severity and to predict prognosis of critically ill patients, but none of them is specific for those patients complicated with AKI. Moreover, although various biomarkers are frequently used to predict short-term prognosis and in-hospital mortality of critically ill patients with AKI, including urinary neutrophil gelatinase-associated lipocalin4, right ventricular longitudinal strain5, microRNAs6 and so forth, long-term prognosis is scarcely evaluated due to the difficulty in long-term follows-up. Since AKI may result in permanent injuries of kidney and the long-term risk of death is twice as high in patients with AKI as those without AKI7, prediction of long-term prognosis and early intervention for AKI patients are of utmost importance.
The red blood cell distribution width (RDW) represents the variability in size of circulating erythrocytes8. Clinically, RDW is useful for the prognostic prediction of acute disease like sepsis9 and pancreatitis10. Although several studies reported the prognostic efficiency of RDW on AKI, conclusions are inconsistent. Wang et al. reported that the short-term prognostic value of RDW was more accurate than Acute Physiology Score III (APS III) and the Sequential Organ Failure Assessment (SOFA) in critically ill patients with AKI11. Hu et al. also demonstrated that RDW was an independent predictor for AKI and mortality in patients in the coronary care unit8. Instead, Elhosseiny et al. found that RDW did not correlate with the development of contrast-induced AKI12. Besides, all previous studies were focused on the short-term prediction efficiency of RDW on AKI. Whether RDW can be used to predict the long-term prognosis in AKI patients and the efficiency of RDW therein as compared with conventional scales merit further investigation.
In this study, data of critically ill patients complicated with AKI during 48 hours after admission to ICU were extracted. We hypothesized that RDW is useful in the prediction of long-term prognosis of critically ill patients with AKI. We analyzed the relationship between RDW and the survival time of patients at different AKI stages. The prognostic value of 4-year mortality as measured by RDW and/or RDW combined with several severity scales was evaluated.
Subjects and methods
Data source
Data were extracted from the Medical Information Mart for Intensive Care III (MIMIC-III) database, which recorded the demographic data, vital signs, medications and other important items of 53,423 adult admissions to ICUs in the Beth Israel Deaconess Medical Center in Boston from 2001 to 201213. The establishment of the MIMIC-III database was approved by the Massachusetts Institute of Technology and the Institutional Review Boards. According to the Health Insurance Portability and Accountability Act (HIPAA) standards (www.hhs.gov), eighteen identifying data elements were removed from the MIMIC-III database13. Our study was conducted entirely on publicly available, anonymized data, thus individual patient consents were waived. To get the permission of access to the MIMIC-III database, Linpei Jia passed the Protecting Human Research Participants Exam of National Institutes of Health (Record ID: 27638410).
Inclusion and exclusion criteria
AKI was defined by the Acute Kidney Injury Network (AKIN) criteria, i.e. serum creatinine (Scr) values increasing ≥ 26.5 μmol/L or 1.5-fold of baseline values during 48 hours, or urine out <0.5 mL/(kg∙h) for more than 6 hours14. In our study, we used the AKIN criteria instead of the Kidney Disease: Improving Global Outcomes (KDIGO) criteria for two reasons. One reason is that most data in the MIMIC-III database were recorded before the publication of the KDIGO guideline15. Likewise, some other studies used the AKIN criteria for the same reason16. The other reason is that during the 7-day follow-up, confounding factors, such as antibiotics and hospital infection, may interfere the kidney function, thus renal function was assessed and AKI was diagnosed within 48 hours after admission17. Because Scr values within 3 months before admission were not recorded in the database, the first measurement of Scr within 24 hours after admitted into ICU was set as the baseline18.
Included patients were those: (1) with first admission to ICU during hospital stays; (2) with AKI during 48 hours after admitted to ICU; (3) aged ≥18 years old and ≤89 years old; (4) were followed-up for 4 years by the CareVue system13. Patients without any RDW data within 24 hours after admission or missing >5% indices were not included in our study. Patients with hospitalization duration longer than 100 days were excluded19.
Data extraction
Data of each patient were extracted from the MIMIC-III database by the Structured Query Language, including age, sex, admission type, ethnicity, marriage, status of renal replacement therapy, RDW values, the survival time, days of hospital stay, and days of ICU stay (Additional File 1). If RDW was measured for several times within 24 hours after the ICU entry, data of the first time were used. Comorbidities were defined as per the Implementation of the International Statistical Classification of Disease and Related Health Problems, 10th Revision (ICD-10) coding systems20. APS III21, the Modified Logistic Organ Dysfunction System (MLODS)22, SOFA23, the Oxford Acute Severity of Illness Score (OASIS)24 and the Systemic Inflammatory Response Syndrome (SIRS) status25 were calculated according to the physiological and laboratory parameters in the MIMIC-III database for estimation of prognosis in AKI patients. All methods were carried out in accordance with relevant guidelines to protect the privacy of patients.
Grouping
The data distribution of RDW in all the enrolled subjects was calculated; the lower tertile and the upper tertile were 13.6% and 15.2% respectively. We hence divided all of the subjects into three groups based on the tertiles of RDW values26,27, namely the low RDW group (RDW < 13.6%), the middle RDW group (13.6% ≤ RDW < 15.2%), and the high RDW group (RDW ≥ 15.2%). Thereafter, we divided patients into subgroups according to AKI stages which are based on the AKIN guidelines14. In specific, AKI stage 1 was defined as AKI with an increase of Scr values ≥1.5–1.9 folds or ≥26.5 μmol/L, or urine output <0.5 ml/(kg∙h) for more than 6 hours; AKI stage 2 was defined as AKI with an increase of Scr values ≥2.0–2.9 folds or urine output to <0.5 ml/(kg∙h) for more than 12 hours; AKI stage 3 was defined as AKI with an increase of Scr values ≥ 3.0 folds or ≥354 μmol/L, or urine output <0.3 ml/(kg∙h) for more than 24 hours. Patients with an anuric status >12 hours were also classified as AKI stage 3.
Outcomes
The MIMIC-III database provides the follow-up data by two systems, namely the Philips CareVue Clinical Information System (models M2331A and M1215A; Philips Health-care, Andover, MA) for four years and the iMDsoft MetaVision ICU (iMDsoft, Needham, MA) for 90 days. Because we aimed at investigating the long-term prognostic effects of RDW, the four-year outcomes after ICU admission in the CareVue system were used. The all-cause death was used as the end-point in our study. We extracted the death status recorded in CareVue system. Since the MIMIC-III database was connected with social security database, which ensures the integrity of follow-up data, we also replenished all-cause death data in four years for patients without recording in the CareVue system.
Statistics
The trend test of one-way analysis of variance (ANOVA) was used for the analysis of continuous data, and the trend test of Chi-square test was used for categorical data. The linear regression model, the Kaplan-Meier (K-M) curve and the Cox regression model were utilized to analyze the relationship between RDW and survival in AKI patients. The receiver operating characteristic (ROC) curve analysis was performed to compare the area under the ROC curve (AUC), which represented the prognostic efficiency. Statistical analyses were performed with the SPSS 22.0 software (SPSS, IBM, NY, US), GraphPad Prism 7.0 (GraphPad Software, San Diego, US) and the Medcalc 18.5.0 software (MedCalc Software, Ostend, Belgium). Statistical significance was defined as P < 0.05. Graphs were generated by Medcalc 18.5.0 and GraphPad Prism 7.0 (GraphPad Software, California, US).
Results
Eighteen thousand two hundred and seventy-nine patients were enrolled
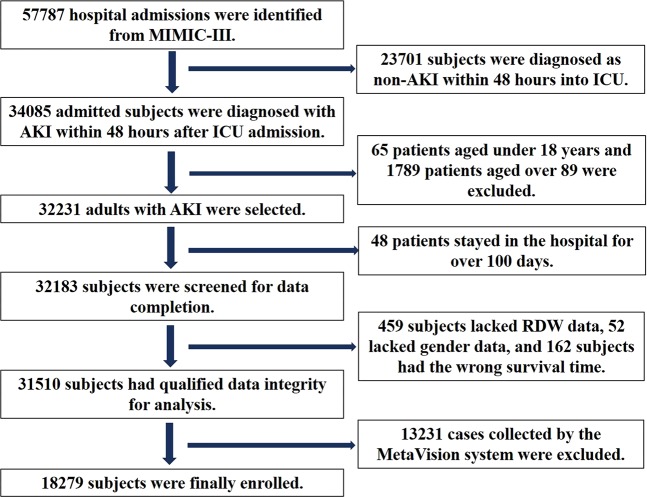
Initially, 57787 subjects were selected from MIMIC-III. After screening, 18279 subjects with mean age of 63.4 ± 16.2 years were enrolled (Fig. 1). The demographic data of the enrolled patients were shown in Table 1. Among these patients, 58.3% were males, and 82.3% were admitted via the emergency room. As the database was constructed in the United States, a majority of the included patients were Caucasian (69.6%). According to the KDIGO guideline, patients at AKI stage 2 accounted for 37.2%, 32.4% of patients were at AKI stage 3, while the others were at AKI stage 1. Only 8.8% of all subjects were treated with renal replacement therapy. Higher severity scale scores represent more severity in critically ill patients with AKI (Table 1). The leading comorbidities were congestive heart failure and chronic pulmonary disease, which accounted for 31.9% and 18.5%, respectively, and 15.9% of the patients were complicated with renal failure. The median hospital stay of all patients was 7.9 days and the median ICU stay was 2.7 days. The all-cause death rate for all subjects was 43.6%.
Figure 1.
Flowchart of subject screening. Initially, data of 57787 subjects were extracted from the Medical Information Mart for Intensive Care III (MIMIC-III) database. Changes of serum creatinine and urine output within 48 hours after admissions to ICUs were calculated. Then 23701 subjects were excluded as non-acute kidney injury (AKI) patients. Because age of patients over 89 years were marked as 300 or more in the database to protect the privacy, we further excluded 1789 senilities for analysis. After filtering out 65 subjects younger than 18-year-old, 32231 adults were further screened for hospital stays. Hence, 32183 subjects with hospital stay less than 100 days were checked for data integrity, and 459 subjects without red blood cell distribution width (RDW), 52 subjects without sex information and 162 subjects with wrong survival time were excluded. Since the MetaVision system only provided 90-day follow-up data, only 18279 patients with complete follow-up data for 4 years were finally enrolled in our study.
Table 1.
Demographic data of study subjects.
| All subjects | Red blood cell distribution width (%) | ||||
|---|---|---|---|---|---|
| <13.6 | 13.6–15.2 | ≥15.2 | P | ||
| (N = 5696) | (N = 6269) | (N = 6314) | |||
| Age (years) | 63.4 ± 16.2 | 59.6 ± 17.7 | 65.3 ± 15.4 | 65.0 ± 14.8 | <0.01 |
| Male | 10661 (58.3%) | 3625 (63.6%) | 3612 (57.6%) | 3424 (54.2%) | <0.01 |
| Admission type | |||||
| Emergency | 15050 (82.3%) | 4574 (80.3%) | 4964 (79.2%) | 5512 (87.3%) | <0.01 |
| Urgent | 641 (3.5%) | 210 (3.7%) | 210 (3.3%) | 221 (3.5%) | |
| Elective | 2588 (14.2%) | 912 (16.0%) | 1095 (17.5%) | 581 (9.2%) | |
| Ethnicity | <0.01 | ||||
| Caucasian | 12714 (69.6%) | 3903 (68.5%) | 4466 (71.2%) | 4345 (68.8%) | |
| Black | 1765 (9.7%) | 334 (5.9%) | 534 (8.5%) | 897 (14.2%) | |
| Asian | 345 (1.9%) | 106 (1.9%) | 121 (1.9%) | 118(1.9%) | |
| Others | 3455 (18.9%) | 1353 (23.8%) | 1148 (18.3%) | 945 (15.1%) | |
| Marriage | <0.01 | ||||
| Married | 9118 (49.9%) | 2893 (50.8%) | 3188 (50.9%) | 3037 (48.1%) | |
| Single | 4235 (23.2%) | 1376 (24.2%) | 1354 (21.6%) | 1505 (23.8%) | |
| Others | 4926 (26.9%) | 1427 (25.1%) | 1727 (27.5%) | 1772 (28.1%) | |
| AKI stage | <0.01 | ||||
| 1 | 5557 (30.4%) | 1751 (30.8%) | 1834 (29.3%) | 1970 (31.2%) | |
| 2 | 6798 (37.2%) | 2233 (39.2%) | 2529 (40.3%) | 2036 (32.2%) | |
| 3 | 5924 (32.4%) | 1710 (30.0%) | 1906 (30.4%) | 2308 (36.6%) | |
| Renal replacement therapy | |||||
| Yes | 1616 (8.8%) | 108 (1.9%) | 334 (5.3%) | 1174 (18.6%) | <0.01 |
| No | 16663 (92.4%) | 5588 (98.1%) | 5935 (94.7%) | 5140 (81.4%) | |
| Severity scale | |||||
| APS III | 44.71 ± 19.71 | 37.87 ± 17.07 | 43.51 ± 18.74 | 52.07 ± 20.38 | <0.01 |
| MLODS | 2.89 ± 2.41 | 2.22 ± 2.18 | 2.83 ± 2.37 | 3.57 ± 2.46 | <0.01 |
| SOFA | 4.52 ± 3.09 | 3.59 ± 2.61 | 4.39 ± 2.92 | 5.51 ± 3.35 | <0.01 |
| OASIS | 32.08 ± 8.01 | 30.79 ± 8.47 | 32.23 ± 8.62 | 33.09 ± 9.13 | <0.01 |
| SIRS | 2.85 ± 1.00 | 2.84 ± 1.02 | 2.88 ± 0.99 | 2.82 ± 0.98 | <0.01 |
| Comorbidity | |||||
| Chronic pulmonary disease | 3389 (18.5%) | 745 (13.1%) | 1265 (20.2%) | 1379 (21.8%) | <0.01 |
| Congestive heart failure | 5831 (31.9%) | 1001 (17.6%) | 2069 (33.0%) | 2761 (43.7%) | <0.01 |
| Liver disease | 1363 (7.5%) | 109 (1.9%) | 338 (5.4%) | 916 (14.5%) | <0.01 |
| Metastatic cancer | 924 (5.1%) | 125 (2.2%) | 269 (4.3%) | 530 (8.4%) | <0.01 |
| Renal failure | 2898 (15.9%) | 216 (3.8%) | 738 (11.8%) | 1944 (30.8%) | <0.01 |
| Time in hospital (days) | 11.3 ± 10.8 | 9.6 ± 9.3 | 11.1 ± 10.4 | 12.9 ± 12.2 | <0.01 |
| Time in ICU (days) | 5.1 ± 7.2 | 4.5 ± 6.5 | 5.1 ± 7.3 | 5.6 ± 7.6 | <0.01 |
| Death | 7973 (43.6%) | 1314 (23.1%) | 2507 (40%) | 4152 (65.8%) | <0.01 |
Note: AKI, acute kidney injury; APS III, acute physiology and chronic health evaluation III; MLODS, modified logistic organ dysfunction system; SOFA, sequential organ failure assessment; OASIS, oxford acute severity of illness score; SIRS, systemic inflammatory response syndrome; ICU, intensive care unit.
RDW values were negatively correlated with survival time of critically ill patients with AKI
Demographic data were compared, and all parameters, including gender, age, admission type, marriage, ethnicity, AKI stage, traditional severity scores, comorbidities, ICU stay and hospital stay, have shown differences among the three RDW groups (all P < 0.01, Table 1). In all subjects, the death rate was increased from low to high RDW groups (23.1% for the low RDW group, 40% for the middle RDW group, 65.8% for the high RDW group, Table 1 and Fig. 2A), while the survival time was decreased from low to high RDW groups (Fig. 2B). Similar trends in death rate and survival time were found at each AKI stage (Fig. 2A,B). In the linear regression model, survival time was negatively correlated to RDW values (r = 0.35, P < 0.01).
Figure 2.
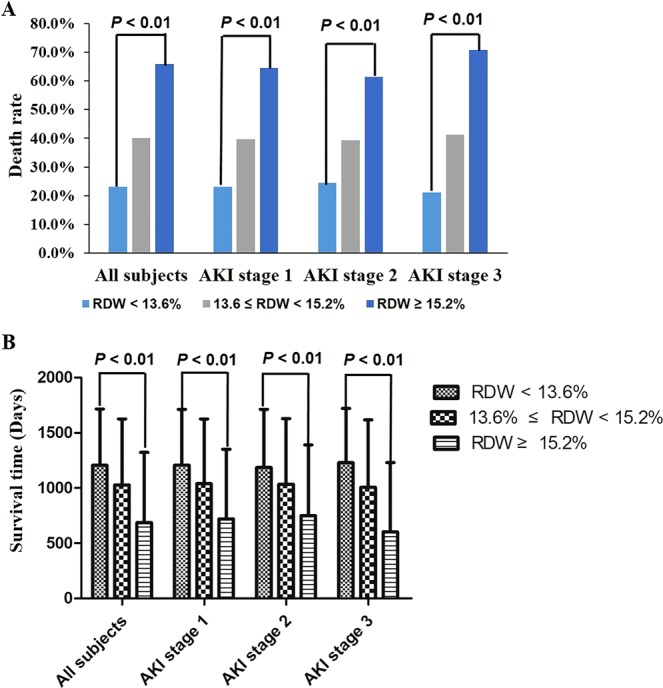
Relationship between red blood cell distribution width (RDW) and all-cause death in critically ill patients with acute kidney injury (AKI). AKI patients were divided into the low (RDW < 13.6%), middle (13.6% ≤ RDW < 15.2%) and high (RDW ≥ 15.2%) RDW groups according to the first RDW measurement. Death rates increased from the low RDW group to the high RDW group for all patients (23.10%, 40.00% and 65.80% for the low, the middle and the high RDW groups respectively, P < 0.01, A). The same relationship was also found at AKI stage 1 (23.20%, 39.70% and 64.50% for the low, middle and high RDW groups respectively, P < 0.01), stage 2 (24.50%, 39.30% and 61.30% for the low, middle and high RDW groups respectively, P < 0.01) and stage 3 (21.10%, 41.20% and 70.80% for the low, middle and high RDW groups respectively, P < 0.01). With the RDW increasing, survival time decreased from 1205.7 ± 509.5 days to 687.0 ± 635.8 days (P < 0.01, B). In subgroup analysis, survival time of each RDW groups were also compared at each AKI stage separately, and AKI patients of low RDW group had the longest survival time at all stages (P < 0.01, B).
RDW was an independent risk factor of 4-year all-cause mortality in critically ill patients with AKI
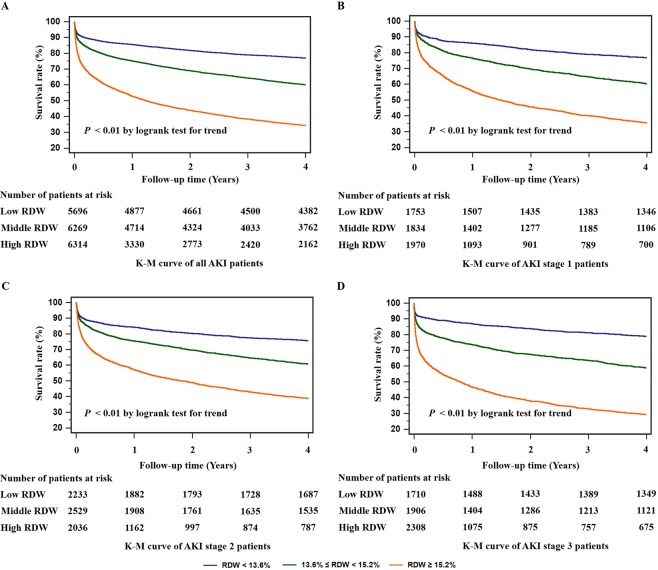
By the K-M analysis, higher RDW values were associated with lower survival rate and shorter survival time (P < 0.01 by logrank test for trend, Fig. 3A). We also conducted subgroup analysis of AKI stage 1, stage 2 and stage 3 groups respectively by the K-M curve, while patients with higher RDW values also showed shorter survival time and higher death rate at each stage (P < 0.01 by logrank test for trend, Fig. 3B–D). Then the Cox regression models were performed for the association between RDW and all-cause mortality independently as well as adjusted by other severity scale scores. RDW was independently related to the all-cause mortality of AKI patients both in unadjusted and adjusted Cox regression models (P < 0.01, Table 2). RDW was also shown as an independent risk factor for all-cause mortality at each AKI stage. In Cox regression models, RDW remained a risk factor of mortality of AKI patients before and after adjustment (Table 3).
Figure 3.
Kaplan-Meier (K-M) survival curves for 4-year all-cause mortalities of critically ill patients with acute kidney injury (AKI). The risk of 4-year overall mortality of each red blood cell distribution width (RDW) group in all participants was shown (A). Patients with RDW ≥ 15.2% had the highest all-cause mortality. In subgroup analysis, the same changes of the Kaplan-Meier curves were observed at AKI stage 1 (B), stage 2 (C) and stage 3 (D).
Table 2.
Relationship between RDW and all-cause mortality in Cox model before and after adjustment.
| All subjects (N = 18279) | AKI stage 1 (N = 5557) | AKI stage 2 (N = 6798) | AKI stage 3 (N = 5924) | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Unadjusted RDW | 1.219 (1.211, 1.228) | <0.01 | 1.214 (1.197, 1.230) | <0.01 | 1.202 (1.185, 1.218) | <0.01 | 1.231 (1.217, 1.245) | <0.01 |
| RDW adjusted for | ||||||||
| APS III | 1.173 (1.164, 1.182) | <0.01 | 1.185 (1.168, 1.202) | <0.01 | 1.172 (1.155, 1.189) | <0.01 | 1.159 (1.144, 1.174) | <0.01 |
| MLODS | 1.194 (1.185, 1.203) | <0.01 | 1.193 (1.176, 1.210) | <0.01 | 1.190 (1.174, 1.207) | <0.01 | 1.185 (1.171, 1.200) | <0.01 |
| SOFA | 1.187 (1.178, 1.196) | <0.01 | 1.189 (1.172, 1.207) | <0.01 | 1.189 (1.173, 1.206) | <0.01 | 1.173 (1.158, 1.187) | <0.01 |
| OASIS | 1.213 (1.204, 1.222) | <0.01 | 1.225 (1.208, 1.242) | <0.01 | 1.199 (1.182, 1.216) | <0.01 | 1.202 (1.188, 1.217) | <0.01 |
| SIRS | 1.222 (1.211, 1.229) | <0.01 | 1.215 (1.198, 1.232) | <0.01 | 1.202 (1.186, 1.219) | <0.01 | 1.228 (1.214, 1.242) | <0.01 |
Note: AKI, acute kidney injury; HR, hazard ratio; CI, confidence interval; SE, standard error; RDW, red blood cell distribution width; APS III, acute physiology and chronic health evaluation III; MLODS, modified logistic organ dysfunction system; SOFA, sequential organ failure assessment; OASIS, oxford acute severity of illness score; SIRS, systemic inflammatory response syndrome.
Table 3.
Relationship between RDW and all-cause mortality of patients without chronic kidney disease in Cox model before and after adjustment.
| All subjects (N = 15381) | AKI stage 1 (N = 4520) | AKI stage 2 (N = 6174) | AKI stage 3 (N = 4687) | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Unadjusted RDW | 1.221 (1.210, 1.231) | <0.01 | 1.212 (1.192, 1.233) | <0.01 | 1.206 (1.188, 1.224) | <0.01 | 1.234 (1.218, 1.250) | <0.01 |
| RDW adjusted for | ||||||||
| APS III | 1.177 (1.166, 1.188) | <0.01 | 1.189 (1.168, 1.211) | <0.01 | 1.177 (1.159, 1.195) | <0.01 | 1.160 (1.143, 1.178) | <0.01 |
| MLODS | 1.202 (1.191, 1.213) | <0.01 | 1.205 (1.184, 1.226) | <0.01 | 1.197 (1.179, 1.216) | <0.01 | 1.190 (1.173, 1.207) | <0.01 |
| SOFA | 1.191 (1.181, 1.202) | <0.01 | 1.199 (1.178, 1.220) | <0.01 | 1.194 (1.176, 1.212) | <0.01 | 1.171 (1.154, 1.188) | <0.01 |
| OASIS | 1.212 (1.202, 1.223) | <0.01 | 1.220 (1.200, 1.242) | <0.01 | 1.201 (1.183, 1.219) | <0.01 | 1.205 (1.188, 1.222) | <0.01 |
| SIRS | 1.220 (1.210, 1.230) | <0.01 | 1.214 (1.193, 1.234) | <0.01 | 1.206 (1.188, 1.224) | <0.01 | 1.230 (1.213, 1.246) | <0.01 |
Note: AKI, acute kidney injury; HR, hazard ratio; CI, confidence interval; SE, standard error; RDW, red blood cell distribution width; APS III, acute physiology and chronic health evaluation III; MLODS, modified logistic organ dysfunction system; SOFA, sequential organ failure assessment; OASIS, oxford acute severity of illness score; SIRS, systemic inflammatory response syndrome.
Effects of RDW on 4-year all-cause mortality were not influenced by the history of renal failure in critically ill patients with AKI
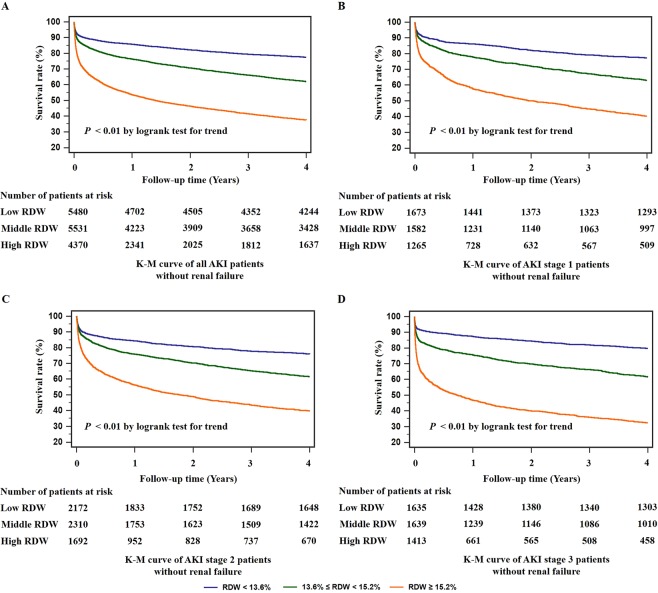
Baseline renal function may have an impact on the incidence and mortality of AKI. Hence, we did the survival analysis of RDW on patients without renal failure. The K-M curves indicated that high RDW values were associated with higher all-cause mortality and shorter survival time in all subjects as well as patients at different AKI stages (P < 0.01, Fig. 4). Thus, regardless of the baseline renal function, RDW was an independent risk factor for long-term mortality in AKI patients.
Figure 4.
Kaplan-Meier (K-M) survival curves for 4-year overall mortalities of critically ill patients with acute kidney injury (AKI) without comorbidity of renal failure. To eliminate the influences of baseline renal function, we did the Kaplan-Meier analysis for each red blood cell distribution width (RDW) groups in patients without renal failure. The survival rate of the low RDW group was significantly higher than the middle and high RDW groups (P < 0.01) for all patients in the 4-year follow-up (A). Higher risk of mortality was also shown in the high RDW group at AKI stage 1 (P < 0.01, B), 2 (P < 0.01, C) and 3 (P < 0.01, D) respectively.
RDW predicted long-term prognosis better than conventional severity scales
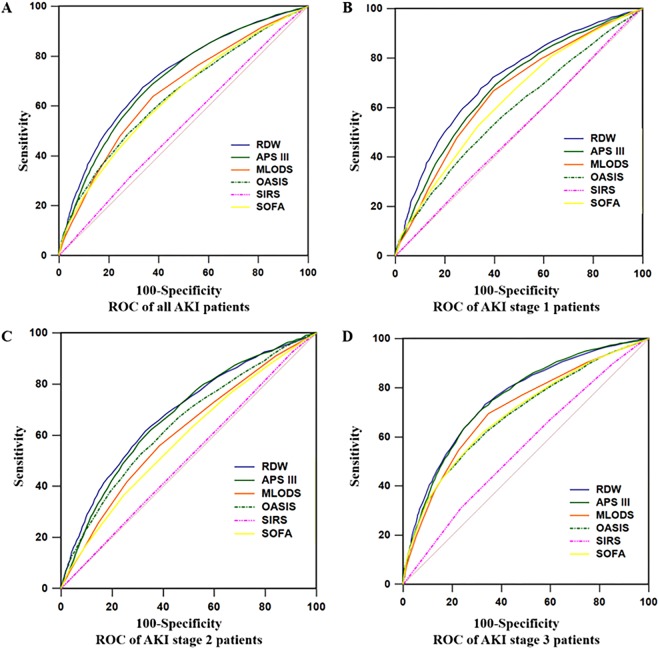
The ROC analysis was performed to evaluate the long-term prognostic value of RDW compared with conventional severity scale scores, including APS III, MLODS, OASIS, SIRS and SOFA. The AUC of RDW for all patients was 0.718 (95% CI, 0.712–0.725), which was significantly higher than other severity scale scores (P < 0.01, Fig. 5A and Table 4). For patients at AKI stage 1, RDW with AUC of 0.713 (95% CI, 0.701–0.725) had a better prognostic value than conventional severity scales (P < 0.01, Fig. 5B and Table 4). However, for patients with AKI stage 2 and 3, AUC of RDW showed no significant difference from APS III (P = 0.297 for AKI stage 2, P = 0.781 for AKI stage 3, Fig. 5C,D and Table 4). When RDW was combined with each severity score, AUCs were significantly increased after combination as compared with APS III, MLODS, OASIS, SOFA or RDW alone, respectively (all P < 0.01, Figure S1 and Table 4). However, no difference of AUC was found after combination of RDW with SIRS (P = 0.080, Figure S1 and Table 4). Taken together, RDW better predicts long-term prognosis of critically ill patients with AKI than traditional severity scoring systems. RDW may also improve the prognostic efficiency of APS III.
Figure 5.
Receiver operating curve (ROC) analyses of predictors of acute kidney injury (AKI) and mortality in intensive care units (ICU) patients. The area under ROC curve (AUC) of red blood cell distribution width (RDW) were compared with Acute Physiology Score III (APS III), the Modified Logistic Organ Dysfunction System (MLODS), the Sequential Organ Failure Assessment (SOFA), the Oxford Acute Severity of Illness Score (OASIS) and the Systemic Inflammatory Response Syndrome (SIRS). RDW values of all subjects (AUC = 0.718, A) and AKI stage 1 subjects (AUC = 0.713, B) were significantly higher than other severity scale scores (P < 0.01). However, at AKI stages 2 and 3, the AUCs of RDW (AUC = 0.684 for stage 2 in C and AUC = 0.757 for stage 3 in D) showed no difference in comparison with APS III (P = 0.297 for stage 2 and P = 0.781 for stage 3).
Table 4.
Area under receiver operating curve of RDW and severity scales at different AKI stages.
| All subjects (N = 18279) | AKI stage 1 (N = 5557) | AKI stage 2 (N = 6798) | AKI stage 3 (N = 5924) | |||||
|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | P | AUC (95% CI) | P | AUC (95% CI) | P | AUC (95% CI) | P | |
| RDW | 0.718 (0.712–0.725) | 0.713 (0.701–0.725) | 0.684 (0.673–0.695) | 0.757 (0.746–0.768) | ||||
| APS III | 0.706 (0.700–0.713) | <0.01 | 0.682 (0.670–0.695) | <0.01 | 0.675 (0.664–0.686) | 0.297 | 0.755 (0.744–0.766) | 0.781 |
| RDW + APS III | 0.758 (0.752–0.764) | <0.01 | 0.741 (0.729–0.752) | <0.01 | 0.725 (0.714–0.735) | <0.01 | 0.802 (0.792–0.812) | <0.01 |
| MLODS | 0.659 (0.652–0.666) | <0.01 | 0.660 (0.647–0.672) | <0.01 | 0.608 (0.596–0.619) | <0.01 | 0.709 (0.698–0.721) | <0.01 |
| RDW + MLODS | 0.741 (0.735–0.748) | <0.01 | 0.734 (0.722–0.745) | <0.01 | 0.700 (0.689–0.711) | <0.01 | 0.787 (0.776–0.797) | <0.01 |
| SOFA | 0.638 (0.631–0.645) | <0.01 | 0.633 (0.620–0.646) | <0.01 | 0.586 (0.574–0.597) | <0.01 | 0.695 (0.683–0.707) | <0.01 |
| RDW + SOFA | 0.732 (0.726–0.739) | <0.01 | 0.724 (0.712–0.736) | <0.01 | 0.689 (0.678–0.700) | 0.115 | 0.780 (0.769–0.791) | <0.01 |
| OASIS | 0.642 (0.635–0.649) | <0.01 | 0.590 (0.577–0.603) | <0.01 | 0.643 (0.631–0.654) | <0.01 | 0.691 (0.679–0.702) | <0.01 |
| RDW + OASIS | 0.750 (0.743–0.756) | <0.01 | 0.726 (0.714–0.738) | <0.01 | 0.727 (0.717–0.738) | <0.01 | 0.792 (0.781–0.802) | <0.01 |
| SIRS | 0.522 (0.514–0.529) | <0.01 | 0.506 (0.493–0.519) | <0.01 | 0.510 (0.498–0.522) | <0.01 | 0.554 (0.542–0.567) | <0.01 |
| RDW + SIRS | 0.720 (0.713–0.726) | 0.080 | 0.713 (0.701–0.725) | 0.964 | 0.685 (0.674–0.696) | 0.402 | 0.760 (0.749–0.771) | 0.018 |
Note: AKI, acute kidney injury; AUC, area under curve; CI, confidence interval; SE, standard error; RDW, red blood cell distribution width; APS III, acute physiology and chronic health evaluation III; MLODS, modified logistic organ dysfunction system; SOFA, sequential organ failure assessment; OASIS, Oxford acute severity of illness score; SIRS, systemic inflammatory response syndrome.
Discussion
Clinically, RDW is a common parameter for evaluation of anemia and inflammation. In recent years, much attention has been paid to the relationship between renal function and RDW. In this study, we explored the association between RDW and long-term outcomes of 18279 critically ill patients with AKI. Higher levels of RDW predicted shorter survival time and higher long-term death rate. Our findings suggest that RDW might be a potential predictor of all-cause mortality of critically ill patients with AKI.
First, we explored the association between RDW and survival time. We demonstrated that higher RDW predicted worse prognosis of AKI patients. Our results were consistent with previous studies on short-term outcomes, which mainly focused on how RDW was related with survival in AKI patients after percutaneous transluminal coronary intervention or with other coronary diseases, especially contrast-induced AKI8,28,29. Survivors of AKI had a lower RDW value. Similar findings about the association between low RDW and high survival rate were also reported in sepsis-induced30 and extracorporeal membrane oxygenation treated patients with AKI. Different from previous studies, however, we emphasized that the baseline RDW values also determined the long-term death rate and survival time in multiple-cause AKI.
To adjust for the confounding of baseline renal function, we further examined the relationship between RDW and long-term all-cause mortalities of AKI patients without comorbidity of renal failure. Elevation of blood RDW levels has been used to predict mortalities in chronic kidney disease (CKD) patients both with and without hemodialysis31,32. For patients with normal baseline renal function, RDW was positively correlated to 4-year survival rates, which was consistent with Odutayo’s results33. Hence RDW may serve as an independent factor of long-term outcomes of critically ill patients with AKI.
We also compared the prognostic value of RDW with traditional severity scores. Oh’s team analyzed the data of 470 renal replacement therapy-treated patients with AKI and found that SOFA with the AUC of 0.694 predicted more accurately than RDW with the AUC of 0.58634. Mizuno and colleagues showed the potential predictive ability of RDW combined with the Mehran risk score but not RDW only for contrast-induced AKI in myocardial infarction patients35. Acute physiology and chronic health evaluation II scores were also confirmed to be better than RDW in predicting in-hospital mortality as well as 2-year mortality8. Inconsistent with previous studies, however, RDW showed a better predictive value of four-year mortality than commonly used severity scoring systems in our study. This inconsistency might be due to differences in sample size and selection of subjects. On one hand, previous studies usually enrolled no more than 1000 subjects, which is far less than the sample size in our study. A large sample could make the results more precise, stable and reliable36. On the other hand, subjects in the present study were critically ill patients with AKI caused by various reasons. Heterogeneous causes of AKI might also influence the prognostic efficiency of RDW. Likewise, different factors that may influence short-term and long-term outcomes should be addressed as well37. Although traditional severity scales are used mainly to evaluate disease severity and short-term prognosis of acute diseases, studies have proved their usefulness in the long-term prognosis prediction. For example, Pekkarinen’s study indicated that SOFA was associated with 1-year outcome and healthcare costs of patients with cardiac arrest38. Hagen’s team found that SIRS could predict poor long-term functional outcome after intracerebral hemorrhage39. In line with these findings, we also showed that either RDW or severity scores could be used to predict the long-term prognosis of AKI patients; RDW appeared to be even more efficient.
Although the predictive value of RDW on all-cause mortalities has been revealed, the mechanism remains unknown. Both inflammation40 and oxidative stress41 may play crucial roles. When inflammation occurs, iron metabolism and bone marrow function are inhibited, and the proliferation and maturation of erythrocytes are inhibited40, leading to an increase of RDW values. Meanwhile, the severity of AKI is related to systematic and intrarenal inflammation42. SIRS mainly represents an inflammation status of whole body, and our results revealed that SIRS values were increased in the high RDW group. Therefore, RDW could predict the prognosis of AKI partially by reflecting the levels of inflammation. AKI patients in ICU are often complicated with activation of oxidative stress including disturbed metabolism, sepsis, and hemodynamic dysregulations43. Oxidative stress plays key roles in the erythroid cell cycle, differentiation and maturation, and RDW has been found associated with various oxidative stress biomarkers, such as serum malondialdehyde and tumor necrosis factor-alpha, in critically ill patients44. Usually, the multi-organ dysfunction is accompanied with severe oxidative stress. Hence, elevations of MLODS and SOFA were shown in the high RDW groups.
The large sample size is the advantage of our study. The complete follow-up data make it possible to investigate the long-term prognosis, which is difficult to be observed in cohort studies. However, limitations should be acknowledged. First, as mentioned in the Subjects and methods section, the AKIN criteria instead of the KDIGO criteria was used due to the intrinsic drawback of the MIMIC-III database. Second, some important information was not recorded in the MIMIC-III database, including Scr during the previous 3 months and the past medical history of end stage renal disease (ESRD), and hence estimation of previousrenal function and exclusion of patients with ESRD were impossible. Third, given the missing items of past medical histories in the MIMIC-III database, patients with hematological diseases or renal anemia were difficult to distinguish and select. Hence, effects of hematological diseases on the prognostic values of RDW were not evaluated. Besides, the missing data of some other RDW-influencing pre-existing conditions, such as serious infections, autoimmune diseases and so forth, also made stratified analyses impossible. Fourth, because the major adverse events after discharge were not followed or recorded, the predictive value of RDW on cardiovascular and cerebrovascular events was not evaluated as well.
Further improvement in the MIMIC-III database may make up for the above-mentioned limitations. Regular follows-up for up to 3 to 6 years are necessary. Detailed past medical history, medication history and the exact cause of death should be recorded, which are helpful to explore the prognostic values of RDW on important complications in critically ill patients with AKI.
Conclusion
Based on the clinical data of 18279 AKI patients in MIMIC-III database, a negative correlation was found between RDW and all-cause mortality. RDW proved to be a predictive parameter of long-term prognosis of critically ill patients with AKI.
Supplementary information
Acknowledgements
We would like to thank all the researchers who built and maintained the MIMIC-III database. At the same time, we appreciated all patients who agreed to offer the data to the MIMIC-III database. The study was supported by grants from Wu Jieping Medical Foundation Clinical Research Funding (No. 320.6750.16050) and Scientific Research Found of Capital Medical University (PYZ2018054).
Author contributions
Linpei Jia extracted data from MIMIC-III database. Linpei Jia, Rufu Jia and Jingyan Yang finished the statistical analysis. Hongliang Zhang and Shijun Cui wrote the manuscript. Hongliang Zhang, Qiang Jia and Lixiao Hao revised the manuscript. All authors have read and approved the final version.
Data availability
All data and material were available at https://mimic.mit.edu/.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Linpei Jia and Shijun Cui.
Contributor Information
Linpei Jia, Email: anny_069@163.com.
Rufu Jia, Email: zxyy5688@126.com.
Hongliang Zhang, Email: drzhl@hotmail.com.
Supplementary information
is available for this paper at 10.1038/s41598-020-61516-y.
References
- 1.Darmon M, et al. Diagnostic work-up and specific causes of acute kidney injury. Intensive Care Med. 2017;43:829–840. doi: 10.1007/s00134-017-4799-8. [DOI] [PubMed] [Google Scholar]
- 2.Hoste EA, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 3.Mehta RL, et al. Sepsis as a cause and consequence of acute kidney injury: Program to Improve Care in Acute Renal Disease. Intensive Care Med. 2011;37:241–248. doi: 10.1007/s00134-010-2089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.da Rocha EP, et al. Urinary Neutrophil Gelatinase-Associated Lipocalin Is Excellent Predictor of Acute Kidney Injury in Septic Elderly Patients. Aging Dis. 2018;9:182–191. doi: 10.14336/ad.2017.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivey-Miranda Juan Betuel, Almeida-Gutiérrez Eduardo, Borrayo-Sánchez Gabriela, Antezana-Castro Javier, Contreras-Rodríguez Alicia, Posada-Martínez Edith Liliana, González-Morales Edith, García-Hernández Nayeli, Romero-Zertuche Diana, Marquez-Gonzalez Horacio, Saturno-Chiu Guillermo. Right ventricular longitudinal strain predicts acute kidney injury and short-term prognosis in patients with right ventricular myocardial infarction. The International Journal of Cardiovascular Imaging. 2018;35(1):107–116. doi: 10.1007/s10554-018-1447-5. [DOI] [PubMed] [Google Scholar]
- 6.Ivan MV, et al. New Molecular and Epigenetic Expressions as Novel Biomarkers in Critically Ill Polytrauma Patients with Acute Kidney Injury (AKI) Clin. Lab. 2018;64:663–668. doi: 10.7754/Clin.Lab.2018.171226. [DOI] [PubMed] [Google Scholar]
- 7.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Y, Liu H, Fu S, Wan J, Li X. Red Blood Cell Distribution Width is an Independent Predictor of AKI and Mortality in Patients in the Coronary Care Unit. Kidney Blood Press. Res. 2017;42:1193–1204. doi: 10.1159/000485866. [DOI] [PubMed] [Google Scholar]
- 9.Han YQ, et al. Red blood cell distribution width predicts long-term outcomes in sepsis patients admitted to the intensive care unit. Clin. Chim. Acta. 2018;487:112–116. doi: 10.1016/j.cca.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Goyal H, Awad H, Hu ZD. Prognostic value of admission red blood cell distribution width in acute pancreatitis: a systematic review. Ann. Transl. Med. 2017;5:342. doi: 10.21037/atm.2017.06.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang B, Lu H, Gong Y, Ying B, Cheng B. The Association between Red Blood Cell Distribution Width and Mortality in Critically Ill Patients with Acute Kidney Injury. Biomed. Res. Int. 2018;2018:9658216. doi: 10.1155/2018/9658216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elhosseiny S, et al. The Value of Adding Red Cell Distribution Width to Mehran Risk Score to Predict Contrast-induced Acute Kidney Injury in Patients with Acute Coronary Syndrome. Cureus. 2018;10:e2911. doi: 10.7759/cureus.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson AE, et al. MIMIC-III, a freely accessible critical care database. Sci. Data. 2016;3:160035. doi: 10.1038/sdata.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta RL, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit. Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Group KDIGOKAKIW. KDIGO clinical practice guideline for acute kidney injury. Kidney Int, Suppl. 2012;2:1–138. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 16.McCullough PA, et al. ABT-719 for the Prevention of Acute Kidney Injury in Patients Undergoing High-Risk Cardiac Surgery: A Randomized Phase 2b Clinical Trial. J. Am. Heart Assoc. 2016;5:e003549. doi: 10.1161/jaha.116.003549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuartero M, Betbese AJ, Nunez K, Baldira J, Ordonez-Llanos J. Does Whole-Blood Neutrophil Gelatinase-Associated Lipocalin Stratify Acute Kidney Injury in Critically Ill Patients? Dis. Markers. 2019;2019:8480925. doi: 10.1155/2019/8480925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin K, Hu Y, Kong G. Predicting in-hospital mortality of patients with acute kidney injury in the ICU using random forest model. Int. J. Med. Inf. 2019;125:55–61. doi: 10.1016/j.ijmedinf.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, Zhu C, Mo L, Hong Y. Effectiveness of sodium bicarbonate infusion on mortality in septic patients with metabolic acidosis. Intensive Care Med. 2018;44:1888–1895. doi: 10.1007/s00134-018-5379-2. [DOI] [PubMed] [Google Scholar]
- 20.Quan H, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 21.Knaus WA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 22.Le Gall JR, et al. The Logistic Organ Dysfunction system. A new way to assess organ dysfunction in the intensive care unit. ICU Scoring Group. JAMA. 1996;276:802–810. doi: 10.1001/jama.1996.03540100046027. [DOI] [PubMed] [Google Scholar]
- 23.Vincent JL, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 24.Johnson AE, Kramer AA. & Clifford, G. D. A new severity of illness scale using a subset of Acute Physiology And Chronic Health Evaluation data elements shows comparable predictive accuracy. Crit. Care Med. 2013;41:1711–1718. doi: 10.1097/CCM.0b013e31828a24fe. [DOI] [PubMed] [Google Scholar]
- 25.Bone RC, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 26.Wasilewski J, et al. Prognostic value of red blood cell distribution width in patients with left ventricular systolic dysfunction: Insights from the COMMIT-HF registry. Cardiol. J. 2018;25:377–385. doi: 10.5603/CJ.a2017.0037. [DOI] [PubMed] [Google Scholar]
- 27.Nam JS, Ahn CW, Kang S, Kim KR, Park JS. Red Blood Cell Distribution Width Is Associated with Carotid Atherosclerosis in People with Type 2 Diabetes. J. Diabetes Res. 2018;2018:1792760. doi: 10.1155/2018/1792760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zbierska-Rubinkiewicz K, et al. Creatine kinase-MB and red cell distribution width as predictors of contrast-induced nephropathy after percutaneous coronary intervention in acute myocardial infarction. Folia Med. Cracov. 2017;57:87–99. [PubMed] [Google Scholar]
- 29.Akin F, et al. Relation of red cell distribution width to contrast-induced acute kidney injury in patients undergoing a primary percutaneous coronary intervention. Coron. Artery Dis. 2015;26:289–295. doi: 10.1097/mca.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 30.Cho AY, Yoon HJ, Lee KY, Sun IO. Clinical characteristics of sepsis-induced acute kidney injury in patients undergoing continuous renal replacement therapy. Ren. Fail. 2018;40:403–409. doi: 10.1080/0886022x.2018.1489288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vashistha T, et al. Red Cell Distribution Width and Mortality in Hemodialysis Patients. Am. J. Kidney Dis. 2016;68:110–121. doi: 10.1053/j.ajkd.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang T, Li J, Lin Y, Yang H, Cao S. Association Between Red Blood Cell Distribution Width and All-cause Mortality in Chronic Kidney Disease Patients: A Systematic Review and Meta-analysis. Arch. Med. Res. 2017;48:378–385. doi: 10.1016/j.arcmed.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Odutayo A, et al. AKI and Long-Term Risk for Cardiovascular Events and Mortality. J. Am. Soc. Nephrol. 2017;28:377–387. doi: 10.1681/asn.2016010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh HJ, et al. Red blood cell distribution width is an independent predictor of mortality in acute kidney injury patients treated with continuous renal replacement therapy. Nephrol. Dial. Transpl. 2012;27:589–594. doi: 10.1093/ndt/gfr307. [DOI] [PubMed] [Google Scholar]
- 35.Mizuno A, Ohde S, Nishizaki Y, Komatsu Y, Niwa K. Additional value of the red blood cell distribution width to the Mehran risk score for predicting contrast-induced acute kidney injury in patients with ST-elevation acute myocardial infarction. J. Cardiol. 2015;66:41–45. doi: 10.1016/j.jjcc.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Jia L, Zhang W, Jia R, Zhang H, Chen X. Construction Formula of Biological Age Using the Principal Component Analysis. Biomed. Res. Int. 2016;2016:4697017. doi: 10.1155/2016/4697017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doyle JF, Forni LG. Acute kidney injury: short-term and long-term effects. Crit. Care. 2016;20:188. doi: 10.1186/s13054-016-1353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pekkarinen PT, et al. Association of extracerebral organ failure with 1-year survival and healthcare-associated costs after cardiac arrest: an observational database study. Crit. Care. 2019;23:67. doi: 10.1186/s13054-019-2359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagen M, et al. Systemic inflammatory response syndrome and long-term outcome after intracerebral hemorrhage. Neurol. Neuroimmunol. Neuroinflamm. 2019;6:e588. doi: 10.1212/nxi.0000000000000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deswal A, et al. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST) Circulation. 2001;103:2055–2059. doi: 10.1161/01.CIR.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 41.Semba RD, et al. Serum antioxidants and inflammation predict red cell distribution width in older women: the Women’s Health and Aging Study I. Clin. Nutr. 2010;29:600–604. doi: 10.1016/j.clnu.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rabb H, et al. Inflammation in AKI: Current Understanding, Key Questions, and Knowledge Gaps. J. Am. Soc. Nephrol. 2016;27:371–379. doi: 10.1681/asn.2015030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pavlakou P, Liakopoulos V, Eleftheriadis T, Mitsis M, Dounousi E. Oxidative Stress and Acute Kidney Injury in Critical Illness: Pathophysiologic Mechanisms-Biomarkers-Interventions, and Future Perspectives. Oxid. Med. Cell Longev. 2017;2017:6193694. doi: 10.1155/2017/6193694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid. Redox Signal. 2008;10:1923–1940. doi: 10.1089/ars.2008.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and material were available at https://mimic.mit.edu/.