Highlights
-
•
Ozone (O3) exposure caused an oxidative stress state in the olfactory bulbs (OB) OB of Swiss Webster mice.
-
•
Similar However, this effect was not observed in C57BL/6J mouse strain.
-
•
The body weight of the all O3 exposed mice was decreased.
-
•
The Cytochrome P450, family 1, subfamily A, polypeptide 1 (CYP1A1) is absent in the C57BL/6J Green fluorescent protein (GFP) O3 exposed mice.
Keywords: 4HNE, CYP1A1, Olfactory bulb, Ozone
Abstract
Environmental ozone (O3) exposure has adverse effects on different body systems. This experimental work aimed to study the effect(s) of O3 exposure on the olfactory bulbs (OB) of Swiss Webster and C57BL/6J mouse strains, using Western blot technique. Both mice strains were exposed to different O3 doses for different number of exposures and durations. The results indicated that O3 exposure caused a significant increase in the level of the proteins involved in the oxidative stress state such as 4-hydroxynonenal (4HNE) and Cytochrome P450, family 1, subfamily A, polypeptide 1 (CYP1A1), in addition to the total OB proteins in Swiss Webster mouse strain. However, this effect was not observed in C57BL/6J mouse strain. Furthermore, CYP1A1 was completely absent in the Green fluorescent protein (GFP) C57BL/6J O3 exposed mice. Moreover, O3 exposure caused a significant decrease in the body weight of the tested mice from the two strains.
1. Introduction
Air pollution is the presence of substances in the atmosphere in certain amount for a particular duration of time, which could be harmful to human life or could lead to particular changes in the weather and climate [1].
The olfactory system receptors are exposed directly to air pollutants making them more susceptive to the harmful effect of toxic substances causing damage to their neural tissue [2]. Furthermore, reviewed articles by Ajmani et al. [3] from 1950 to 2015 focused on human epidemiologic and pathophysiologic studies with some experimental studies on animals; showed a relationship between environmental air pollutant exposure and olfactory function. Moreover, these studies have explained how air pollutants enter the OB system.
The effects of oxidative stress on the olfactory bulbs have taken many researchers attention as a primary site for coding the odourous information. Therefore, any alteration in their components will lead to alteration in the stimulation process; resulting in changing of the olfactory and behavioural responses. Moreover, olfactory bulb is the main organ involved in memory-mindedness and perception of odours [4,5]. In addition, in animal models odour perception has an adaptive value for animal’s survival. Furthermore, some authors found that the plasticity of the nervous system allows individuals to adapt themselves to any changes in the surrounding environment through memory and learning processes [6,7].
The increase of 4-hydroxynonenal (4HNE) level is considered as a biological marker for cellular toxicity due to the toxic effect of environmental O3 exposure [8].
In addition, the role of CYPs genes has been reported as an indicator for tissue damage in mouse skin following O3 exposure. Therefore, in this study, 4HNE and CYP1A1were chosen as indicators for the oxidative stress state which is produced due to O3 exposure [9]. This experimental work aims to study the effect(s) of acute and chronic environmental ozone exposure on mice olfactory bulb from biochemical points of view by assessing the expression level of oxidative stress biomarkers (CYP1A1 and 4HNE) using immune Western blot technique and by measuring the level of plasma proteins. Also, this study aimed to observe the effect of O3 exposure on the body weight of the tested mice.
2. Materials and methods
2.1. Ethical statement
Care and use of animals was conducted according to guidelines established by European Council Directives (609/1986 and 63/2010) and Italian laws (DL 116/92 and D. Lgsl. 26/2014) for the protection of animals used for scientific purposes. The experimental protocols were approved by the Committee for Animal Welfare of the University of Ferrara (OBA), by the Directorate-General for Animal Health of the Ministry of Health, and supervised by the Campus Veterinarian of Ferrara University.
2.2. Experimental animals
In this experimental study, two strains of transgenic mice line expressing the enhanced green florescent protein (e-GFP) protein were used. The first one was Swiss Webster which expresses the e-GFP protein under Calretinin (CR) promoter [10], while the second one wasC57BL/6 strain which expresses the GFP protein under the control of promoter tyrosine hydroxylase (TH) [11].
A total of 49 mice were used. Of whom; 27 were belonged to C57BL/6 J mouse strain and 22 were Swiss Webster mice. Of the 27 C57BL/6 J mic; 17 were the O3 exposed group (ten WT and seven GFP), and 10 were the control group (not exposed to O3). Moreover, of the 22 Swiss Webster mice; 15 were the O3 exposed group while seven were the control group. Furthermore, the O3 exposed groups of the two strains were further subdivided according to the exposure protocol.
The allocation of mice to O3 exposed and control groups was performed randomly. The age of all of the mice was matched and it ranged between two to three months.
2.3. Experimental procedure
According to the study design, the body weight of all mice was measure (O3 and non-O3 exposed group). Then, the exposed group was further subdivided according to the exposure protocols.
Regarding acute O3 exposure protocol, both strains were exposed to 0.5 ppm O3 dose once for either 30 min or 1 h (h). While for chronic exposure protocol, Swiss Webster mice were exposed to 0.2 ppm O3 dose for 2 h, two times for one day or 0.2 ppm O3 dose for 2 h, two times for two days. On the other hand, C57BL/6 J mice were exposed to 0.8 ppm O3 dose for 2 h, two times for successive five days.
Concerning chronic O3 exposure protocol, first the tested mice body weight was measured. Then, the exposed mice were kept in a small cage 32 cm length, 13 cm width and 14 height, then were placed in a closed noiseless chamber with a diffuser connected to a variable-flux ozone generator. Ozone was produced from high-voltage current circulated in a tube; this tube contains aluminum chips and two electrodes that allow the conversion of oxygen into ozone. The O3 concentration in chamber was adjusted according to the selected protocol and was continuously monitored by the O3 sensor.
The first exposure started from 08:30 to 10:30 am. Following the first exposure, the body weight of the tested mice was measured, and the mice were retained to their original cage in the animal facility containing food and water, for two hours. At the end of the two hours, the body weight of the tested mice was measured again. Then, they were exposed to O3 for another two hours. When the time was finished, the tested mice body weight was measured and the mice were retained to their home cage which contains food and water in the animal facility for the second day. Then, the same steps were repeated according to the days of the O3 exposure protocol. In case of exposing C57BL/6 J mice, the steps were repeated for successive four days followed by single exposure on the fifth day. Whereas for acute O3 exposure, the body weight of the O3 exposed mice was measured before and after the exposure.
2.4. Samples
Following O3 exposure, all of the tested mice were sacrificed and approximately 1 ml of blood was collected in 1.5 ml Eppendorf tube (Thermo fisher, Italy), containing 100 μl Na+ citrate (Thermo fisher, Italy), centrifuged by LSE™ Compact Centrifuge (Thomas scientific, united states) at 2500 g at 4 °C for 15 min. Then the plasma was collected in new Eppendorf tube and stored at −20 °C. In addition, olfactory bulbs were collected and stored at −80 °C or freezed directly in liquid nitrogen (Thermo scientific, Italy) at −190 °C for homogenization. Then, 150 μl of lysis buffer (Thermo fisher, Italy), was added to the homogenized olfactory bulb samples, centrifuged at 3500 g at 4 °C for 15 min. Moreover, the supernatant liquid was collected in new Eppendorf tube and stored at −20 °C.
2.5. Bradford protein assay
It was used to measure the protein concentrations in plasma and homogenized olfactory bulb samples.
Briefly, each tested sample was diluted to appropriate dilution. Then, from each diluted sample a volume of 15 μl was taken and was placed in three wells on microtiter plate (Bio Rad) (5 μl for each single well). Also, 5 μl from each protein standard in a concentration of (0, 0.4, 0.6, 0.8, 1, 1.2,2, and 4 μg) was placed in separate well of microtiter plate. After that, 200 μl of 1X Coomassie Brilliant Blue (Biorad, Italy), was added to the diluted sample and protein standard wells. Following that step, the microtiter plate was incubated for 15 min at room temperature in the laboratory shaker, (Benchmark, Brasov, Romania) at 90 RPM speed.
Moreover, by using the spectrophotometer (Thermo Scientific, U.S) the absorbance of the known standard protein samples concentration and of the olfactory bulb and plasma samples (unknown protein concentration) was read at 595 nm.
Then, the standard curve was created by plotting the absorbance of Bovine Serum Albumin (BSA) (Biorad, Italy) standard values (y-axis) versus their concentration in μg/μl (x-axis).
For knowing the concentration of unknown total proteins in each sample (OB and plasma) using the created standard curve of BSA, the following steps were followed: first the mean of each three absorbance values of each single sample was calculated, after that from the curve formula the diluted concentration values were obtained then were multiplied by the appropriated dilution factor for each single sample (OB and plasma).
2.6. Western Blot (WB) technique
By using this technique, 4HNE in plasma and/or OB samples, and CYP1A1 in OB samples were measured in O3 and non-O3 exposed mice.
The total amount of proteins from OB and plasma was determined as 30 ul from each sample to be used for evaluating the level of 4HNE and CYP1A1.
The following antibodies were used to detect the oxidative stress biomarkers:
4HNE antibody (Biorad, Italy1:1000 / 6 ml).
CYP1A1 antibody (Thermo fisher, Italy1:1000–6 ml).
B-actin antibody (Biorad laboratory, Italy1:50000−10 ml).
Anti-Goat antibody used as secondary antibody for4HNE antibody.
Anti-Rabbit antibody (Biorad, Italy1:10000−10 ml) used as secondary antibody for CYP1A1 antibody.
Then the technique was performed according to the Bio Rad manufacture instructions.
2.7. Data analysis
Concerning Western blots results, Image studio lite software was used to measure the level of the tested biomarkers. Whereas, paired, unpaired t-tests and one- way ANOVA were used for the statistical analysis testing, using Graph Pad Prism (version 6) software.
The results of Western blots for each O3 exposed group were compared with the related control group. While, for the effect of O3 exposure on the body weight, the mean of the body weight for each single O3 exposed mice was calculated from the measures of the body weight before and after each single exposure. Then, the values of the obtained means were compared together.
3. Results
This study provides the first report on the effect of acute and chronic environmental O3 exposure on mice olfactory bulb using immune Western blot technique.
3.1. Swiss webster mouse strain
In this study, we aimed to measure the level of 4HNE in OB and/or plasma, CYP1A1 in OB, plasma protein in Swiss Webster and C57BL/6 J mouse strain following acute and chronic O3 exposure doses compared to non-O3 exposed mice. Also, we aimed to observe the effect of O3 exposure on the body weight of the tested mice.
As shown in Table 1, Table 2, Table 3 Fig. 1, the results of the immune Western blot technique (expressed as mean and standard deviation (SD)) showed that acute and chronic O3 exposure caused a significant increase in the OB 4HNE, OB CYP1A1plasma 4HNE and plasma proteins in the O3 exposed mice compared to the non-O3 exposed group. However, mice exposed to 0.5 ppm O3 dose for 30 min once did not show a significant increase in the level of OB 4HNE compared to the control group, Table 2.
Table 1.
Effect of 0.2 ppm/2 h/2times/1day O3 dose on Swiss Webster mice.
| Mice | Mice gender | OB 4HNE level (A.U) * |
OB proteins level (A.U) * |
Decrease in B.W % * | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Control | 5 Males+2 Females | 100 | 24.6 | 100 | 23.3 | – |
| O3 exposed mice | 3 Females | 172.3 | 30.1 | 184.7 | 39.1 | Yes (5.1) |
Table 2.
Effect of 0.5 ppm/30 min/once O3 dose on Swiss Webster mice.
| Mice | Mice gender | OB 4HNE level (A.U) |
OB CYP1A1 level(A.U)* |
OB proteins level(A.U)* |
Decrease in B.W % * | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Control | 5 Males+2 Females | 100 | 13.7 | 100 | 25.7 | 100 | 15.9 | – |
| O3 exposed mice | 2 Females | 111.5 | 21.2 | 221.6 | 43.7 | 146.5 | 23.1 | Yes (1.6) |
Table 3.
Effect of 0.5 ppm/60 min/once O3 dose on Swiss Webster mice.
| Mice | Mice No./ gender | OB 4HNE level(A.U) * |
OB CYP1A1 level (A.U)* |
OB proteins level(A.U) * |
Decrease in B.W % * | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Control | 5 Males+2 Females | 100 | 11.9 | 100 | 18.78 | 100 | 16.3 | – |
| O3 exposed mice | 3 Females | 148.8 | 19.1 | 148.8 | 24.4 | 223.4 | 39.7 | Yes (3.3) |
*: Indicates P< 0.05.
SD: Standard Deviation.
B.W: Body Weight.
OB: Olfactory bulb.
CYP1A1: Cytochrome P450, family 1, subfamily A, polypeptide 1.
No.: Number.
A.U: Arbitrary Unit.
ppm: parts per million.
h: hour.
min: minute.
O3: ozone.
4HNE: 4-hydroxynonenal.
%: percentage.
Control: non-O3 exposed.
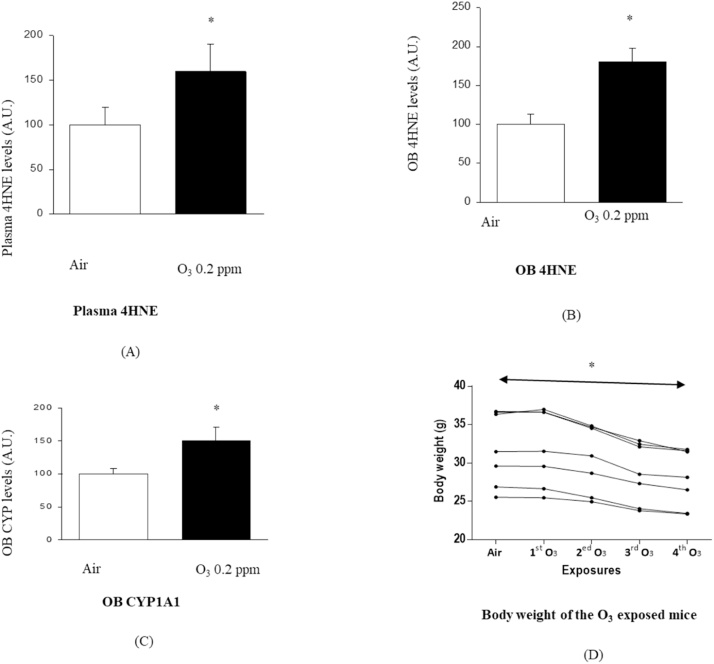
Fig. 1.
Effect of 0.2 ppm O3 dose for 2 h/2times /2days on Swiss Webster mice. (A): Plasma 4HNE level, (B): OB 4HNE level, (C): OB CYP1A1 level in Air (non-O3 exposed) and 0.2 ppm O3 exposed mice. (D): Decrease in the body weight of the O3- exposed mice following O3 exposure, (% of decrease in B W = 11.3).
Air: control (non-O3 exposed group).
A.U: Arbitrary Unit.
*: Indicates P< 0.05.
OB: Olfactory bulb.
CYP1A1: Cytochrome P450, family 1, subfamily A, polypeptide 1.
g: gram.
%: percentage.
3.2. C57BL/6J mouse strain
The effect of ozone exposure was evaluated in C57BL/6 J mouse strain to observe the effect of O3 exposure among different mice stains.
Mice exposed to 0.8 ppm O3 dose were divided by their genotype (WT and GFP), and both of them were exposed two times for two hours in four successive days followed by single exposure on the fifth day.
As shown in Table 4, Table 5, Table 6, Table 7, neither acute nor chronic O3 exposure doses caused a significant increase in the level of 4HNE (OB and plasma), and plasma proteins in both phenotypes. Nevertheless, WT mice exposed to 0.8 ppm O3 dose for two hours, two times for five days, showed a significant increase in the level of OB 4HNE compared with the control group, Table 6.
Table 4.
Effect of 0.5 ppm/30 min/once on C57BL/6 J mice.
| Mice No./genotype | Plasma 4HNE level (A.U) |
OB 4HNE level (A.U) |
OB CYP1A1 level (A.U) |
OB proteins level (A.U) |
||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Control 7 (WT) | 100 | 25.2 | 100 | 13.5 | 100 | 14.5 | 100 | 16.7 |
| O3 exposed 2 (WT) | 96.1 | 31.1 | 106.3 | 24.3 | 113.6 | 29.3 | 89.1 | 11.6 |
Table 5.
Effect of 0.5 ppm/60 min/once on C57BL/6 J mice.
| Mice No./genotype | Plasma 4HNE level (A.U) |
OB 4HNE level (A.U) |
OB CYP1A1 level (A.U) |
OB proteins level (A.U) |
||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Control 7 (WT) | 100 | 19.6 | 100 | 13.4 | 100 | 29.8 | 100 | 16.5 |
| O3 exposed 3 (WT) | 115.3 | 23.7 | 118.1 | 20.9 | 109.6 | 24.6 | 108.5 | 31.8 |
Table 6.
Effect of 0.8 ppm/2 h/2times/5days on C57BL/6 J mice.
| Mice No./genotype | Plasma 4HNE level(A.U) |
OB 4HNE level(A.U) * |
OB CYP1A1 level(A.U) |
OB proteins level(A.U) |
Decrease in B.W % * | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Control 8 (WT) | 100 | 22.0 | 100 | 15.3 | 100 | 48.4 | 100 | 26.6 | – |
| O3 exposed 4 (WT) | 144.4 | 41.1 | 165.3 | 28.4 | 112.3 | 36.8 | 110.9 | 23.6 | Yes (27.8) |
Table 7.
Effect of 0.8 ppm/2 h/2times/5days on C57BL/6 J mice.
| Mice No./genotype | Plasma 4HNE level(A.U) |
OB 4HNE level(A.U) |
OB CYP1A1 level(A.U) |
OB proteins level(A.U) |
Decrease in B.W % * | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Control 8 (WT) | 100 | 18.1 | 100 | 32.1 | 100 | 48.4 | 100 | 42.0 | – |
| 7 (GFP) | 112.3 | 22.4 | 105.1 | 38.6 | Abs. | Abs. | 108.3 | 32.8 | Yes (24.1) |
*: Indicates P< 0.05.
SD: Standard deviation.
B.W: body weight.
OB: olfactory bulb.
CYP1A1: Cytochrome P450, family 1, subfamily A, polypeptide 1.
WT: Wild Type.
No.: Number.
GFP: Green Florescent Protein.
Abs.: Absent.
ppm: parts per million.
h: hour.
min: minute.
O3: ozone.
4HNE: 4-hydroxynonenal.
%: percentage.
Control: non-O3 exposed.
Moreover, the difference in the level of CYP1A1 between WT O3 exposed mice and control mice (WT) was statistically non-significant and it was completely absent in the GFP O3 exposed mice, Table 4, Table 5, Table 6, Table 7.
Moreover, O3 exposure caused a significant decrease in the all of the O3exposed mice regardless their strain and phenotype, Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7 and Fig. 1D.
In addition, the body weight of all of the O3 exposed mice of the two strains was measured before and after each single exposure. Then, the mean was calculated for each single exposure values. As shown in Tables 1–3, 6–7 and Fig. 1D, the statistical analysis result test showed a significant decrease in the body weight of all of the O3 exposed mice regardless their strain and phenotype, Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7 and Fig. 1D.
4. Discussion
In this study, two different mouse strains were used based on the results of other researchers, who studied the effects of O3 exposure on mice and human subjects. Their findings have addressed a significant differences in susceptibility of environmental O3 exposure among different mouse strains [12]. Furthermore, this susceptibility could be due to the differences in mice genetics [13]. Also, they found that, the genetic variability plays an important role in the predisposition of the environmental diseases. In addition, studies on human and animal models revealed that O3 toxicity is species and strain dependent [[14], [15], [16], [17]].
For example, a study by Savov et al. [16] showed differences in O3 susceptibility to lung injury; their results indicated that C3H/HeJ and A/J mice strain were resistant to O3 while, C57BL/6J and 129/SvIm O3 were susceptible strains. Another study by Slade et al. [18] showed a significant difference in susceptibility to O3 exposure in mice; the C3H/HEJ (C3) mice was reported to be less sensitive than C57BL/6J (B6) mice when exposed to the same O3 dose.
4.1. Swiss webster mouse strain
Regarding Swiss Webster mice, the biochemical experiments revealed that O3 caused an increase in the level of the oxidative stress biomarkers 4HNE (in OB and/or plasma samples) and CYP1A1 (in OB samples). Also, the level of OB proteins was elevated.
Our findings concerning the increase in the 4HNE and total proteins levels following O3exposure support what have been published by other researchers. Connor et al. [19] exposed TLR4 mutant C3H/HeJ mice to 0.8 ppm O3 dose for eight hours to study the effects of O3 exposure on the lung. He found an increase in the 4-hydroxynonenal modified protein in the O3 exposed mice compared to the control mice. In addition, another study in human bronchoalveolar lavage cells conducted by Hamilton et al. [20] demonstrated the role of 4HNE in the toxicity of human lung cells after O3 exposure. Furthermore, the results obtained by Fakhrzadeh et al. [21] showed a significant increase in bronchoalveolar lavage fluid protein, lung macrophages and 4-hydroxyalkenals in the lung of 0.8 ppm O3exposed wild-type mice for three hours. Also, another study by Kirichenko et al. [22] demonstrate that HNE is formed in vivo in C3H/HeJ mice following exposure to 2.0 ppm and 0.25 ppm O3 doses for three hours. Furthermore, in vivo experiment in rats, conducted by Valentini et al. [23] who aimed to study the toxic effect of TiO2 NPs using immunohistochemical technique. Their results indicated an increase in the level of 4-Hydroxynonenal due to lipid peroxidation in neuronal cells of hippocampus and cerebellum. Also, Bigagli et al. [24] have reported that, 4-hydroxynonenal and carbonyl residues are the products of protein oxidation; and their level has been increased in vitro model of cellular senescence.
Moreover, regarding the increase of CYP1A1 level following O3 exposure, we obtained similar result to two studies on human epidermal keratinocytes exposed to 0.3 ppm O3 dose, by Afaq et al. [25] and Zaid et al. [26]. Their results showed an increase in protein and mRNA expression of CYP1A1, CYP1A2, and CYP1B1 isoforms.
Based on the above mentioned results, it has been observed that O3 exposure has an adverse effect on mice OB. Which was proved by comparing the levels of oxidative stress biomarkers (4HNE and CYP1A1), plasma proteins between non-O3 and O3 exposed mice, in addition to the marked decrease in body weight following O3 exposure.
From our biochemical results concerning Swiss Webster mice, we could suggest an alteration in the OB cells due to the generated oxidative stress state following O3exposure.This assumption is supported by Colín-Barenque et al. [27] findings. Following O3 exposure, he found an alteration in rates OB mainly in the granular layer.
4.2. C57BL/6J mouse strain
Our findings concerning the biological harmful effect(s) of different O3 doses on C57BL/6 J mice did not match with what have been published by other researchers` work.
For example: Watkinson et al. [28] exposed C57BL/6J (B6) and C3H/HeJ (C3) mice to 2 ppm O3 dose for 2 h to study O3 toxicity in mice. Both strains showed a significant change in the level of O3 toxicity biomarkers. Also, Kleebergeret et al. [29] used less O3 doses (0.12 and 0.30 ppm for 72 h) than we used, to study the susceptibility of mice to ozone-induced inflammation. He found a significant increase in the inflammatory cells after O3 exposure.
Moreover, some in vivo studies tested the effect of different O3 doses on C57BL/6 J mice.
Pulfer et al. [30] exposed the same strain to varying O3 concentrations (0.5–3.0 ppm) for three hours to study the effect of O3 exposure on the lung toxicity. His results indicated that β-epoxideand 6-oxo-3,5-diol have a role in O3 toxicity in the lung of the exposed mice.
On the other hand, the immune Western blot results showed a complete absence of CYP1A1 in the OB samples of GFP O3 exposed mice, although, it was detected in the O3and control groups WT mice. This finding needs further investigation by using more specific techniques.
Furthermore, in our results we observed a significant decrease in the body weight of the O3exposed mice regardless their strain and sex. Therefore, we assumed this finding to the effect of O3 exposure on the peroxidation level of lipids. This presumption is supported by several published experimental findings. As Rivas-Arancibia et al. [[31], [32], [33], [34]] and Pereyra-Muñoz et al. [35] correlated the increase in lipid peroxidation level to the generated oxidative stress state following O3 exposure.
Moreover, comparing the exposure results of mice exposed to 0.5 ppm for 30 and 60 min from the two strains, the result indicates that C57BL/6J mice is more resistant to the harmful effects of environmental O3 exposure compared with Swiss Webster mice strain, regardless the number of the tested mice of the two groups. Therefore, the differences in susceptibility to environmental O3 exposure could be attributed to the differences in genetic background between the two strains. This finding matches what has been published by several researchers. For example: a study by Watkinson et al. [28] who aimed to study the differences in O3 toxicity in C3H/HeJ (C3) and C57BL/6 J (B6) mouse strains. Their findings indicated that, both strains showed a significant difference in the response to environmental O3 exposure, however, following 22 h of O3 exposure B6 mice strain showed a significant increase in BAL fluid protein and cells, however, the number of neutrophils was significantly decreased in comparison with C3 mice strains. Moreover, they assessed the hypothermic response of both strains following O3 exposure, they found that B6 mice were less dynamic than C3 mice strain. Moreover, they observed difference in the relationship between BAL parameters and the induced hypothermic response among the two strains following O3 exposure. In addition, Slade et al. [18] have reported that, the differences in O3 susceptibility among different mice strains could be due to variances in the delivered O3 dose to the lung, which may be correlated to differences in the ability of the mice to lower their body core temperatures following O3 exposure. Furthermore, Kleeberger et al. [29] observed differences in susceptibility to the airway inflammatory responses to subacute O3 exposure (0.12 ppm and 0.30 ppm for 72 h) in B6 and C3 mouse strains. In their study, they found that 0.12 ppm O3 dose caused a significant influx of polymorphonuclear leukocytes (PMNs), lymphocytes, alveolar macrophages and in the total BAL proteins level in both strains. On the other hand, 0.30 ppm O3 dose showed a significant greater inflammatory response; in which B6 mice had high level of BAL proteins and great number of inflammatory cells in comparison to C3 mice strain. Moreover, Another study by Kleeberger et al. [36] was conducted to examined O3-induced airway inflammatory responses using different inbred mouse strains which were exposed to 2 ppm O3 dose for 3 h. Their findings indicated that, the magnitude of the inflammatory responses to O3 was statistically different between B6 and C3 mice strain. Regarding the number of PMNs, the difference was 22-fold after 2 h of O3 exposure and six-fold after 6 h following O3 exposure. While for BAL proteins level, the difference was observed after 6 h of O3 exposure and 24 h after O3 exposure between the two strains.
In addition, the characterization of genetic mechanisms of responses to environmental pollutants could help in identification of susceptibility of individuals to these agents. This was much more clarified by Steven [37], in his study he used nine inbred mouse strains, including: 129/ J, A/ J, AKR/ J, BALB/ cJ, C3H/ HeJ, C57BL/6J, DBA/2J, SJL/J, and SWR/J (male, 5–6 wk) to determine if there is a significant difference in genetic contribution in susceptibility to lung injury and inflammation following exposure to single and repeated exposure to nitrogen dioxide (NO2). His results showed intra-strain variations in epithelial injury, cellular inflammation (polymorphonuclear, epithelial cells, leukocytes and macrophages), lung injury and proteins level due to NO2 exposure. This variation in the response to NO2 indicated a great contribution of genetic background of mice to this agent. Also, he aimed to test whether the susceptibility to NO2 and O3 exposure between B6 and C3 was statistically different or not. The result indicated a significant difference in the response to these two pollutants among the two strains. Moreover, depending on all of his findings, he suggested that, the genetic susceptibility to NO2 is significantly different from O3 among all of the tested mouse strains. Furthermore, published epidemiological studies and clinical data on human showed marked difference in respiratory effect due to O3 exposure among healthy and asthmatic individuals [[38], [39], [40], [41]]. The difference in susceptibility to O3 harmful effect was considered to be due to variation of human genetics [13].
5. Conclusion
In conclusion, Swiss Webster mouse strain is more sensitive to the harmful effects due to O3 exposure than C57BL/6 J mouse strain. In addition, ozone exposure caused an increase in the level of the proteins involved in the oxidative stress state in the OB of Swiss Webster mouse strain. However, in C57BL/6 J mouse strain, this effect was not observed. Moreover, all of the O3 exposed mice showed a significant decrease in the body weight following the exposure. Furthermore, the expression level of CYP1A1 following O3 is affected by mice genotype.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution
The corresponding author Samah Abd-Elrahim Batran has performed the followings: Designed the study, did the mice breeding, exposed mice to O3, prepared the samples, performed the Bradford and Western blot techniques, performed the statistical analysis, did the literature search, performed the statistical analysis, interpreted the results and wrote the manuscript.
Declaration of Competing Interest
The author has no any conflict of interest.
Acknowledgement
Author would like to thank the laboratory of Life Science biochemistry department, Ferrara, Italy for the assistance in carrying out this work.
References
- 1.Lawrence E., Jackson J.M., Jackson A.R.W. Longman; Harlow, Essex, England: 1998. Longman Dictionary of Environmental Science. [Google Scholar]
- 2.Benignus V.A., Prah J.D. Olfaction: anatomy, physiology and behavior. Environ. Health Perspect. 1982;44:15–21. doi: 10.1289/ehp.824415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajmani G.S., Suh H.H., Pinto J.M. Effects of ambient air pollution exposure on olfaction: a review. Environ. Health Perspect. 2016;124:1683–1693. doi: 10.1289/EHP136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kendrick K.M. Formation of olfactory memories mediated by nitric oxide. Nature. 1997;388:670–674. doi: 10.1038/41765. [DOI] [PubMed] [Google Scholar]
- 5.Sánchez-Andrade G., James B.M., Kendrick K.M. Neural encoding of olfactory recognition memory. J. Reprod. 2005;51:547–558. doi: 10.1262/jrd.17031. [DOI] [PubMed] [Google Scholar]
- 6.Carney J.M., Smith C.D., Carney A.M., Butterfield D.A. Aging- and oxygen-induced modifications in brain biochemistry and behavior. Ann. N. Y. Acad. Sci. 1994;738:44–53. doi: 10.1111/j.1749-6632.1994.tb21788.x. [DOI] [PubMed] [Google Scholar]
- 7.Olanow C.W. A radical hypothesis for neurodegeneration. Trends Neurosci. 1993;16:439–444. doi: 10.1016/0166-2236(93)90070-3. [DOI] [PubMed] [Google Scholar]
- 8.Kim Y.K., Koo S.M., Kim K., Uh S.-T., Jang A., Park C.-S. Increased antioxidant activity after exposure of ozone in murine asthma model. Asia Pac. Allergy. 2017;7:163–170. doi: 10.5415/apallergy.2017.7.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaid M.A. Exposure of normal human epidermal keratinocytes to ozone results in increased expression of cytochrome P450 through activation of aryl hydrocarbon receptor. Cancer Res. 2008;68:595. [Google Scholar]
- 10.pubmeddev and F. I. A. et al., ‘Calr etinin-Periglomerular Interneurons in Mice Olfactory Bulb: Cells of Few Words. - PubMed - NCBI’. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/27774053. (Accessed 17 July 2019).
- 11.Chumarina M. Derivation of mouse embryonic stem cell lines from tyrosine hydroxylase reporter mice crossed with a human SNCA transgenic mouse model of Parkinson’s disease. Stem Cell Res. 2017;19:17. doi: 10.1016/j.scr.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein B.D., Lai L.Y., Ross S.R., Cuzzi-Spada R. Susceptibility of inbred mouse strains to ozone. Arch. Environ. Health. 1973;27:412–413. doi: 10.1080/00039896.1973.10666416. [DOI] [PubMed] [Google Scholar]
- 13.Prows D.R., Shertzer H.G., Daly M.J., Sidman C.L., Leikauf G.D. Genetic analysis of ozone-induced acute lung injury in sensitive and resistant strains of mice. Nat. Genet. 1997;17:471–474. doi: 10.1038/ng1297-471. [DOI] [PubMed] [Google Scholar]
- 14.Kleeberger S.R., Reddy S., Zhang L.Y., Jedlicka A.E. Genetic susceptibility to ozone-induced lung hyperpermeability: role of toll-like receptor 4. Am. J. Respir. Cell Mol. Biol. 2000;22:620–627. doi: 10.1165/ajrcmb.22.5.3912. [DOI] [PubMed] [Google Scholar]
- 15.Prows D.R., Shertzer H.G., Daly M.J., Sidman C.L., Leikauf G.D. Genetic analysis of ozone-induced acute lung injury in sensitive and resistant strains of mice. Nat. Genet. 1997;17:471–474. doi: 10.1038/ng1297-471. [DOI] [PubMed] [Google Scholar]
- 16.Savov J.D. Ozone-induced acute pulmonary injury in inbred mouse strains. Am. J. Respir. Cell Mol. Biol. 2004;31:69–77. doi: 10.1165/rcmb.2003-0001OC. [DOI] [PubMed] [Google Scholar]
- 17.Vancza E.M., Galdanes K., Gunnison A., Hatch G., Gordon T. Age, strain, and gender as factors for increased sensitivity of the mouse lung to inhaled ozone. Toxicol. Sci. Off. J. Soc. Toxicol. 2009;107:535–543. doi: 10.1093/toxsci/kfn253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slade R., Watkinson W.P., Hatch G.E. Mouse strain differences in ozone dosimetry and body temperature changes. Am. J. Physiol. 1997;272:L73–77. doi: 10.1152/ajplung.1997.272.1.L73. [DOI] [PubMed] [Google Scholar]
- 19.Connor A.J., Laskin J.D., Laskin D.L. Ozone-induced lung injury and sterile inflammation. Role of toll-like receptor 4. Exp. Mol. Pathol. 2012;92:229–235. doi: 10.1016/j.yexmp.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton R.F., Li L., Eschenbacher W.L., Szweda L., Holian A. Potential involvement of 4-hydroxynonenal in the response of human lung cells to ozone. Am. J. Physiol. 1998;274:L8–16. doi: 10.1152/ajplung.1998.274.1.L8. [DOI] [PubMed] [Google Scholar]
- 21.Fakhrzadeh L., Laskin J.D., Gardner C.R., Laskin D.L. Superoxide dismutase–overexpressing mice are resistant to ozone-induced tissue injury and increases in nitric oxide and tumor necrosis factor-α. Am. J. Respir. Cell Mol. Biol. 2004;30:280–287. doi: 10.1165/rcmb.2003-0044OC. [DOI] [PubMed] [Google Scholar]
- 22.Kirichenko A., Li L., Morandi M.T., Holian A. 4-Hydroxy-2-nonenal–protein adducts and apoptosis in murine lung cells after acute ozone exposure. Toxicol. Appl. Pharmacol. 1996;141:416–424. doi: 10.1006/taap.1996.0307. [DOI] [PubMed] [Google Scholar]
- 23.Valentini X. Morphological alterations induced by the exposure to TiO2 nanoparticles in primary cortical neuron cultures and in the brain of rats. Toxicol. Rep. 2018;5:878–889. doi: 10.1016/j.toxrep.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bigagli E. Long-term Neuroglial Cocultures as a Brain Aging Model: Hallmarks of Senescence, MicroRNA Expression Profiles, and Comparison With In Vivo Models. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71:50–60. doi: 10.1093/gerona/glu231. [DOI] [PubMed] [Google Scholar]
- 25.Afaq F. Aryl hydrocarbon receptor is an ozone sensor in human skin. J. Invest. Dermatol. 2009;129:2396–2403. doi: 10.1038/jid.2009.85. [DOI] [PubMed] [Google Scholar]
- 26.Zaid M.A. Exposure of normal human epidermal keratinocytes to ozone results in increased expression of cytochrome P450 through activation of aryl hydrocarbon receptor. Cancer Res. 2008;68:595. [Google Scholar]
- 27.Colín-Barenque L. Morphologic alteration of the olfactory bulb after acute ozone exposure in rats. Neurosci. Lett. 1999;274:1–4. doi: 10.1016/s0304-3940(99)00639-4. [DOI] [PubMed] [Google Scholar]
- 28.Watkinson W.P., Highfill J.W., Slade R., Hatch G.E. Ozone toxicity in the mouse: comparison and modeling of responses in susceptible and resistant strains. J. Appl. Physiol. Bethesda Md. 1996;80:2134–2142. doi: 10.1152/jappl.1996.80.6.2134. [DOI] [PubMed] [Google Scholar]
- 29.Kleeberger S.R., Levitt R.C., Zhang L.Y. Susceptibility to ozone-induced inflammation. I. Genetic control of the response to subacute exposure. Am. J. Physiol. 1993;264:L15–20. doi: 10.1152/ajplung.1993.264.1.L15. [DOI] [PubMed] [Google Scholar]
- 30.Pulfer M.K., Taube C., Gelfand E., Murphy R.C. Ozone exposure in vivo and formation of biologically active oxysterols in the lung. J. Pharmacol. Exp. Ther. 2005;312:256–264. doi: 10.1124/jpet.104.073437. [DOI] [PubMed] [Google Scholar]
- 31.Rivas-Arancibia S., Vazquez-Sandoval R., Gonzalez-Kladiano D., Schneider-Rivas S., Lechuga-Guerrero A. Effects of ozone exposure in rats on memory and levels of brain and pulmonary superoxide dismutase. Environ. Res. 1998;76:33–39. doi: 10.1006/enrs.1997.3784. [DOI] [PubMed] [Google Scholar]
- 32.Rivas-Arancibia S. Effects of taurine on ozone-induced memory deficits and lipid peroxidation levels in brains of young, mature, and old rats. Environ. Res. 2000;82:7–17. doi: 10.1006/enrs.1999.3996. [DOI] [PubMed] [Google Scholar]
- 33.Rivas-Arancibia S., Dorado-Martínez C., Colin-Barenque L., Kendrick K.M., de la Riva C., Guevara-Guzmán R. Effect of acute ozone exposure on locomotor behavior and striatal function. Pharmacol. Biochem. Behav. 2003;74:891–900. doi: 10.1016/s0091-3057(03)00011-x. [DOI] [PubMed] [Google Scholar]
- 34.Rivas-Arancibia S. Oxidative stress caused by ozone exposure induces loss of brain repair in the hippocampus of adult rats. Toxicol. Sci. Off. J. Soc. Toxicol. 2010;113:187–197. doi: 10.1093/toxsci/kfp252. [DOI] [PubMed] [Google Scholar]
- 35.Pereyra-Muñoz N., Rugerio-Vargas C., Angoa-Pérez M., Borgonio-Pérez G., Rivas-Arancibia S. Oxidative damage in substantia nigra and striatum of rats chronically exposed to ozone. J. Chem. Neuroanat. 2006;31:114–123. doi: 10.1016/j.jchemneu.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Kleeberger S.R., Bassett D.J., Jakab G.J., Levitt R.C. A genetic model for evaluation of susceptibility to ozone-induced inflammation. Am. J. Physiol. 1990;258:L313–320. doi: 10.1152/ajplung.1990.258.6.L313. [DOI] [PubMed] [Google Scholar]
- 37.L.-Y. Z, G. J. J, Kleeberger Steven R. Differential susceptibility to oxidant exposure in inbred strains of mice: nitrogen dioxide versus ozone. Inhal. Toxicol. 1997;9:601–621. [Google Scholar]
- 38.Silverman F. Asthma and respiratory irritants (ozone) Environ. Health Perspect. 1979;29:131–136. doi: 10.1289/ehp.7929131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spektor D.M. Effects of ambient ozone on respiratory function in healthy adults exercising outdoors. Am. Rev. Respir. Dis. 1988;138:821–828. doi: 10.1164/ajrccm/138.4.821. [DOI] [PubMed] [Google Scholar]
- 40.Kreit J.W., Gross K.B., Moore T.B., Lorenzen T.J., D’Arcy J., Eschenbacher W.L. Ozone-induced changes in pulmonary function and bronchial responsiveness in asthmatics. J. Appl. Physiol. 1989;66:217–222. doi: 10.1152/jappl.1989.66.1.217. [DOI] [PubMed] [Google Scholar]
- 41.McBride D.E., Koenig J.Q., Luchtel D.L., Williams P.V., Henderson W.R. Inflammatory effects of ozone in the upper airways of subjects with asthma. Am. J. Respir. Crit. Care Med. 1994;149:1192–1197. doi: 10.1164/ajrccm.149.5.8173759. [DOI] [PubMed] [Google Scholar]