Abstract
Aim
The aim of this study was to study the role of magnetic resonance imaging (MRI) in monitoring hepatic fat content in cases of nonalcoholic fatty liver disease (NAFLD).
Materials and methods
41 adults (mean age: 39 years, 22 males; 19 females) with NAFLD were included after obtaining approval from the institutional ethics committee. The baseline clinical (weight, body mass index [BMI]) and biochemical parameters, fatty liver grade on ultrasonography (USG), and hepatic fat signal fraction (FSF) using dual-echo chemical shift imaging and proton density fat fraction on magnetic resonance spectroscopy (MRS-PDFF) were assessed, before and after intervention (dietary and lifestyle changes and oral vitamin E for six months). They were categorized into Group A (good compliance to intervention) and Group B (poor compliance), and the clinical and imaging parameters were compared between them.
Results
After intervention, Group A (n = 30) showed significant reduction in BMI (28.35 ± 3.25 to 27.14 ± 3.24 kg/m2; P < 0.001), hepatic FSF (19.30 ± 9.09% to 11.18 ± 7.61%; P < 0.05), and MRS-PDFF (18.79 ± 8.53% to 10.64 ± 6.66%). In Group B (n = 11), there was significant increase in BMI (28.85 ± 2.41 to 29.31 ± 2.57 kg/m2; P < 0.001), hepatic FSF (18.96 ± 9.79% to 21.48 ± 11.80%; P < 0.05), and reduction in high-density lipoproteins (P < 0.05). Although there was good correlation between USG and MRS in quantifying liver fat (r = 0.84–0.87; P < 0.001), USG was unable to detect <5.3% change in hepatic fat. There was poor correlation between lipid profile and MRS-PDFF. Change in body weight significantly correlated with change in hepatic fat content (r = 0.76; P < 0.001).
Conclusion
MRI is useful in accurately quantifying and in monitoring hepatic fat content and is better than clinical and biochemical parameters and USG.
Keywords: nonalcoholic fatty liver disease, ultrasonography, magnetic resonance imaging, fatty liver
Abbreviations: BMI, Body Mass Index; CSI, Chemical Shift Imaging; FSF, Fat Signal Fraction; HCC, Hepatocellular Carcinoma; HDL, High Density Lipoproteins; LDL, Low Density Lipoproteins; MRI, Magnetic Resonance Imaging; MRS, Magnetic Resonance Spectroscopy; NAFLD, Non-Alcoholic Fatty Liver Disease; NASH, Non-Alcoholic SteatoHepatitis; PDFF, Proton Density Fat Fraction; USG, Ultrasonography
Nonalcoholic fatty liver disease (NAFLD) is characterized by accumulation of lipid in the liver in the absence of excessive alcohol consumption (defined as alcohol intake <20 g/day). It affects about 10–30% of the population worldwide, with a higher incidence among obese and diabetic patients.1, 2, 3 NAFLD represents a disease spectrum ranging from simple hepatic steatosis to steatohepatitis to fibrosis and cirrhosis. Ultimately, this can lead to end-stage liver failure or the development of hepatocellular carcinomas (HCC), with subsequent need for a liver transplantation.4,5 The risk of occurrence of HCC in patients with nonalcoholic steatohepatitis (NASH)-related cirrhosis is shown to be 0.46% per year.6 Hence, early diagnosis and management is necessary.
Currently, the gold standard for the assessment of hepatic steatosis is liver biopsy.7 However, it is invasive and is associated with bleeding complications, which may be life-threatening.8,9 Furthermore, it cannot be used in the monitoring of these patients. Many noninvasive methods such as body mass index (BMI), waist circumference, and lipid profile (esp. triglycerides) may be used to indirectly assess NAFLD, but are not reliable for accurate assessment of hepatic fat fraction.10, 11, 12, 13 Imaging modalities such as ultrasonography (USG), magnetic resonance imaging (MRI), and magnetic resonance spectroscopy (MRS) are preferred for hepatic fat assessment.14
USG of the liver is a simple, noninvasive, and widely used technique in the detection of fatty infiltration of the liver. The grading of the fatty liver on USG is subjective, and its sensitivity and specificity vary from 60% to 94% and 84%–95%, respectively.15,16 Thus, its use in the monitoring of NAFLD is limited as minor alterations may not be detected. MRI, on the other hand, has shown to be accurate in the quantification of hepatic parenchymal fat and is increasingly being used in patients with NAFLD and NASH.17,18 Chemical shift imaging (CSI) and MRS are good techniques in the quantification of liver fat.19, 20, 21, 22, 23 CSI (dual echo) is a simple and robust technique which provides accurate quantification of liver fat. However, it's values are affected when there is concurrent deposition of iron in the liver, by T1 bias and by multiple fat peaks.22,23 This lead to the exploration of multiecho Dixon techniques, in which the T2* effects are taken into consideration while calculating hepatic fat content. This has shown to be more accurate than the simple dual-echo technique. MRS has shown good correlation with biopsy in estimation of liver fat and has the potential of becoming the gold standard in the future.22,23 A study by Noureddin et al. has suggested that MRI is more sensitive than liver biopsy in assessing changes in liver fat content.24 Although expensive, these techniques have been reliably used in the diagnosis of patients with NAFLD. However, there is limited data on its role in the monitoring of these patients in response to lifestyle modifications and treatment.25
With this background, we aimed to assess the role of MRI in the diagnosis and follow-up of patients with NAFLD, using MRS as gold standard and compare it with USG, lipid profile, and BMI.
Materials and methods
This prospective study was approved by the Institute Ethics Committee (reference number—IESC/T-472/29.11.2013). Treatment-naive adults (>18 years of age) showing fatty liver on screening USG and with a clinical suspicion of NAFLD and were included in the study after obtaining written informed consent. Subjects with known or recently detected diabetes, seropositivity for HIV (human immunodeficiency virus), HBV (hepatitis B virus) and HCV (hepatitis C virus), alcohol intake of >20 g/day, hypothyroidism, and chronic drug intake such as corticosteroids were excluded from the study. BMI was recorded in all cases followed by laboratory investigations which included liver function tests, thyroid-stimulating hormone (to rule out subclinical hypothyroidism), fasting and postprandial blood glucose (to rule out diabetes), and lipid profile (serum cholesterol, triglycerides, low-density lipoproteins [LDL], high-density lipoproteins [HDL]).
After the initial standard grading of fatty liver on USG (by a radiologist with three years' experience, NM), MRI and MRS of the liver were performed in all subjects on a 1.5 T scanner (Achieva, Philips, Best, the Netherlands) using phased-array torso coil. The following sequences were acquired:
-
1.
CSI: T1-weighted in-phase and opposed-phase gradient echo sequence in axial plane (TR 5.8 ms, TE 4.6 ms [in-phase]/2.3 ms [opposed-phase], flip angle 15°, 3D technique, number of slices 70, slice thickness 5 mm, recon voxel size 1.3 mm × 1.3 mm x 2.5 mm, recon matrix 288, number of averages 1, breath hold, time 24sec). This provided hepatic fat signal fraction (FSF).
-
2.
MRS: Single-voxel MRS was performed using point resolved spectroscopy without water suppression from 3 different areas in the right lobe of liver avoiding major vessels in free. The parameters were voxel size 30 × 30 × 30 mm, TE 34 ms, TR 2000 ms, flip angle 90°, number of averages 40, phase cycles 8, time—1min 24 s. This provided proton density fat fraction on magnetic resonance spectroscopy (MRS-PDFF).
The images were evaluated by two radiologists with three and twelve years' experience (NM and KSM, respectively). The hepatic FSF and PDFF were calculated as described in the literature.19,26 For CSI, a region of interest (minimum 1 cm2) was drawn on a homogeneous area in the right lobe of liver avoiding vessels (in the same location where MRS was performed), on both the in-phase and the opposed-phase images. FSF was calculated by the formula (Sip—Sop)/2Sip, where Sip is the signal intensity on the in-phase image and Sop is the signal intensity on the opposed-phase image. Three such ROIs were drawn in three different regions in the right lobe and average FSF was calculated.
The water and fat signals obtained on MRS were postprocessed and PDFF was calculated by using the formula—Afat/(Awater + Afat), where Afat is the area under the spectral peak at 1.3 ppm (fat peak) and Awater is the area under the peak at 4.8 ppm (water peak). Average of the three values obtained from the MRS of three different areas was calculated.
Subsequently, subjects were advised treatment in the form of low-calorie and fat-restricted diet by the physician and the dietician in a self-designed diet chart keeping the energy content of 1000–1200 kcal per day for women and 1200–1600 kcal per day for men.27 Carbohydrates comprised 40–50%, fats <30% (saturated fats < 10 g/day), and proteins 20–30% of the total calories with high fiber content. Subjects were advised to have 4–5 meals per day with not more than 3 h break in between meals.27 They were also advised brisk walking for 60 min a day and daily oral vitamin E (400 mg BD). Role of vitamin E has been found to be effective in NASH but is less clear in NAFLD as it improves both inflammation and steatosis on histology.28 Because histological confirmation was not available in our cases, vitamin E was given to all to maintain the uniformity. The subjects were contacted every two months and their compliance to dietary advice was assessed by 24-h recall method. Compliance to the exercise was assessed by obtaining appropriate history and proper charting of duration of exercise and distance covered. Regular intake of vitamin E was assessed by recall method. Those cases with total calorie intake ±15% of the prescribed amount and following at least four (of six) months of regular exercise and vitamin E intake were considered having a good compliance (Group A). Those cases failing to meet the aforementioned criteria were considered having a poor compliance (Group B). The aforementioned information was collected by a senior registered dietician.
After six months of treatment, all subjects were re-evaluated with BMI, lipid profile, USG (to grade fatty liver), and MRI (with the same protocol to calculate FSF). Based on the compliance to the intervention, the subjects were categorized into compliant (Group A) and noncompliant (Group B) groups at the end of six months.
Statistical analysis was carried out using SPSS 21.0 software (SPSS, IBM, Chicago). Descriptive statistics was used for demographic details. The MRS-PDFF value was taken as the gold standard. The therapeutic response of each of the participants was evaluated by comparing the pretreatment and the posttreatment BMI, laboratory, and imaging parameters (USG grading, hepatic FSF, and MRS-PDFF) using paired t test. Comparison between Group A and Group B was also done using t test. Correlation between the imaging and the clinical factors was done using Pearson's correlation coefficient. A P-value of less than 0.05 was considered significant.
Results
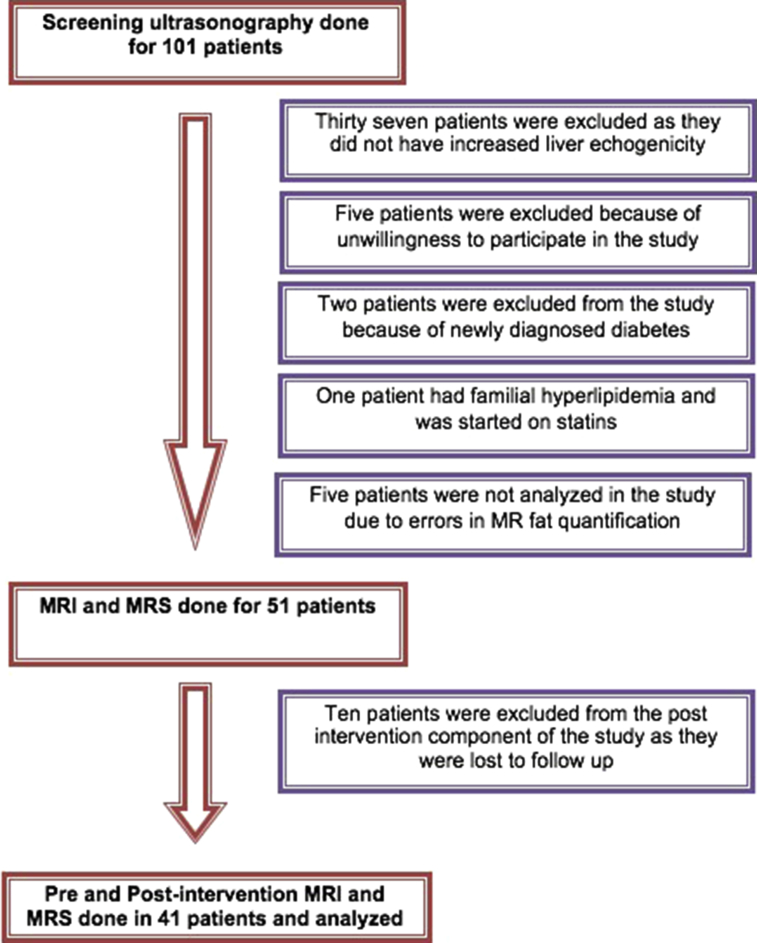
One hundred one nonalcoholic and nondiabetic subjects with a clinical suspicion of NAFLD were referred for USG screening (Figure 1). Of these, 37 subjects did not have fatty liver and were excluded. Eight subjects did not match the inclusion criteria (five were unwilling to participate in the study, two had newly diagnosed diabetes, and one had familial hyperlipidemia). Five subjects were excluded because of technical error in MR fat quantification (which occurred in the initial part of the study). Ten subjects were lost to follow-up. Finally, a total of 41 subjects (22 males, 19 females; mean age: 39 years) were included in the study. There were 30 subjects in Group A and 11 subjects in Group B.
Figure 1.
Flow chart showing inclusion and exclusion of number of cases. MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy.
The demographic characteristics, pretreatment BMI, lipid profile, grade of fatty liver on USG, FSF, and MRS-PDFF in both groups are shown in Table 1. Before intervention, there was no significant difference in BMI, lipid profile, USG grade, MRI-FSF, and MRS-PDFF between the two groups (Table 2). After six months of treatment, there was significant decrease in the body weight, BMI, MRI-FSF, and MRS-PDFF in Group A compared with Group B and the difference was significant (Table 3). Serum transaminases were elevated in only four of the 41 cases which returned to normal after intervention. Hence, they were not evaluated.
Table 1.
Demographic Characteristics of Patients in Both the Groups.
| Patient parameters | Group A (n = 30) | Group B (n = 11) | ||
|---|---|---|---|---|
| Mean age (range) | 38.8 years (20–53 years) | 40 years (27–55 years) | ||
| Sex: Male | 18 | 4 | ||
| Female | 12 | 7 | ||
| Preintervention | Postintervention | Preintervention | Postintervention | |
|---|---|---|---|---|
| BMI (mean) | 28.35 ± 3.25 | 27.14 ± 3.24 | 28.85 ± 2.41 | 29.31 ± 2.57 |
| Lipid profile—serum (mean values) | ||||
| Cholesterol (mg/dL) | 198.51 ± 34.61 | 187.87 ± 29.56 | 179.71 ± 26.43 | 182.64 ± 47.74 |
| HDL (mg/dL) | 44.28 ± 9.48 | 44.97 ± 5.44 | 47.82 ± 10.34 | 42.36 ± 2.77 |
| LDL (mg/dL) | 111.19 ± 33.37 | 111.03 ± 26.12 | 90.89 ± 30.09 | 104.44 ± 41.38 |
| Triglycerides (mg/dL) | 216.60 ± 73.47 | 161.37 ± 55.35 | 205.00 ± 72.35 | 176.82 ± 77.81 |
| USG grade (n) | ||||
| Grade 0 | – | 03 | – | – |
| Grade I | 11 | 16 | 04 | 04 |
| Grade II | 09 | 11 | 06 | 05 |
| Grade III | 10 | – | 01 | 02 |
| Mean MRI-FSF (CSI) | 19.30 ± 9.09 | 11.18 ± 7.61 | 18.96 ± 9.79 | 21.48 ± 11.80 |
| Mean PDFF (MRS) | 18.79 ± 8.53 | 10.64 ± 6.66 | 17.10 ± 9.28 | 19.45 ± 10.48 |
CSI, chemical shift imaging; HDL, high-density lipoproteins; LDL, low-density lipoproteins; MRS, magnetic resonance spectroscopy; USG, ultrasonography; PDFF, proton density fat fraction; FSF, fat signal fraction; BMI, body mass index; MRI, magnetic resonance imaging.
Table 2.
Comparison of Baseline Characteristics Group A and B.
| Patient parameters | Group A (n = 30) | Group B (n = 11) | P-value |
|---|---|---|---|
| Clinical: | |||
| Age (years) | 38.80 ± 7.84 | 40.00 ± 7.25 | 0.330 |
| Weight (kg) | 76.53 ± 8.14 | 74.27 ± 10.48 | 0.235 |
| BMI | 28.35 ± 3.25 | 28.85 ± 2.41 | 0.321 |
| Biochemical: | |||
| Serum cholesterol (mg/dL) | 198.51 ± 34.61 | 179.71 ± 26.43 | 0.055 |
| Serum LDL (mg/dL) | 111.19 ± 33.37 | 90.89 ± 30.09 | 0.042* |
| Serum HDL (mg/dL) | 44.28 ± 9.48 | 47.82 ± 10.34 | 0.154 |
| Serum triglycerides (mg/dL) | 216.60 ± 73.47 | 205.00 ± 72.35 | 0.328 |
| MRI: | |||
| Mean FSF (CSI) | 19.30 ± 9.09 | 18.96 ± 9.79 | 0.458 |
| Mean PDFF (MRS) | 18.79 ± 8.53 | 17.10 ± 9.28 | 0.293 |
*Significant at P-value.
HDL, high-density lipoproteins; LDL, low-density lipoproteins; MRS, magnetic resonance spectroscopy; PDFF, proton density fat fraction; FSF, fat signal fraction; BMI, body mass index; MRI, magnetic resonance imaging.
Table 3.
Comparison of BMI and Hepatic FSF and MRS-PDFF Before and After Intervention in Group A and Group B.
| Preintervention | Postintervention | P-value | |
|---|---|---|---|
| Group A | |||
| BMI (mean) | 28.35 ± 3.25 | 27.14 ± 3.24 | P <0.001 |
| Mean MRI FSF | 19.30 ± 9.09 | 11.18 ± 7.61 | P <0.001 |
| Mean MRS PDFF | 18.79 ± 8.53 | 10.64 ± 6.66 | P <0.001 |
| Group B | |||
| BMI (mean) | 28.85 ± 2.41 | 29.31 ± 2.57 | P <0.001 |
| Mean MRI FSF | 18.96 ± 9.79 | 21.48 ± 11.80 | P = 0.097 |
| Mean MRS PDFF | 17.10 ± 9.28 | 19.45 ± 10.48 | P <0.001 |
BMI, body mass index; MRS, magnetic resonance spectroscopy; PDFF, proton density fat fraction; FSF, fat signal fraction.
Comparison of USG with MRS-PDFF
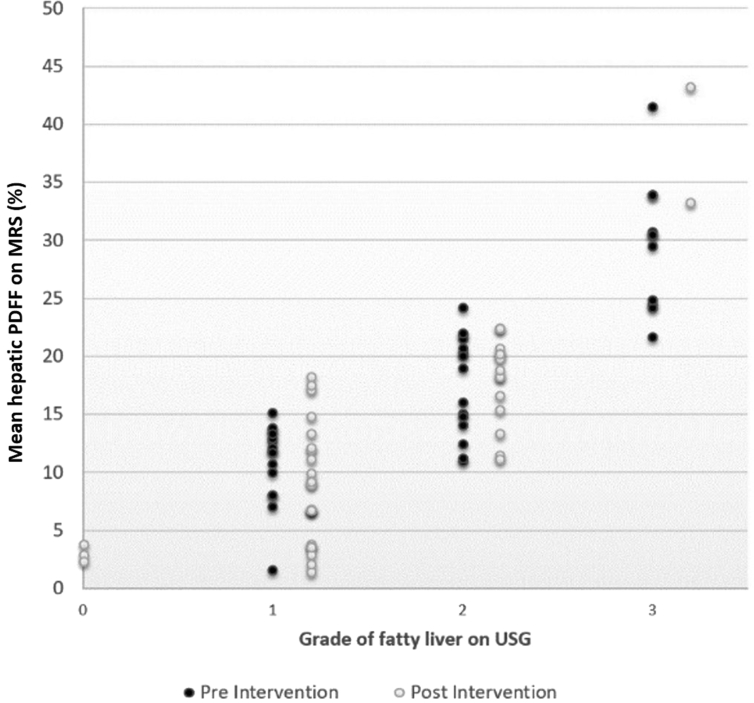
Before intervention: In the cases (Groups A and B combined) with grade 1 fatty liver on USG, the mean PDFF was 10.8% (range 1.5%–15%). Similarly, in patients with grade 2 and grade 3 fatty liver on USG, the mean PDFF were 17.6% (range 11.2%–24.1%) and 29.5% (range 21.6%–41.5%), respectively. There was good correlation between the grade of fatty liver on USG and PDFF (r = 0.87; P < 0.001) (Figure 2).
Figure 2.
Scatter plot comparing grades of fatty liver on USG with hepatic PDFF on MR spectroscopy, before and after intervention. Wide range in liver fat percentage was seen in each grade on USG with overlap between grades. MR, magnetic resonance; USG, ultrasonography; PDFF, proton density fat fraction.
After intervention: In three cases, where there was no fatty liver on USG, the mean PDFF was 3% (range: 2.3%–3.8%). In cases with grade 1, grade 2, and grade 3 fatty liver on USG, the mean PDFF were 8.1% (1.45%–17.5%), 17.7% (11.15%–22.4%), and 38.3% (33.3–43.3%), respectively. There was good correlation between USG and PDFF (r = 0.84; P < 0.001) (Figure 2).
However, there was overlap in MRS-PDFF between different grades of fatty liver on USG before and after intervention (Figure 2).
We further compared the change in the grade of fatty liver on USG with the change in the mean PDFF after intervention. The results are shown in Figure 3 and Table 4. It showed that USG was unable to detect <5.3% change in PDFF. A mean change of 9.45% in the PDFF was needed for the change to be detected by USG.
Figure 3.
Comparison between change (Δ) in USG grade of fatty liver and change (Δ) in hepatic PDFF after 6 months of intervention. USG, ultrasonography; PDFF, proton density fat fraction; MRS, magnetic resonance spectroscopy.
Table 4.
Comparison Between Change (Δ) in USG Grade and MRS-PDFF After 6 Months of Intervention.
| Change in USG grade after intervention | Mean (range) change in MRS-PDFF after intervention | P-value |
|---|---|---|
| Increase by 1 unit (n = 1) | Increase by 11.20% | <0.001 |
| No change (n = 21) | Change by 1.97% (−2.53 to +6.47%) | <0.001 |
| Reduction by 1 unit (n = 17) | Reduction by 9.45% (+5.30 to +13.60%) | <0.001 |
| Reduction by 2 units (n = 2) | Reduction by 13.08% (+10.43 to +15.73%) | <0.001 |
Figure 4 (before intervention) and Figure 5 (after intervention) show an example of a case with good compliance to lifestyle modifications, who showed reduction in hepatic fat content.
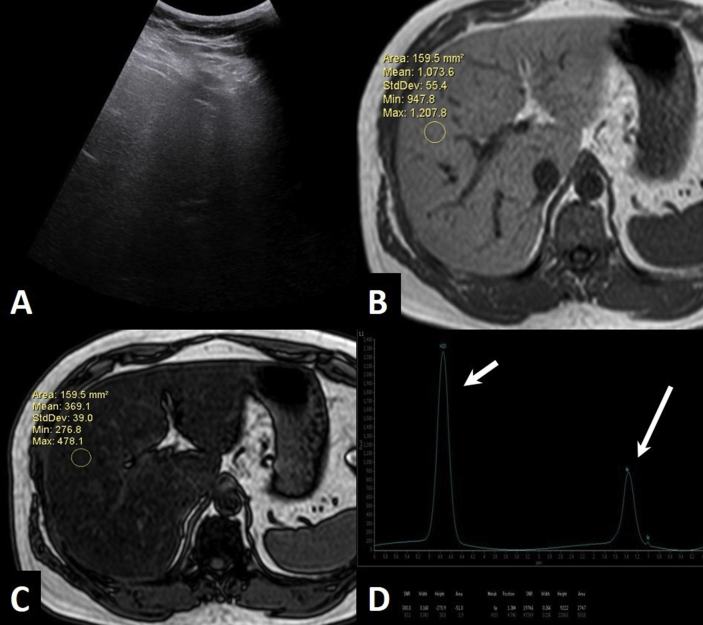
Figure 4.
USG and MRI of a 30-year-old male with weight 86.5 kg and BMI 27.9 kg/m2. (A)—USG shows Grade 3 fatty liver. (B & C) In-phase (B) and opposed-phase (C) T1-weighted images shows a calculated fat signal fraction of 32.81%. (D)—MR spectroscopy shows area under the curve of lipid peak (long arrow) of 274.7 and water peak (short arrow) of 583.8. The calculated proton density fat fraction was 31.9%. BMI, body mass index; MRI, magnetic resonance imaging; USG, ultrasonography; PDFF, proton density fat fraction.
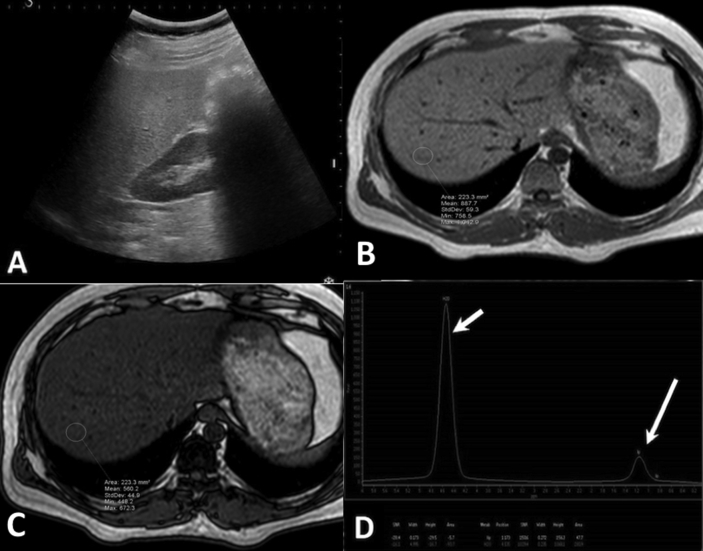
Figure 5.
USG and MRI of the same patient in Figure 4, after 6 months of intervention, with weight—81 kg and BMI 26.14 kg/m2 (good compliance). (A)—USG shows Grade 2 fatty liver. (B & C) In-phase (B) and opposed-phase (C) T1-weighted images shows a calculated fat signal fraction of 18.4%. (D) MR spectroscopy shows reduced area under the curve of lipid peak (long arrow) with a calculated proton density fat fraction of 14.7%. This patient showed significant reduction in liver fat content on both USG and MRI after intervention. BMI, body mass index; MRI, magnetic resonance imaging; USG, ultrasonography.
Comparison of BMI and lipid profile with MRS-PDFF
Mean hepatic PDFF in different BMI categories in each group, before and after intervention, was assessed and is shown in Table 1. The PDFF showed poor correlation with BMI in both groups, before and after intervention (correlation coefficient: 0.1 to 0.4).
However, Figure 6 shows good linear correlation between change in the body weight and in mean PDFF after intervention (r = 0.76; P < 0.001). On regression analysis, we found that a decrease of 1 kg in the body weight is associated with an approximately 3.4% reduction in the hepatic PDFF. Similarly, an increase in the body weight of 1 kg resulted in 2.5% increase in the hepatic PDFF. Conversely, a 5% reduction in the hepatic PDFF was associated with a reduction of 1.8 kg of body weight and an increase in 5% hepatic PDFF was associated with an increase of 0.7 kg in the body weight.
Figure 6.
Scatter plot comparing change (Δ) in mean hepatic PDFF by MRS and change (Δ) in the body-weight in all patients (Group A and B combined). MRS, magnetic resonance spectroscopy; PDFF, proton density fat fraction.
There was poor correlation between hepatic MRS-PDFF and serum cholesterol, serum LDL, HDL, and triglyceride levels in both the groups, both before and after intervention (Table 5).
Table 5.
Correlation Between Hepatic PDFF and Lipid Profile.
| Hepatic PDFF (on MRS) | TC | TG | LDL | HDL | |
|---|---|---|---|---|---|
| Preintervention (n = 41) | r | −0.11 | −0.25 | 0.02 | 0.07 |
| P | 0.51 | 0.12 | 0.88 | 0.66 | |
| Postintervention (n = 41) | r | 0.07 | −0.02 | 0.13 | −0.15 |
| P | 0.64 | 0.90 | 0.41 | 0.36 |
PDFF, proton density fat fraction; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MRS, magnetic resonance spectroscopy; TC, total cholesterol; TG, triglycerides; r, correlation coefficient; p, P-value.
Comparison of MRI-FSF with USG, BMI, and MRS-PDFF
Mean hepatic FSF was also compared with USG, BMI, and MRS-PDFF in all the cases, before as well as after intervention. On correlating it with USG grade, the results were as follows. Before intervention, the mean hepatic FSF in cases with grade 1, grade 2, and grade 3 fatty liver on USG were 11.01% (range 1.6%–14.6%), 19.20% (range 12.3%–33.4%), and 30.40% (range 24.3%–35%), respectively. Similarly, after intervention, the mean hepatic FSF in cases with grade 0, grade 1, grade 2, and grade 3 fatty liver on USG were mean FSF of 1.67% (range: 1.4%–2.1%), 8.6% (1.45%–17.5%), 19.3% (11.15%–22.4%), and 41.8% (33.3–43.3%), respectively. There was good correlation between the grade of fatty liver on USG and the FSF, both before and after intervention (r = 0.83–0.84; P < 0.001).
The hepatic FSF showed poor correlation with BMI in both the groups, before and after intervention (correlation coefficient, r = 0.20 and 0.49, respectively).
We also compared the hepatic FSF measured by CSI and hepatic PDFF by MRS (used as gold standard) and found a very good linear correlation between them, both before and after intervention (P < 0.001, Pearson's correlation coefficient r = 0.965) (Figure 7).
Figure 7.
Scatter plot comparing mean FSF calculated by chemical shift imaging and PDFF by MR spectroscopy, before and after intervention. Excellent linear correlation was seen. MR, magnetic resonance; PDFF, proton density fat fraction; FSF, fat signal fraction.
Discussion
In this study, we found that MRI is effective in assessing the changes in liver fat fraction in cases of NAFLD after intervention. The results were significantly different in subjects who were compliant to intervention than those who were not (P < 0.001). The only correlation which was found was between change in liver fat content and change in body weight. Although USG grade showed correlation with MRS-PDFF, it was not sensitive to minor changes.
Quantification of liver fat by MRS has shown consistent correlation with histological quantification, and many have started considering it as equivalent to liver biopsy.21, 22, 23,29 We also used MRS as gold standard in this study and could reliably assess the change in the liver fat fraction (PDFF) in response to treatment.
As expected, the hepatic PDFF decreased in the group compliant to treatment (by 43.4%) and increased in the noncompliant group (by 13.7%). Noureddin et al., in a longitudinal study, compared change in liver fat (assessed by CSI and MRS) with a change in body weight after 24 weeks of intervention, using liver histology as gold standard. Both techniques showed statistically significant correlation with each other and with histology (P < 0.05).24 Our study also showed good correlation of FSF by CSI with PDFF (r = 0.965).
Koh et al. evaluated the dual-echo and triple-echo MRI in quantifying liver fat during monitoring of pediatric patients with NAFLD before and after one year of treatment. They found a significant decrease in the liver fat fraction in the compliant group (−19.2% on dual echo; −13.4% on triple echo) and a significant increase in the liver fat fraction in the noncompliant group (+4.6% on dual echo; + 3.5% on triple echo).25 They, however, used only dual-echo and triple-echo gradient echo sequences at 3T and not MRS. Middleton et al. also showed that liver fat quantified by multiecho gradient echo sequence (MRI-PDFF) correlated with histological grade before and 72 weeks after intervention in patients with NASH. The MRI-PDFF showed an area under the receiver operating characteristic of 0.81 for histological change in steatosis grade. The authors suggested that MRI-PDFF is accurate as a biomarker in the monitoring such patients.21 Although dual-echo MRI is easier to perform, the results may be inaccurate when there is concomitant deposition of iron in the liver parenchyma.
In a randomized control trial of treatment of cases of biopsy-proven NASH by ezetemibe vs placebo, Lin et al. compared change in hepatic fat fraction assessed by multiecho MRI (MRI-PDFF) with change in histologic steatosis grade, total liver volume (TLV), and total liver fat index (TLFI).30 They showed that >10% reduction in hepatic fat fraction was associated with significant reduction in TLV (correlation coefficient 0.6, P < 0.001). No significant correlation was seen with change in steatosis as well as TLFI. Triple-echo gradient MRI and MRS have been shown to best correlate with histological liver fat content and are better than dual-echo technique.31, 32, 33 This justifies our use of MRS as gold standard.
The main limitation of MRS, however, is that it assesses only a small area of the liver and thus may not be representative.34 Similar limitation exists with biopsy too. Hence, quantification with triple-echo or multiecho gradient sequence may be a better option. A recent study by Cunha et al. used an abbreviated MRI protocol (including multiecho gradient MRI for hepatic PDFF, MR elastography for liver stiffness, and 3D 3-point Dixon technique for visceral fat) for the evaluation and follow-up of 22 patients with NAFLD and showed that it is a feasible and reliable option in screening and monitoring patients with NAFLD.35
USG, although a simple technique for evaluation of fatty liver, is not sensitive to minor changes in liver fat content.36 Even for severe fatty liver (>30%), the sensitivity of USG is in the range of 67–84%.37,38 In this study, although USG had good correlation with MRI, could detect changes in the grade when there was at least a 5.3% change in the hepatic MRS-PDFF. Furthermore, USG grading may be erroneous in patients who are obese, as most are, thus limiting its routine use for accurate monitoring.37 We also feel that, although USG is very useful as a screening modality, it is less useful in monitoring changes in liver fat. The routine use of CT scan to assess hepatic fat is limited by its effects of radiation. Both, single-energy and dual-energy CT scan are comparable with each other and with histological results.39,40 The controlled attenuation parameter obtained during transient elastography (FibroScan) of liver has been used to quantify liver fat.41 Although it is sensitive in detecting fatty liver, there is overlap in the attenuation values between different histological grades and is still under investigation.42
Studies in literature have shown that there is no correlation of liver fat content with BMI.23,43 There was weak correlation between the body weight and BMI and the hepatic FSF in this study. However, we found that change in the body weight and the BMI after therapeutic intervention correlated well with the change in hepatic FSF. The results showed that 1 kg reduction in the body weight corresponded to 3.4% reduction in the hepatic FSF. Thus, body weight may be used as an indirect parameter to predict a change in the liver fat content. Similar results were seen in the study by Patel et al.44 They found that at least 5% reduction in the BMI was associated with significant reduction (relative decrease by 25%) in the liver fat and volume in patients of NASH. However, results of the study by Koh et al. showed no correlation between change in the hepatic fat fraction and the BMI and body weight.25 This could be due to the fact that their study population was pediatric and smaller in number (n = 27). More studies are thus necessary to validate these results.
There were a few limitations in the study. The study number was small. There was no histological confirmation in any of the study subjects. Presence of steatohepatitis (compared with only steatosis) which can alter the results of fat quantification can be confirmed on biopsy or by MR elastography. In a recent study by Costa et al., MR elastography showed significant differences in stiffness values between patients with simple steatosis and definite steatohepatitis.45 However, none of the patients had clinical indication for performing a biopsy, and hence, it was not done. The voxel for MRS was placed only in the right lobe of liver. This may produce inconsistent results as NAFLD often causes inhomogeneous fat deposition. But liver biopsy also has the same limitation. The follow-up duration was short (6 months). Longer follow-up will help in assessing the role of MRI, USG, and BMI better.
In conclusion, MRI, using dual-echo CSI and MRS, is shown to be a useful technique in the monitoring of patients of NAFLD, with dual-echo CSI measured hepatic FSF correlating well with MRS-PDFF. Thus, invasive liver biopsy can be avoided. This technique is better than assessment by USG, lipid profile, and BMI measurement.
Conflicts of interest
The authors have none to declare.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Lee R.G. Nonalcoholic steatohepatitis: tightening the morphological screws on a hepatic rambler. Hepatology. 1995;21:1742–1743. doi: 10.1002/hep.1840210636. [DOI] [PubMed] [Google Scholar]
- 3.Duseja A. Non-alcoholic fatty liver disease: a lot done, yet more required. Indian J Gastroenterol. 2010;29:217–225. doi: 10.1007/s12664-010-0069-1. [DOI] [PubMed] [Google Scholar]
- 4.Ford E.S., Giles W.H., Mokdad A.H. Increasing prevalence of the metabolic syndrome among U.S. adults. Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 5.Starley B.Q., Calcagno C.J., Harrison H.A. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2013;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 6.Amarapurkar D., Dharod M., Gautam S. Risk of development of hepatocellular carcinoma in patients with NASH-related cirrhosis. Trop Gastroenterol. 2013;34:159–163. doi: 10.7869/tg.120. [DOI] [PubMed] [Google Scholar]
- 7.Adani G.L., Baccarani U., Sainz-Barriga M. The role of hepatic biopsy to detect microvacuolar steatosis during liver procurement. Transplant Proc. 2006;38:1404–1406. doi: 10.1016/j.transproceed.2006.02.111. [DOI] [PubMed] [Google Scholar]
- 8.Ratziu V., Charlotte F., Heurtier A. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 9.Bravo A.A., Sheth S.G., Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 10.Margariti E., Deutsch M., Manolakopoulos S., Papatheodoridis G.V. Non-alcoholic fatty liver disease may develop in individuals with normal body mass index. Ann Gastroenterol. 2012;25:45–51. [PMC free article] [PubMed] [Google Scholar]
- 11.Bhusal K.R., Simkhada R., Nepal P. Lipid profile in different grades of ultrasonic non-alcoholic fatty liver disease. JCMS Nepal. 2017;13:258–261. [Google Scholar]
- 12.Sen A., Kumar J., Misra R.P., Uddin M., Shukla P.C. Lipid profile of patients having non-alcoholic fatty liver disease as per ultrasound findings in north Indian population: a retrospective observational study. J Med Allied Sci. 2013;3:59–62. [Google Scholar]
- 13.Mahaling D.U., Basawaraj M.M., Bika A.J. Comparison of lipid profile in different grades of non-alcoholic fatty liver disease diagnosed on ultrasound. Asian Pac J Trop Biomed. 2013;3:907–912. [Google Scholar]
- 14.Adams L.A., Talwalkar J.A. Diagnostic evaluation of non-alcoholic fatty liver disease. J Clin Gastroenterol. 2006;40:S34–S38. doi: 10.1097/01.mcg.0000168642.38945.f1. [DOI] [PubMed] [Google Scholar]
- 15.Foster K.J., Dewbury K.C., Griffith A.H., Wright R. The accuracy of ultrasound in the detection of fatty infiltration of the liver. Br J Radiol. 1980;53:440–442. doi: 10.1259/0007-1285-53-629-440. [DOI] [PubMed] [Google Scholar]
- 16.Debongnie J.C., Pauls C., Fievez M., Wibin E. Prospective evaluation of the diagnostic accuracy of liver ultrasonography. Gut. 1981;22:130–135. doi: 10.1136/gut.22.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reeder S.B., Sirlin C.B. Quantification of liver fat with magnetic resonance imaging. Magn Reson Imag Clin N Am. 2010;18:337–357. doi: 10.1016/j.mric.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caussy C., Reeder S.B., Sirlin C.B., Loomba R. Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoing in NASH trials. Hepatology. 2018;68:763–772. doi: 10.1002/hep.29797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang J.S., Taouli B., Salibi N., Hecht E.M., Chin D.G., Lee V.S. Opposed-phase MRI for fat quantification in fat-water phantoms with 1H MR spectroscopy to resolve ambiguity of fat or water dominance. AJR Am J Roentgenol. 2006;187:W103–W106. doi: 10.2214/AJR.05.0695. [DOI] [PubMed] [Google Scholar]
- 20.Thomsen C., Becker U., Winkler K., Christoffersen P., Jensen M., Henriksen O. Quantification of liver fat using magnetic resonance spectroscopy. Magn Reson Imaging. 1994;12:487–495. doi: 10.1016/0730-725x(94)92543-7. [DOI] [PubMed] [Google Scholar]
- 21.Middleton M.S., Heba E.R., Hooker C.A. Agreement between magnetic resonance imaging proton density fat fraction measurements and pathologist-assigned steatosis grades of liver biopsies from adults with nonalcoholic steatohepatitis. Gastroenterology. 2017;153:753–761. doi: 10.1053/j.gastro.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Idilman I.S., Keskin O., Celik A. A comparison of liver fat content as determined by magnetic resonance imaging-proton density fat fraction and MRS versus liver histology in non-alcoholic fatty liver disease. Acta Radiol. 2016;57:271–278. doi: 10.1177/0284185115580488. [DOI] [PubMed] [Google Scholar]
- 23.Kramer H., Pickhardt P.J., Kliewer M.A. Accuracy of liver fat quantification with advanced CT, MRI and ultrasound techniques: prospective comparison with MR spectroscopy. AJR Am J Roentgenol. 2017;208:92–100. doi: 10.2214/AJR.16.16565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noureddin M., Lam J., Peterson M.R. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930-1940. doi: 10.1002/hep.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koh H., Kim S., Kim M.J., Kim H.G., Shin H.J., Lee M.J. Hepatic fat quantification magnetic resonance for monitoring treatment response in pediatric non-alcoholic steatohepatitis. World J Gastroenterol. 2015;21:9741–9748. doi: 10.3748/wjg.v21.i33.9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borra R.J., Salo S., Dean K. Nonalcoholic fatty liver disease: rapid evaluation of liver fat content with in-phase and out-of-phase MR imaging. Radiology. 2009;250:130–136. doi: 10.1148/radiol.2501071934. [DOI] [PubMed] [Google Scholar]
- 27.Kargulewicz A., Stankowiak-Kulpa H., Grzymisławski M. Dietary recommendations for patients with non-alcoholic fatty liver disease. Prz Gastroenterol. 2014;9:18–23. doi: 10.5114/pg.2014.40845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perumpail B.J., Li A.A., John N. The role of vitamin E in the treatment of NAFLD. Diseases. 2018;6:E86. doi: 10.3390/diseases6040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reeder S.B., Hu H.H., Sirlin C.B. Proton density fat fraction: a standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging. 2012;36:1011–1014. doi: 10.1002/jmri.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin S.C., Heba E., Bettencourt R. Assessment of treatment response in non-alcoholic steatohepatitis using advanced magnetic resonance imaging. Aliment Pharmacol Ther. 2017;45:844–854. doi: 10.1111/apt.13951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qayyum A., Goh J.S., Kakar S., Yeh B.M., Merriman R.B., Coakley F.V. Accuracy of liver fat quantification at MR imaging: comparison of out-of-phase gradient-echo and fat-saturated fast spin-echo techniques—initial experience. Radiology. 2005;237:507–511. doi: 10.1148/radiol.2372040539. [DOI] [PubMed] [Google Scholar]
- 32.Wu C.H., Ho M.C., Jeng Y.M. Quantification of hepatic steatosis: a comparison of the accuracy among multiple magnetic resonance techniques. J Gastroenterol Hepatol. 2014;29:807–813. doi: 10.1111/jgh.12451. [DOI] [PubMed] [Google Scholar]
- 33.Tang A., Tan J., Sun M. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267:422–431. doi: 10.1148/radiol.12120896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwenzer N.F., Springer F., Schraml C., Stefan N., Machann J., Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433–445. doi: 10.1016/j.jhep.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 35.Cunha B.M., Villela-Nogueira C.A., Bergman A. Lobo Lopes FPP. Abbreviated mpMRI protocol for diffuse liver disease: a practical approach for evaluation and follow-up of NAFLD. Abdom Radiol. 2018;43:2340–2350. doi: 10.1007/s00261-018-1504-5. [DOI] [PubMed] [Google Scholar]
- 36.Lall C.G., Aisen A.M., Bansal N., Sandrasegaran K. Nonalcoholic fatty liver disease. AJR Am J Roentgenol. 2008;190:993–1002. doi: 10.2214/AJR.07.2052. [DOI] [PubMed] [Google Scholar]
- 37.Graif M., Yanuka M., Baraz M. Quantitative estimation of attenuation in ultrasound video images: correlation with histology in diffuse liver disease. Investig Radiol. 2000;35:319–324. doi: 10.1097/00004424-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Saadeh S., Younossi Z.M., Remer E.M. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 39.Hur B.Y., Lee J.M., Hyunsik W. Quantification of the fat fraction in the liver using dual-energy computed tomography and multimaterial decomposition. J Comput Assist Tomogr. 2014;38:845–852. doi: 10.1097/RCT.0000000000000142. [DOI] [PubMed] [Google Scholar]
- 40.Hyodo T., Yada N., Hori M. Multimaterial decomposition algorithm for the quantification of liver fat content by using fast-kilovolt-peak switching dual-energy CT: clinical evaluation. Radiology. 2017;283:108–118. doi: 10.1148/radiol.2017160130. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Fan Q., Wang T. Controlled attenuation parameter for assessment of hepatic steatosis grades: a diagnostic meta-analysis. Int J Clin Exp Med. 2015;8:17654–17663. [PMC free article] [PubMed] [Google Scholar]
- 42.Jun B.G., Park W.Y., Park E.J. A prospective comparative assessment of the accuracy of the fibroscan in evaluating liver steatosis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joshi M., Dillman J.R., Singh K. Quantitative MRI of fatty liver disease in a large pediatric cohort: correlation between liver fat fraction, stiffness, volume and patient-specific factors. Abdom Radiol. 2018;43:1168–1179. doi: 10.1007/s00261-017-1289-y. [DOI] [PubMed] [Google Scholar]
- 44.Patel N.S., Doycheva I., Peterson M.R. Effect of weight loss on magnetic resonance imaging estimation of liver fat and volume in patients with non-alcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2015;13:561–568. doi: 10.1016/j.cgh.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costa-Silva L., Ferolla S.M., Lima A.S., Vidigal P.V.T., Ferrari T.C.A. MR elastography is effective for the non-invasive evaluation of fibrosis and necroinflammatory activity in patients with nonalcoholic fatty liver disease. Eur J Radiol. 2018;98:82–89. doi: 10.1016/j.ejrad.2017.11.003. [DOI] [PubMed] [Google Scholar]