Abstract
Objective:
The objective of this study was to determine whether the trabecular volumetric Bone Mineral Density (vBMD) of the middle, body and angle of the mandible correlates with vBMD of the cervical and lumbar vertebrae in a Chinese population.
Methods and materials:
661 subjects (270 males, 391 females), ranging from 20 to 59 years of age, were recruited for vBMD measurements by quantitative CT (QCT). Basic information (age, height and weight), vBMD of the mandible (middle, body and angle sites), and vBMD of the cervical and lumbar vertebrae were recorded. Spearman’s rank correlation test was used to investigate the association of mandibular with vertebral vBMD.
Results:
The study cohort comprised 661 subjects: 270 (41%) males, 391 (59%) females. Median age in males was 40 (range, 21–59) years. Median age in females was 41 (range, 20–59) years. Values of the Spearman correlation coefficient between mandibular and vertebral vBMD ranged from R = 0.048 to 0.141. In males, the three correlation coefficients between mandibular and cervical vBMD (middle: R = 0.138; body: R = 0.126; angle: R = 0.122) were all statistically significant (p < 0.05). In females, the correlation between the middle mandibular site and cervical site was statistically significant (R = 0.141, p < 0.01). None of the other correlations examined were statistically significant.
Conclusion:
In this study population, mandibular vBMD was at best weakly correlated with cervical and lumbar vertebral vBMD, indicating that mandibular vBMD should be measured independently for the assessment of mandibular bone status.
Keywords: Computed tomography, Bone density, Mandible, Spine
Introduction
Osteoporosis is a common metabolic disease characterized by reduced bone mass and thinning of cancellous architecture, leading to increased bone fragility and frequently resulting in fractures of the vertebrae, hip, or forearm.1 According to the World Health Organization (WHO) Study Group, osteoporosis is the second most common disorder following cardiovascular diseases.2 It is a very common disease in countries around the world with a moderate climate, and is directly related to age.3 With the aging of the world's population, osteoporosis has become one of the major diseases affecting the health of the elderly and leading to poorer quality of life. One-third of females and one-fifth of males over 50 years old will experience an osteoporotic fracture.4 Measurement of bone mineral density (BMD) is the principal method of diagnosing osteoporosis because patients with low BMD values have an elevated risk of fracture.5 Although osteoporosis is a disease that involves the entire skeleton, the most widespread osteoporosis-related fractures are vertebral, hip, and distal-third forearm fractures.6 The WHO Study Group established that the diagnosis of osteoporosis can be determined by bone densitometry when the values of BMD of lumbar spine and femoral neck are lower than normal.7 These sites should be analyzed in all patients with clinical risk factors for osteoporosis.
Since the mandible is rich in cancellous bone and its metabolism should be consistent with other similar areas in the skeleton such as the cervical and lumbar vertebrae, it has been proposed by different authors to use the evaluation of changes in mandibular BMD to estimate systemic bone loss.8–10
Quantitative CT (QCT) has been considered as an important approach in the assessment of osteoporosis, and measures BMD from CT images with the use of a calibration phantom.11 Volumetric BMD (vBMD) measured by QCT is based on three-dimensional imaging techniques and is different to the conventional two-dimensional areal BMD (aBMD) measured by dual-energy X-ray absorptiometry (DXA). Unlike DXA, QCT can evaluate the cancellous bone of a certain volume independently without the influence of cortical bone and other extraosseous tissues.12
The purpose of this study was to determine whether mandibular vBMD is associated with cervical or lumbar vertebral vBMD in a Chinese population.
Methods and materials
Study subjects
The subjects included in this study were participants since June 2014 in an ongoing study of degeneration of the spine and knee. The present study analyzed existing data from previous studies, which were approved by the Ethics Committee of Beijing Jishuitan Hospital, Peking University Fourth School of Clinical Medicine, China.13,14 Details of these studies have been reported previously.13,14 The criteria for inclusion were: healthy adults, aged 20–65 years. Participants on medications that have an influence on bone metabolism were excluded. The exclusion criteria also included: diabetes, thyroid and parathyroid disease, or other chronic illnesses affecting BMD. All the subjects signed informed consent before examination. In brief, 680 healthy subjects were enrolled. In nine subjects, vBMD values for the mandible were missing for the following reasons: periodontitis, mandibular metal implants, artifacts caused by dentures and motion. In further four subjects, values for the cervical spine were missing due to artifacts caused by the lead-based shielding vestment used to protect subjects against radiation. In addition, six subjects declined to undergo the lumbar spine QCT examination. Results for 661 individuals were available for statistical analysis including 270 males (range 21–59 years old) and 391 females (range 20–59 years old). Demographic information including age (years), weight (kg), height (cm) and body mass index (BMI, kg/m2) were recorded before scanning.
Mandible, cervical and lumbar vertebrae scanning by QCT
As part of the study protocol, the cervical vertebrae from C2 to C7 and lumbar vertebrae from L2 to L4 were scanned as well as the mandible. QCT scans were obtained using a Toshiba CT scanner (Aquilion PRIME ESX-302A, Toshiba Medical Systems Corporation, Otawara, Japan), as previously described.13,14 A Model three five-rod QCT calibration phantom (Mindways Inc., Austin, TX) with an aqueous K2HPO4 bone density standard was placed beneath the scanning table and scanned simultaneously according to the standard protocol of Lang et al15. The scanning parameters were as follows: 120 kV, 187 mAs, 1 mm slice thickness, 40 cm field of view (SFOV), and 512 × 512 matrix in spiral reconstruction and standard reconstruction algorithms. The precision error of this measurement method is reportedly less than 1.5%.15,16
Volumetric BMD (vBMD) measurement
After CT scanning, the images were transferred to a QCT workstation for further analysis with the QCT Pro 5.0.3 (Mindways Inc.) analysis software. Round or oval-shaped regions of interest (ROIs) were defined in the middle of the left mandible (beneath the incisors), the body (beneath the canines) and the angle (beneath the first and second molars).14 The ROIs were 20–40 mm2 in area and 5 mm in height, and excluded cortical bone. The ROIs in the spine were defined as the largest oval-shaped areas that could be set in the cervical and lumbar vertebrae trabecular bone with a height of 9 mm, excluding cortical bone and the basivertebral plexus.13,17 The vBMD values were recorded for statistical analysis (Figure 1).
Figure 1.
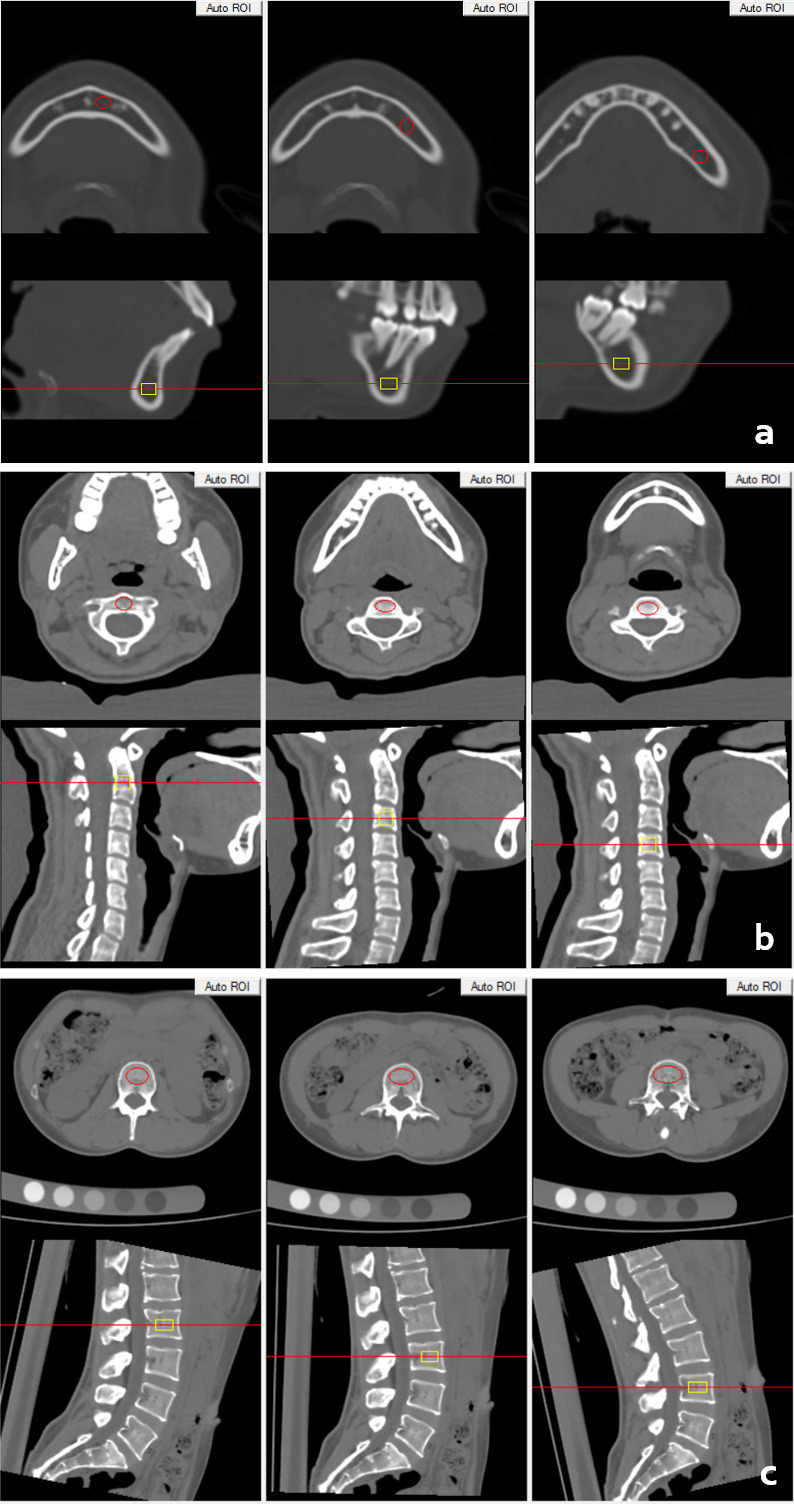
Volumetric BMD measurement of mandible (a), cervical (b) and lumbar spine (c) in a 28-year-old female. BMD, bone mineral density.
Statistical analysis
SPSS Statistics for Windows v. 20.0 (IBM SPSS Inc., Chicago, IL) was used for statistical analysis. A descriptive study was made of each variable. The baseline characteristics of the subjects were calculated as means ± standard deviation (SD). The vBMD data were grouped based on sex and age (10 year intervals) and expressed as the median and interquartile range. Non-parametric test was used to compare the vBMD of different groups. The Spearman rank correlation test was used to compare mandibular and spine vBMD in different age groups. p < 0.05 was considered statistically significant.
Results
Study population characteristics
661 healthy subjects (270 males, mean age 40 years; 391 females, mean age 41 years) were recruited to this study. Baseline characteristics of the subjects are shown in Table 1. There were 102 participants in the 20–29 year age group (38 males, 64 females), 213 in the 30–39 year age group (96 males, 117 females), 236 in the 40–49 year age group (98 males, 138 females) and 110 in the 50–59 year age group (38 males, 72 females). The age difference between males and females was not statistically significant (p = 0.249). Males were taller and heavier than females and the BMI difference between the sexes was statistically significant (Table 1). The cervical and lumbar vertebrae vBMD in females were statistically significantly higher than in males. The vBMD of the mandibular body and angle sites were also statistically significantly higher in females than in males. There was no significant difference in vBMD of the mandibular middle site between males and females.
Table 1.
Characteristics of the subjects
| Parameters | Males | Females | p value |
|---|---|---|---|
| Sample size | 270 | 391 | |
| Age (years) | 39.4 ± 8.6 | 40.2 ± 9.2 | 0.249 |
| Height (cm) | 172.4 ± 5.9 | 160.4 ± 7.4 | <0.001 |
| Weight (kg) | 78.1 ± 11.8 | 62.1 ± 10.2 | <0.001 |
| BMI (kg/m2) | 26.2 ± 3.6 | 24.5 ± 8.5 | <0.001 |
| Mandibular middle vBMD (mg/cm3) | 234.9 (173.3, 323.2) | 238.6 (159.5, 340.7) | 0.531 |
| Mandibular body vBMD (mg/cm3) | 143.4 (89.8, 241.0) | 176.9 (113.8, 255.6) | 0.003 |
| Mandibular angle vBMD (mg/cm3) | 121.6 (66.5, 219.2) | 148.3 (89.4, 226.4) | 0.014 |
| C2-7 vBMD (mg/cm3) | 266.3 (228.3, 297.7) | 302.9 (257.1, 346.3) | <0.001 |
| L2-4 vBMD (mg/cm3) | 146.5 (127.0, 167.1) | 163.3 (138.6, 184.8) | <0.001 |
Results for age, height, weight and BMI are the mean and standard deviation. Results for mandibular and vertebral vBMD are the median and interquartile range.BMD, bone mineral density; BMI: body mass index.
Distribution trend of BMD in mandible, cervical and lumbar spine in males and females
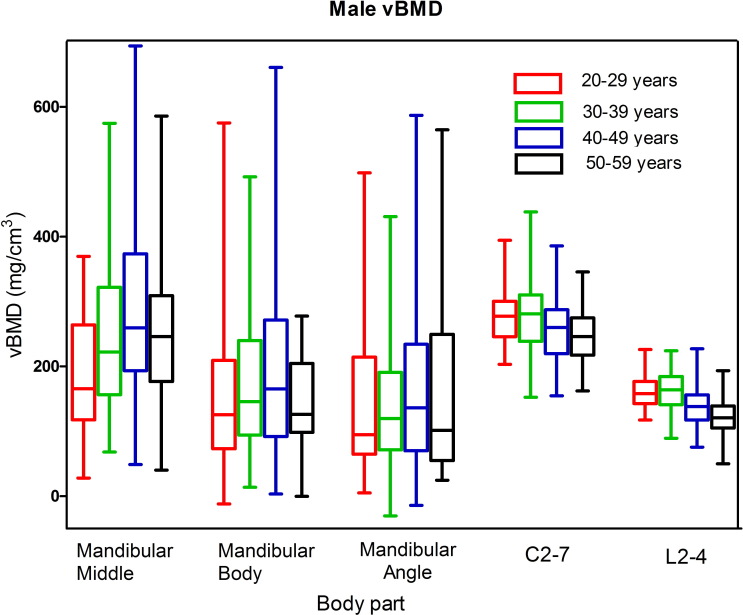
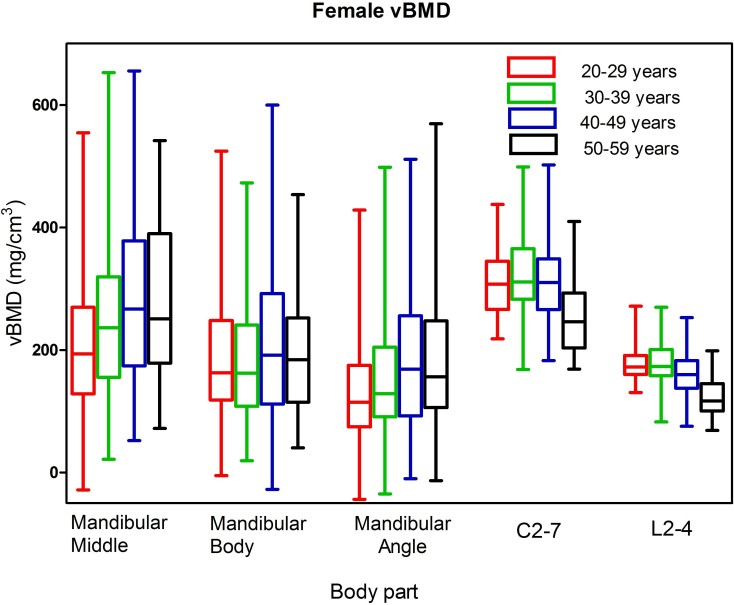
Median vBMD values and interquartile ranges for males and females are plotted in the box-and-whisker plots in Figures 2 and 3 respectively. For males, median vBMD in the spine reached the peak value in the 30–39 year group and then started to decline gradually in 40–49 and 50–59 year groups. Median vBMD in the mandible increased with age, and was highest in the 40–49 year group, before decreasing in the 50+ year group. In females, the age related trends of spinal and mandibular vBMD were consistent with those in males. In addition, the vBMD of cervical vertebrae were significantly higher than lumbar vertebrae for both males and females (p﹤0.01). The vBMD of vertebrae in females was significantly higher than males at the three age groups below 50 years for both the cervical and lumbar spine (p﹤0.01), and there were no significant gender difference at 50–59 year group for the vBMD of either cervical or lumbar vertebrae with p = 0.930 and 0.571, respectively. The median vBMD at the mandibular middle site was higher than the other two mandibular sites for both males and females.
Figure 2.
Box and whisker plot showing the median, interquartile range, maximum and minimum vBMD values for the mandible, cervical and lumbar spine in males. BMD, bone mineral density.
Figure 3.
Box and whisker plot showing the median, interquartile range, maximum and minimum vBMD values for the mandible, cervical and lumbar spine in females. BMD, bone mineral density.
Correlation between cervical, lumbar vertebrae and mandible vBMD for Males and Females
Values of the Spearman correlation coefficient between mandibular and vertebral vBMD ranged from R = 0.048 to 0.141 (Table 2). In males, the three correlation coefficients between mandibular and cervical vBMD (middle: R = 0.138; body: R = 0.126; angle: R = 0.122) were all statistically significant (p < 0.05). In females, the correlation between the middle mandibular site and cervical site was statistically significant (R = 0.141, p < 0.01). Of the other eight correlations examined, none was statistically significant.
Table 2.
Spearman rank correlation coefficients between vBMD measurements for males and females
| Sections | Males | Females | ||||
|---|---|---|---|---|---|---|
| Number | C2-7 | L2-4 | Number | C2-7 | L2-4 | |
| Middle | 270 | 0.138a | 0.057 | 391 | 0.141b | 0.048 |
| Body | 270 | 0.126a | 0.119 | 391 | 0.078 | 0.070 |
| Angle | 270 | 0.122a | 0.083 | 391 | 0.095 | 0.068 |
p values were for the correlation between mandible and spine analyzed using the Spearman rank correlation coefficient.
The correlation was statistically significant (p<0.05).
The correlation was statistically significant (p<0.01).
Discussion
In theory, the mandible should have similar metabolism with other cancellous rich sites such as the vertebral bodies. However, due to the unique characteristics of mandible anatomy, phylogenetic factors, complex motions from the surrounding muscles and tooth occulsions, mandibular bone mineral density has its own particularity.
Differences in the measurement of BMD using DXA and QCT are widely accepted and are related to the different treatment of cancellous and cortical bone.18–20 As a two-dimensional projection measurement, DXA measures all the bone within a specific area of interest and, unlike QCT, cannot differentiate between cortical and cancellous bone. This may affect pre-treatment evaluations of the condition of bone and future treatment plans. However, QCT is more advanced because it selectively measures the volumetric trabecular bone (cancellous bone only) without any superposition of cortical bone or other surrounding tissues.21–23
Previous studies have investigated the correlation between mandibular BMD and osteoporosis using different techniques.8,13 Miliuniene et al used DXA and panoramic radiographs to demonstrate a high probability of osteoporosis in cases where the cortical bone height of the mandibular angle was low.8 Li et al reported a correlation between bone loss at the manibular angle site and lumbar vertebrae bone loss using DXA.24 Lin et al showed that senile osteoporotic patients had significant mandibular cortical bone loss.25 Osteoporosis had no significant effect on healthy alveolar bone and the alveolar bone loss was mainly an age-related change.25 Allen et al found no significant correlation between panoramic radiograph-determined mandibular cortical thickness and the QCT-derived BMD of the lumbar vertebrae in survivors of childhood acute lymphoblastic leukemia.26 These studies of the mandible used panoramic radiographs and DXA, and were unable to distinguish between trabecular and cortical bone in the mandible. Analysis of cortical bone thickness by mandibular panoramic radiograph is a simple classification of changes in the cortex and is not able to distinguish between normal and osteopenic/osteoporotic postmenopausal edentulous females. The efficacy of the panoramic-based mandibular indices in diagnosing osteopenia/osteoporosis is low to moderate.27
The lumbar spine is regarded as the most sensitive site for BMD measurements for the diagnosis of osteoporosis.28 We investigated the trabecular vBMD of the mandibular middle, body, angle and the cervical and lumbar vertebrae. Values of the Spearman rank correlation coefficient in Table 2 were very low and the majority of the correlations between mandibular and vertebral vBMD were not statistically significant. We considered that there was little or no significant association between the mandibular vBMD and the axial bone BMD in a healthy population including subjects with osteopenia. The important question, however, is whether mandibular bone loss with age is similar to that in vertebral bone or whether it is subtly different. Mandibular bone develops as a membrane bone, whereas the vertebrae develop as endochondral bones. The mandible is of neural crest origin whereas the vertebral columns are of mesodermal origin. There are minor phenotypic differences between osteoblasts depending on their site of origin and anatomical location, which can be demonstrated biochemically.29–31 The mandible is formed by the complicated processes of ossification. However, aside from the spine BMD, additional undefined factors, including mechanical stress from occlusion, may be involved in maintaining mandibular BMD. Mandibular vBMD does not change synchronously with spine vBMD and is not a good predictor of spine vBMD. Therefore, mandibular vBMD measurements have limited value for the evaluation of systemic bone loss and should not replace spine vBMD in clinical practice for the assessment of osteoporotic fracture risk, for which the lumbar spine is still the first choice of BMD measurement site.
In conclusion, the results from our study showed that the correlations between mandibular vBMD and cervical and lumbar vertebral vBMD were poor and generally not statistically significant. These findings indicate that the change of mandibular vBMD often differs from that in the spine, and that mandibular vBMD should be measured independently for the assessment of mandibular bone status.
Footnotes
The authors Zhe Guo and Xia Du contributed equally to the work.
Contributor Information
Zhe Guo, Email: guoz1982@163.com.
Giuseppe Guglielmi, Email: giuseppe.guglielmi@unifg.it.
Khrystyna Zhurakivska, Email: khrystyna.zhurakivska@gmail.com.
Lorenzo Lo Muzio, Email: lorenzo.lomuzio@unifg.it.
Glen M Blake, Email: glen.blake@kcl.ac.uk.
Xiaoguang Cheng, Email: xiao65@263.net.
REFERENCES
- 1. Peck W . Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis . Am J Med 1993. ; 94 : 646 – 50 . doi: 10.1016/0002-9343(93)90218-e [DOI] [PubMed] [Google Scholar]
- 2. Organization WH . Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO study group [meeting held in Rome from 22 to 25 June 1992] ; 1994. . [PubMed]
- 3. Slaidina A , Soboleva U , Daukste I , Zvaigzne A , Lejnieks A . Postmenopausal osteoporosis and tooth loss . Stomatologija 2011. ; 13 : 92 – 5 . [PubMed] [Google Scholar]
- 4. Melton LJ , Chrischilles EA , Cooper C , Lane AW , Riggs BL . Perspective. How many women have osteoporosis? J Bone Miner Res 1992. ; 7 : 1005 – 10 . doi: 10.1002/jbmr.5650070902 [DOI] [PubMed] [Google Scholar]
- 5. Lee BD , White SC . Age and trabecular features of alveolar bone associated with osteoporosis . Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005. ; 100 : 92 – 8 . doi: 10.1016/j.tripleo.2004.11.020 [DOI] [PubMed] [Google Scholar]
- 6. Pérez JAM , Garcia FC , Palacios S , Pérez M . Epidemiology of risk factors and symptoms associated with menopause in Spanish women . Maturitas 2009. ; 62 : 30 – 6 . doi: 10.1016/j.maturitas.2008.10.003 [DOI] [PubMed] [Google Scholar]
- 7. Kanis JA , Kanis JA . Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a who report. who Study Group . Osteoporos Int 1994. ; 4 : 368 – 81 . doi: 10.1007/BF01622200 [DOI] [PubMed] [Google Scholar]
- 8. Miliuniene E , Alekna V , Peciuliene V , Tamulaitiene M , Maneliene R . Relationship between mandibular cortical bone height and bone mineral density of lumbar spine . Stomatologija 2008. ; 10 : 72 – 5 . [PubMed] [Google Scholar]
- 9. Jeffcoat MK , Lewis CE , Reddy MS , Wang CY , Redford M . Post-Menopausal bone loss and its relationship to oral bone loss . Periodontol 2000 2000. ; 23 : 94 – 102 . doi: 10.1034/j.1600-0757.2000.2230109.x [DOI] [PubMed] [Google Scholar]
- 10. Southard KA , Southard TE , Schlechte JA , Meis PA . The relationship between the density of the alveolar processes and that of post-cranial bone . J Dent Res 2000. ; 79 : 964 – 9 . doi: 10.1177/00220345000790041201 [DOI] [PubMed] [Google Scholar]
- 11. Oei L , Koromani F , Rivadeneira F , Zillikens MC , Oei EHG . Quantitative imaging methods in osteoporosis . Quant Imaging Med Surg 2016. ; 6 : 680 – 98 . doi: 10.21037/qims.2016.12.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Engelke K . Quantitative computed Tomography-Current status and new developments . J Clin Densitom 2017. ; 20 : 309 – 21 . doi: 10.1016/j.jocd.2017.06.017 [DOI] [PubMed] [Google Scholar]
- 13. Zhang Y , Zhou Z , Wu Cheng'ai , Zhao D , Wang C , Cheng X , et al. . Population-Stratified analysis of bone mineral density distribution in cervical and lumbar vertebrae of Chinese from quantitative computed tomography . Korean J Radiol 2016. ; 17 : 581 – 9 . doi: 10.3348/kjr.2016.17.5.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Du X , Jiao J , Cheng X , Wang L , Li K , Liu H , et al. . Age-Related changes of bone mineral density in mandible by quantitative computed tomography . J Biol Regul Homeost Agents 2017. ; 31 : 997 – 1003 . [PubMed] [Google Scholar]
- 15. Lang TF , Li J , Harris ST , Genant HK . Assessment of vertebral bone mineral density using volumetric quantitative CT . J Comput Assist Tomogr 1999. ; 23 : 130 – 7 . doi: 10.1097/00004728-199901000-00027 [DOI] [PubMed] [Google Scholar]
- 16. Bligh M , Bidaut L , White RA , Murphy WA , Stevens DM , Cody DD . Helical multidetector row quantitative computed tomography (QCT) precision . Acad Radiol 2009. ; 16 : 150 – 9 . doi: 10.1016/j.acra.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 17. Wang L , Wang W , Xu L , Cheng X , Ma Y , Liu D , et al. . Relation of visceral and subcutaneous adipose tissue to bone mineral density in Chinese women . Int J Endocrinol 2013. ; 2013 : 1 – 5 . doi: 10.1155/2013/378632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pickhardt PJ , Bodeen G , Brett A , Brown JK , Binkley N . Comparison of femoral neck BMD evaluation obtained using Lunar DXA and QCT with asynchronous calibration from CT colonography . J Clin Densitom 2015. ; 18 : 5 – 12 . doi: 10.1016/j.jocd.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 19. Emohare O , Dittmer A , Morgan RA , Switzer JA , Polly DW . Osteoporosis in acute fractures of the cervical spine: the role of opportunistic CT screening . J Neurosurg 2015. ; 23 : 1 – 7 . doi: 10.3171/2014.10.SPINE14233 [DOI] [PubMed] [Google Scholar]
- 20. Setiawati R , Di Chio F , Rahardjo P , Nasuto M , Dimpudus FJ , Guglielmi G . Quantitative assessment of abdominal aortic calcifications using lateral lumbar radiograph, dual-energy X-ray absorptiometry, and quantitative computed tomography of the spine . J Clin Densitom 2016. ; 19 : 242 – 9 . doi: 10.1016/j.jocd.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 21. Grampp S , Jergas M , Glüer CC , Lang P , Brastow P , Genant HK . Radiologic diagnosis of osteoporosis. current methods and perspectives . Radiol Clin North Am 1993. ; 31 : 1133 – 45 . [PubMed] [Google Scholar]
- 22. Bruno AG , Broe KE , Zhang X , Samelson EJ , Meng C-A , Manoharan R , et al. . Vertebral size, bone density, and strength in men and women matched for age and areal spine BMD . J Bone Miner Res 2014. ; 29 : 562 – 9 . doi: 10.1002/jbmr.2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheng X-G , Li K , Ou S-X , Tang G-Y , Wang Q-Q , Wang C , et al. . Heterogeneity in spinal bone mineral density among young adults from three eastern provincial capital cities in mainland China . J Clin Densitom 2017. ; 20 : 198 – 204 . doi: 10.1016/j.jocd.2016.03.009 [DOI] [PubMed] [Google Scholar]
- 24. Li N , Jing H , Li J , Zhou F , Bu L , Yang X . Study of mandible bone mineral density of Chinese adults by dual-energy X-ray absorptiometry . Int J Oral Maxillofac Surg 2011. ; 40 : 1275 – 9 . doi: 10.1016/j.ijom.2011.05.012 [DOI] [PubMed] [Google Scholar]
- 25. Lin Z-tong , Wang T-mei , Ge J-yu , Lin H , Zhu X-fen . Analysis of mandibular bone mineral density of senile osteoporosis patients . Zhonghua Kou Qiang Yi Xue Za Zhi 2010. ; 45 : 214 – 8 . [PubMed] [Google Scholar]
- 26. Allen B , Migliorati C , Rowland C , An Q , Shintaku W , Donaldson M , et al. . Comparison of mandibular cortical thickness and QCT-derived bone mineral density (BMD) in survivors of childhood acute lymphoblastic leukemia: a retrospective study . Int J Paediatr Dent 2016. ; 26 : 330 – 5 . doi: 10.1111/ipd.12203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Drozdzowska B , Pluskiewicz W , Tarnawska B . Panoramic-based mandibular indices in relation to mandibular bone mineral density and skeletal status assessed by dual energy X-ray absorptiometry and quantitative ultrasound . Dentomaxillofac Radiol 2002. ; 31 : 361 – 7 . doi: 10.1038/sj.dmfr.4600729 [DOI] [PubMed] [Google Scholar]
- 28. Miller PD . Bone mineral density--clinical use and application . Endocrinol Metab Clin North Am 2003. ; 32 : 159 – 79 . doi: 10.1016/S0889-8529(02)00086-5 [DOI] [PubMed] [Google Scholar]
- 29. Cheng A , Daly CG , Logan RM , Stein B , Goss AN . Alveolar bone and the bisphosphonates . Aust Dent J 2009. ; 54 ( Suppl 1 ): S51 – 61 . doi: 10.1111/j.1834-7819.2009.01143.x [DOI] [PubMed] [Google Scholar]
- 30. Meikle MC . Craniofacial development, growth and evolution: Bateson ; 2002. . [Google Scholar]
- 31. Zhuang X-M , Yu B-S , Zheng Z-M , Zhang J-F , Lu WW , BS Y , WW L . Effect of the degree of osteoporosis on the biomechanical anchoring strength of the sacral pedicle screws: an in vitro comparison between unaugmented bicortical screws and polymethylmethacrylate augmented unicortical screws . Spine 2010. ; 35 : E925 – 31 . doi: 10.1097/BRS.0b013e3181c5fb21 [DOI] [PubMed] [Google Scholar]