Abstract
Follicular thyroid cancer (FTC) is a less common form of differentiated thyroid cancer. Liver metastasis of differentiated thyroid cancer frequently occurs in the late onset of the metastatic disease, are often unrescetable and noniodine avid, leading to a poor prognosis. A 69-year-old man with a 14-year history of multi-metastatic follicular thyroid cancer was treated iteratively with 131-Iodine allowing to maintain a stable disease. Upon a recent exponential increase of the thyroglobulin, a peritoneal mass and a voluminous hepatic metastasis were discovered, comorbidities and an insufficient future remnant liver function excluded liver surgical resection. The tumour board proposed a resection of the peritoneal mass followed by selective internal radiation therapy of the liver mass. Due to the already impaired liver function, personalized dosimetry allowed a safe treatment delivering low activity to the nontumoral liver followed by a clinical and imaging response of the liver mass at 3 months. At our knowledge, this is the first case of thyroid liver metastasis treated by selective internal radiation therapy.
Keywords: Liver metastasis, Personalized dosimetry, Radioembolization, Thyroid Cancer
Introduction
Follicular thyroid cancer (FTC) is a less common form of differentiated thyroid cancer and spreads via haematogenous dissemination with distant metastases occurring in 10 to 15 percent of patients most commonly located in bone or lungs. According to the American thyroid association guidelines, treatment of FTC distant metastases requires 131I as long as iodine avidity is conserved. We report the first case of a patient presenting a progressive non resectable and iodine nonavid hepatic metastasis of FTC treated by radioembolization using hepatic selective internal radiation therapy (SIRT).
Case presentation
A 69-year-old man presented with follicular thyroid cancer (T4N1M1) initially treated by total thyroidectomy and ablation of one bone metastasis (cervical) followed by a first dose of radioactive 131-iodine (131I). During 14 years follow-up, the patient presented repeatedly several recurrences in bones, lungs, mediastinal lymph nodes and peritoneum treated iteratively with 131I (cumulative administered activity: 40.7 GBq in 6 sessions) each time achieving temporary disease control.
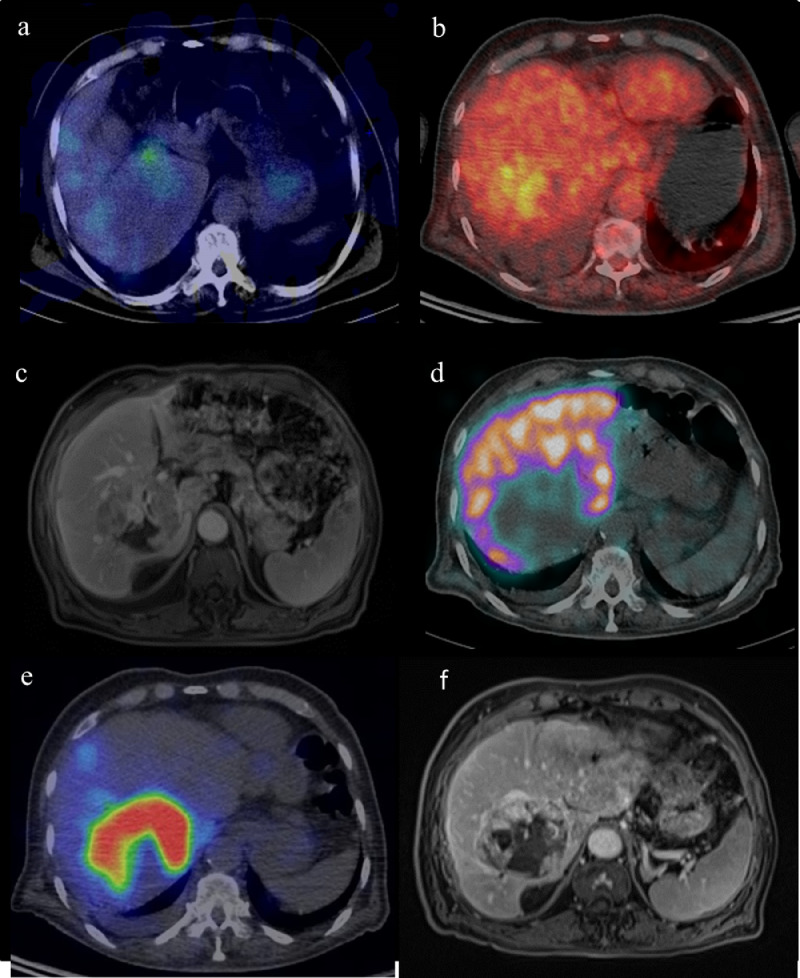
In 2018, after 3-years of loss of follow-up, the patient presented at the emergency unit for pyrexia. An increase of thyroglobulin (13076 µg/L) was noted. A diagnostic 131I-single photon emission computed tomography (SPECT/CT) (192 MBq) (Fig. 1a) was performed and showed a progressive disease with an intense uptake in a peritoneal mass without significant uptake of the liver mass. [18]F-fluorodeoxyglucose positron emission tomography (18F-FDG PET/CT) showed an increased glucose metabolism of the peritoneal mass, a mildly hypermetabolic liver metastasis and stable disease in lung and mediastinal lymph node (Fig. 1b). Magnetic resonance imaging (MRI) showed a 76 mm liver tumour in segments I, V, VI, VII, VIII (Fig. 1c) and a liver biopsy confirmed the presence of follicular carcinoma cells consistent with the known primary tumour. As part of preoperative work-up a hepatobiliary scintigraphy using 99mTechnitium-mebrofenine (99mTc) was performed to determine hepatic function (HF) and assess future remnant liver function. Briefly, it consists in a dynamic planar acquisition followed by a SPECT/CT (Fig. 1d). Despite normal liver blood tests and no history of prior hepatic disease, the measured HF was 4.4 %/min/m² (normal value >7%/min/m², with the lowest acceptable HF cut-off limit for future liver remnant set at 2.7 %/min/m²) [1,2]. On this basis, the liver mass was considered as initially nonresectable, requiring preoperative liver volumes modulation. Due to the uncertainty of the benefit, the impaired liver function and the expected morbidity of such major resection, the thyroid and digestive multidisciplinary tumour board decided to perform a complete resection of the peritoneal mass followed by radioembolization of the liver metastasis. The radioembolization was realized with 90-Yttrium (90Y) labelled resin microspheres, supra-selectively, in the segmental artery covering the lesion in order to keep as much as possible liver parenchyma out of the field of therapy.
Fig. 1.
(a) Axial fused 5-days post-administration (192MBq) 131I-SPECT-CT showing low avidity of the liver metastasis for iodine (b) Axial fused 18F FDG-PET-CT showing a moderate uptake of FDG (c) Axial MRI T1-VIBE- fat sat before radioembolization (d) Axial fused 99mTc–mebrofenin hepatobiliary SPECT-CT showing no uptake in the lesion territory (e) Axial fused 99mTc–macro-aggregates of albumin SPECT-CT showing a high specific accumulation in the liver metastasis (f) Axial MRI T1-DIXON-fat sat 3 months after radioembolization
SIRT simulation was performed with 2 supra-selective injections of 99mTc–macro-aggregates of albumin respectively in the arteries vascularizing segment I and segments V to VIII (Fig. 1e). The field of treatment represented 85% of the whole liver and high selective uptake was noted (Tumour to nontumour uptake ratio: 3.54). No extrahepatic activity was observed. Personalized predictive voxel-based dosimetry was performed on macro-aggregates of albumin SPECT/CT images using a dedicated SIRT treatment planning system (Planet Onco dose, Dosisoft). Activity was prescribed to reach mean absorbed doses of 95 Gy and 35 Gy for the liver lesion and for the non-tumoural liver within the treatment field, respectively [3].
One week later, SIRT was performed at the exact same catheter positions as during the simulations without any immediate complication. A 90Y-PET/CT was acquired at day 1 post-SIRT, and post-treatment voxel-based 3D dosimetry was performed. Computed mean absorbed doses to the lesion and to nontumoural liver were 85 and 24 Gy, respectively.
Outcome and follow-up
Eight weeks post-RE, the patient was clinically doing well with no clinical or biological signs of radioembolization-induced liver disease (REILD). REILD is defined as a symptomatic post-radioembolization deterioration in the ability of the liver to maintain its (normal or preprocedural) synthetic, excretory, and detoxifying functions. It is characterized by jaundice and the development of or increase in ascites, hyperbilirubinemia, and hypoalbuminemia developing at least 2 weeks to 4 months after radioambolization, in the absence of tumor progression or biliary obstruction [4]. After 3 months, the size of the lesion was stable on MRI (Fig. 1f) with an overall increase in apparent diffusion coefficient values and a partial devascularization of the liver lesion. Thyroglobulin decreased from 4844 µg/l to 3667 µg/L, only partial remission as explained by the persistence of a residual extrahepatic PTC (mediastinal nodes and lung).
Discussion
Liver metastases of differentiated thyroid cancer (LMDTC), occurs in approximatively 0.5% to 1.7% of the population with thyroid cancer [5,6]. LMDTC generally appears after the onset of metastases at other sites [5,7]. Because of a frequent dedifferentiation process (decreased expression and/or function of the sodium iodide symporter), most cases of LMDTC are not iodine-avid, limiting the effectiveness of radioiodine therapy [8]. Most patients are not eligible for surgery due to multiple LMDTC not accessible to complete surgical excision, disseminated metastases, or significant comorbidities. In this case the patient had already received a cumulative administered activity of 40.7 GBq of 131I, which could classify this disease as a 131I refractory disease [9]. Tyrosine kinase inhibitors have been reported as an effective option in patients with inoperable or refractory recurrent disease [6,9]. Due to the already impaired liver function, predictive activity prescription was indicated in order to optimize treatment efficacy and avoid the risk of REILD. The loss of liver function could be related to the multiple prior iodine-131 therapies. Should the classical modified body surface area (mBSA)-method have been used for SIRT activity prescription in this patient, the given activity would have been double (1450 MBq instead of 728 MBq) which would have resulted in a mean absorbed dose of the normal liver superior to 40 Gy associated with a very high risk of REILD [3,10,11].
Conclusion
To our knowledge, this is the first case of a thyroid liver metastasis successfully treated by SIRT using 90Y-microspheres as part of a multimodal treatment strategy. This case also highlights the importance of pretherapeutic work plan and predictive dosimetry to optimize personalized treatment to minimize the risk of REILD in an already impaired liver.
Statement of ethics
All data about the patient's privacy is blinded.
The patient gave his informed consent for the publication of this case report.
References
- 1.Cieslak KP., Bennink RJ., de Graaf W., van Lienden KP., Besselink MG., Busch ORC. Measurement of liver function using hepatobiliary scintigraphy improves risk assessment in patients undergoing major liver resection. HPB (Oxford) 2016;18(9):773–780. doi: 10.1016/j.hpb.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Graaf W., van Lienden KP., Dinant S., Roelofs JJTH., Busch ORC., Gouma DJ. Assessment of future remnant liver function using hepatobiliary scintigraphy in patients undergoing major liver resection. J Gastrointest Surg. 2010;14(2):369–378. doi: 10.1007/s11605-009-1085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brient C., Mucci S., Taïeb D., Mathonnet M., Menegaux F., Mirallié E. Differentiated thyroid cancer with liver metastases: lessons learned from managing a series of 14 patients. Int Surg. 2015;100(3):490–496. doi: 10.9738/INTSURG-D-14-00026.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braat Manon N.G.J.A., van Erpecum Karel J., Zonnenberg Bernard A., van den Bosch Maurice A.J., Lam Marnix G.E.H. Radioembolization-induced liver disease: a systematic review. Eur J Gastroenterol Hepatol. 2017;29(2):144–152. doi: 10.1097/MEG.0000000000000772. [DOI] [PubMed] [Google Scholar]
- 5.Shah DH, Samuel AM. Metastasis to the liver in well-differentiated carcinoma of the thyroid. Thyroid. 1996;6(6):607–611. doi: 10.1089/thy.1996.6.607. [DOI] [PubMed] [Google Scholar]
- 6.Saito Y., Sugino K., Takami H., Matsuzu K., Uruno T., Ohkuwa K. Clinical status and treatment of liver metastasis of differentiated thyroid cancer using tyrosine kinase inhibitors. World J Surg. 2018;42(11):3632–3637. doi: 10.1007/s00268-018-4676-9. [DOI] [PubMed] [Google Scholar]
- 7.Farina E., Monari F., Tallini G., Repaci A., Mazzarotto R., Giunchi F. Unusual thyroid carcinoma metastases: a case series and literature Review. Endocr Pathol. 2016;27(1):55–64. doi: 10.1007/s12022-015-9410-7. [DOI] [PubMed] [Google Scholar]
- 8.Garin E., Lenoir L., Rolland Y., Edeline J., Mesbah H., Laffont S. Dosimetry Based on 99mTc-Macroaggregated Albumin SPECT/CT Accurately Predicts Tumor Response and Survival in Hepatocellular Carcinoma Patients Treated with 90Y-Loaded Glass Microspheres: Preliminary Results. J Nucl Med. 2012;53(2):255–263. doi: 10.2967/jnumed.111.094235. [DOI] [PubMed] [Google Scholar]
- 9.Flamen P., Vanderlinden B., Delatte P., Ghanem G., Ameye L., Van Den Eynde M. Multimodality imaging can predict the metabolic response of unresectable colorectal liver metastases to radioembolization therapy with Yttrium-90 labeled resin microspheres. Phys Med Biol. 2008;53(22):6591–6603. doi: 10.1088/0031-9155/53/22/019. [DOI] [PubMed] [Google Scholar]
- 10.Schlumberger M., Tahara M., Wirth LJ., Robinson B., Brose MS., Elisei R. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621–630. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 11.Ho S., Lau WY., Leung TWT., Chan M., Ngar YK., Johnson PJ. Partition model for estimating radiation doses from yttrium-90 microspheres in treating hepatic tumours. Eur J Nucl Med. 1996;23(8):947–952. doi: 10.1007/BF01084369. [DOI] [PubMed] [Google Scholar]