Abstract
Background & aims
Although serological markers of disease severity improve after hepatitis C virus (HCV) treatment, it is unclear if all patients experience sustained improvement. We aim to evaluate longitudinal changes in aspartate (AST), alanine (ALT) aminotransferase, platelet count (PLT), and fibrosis-4 (FIB-4) after HCV treatment.
Methods
All adult chronic HCV patients who received antiviral therapy from January 2011 to February 2017 at four large urban hospital systems were evaluated to assess changes in AST, ALT, PLT, and FIB-4 from pre-treatment to post-treatment annually up to 4 years after HCV therapy. Comparisons used Student's t-test and analysis of variance, and were stratified by sex, race, ethnicity, age, body mass index (BMI), and diabetes mellitus.
Results
Among 2691 patients (62.2% men, 76.9% aged 45–65 years, 56.5% white), all markers of disease severity demonstrated sustained improvements from pre-treatment to 4 years post-treatment (AST 53 U/L to 27.5 U/L, ALT 53 U/L to 29 U/L, PLT 168 × 103 to 176 × 103, FIB-4 2.51 to 1.68). However, Hispanics and patients with BMI >30 kg/m2 experienced rebound increases in AST, ALT, and FIB-4 at 4 years post-treatment after experiencing initial improvements in these serological markers in the first-year post-treatment. Sustained improvements in PLT were observed in all groups, including Hispanics and patients with BMI >30 kg/m2.
Conclusion
HCV treatment in a large community-based cohort demonstrated sustained improvements in AST, ALT, PLT, and FIB-4. Rebound increases in AST, ALT, and FIB-4 observed in Hispanics and those with BMI >30 kg/m2 may reflect persisting nonalcoholic fatty liver disease.
Keywords: HCV, hispanic, diabetes, fibrosis, obesity
Abbreviations: BMI, body mass index; EHR, electronic health records; FIB-4, fibrosis-4; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; VA, Veterans Administration
The current era of antiviral therapies for the treatment of chronic hepatitis C virus (HCV) infection offers safer, shorter, and more effective options for HCV.1 Existing studies have demonstrated the beneficial impact of HCV treatment, such that achieving cure translates into reduced risk of hepatocellular carcinoma (HCC), improved patient-reported outcomes, and improved overall mortality.2, 3, 4 Improvements in histologic fibrosis after HCV treatment can be monitored with longitudinal changes in noninvasive markers, which correlate with overall mortality as well.5, 6, 7, 8, 9
Existing studies have also demonstrated different rates of fibrosis progression among untreated chronic HCV patients.10,11 For example, among chronic HCV patients in the Veterans Administration (VA) HCV Clinical Case Registry, Hispanic HCV patients had a 28% higher risk of developing cirrhosis, whereas African American HCV patients had a 42% lower risk of developing cirrhosis.10 Few studies have specifically evaluated trends and disparities in improvement of serological markers of disease severity after chronic HCV treatment. Identifying groups with suboptimal improvements or rebound increases in aspartate (AST) or alanine (ALT) aminotransferase or platelet count (PLT) and markers of fibrosis after HCV treatment will help clinicians focus on these subpopulations to more aggressively target modifiable factors to further prevent disease progression (e.g. weight loss to reduce concurrent nonalcoholic fatty liver disease [NAFLD], alcohol abstinence programs). We aim to evaluate differences in the AST, ALT, PLT, and fibrosis-4 (FIB-4) index12 after HCV treatment among a large multicentered cohort of community-based health systems.
Methods
Study Population
All adult chronic HCV patients who received HCV antiviral therapies from January 1, 2011 to February 28, 2017 at four large urban hospital systems in California, Louisiana, Texas, and Virginia were included. Chronic HCV patients were identified via electronic health record (EHR) query using ICD-9 and ICD-10 codes (070.70, 070.71, 070.41, 070.44, 070.54, 070.6, V02.62, B19.2, or B18.2) as well as manual review of the medical records. Additional confirmation of chronic HCV status was performed via assessment of HCV RNA. Sociodemographics, comorbidities, insurance status, severity of liver disease, and other patient characteristics were collected at the time of study entry. Patients were followed up for a minimum of 3 months or until the end of the study period (February 28, 2017).
Data Sources
Alameda collected data through manual extraction of patient records using a detailed study protocol. At this site, HCV patients who had received antiviral therapy were identified using an existing prospective longitudinal data registry and laboratory data on all HCV antibody positive patients with positive HCV RNA. A coordinator extracted data manually from the EHRs using a standard data extraction dictionary used by all sites. Data validation by a second reviewer was performed by chart review of randomly selected 10% of charts. Other organizations completed the study protocol using a query of EHR for the variables and outcomes of interest.
Variables of Interest
In addition to patient demographics (e.g. age at diagnosis, sex, race/ethnicity) and disease-specific characteristics (e.g. presence of cirrhosis-related complications such as HCC, HCV genotype, HCV RNA), we specifically focused on AST, ALT, PLT, and FIB-4 scores. Comorbidities and liver-related complications noted above were identified using ICD-9 or ICD-10 diagnosis codes. Missing data were minimized to the extent possible by manual record abstraction if the EHR was incomplete.
Statistical Approach
To assess changes in AST, ALT, PLT, and FIB-4 score after HCV treatment, we used laboratory values one year before index date of HCV treatment (pre-treatment) and monitored post-treatment laboratory values annually up to 4 years post-treatment using the first available set of values each year. AST, ALT, PLT, and FIB-4 scores were presented as median and interquartile ranges and were stratified by sex (male, female), race (white, black, other), ethnicity (Hispanic, non-Hispanic), age (<45 years, 45–65 years, > 65 years), body mass index (BMI), and diabetes mellitus status (BMI < 30 kg/m2 without diabetes, <30 kg/m2 with diabetes, >30 kg/m2 without diabetes, and >30 kg/m2 with diabetes), and FIB-4 categories (FIB-4 < 1.45, 1.45–3.25, and >3.25). Statistical tests for the comparison of AST, ALT, PLT, and FIB-4 score changes between groups used Student's t-test for comparison between two groups and analysis of variance when comparing among more than two groups. Statistical analyses were performed using SAS 9.4 with a two-tailed P-value <0.05 indicating statistical significance. This study was approved by the institutional review boards of each of the participating sites.
Results
Patient Characteristics
Among 2691 patients who received HCV therapy during the study period, most patients were men (62.2%, n = 1674), aged 45–65 years (76.9%, n = 2070), non-Hispanic ethnicity (93.2%, n = 2508), and white race (56.5%, n = 1519) (Table 1). Most patients were HCV genotype 1 (68.0%) and few patients had liver-related complications at the time of treatment initiation (9.8% with concurrent HCC, 11.0% with hepatitis B virus co-infection, 7.5% with concurrent NAFLD, 12.6% with ascites, 10.6% with hepatic encephalopathy, 1.8% with history of variceal bleeding, and 13.5% had undergone liver transplantation). When stratified by FIB-4 categories, 22.7% had FIB-4 <1.45 before HCV treatment, 39.8% had FIB-4 1.45–3.25, and 37.5% had FIB-4 >3.25. Overall, 29.2% of patients had concurrent diabetes mellitus and 27.2% had obesity (defined as BMI > 27.5 kg/m2 for Asians; and BMI > 30 kg/m2 for all other race/ethnic groups; Table 1). Overall, 18.5% of patients were treated with nondirect acting antivirals (DAA) and 81.5% were treated with DAAs. Overall sustained virologic response (SVR) was 80.1%, which was significantly higher in patients treated with DAAs vs. non-DAA therapies (85.1% vs. 59.4%, P < 0.001).
Table 1.
Characteristics of the Study Cohort.
| N | % | |
|---|---|---|
| Total | 2691 | 100.0 |
| Sex | ||
| Male | 1674 | 62.2 |
| Female | 1017 | 37.8 |
| Age (years) | ||
| 18–24 | 12 | 0.4 |
| 25–44 | 204 | 7.6 |
| 45–54 | 2070 | 76.9 |
| 65 and older | 405 | 15.1 |
| Race | ||
| White | 1519 | 56.4 |
| Black | 1002 | 37.2 |
| Asian | 66 | 2.5 |
| Native American | 14 | 0.5 |
| Other | 39 | 1.4 |
| Unknown | 51 | 1.9 |
| Hispanic ethnicity | ||
| No | 2509 | 93.2 |
| Yes | 127 | 4.8 |
| Unknown | 55 | 2.0 |
| FIB-4 categories | ||
| <1.45 | 611 | 22.7 |
| 1.45–3.25 | 1071 | 39.8 |
| >3.25 | 1009 | 37.5 |
| Liver-related comorbidities | ||
| Hepatocellular carcinoma | 264 | 9.8 |
| Ascites | 339 | 12.6 |
| Concurrent NAFLD | 202 | 7.5 |
| Hepatic encephalopathy | 284 | 10.6 |
| Variceal bleeding | 48 | 1.8 |
| Hepatorenal syndrome | 19 | 0.7 |
| Post-liver transplant | 363 | 13.5 |
| Other comorbidities | ||
| HIV | 75 | 2.8 |
| Cardiovascular disease | 1954 | 72.6 |
| Hypertension | 1592 | 59.2 |
| Diabetes | 786 | 29.2 |
| Body Mass Index | ||
| BMI ≥ 30 kg/m2 | 729 | 27.1 |
| Alcohol use history | ||
| Current/past alcohol use | 973 | 36.2 |
| Unsure but probably | 247 | 9.2 |
| No evidence | 1397 | 51.9 |
| Unknown | 74 | 2.7 |
| Drug use history | ||
| Current/past drug use | 424 | 15.8 |
| Unsure but probably | 557 | 20.7 |
| No evidence | 1629 | 60.5 |
| Unknown | 81 | 3.0 |
| Treatment type | ||
| Non-DAA | 498 | 18.5 |
| DAA | 2193 | 81.5 |
BMI, body mass index; DAA, direct acting antiviral; FIB-4, fibrosis-4; HIV, human immunodeficiency virus; NAFLD, nonalcoholic fatty liver disease.
Overall Trends after Treatment
Overall, median AST decreased from 53 U/L pre-treatment to 28 U/L 1-year post-treatment, which was sustained at 27.5 U/L at 4 years post-treatment (trend analysis, P < 0.001; Figure 1). Similarly, median ALT decreased from 53 U/L pre-treatment to 30 U/L 1-year post-treatment, which was sustained at 29 U/L at 4 years post-treatment (trend analysis, P < 0.001). PLT demonstrated continued improvement after HCV treatment from 168 × 103 to 176 × 103 at 4 years post-treatment (trend analysis, P = 0.212). Overall, FIB-4 scores improved from 2.51 pre-treatment to 1.68 at 4 years post-treatment (trend analysis, P < 0.001).
Figure 1.
Overall changes in AST, ALT, PLT, and FIB-4 scores after HCV treatment. ALT, alanine; AST, aspartate; FIB-4, fibrosis-4; HCV, hepatitis C virus; PLT, platelet count.
AST and ALT Aminotransferase Levels
Both men and women experienced significant and sustained decreases in AST and ALT after HCV treatment (men: median ALT decreased from 55 U/L pre-treatment to 31 U/L 1-year post-treatment, which was sustained at 29 U/L at 4 years post-treatment; women: median ALT decreased from 50 U/L pre-treatment to 29 U/L at 1-year post-treatment to 28 U/L at 4 years post-treatment; Figure 2, Figure 3). Similarly, sustained improvements in both AST and ALT after HCV treatment were observed when evaluating by age groups. When stratified by race, while whites had higher AST and ALT than blacks pre-treatment, both whites and blacks with HCV experienced significant and sustained decreases in AST and ALT. When stratified by ethnicity, Hispanics had significantly higher AST and ALT levels pre-treatment when compared with non-Hispanics. Although both groups experienced significant and sustained decreases in AST and ALT after HCV treatment, at 4 year post-treatment, Hispanics had a trend towards higher AST (46 U/L vs. 25 U/L, P = 0.288) and ALT (36 U/L vs. 29 U/L, P = 0.167) compared with non-Hispanics. When stratified by pre-treatment FIB-4 score, as expected, higher FIB-4 scores correlated with higher pre-treatment AST and ALT levels (Figure 2, Figure 3). After HCV treatment, patients in all FIB-4 groups experienced improvements in AST and ALT, with the greatest improvement seen in those with the highest pre-treatment FIB-4 score, such that by 4 years post-treatment, AST and ALT levels were similar across all patients. When stratified by BMI and presence of diabetes, all patients experienced significant improvements in AST and ALT at 1-year post-treatment (Figure 2, Figure 3). However, when evaluating the sustainability of these improvements, the presence of BMI >30 kg/m2 and diabetes was associated with an increase in AST in the subsequent years. For example, median AST in patients with combined BMI >30 kg/m2 and diabetes improved from 46 U/L pre-treatment to 28 U/L 1-year post-treatment, but AST subsequently increased to 42.5 U/L at 4 years post-treatment, higher than patients with BMI <30 kg/m2 without diabetes (24 U/L) or patients with BMI >30 kg/m2 without diabetes (29.5 U/L).
Figure 2.
Changes in serum AST levels after HCV treatment.
Figure 3.

Changes in serum ALT levels after HCV treatment.
PLT Levels
PLTs in men and women remained stable up to 4 years post-treatment (Figure 4). When stratified by race, PLT in whites demonstrated continued improvement in the years after HCV treatment (170 × 103 at 4 years post-treatment vs. 157 × 103 pre-treatment, P = 0.116), whereas PLTs remained stable in blacks. Increasing trends of PLT were also observed in Hispanics and non-Hispanics. When stratified by age groups, HCV patients aged >65 years experienced continued decreases in PLT despite HCV treatment, whereas all younger patients experienced increasing post-treatment PLTs (Figure 4). When stratified by pre-treatment FIB-4 score, patients with FIB-4 >3.25 pre-treatment experienced a sustained and continued improvement in PLT after treatment (135 × 103 at 4 years post-treatment vs. 106 × 103 pre-treatment, P < 0.001). When stratified by BMI and diabetes, HCV patients with BMI >30 kg/m2 with (211 × 103 at 4 years post-treatment vs. 179 × 103 pre-treatment, P = 0.684) and without diabetes (222 × 103 at 4 years post-treatment vs. 174 × 103 pre-treatment, P = 0.039) demonstrated the greatest improvements in PLT after treatment (Figure 4).
Figure 4.
Changes in serum PLT levels after HCV treatment.
FIB-4 Scores
Both men and women had similar proportional declines in median FIB-4 that was sustained at 4 years post-treatment (Figure 5). When stratified by age, although older HCV patients experienced the greatest decline in FIB-4 score in the first year post-treatment, FIB-4 in patients aged 65 years and older increased in the subsequent years, such that the overall improvement at 4 years after HCV treatment was minimal in this older group (Figure 5). When stratified by race, both white and black patients demonstrated sustained improvements in FIB-4 at 4 years. Although both Hispanic and non-Hispanic patients experienced declines in FIB-4, pre-treatment FIB-4 was higher in Hispanics, and this trend in higher FIB-4 persisted even at 4 year post-treatment (median FIB-4 at 4 years post-treatment: 2.09 in Hispanics, 1.66 in non-Hispanics). When stratified by BMI and diabetes, although all groups experienced declines in FIB-4 in the first year post-treatment, HCV patients with combined BMI >30 kg/m2 and diabetes experienced a rebound in median FIB-4 in the subsequent years (Figure 5).
Figure 5.
Changes in FIB-4 scores after HCV treatment.
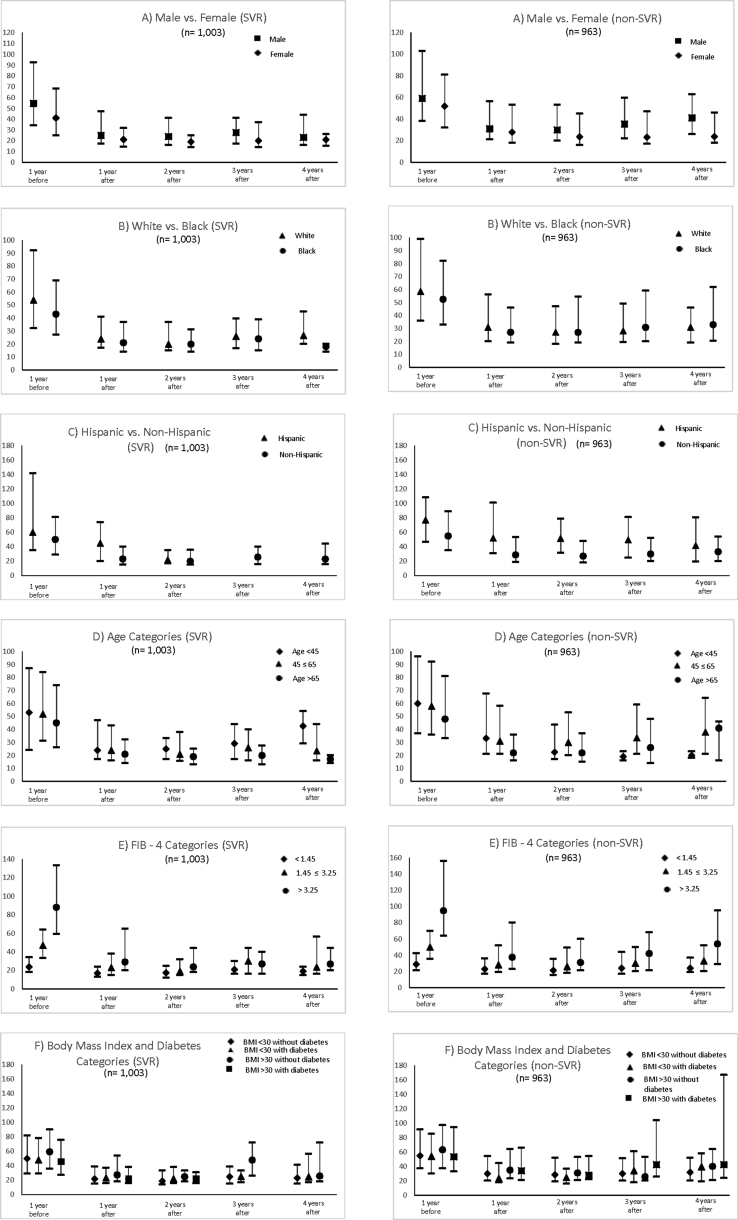
Analyses Based on Treatment Type and Treatment Response
We further performed stratified analyses focusing on longitudinal changes in AST, ALT, PLT, and FIB-4 scores by treatment type and treatment response. As illustrated by Figure 6, overall changes in the aforementioned laboratory markers were similar between patients who were treated with DAA vs. non-DAA therapies. Notably, FIB-4 score declined for both groups after HCV treatment and the lower FIB-4 score was sustained at 4 years post-treatment for both patients treated with DAA or non-DAA therapies. We also stratified our outcomes by whether patients achieved SVR vs. no SVR (Figure 7). Although both SVR and non-SVR patients experienced sustained improvements in all markers of liver disease severity, HCV patients who achieved SVR had slightly better AST, ALT, platelets, and FIB-4 score at 4 years post-treatment compared with non-SVR, but these differences did not reach statistical significance. To provide a more granular assessment of these changes, we performed sub-set analyses of longitudinal changes in AST, ALT, PLT, and FIB-4 stratified by sex, race (white versus black), ethnicity (Hispanic versus non-Hispanic), age groups, baseline FIB-4 score, and BMI and diabetes categories for patients treated with DAA vs. non-DAA and patients who achieved SVR vs. non-SVR (Supplementary Figures 1–8).
Figure 6.
Changes in FIB-4 scores, AST, ALT, and PLT stratified by treatment with DAA vs. non-DAA. DAA, direct acting antiviral.
Figure 7.
Changes in FIB-4 scores, AST, ALT, and PLT stratified by patients who achieved SVR vs. non-SVR. SVR, sustained virologic response.
Discussion
Among our multicentered cohort of 2691 patients who were successfully treated for chronic HCV, overall sustained improvements were observed in AST, ALT, PLT, and FIB-4 scores. Few studies have specifically evaluated longitudinal changes in serological makers after HCV treatment in the DAA era. Our study also provides interesting data on disparate changes in these serological markers in different subgroups.
Although our race/ethnicity-specific analyses demonstrated similar improvements in serological markers post-treatment in whites and blacks with HCV, significant differences were observed between Hispanics vs. non-Hispanics. For example, Hispanics had significantly higher AST, ALT, and FIB-4 scores pre-treatment compared with non-Hispanics. Although all groups experienced improvement in serological markers post-treatment, higher AST, higher ALT, and higher FIB-4 scores persisted in Hispanics compared with non-Hispanics, even out to 4 years post-treatment. Although this Hispanic ethnicity-specific disparity has been previously reported in untreated HCV patients,10,13 our study is novel in reporting the persistence of these disparities post-HCV treatment using serological noninvasive markers of disease severity. Previous studies have reported higher risk of cirrhosis among untreated Hispanic HCV patients: 28% higher than non-Hispanic whites in a VA population,10 37% higher than non-Hispanic whites in a multicentered community-based/academic hospital-based study.13 Although potential explanations for these findings included disparate access to HCV care in Hispanic populations leading to disease progression and HCC,10,13, 14, 15 our study specifically focuses on post-treatment differences and continued to observe Hispanic ethnicity-specific disparities. The higher rates of disease progression among Hispanics has also been attributed to higher rates of concurrent NAFLD among this group, which would potentiate underlying liver disease leading to more aggressive disease progression.11,16, 17, 18, 19 In our population, we observed that Hispanics were generally younger than non-Hispanics at the time of HCV treatment (15.0% <45 years in Hispanics vs. 7.7% in non-Hispanics, P < 0.01) (Supplementary Table 1). Although Hispanics had lower rates of concurrent NAFLD compared with non-Hispanics, this lower prevalence is likely due to under-diagnosis given that Hispanics had higher rates of concurrent diabetes and BMI >30 kg/m2 when compared with non-Hispanics. Hispanics were also observed to have higher rates of HIV coinfection compared with non-Hispanics. Although our observational approach does not establish causation, these observations may partly explain the more severe markers of disease severity observed in Hispanics.
To specifically evaluate the potential impact of concurrent obesity and diabetes on post-treatment serological markers of liver disease, we used the aforementioned four categories of BMI and diabetes status. Although all patients experienced improvements in AST and ALT in the first year after treatment, patients with BMI >30 kg/m2 demonstrated a rebound increase following this initial decline. The group with combined BMI >30 kg/m2 and diabetes also had a rebound increase in FIB-4 in years 3 and 4 post-treatment, despite demonstrating sustained improvement in PLT counts. These observations may reflect the increased risk of concurrent NAFLD that is associated with presence of obesity, diabetes, and metabolic syndrome.20, 21, 22, 23 However, it is important to note that while concurrent obesity and diabetes does significantly increase risks of NAFLD, there are inherent assumptions and limitations in this association. Although concurrent metabolic syndrome components and NAFLD can potential HCV disease progression,11,24, 25, 26 curing HCV does not eliminate NAFLD risk. Therefore, these data highlight the importance of continued awareness and optimization of NAFLD risk factors in at risk patients despite successful HCV treatment.
Our large sample size with 4 years of longitudinal post-treatment follow-up, spanning across four diverse states within the United States is a strength. Furthermore, our cohort includes a large proportion of ethnic minorities, including two safety-net hospitals, which improves the generalizability of our findings. However, certain limitations typical of observational studies should be acknowledged. Although our study group was meticulous in sorting ICD-9 and ICD-10 codes to accurately identify chronic HCV as well as the different variables included in our study, potential errors in diagnostic coding may have contributed to misclassification bias, which was minimized with consistent methodology across sites. Our assessment of fibrosis in this retrospective cohort study used FIB-4 scores only, which may have introduced some degree of misclassification bias. As such, we attempted to provide a more comprehensive perspective by also analyzing AST, ALT, and PLT. More accurate measures of hepatic fibrosis such as liver biopsy or transient elastography were not routinely evaluated or available in all patients. In addition, given the retrospective observational nature of our study design that was primarily based on ICD-9/10 coding, certain more granular data such as treatment protocols and treatment compliance were not feasible to incorporate into our analyses. Follow-up time was also nonstandardized, which while typical of retrospective study designs were by different patients may have entered the study cohort at different time points, it is important to acknowledge this limitation when interpreting the overall results. Our data set did not have data on liver biopsy histology or transient elastography and thus were unable to correlate these findings with other markers of hepatic fibrosis. Furthermore, while we use the presence of elevated BMI and concurrent diabetes to assess potential impact of NAFLD, this presumed association should be interpreted with caution in the absence of more definitive data from biopsy or elastography to confirm this diagnosis. Furthermore, although our study specifically focused on longitudinal changes in laboratory-based markers of disease severity, the retrospective and observational nature of our study precluded longitudinal analyses to determine incidence of developing liver and non-liver comorbidities that are based on ICD-9/10 coding definitions. Although we could not accurately assess long-term impact of alcohol consumption given lack of available data, assumption of alcohol abstinence during and in the immediate post-treatment period seems reasonable given sobriety requirements of payers.14
In conclusion, among a large multicentered HCV cohort in the United States, sustained post-treatment improvements in AST, ALT, PLT, and FIB-4 were observed. However, Hispanics and patients with combined BMI >30 kg/m2 and diabetes demonstrated rebound increase in markers of liver disease severity after the initial improvements seen in the first-year post-treatment. These changes may reflect the risk of NAFLD in these cohorts.
Disclosures
RJW receives research funding from Gilead Sciences and Abbvie, has served as a consultant and member of the advisory board for Gilead Sciences, and serves on the speaker's bureau for Gilead Sciences, Salix, and Bayer. RJW is also funded by an AASLD Foundational Clinical and Translational Research Award in Liver Diseases.
MKJ receives research funding from Gilead Sciences, Merck, Janssen, and GlaxoSmithKline / ViiV Healthcare and has served as an advisor for GlaxoSmithKline.
MLS receives grant funding from Abbvie, Bristol Myers-Squibb, Conatus, CymaBay, Exalenz, Galectin, Genfit, Gilead, Intercept, Immuron, Merck, NGMBio, Novartis, and Shire. He is also an advisor/speaker for Abbvie, Bayer, Bristol Myers-Squibb, Daiichi Sankyo, Gilead, Intercept, Merck, and Salix and serves as a consultant for Optum Rx.
Authors' contribution
RJW and MT—study concept and design; RJW, MKJ, GT, MLS, OK, CC, and MT—acquisition of data; RJW, MKJ, GT, MLS, OK, CC, and MT—analysis and interpretation of data; OK and MT—statistical analysis; MKJ and MT—drafting of the manuscript; RJW, MKJ, GT, MLS, OK, CC, and MT—critical revision of the manuscript for important intellectual content; RJW and MT—study supervision; RJW and MT had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
Conflicts of interest
The authors have none to declare.
Funding/Support
This study was supported by an investigator-initiated study research grant from Gilead Sciences. Robert Wong is supported by an AASLD Foundation Clinical and Translational Research Award in Liver Diseases.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2019.09.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
figs5.
figs6.
figs7.
figs8.
References
- 1.Falade-Nwulia O., Suarez-Cuervo C., Nelson D.R. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166:637–648. doi: 10.7326/M16-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backus L.I., Belperio P.S., Shahoumian T.A. Impact of sustained virologic response with direct-acting antiviral treatment on mortality in patients with advanced liver disease. Hepatology. 2018;68:827–838. doi: 10.1002/hep.29811. [DOI] [PubMed] [Google Scholar]
- 3.Ioannou G.N., Green P.K., Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.08.030. pii: S0168-8278(17)32273-0 [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi Z.M., Stepanova M., Gordon S. Patient-reported outcomes following treatment of chronic hepatitis C virus infection with sofosbuvir and velpatasvir, with or without voxilaprevir. Clin Gastroenterol Hepatol. 2018;16:567–574 e6. doi: 10.1016/j.cgh.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Lu M., Li J., Zhang T. Serum biomarkers indicate long-term reduction in liver fibrosis in patients with sustained virological response to treatment for HCV infection. Clin Gastroenterol Hepatol. 2016;14:1044–1055 e3. doi: 10.1016/j.cgh.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George S.L., Bacon B.R., Brunt E.M. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology. 2009;49:729–738. doi: 10.1002/hep.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachofner J.A., Valli P.V., Kroger A. Direct antiviral agent treatment of chronic hepatitis C results in rapid regression of transient elastography and fibrosis markers fibrosis-4 score and aspartate aminotransferase-platelet ratio index. Liver Int. 2017;37:369–376. doi: 10.1111/liv.13256. [DOI] [PubMed] [Google Scholar]
- 8.Jain M.K., Seremba E., Bhore R. Change in fibrosis score as a predictor of mortality among HIV-infected patients with viral hepatitis. AIDS Patient Care STDS. 2012;26:73–80. doi: 10.1089/apc.2011.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vergniol J., Boursier J., Coutzac C. Evolution of noninvasive tests of liver fibrosis is associated with prognosis in patients with chronic hepatitis C. Hepatology. 2014;60:65–76. doi: 10.1002/hep.27069. [DOI] [PubMed] [Google Scholar]
- 10.El-Serag H.B., Kramer J., Duan Z. Racial differences in the progression to cirrhosis and hepatocellular carcinoma in HCV-infected veterans. Am J Gastroenterol. 2014;109:1427–1435. doi: 10.1038/ajg.2014.214. [DOI] [PubMed] [Google Scholar]
- 11.Dyal H.K., Aguilar M., Bhuket T. Concurrent obesity, diabetes, and steatosis increase risk of advanced fibrosis among HCV patients: a systematic review. Dig Dis Sci. 2015;60:2813–2824. doi: 10.1007/s10620-015-3760-3. [DOI] [PubMed] [Google Scholar]
- 12.Vallet-Pichard A., Mallet V., Nalpas B. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 13.Le A.K., Zhao C., Hoang J.K. Ethnic disparities in progression to advanced liver disease and overall survival in patients with chronic hepatitis C: impact of a sustained virological response. Aliment Pharmacol Ther. 2017;46:605–616. doi: 10.1111/apt.14241. [DOI] [PubMed] [Google Scholar]
- 14.Wong R.J., Jain M.K., Therapondos G. Race/ethnicity and insurance status disparities in access to direct acting antivirals for hepatitis C virus treatment. Am J Gastroenterol. 2018;113:1329–1338. doi: 10.1038/s41395-018-0033-8. [DOI] [PubMed] [Google Scholar]
- 15.Kuniholm M.H., Jung M., Del Amo J. Awareness of hepatitis C virus seropositivity and chronic infection in the hispanic community health study/study of latinos (HCHS/SOL) J Immigr Minority Health. 2016;18:1257–1265. doi: 10.1007/s10903-016-0350-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong R.J., Liu B., Bhuket T. Significant burden of nonalcoholic fatty liver disease with advanced fibrosis in the US: a cross-sectional analysis of 2011-2014 National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2017;46:974–980. doi: 10.1111/apt.14327. [DOI] [PubMed] [Google Scholar]
- 17.Schneider A.L., Lazo M., Selvin E. Racial differences in nonalcoholic fatty liver disease in the U.S. population. Obesity (Silver Spring) 2014;22:292–299. doi: 10.1002/oby.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rich N.E., Oji S., Mufti A.R. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16:198–210 e2. doi: 10.1016/j.cgh.2017.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill C., Vatcheva K.P., Pan J.J. Frequency of nonalcoholic fatty liver disease and subclinical atherosclerosis among young Mexican Americans. Am J Cardiol. 2017;119:1717–1722. doi: 10.1016/j.amjcard.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dowman J.K., Tomlinson J.W., Newsome P.N. Systematic review: the diagnosis and staging of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2011;33:525–540. doi: 10.1111/j.1365-2036.2010.04556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ioannou G.N., Weiss N.S., Boyko E.J. Contribution of metabolic factors to alanine aminotransferase activity in persons with other causes of liver disease. Gastroenterology. 2005;128:627–635. doi: 10.1053/j.gastro.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Pagano G., Pacini G., Musso G. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002;35:367–372. doi: 10.1053/jhep.2002.30690. [DOI] [PubMed] [Google Scholar]
- 23.Rinella M.E. Nonalcoholic fatty liver disease: a systematic review. J Am Med Assoc. 2015;313:2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 24.Dyal H.K., Aguilar M., Bartos G. Diabetes mellitus increases risk of hepatocellular carcinoma in chronic hepatitis C virus patients: a systematic review. Dig Dis Sci. 2016;61:636–645. doi: 10.1007/s10620-015-3983-3. [DOI] [PubMed] [Google Scholar]
- 25.Adinolfi L.E., Gambardella M., Andreana A. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358–1364. doi: 10.1053/jhep.2001.24432. [DOI] [PubMed] [Google Scholar]
- 26.Fartoux L., Poujol-Robert A., Guechot J. Insulin resistance is a cause of steatosis and fibrosis progression in chronic hepatitis C. Gut. 2005;54:1003–1008. doi: 10.1136/gut.2004.050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.