Abstract
Objectives
The therapeutic value of corticosteroid bursal injection after ultrasound-guided irrigation and lavage for the treatment of shoulder calcific tendinosis has not been established yet in the long term.
Methods
41 patients suffering from chronic symptomatic rotator cuff calcific tendinopathy were recruited for this study. Group A (20 patients) received a double needle ultrasound-guided irrigation and lavage of the calcification with xylocaine injection, while group B (21 patients) underwent a double needle ultrasound-guided irrigation and lavage of the calcification with a xylocaine and betamethazone bursal injection.
Results
After twelve months, we documented full –or almost full- decline (VAS: 0–20/100) of the symptoms in 70% of the group A patients and in 61.9% of the group B patients. There was no statistical difference (chi square, p < 0.05) in group success ratio. We also did not find any statistical difference as for the mean Q-DASH difference between the two groups (t-test).
Conclusions
It was proven that the additional use of corticosteroid bursal injection did not provide with any additional short- to mid-term therapeutic benefit those patients with shoulder calcific tendinopathy who were treated with ultrasound-guided aspiration.
Keywords: Shoulder calcific, Rotator cuff tendinopathy, Ultrasound guided irrigation and lavage, Calcific aspiration, Corticosteroid shoulder injection
1. Introduction
Shoulder calcific tendinopathy is considered to be a chronic, recurrent, self-limited condition without a clear etiology.1 It is characterized by calcium deposition within the mass of rotator cuff, most often in the supraspinatus tendon.2,3 The incidence of rotator cuff (R/C) calcific tendinopathy in the general population ranges between 2.7% and 20%, while it seems to occur more frequently to females between 31 and 55 years of age.4, 5, 6 The most usual clinical symptom requiring medical advice in relation to shoulder calcific tendinopathy is undoubtedly pain. Other clinical findings could be tenderness and reduced range of motion (RoM).
The X-ray's are the main diagnostic modality for the diagnosis of shoulder calcific tendinopathy. Musculoskeletal ultrasound could be useful for the differential diagnosis of rotator cuff pathology, especially when the initial radiographic control is negative.7 Except for the diagnostic use, ultrasonography has been also established as an effective and accurate imaging tool for the guidance of variable interventional techniques, like “barbotage” or dry needling with lavage, aspiration and, finally, therapeutic injections.8, 9, 10, 11, 12
The treatment of calcific tendinopathy is mainly based on conservative methods with a reporting 90% success ratio.13 Conservative treatment involves non-steroidal anti-inflammatory drugs (NSAID's), physiotherapy, modification of activity and extracorporeal shockwave therapy (ESWT).13 In the case that conservative means fail, another possible solution might be the various ultrasound-guided or “blind” empirical infiltrations. There have been different types of injections for the treatment of R/C calcific tendinopathy, like Platelet – rich Plasma (PRP), stem cells, hyaluronic acid and corticosteroid infiltrations.14 However, the clinical efficacy of the different injections remains controversial.14,15 For example, the use of PRP for rotator cuff tendinopathy has recently gained popularity as a biological therapeutic option, whereas some authors have reported significant clinical improvement.16, 17, 18 Despite that, a relative systematic review supported that the positive results of the laboratory studies were not followed by analogous clinical improvement.19
Apropos of shoulder corticosteroid injections, Wolf et al. suggested that they could be an effective treatment whenever the clinical symptoms derive from the mechanical subacromial impingement (like an organized rotator cuff's calcification).20 It is true that corticosteroid injections are widely used, because they combine symptomatic relief in the inflammatory phase of the disease with a reported low risk of side effects.21 Nevertheless, a recent systematic review showed significant long-term harms to tendon tissue and cells associated with corticosteroid injections 22, while another study supported that the therapeutic impact of corticosteroid injections is rather minor and transient.23
As for chronic recurrent cases, the use of ultrasound-guided aspiration after dissolution and lavage of the calcific deposits might be a very effective treatment.9,24 In a way, this treatment is the “Middle Earth” between conservative and operative therapeutic options, like the arthroscopic or mini-open removal of the calcification. According to a recent meta-analysis, ultrasound-guided needling should be considered as a safe procedure but it has yet not been proven more effective than the well-established, ultrasound-guided, subacromial, corticosteroid injection.1 Another study illustrated that the US-guided injection of xylocaine alone provided a prolonged pain relief period in comparison to the mixture of xylocaine and corticosteroids for patients with rotator cuff calcific tendinosis.25
We conducted a study to investigate whether a subsequent corticosteroid injection after an ultrasound-guided, double-needle irrigation and lavage may have confounded the long-term results or may play no significant role in patients with symptomatic shoulder calcific tendinopathy. Our hypothesis was that the guided consecutive aspirations after the lavage are more than enough to treat these patients effectively in long-term. In other words, we hypothesized that the corticosteroid injection does not offer any additional benefit and is rather unnecessary for the afore-mentioned category of patients.
2. Materials and methods
41 patients suffering from chronic symptomatic calcific tendinopathy of the supraspinatus tendon, non responsive to NSAID's, physiotherapy and ice-therapy, were recruited for this study. These patients were = treated in an orthopaedic academic center from September 2013 until January 2016. They were all clinically examined and radiographically evaluated with an anteroposterior plain view and a routine shoulder ultrasound.
The ultrasonography was performed with a portable grey scale ultrasound (frequency of 10–12 Mhz, A6 Portable Ultrasonic Diagnostic System, Sonoscape Company Limited, Shenzen, P.R. China) by the same operator, an orthopaedic surgeon proficient in MSK U/S. This research complied with the World Medical Association Declaration of Helsinki — Ethical Principles for Medical Research Involving Human Subjects.
Our inclusion criteria were patients older than 18 years old, with radiological establishment of rotator cuff calcific tendinopathy and persistent clinical symptoms (more than 3 months). We excluded from our study patients with uncontrolled hypothyroidism, hormonal disorders, alcohol abuse, per os use of corticosteroid, smoking, autoimmune diseases, obesity, chronic kidney insufficiency, cervical spondylosis, thoracic outlet syndrome, overuse injury, former injuries or/and surgeries or injections at the ipsilateral shoulder, psychiatric disorders or serious mental stress, upper limb pain syndrome, chronic lateral epicondylitis. Pregnant patients or patients with symptoms of ipsilateral limb entrapment syndrome or cervical radiculopathy were also excluded, as were those who had undergone local corticosteroid injection in the past six months. As a result, from the initial sample of the 66 patients, 25 were excluded from our study due to the afore-mentioned criteria, so that, finally, we included 41 patients (26 women: 15 men, mean age: 52 years).
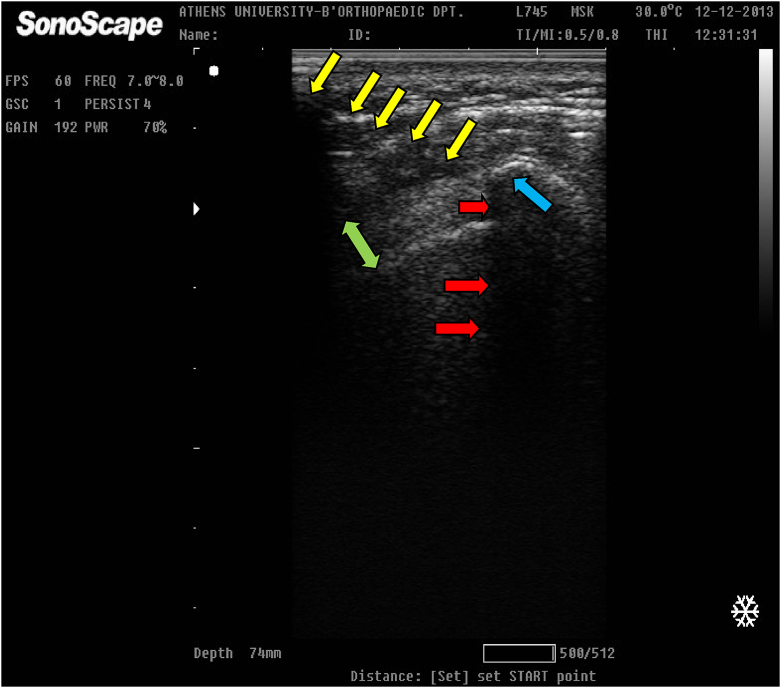
A sitting position of the patient during the interventional procedure was selected. After the initial xylocaine injection (5 ml) in the subcutaneous tissue and the subacromial bursa under sterile conditions, group A (20 patients) received a double needle ultrasound-guided irrigation and lavage with consecutive aspirations of the calcification (Fig. 1).
Fig. 1.
Ultrasound-guided aspiration (needle: under the yellow arrows, acoustic shadowing: red arrows) and lavage of a well organized calcification (light blue arrow) into the supraspinatus tendon (green double arrow).
We attached a syringe including 10 ml of normal saline (N/S) at the first needle and then we injected targeting the calcium deposit. Then we tried to achieve consecutive aspirations through the second needle. Repeated punctures were utilized without removing the needles from the initial puncture site. No attempt of dry needling was performed within the areas of tendon degeneration.
Group B (21 patients) underwent also a double needle ultrasound-guided irrigation and lavage of the calcification after the initial local anesthesia. The technique was identical in the two groups apart from the last step. The critical difference in comparison to group A was that at the end of the consecutive aspirations, group B patients received additionally a betamethazone bursal injection (2 ml).
The ultrasound guidance was deployed under sterile conditions and coverage of the linear probe of the ultrasound device with a sterilized surgical glove, while we used a sterile ultrasound-transmitting gel. The whole procedure, including the ultrasound-guidance, the initial xylocaine infiltration, the dry needling, lavage and the consecutive aspirations of the calcification, lasted approximately 12 min for each patient of both groups. All patients were blinded to the technique used (corticosteroid or not). In contrast, the physician who performed the interventional procedure was not blinded to the technique.
After 48 h of limb resting, all patients were encouraged to resume to their daily activities following a set schedule of physiotherapies. In the follow-up, patients were evaluated by an independent researcher other than the researcher who did the initial assessment as well as the physician who performed the intervention. This third doctor was blinded both to the chosen procedure (corticosteroid or not) and the pre-injection scores of the patients.
The subjective clinical scores used were the visual analogue scale (VAS) 100/100 and the shortened disabilities of the arm, shoulder and hand questionnaire (Q-DASH score): a. before, c. three months and d. twelve months after the injection. In addition, we asked our patients whether they were satisfied with their treatment (rated as “good”, “very good” or “excellent”) and if they would be eager to undergo this procedure again if they experienced recurrent symptoms.
The success ratio was primarily calculated by a. the number of patients who had a final end-point VAS <20/100 per group. Differences between groups were evaluated using Student's t-test and chi-square test. The mean ± standard deviation (SD) was calculated and all analyses were conducted using the SPSS statistical package (Statistical Package for Social Sciences v. 17, Chicago, IL, USA).
3. Results
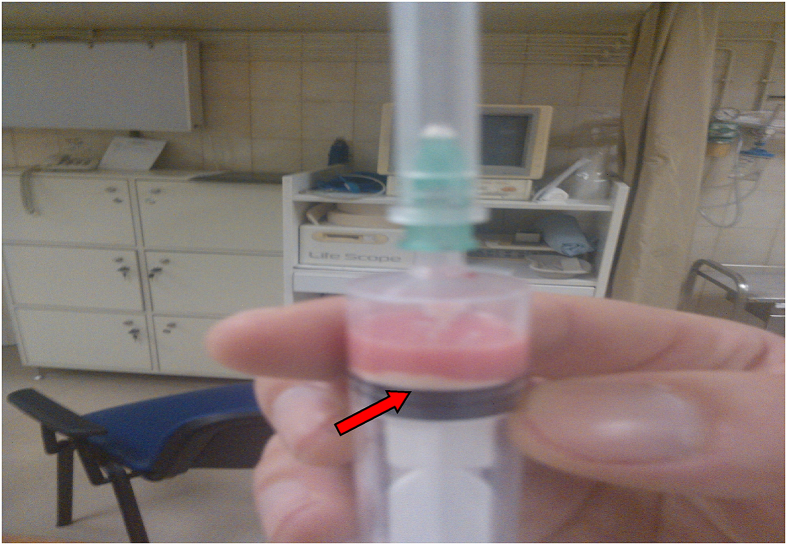
No one of the patients included in the study was lost or exempt during the follow-up. No serious side-effects or complications, such as bleeding, infection, or ligament tear were noticed, so that the technique was in general well tolerated. The baseline US evaluation revealed a single calcific deposit in almost all patients, while none of the calcifications was in touch with the adjacent humeral head bone (100% intratendinous position). In most cases we recorded a noticeable amount of aspirated milky or mixed white-red coloured fluid at the bottom of the syringe after the calcific dissolution (Fig. 2).
Fig. 2.
Successful irrigation and lavage. It is observed a mixed white-red coloured fluid at the bottom of the syringe (red arrow) after the dissolution.
The power analysis of the study was estimated 95% (Gpower computer program, Faul & Erdfelder, 1998). There were no statistical differences between the characteristics of the two groups, such as age, sex, initial VAS, Q-DASH (chi square test, t-test, mean, standard deviation).
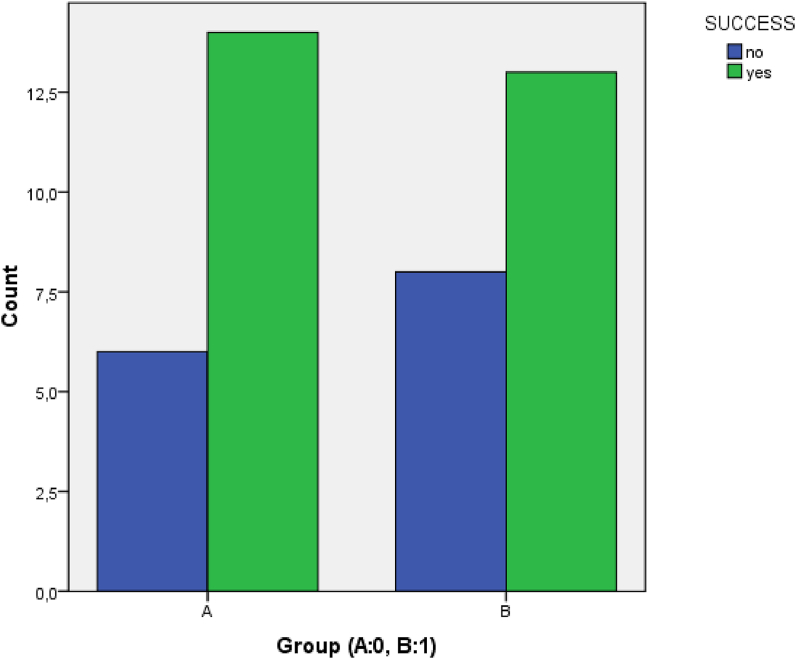
After twelve months, we documented full –or almost full- decline (VAS: 0–20/100) of the symptoms in 70% of the group A patients (14 out of 20 patients) and in 61.9% of the group B patients (13 out of 21 patients). There was no statistical difference (chi square, p < 0.05) in group success ratio between the two groups (Fig. 3).
Fig. 3.
There was no difference concerning the success rate between the two groups.
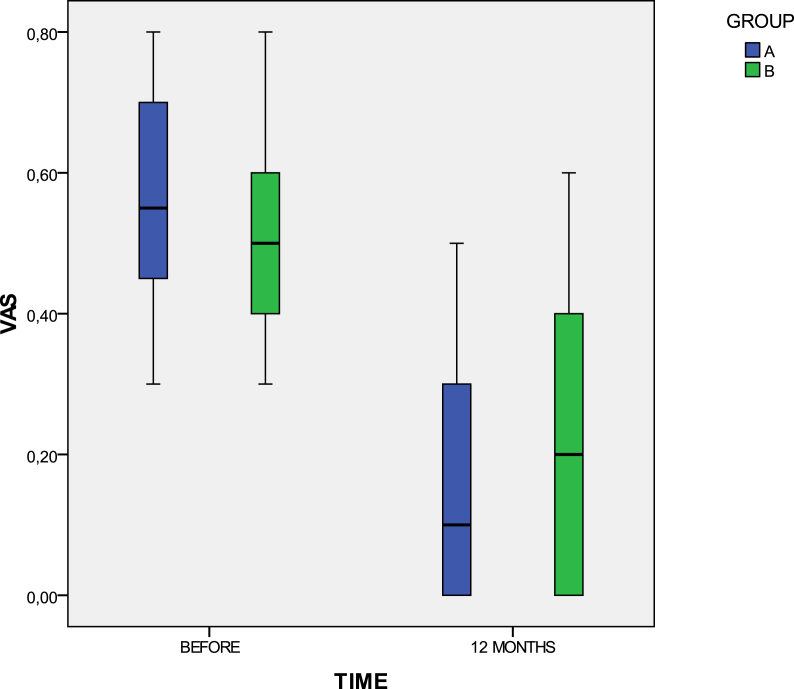
As for group A patients, the mean group VAS, from an initial preinjected score 55/100 (SD: ±15.7), was sharply decreased to 17/100 (SD: ±15.3) and 15/100 (SD: ±18.2) after 3 and 12 months, respectively. Concerning group B patients, from an initial mean group VAS value 51.9/100 (SD: ±12.5), they reached to mean follow-up values of 20.5/100 (SD: ±20.1) and 21/100 (SD: ±20), after 3 and 12 months, respectively (Fig. 4).
Fig. 4.
A significant improvement for both groups was recorded after the treatment regarding the mean group VAS score.
In addition, group A patients reached from an initial mean Q-DASH 44 (SD: ±23.7) to 15.8 (SD: ±16.3) after 3 months and 15.8 (SD: ±16.8) in the 12-month end-point. Group B patients were found with an initial mean Q-DASH 40.5 (SD: ±21.2), whereas they had a 3-month mean Q-DASH 17.6 (SD: ±16.6) and a 12-month final mean Q-DASH 18 (SD: ±16.8) (Fig. 5).
Fig. 5.
The final Q-DASH was significantly improved for both groups, when compared with the initial pre-injected value. Despite that there was no statistical difference between the two groups.
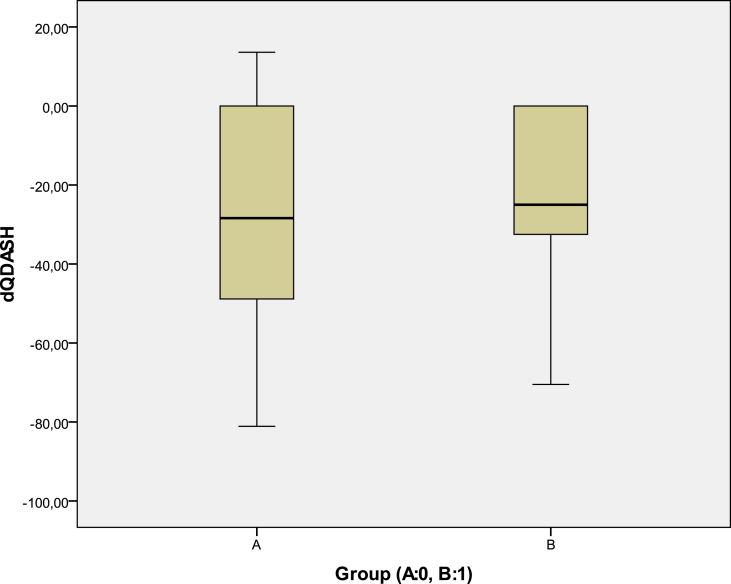
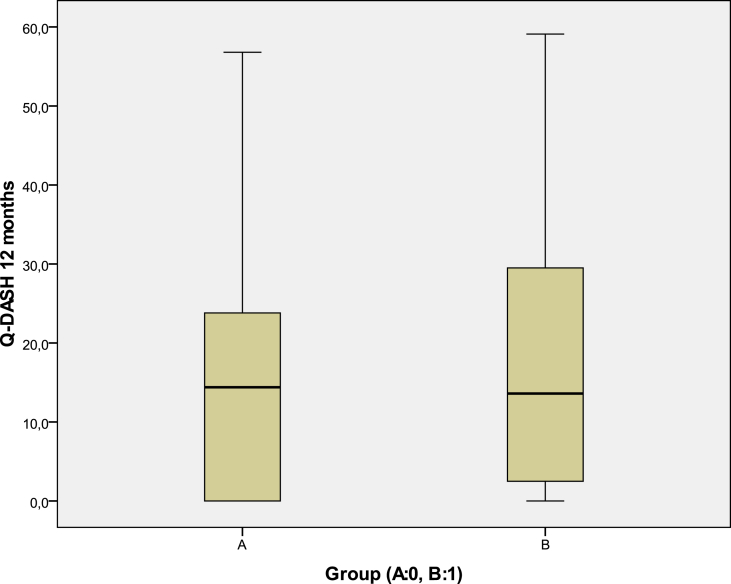
There were also no significant statistical differences regarding the mean total Q-DASH difference between the two groups (Fig. 6) as well as the final 12-month mean Q-DASH values per group (t-test) (Fig. 7).
Fig. 6.
The difference of the mean group final 12-month Q-DASH score minus the pre-injected Q-DASH score was not significant between the two groups.
Fig. 7.
No statistical difference in relation to the 12-month final mean Q-DASH scores between the two groups.
The majority of the patients in both groups was satisfied with their treatment (80% of group A and 76.1% of group B) and would be eager to undergo it again if it was necessary (60% of group A and 57.1% of group B). So, there was no statistical difference between the two groups in relation to final patients’ satisfaction (Table 1) and willingness to repeat the treatment (Table 2).
Table 1.
Patient's satisfaction was similar between group A and group B.
| Patients' Satisfaction | ||||||
|---|---|---|---|---|---|---|
| Frequency | Percent | Valid Percent | Cumulative Percent | |||
| Valid | No | A | 4 | 20.0 | 20.0 | 20.0 |
| B | 5 | 23.8 | 23.8 | 23.8 | ||
| Yes | A | 16 | 80.0 | 80.0 | 100.0 | |
| B | 16 | 76.2 | 76.2 | 100.0 | ||
| Total | A | 20 | 100,0 | 100,0 | ||
| B | 21 | 100,0 | 100,0 | |||
Table 2.
More than half of the patients per group were willing to repeat the procedure if necessary.
| Willingness to Repeat it | ||||||
|---|---|---|---|---|---|---|
| Frequency | Percent | Valid Percent | Cumulative Percent | |||
| Valid | No | A | 8 | 40.0 | 40.0 | 40.0 |
| B | 9 | 42.9 | 42.9 | 42.9 | ||
| Yes | A | 12 | 60.0 | 60.0 | 100.0 | |
| B | 12 | 57.1 | 57.1 | 100.0 | ||
| Total | A | 20 | 100.0 | 100.0 | ||
| B | 21 | 100.0 | 100.0 | |||
4. Discussion
The most important finding of our study was that group B (corticosteroid) had similarly reduced levels of pain with group A (no corticosteroid) after three and twelve months. So, the ultrasound-guided xylocaine-corticosteroid bursal infiltration yielded no statistically superior results than the xylocaine-only infiltration after three months' and one year's follow-up.
Needle irrigation and lavage (“barbotage”) of shoulder calcific deposits was formerly performed under radiographic guidance.26,27 More recently, musculoskeletal ultrasound has been established as the gold standard imaging modality to guide this type of interventions.10, 11, 12,28 The ultrasound-guided approach is radiation-free, while it enables easy localization of the calcification, accurately guiding of the subacromial injection and detailed assessment of the rotator cuff pathology.29 In general, needle irrigation and lavage of shoulder calcific tendinopathy is considered to be a safe and well tolerated interventional procedure which can be performed in the outpatient clinic under local anesthesia. The technique includes single- or double-needle lavage and aspiration.30 Still, there is no consensus regarding the best size or number of needles needed to achieve optimal results. A number of authors are in favour of small diameter needles and limited number of punctures to prevent excessive tendon damage,8,31 whereas others prefer multiple punctures or larger diameter needles to stimulate acute inflammation, neovascularization, and consecutive calcific absorption.28
Galletti et al. showed that the vast majority of the afore-mentioned patients had a noticeable symptomatic relief within a few days after an U/S-guided aspiration of their calcification.11 A nonrandomized controlled trial by Serafini et al., comparing the clinical outcome of patients who underwent double-needle U/S-guided percutaneous aspiration with a second group who refused treatment, reported significant long-term pain relief as for the first group.8 In addition, a study by del Cura et al. supported that almost all patients have completely or nearly completely radiographic resolution of their calcification one year after percutaneous aspiration and lavage.32
Furthermore, Yoo et al. demonstrated that ultrasound-guided needle decompression with subacromial steroid injection is clinically effective in more than seven out of ten patients with calcific tendinitis.33 De Witte et al. compared U/S-guided needling and lavage combined with an US-guided corticosteroid subacromial bursal injection with an isolated “blind” subacromial injection.9 Both groups were recorded having statistically significant improvement after 1-year follow-up. However, it was shown that the clinical and radiological results were significantly better in the “barbotage” group.9 In a second study by the same team, who investigated the 5-year clinical and radiological outcome of the same patients, no more significant differences between the two groups were found.34
The necessity of a local corticosteroid injection after U/S-guided mechanical irrigation and lavage remains controversial. It is accepted that a single xylocaine infiltration provides short-term pain relief due to its local anesthetic effect, while corticosteroids offer long-term relief by affecting diverse types of cells and endogenous molecules involved in the inflammation process.35 In a meta-analysis dealing with shoulder injections, it was reported that local corticosteroid injections proved to be three times more effective compared than placebo injections and one and a half time more effective than oral NSAID's.36 On the other hand, it has been shown that the local administration of glucocorticoid has significant negative effects on tendon cells in vitro, including reduced cell viability, cell proliferation and collagen synthesis.22 There is increased collagen disorganisation and necrosis as shown by in vivo studies.22 The mechanical properties of tendon are also significantly reduced.22
To our knowledge, our study was the first trial which investigated in the long term the clinical outcome of a xylocaine-only injection group against a combined xylocaine-corticosteroid group of patients, who were initially treated with an U/S-guided needle irrigation and lavage. There is only one clinical trial comparing the short-term results of xylocaine-only with the combined xylocaine-corticosteroid bursal injection after an U/S-guided needle irrigation and lavage for patients with shoulder calcific tendinopathy.25 Lin et al. reported that a single injection of xylocaine alone after US-guided percutaneous treatment of rotator cuff calcific tendinopathy provided longer lasting pain relief than the combined xylocaine-corticosteroid injection.25 The final follow-up of that study was set at 3 months and the only clinical outcome assessed was the VAS score. On the contrary, our study evaluated the clinical outcome in long term and by using four different subjective clinical variables (VAS, Q-DASH, patients’ satisfaction, willingness to repeat the procedure). Despite that, both studies illustrated that the additional use of a corticosteroid injection is not necessary when an ultrasound-guided mechanical irrigation and lavage of the intratendinous calcification is performed.
Our results indicated that both groups showed statistically significant and clinically relevant improvement during the follow-up of 3 and 12 months. Independently of the corticosteroid use (or not), it was proven that ultrasound-guided irrigation and lavage under local anesthesia is an effective treatment for patients suffering from symptomatic shoulder calcific tendinopathy. An interesting point of our trial was that the xylocaine-only group experienced less pain than the combined xylocaine-corticosteroid group at three months after treatment (in the short-term). However, this difference was not statistically significant. Finally, it is important to be noted that the biggest improvement was noticed within the first three months, whereas only slight further improvement was observed at the final, 12-month end point of our survey.
Our study was not without limitations. First, it was a single-center study involving a rather small number of patients. No ad hoc analysis was utilized. Moreover, the alleged benefit of using two different needles for irrigation and lavage has not yet been verified. Additionally, this was a retrospective analysis of prospectively collected data. On the other hand, patients were randomly treated by one of the two methods, without any other criteria, by the same treating physician. The post hoc power analysis which was higher than 90%, the strict inclusion-exclusion criteria and the fact that the follow-up was completed in all patients (100%), improved the validity of our results.
In summary, it was proven that the additional use of corticosteroid bursal injection did not provide with any additional short- to mid-term therapeutic benefit those patients with shoulder calcific tendinopathy who were treated with ultrasound-guided aspiration.
Declaration of competing interest
The authors declare that there is no conflict of interest related to this manuscript.
Acknowledgements
The authors wish to thank Dr. Chrysanthi Papanastasopoulou-Kaza, Ph.D., M.D., for her valuable contribution to the statistical analysis of our study.
Contributor Information
Vasileios Raoulis, Email: v_raoulis@yahoo.gr.
Nikolaos Vergados, Email: nikosvergados11@gmail.com.
Vasileios S. Nikolaou, Email: vassilios.nikolaou@gmail.com.
References
- 1.Louwerens J.K., Sierevelt I.N., van Noort A., van den Bekerom M.P. Evidence for minimally invasive therapies in the management of chronic calcific tendinopathy of the rotator cuff: a systematic review and meta-analysis. J Shoulder Elb Surg. 2014 Aug;23(8):1240–1249. doi: 10.1016/j.jse.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Sansone V., Maiorano E., Galluzzo A., Pascale V. Calcific tendinopathy of the shoulder: clinical perspectives into the mechanisms, pathogenesis, and treatment. Orthop Res Rev. 2018 Oct 3;10:63–72. doi: 10.2147/ORR.S138225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loew M., Jurgowski W., Mau H.C., Thomsen M. Treatment of calcifying tendinitis of rotator cuff by extracorporeal shock waves: a preliminary report. J Shoulder Elb Surg. 1995;4:101–106. doi: 10.1016/s1058-2746(05)80062-x. [DOI] [PubMed] [Google Scholar]
- 4.Welfling J., Kahn M.F., Desroy M., Paolaggi J.B., de Seze S. Calcifications of the shoulder. II. The disease of multiple tendinous calcifications [in French] Rev Rheum Mal Osteoartic. 1965;32:325–334. [PubMed] [Google Scholar]
- 5.DePalma A.F., Kruper J.S. Long term study of shoulder joints afflicted and treated for calcific tendinitis. Clin Orthop. 1961;20:61–72. [PubMed] [Google Scholar]
- 6.Uhthoff H.K., Loehr J.W. Calcific tendinopathy of the rotator cuff: pathogenesis, diagnosis and management. J Am Acad Orthop Surg. 1997;5:183–191. doi: 10.5435/00124635-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Hartig A., Huth F. Neue Aspekte zur Morphologie und Therapie der Tendinosis calcarea der Schultergelenke. Artroskopie. 1995;8:117–122. [Google Scholar]
- 8.Serafini G., Sconfienza L.M., Lacelli F., Silvestri E., Aliprandi A., Sardanelli F. Rotator cuff calcific tendonitis: short-term and 10-year out- comes after two-needle US-guided percutaneous treatment. Nonrandomized controlled trial. Radiology. 2009;252(1):157–164. doi: 10.1148/radiol.2521081816. [DOI] [PubMed] [Google Scholar]
- 9.de Witte P.B., Selten J.W., Navas A. Calcific tendinitis of the rotator cuff: randomized controlled trial of ultrasound guided needling and lavage versus subacromial corticosteroids. Am J Sports Med. 2013;41:1665. doi: 10.1177/0363546513487066. [DOI] [PubMed] [Google Scholar]
- 10.Cooper G., Lutz G.E., Adler R.S. Ultrasound-guided aspiration of symptomatic rotator cuff calcific tendonitis. Am J Phys Med Rehabil. 2005;84(1):81. doi: 10.1097/01.phm.0000150821.55552.2c. [DOI] [PubMed] [Google Scholar]
- 11.Galletti S., Magnani M., Rotini R. The echo-guided treatment of calcific tendinitis of the shoulder. Chir Organi Mov. 2004;89:319–323. [PubMed] [Google Scholar]
- 12.Aina R., Cardinal E., Bureau N.J., Aubin B., Brassard P. Calcific shoulder tendinitis: treatment with modified US-guided fineneedle method. Radiology. 2001;221(2):455–461. doi: 10.1148/radiol.2212000830. [DOI] [PubMed] [Google Scholar]
- 13.Kachewar S.G., Kulkarni D.S. Calcific tendinitis of the rotator cuff: a review. J Clin Diagn Res. 2013 Jul;7(7):1482–1485. doi: 10.7860/JCDR/2013/4473.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uhthoff H.K., Sarkar K. Calcifying tendinitis. In: Rockwood C.R. Jr., Matsen F.A. III, editors. vol. 2. WB Saunders; Philadelphia: 1990. pp. 774–790. (The Shoulder). [Google Scholar]
- 15.Noel E., Carillon Y., Gaillard T., Bouvier M. Needle aspiration irrigation in calcifying tendinitis of rotator cuff. In: Gazielly D.F., Gleyze P.T.T., editors. The Cuff. Elsevier; Paris: 1997. pp. 152–157. [Google Scholar]
- 16.Scarpone M., Rabago D., Snell E. Effectiveness of platelet-rich plasma injection for rotator cuff tendinopathy: a prospective open-label study. Glob Adv Health Med. 2013 Mar;2(2):26–31. doi: 10.7453/gahmj.2012.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seijas R., Ares O., Alvarez P., Cusco X., Garcia-Balletbo M., Cugat R. Platelet-rich plasma for calcific tendinitis of the shoulder: a case report. J Orthop Surg. 2012 Apr;20(1):126–130. doi: 10.1177/230949901202000128. [DOI] [PubMed] [Google Scholar]
- 18.Tahririan M.A., Moezi M., Motififard M., Nemati M., Nemati A. Ultrasound guided platelet-rich plasma injection for the treatment of rotator cuff tendinopathy. Adv Biomed Res. 2016 Dec 27;5:200. doi: 10.4103/2277-9175.190939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kesikburun S., Tan A.K., Yilmaz B., Yaşar E., Yazicioğlu K. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy: a randomized controlled trial with 1-year follow-up. Am J Sports Med. 2013 Nov;41(11):2609–2616. doi: 10.1177/0363546513496542. [DOI] [PubMed] [Google Scholar]
- 20.Wolf W.B., 3rd Shoulder tendinoses. Clin Sports Med. 1992;11(4):871–890. [PubMed] [Google Scholar]
- 21.DE Carli A., Pulcinelli F., Rose G.D., Pitino D., Ferretti A. Calcific tendinitis of the shoulder. Joints. 2014 Aug 1;2(3):130–136. doi: 10.11138/jts/2014.2.3.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dean B.J., Lostis E., Oakley T., Rombach I., Morrey M.E., Carr A.J. The risks and benefits of glucocorticoid treatment for tendinopathy: a systematic review of the effects of local glucocorticoid on tendon. Semin Arthritis Rheum. 2014 Feb;43(4):570–576. doi: 10.1016/j.semarthrit.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Mohamadi A., Chan J.J., Claessen F.M., Ring D., Chen N.C. Corticosteroid injections give small and transient pain relief in rotator cuff tendinosis: a meta-analysis. Clin Orthop Relat Res. 2017 Jan;475(1):232–243. doi: 10.1007/s11999-016-5002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy O. Ultrasound-guided barbotage in addition to ultrasound-guided corticosteroid injection improved outcomes in calcific tendinitis of the rotator cuff. J Bone Joint Surg Am. 2014 Feb 19;96(4):335. doi: 10.2106/JBJS.9604.ebo887. [DOI] [PubMed] [Google Scholar]
- 25.Lin Y.H., Chiou H.J., Wang H.K., Lai Y.C., Chou Y.H., Chang C.Y. Comparison of the analgesic effect of xylocaine only with xylocaine and corticosteroid injection after ultrasonographically-guided percutaneous treatment for rotator cuff calcific tendonosis. J Chin Med Assoc. 2015 Feb;78(2):127–132. doi: 10.1016/j.jcma.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Moutounet J., Chevrot A., Wybier M., Godefroy D. X-ray guided puncture-aspiration of refractory calcifications of the shoulder. Ann Radiol. 1992;35(3):156–159. [PubMed] [Google Scholar]
- 27.Patterson R.L., Darrach W. Treatment of acute bursitis by needle irrigation. J Bone Joint Surg Am. 1937;19:993–1002. [Google Scholar]
- 28.Farin P.U., Rasanen H., Jaroma H., Harju A. Rotator cuff calcifications: treatment with ultrasound-guided percutaneous needle aspiration and lavage. Skelet Radiol. 1996;25(6):551–554. doi: 10.1007/s002560050133. [DOI] [PubMed] [Google Scholar]
- 29.Farin P.U., Jaroma H., Soimakallio S. Rotator cuff calcifications: treatment with US-guided technique. Radiology. 1995;195:841–843. doi: 10.1148/radiology.195.3.7754018. [DOI] [PubMed] [Google Scholar]
- 30.Tagliafico A., Russo G., Boccalini S. Ultrasound-guided interventional procedures around the shoulder. Radiol Med. 2014 May;119(5):318–326. doi: 10.1007/s11547-013-0351-2. [DOI] [PubMed] [Google Scholar]
- 31.Krasny C., Enenkel M., Aigner N., Wlk M., Landsiedl F. Ultrasound guided needling combined with shock-wave therapy for the treatment of calcifying tendonitis of the shoulder. J Bone Joint Surg Br. 2005;87:501–507. doi: 10.1302/0301-620X.87B4.15769. [DOI] [PubMed] [Google Scholar]
- 32.del Cura J.L., Torre I., Zabala R., Legórburu A. Sonographically guided percutaneous needle lavage in calcific tendinitis of the shoulder: short- and long-term results. AJR Am J Roentgenol. 2007 Sep;189(3):W128–W134. doi: 10.2214/AJR.07.2254. [DOI] [PubMed] [Google Scholar]
- 33.Yoo J.C., Koh K.H., Park W.H., Park J.C., Kim S.M., Yoon Y.C. The outcome of ultrasound-guided needle decompression and steroid injection in calcific tendinitis. J Shoulder Elb Surg. 2010 Jun;19(4):596–600. doi: 10.1016/j.jse.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 34.de Witte P.B., Kolk A., Overes F., Nelissen R.G.H.H., Reijnierse M. Rotator cuff calcific tendinitis: ultrasound-guided needling and lavage versus subacromial corticosteroids: five-year outcomes of a randomized controlled trial. Am J Sports Med. 2017 Dec;45(14):3305–3314. doi: 10.1177/0363546517721686. [DOI] [PubMed] [Google Scholar]
- 35.Tsai M.J., O'Malley B.W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451e86. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 36.Arroll B., Goodyear-Smith F. Corticosteroid injections for painful shoulder: a meta-analysis. Br J Gen Pract. 2005;55:224–228. [PMC free article] [PubMed] [Google Scholar]