Abstract
Background
Granulocyte colony-stimulating factor (GCSF) has been utilized in decompensated cirrhosis (DC) for improving transplant-free survival (TFS). Data from multiple centers are conflicting with regard to patient outcomes. In this retrospective study, we present our ‘real-world experience’ of GCSF use in a large group of DC.
Methods
From September 2016 to September 2018, 1231 patients with cirrhosis were screened, of which 754 were found to have decompensation(s). Seventy-three patients with active ascites, jaundice, or both completed GCSF treatment (10 mcg/kg per day for 5 days, followed by 5 mcg/kg/day once every third day for total 12 doses). Per-protocol analysis (n = 56) was performed to study clinical events, liver disease severity, and outcomes at 3, 6, and 12 months after treatment. Modified intention-to-treat (mITT, n = 100) analysis was performed to study overall survival at 180 days. Outcomes were compared with a matched historical control (HC) group (n = 24).
Results
Nine (16%, n = 56), 24 (43%, n = 56), and 36 (75%, n = 48) patients died at 3, 6, and 12-month follow-up after GCSF. The commonest cause of death was sepsis (53%) followed by progressive liver failure (33%). Nine percent of patients developed hepatocellular carcinoma on follow-up at the end of 1 year. Acute variceal bleeds, overt hepatic encephalopathy, intensive unit admissions, and liver disease severity scores were higher after treatment at the end of 1 year. The Child–Pugh score >11 and model for end-stage liver disease-sodium score >25 and > 20 predicted worse outcomes at all time points and at 6 and 12 months after GCSF, respectively. Compared to a matched HC group, patients receiving GCSF had higher mortality (75% vs 46%, P = 0.04) at one year. mITT analysis revealed poor overall survival at 6 months compared to HCs (48% vs 75%, P = 0.04).
Conclusion
Survival in DC was shorter than what was expected in the natural history of the disease after GCSF use.
Keywords: growth factor, encephalopathy, HCC, erythropoietin, hyponatremia
Abbreviations: AKI, acute kidney injury; AUC, area under the receiver operating curve; AVB, acute variceal bleeding; BMSCs, Bone marrow–derived stem cells; CTP score, Child–Pugh score; DC, decompensated cirrhosis; DP, darbepoetin; GCSF, granulocyte colony-stimulating factor; HC, historical control; HCC, hepatocellular carcinoma; HE, hepatic encephalopathy; ICU, intensive care unit; INR, international normalized ratio; LT, liver transplantation; MELD-Na, model for end-stage liver disease-sodium; NASH, nonalcoholic steatohepatitis; RCT, randomized controlled trial; SBP, spontaneous bacterial peritonitis; SMT, standard medical treatment; TFS, transplant free survival
The median survival for patients with compensated cirrhosis is 12 years and for those with decompensated cirrhosis (DC), 2 years with an overall survival probability of 66% at one year.1, 2 Cirrhosis and its natural course are classified according to stages—I, compensated cirrhosis without esophagogastric varices; II, compensated cirrhosis with varices; III, bleeding (acute variceal bleeding [AVB]) without other complications; IV, first nonbleeding decompensation, and V, any second decompensating event. The median 1-year survival for stages 3 and 4 was found to be 80%, and 43%, respectively.3, 4 Liver transplantation (LT) is the curative treatment for DC. In developing countries, where organ transplantation resources are scare, in the absence of centralized organ allocation systems and penurious acceptance of LT within patient community, treatments aimed at transplant-free survival (TFS) are an unmet need. Regenerative therapy utilizing granulocyte colony-stimulating factor (GCSF) has been shown to improve TFS in DC in randomized controlled trials. In this study, we retrospectively analyzed survival benefits of GCSF therapy in a large cohort of DC and attempted to shed light on patient outcomes, significant predictors of outcomes and propose future directions based on our striking findings.
Patients and Methods
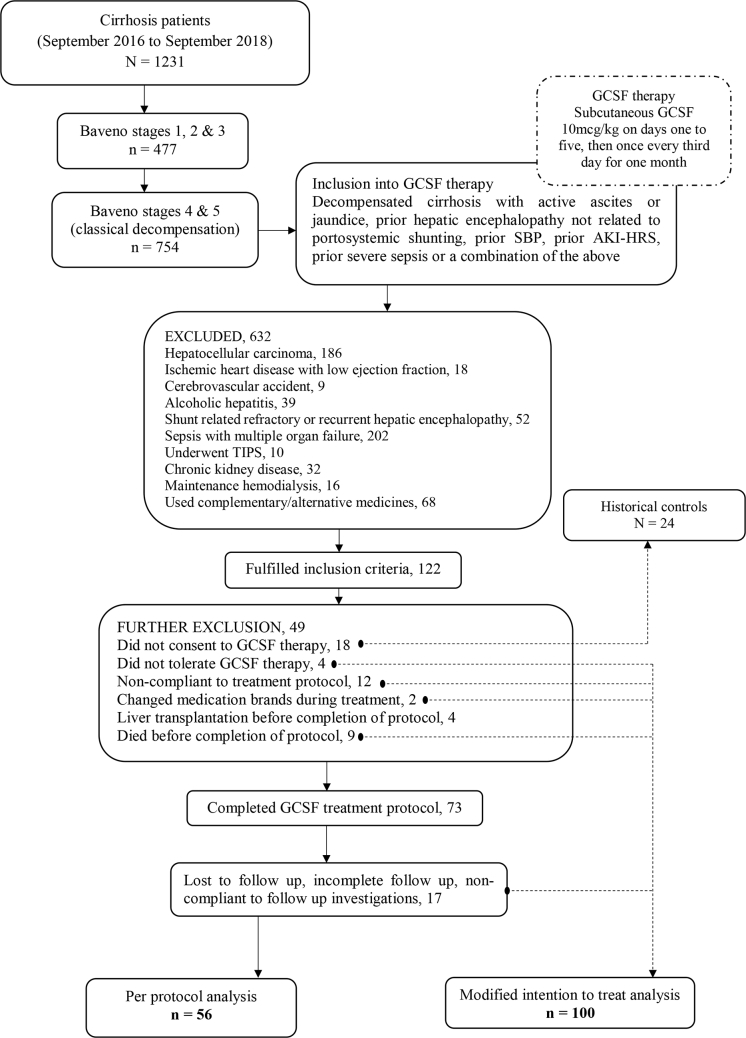
From September 2016 to September 2018, of a total of 1231 cirrhotics, 477 patients (38.7%) had Baveno stage I, II, and III cirrhosis (compensated cirrhosis and those with only AVB as decompensation), while 754 patients (61.2%) were in stages IV and V of cirrhosis. In the latter group, patients with active ascites or jaundice or both (not fulfilling definition of acute on chronic liver failure as per the European Association for the Study of Liver-Chronic Liver Failure)5 with prior hepatic encephalopathy (HE) not related to portosystemic shunting or prior AVB, prior spontaneous bacterial peritonitis (SBP), prior acute kidney injury (AKI), prior severe sepsis requiring ≥ 3 days of hospital stay, or a combination of the above were included for receiving GCSF therapy. Patients received subcutaneous GCSF (Filgrastim; Grafeel®, Dr Reddy's Laboratories, Hyderabad, India) at 10 mcg/kg in two divided doses for 5 days, followed by 5 mcg/kg once every third day for a total of 12 doses. We utilized this dosing schedule based on the fact that two-dose induction schedule was found to be more efficient in mobilizing peripheral blood stem cells.6 After exclusions due to various reasonable reasons, finally 73 patients were found to have completed GCSF treatment (Figure 1). Seventeen patients who were lost to follow-up were excluded, and 56 patients were included in the final analysis. Twenty-four patients (including 18 who fulfilled inclusion criteria for GCSF but did not consent for therapy) matched for age, sex, liver disease severity, sepsis events within three months prior to start of therapy, and etiology of liver disease from the same study time period were chosen as historical controls (HCs) in an approximate ratio of 1:2 for per-protocol analysis and 1:4 for modified intention-to-treat (mITT) analysis. Past clinical events were defined if they occurred before 3 months of inclusion into protocol, whereas recent clinical events occurred within 3 months. Ascites was graded as 1 (mild, detected on imaging), 2 (moderate, presence of shifting dullness), and 3 (severe, presence of fluid thrill). Liver disease severity was ascertained as per the Child–Pugh (CTP) and the model for end-stage liver disease-sodium (MELD-Na) score. Patient outcomes were studied at the end of 3, 6, and 12 months after completion of GCSF treatment. Primary sepsis was defined when patients had evidence of sepsis at admission, and secondary sepsis, when patients developed sepsis in hospital as a consequence of a primary liver or portal hypertensive event. Informed consent was obtained from those patients who were willing for GCSF therapy. The study protocol confirmed to the ethical guidelines of the 1975 Declaration of Helsinki and with approval for retrospective analysis by the hospital's institutional review board committee.
Figure 1.
CONSORT flow diagram. GCSF, granulocyte colony-stimulating factor; SBP, spontaneous bacterial peritonitis; AKI, acute kidney injury; HRS, hepatorenal syndrome; TIPS, transjugular intrahepatic portosystemic shunt.
Statistical Analysis
Statistical analysis was performed using MedCalc Statistical Software (Ostend, Belgium).
Per-protocol analysis was utilized to study efficacy, hepatic and extrahepatic events, and liver disease severity at follow-up periods from the baseline as well as outcomes. Additionally, mITT analysis was performed, excluding those who underwent LT during the treatment period. Patients undergoing LT before completion of treatment were excluded from mITT population because this event could not be predicted at the time of initiation of therapy and would have introduced a survival bias at 180 days. We analyzed survival at 180 days compared to the HC group as the credible end-point on mITT analysis. Patients who were lost to follow-up beyond three months after completion of treatment were considered to have died. Data are given as mean and standard deviation (SD) or as median and range between brackets as applicable. If the data of a variable not normally distributed, logarithmic transformation was applied. Chi-squared test was utilized to analyze significance between proportions of dichotomous data at different time points. Repeated-measures analysis of variance, on log-transformed nominal data, was performed when the same parameter was measured at different time points on the same subjects. Bonferroni correction to counteract multiple comparisons was applied for P-values and confidence intervals. Backward multiple regression analysis with automatic weighted regression procedure to correct for heteroscedasticity was used to examine the significant predictors associated with outcome at different time points. If there were more than one significant independent variable and the dependent variable was dichotomous, then logistic regression was utilized. The power of the regression model's predicted values was quantified by the area under the receiver operating curve (AUC). The Hosmer–Lemeshow test was used to assess goodness of fit of predictive variables on logistic regression. The Cox regression was used to analyze the effect of several variables on survival. The probability of survival at set time periods and between grouped independent variables was calculated using the Kaplan–Meier method. Comparison of survival curves was done using the log-rank test, and p < 0.05 was considered significant.
Results
Patient Demographics and Baseline Clinical and Investigational Characteristics
Males predominated (n = 54, 96.4%) with mean age (±SD) 52.4 ± 8.9 years. Commonest etiology of cirrhosis was nonalcoholic steatohepatitis (NASH, n = 28, 50%) followed by alcoholic cirrhosis (n = 26, 46.4%). 78.6% patients had diabetes, and 23% were obese cirrhotics. Ascites in the past was noted in 55.4%, AKI in 18%, AVB in 71%, and HE in 43%, while 20% had past history of jaundice. 77% (n = 44) and 14% (n = 8) had history of intensive care unit admission for management of complications of cirrhosis and sepsis in the past, respectively. Recent AKI, AVB, HE, intensive care unit admission, and sepsis were seen in 37.5% (n = 21), 14.3%,8 73.2% (41), 62.5% (35), and 41%,23 respectively. At the time of initiation of GCSF therapy, 87.5% (n = 49) and 76.8% (n = 43) had ascites and jaundice, respectively. The mean CTP and MELD-Na score at start of therapy were 11.3 ± 1.6 and 23.7 ± 4.7, respectively. Fifty-six each and 48 patients completed 3, 6, and 12-month follow-up, respectively. Patients were followed up for median 180 (min–max, 42–398) days. The baseline and follow-up investigational parameters are shown in Table 1.
Table 1.
Investigations at Baseline and After GCSF Treatment in Patients.
| Parameters | N | Minimum | Maximum | Mean | Median | SD |
|---|---|---|---|---|---|---|
| Age (in years) | 56 | 34 | 73 | 52.4 | 53 | 8.92 |
| Hemoglobin (g/dl) at baseline | 56 | 2.7 | 14.4 | 10.2 | 10.3 | 2.01 |
| Hemoglobin at 3 months | 56 | 5.5 | 14.3 | 10.1 | 10 | 1.6 |
| Hemoglobin at 6 months | 47 | 6.8 | 14 | 10 | 9.9 | 1.56 |
| Hemoglobin at 12 months | 23 | 6.8 | 14.7 | 10.1 | 10.2 | 1.91 |
| Total counts at baseline (x103 per cubic mm) | 56 | 1.1 | 17 | 5.4a | 5.5a | |
| Total counts at 3 months | 56 | 1.6 | 21.9 | 9.9 | 9.8 | 3.8 |
| Total counts at 6 months | 47 | 2.9 | 22.3 | 9.03a | 9.7a | |
| Total counts at 12 months | 23 | 4.6 | 17.8 | 8.6a | 8.7a | |
| Platelets (in lakhs/cubic mm) at baseline | 56 | 36 | 244 | 89.7a | 90.5a | |
| Platelets at 3 months | 56 | 15 | 212 | 96.2 | 94.5 | 31.8 |
| Platelets at 6 months | 47 | 20 | 214 | 83.8a | 94a | |
| Platelets at 12 months | 23 | 56 | 139 | 89.4 | 92 | 22.1 |
| Total bilirubin (mg/dl) at baseline | 56 | 1.2 | 16.7 | 4.93a | 4.9a | |
| Total bilirubin at 3 months | 56 | 1.1 | 17.8 | 5.1a | 5.2a | |
| Total bilirubin at 6 months | 47 | 1.1 | 24.4 | 5.01a | 4.2a | |
| Total bilirubin at 12 months | 23 | 1.4 | 31.8 | 4.3a | 3.2a | |
| Aspartate transaminase (IU/L) at baseline | 56 | 25 | 213 | 60.5a | 56a | |
| Alanine transaminase (IU/L) at baseline | 56 | 22 | 132 | 48.8a | 50.9a | |
| Serum albumin (g/dl) at baseline | 56 | 2.1 | 3.7 | 2.7a | 2.8a | |
| Serum albumin at 3 months | 56 | 2 | 3.6 | 2.8 | 2.9 | 0.3 |
| Serum albumin at 6 months | 47 | 1.8 | 3.6 | 2.8 | 2.9 | 0.4 |
| Serum albumin at 12 months | 23 | 1.9 | 3.6 | 2.9 | 3.1 | 0.5 |
| Blood urea nitrogen (mg//dl) at baseline | 56 | 5 | 144 | 23.4a | 22.5a | |
| Serum creatinine (mg/dl) at baseline | 56 | 0.4 | 2.3 | 1.02a | 1a | |
| Serum creatinine at 3 months | 56 | 0.4 | 3.4 | 1.01a | 1a | |
| Serum creatinine at 6 months | 47 | 0.4 | 5.6 | 1.2a | 0.9a | |
| Serum creatinine at 12 months | 23 | 0.4 | 7.8 | 1.1a | 1a | |
| International normalized ratio at baseline | 56 | 1.1 | 2.8 | 1.9 | 1.8 | 0.4 |
| International normalized ratio at 3 months | 56 | 1.4 | 3.9 | 1.9a | 1.8a | |
| International normalized ratio at 6 months | 47 | 1.14 | 4.3 | 2.1a | 1.8a | |
| International normalized ratio at 12 months | 23 | 1.34 | 4.9 | 1.9a | 1.5a | |
| Serum sodium (mEq/L) at baseline | 56 | 117 | 147 | 134.3 | 134 | 6.3 |
| Serum sodium at 3 months | 56 | 109 | 145 | 133.2 | 134 | 6.1 |
| Serum sodium at 6 months | 47 | 122 | 146 | 133.7 | 135 | 5.6 |
| Serum sodium at 12 months | 23 | 118 | 145 | 133.3 | 136 | 7.4 |
| Serum potassium (mEq/L) at baseline | 56 | 2.8 | 5.1 | 4.1 | 4.1 | 0.4 |
| Child–Pugh score at baseline | 56 | 7 | 14 | 11.3 | 11 | 1.6 |
| Child–Pugh score at 3 months | 56 | 7 | 14 | 10.a | 11a | |
| Child–Pugh score at 6 months | 47 | 6 | 15 | 9.8a | 10a | |
| Child–Pugh score at 12 months | 23 | 5 | 14 | 9a | 8a | |
| Model for end-stage liver disease-sodium score at baseline | 56 | 13 | 34 | 23 | 24 | 4.7 |
| Model for end-stage liver disease-sodium score at 3 months | 56 | 14 | 40 | 23a | 23.5a | |
| Model for end-stage liver disease-sodium score at 6 months | 47 | 13 | 40 | 23.4a | 21a | |
| Model for end-stage liver disease-sodium score at 12 months | 23 | 13 | 40 | 21.8a | 20a | |
| Follow-up (in days) | 56 | 42 | 398 | 180.a | 180a | |
| HCC diagnosis day on follow-up | 5 | 108 | 218 | 161.2 | 144 | 45.4 |
HCC, hepatocellular carcinoma; GCSF, granulocyte colony-stimulating factor; SD, standard deviation.
Log transformed.
Patient Outcomes
From the baseline, 9 (16%, n = 56), 24 (43%, n = 56), and 36 (75%, n = 48) patients died at 3, 6, and 12-month follow-up after GCSF treatment. The commonest cause of death was primary sepsis (53%, n = 19) followed by progressive liver failure (33%, n = 12). SBP (40%, n = 10 of 25), pneumonia (24%, 6), and bacteremia (14%, 5) were the most common sites of infection. Five patients (9%, n = 56; etiology: NASH in three, alcohol in one, and hepatitis B in one) developed hepatocellular carcinoma (HCC) on follow-up at the end of 1-year. The median time to detection of HCC was 144 (108–218) days. Two other surviving patients had alfafeto protein (AFP) >100 (both NASH etiology) on follow-up but without evidence of HCC on serial contrast imaging. Only one patient (4%, NASH etiology) developed HCC on retrospective analysis of an HC group (n = 24) matched for age, sex, liver disease severity, sepsis events within three months prior to start of therapy, and etiology of liver disease (NASH, n = 14, 58%) at end of one year which did not reach statistical significance.
Trends
Clinical Events
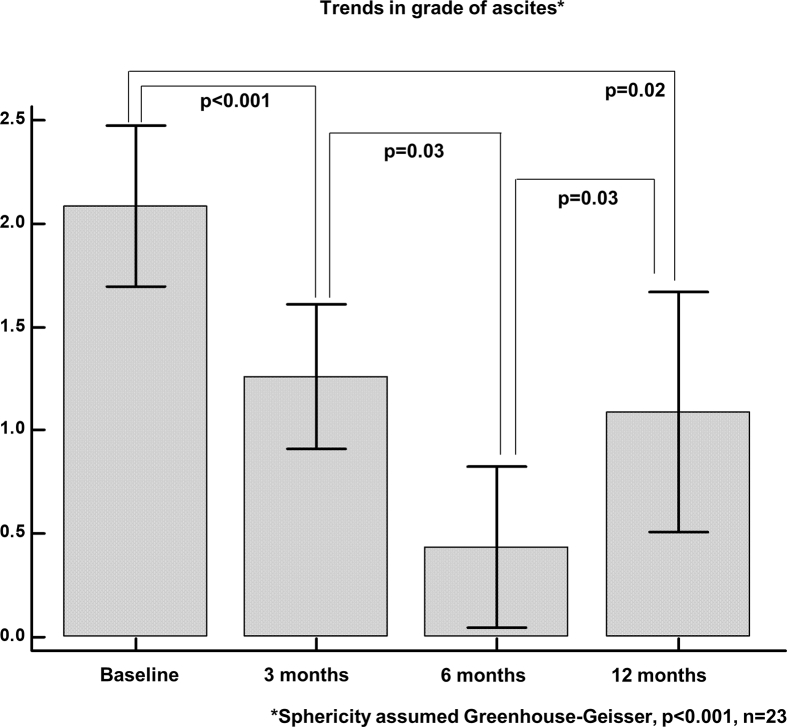
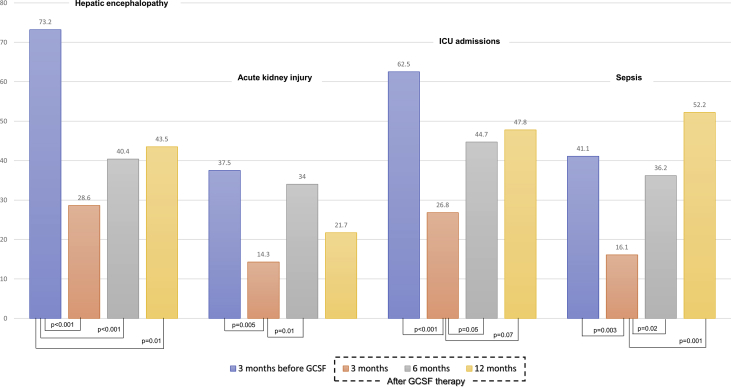
52% (n = 29 of 56) and 30% (n = 17) patients had grade 2 and 3 ascites at baseline, respectively, improving to 35%,20 and 5%4 at 3 months. However, the proportion of patients with grade 3 ascites increased beyond 3 months to 6% (3 of 47) and 26% (6 of 23) at 6 months and 1 year among survivors, respectively. Significant reduction in ascites was noted from baseline at 3 (P < 0.001), 6 (P < 0.001), and 12 months (P = 0.02), while significant worsening was noted from 6 to 12 months (P = 0.03) [Figure 2]. In 3 months prior to GCSF initiation 14% (8 of 56) patients had AVB. Fifteen (27%, n = 56) patients developed AVB after GCSF treatment at 1 year. Two (4%, n = 56), 8 (17%, n = 47), and 5 (22%, n = 23) patients developed AVB at 0–3, 3–6, and 6–12 months of follow-up; of which, 5 patients (14%, n = 36) died due to refractory bleed, respectively. Even though the incidence of post-treatment AVB was higher at 6 and 12 months, pairwise comparisons revealed only trend towards statistical significance (P = 0.07 and 0.08 respectively), but the overall change in continuous outcome of AVB within subjects was found to be significant (P = 0.02) showing significant linear trend in AVB incidence with time (P = 0.04). Prior to GCSF therapy, 73.2% (41 of 56) patients had experienced an episode of overt HE. After treatment, only 28.6% (16 of 56) had overt HE at the end of 3 months. This increased to 40.4% (N = 47) and 43.5% (N = 23) at 6 and 12 months, respectively. From baseline, at all time points, statistically significant reduction in HE was seen, but between time points, this significant reduction was not maintained with trend towards increase in overt HE. AKI was noted in 37.5% (N = 56) patients before GCSF initiation. The incidence decreased to 14.3% at 3 months and increased to 34% with further reduction to 22% at 6 and 12 months, respectively. The decrease in AKI at 3 and increase at 6 months were statistically significant (P = 0.005 and 0.01, respectively). Intensive care admissions in treated patients in 3 months prior to GCSF initiation were 62.5%, which reduced to 26.8%, with further increment to 44.7% and 47.8% at 6 and 12 months, respectively. The decrease in ICU admissions at 3 months from baseline was statistically significant (P < 0.001), while the increased incidence of sepsis at 6 and 12 months from the end of 3 months after GCSF therapy showed trend towards statistical significance (P = 0.05 and 0.07, respectively). The incidence of sepsis in patients with DC completing GCSF was higher at all time points from the third month onwards after initial significant reduction in the same (Figure 3).
Figure 2.
Trends in ascites in patients with decompensated cirrhosis completing GCSF treatment, from baseline at different time periods until one-year of follow-up.
Figure 3.
Clinical events in patients with decompensated cirrhosis receiving GCSF therapy from baseline up to one-year follow-up. GCSF, granulocyte colony-stimulating factor.
Investigational Parameters
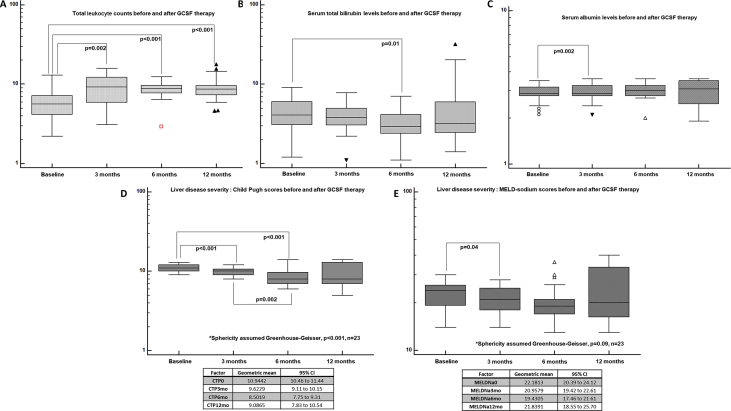
After completion of GCSF therapy, there was no significant change noted in hemoglobin levels at all time points until 1 year. However, the total leukocyte counts showed a steady and statistically significant increase after treatment at all time points from baseline but not between follow-up time periods (Greenhouse-Geisser sphericity, P < 0.001). The total bilirubin showed statistically significant decrease from baseline (geometric mean 4.2 mg/dl, 95% confidence interval [CI; 3.42–5.18]) to 6 months (3.18, 95% CI [2.64–3.82]) after GCSF therapy (P = 0.012). There was, however, no improvement in hyperbilirubinemia after 6 months, and on the contrary, a mild increase in mean serum bilirubin was noted among survivors at 12 months. International normalized ratio (INR) did not show significant changes from baseline at follow-up time points after GCSF treatment in DC, even though the mean increment in INR was higher at the end of 12 months from baseline (1.81 vs 1.93). There were no significant changes in the serum sodium level before and after GCSF treatment. Significant improvement in serum albumin levels was seen at 3 months after GCSF use, which was maintained until the end of 1 year (Figure 4).
Figure 4.
Investigational parameters in patients with decompensated cirrhosis before and after GCSF treatment from baseline. GCSF, granulocyte colony-stimulating factor; MELD, model for end-stage liver disease; CI, confidence interval; CTP, Child–Pugh.
Disease Severity and Predictors of Outcome in DC on Growth Factor Therapy
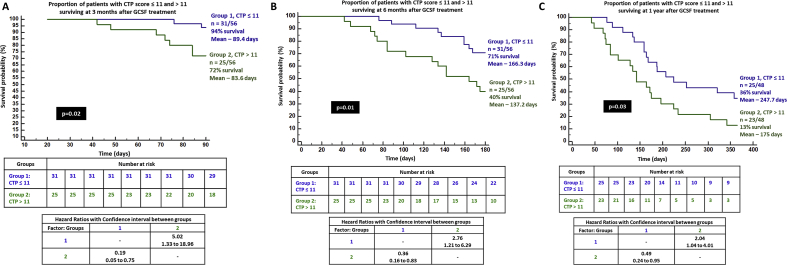
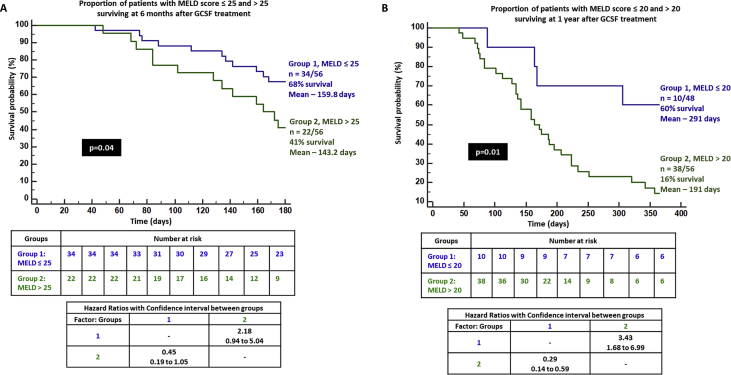
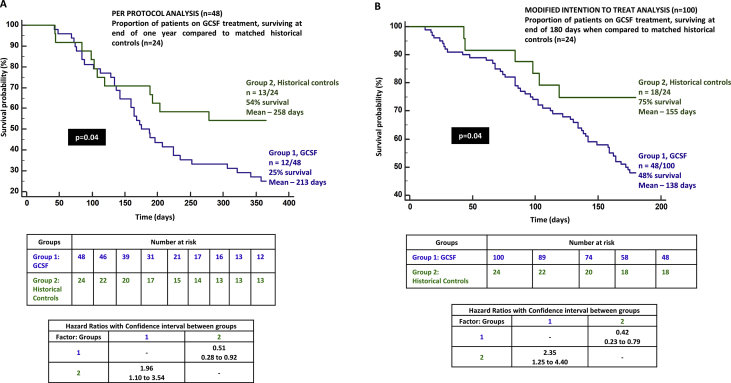
The CTP score improved significantly from baseline at 3, 6, and 12 months. Nevertheless, it was higher at 1 year when compared to 6 months, without statistical significance. The MELD-Na score also showed similar significant improving trends from baseline, although the 1-year MELD-Na score was higher than that at 3 and 6 months. On logistic regression, only the absence of the past AKI significantly predicted 3-month survival in patients with DC on GCSF treatment (P = 0.03, AUC 0.935, 95% CI [0.836–0.983]). On Cox regression, patients without AKI in 3 months prior to the start of GCSF treatment had trend towards better chance of survival at 3 months compared to those with AKI (P = 0.06, hazard ratio 0.061, 95% CI [0.003–1.136]). Sepsis within 3 months prior to GCSF therapy significantly predicted mortality at 6 months in patients undergoing growth factor therapy (P = 0.006, AUC 0.848, 95% CI [0.727–0.930]). Patients with recent sepsis were found to have 3.4 and 2.3 times higher risk of death at 6 and 12 months when compared to those without sepsis, receiving GCSF treatment (P = 0.01, 95% CI [1.23–9.2] and P = 0.04, [1.05–5.31], respectively). CTP>11 at baseline predicted mortality at 1 year (P = 0.013, AUC 0.698, 95% CI [0.548–0.822], sensitivity 58%, specificity 75%). On grouping patients who received GCSF based on CTP scores ≤11 and > 11, the proportion of patients surviving in the latter group were much lower at 3, 6, and 12 months (72%, 40%, and 13%, respectively) reaching statistical significance [Figure 5]. In patients with MELD-Na>25, the 6-month mortality was substantially higher compared to those with MELD-Na≤25 (40% vs 68%, P = 0.04). In those who received GCSF treatment with baseline MELD-Na>20, significantly higher proportion of patients died at the end of 1 year in comparison to those with MELD-Na ≤20 (16% vs 60%, P = 0.01) [Figure 6]. When compared to a matched HC group (n = 24, NASH 58%, male sex 86%, sepsis events in preceding 3 months in 45%, mean CTP 10.9 ± 2.3 and MELD-Na 24 ± 6.3) on standard medical care, we found that patients receiving GCSF treatment had a higher mortality than the control group at one year (75% vs 46%, P = 0.04; Figure 7a).
Figure 5.
Kaplan–Meier survival analysis in patients with decompensated cirrhosis and Child–Pugh score ≤11 and > 11 receiving GCSF therapy at 3 months (A), 6 months (B), and 1 year (C). GCSF, granulocyte colony-stimulating factor; CTP score, Child–Pugh score.
Figure 6.
Kaplan–Meier survival analysis in patients with decompensated cirrhosis and (A) model for end-stage liver disease (MELD) score ≤ 25 and > 25 and (B) MELD score ≤ 20 and > 20 receiving GCSF therapy at 6 months and 1 year respectively. GCSF, granulocyte colony-stimulating factor.
Figure 7.
Kaplan–Meier survival curve of (A) per-protocol analysis (n = 48) and (B) modified intention-to-treat analysis (n = 100) between patients receiving GCSF treatment and matched historical controls. GCSF, granulocyte colony-stimulating factor.
mITT Analysis
Patient Outcome at 180 Days
In the mITT cohort (n = 100), mean age was 55.6 ± 8.9 years, males predominated (88%), and NASH (50%), followed by ALD (42%) were common etiologies. The mean hemoglobin level was 10.1 ± 1.8 g/L, total leukocyte counts 6.1 ± 2.6 (x 103 per milliliter), median platelet counts 91.0 x 103/L, total bilirubin level 4.65 mg/dl, serum albumin level 2.7 mg/dl, INR 1.88, serum sodium level 132 mEq/L, serum creatinine level 1.2 mg/dl, and mean CTP and MELD-Na score were 11.2 ± 1.4 and 25.2 ± 4.6, respectively. The overall survival at 180 days was 48%. Significantly, higher proportion of patients died in the whole mITT cohort when compared to HCs (n = 52 of 100, 52% vs n = 6 of 24, 25%, respectively, P = 0.004; Figure 7b).
Discussion
We report outcomes of using GCSF therapy in a large group of DC. Our findings shed light on the need for deeper quality ‘bench’ work before ‘bedside’ use of GCSF. Bone marrow–derived stem cells (BMSCs) can contribute to scar tissue development through myofibroblast activity; concerns of progressive liver fibrosis and tumorigenesis, driven by GCSF-associated pathways through BMSCs and hepatic oval cells as well as GCSF-secreting HCCs, have been demonstrated in literature.7, 8, 9, 10, 11 Our study findings are in line with these observations and concerns. Nakamura et al.12 studied clinical benefits of GCSF-utilized CD34 + bone marrow–derived cells in patients with DC and found that all treated patients showed stable CTP scores, improvement in the MELD, and MELD-Na scores at 6 months but without statistical significance or significant improvement in hepatic function. Kedarisetty et al. studied the therapeutic benefits of GCSF injections along with darbepoetin (DP) in patients with DC compared to placebo. The cumulative probability of survival at 12 months was 68.6% in the GCSF+DP group and 26.9% in controls. Sepsis events were lower in the GCSF+DP group compared to controls. The most important weakness in this study, because of which strong conclusions on the efficacy of GCSF cannot be made, was the absence of a GCSF-only treatment arm.13
Seehofer et al.14, in an animal model of chronic liver disease, found that hepatic regeneration was slightly inhibited in the GCSF group. In our patients, mortality was higher with GCSF use and sepsis, followed by progressive liver failure were major causes of death. Data from multiple studies do not support the use of GCSF in sepsis. GCSF has immunosuppressive effects on monocytes, macrophages, dendritic cells, and T lymphocytes when exogenously administered. And high levels of GCSF negatively regulate IL-17 production, worsening sepsis, while the high level of GCSF at baseline was associated with poor outcome in sepsis (15, 16, 17, 18). Stephens et al.19 showed a higher rate of liver dysfunction and elevation of troponin in the GCSF-treated group and concluded that growth factor use was detrimental in septic patients. We found that DC patients with at least one episode of severe sepsis within 3 months before the start of GCSF therapy had worse outcomes at 6 and 12 months and higher incidence of sepsis. Our findings agree with published literature on adverse events noted with GCSF use in patients with sepsis. A study on effect of GCSF in liver fibrosis found that it significantly decreased the survival rate of mice.20 Verma et al. studied outcomes of GCSF with/without growth hormone in patients with DC compared to standard medical treatment (SMT). Patients with MELD of 10, who did not require listing for LT, were also included. The mean survival of patients on GCSF was 11.8 months with cumulative probability of TFS of 95.2%. There was an alarming decrease in liver stiffness measurements in patients who received GCSF; the reasons for this remain unknown because fibrosis reversal is a complex process with multiple pathogenic mechanisms. The authors demonstrate increase in CD34 + cells in peripheral blood of patients and link the same to improvement in liver-related events, similar to what was shown in the blood and serial liver biopsies by Kedarisetty et al.21, 13 The assumption that bone marrow–derived CD34 + cells could explain everything with hepatic regeneration is not completely accurate as there is much more than meets the eye in liver regeneration. To convincingly show that CD34 + hematopoietic stem cells differentiate into hepatocytes, several liver transcription factors and cytoplasmic proteins that are selectively expressed during the differentiation of mature hepatocyte markers need to be assessed—a practical concept that seems to be lacking in all human trials on GCSF in DC.22 Evidence that GCSF reverses fibrosis is still lacking in preclinical trials. When healthy volunteers were given daily GCSF injections, the number of mobilized CD34 + cells was the greatest on day 5, slightly less on day 6, and the least on day 4. With multiple GCSF injections spaced out like what was shown in the study by Verma et al., CD34 + cell mobilization and its singular role in amelioration of liver-related events is difficult to explain.23 Hence, without proper elucidation of GCSF-mediated significant pathways that promote or ameliorate liver damage, studying only CD34 + cells in DC does not improve our understanding of the disease and its mechanisms and cannot be extrapolated to outcomes in such patients. In animal studies on GCSF, an improvement in serum albumin has been shown, with doubtful long-term effect, while BMSC engraftment within the liver appears to be a temporary phenomenon.24 In our patients, similar findings as those described in preclinical studies were reproduced. Serum albumin levels improved in the short term in our patients and remained stable. Initial benefits of amelioration in liver-related events were short lived. Both per-protocol and mITT survival analyses were comparable in our cohort of patients, proving robustness of our study outcomes.
Newsome et al.25 assessed the safety and efficacy of GCSF and CD133 + hemopoietic stem cell infusions in patients with liver cirrhosis and found that singular or combination use of either did not improve liver dysfunction or fibrosis but was associated with higher frequency of adverse events compared with standard care. Our study findings echo those of Newsome et al. Whatever little benefit GCSF had to offer was short lived and, furthermore, worsened survival in patients in the long term. We show that AKI in the past predicted poor short-term outcomes with GCSF use and, CTP and MELD scores at baseline predicted intermediate- and long-term mortality with the possibility of HCC development. Finally, a recent study by Anand et al.26 showed that addition of erythropoietin to GCSF led to better regenerative response than GCSF monotherapy (in patients with MELD<16). However, the need for regenerative therapy in such low MELD group is debatable.
The major limitation of our study is that it was based at a single center and retrospectively analyzed, which makes it an uncontrolled design. However, we aimed to study only patient outcomes with respect to GCSF use in a ‘real-world’ situation and in patients truly deserving TFS. Compared to HCs with similar characteristics, those receiving GCSF had worse survival. The associated complications in cirrhosis, longitudinally presented in this study, may have been related to the natural history of cirrhosis and not GCSF use. However, compared to a matched HC group, GCSF-treated patients lacked survival benefit as the important aspect of therapy was to prevent liver-related complications, which was not evident. Over 70% of patients were treated in the intensive unit for sepsis and other complications of cirrhosis, and it might seem that rather an end-stage cohort was selected for GCSF use. Studies on GCSF in DC in literature state improvement in sepsis- and liver-related events. We specifically studied those patients with DC in whom sepsis- and liver-related events needed to be reduced/controlled but found the contrary with GCSF therapy. We considered patients with ‘cryptogenic’ cirrhosis as burned out NASH, and hence, the representation of NASH-related cirrhosis was higher in our study cohort. Liver cancer incidence was higher in patients on GCSF compared to HCs. NASH etiology and the possibility of preexistent preneoplastic lesions in the liver could have been the reason for the high rate of HCC, and the role of GCSF in such scenario needs to be studied in a larger group of patients with longer follow-up. We did not perform serial liver biopsies in view of advanced liver disease and poor patient participation for the same. Our study findings need to be validated by other groups for strengthening outcome predictions.
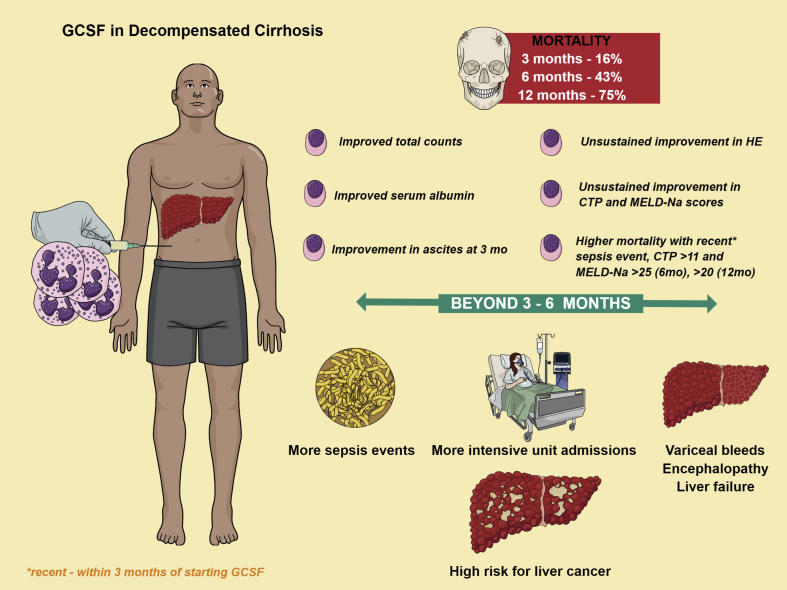
GCSF use in DC worsens survival and promotes sepsis, and severity scores and clinical events in the recent past predict poor outcomes (summary, Figure 8). Bone marrow–derived cells may have the potential to contribute significantly to fibrosis. Even though clinical trials paint a pretty picture of GCSF use in cirrhotics, this must be taken with a pinch of salt until further preclinical work sheds light on this phenomenon with regard to fate of induced stem cells through available cell-tracing techniques, liver histology changes, and reversal of fibrotic mechanisms that modulate the liver microenvironment towards a beneficial outcome. The choice of using GCSF in patients with advanced DC needs caution as it may shorten the natural history of the disease and predispose to sepsis events and liver cancer.
Figure 8.
Summary of the study. GCSF, granulocyte colony-stimulating factor; HE, hepatic encephalopathy; CTP score, Child–Pugh score; MELD-Na, model for end-stage liver disease-sodium.
Author contributions
C.A.P designed the study, performed analysis, and drafted the manuscript; P.A., S.R., R.A, R.P.,G.P., G.C.V. and S.K.J. made critical revisions to the manuscript; R.Z., R.P., and G.P., collected data and prepared graphical data; all authors approved the final version of the full manuscript.
Conflicts of Interest
The authors have none to declare.
References
- 1.D'Amico G., Garcia-Tsao G., Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Fleming K.M., Aithal G.P., Card T.R., West J. All-cause mortality in people with cirrhosis compared with the general population: a population-based cohort study. Liver Int. 2012;32:79–84. doi: 10.1111/j.1478-3231.2011.02517.x. [DOI] [PubMed] [Google Scholar]
- 3.D'Amico G., Pasta L., Morabito A. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther. 2014;39:1180–1193. doi: 10.1111/apt.12721. [DOI] [PubMed] [Google Scholar]
- 4.Muir A.J. Understanding the complexities of cirrhosis. Clin Ther. 2015;37:1822–1836. doi: 10.1016/j.clinthera.2015.05.507. [DOI] [PubMed] [Google Scholar]
- 5.Hernaez R., Solà E., Moreau R., Ginès P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541–553. doi: 10.1136/gutjnl-2016-312670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee V., Li C.K., Shing M.M. Single vs twice daily G-CSF dose for peripheral blood stem cells harvest in normal donors and children with non-malignant diseases. Bone Marrow Transplant. 2000;25:931–935. doi: 10.1038/sj.bmt.1702338. [DOI] [PubMed] [Google Scholar]
- 7.Dalakas E., Newsome P.N., Harrison D.J. Hematopoietic stem cell trafficking in liver injury. FASEB J. 2005;19:1225–1231. doi: 10.1096/fj.04-2604rev. [DOI] [PubMed] [Google Scholar]
- 8.Wu X.Z., Chen D. Origin of hepatocellular carcinoma: role of stem cells. J Gastroenterol Hepatol. 2006;21:1093–1098. doi: 10.1111/j.1440-1746.2006.04485.x. [DOI] [PubMed] [Google Scholar]
- 9.Dumble M.L., Croager E.J., Yeoh G.C. Generation and characterisation of p53 null transformed hepatic progenitor cells: oval cells give rise to hepatocellular carcinoma. Carcinogenesis. 2002;23:435–445. doi: 10.1093/carcin/23.3.435. [DOI] [PubMed] [Google Scholar]
- 10.Kohno M., Shirabe K., Mano Y. Granulocyte colony-stimulating-factor-producing hepatocellular carcinoma with extensive sarcomatous changes: report of a case. Surg Today. 2013;43:439–445. doi: 10.1007/s00595-012-0202-0. [DOI] [PubMed] [Google Scholar]
- 11.Nagata H., Komatsu S., Takaki W. Granulocyte colony-stimulating factor-producing hepatocellular carcinoma with abrupt changes. World J Clin Oncol. 2016;10:380–386. doi: 10.5306/wjco.v7.i5.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura T., Torimura T., Iwamoto H. CD34(+) cell therapy is safe and effective in slowing the decline of hepatic reserve function in patients with decompensated liver cirrhosis. J Gastroenterol Hepatol. 2014;29:1830–1838. doi: 10.1111/jgh.12622. [DOI] [PubMed] [Google Scholar]
- 13.Kedarisetty C.K., Anand L., Bhardwaj A. Combination of granulocyte colony-stimulating factor and erythropoietin improves outcomes of patients with decompensated cirrhosis. Gastroenterology. 2015;148:1362–1370. doi: 10.1053/j.gastro.2015.02.054. [DOI] [PubMed] [Google Scholar]
- 14.Seehofer D., Neumann U.P., Schirmeier A. Synergistic effect of erythropoietin but not G-CSF in combination with curcumin on impaired liver regeneration in rats. Langenbeck's Arch Surg. 2008;393:325–332. doi: 10.1007/s00423-008-0290-x. [DOI] [PubMed] [Google Scholar]
- 15.Mathias B., Szpila B.E., Moore F.A., Efron P.A., Moldawer L.L. A review of GM-CSF therapy in sepsis. Medicine (Baltim) 2015;94 doi: 10.1097/MD.0000000000002044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohammad R.A. Use of granulocyte colony-stimulating factor in patients with severe sepsis or septic shock. Am J Health Syst Pharm. 2010;67:1238–1245. doi: 10.2146/ajhp090325. [DOI] [PubMed] [Google Scholar]
- 17.Martins A., Han J., Kim S.O. The multifaceted effects of granulocyte colony-stimulating factor in immunomodulation and potential roles in intestinal immune homeostasis. IUBMB Life. 2010;62:611–617. doi: 10.1002/iub.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishikawa K., Tanaka H., Nakamori Y. J. Difference in the responses after administration of granulocyte colony-stimulating factor in septic patients with relative neutropenia. Trauma. 2000;48:814–824. doi: 10.1097/00005373-200005000-00004. discussion 824-5. [DOI] [PubMed] [Google Scholar]
- 19.Stephens D.P., Thomas J.H., Higgins A. Randomized, double-blind, placebo-controlled trial of granulocyte colony-stimulating factor in patients with septic shock. Crit Care Med. 2008;36:448–454. doi: 10.1097/01.CCM.0B013E318161E480. [DOI] [PubMed] [Google Scholar]
- 20.Ogiso T., Nagaki M., Takai S. Granulocyte colony-stimulating factor impairs liver regeneration in mice through the up-regulation of interleukin-1beta. J Hepatol. 2007;47:816–825. doi: 10.1016/j.jhep.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Verma N., Kaur A., Sharma R. Outcomes after multiple courses of granulocyte colony-stimulating factor and growth hormone in decompensated cirrhosis: a randomized trial. Hepatology. 2018;68:1559–1573. doi: 10.1002/hep.29763. [DOI] [PubMed] [Google Scholar]
- 22.Jang Y.Y., Collector M.I., Baylin S.B., Diehl A.M., Sharkis S.J. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2008;6:532–539. doi: 10.1038/ncb1132. [DOI] [PubMed] [Google Scholar]
- 23.Stroncek D.F., Clay M.E., Herr G. The kinetics of G-CSF mobilization of CD34+ cells in healthy people. Transfus Med. 1997;7:19–24. doi: 10.1046/j.1365-3148.1997.d01-75.x. [DOI] [PubMed] [Google Scholar]
- 24.Hua L., Aoki T., Jin Z. Elevation of serum albumin levels in nagase analbuminemic rats by allogeneic bone marrow cell transplantation. Eur Surg Res. 2005;37:111–114. doi: 10.1159/000084542. [DOI] [PubMed] [Google Scholar]
- 25.Newsome P.N., Fox R., King A.L. Granulocyte colony-stimulating factor and autologous CD133-positive stem-cell therapy in liver cirrhosis (REALISTIC): an open-label, randomized, controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3:25–36. doi: 10.1016/S2468-1253(17)30326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anand L., Bihari C., Kedarisetty C.K. Early cirrhosis and a preserved bone marrow niche favour regenerative response to growth factors in decompensated cirrhosis. Liver Int. 2019;39:115–126. doi: 10.1111/liv.13923. [DOI] [PubMed] [Google Scholar]