Abstract
The use of immunotherapy in transplant recipients is considered a contraindication because of very high risks for graft loss. The graft loss is to be expected because cytotoxic T-lymphocyte–associated protein-4 and programmed death 1 pathways are implicated in graft tolerance. In this case report, we describe a woman with recurrent, disseminated hepatocellular carcinoma who was successfully treated with nivolumab, an immune checkpoint inhibitor.
Keywords: post liver transplant, recurrent HCC, immune checkpoint inhibitor
Abbreviations: CPI, immune checkpoint inhibitor; HCC, Hepatocellular carcinoma; PD- L1, programmed death -ligand 1; PD-1, programmed death -1
Recurrence of hepatocellular carcinoma (HCC), around 10–20%, after liver transplantation (LT) is associated with close to 100% short-term mortality. Locoregional or systemic therapies have shown only limited benefit in recurrent HCC. Immunotherapy is contraindicated or at the very best controversial in transplant recipients.1, 2, 3 We describe a woman with disseminated HCC who was successfully treated with nivolumab, an immune checkpoint inhibitor (CPI).
Case report
A 62-year-old black woman received a LT in November 2016 for a single nodule (2 cm) HCC and hepatitis C (HCV) cirrhosis. She had successful HCV treatment and transarterial chemoembolization (TACE) before LT. Explanted liver showed a well differentiated HCC (0.9 cm) with no extrahepatic disease. The immediate recovery after LT was uneventful, but she had two episodes of acute cellular rejection, cytomegalovirus (CMV) viremia, and an anastomotic biliary stricture during the first 6 months after LT. Her immunosuppressive regimen included tacrolimus and mycophenolate. She underwent 3-month HCC surveillance protocol. The magnetic resonance imaging (MRI) performed at 12 months (November 2017) showed a 4.5 cm lesion in the right dome of the liver with several additional subcentimeter lesions, and HCC was confirmed by biopsy (Figure 1A). Immunohistochemical analysis showed that tumor was positive for programmed death -ligand 1 (PD-L1) (tumor proportion score 25%) with a high tumor mutational burden (29 Mutations/Mb). She was initially treated by TACE, but follow-up MRI 4 weeks later showed multifocal HCC with invasion of anterior diaphragm with right side malignant pleural effusion (Figure 1, Figure 2B) and enhancing foci in the right pericardium and internal mammary areas (Figure 2B). The bone scan showed numerous metastases in the thoracic spine, sacrum, left sacroiliac joint, skull, and left sixth rib. Despite the disseminated HCC, the patient maintained excellent functional and performance status.
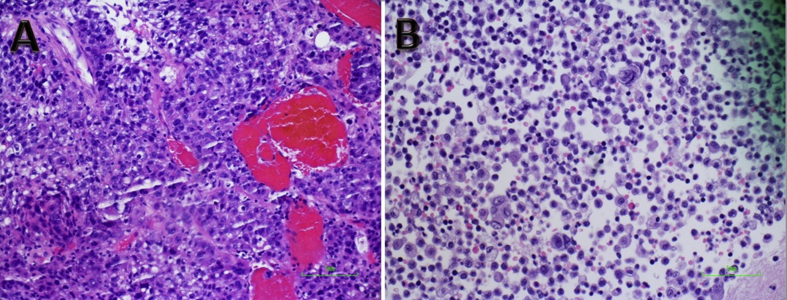
Figure 1.
A) Liver core biopsy shows a poorly differentiated carcinoma with granular to clear cytoplasm, increased N/C ratio, and arranged in solid and thickened trabecular growth pattern. Abundant apoptosis and brisk mitosis were seen. The sinusoid is congested. The tumor cell is immunoreactive to Glypican 3 and Arginase 1 (not shown), supporting poorly differentiated hepatocellular carcinoma. (B) Pleural fluid showed atypical cells, morphologically identical to that is seen in liver core biopsy, consistent with hepatocellular carcinoma.
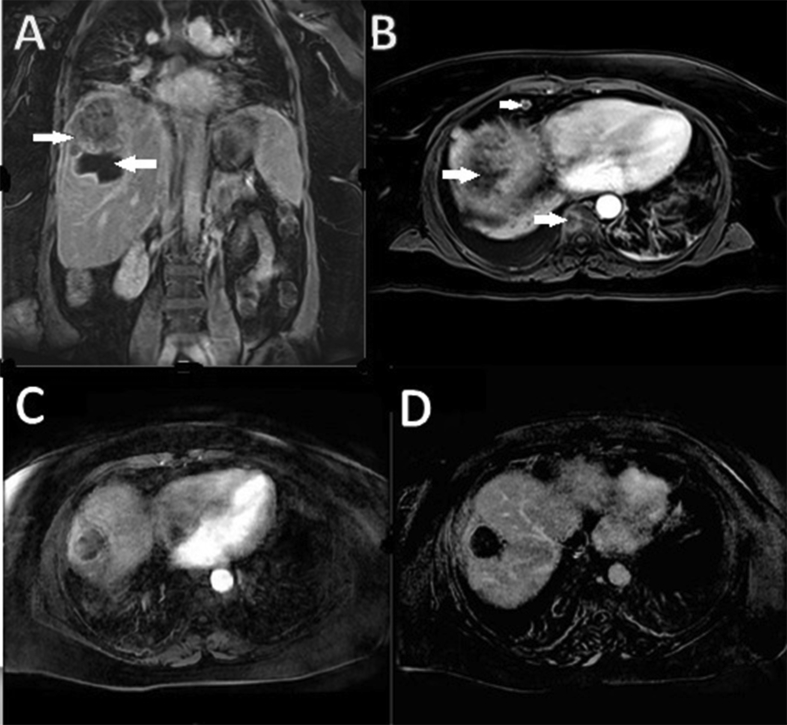
Figure 2.
(A) MRI T1 postcontrast coronal view shows thick rimmed necrotic cavity with solid tumor mass superior to necrotic cavity. (B) MRI T1 postcontrast axial view shows enhancing epicardial lymph node and thoracic bone lesion. (C) MRI T1 postcontrast axial view shows no enhancing tumor and (D) MRI T1 postcontrast axial view shows complete necrosis. Enhancing epicardial lymph node and bone lesions were not seen. MRI, magnetic resonance imaging.
Considering the dismal prognosis, the patient was started on nivolumab in March 2018 after discussing the risks including graft loss. Bone pain from spine metastasis was treated with local radiation. At week-12 into treatment, liver enzymes were elevated, and this was managed by withholding nivolumab for 3 weeks and treatment with tapering dose of prednisone. After 6 months of treatment, liver lesions and all metastatic lesions were either necrotic or no longer visible and the pleural effusion resolved (Figures 2C, 2D). The patient was continued on nivolumab and as of November 2019 (24 months after the diagnosis of disseminated HCC), there is no evidence of recurrent HCC on MRI, bone, or positron emission tomography (PET) scan. Moreover, the patient remains in excellent health with near normal liver enzymes.
Discussion
The use of nivolumab in transplant recipients is considered a contraindication because of very high risks for graft loss which is to be expected because cytotoxic T-lymphocyte–associated protein-4 and programmed death 1 pathways are implicated in graft tolerance.4,5 Nivolumab, a PD-1 receptor inhibitor, is effective in less than 20% of patients with advanced HCC. There have been many reviews examining the role of CPIs in organ transplant recipients.1, 2, 3 Yet, to date, only 48 transplant recipients (19 LT, 29 renal) had been treated with CPI. Of these, 37% of patients with LT and 45% of patients with renal transplantation experienced rejection, and only 21% had antitumor response with durable graft function.2 According to one systematic review, only 4 patients with LT with recurrent HCC had been treated previously with CPI.1 It is difficult to draw any conclusions regarding efficacy, the benefit of tumor tissue staining for PD-L1 before treatment initiation, or the relative risks/benefits of various Food and Drug Administration–approved CPIs from the published anecdotal reports. Our case report illustrates the potential benefit in some patients who otherwise may not have any other treatment options that could prolong their life. A prospective national database may help to learn how and when to use CPI in LT recipients with advanced cancers.5
Conflicts of interest
All authors have none to declare.
References
- 1.Abdel-Wahab N., Safa H., Abudayyeh A. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of literature. J Immunother Cancer. 2019;7:106. doi: 10.1186/s40425-019-0585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Bruyn P., Van Gestel D., Ost P. Immune checkpoint blockade for organ transplant patients with advanced cancer: how far can we go? Curr Opin Oncol. 2019;31:54–64. doi: 10.1097/CCO.0000000000000505. [DOI] [PubMed] [Google Scholar]
- 3.Munker S., De Toni E.N. Use of checkpoint inhibitors in liver transplant recipients. United European Gastroenterology Journal. 2018;6:970–973. doi: 10.1177/2050640618774631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kittai A.S., Oldham H., Cetnar J., Taylor M. Immune checkpoint inhibitors in organ transplant patients. J Immunother. 2017;40:277–281. doi: 10.1097/CJI.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 5.Ho C.M., Chen H.L., Hu R.H., Lee P.H. Harnessing immunotherapy for liver recipients with hepatocellular carcinoma: a review from a transplant oncology perspective. Ther Adv Med Oncol. 2019;11:1–11. doi: 10.1177/1758835919843463. [DOI] [PMC free article] [PubMed] [Google Scholar]