Abstract
We currently obtain pre- and post-contrast enhanced whole brain 3D-real inversion recovery images for the evaluation of endolymphatic hydrops. We noticed that the space between the pial sheath surrounding the cortical veins and the cortical venous wall is enhanced and this enhancement seems to connect to the meningeal lymphatics along superior sagittal sinus. This new anatomical concept regarding the outflow from the glymphatic system might be important for the future research in neuroscience.
Keywords: glymphatic, magnetic resonance imaging, meningeal lymphatics, pial sheath
Low concentration gadolinium-based contrast agents (GBCAs) in fluid can be detected using 3D-real inversion recovery (IR) sequence.1 It has been reported that intravenously administered GBCAs distribute around the cortical veins in the subarachnoid space even in subjects without blood-brain barrier (BBB) disruption in whole brain 3D-real IR images.2 The GBCA around the cortical veins spreads into the cerebrospinal fluid (CSF) space in elderly subjects by 4 h following intravenous (IV) administration.2 In the anatomical literature, the perivenous space, which is the efflux route of the glymphatic system, is described to be continuous with the space between the pial sheath surrounding the cortical veins and the venous wall.3
The meningeal lymphatics were discovered in 2015 in animals.4 Prior to that discovery, it was believed that the central nervous system (CNS) lacked lymphatic channels. The meningeal lymphatic channels are thought to be involved in the drainage of interstitial fluid and immune cells from the brain, and in CSF resorption.5–7 Recently, the meningeal lymphatic channels parallel to the superior sagittal sinus (SSS) were visualized in human and non-human primates with contrast enhanced MR imaging.5 The meningeal lymphatic channels were visualized mostly at the bilateral superior lateral corners of the triangular shaped SSS on coronal contrast enhanced fluid-attenuated inversion recovery (FLAIR) images. On the FLAIR images, the meningeal lymphatic channels are visualized as enhanced structures, but the dura is not enhanced. In this study, the FLAIR images were obtained at 6–10 min after IV-GBCA. Subsequently obtained black-blood T1-weighted images also showed enhancement of the meningeal lymphatic channels5 On the T1-weighted image with magnetization prepared rapid gradient-echo (MP-RAGE), the dural enhancement was prominent, but the enhancement of the meningeal lymphatic channels was not conspicuous due to a strong enhancement of the adjacent venous sinus lumen. The authors also showed that an intravascular-type GBCA (gadofosveset, which is a serum albumin-binding contrast agent) failed to visualize the enhancement of these parasagittal meningeal lymphatic channels. Additionally, they showed that the meningeal lymphatics display a typical panel of lymphatic endothelial markers by immunohistochemistry.5
The connections between the parasagittal meningeal lymphatic channels, brain parenchyma, perivascular space, arachnoid granulations, and venous lacunae have not been elucidated.7 The detailed anatomy of these regions is very important to understand the mechanisms of waste clearance, particularly the outflow from the glymphatic system. There is a speculation that congestion of the venoglymphatic connections is the basis for idiopathic intracranial hypertension.7 In this study, the authors speculated that in physiologic conditions, the CSF is driven from the perivenous space of the cortical veins into the lumen of the venous sinus through the CSF-venous blood barrier. The decrease in permeability of the CSF-venous blood barrier could be due to an alteration of aquaporin function. This alteration could lead to growth of arachnoid granulations inside the venous sinus at the entry of the cortical vein, and might result in intrinsic stenosis of the venous sinus.7 Therefore, a detailed analysis of the connection between the cortical veins and the sinus might provide new insight into the pathophysiology of neurological disease.
The aim of this brief communication is to present a new concept to the MR and neuroscience communities regarding the anatomy of the outflow from the glymphatic system: the area of potential connection between the meningeal lymphatic channels and the sub-pial space surrounding the cortical veins.
We currently obtain whole brain 3D-real IR images based on sampling perfection with application-optimized contrasts using different flip angle evolutions before, and at 10 min, 4 and 24 h after IV-GBCA for the assessment of endolymphatic hydrops of the inner ear as well as to assess the permeability of the blood-perilymph barrier.1 This sequence is highly sensitive to low concentrations of GBCA in fluid similar to heavily T2-weighted 3D-FLAIR imaging, while avoiding the risk of paradoxical signal decrease by GBCA.1 We noticed a linear enhancement around the cortical veins at 10 min after IV-GBCA, which was presumed to be leakage of the GBCA into the space between the pial sheath and the venous wall that is filled with interstitial fluid. This space connects to the meningeal lymphatic channels in the parasagittal region (Fig. 1) The parasagittal linear structure on the 3D-real IR image shows a signal increase at 10 min after IV-GBCA and the enhancement persists for 4 h. At 24 h after IV-GBCA, the signal enhancement of the presumed meningeal lymphatic channels became comparable to that on the pre-contrast image (Fig. 2).
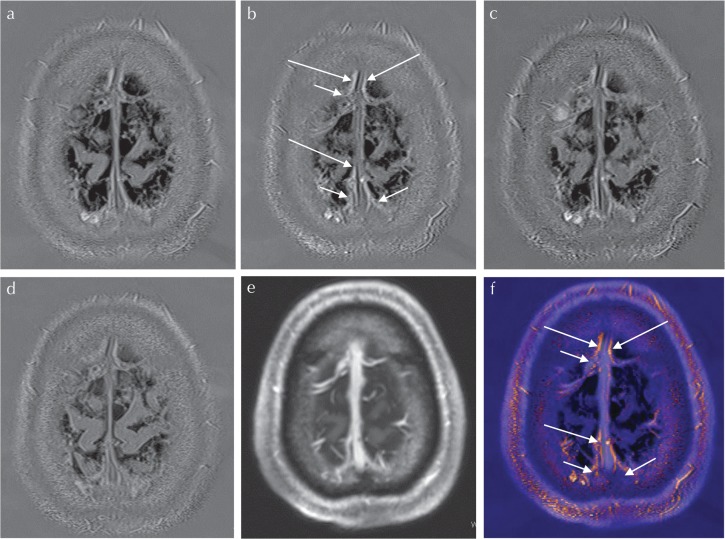
Fig. 1.
A 67-year-old man with suspected endolymphatic hydrops underwent a pre- and post-contrast enhanced MR study. 3D-real inversion recovery (IR) images (TR of 15130 ms, TE of 549 ms, inversion delay [TI] of 2700 ms, voxel size of 0.5 × 0.5 × 1.0 mm3, 10 min of acquisition time) were obtained before (a), and at 10 min (b), 4 h (c) and 24 h (d) after intravenous administration of a single dose of gadolinium-based contrast agent (IV-GBCA). The window display level and width were kept constant among (a–d). After 10 min of IV-GBCA, the linear enhancement along the cortical veins and superior sagittal sinus (SSS) can be seen. The linear enhancement along the cortical veins (arrows, b) is presumed to be enhancement of the pial sheath along the cortical veins. The linear enhancement along the SSS (long arrows, b) is presumed to be the previously reported meningeal lymphatics. Magnetization prepared rapid gradient-echo (MP-RAGE, TR of 1570 ms, TE of 2.2 ms, TI of 800 ms, voxel size of 1.0 × 1.0 × 1.0 mm3, flip angle of 15°, 3 min of acquisition time) images were obtained at 7 min after IV-GBCA (e). On the MP-RAGE image, the venous lumen is strongly enhanced. A fusion image between (b) and (e) is shown in (f). The enhanced area of the 3D-real IR is colored. On the fusion image, the linear colored enhancement (arrows, f) is located along the cortical veins and the SSS. The linear colored enhancement is located outside of the venous lumen. The colored linear enhancement along the cortical vein is presumed to be the space between the pial sheath and the cortical venous wall (arrows, f), and appears to continue to the colored enhancements along the SSS (presumed to be the meningeal lymphatics, long arrows, f). This suggests that the space between the pial sheath and the cortical venous wall is connected to the meningeal lymphatics.
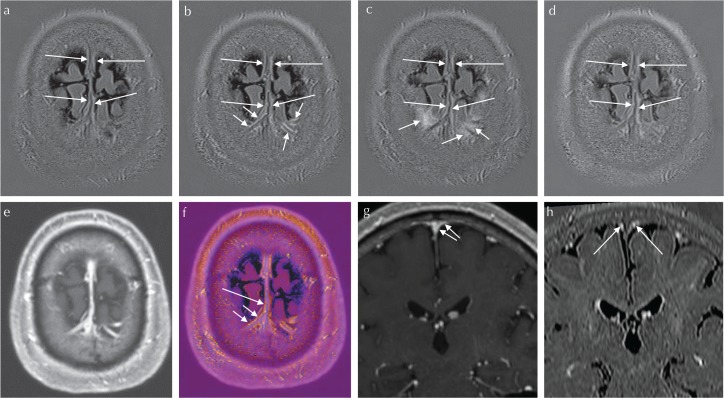
Fig. 2.
A 55-year-old man suspected of endolymphatic hydrops underwent a pre- and post-contrast enhanced MR study. Scan parameters are the same as in Fig. 1. 3D-real IR images were obtained before (a), and at 10 min (b), 4 h (c) and 24 h (d) after intravenous administration of a single dose of gadolinium-based contrast agent (IV-GBCA). The window display level and width were kept constant among (a–d). At 10 min after IV-GBCA, the linear enhancement along the cortical veins and the superior sagittal sinus (SSS) can be seen (arrows and long arrows, b). The linear enhancement along the cortical veins is presumed to be the enhancement in the space between the pial sheath and the wall of the cortical vein (arrows, b). The linear enhancement along the SSS is presumed to be the previously reported meningeal lymphatics (long arrows, a–d). Note the linear enhancement around the cortical vein at 10 min after IV-GBCA (arrows, b) that spreads to the surrounding cerebrospinal fluid (CSF) space at 4 h after IV-GBCA (arrows, c). The enhancement of the meningeal lymphatics along the SSS can be appreciated on images obtained at 10 min after IV-GBCA (b) as well as 4 h after IV-GBCA (c). Magnetization prepared rapid gradient-echo (MP-RAGE) images were obtained at 7 min after IV-GBCA (e). On the MP-RAGE image, the venous lumen is enhanced. The fusion image between (b) and (e) is shown in (f). The enhanced area of the 3D-real IR is colored. On the fusion image, the linear colored enhancement is located along the cortical veins (arrows, f) and the SSS (long arrow, f). The linear colored enhancement is located outside of the venous lumen. The colored linear enhancement along the cortical vein is presumed to be the space between the pial sheath and the cortical venous wall (arrows, f), and appears to continue to the colored enhancement along the SSS (presumed to be the meningeal lymphatics, long arrow, f). This suggests that the space between the pial sheath and the cortical venous wall connects to the meningeal lymphatics. On reformatted coronal MP-RAGE images obtained at 7 min after IV-GBCA (g), a cortical vein drains into the lower part of the SSS (arrows, g). On reformatted coronal 3D-real IR image obtained at 10 min after IV-GBCA, meningeal lymphatics are located at the superior edges of the SSS (arrows, h).
To date, this potential connection between the pial sheath and the meningeal lymphatics has not been reported. Although we have not confirmed this connection histologically, we think confirmation of this connection would be very important for future neuroscience research. In this brief communication, we present the demonstrative images of two patients including fusion images between the contrast enhanced 3D-real IR image and contrast enhanced MP-RAGE image to show the potential connection as described above.
The reason that GBCA leaks into the surrounding CSF space from the pial sheath at 4 h after IV-GBCA in aged subjects is also not known. We can speculate that the permeability of the stomata in the pial sheath may increase with aging,3 or congestion might occur in the meningeal lymphatics, or possibly stenosis in the connection point between the pial sheath and the meningeal lymphatics may develop. It would be quite interesting to evaluate the number and size of the arachnoid granulations in the sinus and the degree of GBCA leakage into the surrounding CSF space.
The cortical veins drain into the lower part of the SSS, but the meningeal lymphatics lay in the bilateral superior lateral part of the SSS.8 Therefore, the pial sheath and the cortical veins should separate before the point where the cortical veins pass the dura. The MR images shown in this manuscript agree with this speculation. Of course, the relationship between the classic concept of the venous lacunae and the meningeal lymphatics has not been fully elucidated.9 It was reported that the cross-sectional dimension of the visualized lymphatic vessels was difficult to measure but was approximately 0.5 mm, similar in size to the resolution of high-resolution imaging sequences. This estimate is in line with that observed by MRI and histology in the previous work by Absinta et al.5 The meningeal lymphatics are the drainage route of waste and CSF from the brain.10 Therefore, the concepts presented in this brief communication might open the possibility of new research for Alzheimer’s disease,10 Parkinson’s disease, stroke,11 normal pressure hydrocephalus and sleep disorders, for which the etiology may be related to a failure of the waste clearance system in the brain. This idea might be also important to develop new methods for drug delivery to the central nervous system.
Footnotes
Funding
This study was supported in part by Grants-in-Aid for scientific research from the Japanese Society for the Promotion of Science (JSPS KAKENHI, numbers 17H04259) to S.N.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Naganawa S, Kawai H, Taoka T, Sone M. Improved 3D-real inversion recovery: a robust imaging technique for endolymphatic hydrops after intravenous administration of gadolinium. Magn Reson Med Sci 2019; 18:105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naganawa S, Nakane T, Kawai H, Taoka T. Age dependence of gadolinium leakage from the cortical veins into the cerebrospinal fluid assessed with whole brain 3D-real inversion recovery MR imaging. Magn Reson Med Sci 2019; 18:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang ET, Inman CB, Weller RO. Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J Anat 1990; 170:111–123. [PMC free article] [PubMed] [Google Scholar]
- 4.Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015; 523:337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Absinta M, Ha SK, Nair G, et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife 2017; 6:e29738. doi: 10.7554/eLife.29738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 2015; 212:991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenck S, Radovanovic I, Nicholson P, Hodaie M, Krings T, Mendes-Pereira V. Idiopathic intracranial hypertension: the veno glymphatic connections. Neurology 2018; 91:515–522. [DOI] [PubMed] [Google Scholar]
- 8.Fox RJ, Walji AH, Mielke B, Petruk KC, Aronyk KE. Anatomic details of intradural channels in the parasagittal dura: a possible pathway for flow of cerebrospinal fluid. Neurosurgery 1996; 39:84–90; discussion 90–91. [DOI] [PubMed] [Google Scholar]
- 9.Coles JA, Myburgh E, Brewer JM, McMenamin PG. Where are we? The anatomy of the murine cortical meninges revisited for intravital imaging, immunology, and clearance of waste from the brain. Prog Neurobiol 2017; 156:107–148. [DOI] [PubMed] [Google Scholar]
- 10.Da Mesquita S, Louveau A, Vaccari A, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 2018; 560:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanev P, Poinsatte K, Hominick D, et al. Impaired meningeal lymphatic vessel development worsens stroke outcome. J Cereb Blood Flow Metab 2019. 9 January doi: 10.1177/0271678X18822921. [Epub ahead of print] [DOI] [PMC free article] [PubMed]