Abstract
Aims
The purpose of this study is to develop a FE model of the temporomandibular joint (TMJ) to investigate a musculoskeletal System of forces able to taking into account the effect of all the muscles on the TMJ in terms of stress evaluated on the bone.
Methods
A 3-dimensional finite element model of the mandible was constructed from the images generated by cone-beam computed tomography of a patient undergoing fixed orthodontic treatment. In order to define the loading force system an exustive study was developed to investigated the entity of the Lateral pterygoid, Masseter, medial pterygoid, Temporalis, and Geniohoid digastric, muscles.
Results
Stresses in the TMJ components (disc, mandible condyle and the fossa eminence on the skull) were obtained. The results have shown stress distribution during normal occlusion.
Conclusion
An appreciation of the anatomical and mechanical features associated with the TMJ can serve as a foundation for understanding a patient's clinical presentation. Performance of a thorough patient history and clinical examination can guide the clinician toward an improved diagnostic process.
Keywords: Biomechanical behavior, FE analysis, Temporomandibular joint, Von mises stress
1. Introduction
Temporomandibular joint disorders (TMDs) are common in adults, and one-third of adults report having one or more symptoms, which include joint pain, headache, and clicking or grating within the joint.1, 2, 3 It is referred to as a cluster of conditions characterized by pain in the temporomandibular joint (TMJ) during motion. To examine the biomechanical behavior of TMDs, one must focus on the magnitude and location of the maximum stresses under physiological loading. The TMJ is located just anterior to the external auditory meatus, consists superiorly of the temporalis bone and inferiorly of the mandible, contains an intraarticular disk within the joint capsule, and its contractile tissues are the muscles of mastication.4 The mandibular condyle and glenoid fossa of the temporalis bone form the foundation of the TMJ,.5 A biconcave intraarticular disk divides the joint into upper (disco temporal) and lower (disco mandibular) joint spaces. Under normal circumstances, the mandibular condyle can have variable shapes6 and can be asymmetrical side-to-side. Condyle shapes have been previously described as convex, flat, angular, and rounded.7 During childhood, the mandibular condyle undergoes significant changes in size and shape.8 Under pathological circumstances, mandibular condyle shape can vary to greater extents. Certain mandibular abnormalities are only visible upon imaging or entry into the joint whereas others are profound enough to cause distortion of facial features. One classification method involves naming the relative bone growth in terms of aplasia, hypoplasia, or hyperplasia.9 The articular surfaces of the TMJ are highly incongruent and consist of fibrocartilage, not hyaline cartilage like other synovial joints.10 The TMJ is subject to degenerative changes, though the temporal bone and upper joint space generally undergo less degeneration relative to the mandibular condyle and lower joint space. Both local and extensive degenerative changes can occur. When local changes occur, the lateral aspect of the articular tubercle of the temporal bone is most likely to be affected but no one part of the mandibular condyle is at greater risk.11 Extensive changes can culminate in a total loss of articular cartilage. There is no relationship between degenerative changes seen on radiographic imaging and verbal reports of TMJ pain, palpable tenderness of the TMJ, mandibular mobility, and pressure pain thresholds.12 The intraarticular disk attaches both to the medial and lateral aspects of the mandibular condyle. It has direct connections to the surrounding ligamentous capsule (disco capsular complex) and musculature that ensure the disk and condyle move together under the temporal bone when tissue is taut. Additionally, the disk attaches anteriorly to the capsule and posteriorly to the retro discal tissue.13 The inferior surface of the disk undergoes degenerative changes roughly 3.3 times more frequently than the superior aspect. The intraarticular disk can displace in the anterior, medial, lateral, or posterior directions.14,15 Attempts to relate disk position (normal vs displaced) and the presence of degenerative changes have not been successful.16 The joint capsule is lined by a synovial membrane, contains synovial fluid, and possesses a lateral ligamentous thickening (temporomandibular ligament) that reinforces the joint. The capsular pattern of the TMJ has been reported as opening, protrusion, and lateral deviation but no scientific evidence exists to verify this claim. Under normal circumstances, the capsule of the lower joint space does not extend past the mandibular condyle.
Accordingly, under normal conditions the upper joint space extends farther forward than the lower joint space. This relative relationship is likely due to arthro kinematic rolling in place of the lower joint space whereas the upper joint space translates anteriorly. In joints with anterior disk displacement, the anterior joint capsule of the lower joint space extends as far anterior as the disk displaces, which is considerably past the margin of the mandibular condyle. This represents a significant alteration in the joint capsule. Musculature located in the head, face, and cervical spine contributes to movement and stability of the TMJ.17 Muscles of mastication are split into two groups: openers and closers. Opening is sometimes referred to as mandibular depression whereas closing is sometimes referred to as mandibular elevation.
The lateral pterygoid is the primary opener and is the strongest contributor to both protrusion and medial/lateral deviation of the jaw, both of which are required for normal mastication. Other muscles including the geniohyoid, mylohyoid, and the digastric muscles assist in opening.18 The primary closers include the temporalis, masseter, and medial pterygoids.19 These muscles originate on the cranium and insert on the mandible. Finite element analysis is a useful tool in order to better investigate different patologies, for example computational fluid dynamic analysis has been to investigate carotid artery stenosis.20 In this case FE nalysis has been used to quantify the stress distribution in the TMJ and surrounding tissues, and it has been previously used in the study of biomechanical behavior of orthopedic devices, including hip, knee, and spinal implants,21, 22, 23, 24 under various loading conditions.25, 26, 27, 28 Therefore, the aim of this study was to develop a 3D finite element model, reconstructed from in vivo image data, to quantify the stress distribution in the mandibular condyle, disc, and articular eminence.
2. Materials and methods
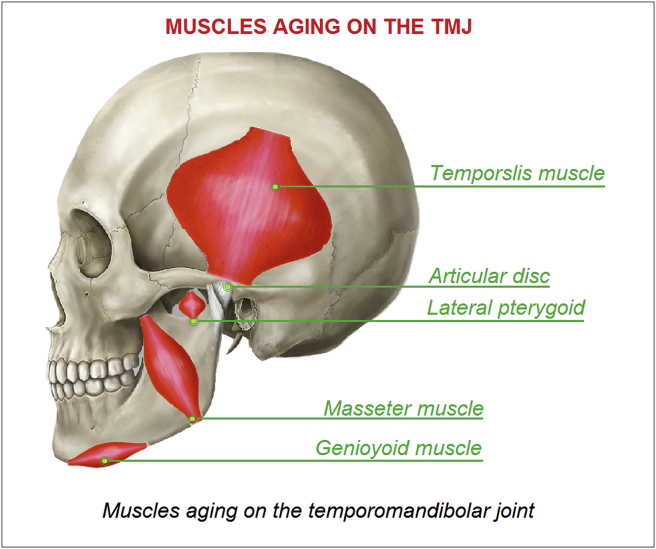
The purpose of this study is to develop a FE model of the temporomandibular joint (TMJ) to investigate a musculoskeletal System of forces able to taking into account the effect of all the muscles on the TMJ in terms of stress evaluated on the bone. In order to define the musculoskeletal system of forces it is necessary to investigate the effect and the typology of muscles aging on the temporomandibular joint, see Fig. 1.
Fig. 1.
Muscles aging on the temporomandibolar joint.
2.1. Temporalis muscle
In humans, it arises from the temporal fossa and the deep part of temporal fascia, passing medial to the zygomatic arch and forms a tendon which inserts onto the coronoid process of the mandible, with its insertion extending into the retromolar fossa posterior to the most distal mandibular molar. The temporal muscle is the most powerful muscle of the temporomandibular joint and can be divided into two functional parts: anterior and posterior. The anterior portion runs vertically and its contraction results in elevation of the mandible (closing the mouth). The posterior portion has fibers, which run horizontally, and contraction of this portion results in retrusion of the mandible.
2.2. Masseter muscle
The masseter is a thick quadrilateral muscle, consisting of two heads, superficial and deep. The superficial head arises by a thick, tendinous aponeurosis from the temporal process of zygomatic bone, and from the anterior two-thirds of the inferior border of the zygomatic arch. The deep head is much bigger; it arises from the posterior third of the lower border and from the whole of the medial surface of the zygomatic arch. The deep head of the muscle is partly concealed, anteriorly, by the superficial portion. The masseter is innervated on the anterior division of the mandibular division of the trigeminal nerve. The action of the muscle is to elevate the mandible, raising the lower jaw.
2.3. Medial pterygoids (the p is silent)
It consists of two heads.
-
•
The bulk of the muscle arises as a deep head from just above the medial surface of the lateral pterygoid plate.
-
•
The smaller, superficial head originates from the maxillary tuberosity and the pyramidal process of the palatine bone.
The insertion joins the masseter muscle to form a common tendinous sling, which allows the medial pterygoid and masseter to be powerful elevators of the jaw. Medial pterygoid is innervated on the tensor tympani and tensor veli palatini. Its functions include elevation of the mandible, and minor contribution to protrusion.
2.4. Lateral pterygoids
The upper/superior head originates on the infratemporal surface and infratemporal crest of the greater wing of the sphenoid bone and inserts onto the articular disc and fibrous capsule of the temporomandibular joint. The lower/inferior head originates on the lateral surface of the lateral pterygoid plate and inserts onto the neck of condyloid process of the mandible. The mandibular trigeminal nerve innervates the lateral pterygoid muscle. The primary function of the lateral pterygoid muscle is to pull the head of the condyle out of the mandibular fossa along the articular eminence to protrude the mandible.
2.5. Geniohyoid muscle
It arises from the inferior mental spine, on the back of the mandibular symphysis, and runs backward and downward, to be inserted into the anterior surface of the body of the hyoid bone. The geniohyoid muscle is innervated on the first cervical nerve travelling alongside the hypoglossal nerve.
2.6. Numerical model
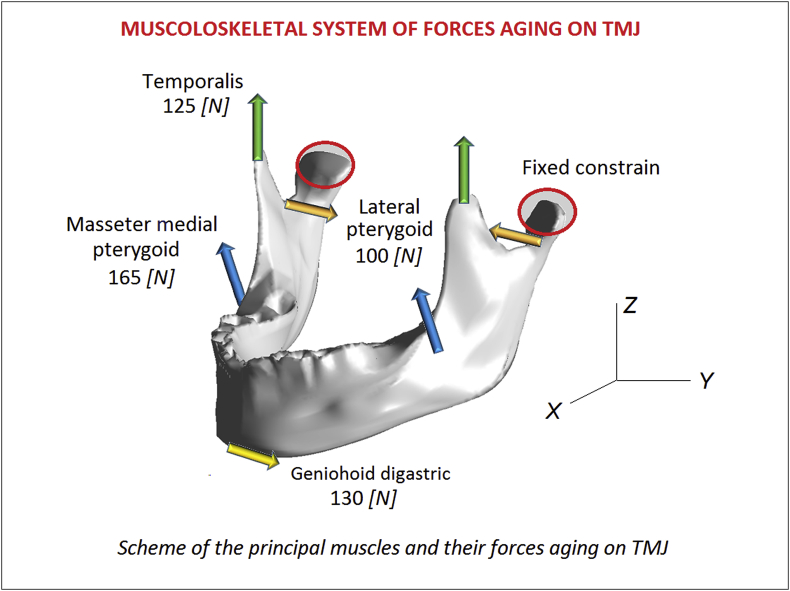
For the reconstructed model, magnetic resonance imaging (MRI, slices at 1 mm intervals) and computed tomography (CT, slices at 1 mm intervals) examinations were conducted with closed and maximum open mouth positions in the parasagittal section. The images were segmented for data extraction to describe the surfaces of the condyle, disc, and articular eminence. The 3D image reconstruction and solid modeling were conducted using ANSYS Workbench 12.1 (ANSYS, Inc., USA) finite element software. In Fig. 2 is reported the numerical model of the TMJ realized with 95.425 and 132.234 nodes. To simulate the frictionless contact between the joint surfaces, ABAQUS automated surface-to-surface contact option was used.29,30 The linear elastic material law, the Young's modulus and Poisson's ratio for the bony structures were assigned as 7300 MPa and 0.3. 31,32 The three muscles used during mastication, masseter, medial pterygoid, and temporalis, are activated to close the mouth, while the lateral pterygoid is used for mouth opening. It is necessary to know the cross-sectional area (CSA) to determine the maximum muscle force via the following mathematical function.33 The maximum muscle forces evaluated with this method are reported in Table 1. In Fig. 2 is reported the numerical setup of the analyses.
Fig. 2.
Scheme of the principal muscles and their forces aging on TMJ.
Table 1.
Musculoskeletal system of forces.
| Muscles | Force [N] | Fx [N] | FY [N] | Fz [N] |
|---|---|---|---|---|
| Lateral pterygoid | 100 | 0 | 100 | 0 |
| Masseter medial pterygoid | 165 | 0 | 117 | 117 |
| Temporalis | 125 | 0 | 0 | 125 |
| Geniohoid digastric | 130 | −65 | 0 | −112 |
3. Results
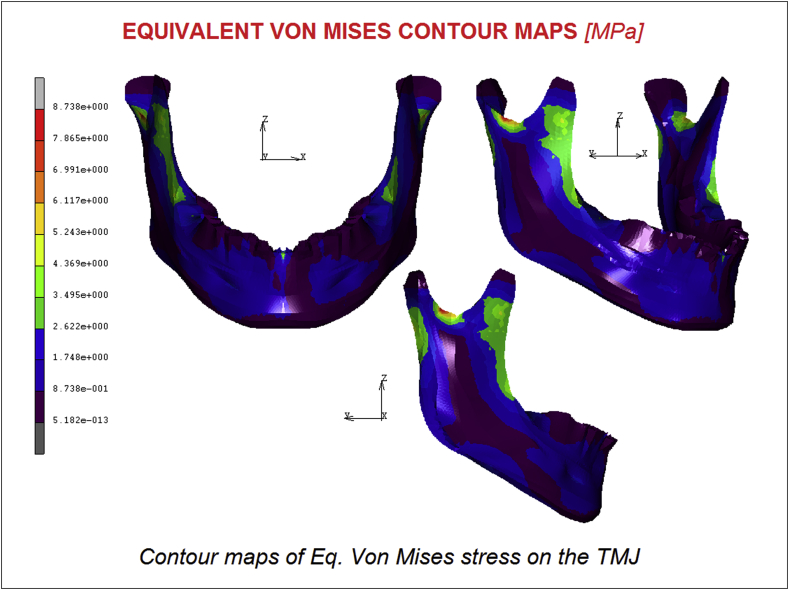
Fig. 3 displays the stress distribution of the model; the highest von Mises stress was of 8.73 MPa. The maximum stresses were localized usually in the antero-posterior direction for all regions. In the mandibular condyle region, the highest stress varied from 3.34 to 8.73 MPa, and the highest stress was observed in the central mandibular condyle. The maximum stresses varied from 0.87 to 2.62 MPa in the articular disc region. For the articular eminence, the highest von Mises stress was 0.87. On the masseter muscle-acting zone, it is possible to notice a significative stress of about 4.36 MPa localized on the antero posterior direction. The chin area is quite uncharged, as the effect of the Geniohoid digastric muscle has not a remarkable effect.
Fig. 3.
Contour maps of Eq. Von Mises stress on the TMJ.
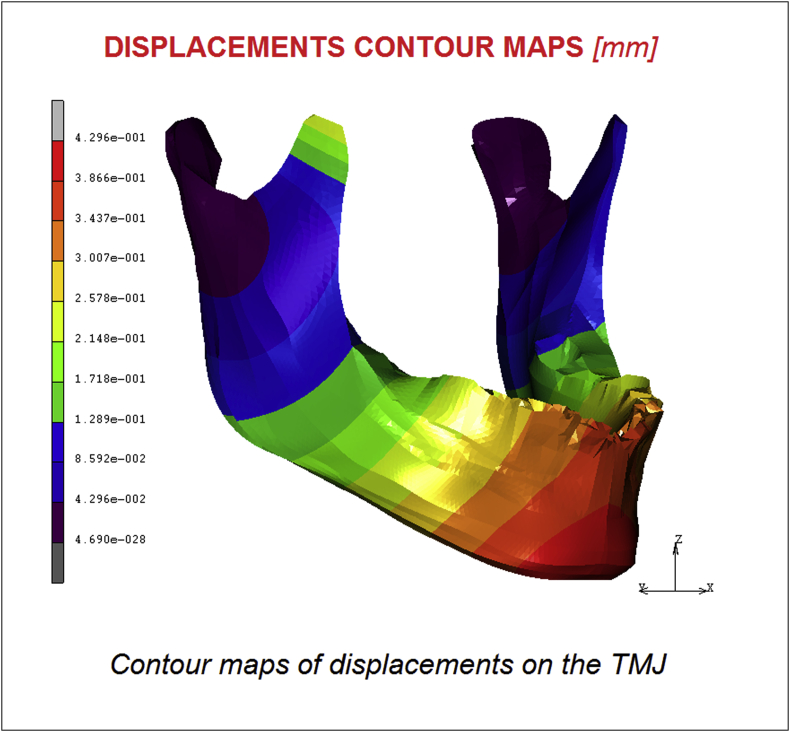
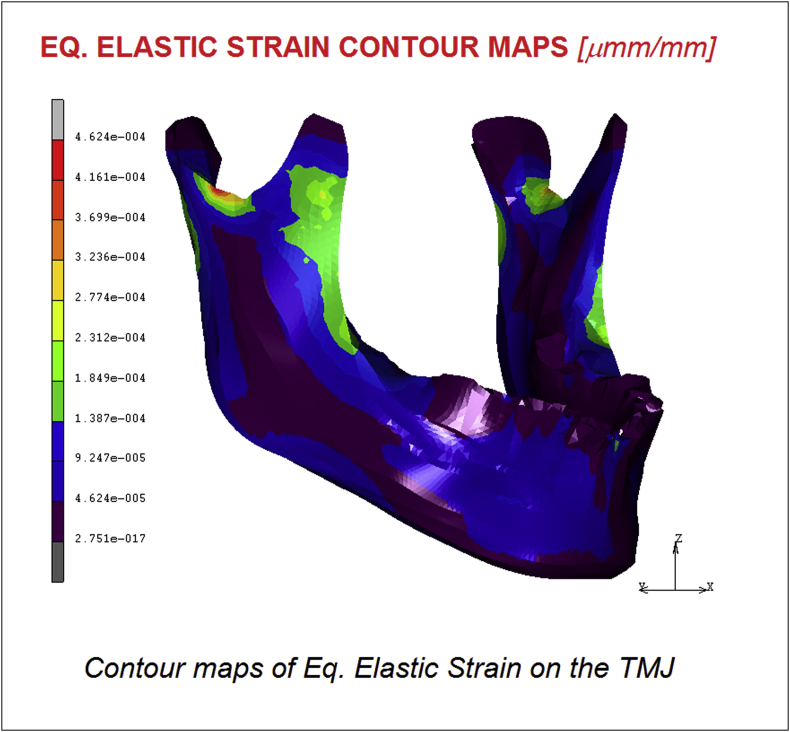
In Fig. 4, Fig. 5 are reported respectively the displacements and the Equivalent elastic strain contour maps. Results confirm a maximum value of 0.42 mm of displacement registered on the chin and a value of 4.62 E−004 μmm/mm for the equivalent elastic strain following the same trend of the stress shielding.
Fig. 4.
Contour maps of displacements on the TMJ.
Fig. 5.
Contour maps of Eq. Elastic Strain on the TMJ.
4. Discussion
The anterior, central, and medial regions of the mandibular condyle are considered important functional regions that sustain masticatory loading. Treatment could release abnormal focusing stresses on these important regions. Decreasing the load would lead to severe resorption, while a mechanical stimulus or stress in the skeleton is one of the major factors in the maintenance of a normal balance between the dynamic processes of bone formation and resorption. The abnormal effects of stress shielding are known to persist. This could promote the stress shielding problem in the posterior and lateral regions as a result of treatment. Several researchers have attempted to assess or measure the mechanical loads directly in the TMJ during jaw movement by a strain gauge technique, which indicates that substantial forces are induced and, hence, the TMJ is acting as a load-bearing member. However, these experimental approaches have shortcomings, such as surgical invasion of the TMJ structures. On the other hand, the analytical techniques of the finite element method have been used to investigate stresses and forces in the TMJ by mathematical models for a long time. Researchers in the biomechanics field have successfully used these analytical techniques, which have many advantages. These techniques measure the internal stresses in the model and can easily simulate conditions with different magnitude forces or bone densities. Another important factor, which governs the results of the analyses, is the mechanical and physical properties of the model compared with the actual object.
The orientation of muscle forces, on the other hand, appears to be rather important. Not only strain directions, but also strain magnitudes are affected by a relatively small change in the force orientation. Great care should therefore be taken when determining the orientation of muscle forces. If the lines of action of the masticatory muscles cannot be determined based on the muscles of the same individual, they could be estimated based on the morphology of the cranium of the same individual or at least of a cranium with comparable size and morphology. The inclusion of simplified TMJ models with blocks of temporal bone and soft tissue also has a major effect on the results. It is one way to avoid over-constraining FE mandible models at the joints. As the condyles are thus less constrained, wish boning of the mandible is possible, which is the main deformation type of the mandible during mastication in non-human primates. The inclusion of simplified TMJ models with soft tissue, which is common in FE models of human mandibles, appears to be a good way of allowing some displacement of the condyles without over-constraining the model.
The purpose of this study was to develop a FE analysis of the temporomandibular joint (TMJ) and a musculoskeletal System of forces considering a physiologic model able to represent the effect of all the components, bones and muscles of the TMJ. The obtained stress distribution evidenced a maximum value of 8.73 MPa in the mandibular condyle region. The masseter muscle effect is significant and the stress in its area reached 4.36 MPa. Displacements and Equivalent elastic strain reached maximum values of 0.42 mm and 4.62 E−004 μmm/mm respectively.
5. Conclusions
Future studies could investigate the musculoskeletal schematization by comparing experimental results in order to develop more realistic TMJ models. The present study contains some limitations as uniform muscle forces have been considered, the viscoelasticity of bone was not taken into account, more components (bones, soft tissues, muscles, etc.) should be considered. Moreover, errors or biased results due to the location of sampling points could arise. In conclusion by coupling experimental and numerical analyses it is really possible to develop very interesting techniques of investigation.
References
- 1.Buescher J.J. Temporomandibular joint disorders. Am Fam Physician. 2007;76:1477–1482. [PubMed] [Google Scholar]
- 2.Kalpakci K.N., Willard V.P., Wong M.E., Athanasiou K.A. An interspecies comparison of the temporomandibular joint disc. J Dent Res. 2010;90:193–198. doi: 10.1177/0022034510381501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durham J., Steele J.G., Wassell R.W., Exley C. Living with uncertainty: temporomandibular disorders. J Dent Res. 2010;89:827–830. doi: 10.1177/0022034510368648. [DOI] [PubMed] [Google Scholar]
- 4.Gray R.J., Al-Ani M.Z. Wiley-Blackwell; Ames, IA, USA: 2011. Temporomandibular Disorders: A Problem-Based Approach. [Google Scholar]
- 5.Alomar X., Medrano J., Cabratosa J. Anatomy of the temporomandibular joint. Semin Ultrasound CT. 2007;MR28:170–183. doi: 10.1053/j.sult.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Yale S.H., Allison B.D., Hauptfuehrer J.D. An epidemiological assessment of mandibular condyle morphology. Oral Surg Oral Med Oral Pathol. 1996;21:169–177. doi: 10.1016/0030-4220(66)90238-6. [DOI] [PubMed] [Google Scholar]
- 7.Karlo C.A., Stolzmann P., Habernig S., Müller L., Saurenmann T., Kellenberger C.J. Size, shape and age-related changes of the mandibular condyle during childhood. Eur Radiol. 2010;20:2512–2517. doi: 10.1007/s00330-010-1828-1. [DOI] [PubMed] [Google Scholar]
- 8.Kaneyama K., Segami N., Hatta T. Congenital deformities and developmental abnormalities of the mandibular condyle in the temporomandibular joint. Congenital Anom. 2008;48:118–125. doi: 10.1111/j.1741-4520.2008.00191.x. [DOI] [PubMed] [Google Scholar]
- 9.Kuroda S., Tanimoto K., Izawa T., Fujihara S., Koolstra J.H., Tanaka E. Biomechanical and biochemical characteristics of the mandibular condylar cartilage. Osteoarthr Cartil. 2009;17:1408–1415. doi: 10.1016/j.joca.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 10.Flygare L., Rohlin M., Akerman S. Microscopy and tomography of erosive changes in the temporomandibular joint. An autopsy study. Acta Odontol Scand. 1995;53:297–303. doi: 10.3109/00016359509005991. [DOI] [PubMed] [Google Scholar]
- 11.Nordahl S., Alstergren P., Appelgren A., Appelgren B., Eliasson S., Kopp S. Pain, tenderness, mandibular mobility, and anterior open bite in relation to radiographic erosions in temporomandibular joint disease. Acta Odontol Scand. 1997;55:18–22. doi: 10.3109/00016359709091935. [DOI] [PubMed] [Google Scholar]
- 12.Tasaki M.M., Westesson P.L. Temporomandibular joint: diagnostic accuracy with sagittal and coronal MR imaging. Radiology. 1993;186:723–729. doi: 10.1148/radiology.186.3.8430181. [DOI] [PubMed] [Google Scholar]
- 13.Brooks S.L., Westesson P.L. Temporomandibular joint: value of coronal MR images. Radiology. 1993;188:317–321. doi: 10.1148/radiology.188.2.8327672. [DOI] [PubMed] [Google Scholar]
- 14.Chossegros C., Cheynet F., Guyot L., Bellot-Samson V., Blanc J.L. Posterior disk displacement of the TMJ: MRI evidence in two cases. Cranio19. 2001;289–93 doi: 10.1080/08869634.2001.11746180. [DOI] [PubMed] [Google Scholar]
- 15.Kondoh T., Westesson P.L., Takahashi T., Seto K. Prevalence of morphological changes in the surfaces of the temporomandibular joint disc associated with internal derangement. J Oral Maxillofac Surg. 1998;56:339–343. doi: 10.1016/s0278-2391(98)90111-2. [DOI] [PubMed] [Google Scholar]
- 16.Bravetti P., Membre H., El Haddioui A. Histological study of the human temporo-mandibular joint and its surrounding muscles. Surg Radiol Anat. 2004;26:371–378. doi: 10.1007/s00276-004-0248-9. [DOI] [PubMed] [Google Scholar]
- 17.Kraus SL. Physical therapy management of TMJ dysfunction In: Kraus SL, editors. TMJ Disorders: Management of the Craniomandibular Complex New York, NY, USA: Churchill Livingstone; p. 139–741988.
- 18.Dolwick M.F., Lipton J.S., Warner M.R., Williams V.F. Sagittal anatomy of the human temporomandibular joint spaces: normal and abnormal findings. J Oral Maxillofac Surg. 1983;41:86–88. doi: 10.1016/0278-2391(83)90213-6. [DOI] [PubMed] [Google Scholar]
- 19.Boering G. Anatomical and physiological considerations regarding the temporomandibular joint. Int Dent J. 1979;29:245–251. [PubMed] [Google Scholar]
- 20.Filardi V. Carotid artery stenosis near a bifurcation investigated by fluid dynamic analyses. Neuroradiology Journal. 2013;26(4):439–453. doi: 10.1177/197140091302600409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filardi V. FE analysis of stress and displacements occurring in the bony chain of leg. J Orthop. 2014;11(4):157–165. doi: 10.1016/j.jor.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filardi V., Simona P., Cacciola G.…Milardi D., Alessia B. Finite element analysis of sagittal balance in different morphotype: forces and resulting strain in pelvis and spine. J Orthop. 2017;14(2):268–275. doi: 10.1016/j.jor.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filardi V., Milardi D. Experimental strain analysis on the entire bony leg compared with FE analysis. J Orthop. 2017;14(1):115–122. doi: 10.1016/j.jor.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filardi V. Stress shielding in the bony chain of leg in presence of varus or valgus knee. J Orthop. 2015;12(2):102–110. doi: 10.1016/j.jor.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filardi V. Characterization of an innovative intramedullary nail for diaphyseal fractures of long bones. Med Eng Phys. 2017;49(1):94–102. doi: 10.1016/j.medengphy.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Filardi V. Healing of femoral fractures by the meaning of an innovative intramedullary nail. J Orthop. 2018;15(1):73–77. doi: 10.1016/j.jor.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filardi V., Montanini R. Measurement of local strains induced into the femur by trochanteric Gamma nail implants with one or two distal screws. Med Eng Phys. 2007;29(1):38–47. doi: 10.1016/j.medengphy.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Montanini R., Filardi V. In vitro biomechanical evaluation of antegrade femoral nailing at early and late postoperative stages. Med Eng Phys. 2010;32(8):889–897. doi: 10.1016/j.medengphy.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Filardi V. The healing stages of an intramedullary implanted tibia: a stress strain comparative analysis of the calcification process. J Orthop. 2015;12:51–61. doi: 10.1016/j.jor.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Filardi V. Numerical comparison of two different tibial nails: expert tibial nail and innovative nail. Int J Interact Des Manuf. 2018;12(4):1435–1445. [Google Scholar]
- 31.Filardi V. Flatfoot and normal foot a comparative analysis of the stress shielding. J Orthop. 2018;15(3):820–825. doi: 10.1016/j.jor.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filardi V. Finite element analysis of the foot: stress and displacement shielding. J Orthop. 2018;15(4):974–979. doi: 10.1016/j.jor.2018.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weijs A., Hillen B. Relationship between the physiological cross-section of the human jaw muscles and their cross-sectional area in computer tomograms. Acta Anat. 1984;118:129–138. doi: 10.1159/000145832. [DOI] [PubMed] [Google Scholar]