Abstract
Background
Stereotactic body radiotherapy (SBRT) delivers high-dose radiation to tumor tissues in few fractions, thereby reducing radiation damage to at-risk organs. There are more potential effects of SBRT owing to the higher biological equivalent dose delivered. Herein, we retrospectively analyzed its effectiveness and toxicity at our institution.
Methods
Data from patients with hepatocellular carcinoma (HCC; n = 10) and liver metastases (n = 10) who underwent SBRT (total dose of 30–50 Gy in 5–10 fractions) between 2013 and 2016 were analyzed. Adverse events were recorded at the end of RT, 6 months after treatment, or upon death. Overall survival (OS) was calculated according to the biological effective dose (BED α/β = 10) and liver function (Child–Pugh [CP] classification 5 or 6 vs. 7 or 8) after SBRT, using Kaplan–Meier analyses.
Results
Of the 20 patients, 6 declined the CP classification score after SBRT; grade 3 adverse events were not seen in any patient. A higher OS rate was seen in patients receiving a higher BED and in those with better CP classification after SBRT. Kaplan–Meier survival analysis yielded a median OS of 401 days and 1- and 2-year OS of 45% and 15%, respectively.
Conclusion
The higher BED was significantly associated with tumor control, and there were no differences in the tumor control rate between HCC and metastatic tumors. Changes in CP scores after SBRT also affected the survival rate. Good liver function may permit multiple rounds of SBRT.
Keywords: biological effective dose, hepatocellular carcinoma, liver metastasis, radiotherapy, tumor control
Abbreviations: BED, biological effective dose; CP, Child–Pugh; HCC, hepatocellular carcinoma; OS, overall survival; SBRT, stereotactic body radiotherapy
Liver tumors, including hepatocellular carcinoma (HCC) and metastatic tumor, are relatively common in Asia and have various treatment methods. Stereotactic body radiotherapy (SBRT) for liver tumors is an alternative strategy to standard treatment modalities such as surgical resection and radiofrequency ablation (RFA).1, 2, 3, 4 Transarterial chemoembolization (TACE) is commonly used to treat HCC; however, it has difficulty reaching larger tumors (more than 3–5 cm) with complete ischemia or necrosis. Most hepatic cancers are supplied by two blood vessels, the hepatic artery and the portal vein. Therefore, although the supplying artery is completely embolized using TACE, the portal vein continues to supply blood to the residual tumor.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 Thus, blood supply to metastatic liver tumors is diverse. TACE may occasionally fail because the hepatic artery does not supply blood flow to the tumor.18, 19, 20, 21, 22 SBRT includes the use of a small number of significantly large fraction sizes of radiation dose targeted at a specific area (tumor tissue) while reducing exposure to adjacent normal organs.23 Compared with the three-dimensional RT, SBRT has a high-dose concentration and carries lower risk of radiation-induced liver disease. Therefore, we retrospectively analyzed the effectiveness of SBRT and the adverse events after SBRT for liver tumors.
METHODS
Patients
From 2013 to 2016, 46 patients underwent SBRT at our hospital, and 20 patients who could be followed up until their death were retrospectively enrolled. This study was approved by the internal review board and was registered at the National Clinical Trial Register with the number UMIN000034389. We retrieved data from the electric medical records of all patients from the beginning to death. Important data are shown in Table 1. Hematologic data were retrieved for all patients, but there was no association with liver function; some patients underwent indocyanine green (ICG) test and liver reserve scintigraphy, but their data were small. Therefore, Child–Pugh (CP) classification was introduced because it has been widely used in the world. The scores of patients B7, B8, and B9 were 2, 2, and 0 before the treatment, respectively; after RT, the scores of patients B7, B8, and B9 were 4, 2, and 0, respectively. None of the patients scored more than 1 point, and none of the patients had a CP reduction score of 2. Liver volumetry was performed in all the patients and compared between the HCC and metastatic lesion groups.
Table 1.
Univariate Analysis of Overall Survival.
| Factor | Median | SD | P value |
|---|---|---|---|
| Gender: male/female | 2/18 | 0.3 | 0.33 |
| Alpha-fetoprotein | 563.4 | 102.6 | 0.41 |
| PIVKA2 | 87.3 | 24.6 | 0.56 |
| Age | 73 | 11.2 | 0.21 |
| Dose (Gy) | 40 | 9.8 | 0.09 |
| No. of fraction | 7 | 2.9 | 0.03 |
| Biological effective dose | 160 | 38.6 | 0.04 |
| Tumor size (cm) | 49 | 53.4 | 0.70 |
| Pathology: HCC/others | 14/6 | 0.9 | 0.10 |
| Place: central*/distal | 12/8 | 1.1 | 0.37 |
| Performance status: 0–1/2 | 16/4 | 0.7 | 0.87 |
| No. of treatments | 2 | 1.9 | 0.08 |
| Surgery: yes/no | 10/10 | 0.5 | 0.19 |
| Child–Pugh classification (score) before treatment | 5.80* | 1.1 | 0.36 |
| Child–Pugh classification (score) after treatment | 6.40* | 1.3 | 0.03 |
HCC, hepatocellular carcinoma; SD; standard deviation.
PIVKA2; protein induced by vitamin K absence or antagonists-II.
*Mean.
*Central was defined as a tumor that presented at a distance within 2 cm from the porta hepatis.
Bold indicates statistically significant difference (P<0.05).
We cross-checked the retrieved data, and Excel software (Microsoft Office 2013, USA) was used to store the data. We opted the retrospective analysis on the home page of our hospital.
Inclusion and Exclusion Criteria
The inclusion criteria were patients with liver tumor, who were unwilling for other therapies (i.e., surgery, RFA, and TACE) or in whom the place of the tumor was difficult to be treated by such aforementioned therapy. Our series included 10 patients with HCC and 10 patients with liver metastasis. The primary lesion of liver metastases was 8 rectal cancers and 2 stomach cancers, and the primary site was under control. The values of CP classification of metastatic liver tumor were 5 and 6 for 8 and 2 patients, respectively (metastatic liver cancer is not classified as a CP classification) The 2 patients with metastatic liver with a CP score of 6 had only mild albumin levels. The exclusion criteria were if the CP score of patients was 9 or more and the tumor was near the porta hepatis. Patients with extrahepatic metastasis were excluded.
Important data are shown in Table 1. Hematologic data were also retrieved from all patients (hemoglobin, aspartate transaminase (AST), alanine transaminase (ALT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (γ-GTP), bilirubin, protein, albumin, alpha-fetoprotein, PIVKA2 levels, and white blood cell and platelet counts). Some patients underwent ICG test before SBRT. The CP classification was introduced as it has been widely used throughout the world. The patients were classified based on CP scores; CP scores were 5, 6, 7, and 8 for 10, 6, 2, and 2 patients, respectively (10 patients were at the score of 5).
The patients with liver tumor were diagnosed as HCC or metastatic tumor by pathology or medical imaging. Recurrent and residual tumors were defined as the existence of the tumor on contrast-enhanced computed tomograhphy/magnetic resonance imaging (CT/MRI) after RFA and/or TACE. In the surveillance for HCC in patients with chronic liver disease or cirrhosis, ultrasonography and tumor marker tests play a central role and are presently widely performed. We obtained dynamic triple-phase CT and gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (EOB-DTPA) MRI in all cases. Ultrasound examinations of patients with HCC were taken every 3 months as part of the screening. The patients after radical surgery were followed up every 3 months by conducting blood examination; CT was performed if the tumor marker was elevated. In general, RT was not performed when the extrahepatic lesion was noticed. We treated one lesion at a time (local recurrence and previously treated lesions were included as criteria). The tumor size was less than 5 cm in diameter.
Radiotherapy Planning
The fiducial marker placement was used in 13 of 20 patients when lipiodol or calcification was not recognized on onboard imaging. Cone beam CT was used throughout. Radiotherapy was planned using breath-hold gating systems: Elekta Synergy® with a micro-multileaf collimator (Elekta AB Box 7593 SE-103 93, Stockholm, Sweden).
The gross target volume (GTV) was defined as tumor visibility on CT and MRI scans using fusion methods. The GTV was expanded by approximately 5 mm to form the clinical target volume (CTV). Next, the CTV was expanded by 3–5 mm to form the planning target volume (PTV). The mean value of the major diameter of the target was 49.1 cm (range: 10–77.6 cm). All patients were treated with a total dose of 30–50 Gy in 5–10 fractions. The prescribed dose corresponded to the isodose line that encompassed >95% of the PTV. The organs at risk were as follows: esophagus, spinal cord, the remaining healthy liver, stomach, intestine, kidneys, lung, and heart. For the intestine, the maximum point dose was <20 Gy. The mean exposure dose for the entire liver was <20 Gy and that of the bilateral kidneys was <15 Gy.2
Adverse event occurrence was recorded at the end of RT and at 6 months after treatment, according to the recommendations of the Common Terminology Criteria for Adverse Events version 4.0. The change in CP classification was evaluated as the difference between CP scores at SBRT initiation and 6 months after SBRT. Liver function in metastatic tumor was also scored according to the CP classification. If death occurred within 6 months of SBRT, it was scored as such immediately. Survival rates were calculated from the date of SBRT initiation. Kaplan–Meier survival analysis was used to estimate overall survival (OS). All statistical analyses used Excel Bell curve (SSRI corp., Tokyo, Japan), and a P value < 0.05 was considered statistically significant.
RESULTS
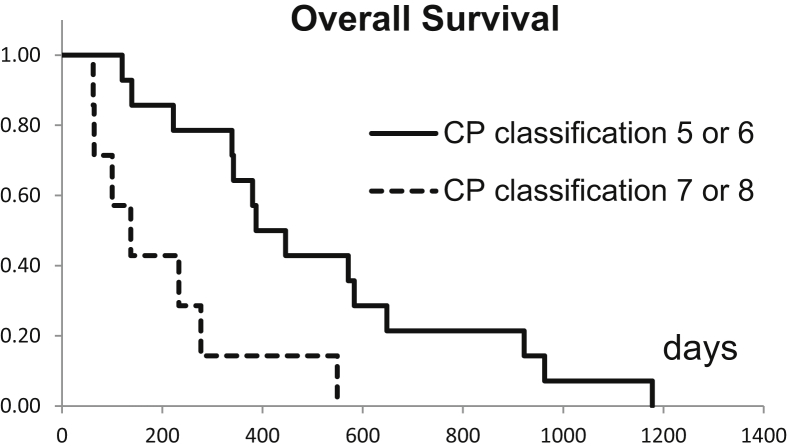
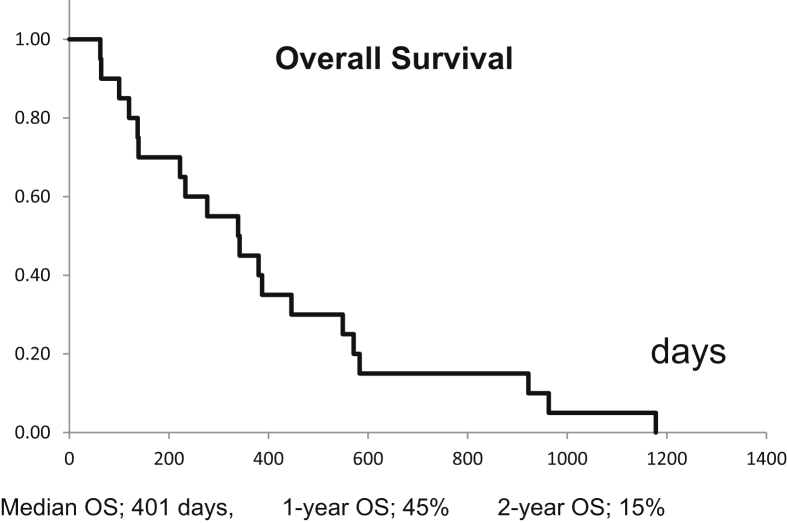
Table 1 shows the results of the univariate analysis. The size of the liver of patients with HCC was 1350 (range: 1160–1560) cm3 on an average, and the size of the metastasized liver was 1140 (range: 1010–1340) cm3. No significant difference for OS was observed. The number of lesions was one for each treatment. A higher survival rate was associated with a smaller number of radiation fractions, a higher biological effective dose (BED), and a lower score in CP classification after SBRT. The OS rate was significantly higher in patients with good liver function after SBRT (Figure 1) than in those with poor liver function. No factor was found to significantly affect the OS rate during univariate analysis. Hematologic data were not statistically associated with liver function (univariate analysis). After the treatment, CP scores were 5, 6, 7, and 8 for 8, 6, 4, and 2 patients, respectively. Grade 3 adverse events did not occur in any patient. Kaplan–Meier survival analysis yielded a median OS of 401 days with 1- and 2-year OS rates of 45% and 15%, respectively (Figure 2).
Figure 1.
Overall survival comparison of the Child–Pugh (CP) classification score: 5 or 6 and 7 or 8. Kaplan–Meier survival analysis.
Figure 2.
Overall survival of all patients. The median OS was 401 days; 1-year and 2-year OS was 45% and 15%, respectively.
DISCUSSION
Many research studies on the results and side effects of SBRT for liver tumors have been published. The curative treatment options for HCC include surgical resection, liver transplantation, and RFA. Surgery cannot be performed without adequate liver function. Although RFA is highly safe, it is impossible in the vicinity of the diaphragm and caudate lobe. Therefore, SBRT is the choice of a noninvasive local treatment method for HCC, wherein an established treatment therapy cannot be applied.
In a phase II study of 50 inoperable patients previously treated with TACE 1–5 times, 38.3% of patients receiving SBRT achieved a partial response or Complete response (CR) at 6 months. The 2-year control rate, OS, and Progress free survival (PFS) were 94.6%, 68.7%, and 33.8%, respectively.19 Bujold et al. have reported that of 102 patients with HCC unsuitable for local therapy, 61% had multiple lesions, 55% had tumor vessel thrombosis, and 12% had extrahepatic tumor. All patients with a local control rate of 87% at 1 year and a median OS of 17.0 months were treated with SBRT.21 Scorsetti et al.17 in their study on HCC, have reported that the BED>100 Gy and GTV size were significant prognostic factors for local control (LC) in univariate analysis (P < 0.01 and P < 0.02). Univariate analysis showed that OS is correlated with LC (P < 0.04), BED>100 Gy (P < 0.05), and cumulative GTV<5 cm (P < 0.04). The number of fraction is correlated with BED. The BED increases when we increase dosages per fraction; many institutes use the dosage of 40–60 Gy per 3–5 fractions.
Many cancers often metastasize to the liver at the onset of systemic disease.18, 19, 20, 21, 22 Thus, SBRT is the current treatment of choice in patients with good clinical conditions. Zhang et al. have reported SBRT for liver metastasis and have proved it to be effective and safe with LC rates ranging from 70% to 100% within 1 or 2 years and 2-year OS rates ranging from 30% to 38%. Onal et al.20 have reported that in 22 patients with 29 breast cancer liver metastatic (BCLM) lesions treated with liver SBRT, the 1- and 2-year LC rates were 100% and 88%, respectively. They concluded that SBRT is feasible for patients with BCLM lesions. Liver SBRT is a conservative approach with excellent LC and limited toxicities.20 Joo et al.22 have reported that in 70 patients with 103 lesions from colorectal cancer, the prescribed dose was 45–60 Gy in 3–4 fractions and the 2-year OS was 75%. In subgroups, the 2-year LC rates for BED ≤80 Gy (group 1), 100–112 Gy (group 2), and ≥132 Gy (group 3) were 52%, 83%, and 89%, respectively.
In our series, OS was not different significantly between patients with HCC and liver metastasis (LM). CP classification makes quantitative judgment difficult because of the subjective items such as ascites and encephalopathy. The ICG test is used for quantitative assessment of liver reserve but is an assessment of the entire liver. This time, evaluation for liver metastases was performed using the CP classification. However, liver cirrhosis is not often seen in liver metastasis, and the residual liver reserve after local treatment is expected to be high compared with that of HCC. Three-dimensional evaluation of liver function before and after SBRT can be performed using 99m-Technetium Galactosyl Human Serum Albumin Scanning single-photon emission computed tomography.
Post-RT CP classification scores were associated with OS, with lower scores leading to longer survival rates. Conversely, in the current analysis, we only included patients with CP scores of 5–8. In liver SBRT, a CP classification score of 5 or 6 is considered desirable.3 However, RT may be necessary when the tumor invades the portal veins of the tumor or when liver function is poor. We have used a radiation dose of 30 Gy 10 times in three patients with an initial CP score of 8. In one such patient, the CP score remained 8 after treatment, whereas in two cases, it worsened to 9, and the OS was 62 days (HCC), 100 days (HCC), and 549 days (metastatic liver tumor).
If the liver tumor is metastatic and the primary site (this case was colon cancer) tumor is under control, SBRT might be a better option than liver resection.18, 19, 20, 21, 22
Tumor locations of the liver amenable to SBRT are limited; for instance, RFA is not suitable when the tumor exists under the diaphragm. In the liver, when the tumor is marginal, RT is easy but needs to be balanced with organ at risk (OAR) when the tumor is located at the center of the liver.6 Takeda et al. reported that centrally located tumors have a lower control rate.
Single liver metastasis is curable by surgery. Therefore, SBRT shows similar results to surgery. However, SBRT is chosen when the liver could not be operated. Therefore, this time, our results are not better than surgery. However, depending on the case, the outcome may yield similar results to that of surgery. Thus, it is necessary to recognize SBRT and RFA. Yeung et al. reported in their series of 34 patients with HCC that 1-year LC and OS were 94% and 84%, respectively. However, 6 patients (19%) had worsened the CP score by 2 or more points during follow-up; overall, 32% of patients experienced ≥ grade 3 + toxicities. Strong treatment also has numerous side effects.24
Compared with their analysis, our results were not good. The reasons were as follows: (1) our target size included 5 cm in diameter, (2) the median number of treatments was two, and (3) we were targeting only patients who had seen it until the end. Because the family doctor keeps track of the patients who were cured, we noticed many cases with poor conditions.
BED is significantly associated with tumor control, and no significant differences in tumor control rates between HCC and metastatic tumors were noted. The CP classification score after RT affects the survival rate. When liver function is good before treatment, multiple rounds of SBRT are possible.
Conflicts of interest
The authors have none to declare.
Footnotes
National clinical study registered number UMIN000034389 (UMIN-CTR, Japan).
References
- 1.Sapisochin G., Barry A., Doherty M. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol. 2017;67:92–99. doi: 10.1016/j.jhep.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Bettinger D., Gkika E., Schultheiss M. Comparison of local tumor control in patients with HCC treated with SBRT or TACE: a propensity score analysis. BMC Canc. 2018;18:807. doi: 10.1186/s12885-018-4696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeda A., Sanuki N., Tsurugai Y. Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer. 2016;122:2041–2049. doi: 10.1002/cncr.30008. [DOI] [PubMed] [Google Scholar]
- 4.Kang J.K., Kim M.S., Cho C.K. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. 2012;118:5424–5431. doi: 10.1002/cncr.27533. [DOI] [PubMed] [Google Scholar]
- 5.Toesca D.A.S., Osmundson E.C., von Eyben R., Shaffer J.L., Koong A.C., Chang D.T. Assessment of hepatic function decline after stereotactic body radiation therapy for primary liver cancer. Pract Radiat Oncol. 2017;7:173–182. doi: 10.1016/j.prro.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Weiner A.A., Olsen J., Ma D. Stereotactic body radiotherapy for primary hepatic malignancies - report of a phase I/II institutional study. Radiother Oncol. 2016;121:79–85. doi: 10.1016/j.radonc.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray L.J., Dawson L.A. Advances in stereotactic body radiation therapy for hepatocellular carcinoma. Semin Radiat Oncol. 2017;27:247–255. doi: 10.1016/j.semradonc.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Lazarev S., Hardy-Abeloos C., Factor O., Rosenzweig K., Buckstein M. Stereotactic body radiation therapy for centrally located hepatocellular carcinoma: outcomes and toxicities. J Cancer Res Clin Oncol. 2018;144:2077–2083. doi: 10.1007/s00432-018-2729-y. [DOI] [PubMed] [Google Scholar]
- 9.McPartlin A.J., Dawson L.A. Stereotactic body radiotherapy for hepatocellular carcinoma. Cancer J. 2016;22:296–301. doi: 10.1097/PPO.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 10.Caivano D., Valeriani M., Russo I. Stereotactic body radiation therapy in primary and metastatic liver disease. Anticancer Res. 2017;37:7005–7010. doi: 10.21873/anticanres.12169. [DOI] [PubMed] [Google Scholar]
- 11.Gerum S., Heinz C., Belka C. Stereotactic body radiation therapy (SBRT) in patients with hepatocellular carcinoma and oligometastatic liver disease. Radiat Oncol. 2018;13:100. doi: 10.1186/s13014-018-1048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bibault J.E., Dewas S., Claire V.D. Stereotactic body radiation therapy for hepatocellular carcinoma: prognostic factors of local control, overall survival, and toxicity. PLoS One. 2013;8:e77472. doi: 10.1371/journal.pone.0077472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee K., Yu J., Park H.C. Is higher dose always the right answer in stereotactic body radiation therapy for small hepatocellular carcinoma? Radiat Oncol J. 2018;36:129–138. doi: 10.3857/roj.2017.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nair V., Pantarotto J. Treatment of metastatic liver tumors using stereotactic ablative radiotherapy. World J Radiol. 2014;6:18–25. doi: 10.4329/wjr.v6.i2.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon J.H., Bae S.H., Kim J.Y. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection: stereotactic radiotherapy for liver cancer. BMC Canc. 2010;10:475. doi: 10.1186/1471-2407-10-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bujold A., Massey C.A., Kim J.J. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631–1639. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]
- 17.Scorsetti M., Comito T., Cozzi L. The challenge of inoperable hepatocellular carcinoma (HCC): results of a single-institutional experience on stereotactic body radiation therapy (SBRT) J Cancer Res Clin Oncol. 2015;141:1301–1309. doi: 10.1007/s00432-015-1929-y. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S.Y., Zhu G.Y., Li G., Zhang Y.B., Geng J.H. Application of stereotactic body radiation therapy to cancer liver metastasis. Cancer Lett. 2016;379:225–229. doi: 10.1016/j.canlet.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 19.Aitken K.L., Hawkins M.A. Stereotactic body radiotherapy for liver metastases. Clin Oncol. 2015;27:307–315. doi: 10.1016/j.clon.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 20.Onal C., Guler O.C., Yildirim B.A. Treatment outcomes of breast cancer liver metastasis treated with stereotactic body radiotherapy. Breast. 2018;42:150–156. doi: 10.1016/j.breast.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Goodman K.A., Kavanagh B.D. Stereotactic body radiotherapy for liver metastases. Semin Radiat Oncol. 2017;27:240–246. doi: 10.1016/j.semradonc.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Joo J.H., Park J.H., Kim J.C. Local control outcomes using stereotactic body radiation therapy for liver metastases from colorectal cancer. Int J Radiat Oncol Biol Phys. 2017;99:876–883. doi: 10.1016/j.ijrobp.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 23.Bentzen S., Constine L.S., Deasy J.O. Quantitative analyses of normal tissue effects in the clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys. 2010;76 doi: 10.1016/j.ijrobp.2009.09.040. S3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeung R., Beaton L., Rackley T. Stereotactic body radiotherapy for small unresectable hepatocellular carcinomas. Clin Oncol. June 2019;31:365–373. doi: 10.1016/j.clon.2019.01.012. [DOI] [PubMed] [Google Scholar]