Abstract
Background
Medium-term clinical results and survival of the Copeland resurfacing hemiarthroplasty of the shoulder (CRHA) in a large consecutive group are presented with a comparison of outcomes for underlying pathologies.
Methods
A consecutive series of patients undergoing CRHA over 14 years was retrospectively analysed with no exclusions. Patients had a minimum 2-year follow-up by an independent assessor. Functional outcome was assessed using the Oxford Shoulder Score (OSS) and Constant-Murley Score (CMS). Pain and satisfaction was assessed using a visual analogue score.
Results
279 CRHAs were performed in 242 patients between 2002 and 2016. The mean follow-up was 6 years. The indication for surgery was osteoarthritis (OA) in 212, inflammatory arthropathy (RA) in 35, rotator cuff tear arthropathy (CTA) in 22 and avascular necrosis (AVN) in 2. For the OA group, 5-year survival was 90%, 10-year survival was 83% and mean survival was 13.2 years (95% CI 12.5–13.9). The mean OSS was 35.0 and mean CMS 49.9. CRHA for CTA had significantly poorer (p < 0.001) 5-year survival (55%), 10-year survival (41%) and mean survival (5.9 years, 95% CI 4.7–7.2). Mean OSS was 23.6 and mean CMS 30.3, which was poorer than for OA (p < 0.001). A subgroup analysis of OA patients found significantly better survival (p = 0.013) in those aged over 65 years but no difference in functional outcome.
Conclusion
CRHA remains a reasonable option for OA in patients with an intact rotator cuff and with sufficient bone stock, especially in those aged over 65 years. With poorer functional outcomes and survival, CRHA should not be offered in those with CTA.
Level of evidence
Level III (retrospective comparative study)
Keywords: Copeland, Resurfacing, Hemiarthroplasty, Shoulder
1. Introduction
Resurfacing hemiarthroplasty of the shoulder has several benefits including avoiding the need to replace the glenoid and bone preservation of the humeral head and medullary canal. The procedure is also quicker and simpler than other designs of arthroplasty.1
The Copeland resurfacing hemiarthroplasty (CRHA) aims to restore normal anatomy by replacing only the damaged joint bearing surfaces with minimal bony resection involving trimming of peripheral osteophytes and reshaping the humeral head only. Version, the centre of rotation and offset are maintained and no intramedullary reaming is required. First designed in 1979 the CRHA has evolved from a design with a central peg secured by a screw (Mark I) to a modern fluted taper fit prosthesis without a screw (Mark II, Zimmer, Swindon, UK). Hydroxyapatite coating was added in 1993 (Mark III, Biomet Merck, Swindon, UK) in order to promote biological fixation with bony in-growth.2 Copeland originally described indications as any pathology with sufficient humeral bone stock including osteoarthritis (OA), inflammatory arthropathy such as rheumatoid arthritis (RA), cuff tear arthropathy (CTA) and avascular necrosis (AVN).3
Recent studies have reported mixed results of CRHA with 5- year survival estimated as low as 79%4 and as high as 97.5%.5,6 These studies included relatively small numbers of patients and used strict inclusion and exclusion criteria therefore may not be representative of the entire population.
The purpose of this study is to evaluate the medium term outcomes and survival of the CRHA and compare these for the major underlying pathologies in a consecutive series of patients in an independent centre. We provide the largest study group to date. We hypothesise that patients undergoing CRHA for CTA have poorer survival.
2. Methods
2.1. Study design and study population
A retrospective cohort study was performed. Inclusion criteria were all CRHAs implanted in a single hospital trust between January 2002 and July 2016. No patients were excluded.
All patients had completed a minimum 12 months of non-surgical management comprising oral analgesia, intra-articular corticosteroid injection and physiotherapy.
2.2. Procedure
Patients were identified using a prospectively collected electronic database for all patients passing through the shoulder and elbow unit, collecting data on diagnosis and procedures from 2002 onwards. Diagnosis and procedural information entered into the database was crosschecked on a case-by-case basis by a data entry team using patient notes and hospital computer records. The database was searched for all records of shoulder arthroplasty and further information on complications and outcome was extracted from patient case notes. The indication for the procedure was noted on this database from clinic letters. The rotator cuff tendon was assessed radiologically either by an ultrasound scan or by magnetic resonance imaging. Cases where a rotator cuff tear was noted intra-operatively were also included in the CTA group. Patients who had not had recent follow up were individually contacted by an arthroplasty physiotherapist and invited back to clinic for review. Deaths were recorded.
Postoperative assessment scores were performed independently by an upper limb arthroplasty physiotherapist who began reviewing patients in 2013. Patients had routine clinical follow-up at 3 months, 1 year, 2 years, 5 years and 10 years. Patients were also reviewed by the treating surgeon in the outpatient clinic. Before the introduction of the arthroplasty physiotherapist, only OSS was recorded by the patient whilst in the clinic waiting room. The most recent available outcome scores were used for this study.
2.3. Outcome measures
All patients were routinely assessed with postoperative clinical and functional outcomes measured by an arthroplasty physiotherapist using the Constant-Murley Score (CMS)7 and Oxford Shoulder Score (OSS).8,9 The CMS is a validated 100 point scoring system that is patient-reported and clinician-assessed and covers pain (15 points), function (20 points), range of motion (40 points) and strength (25 points) with a higher score indicating a better outcome. The OSS is a validated 12-question patient-reported scoring system evaluating pain (16 points) and function (32 points). Scores range from 0 to 48, with 48 being the best outcome. A visual analogue score (VAS) was recorded on which patients were asked how better they felt (improved) on a scale of 1–100 and how much pain they currently had. They were also asked a simple yes/no questionnaire as to whether they would have the procedure again or recommend it to a friend or relative. Revision surgery was considered the end point for survival analysis.
2.4. Surgical procedure
The Copeland Mark III (Biomet Merck, Swindon, UK) hydroxyapatite coated prosthesis was used for all cases. All operations were performed by consultant surgeons or senior trainees under direct consultant supervision. An inter-scalene block was performed in the majority of cases to provide postoperative analgesia. Patients were placed in the beach chair position close to the edge of the operating table, allowing the shoulder to be extended and adducted to allow delivery of the humeral head through the incision. The deltopectoral approach was used for all cases with exposure of the proximal humerus sufficient to allow complete visualisation of the articular surface and to remove all osteophytes. The approach included a subscapularis tenotomy 1 cm from its insertion and a biceps tenodesis below its groove. The rotator cuff tendon integrity and quality was noted at the time of surgery. The conjoint tendon and pectoralis major tendons were not released. The glenoid was inspected but not drilled, as suggested by Copeland.10 Humeral preparation consisted of circumerfential removal of osteophytes and insertion of a drill guide with its free edge parallel to the anatomic neck to achieve central wire placement. The humeral surface reamer was used over the central guide wire to remove articular cartilage. A spade-cutting drill was used to prepare the central peg hole and a trial component was inserted in order to assess stability and range of motion. The component size was carefully assessed in order to avoid oversizing and overstuffing of the joint. Cancellous bone was removed from the reamer and placed on the inner surface of the prosthesis before implantation. The subscapularis was repaired with No. 2 braided non-absorbable suture. The patient was placed into a broad arm sling following wound closure and dressing application. Patients were discharged after physiotherapy assessment the following day. A standardised physiotherapy protocol was used for rehabilitation starting on day 1 with passive assisted mobilisation, progressing to active-assisted mobilisation at 2 weeks and concentric strengthening at 6 weeks. The sling was worn for 6 weeks, after which the patients were allowed full, unrestricted range of motion exercises.
In line with our theory that CRHA for CTA had poorer survival, the use of the implant tailed off since 2012 and its use ceased for this indication in 2014.
No institutional review board or ethical approval was required for this study as data was prospectively collected as part of normal practice and service evaluation in our department with retrospective analysis performed.
2.5. Statistical analysis
Descriptive statistical analysis was performed using Statistical Package for the Social Sciences (SPSS Statistics for Mac, version 23.0; SPSS, Inc., Chicago, IL, USA).11 Survival analysis followed by using the Chi-squared test to compare the survival curves for different groups using GraphPad Prism version 7.0 for Mac (GraphPad Software, La Jolla California USA).12 One-way ANOVA testing with post hoc Bonferroni correction was performed to compare the range of OSS, CMS, pain VAS, improvement VAS and range of movement for OA, RA and CTA. Fisher's exact test was used to compare survey outcomes for the yes/no questions (if the patients would have the surgery again and if they would recommend it). A subgroup analysis of patients aged 65 years or below versus over 65 was performed. The OSS and CMS were compared using an unpaired t-test, the survival curves were compared with the Chi-squared test. p < 0.05 was considered statistically significant.
3. Results
279 Consecutive CRHAs were implanted by 5 consultant surgeons from January 2002 to July 2016 in 242 patients. Thirty-seven patients had bilateral implants. 73 were male and 206 were female. The median age of patients at the time of surgery was 71 (Table 1). The indications for operation were primarily pain and loss of function. The underlying diagnosis included osteoarthritis (OA) in 228 shoulders, rheumatoid arthritis or other inflammatory arthropathy (RA) in 42 shoulders, rotator cuff tear arthropathy (CTA) in 22 shoulders and avascular necrosis (AVN) in 2 shoulders. The integrity of the rotator cuff was assessed clinically, radiologically and intra-operatively. All patients with a full thickness tear of the rotator cuff tendon were included in the CTA group regardless of migration of the humeral head.
Table 1.
Demographics. OA = osteoarthritis RA = inflammatory arthropathy (mainly rheumatoid arthritis), CTA = rotator cuff tear arthropathy, AVN = avascular necrosis.
| Group | Cases | Gender |
Median age | IQR | Range | Mean Years Follow-up (SD) | Range | |
|---|---|---|---|---|---|---|---|---|
| M | F | |||||||
| OA | 212 | 55 | 157 | 71.0 | 64.0–78.0 | 38–90 | 6.3 (3.2) | 2–15 |
| RA | 35 | 10 | 28 | 65.5 | 56.0–71.0 | 36–84 | 6.8 (3.6) | 2–15 |
| CTA | 22 | 7 | 20 | 74.0 | 66.0–78.3 | 56–84 | 4.4 (2.5) | 2–9 |
| AVN | 2 | 1 | 1 | 56.0 | 38.0–56.0 | 38–74 | 2.6 (0.5) | 2–3 |
| Overall | 279 | 67 | 194 | 71.0 | 64.0–77.0 | 36–90 | 6.1 (3.2) | 2–15 |
13 Cases (5%) did not achieve a minimum 2-year follow-up and therefore were excluded. 6 of these cases died and 7 were lost to follow up.
A minimum 2-year postoperative follow-up was achieved in the remaining 266 consecutive cases. Mean follow up was 6.1 years (Table 1). Clinical and radiographical follow up was performed by an independent arthroplasty physiotherapist with completed OSS and CMS in 174 cases (65%). 25 Cases (9%) were followed up only by a doctor as part of routine clinical and plain radiographical follow up and completed OSS but not CMS. 54 Cases (20%) were followed up only by a doctor as part of routine clinical and radiographical follow up and did not complete outcome scores.
Postoperative functional scores were completed in 174 patients (67%). The mean postoperative OSS and CMS (with standard deviations) are shown in Table 2. Since there were only two cases of AVN and only one had available outcome scores, means could not be calculated for this group. One way ANOVA test with post hoc Bonferroni correction revealed a significant difference in the total OSS and the pain and function components of the OSS between the groups (p < 0.0001).
Table 2.
Functional outcome scores (Means with standard deviations). p values for one way ANOVA comparison between groups with post hoc Bonferroni correction, values < 0.05 marked with *. OA = osteoarthritis RA = inflammatory arthropathy (mainly rheumatoid arthritis), CTA = rotator cuff tear arthropathy, AVN = avascular necrosis.
| Oxford Shoulder Score |
Constant Murley Score |
|||||||
|---|---|---|---|---|---|---|---|---|
| Pain | Function | Total | Pain | ADL | ROM | Strength | Total | |
| OA | 10.9 (4.3) | 23.7 (8.0) | 35.0 (11.4) | 9.0 (4.8) | 16.2 (3.9) | 21.4 (7.6) | 4.1 (5.9) | 49.9 (18.0) |
| RA | 10.6 (3.7) | 18.8 (7.8) | 29.4 (9.6) | 9.4 (4.6) | 13.7 (4.0) | 13.9 (7.5) | 1.0 (2.8) | 37.2 (16.3) |
| CTA | 7.1 (4.1) | 16.6 (8.5) | 23.6 (11.6) | 4.6 (4.2) | 11.4 (4.7) | 13.1 (5.5) | 1.4 (3.8) | 30.3 (15.1) |
| AVN | NA | NA | NA | NA | NA | NA | NA | NA |
| Significance | 0.092* | 0.001* | 0.002* | 0.108 | 0.001* | 0.001* | 0.010* | 0.001* |
| Overall | 10.5 (4.3) | 22.2 (8.3) | 33.1 (11.5) | 8.7 (4.8) | 15.3 (4.3) | 19.4 (8.3) | 3.3 (5.5) | 46.1 (18.9) |
Range of movement was compared using one way ANOVA with post hoc Bonferroni correction and revealed significant differences between all groups for active flexion (p < 0.001), active abduction (p < 0.001) but no difference in active external rotation (p = 0.77) (Table 3).
Table 3.
Range of Movement (means with standard deviations, median for internal rotation). p values for one way ANOVA comparison between groups with post hoc Bonferroni correction, values < 0.05 marked with *. OA = osteoarthritis RA = inflammatory arthropathy (mainly rheumatoid arthritis), CTA = rotator cuff tear arthropathy, AVN = avascular necrosis.
| Flexion | Abduction | External Rotation | Internal Rotation | |
|---|---|---|---|---|
| OA | 98.1 (26.2) | 84.4 (28.7) | 39.9 (17.1) | L3 |
| RA | 81.2 (28.8) | 67.2 (22.1) | 38.2 (19.6) | Sacrum |
| CTA | 71.3 (31.3) | 63.8 (23.1) | 36.3 (19.6) | Sacrum |
| AVN | NA | NA | NA | NA |
| Significance | 0.002* | 0.01* | 0.91 | 0.02* |
| Overall | 95.0 (28.5) | 80.5 (28.5) | 39.7 (17.4) | L3 |
Survey outcomes showed no difference between groups for pain VAS (p = 0.28) (Table 4). There was a tendency towards better improvement VAS for the OA group but this was not significant (p = 0.056). There was a significant difference in patients that would have the surgery again, with the CTA group scoring poorer at 75% versus 91% for OA and 100% for RA. There was no significant different in whether the patients would recommend the surgery (p = 0.058)
Table 4.
Survey outcomes (means with standard deviations). VAS = visual analogue score (scale 0–100). p values for one way ANOVA comparison between groups with post hoc Bonferroni correction for VAS and Fisher's Exact test for Have again and recommend. OA = osteoarthritis RA = inflammatory arthropathy (mainly rheumatoid arthritis), CTA = rotator cuff tear arthropathy, AVN = avascular necrosis.
| Pain VAS | Improvement VAS | Have again | Recommend | |
|---|---|---|---|---|
| OA | 27.9 (24.9) | 71.4 (30.3) | 99/107 (93%) | 93/105 (89%) |
| RA | 33.5 (28.9) | 69.1 (26.1) | 22/22 (100%) | 20/22 (91%) |
| CTA | 39.3 (30.7) | 47.5 (41.1) | 6/8 (75%) | 4/7 (57%) |
| AVN | NA | NA | 1/1 (100%) | 1/1 (100%) |
| Significance | 0.410 | 0.101 | 0.119 | 0.066 |
| Overall | 32.2 (26.6) | 69.1 (30.6) | 128/138 (93%) | 118/135 (87%) |
45 patients underwent revision surgery (Table 5). The indications were pain and stiffness secondary to glenoid erosion in 44 cases and aseptic loosening in one case. There were no revisions for infection or fracture. The revision implant was a reverse total shoulder replacement in 27 cases, stemmed hemiarthroplasty in 6 cases, anatomical total shoulder replacement in 12 cases.
Table 5.
Summary of revision cases with mean survival analysis. OA = osteoarthritis RA = inflammatory arthropathy (mainly rheumatoid arthritis), CTA = rotator cuff tear arthropathy, AVN = avascular necrosis. Chi-squared used for significance.
| Cases | Revised | Mean Years Survival (95%CI) | 5 Year Survival (Std. Error) | 10 Year Survival (Std. Error) | Significance | |
|---|---|---|---|---|---|---|
| OA | 212 | 26 | 13.2 (12.5–13.9) | 90% (2%) | 83% (4%) | |
| RA | 38 | 6 | 12.7 (11.0–14.3) | 93% (4%) | 70% (12%) | |
| CTA | 27 | 12 | 5.9 (4.7–7.2) | 55% (11%) | 41% (12%) | |
| AVN | 2 | 1 | 2.6 (2.1–3.2) | NA | NA | |
| Overall | 279 | 45 | 12.6 (11.9–13.2) | 86% (2%) | 78% (4%) | p < 0.0001 |
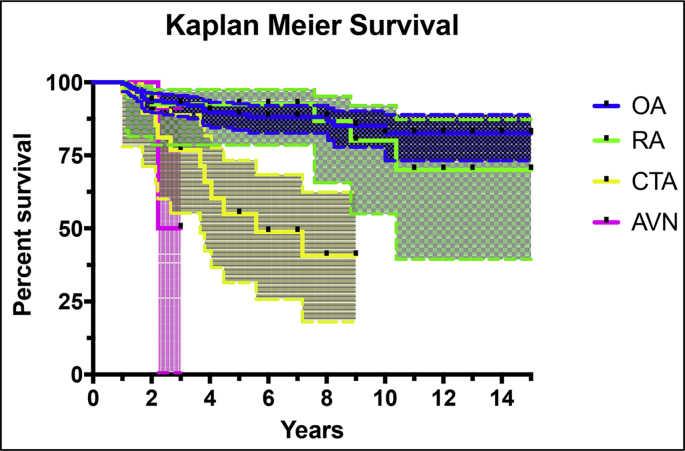
Kaplan Meier survival analysis is shown in Fig. 1 and Table 5. Chi-squared test showed a significant difference in the curves for OA, RA and CTA (p < 0.001). Since there were only two cases of AVN, a statistical analysis of the survival curve was not performed.
Fig. 1.
Kaplan Meier Survival analysis (with 95% confidence intervals). OA = osteoarthritis RA = inflammatory arthropathy (mainly rheumatoid arthritis), CTA = rotator cuff tear arthropathy, AVN = avascular necrosis.
A subgroup analysis comparing patients with OA aged 65 years or less with those aged more than 65 years revealed a significantly lower mean survival (10.9 years versus 14 years, p = 0.013) in the younger patients (Table 6). There were no differences in total OSS (33.8 vs 35.8, p = 0.302) or CMS (50.6 vs 50.2, p = 0.906).
Table 6.
Subgroup analysis of outcomes in patients older and younger than 65 with osteoarthritis.
| Age <65 | Age >65 | Test | Significance | |
|---|---|---|---|---|
| Cases | 53 | 99 | ||
| OSS | 33.8 (13.3) | 35.8 (10.0) | unpaired t-test | p = 0.302 |
| CMS | 50.6 (21.8) | 50.2 (16.2) | unpaired t-test | p = 0.906 |
| Mean Survival | 10.9 (9.8–12.0) | 14.0 (13.3–14.6) | Chi-squared | p = 0.013 |
| 5y Survival (Std. Error) | 95.4% (1.5%) | 100% (0%) | ||
| 10y Survival (Std. Error) | 90.6% (3.7%) | 99.3% (0.7%) |
There was one deep infection in a 62-year-old female patient with RA 2 years post surgery. She was being treated with immunosuppressant medication due to a previous renal transplant. She was treated successfully treated with implant retention and an arthroscopic washout followed by 6 weeks of intravenous antibiotics. Microbiological culture of tissue samples at washout grew a penicillin sensitive Staphylococcus aureus. No subsequent evidence of infection or indication for revision surgery was evident at the termination of this study 1 year later. There were two cases of postoperative stiffness, one required arthroscopic capsular release (14.5 months post CRHA for OA), one case required open release (61.5 months post CRHA for RA). There were two periprosthetic fractures and both were successfully managed non-operatively. There were no neurovascular injuries and no dislocations. There were no cases of lysis on radiographs.
4. Discussion
The purpose of this study was to evaluate the medium term outcomes and survival of CRHA and compare the major underlying pathologies. We present mean 6-year follow up data from 279 CRHAs. 5-year survival for those with OA was 90% and 10-year survival was 83% with good functional outcomes assessed by OSS (35.0) and CMS (49.9). 5-year survival for CTA was poor at only 55% and 41% at 10 years with significantly lower (p = 0.001) OSS (23.6) and CMS (30.3). Range of movement was also significantly better in the OA group; however, VAS for improvement and pain were no different. Patients with OA were more likely to have the surgery again (93%) than with CTA (75%).
There remains controversy as to whether resurfacing hemiarthroplasty or traditional hemiarthroplasty (HA) are optimum treatments for the younger patient. HA requires more bony resection even in stemless variants and also risks failure in a similar mode to resurfacing hemiarthroplasty due to glenoid erosion.13 In 2018 the National Joint Registry the United Kingdom (NJR)14 reported a decline in the proportion of resurfacings (both total and partial) with resurfacing hemiarthroplasty accounting for only 3.3% of implants in 2017 as compared to 6.5% for stemmed HA and 2.5% for stemless HA. The indication in 76.1% was OA with 6.5% for CTA and the remainder for trauma, inflammatory arthropathy and AVN. The NJR has published 5-year survival rates for shoulder arthroplasty and shows a revision rate of 7.9% (6.6–9.6) for resurfacing as compared with 8.6% (5.9–12.6) for stemless HA and 5.9% (4.6–7.7) for stemmed HA. Data from the Norwegian Arthroplasty Register in a recent study found 5-year survival of 94% for HA and 96% for resurfacing HA.15 Authors from a designer centre have reported in patients aged under 50 years receiving CRHA for OA having a 5-year and 11-year survival of 97%.16 This study has demonstrated survival at 5 years of 90% and at 10 years of 83% for those implanted for OA of all ages. For patients aged over 65 years with CRHA implanted for OA, survival rates were improved with 100% 5-year survival and 99% 10-year survival with no significant differences in functional outcomes between this age group and the younger age group. Different populations as well as patient selection specifically with reference to age may therefore explain the differing survival rates from other sources to some extent.
Functional outcome as measured by the OSS was overall marginally above the validated threshold for an acceptable symptom state of 3417; however, for indications other than OA the outcomes were below this threshold. In particular outcomes from the CTA group were significantly poorer than the OA group. Outcome scores for OA were lower than those from another study of CRHA for OA in 2012 which found postoperative OSS of 42 (versus 35 in this study) and Constant score of 62.5 (versus 49.9 in this study), although this was a small single surgeon series.18 Functional outcomes for HA have not been proven superior to resurfacing with a recent study publishing a 5-year mean OSS of 32.719 although the Oxford group found the minimum 3-year (53.5 months mean follow-up) OSS of 38.8 for HA in OA with an intact rotator cuff.20 A Danish randomized trial found similar CMS in OA patients treated with resurfacing hemiarthroplasty (48.9) to this study (49.9) although this was not using the CRHA prosthesis.21
Reverse shoulder arthroplasty (RSA) has now emerged as the preferred choice of arthoplasty when surgically treating cuff arthropathy. Prior to the popularity of RSA systems CHRA was still considered as an option in cuff related arthropathy with the resurfacing implant positioned in a valgus orientation to provide a smooth articulation against the gleno-acromial arc.22 The ‘extended articular arc’ resurfacing system evolved to address the cuff deficient compensated shoulder.23 Our study highlights this issue as seen in the worse functional outcomes and survival in the cuff deficient group. While we used the CHRA for this indication initially, the use tailed off from 2012 and the implant was no longer used from 2014.
This study has a number of strengths including that postoperative outcomes were prospectively collected and assessed independently by an independent arthroplasty physiotherapist rather than the operating surgeon, avoiding measurement bias. Patients were also assessed in person rather than being sent a questionnaire by mail. Loss to follow up was minimal (7 cases) since patients without recent follow up data were individually contacted by the arthroplasty physiotherapist.
Limitations of this study include that this was a retrospective review with no true control group and no randomization, making it prone to confounding factors and selection bias. Although an independent arthroplasty physiotherapist assessed most patients, some were seen by the treating surgeon and this may introduce bias. The surgeon did not influence the completion of the OSS, however, which was recorded by the patient without assistance. Preoperative functional scores were not available for a significant proportion of patients therefore were not included as part of this study. Postoperative functional scores were only available for 69% of patients. This was because those patients without scores had been performed in our unit before the introduction of the arthroplasty physiotherapist and therefore scores had not been collected. Although patients had plain film radiographs performed at follow up these were not qualitatively analysed and not presented in this study. Because of this, the severity of the glenoid arthrosis and glenoid type was not recorded which limits the generalizability of the data.
The indication for CRHA has diminished in the recent years with the increase in utilisation of reverse total and anatomical total shoulder arthroplasty. Our study shows that CRHA remains a reasonable option for OA in patients with an intact rotator cuff and with sufficient bone stock, especially in those aged over 65 years. With poorer functional outcomes and survival, CRHA should not be offered in those with rotator cuff tears.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcot.2019.05.014.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Levy O., Copeland S.A. Cementless surface replacement arthroplasty (Copeland CSRA) for osteoarthritis of the shoulder. J Shoulder Elb Surg. 2004;13:266–271. doi: 10.1016/j.jse.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Levy O., Copeland S.A. Cementless surface replacement arthroplasty of the shoulder. 5- to 10-year results with the Copeland mark-2 prosthesis. J Bone Jt. Surg. 2001;83:213–221. doi: 10.1302/0301-620x.83b2.11238. British volume. [DOI] [PubMed] [Google Scholar]
- 3.Copeland S.A., Levy O., Brownlow J.C. Resurfacing arthroplasty of the shoulder. Tech Shoulder Elbow Surg. 2003;4:199–210. [Google Scholar]
- 4.Hwang N., Modi C.S., Drew S.J., Turner S.M. Mid-term results of Copeland shoulder cementless surface replacement arthroplasty from an independent centre. Shoulder Elbow. 2014;6:75–80. doi: 10.1177/1758573213517227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rai P., Davies O., Wand J., Bigsby E. Long-term follow-up of the Copeland mark III shoulder resurfacing hemi-arthroplasty. J Orthop. 2016;13:52–56. doi: 10.1016/j.jor.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verstraelen F.U., Horta L.A., Schotanus M.G.M., Kort N.P., Samijo S.K., Jansen E.J.P. Clinical and radiological results 7 years after Copeland shoulder resurfacing arthroplasty in patients with primary glenohumeral osteoarthritis: an independent multicentre retrospective study. Eur J Orthop Surg Traumatol. 2018;28:15–22. doi: 10.1007/s00590-017-2023-8. [DOI] [PubMed] [Google Scholar]
- 7.Constant C.R., Murley A.H. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987:160–164. [PubMed] [Google Scholar]
- 8.Dawson J., Fitzpatrick R., Carr A. The assessment of shoulder instability. The Development and Validation of a Questionnaire. The Journal of bone and joint surgery. British volume. 1999 May;81-B:420–426. doi: 10.1302/0301-620x.81b3.9044. [DOI] [PubMed] [Google Scholar]
- 9.Dawson J., Rogers K., Fitzpatrick R., Carr A. The Oxford shoulder score revisited. Arch Orthop Trauma Surg. 2009;129:119–123. doi: 10.1007/s00402-007-0549-7. [DOI] [PubMed] [Google Scholar]
- 10.Copeland S., Funk L., Levy O. Surface-replacement arthroplasty of the shoulder. Curr Orthop. 2002;16:21–31. (iii) [Google Scholar]
- 11.IBM Corp . ed. IBM Corp; Armonk, N.Y., USA: 2015. IBM SPSS Statistics for Mac. Version 23.0. Released. [Google Scholar]
- 12.GraphPad Prism. Version 7.0 for Mac ed: La Jolla California USA..
- 13.Widnall J.C., Dheerendra S.K., Macfarlane R.J., Waseem M. The use of shoulder hemiarthroplasty and humeral head resurfacing: a review of current concepts. Open Orthop J. 2013;7:334–337. doi: 10.2174/1874325001307010334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Joint Registry 15th Annual Report: National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 2018. [Google Scholar]
- 15.Fevang B.T., Nystad T.W., Skredderstuen A., Furnes O.N., Havelin L.I. Improved survival for anatomic total shoulder prostheses. Acta Orthop. 2015;86:63–70. doi: 10.3109/17453674.2014.984113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy O., Tsvieli O., Merchant J. Surface replacement arthroplasty for glenohumeral arthropathy in patients aged younger than fifty years: results after a minimum ten-year follow-up. J Shoulder Elb Surg. 2015;24:1049–1060. doi: 10.1016/j.jse.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 17.Christie A., Dagfinrud H., M Garratt A., Ringen Osnes H., Hagen K. 2011. Identification of Shoulder-specific Patient Acceptable Symptom State in Patients with Rheumatic Diseases Undergoing Shoulder Surgery. [DOI] [PubMed] [Google Scholar]
- 18.Al-Hadithy N., Furness N., Patel R. Cementless surface replacement hemiarthroplasty for primary glenohumeral osteoarthritis: results of over 5-year follow-up in patients with or without rotator cuff deficiency. Shoulder Elbow. 2015;7:237–243. doi: 10.1177/1758573215573456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Merwe M., Boyle M.J., Frampton C.M.A., Ball C.M. Reverse shoulder arthroplasty compared with hemiarthroplasty in the treatment of acute proximal humeral fractures. J Shoulder Elb Surg. 2017;26:1539–1545. doi: 10.1016/j.jse.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Rees J.L., Dawson J., Hand G.C. The use of patient-reported outcome measures and patient satisfaction ratings to assess outcome in hemiarthroplasty of the shoulder. J Bone Jt. Surg. 2010;92:1107–1111. doi: 10.1302/0301-620X.92B8.22860. British volume. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen J.V., Olsen B.S., Sorensen A.K., Hrobjartsson A., Brorson S. Resurfacing hemiarthroplasty compared to stemmed hemiarthroplasty for glenohumeral osteoarthritis: a randomised clinical trial. Int Orthop. 2015;39:263–269. doi: 10.1007/s00264-014-2505-9. [DOI] [PubMed] [Google Scholar]
- 22.Pape G., Bruckner T., Loew M., Zeifang F. Treatment of severe cuff tear arthropathy with the humeral head resurfacing arthroplasty: two-year minimum follow-up. J Shoulder Elb Surg. 2013;22:e1–e7. doi: 10.1016/j.jse.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Biomet Manufacturing Corp W, IN (US) Extended Articular Surface Resurfacing Head. United States Patent2003..
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.