Abstract
Identification of reliable characteristics of follicle quality and developmental competence has been pursued in numerous studies, but with inconsistent outcomes. Here, we aimed to identify these characteristics by analysis of the follicular fluid (FF) steroid profile in relation to cumulus-oocyte complex (COC) morphology and follicle size, followed by molecular substantiation. Multiparous sows at weaning were used to facilitate analysis at the start of the follicular phase of the oestrus cycle. Sows with a higher average follicle size (≥5 mm vs. < 5 mm) had a higher follicular fluid β-estradiol concentration, but did not differ in other measured steroids. Sows with high compared to low percentage high-quality COCs (<70% vs. ≥70% high-quality) had follicular fluid with a higher concentration of β-estradiol, 19-norandrostenedione, progesterone, and α-testosterone, while the concentration of cortisol was lower. Transcriptome analysis of granulosa cells of healthy follicles of sows with a high percentage high-quality COCs showed higher abundance of transcripts involved in ovarian steroidogenesis (e.g., CYP19A2 and 3, POR, VEGFA) and growth (IGF1) and differential abundance of transcripts involved in granulosa cell apoptosis (e.g., GADD45A, INHBB). Differences in aromatase transcript abundance (CYP19A1, 2 and 3) were confirmed at the protein level. In addition, sows with a high percentage high-quality COCs lost less weight during lactation and had higher plasma IGF1 concentration at weaning, which may have affected COC quality. To the best of our knowledge, this study is also the first to report the relation between FF steroid profile and COC quality.
Keywords: steroid hormones, granulosa cells, metabolism, insulin-like growth factor, porcine, follicle
Sows with a higher percentage high-quality COCs have a distinct follicular fluid steroid profile and concomitant granulosa cell transcriptome.
Introduction
During ovarian follicular development, most follicles degenerate via follicular atresia, while only a few follicles reach the pre-ovulatory state from which an oocyte ovulates to produce viable offspring [1]. Hence, from a reproductive point of view, selection of the best quality follicles is of utmost importance. It is therefore not surprising that a vast number of studies have been dedicated to identifying reliable predictors of follicle and oocyte quality, which could be useful for the selection of oocytes with high developmental competence in in vitro fertilization (IVF) procedures [2, 3].
Some studies have investigated relationships between morphological characteristics of follicles or cumulus-oocyte complexes (COCs) and oocyte developmental competence, with very variable results. A number of these studies reported the presence of relations between antral follicle size and oocyte developmental competence, where oocytes from larger antral follicles show an increased blastocyst formation rate and implantation rate [4, 5]. Other studies did not find such relations [6, 7]. Distinct morphological classifications have also been used to assess oocyte competence, such as the number of cumulus cell layers [8], darkness of the ooplasm [9] and number of oocyte anomalies [10], again with variable outcomes [11]. Follicular fluid (FF) steroid profiling could provide a new tool to identify reliable markers for follicle quality and developmental competence, as FF steroid composition determines the microenvironment in which the oocyte develops. For example, estradiol and progesterone can improve oocyte developmental competence during in vitro maturation (IVM) in swine and cattle, when administered using optimal timing and dosing [12, 13]. Also, in swine, greater cleavage and blastocyst formation rates were found when oocytes were matured in FF with higher testosterone and androstenedione concentrations during IVM [14]. FF steroid concentrations can therefore influence oocyte developmental competence.
The aim of this study was to identify potential markers for follicle quality, by studying relations between FF steroid profile, COC morphology, and follicle size. As FF composition is also dependent on the steroidogenic activity of granulosa cells [15], we additionally analyzed granulosa cell transcript abundance using whole-genome transcriptome analysis to explain underlying mechanisms of sow differences in COC morphology and FF steroid profiles. We therefore hypothesized that the follicular fluid steroid profile and granulosa cell transcriptome differ between sows with a high vs. low percentage high-quality COCs, already at the start of the follicular phase onwards.
Studies on follicle and oocyte developmental capacity have mainly used widely available pre-pubertal porcine or bovine ovaries from the slaughterhouse, where the stage of the oestrus cycle is not well controlled or absent. Therefore, we used sows at the moment of weaning, as sows have a well-defined start of the follicular phase at the end of lactation [16]. In many animal species, but especially in sows, the metabolic state highly influences follicular development and FF content [17, 18]. Therefore, we additionally analyzed the sows’ metabolic state and its relations with follicular development and FF content.
Materials and methods
The experiment was approved by the Animal Care and Use Committee of Wageningen University (DEC2016036) and performed according to national and EU guidelines.
Animals
A total of 29 multiparous Dutch Landrace sows (parity 3 to 5; Topigs Norsvin, Vught, the Netherlands) with an average parity of 3.8 ± 0.2 were used. The sows were weighed approximately 1 week before parturition and immediately after weaning. Sow weight after parturition was estimated to calculate body weight loss during lactation, as described in Costermans et al. [19].
Blood and ovary collection
After a lactation of 26.1 ± 0.2 days, sows were killed by stunning and exsanguination within 2 h after weaning. Blood was collected in 9 ml ice-cold EDTA activator tubes (Greiner Bio-One, Monroe, NC, USA) and centrifuged at 3000 × g for 10 min at 4°C to collect plasma. The samples were stored at −20°C until further analysis. Left ovaries were immediately placed in an insulated container and after arrival in the lab placed in PBS pH 7.4 in a water bath at 37°C until follicle aspiration. Right ovaries were immediately snap-frozen in liquid N2 and stored in −80°C until follicle size, follicle health, and transcriptome analyses were performed.
Measurements
The experimental procedure used to assess ovarian measurements is summarized in Figure 1.
Figure 1.
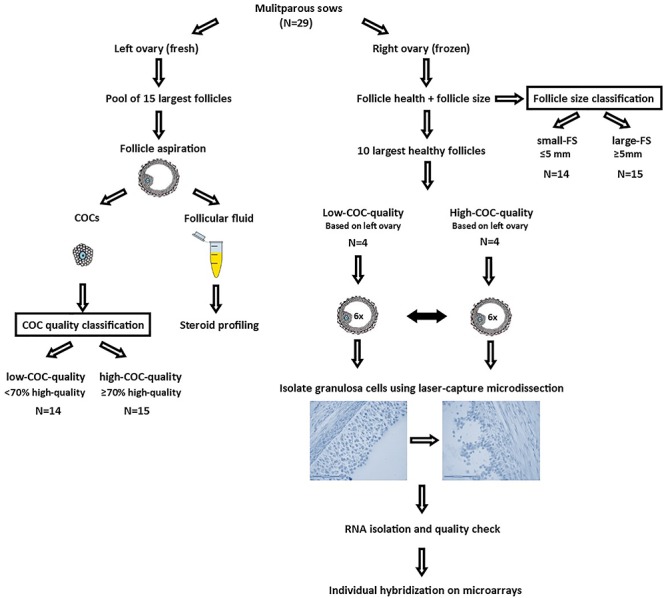
Experimental set-up of a total of 29 multiparous sows; left ovaries were used for follicle aspiration of the 15 largest follicles. Follicular fluid was used for steroid profiling using a modified UHPLC-MS/MS method, and cumulus-oocyte complexes (COCs) were morphologically classified as high-quality or low-quality to determine the percentage of high-quality COCs for each sow. Sows with < 70% high-quality COCs were classified as low-COC-quality and sows with ≥70% high-quality COCs as high-COC-quality. Right ovaries were used to determine follicle size and health status of all visible follicles of the right ovary, by using a cleaved-caspase 3 staining. Average follicle size of the 15 largest follicles was used to determine the follicle size class (small-FS < 5 mm vs. large-FS ≥ 5 mm). A subset of 8 out of 29 sows was used for whole-genome transcriptome analysis of granulosa cells, of which 4 were classified as low-COC-quality (54 ± 12% high-quality COCs) and 4 were classified as high-COC-quality (93 ± 15% high-quality COCs), as based on the left ovary. For each sow, granulosa cells of 6 healthy follicles of the pool of 10 largest healthy follicles were isolated using laser capture microdissection, after which samples were individually hybridized onto whole-genome microarrays for whole- genome transcriptome analysis.
Left ovary
COC morphology + amount of follicular fluid
The 15 largest follicles of the left ovary were used for follicle aspiration within 5 h from slaughter. The contents of the 15 follicles were pooled and collected in a tube and allowed to settle for 5 min. The supernatant was removed and centrifuged at 1900 × g at 4°C for 30 min to separate cells from the follicular fluid. The total volume of follicular fluid was assessed using reverse pipetting and subsequently stored at −20°C until further analysis. The recovered cumulus-oocyte complexes (COCs) were morphologically classified under a dissection microscope as high-quality (intact cumulus and normal-shaped oocyte) or low-quality (degraded cumulus or degenerated oocyte) similar to Alvarez et al. [20]. For each sow, the percentage high-quality COCs was calculated.
Steroid profiling
The follicular fluid of the 15 largest follicles of the left ovary was pooled to obtain a sufficient sample volume needed for endogenous steroid hormone profiling. A modified UHPLC-MS/MS method as described in Blokland et al. [21] was used to detect endogenous aglycons in FF. In short: 900 μl water was added to 100 μl follicular fluid and followed by solid phase extraction using an Oasis HLB 96-well SPE plate (Waters, Milford, CA, USA), derivatization of aglycons was performed with picolinic acid. Chromatographic separation of the aglycons was achieved on a Waters BEH C18 column (Waters) followed by analysis on a Xevo TQS mass spectrometer (Waters) in positive ESI mode.
Right ovary
Follicle size + follicle health
Right frozen ovaries were held against a ruler and photographed from different sides to measure the size of the 15 largest follicles using ImageJ (version 1.51f, National Institutes of Health). These 15 largest follicles were assumed to represent approximately half of the ovulatory follicle pool, as ovulation rates in modern sows are around 25–30 [22]. Subsequently, follicle health was assessed using an immunohistochemical staining against the apoptotic marker cleaved-Caspase 3 (cCASP), as described in Costermans et al. [19]. Follicles were classified as atretic when they had > 5% positively stained granulosa cells.
Granulosa cell transcriptomics
A subset of 8 out of 29 sows was used for whole-genome transcriptome analysis of granulosa cells, of which 4 were classified as low-COC-quality (54 ± 12% high-quality COCs of the largest 15 follicles) and 4 were classified as high-COC-quality (93 ± 15% high-quality COCs), based on left ovary classification. The right ovary was used for transcriptome analysis as it is essential for this analysis to obtain high quality intact RNA, for which the ovaries needed to be immediately frozen. The subset of sows which was used for transcriptome analysis had a similar average follicle size (5.1 ± 0.1 mm vs. 5.0 ± 0.1 mm in low-COC-quality and high-COC-quality, respectively). In short, for each of the 8 sows, granulosa cells of 6 individual healthy follicles of the pool of 10 largest healthy follicles were isolated using laser-capture microdissection and individually hybridized onto 4 × 44K Agilent whole-porcine genome arrays (G2519F-026440, Agilent Technologies Inc., Santa Clara, CA, USA) as described in Costermans et al. [23]. Microarray data have been deposited in NCBI Gene Expression Omnibus (GEO) under accession number GSE125189. Fold-change is expressed as the ratio of the normalized expression of sows with low-COC-quality (<70% high-quality COCs) over sows with high-COC-quality (≥70% high-quality COCs).
Aromatase immunostaining
The right ovary of the sows was used for quantification of aromatase protein abundance. A subset of 12 out of 29 sows was selected for analysis, 6 sows with low-COC-quality (37 ± 19%) and 6 sows with high-COC-quality (98 ± 5%), based on left ovary classification. For each of the 12 sows, both the largest and smallest follicles of the 10 largest healthy follicles were analyzed. Cryosections were mounted on Superfrost plus glass slides (Menzel- Gläser, Braunschweig, Germany), air-dried for 30 min, and fixed in 4% PAF for 10 min. Slides were subsequently washed in H2O, microwaved in sub-boiling 0.1 M sodium citrate buffer (pH 6) for 10 min for epitope antigen retrieval, cooled down to room temperature, and next rinsed with PBS pH 7.4. Aldehyde residues were blocked with 0.3% glycine in PBS for 10 min. After rinsing with PBS, sections were pre-incubated with 5% (wt/v) normal goat serum in PBS for 60 min at room temperature. Subsequently, the sections were incubated overnight at 4°C in a humid chamber with anti-aromatase primary antibody (ab18995, lotnr:GR3231482–1, Abcam, Cambridge, UK) diluted 1:50 in PBS-BSA-c (Aurion, Wageningen, The Netherlands). Next, sections were rinsed with PBS and incubated in the dark with a secondary Alexa fluor 488 labeled goat-anti-rabbit antibody (A-11008, ThermoFisher Scientific, Waltham, MA, USA) diluted 1:200 (v/v) in PBS-BSA-c for 1 h at room temperature. Sections were counterstained with DAPI (1 μg/ml; Sigma-Aldrich, Saint Louis, MO, USA) for 10 min. Sections were imaged at 10 times magnification using a fluorescence microscope (Leica DM6B), a digital camera (DFC365 FX), and imaging software (LasX; all Leica Microsystems, Amsterdam, The Netherlands). Mean staining intensity was determined using ImageJ for the complete intact granulosa cell layer. Those parts of the granulosa layer that were visually not intact were excluded from the analysis.
IGF1 measurement
Plasma insulin-like growth factor 1 (IGF1) content was measured by an immunoradiometric assay according to the manufacturer’s protocol (A15729, Beckman Coulter, Woerden, The Netherlands) supplemented with an additional acid-ethanol extraction (87.5%v/v EtOH and 2.9 v/v 12 N HCl). Samples were measured in duplicate and intra-assay coefficient of variation was ≤ 6% for all samples.
Statistical analyses
Distributions of the means and residuals were examined to verify model assumptions of normality and homogeneity of variance. Concentrations of β-Estradiol, progesterone, DHEA, α-testosterone, β-testosterone, 11-deoxycorticosterone, 11-deoxycortisol, cortisone, cortisol, DHT, 17α-OH-progesterone, and corticosterone were log transformed to obtain normality.
The presence of outliers was tested by calculating the studentized residuals using the REG procedure (SAS 9.4, Cary, NC, USA); identified outliers were removed from further analyses. One outlier was removed for β-estradiol, progesterone, dehydroepiandrostenedion (DHEA), α-testosterone, 11-deoxycortisol, 4-androsten-3,17-dione, and 17α-OH-progesterone. Differences between follicle size (FS) classes (average follicle size of 15 largest follicles: small-FS < 5 mm (N = 14) and large-FS ≥ 5 mm (N = 15)) and COC quality classes (percentage high-quality COCs of the 15 largest follicles: low-COC-quality<70% (N = 14) and high-COC-quality≥70% (N = 15)) were analyzed in the same model using the procedure GLM in models that also contained the factor PAR (PAR3 (parity 3, N = 14) and PAR4 + 5 (parity 4 and 5, N = 15)) and the interactions with PAR. The interactions were excluded from the models when not significant. All values are presented as LS means ± SE unless otherwise stated.
For microarray analysis, differences in transcript abundance were tested with models which included sow COC quality class (high-COC-quality or low-COC-quality) with sow as repeated subject, using the GENMOD procedure in SAS 9.4 (Cary, NC, USA). A false discovery rate (FDR) adjustment for multiple testing correction was done, according to Benjamini-Hochberg.
Fold-change (FC) is defined as the normalized expression of high-COC-quality over low-COC-quality. All transcripts with a FDR adjusted P ≤ 0.2 and absolute FC ≥ 1.2 were considered significantly different. Analysis of differences in aromatase staining intensity was performed using a 2-way ANOVA (Graphpad Prism version 5.04, GraphPad Software, La Jolla, CA, USA) using sow COC quality class (high-COC-quality (N = 4) and low-COC-quality (N = 4) and follicle size class (large or small) as factors. The interaction between sow COC quality class and follicle size class was not significant.
Results
High-COC-quality sows had lost less body weight on a percentage basis during lactation and had a higher IGF1 plasma concentration at weaning as compared to low-COC-quality sows, but did not differ in follicle size or volume (see Table 1). In follicles of high-COC-quality sows, follicular fluid β-estradiol, progesterone, 19-norandrostenedione, and α-testosterone concentrations were higher as compared to low-COC-quality sows, while cortisol concentration was lower (Figure 2). Concentrations of other steroids did not differ (Supplemental Table S1).
Table 1.
Effects of follicle size (av. follicle size of 15 largest follicles: Small-FS < 5 mm, N = 14 and Large-FS > 5 mm, N = 15) and COC quality class (percentage high-quality COCs: low-COC-quality<70%, N = 14 and high-COC-quality>70%, N = 15) on lactation characteristics, body condition, metabolic parameters, and follicular parameters.
| Follicle size | COC quality | P-values | ||||
|---|---|---|---|---|---|---|
| Small | Large | Low | High | FS | COC | |
| Lactation | ||||||
| Number of piglets weaned | 12.6 ± 0.3 | 12.7 ± 0.2 | 12.4 ± 0.3 | 12.9 ± 0.2 | 0.99 | 0.12 |
| Litter growth (kg) | 71 ± 3 | 73 ± 2 | 72 ± 3 | 72 ± 2 | 0.47 | 0.99 |
| Body condition | ||||||
| Weight parturition (kg) | 254 ± 6 | 243 ± 5 | 253 ± 6 | 242 ± 5 | 0.12 | 0.14 |
| Weight weaning (kg)a | 225 ± 4 | 220 ± 4 | 222 ± 4 | 224 ± 4 | 0.53 | 0.82 |
| Weight loss lactation (%) | 11 ± 2 | 10 ± 2 | 12 ± 2 | 7 ± 2 | 0.92 | 0.03 |
| IGF-1 (ng/ml) | 150 ± 17 | 138 ± 15 | 120 ± 16 | 167 ± 15 | 0.62 | 0.05 |
| Follicular parameters | ||||||
| Av. follicle size (mm) | 4.4 ± 0.1 | 5.6 ± 0.1 | 5.0 ± 0.1 | 5.1 ± 0.1 | <0.001 | 0.84 |
| Amount of follicular fluid (μl) | 334 ± 40 | 405 ± 38 | 363 ± 40 | 376 ± 38 | 0.22 | 0.82 |
| High-quality COCs (%) | 67 ± 4 | 70 ± 4 | 49 ± 4 | 89 ± 4 | 0.67 | <0.001 |
All values are presented as LS means±SE. COC = cumulus-oocyte complex. Follicle size was not related to percentage high-quality COCs.
aLS means estimates for the interaction FS*PAR (P < 0.01): Small-FS*PAR3: 228 ± 8, Small-FS*PAR4 + 5: 259 ± 6, Large-FS*PAR3: 252 ± 6, LargeFS*PAR4 + 5: 239 ± 6.
Figure 3.

Follicular fluid steroid concentrations (ng/ml) of sows with a small average follicle size (small-FS: average follicle size of the 15 largest follicles of the right ovary ≤ 5 mm, N = 14) vs. sows with a large follicle size (large-FS: average follicle size ≥5 mm, N = 15). *P-value < 0.05.
Figure 2.

Follicular fluid concentrations of progesterone (A), 19-norandrostenedione (B), α-testosterone (C), β-estradiol (D), and cortisol (E) ( ng/ml) of the 15 largest follicles of the left ovary of sows with high-COC-quality (≥70% high-quality COCs, N = 14) vs. sows with low-COC-quality (<70% high-quality COCs, N = 15). *P-value < 0.05.
In large-FS sows, average follicle size of the 15 largest follicles were larger as compared to small-FS sows (P < 0.001). No significant effects of follicle size were found in any of the lactational or metabolic measurements (see Table 1). Large-FS sows had a higher concentration of β-estradiol in follicular fluid (5.63 ± 0.60 vs. 3.51 ± 0.64 ng/ml, P = 0.03) compared to small-FS sows, but did not differ in any of the other measured steroid concentrations (Figure 3).
Subsequently, whole-genome granulosa cell transcriptomes of healthy antral follicles of the right ovary were assessed in a subset of 8 sows of which 4 were classified as high-COC-quality and 4 were classified as low-COC-quality. The analysis showed a total of 337 unique transcripts, which were differentially expressed. Transcript analysis showed an increased abundance of transcripts involved in ovarian steroidogenesis in sows with high-COC-quality, e.g., CYP19A2 and CYP19A3 (FC = 1.46 and FDR = 0.09 and FC = 1.43 and FDR = 0.03, respectively) and P450 oxidoreductase (POR; FC = 1.22, FDR = 0.03) and vascular endothelial growth factor A (VEGFA; FC = 1.22, FDR = 0.15) (see Table 2 for details). Next to genes involved in steroidogenesis, the top 10 regulated transcripts with a higher abundance in high-COC-quality sows included, e.g., inhibin subunit beta B (INHBB; FC = 1.64 and FDR = 0.05) and metallothionein 1A (MT1A; FC = 1.52 and FDR = 0.07) while the top 10 transcripts with a lower abundance in high-COC-quality sows include, e.g., growth arrest and DNA damage inducible alpha (GADD45A; FC = −1.63 and FDR = 0.08) (for details see Supplemental Table S2). In addition, granulosa cells of antral follicles in high-COC-quality sows had higher IGF1 transcript abundance (FC = 1.21 and FDR = 0.02). Pigs express multiple homologous (>99%) isoforms of aromatase, CYP19A1, 2 and 3, which are mainly expressed in ovary, placenta, and embryonic tissues, respectively [24]. As CYP19A2 and CYP19A3 were regulated, we additionally analyzed aromatase (CYP19A1–3) protein abundance. Sows with high-COC-quality had higher aromatase protein abundance as compared to sows with low-COC-quality (P < 0.01). Aromatase protein abundance did not differ between the large and small healthy antral follicles (P = 0.27) (Figure 4, Supplemental Figure S1).
Table 2.
Significantly different genes (absolute FC ≥ 1.2, FDR ≤ 0.2) involved in ovarian steroidogenesis (ref: Ovarian Kaleidoscope Database) between sows with high-COC-quality (93 ± 15% high-quality COCs (N = 4)) vs. sows with low-COC-quality (54 ± 12% high-quality COCs (N = 4))
| Gene symbol | Gene description | Accession no | FC | FDR |
|---|---|---|---|---|
| CYP19A2 | Cytochrome P450 19A2 | NM_214430 | 1.46 | 0.09 |
| CYP19A3 | Cytochrome P450 19A3 | NM_214431 | 1.43 | 0.03 |
| CYP51 | Cytochrome P450, family 51, subfamily A, polypeptide 1 | XM_005667583 | 1.28 | 0.05 |
| ENSSSCT00000010858 | TP53 regulated inhibitor of apoptosis 1 | - | 1.25 | <0.01 |
| HSD17B7 | Hydroxysteroid 17-beta dehydrogenase 7 | NM_001185137 | 1.25 | 0.05 |
| HSD17B4 | Hydroxysteroid 17-beta dehydrogenase 4 | XM_021081514 | 1.24 | 0.13 |
| POR | P450 oxidoreductase | NM_001129959 | 1.22 | 0.03 |
| VEGFA | Vascular endothelial growth factor A | NM_214084 | 1.22 | 0.15 |
| EPHX1 | Epoxide hydrolase 1 | NM_214355 | 1.21 | <0.01 |
| IHH | Indian hedgehog signaling molecule | NM_001244470 | −1.99 | 0.10 |
| PTCH1 | Patched 1 | XM_013980137 | −1.46 | 0.09 |
| PPARG | Peroxisome proliferator activated receptor gamma | NM_214084 | −1.41 | <0.01 |
| LGALS1 | Galectin 1 | NM_001001867 | −1.24 | 0.08 |
| GHR | Growth hormone receptor | NM_214254 | −1.24 | 0.20 |
| KLF13 | Kruppel-like factor 13 | NM_001011505 | −1.21 | 0.04 |
For each gene, the fold-change (FC) of high–COC–quality sows vs. low–COC–quality sows and significance (FDR) are shown.
Figure 4.
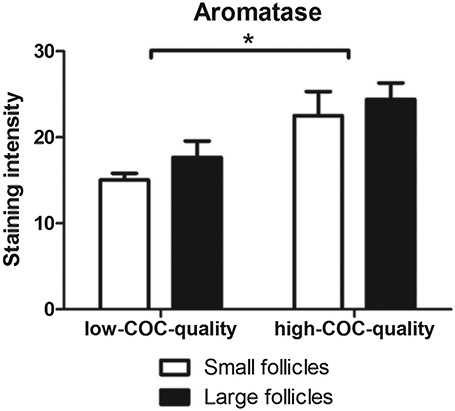
Aromatase protein abundance in granulosa cells of healthy follicles of sows with high-COC-quality (98 ± 5% high-quality COCs (N = 6)) vs. low-COC-quality (37 ± 19% high-quality COCs (N = 6)) in the smallest and largest follicle of the 10 largest healthy follicles. Data are presented as average ± SD. *P-value < 0.05.
Discussion
The aim of this study was to identify potential markers for antral follicle quality. We investigated relations between follicular fluid steroid concentrations, follicular development, and COC morphology in multiparous sows at the onset of the follicular phase of the oestrus cycle. To our knowledge, this is the first study in which porcine follicular fluid steroid profiling is reported, let alone in follicles at a specific stage of the estrous cycle.
Sows at the start of the follicular phase of the oestrus cycle with a large vs. small average follicle size had higher follicular fluid β-estradiol concentrations. In pigs, granulosa cell CYP19 abundance and subsequent β-estradiol production increases from the start of the follicular phase until the pre-ovulatory stage approximately 5 days later [25], and consequently as follicles develop, follicular fluid β-estradiol concentration increases with follicular diameter. In most studies, effects of follicle size on steroid concentrations were either not investigated in ovaries which were in the same stage of the oestrus cycle [26–28] or were only investigated at the final stages of follicular development, from pro-oestrus to late oestrus [29]. The results of our study are the first to indicate that from the pool of antral follicles, the larger subset of follicles has an increased β-estradiol concentration in follicular fluid already at the start of the follicular phase. Having said this, follicle size is not related to any of the other measured follicular fluid steroids. Follicular progesterone production is highest in the early luteal stage and only starts to increase in the latest stages of the follicular phase [27, 30], which can explain why we did not find a relation between follicular size and follicular fluid progesterone concentration. Although high levels of testosterone and androstenedione are present during the entire follicular phase [26, 30], these androgens can also be quickly aromatized into β-estradiol, which might explain why we did not find a relation between follicle size and testosterone and androstenedione steroid concentrations. Next, we compared the follicular fluid steroid profile of sows with high vs. low percentage of high-quality COCs. COC morphology was used as a marker for oocyte quality as COCs with an intact cumulus layer and normal shaped oocytes have the highest developmental capacity in vitro [20]. Sows with a high percentage high-quality COCs had a higher concentration of FF β-estradiol, progesterone, 19-norandrostenedione, and α-testosterone as compared to sows with a low percentage of high-quality COCs at the start of the follicular phase. Similarly, studies in pigs have shown that in early atretic antral follicles, transcript abundance of follicle-stimulating hormone receptor (FSHR), luteinizing hormone receptor (LHCGR), and CYP19 is lower [31, 32], resulting in lower FSH-induced estradiol production [33, 34] and lower in vitro cumulus cell progesterone production [35]. It therefore seems reasonable to assume that the follicle pool of 15 largest follicles from which the COCs were isolated and where we did not discriminate between healthy and atretic follicles contained a higher fraction of atretic follicles in sows with a low percentage high-quality COCs. Besides a higher concentration of follicular fluid β-estradiol, progesterone, 19-norandrostenedione, and α-testosterone, we found a lower concentration of cortisol in sows with a higher percentage of high-quality COCs. As cortisol is an important regulator of stress-related apoptosis of granulosa cells and oocytes via the FAS system [36], differences in FF cortisol concentration can at least partly explain the observed differences in COC quality.
To better understand differences in COC quality and FF fluid steroid content in sows, we performed whole-genome transcriptome analysis in granulosa cells. We used a subset of eight sows to analyze mural granulosa cell transcriptomes in healthy follicles only. This transcriptome analysis revealed an increased abundance of two transcripts (CYP19A2 and CYP19A3) encoding for cytochrome P450 aromatase, the granulosa cell terminal enzyme in the estrogen biosynthetic pathway responsible for the conversion of androgens into estradiol [29]. Immunostaining against aromatase (CYP19A1–3) confirms the increased aromatase abundance in granulosa cells of sows with a high percentage of high-quality COCs at the protein level. This increased granulosa cell aromatase transcript and protein abundance may explain the higher FF β-estradiol concentration in sows with high COC quality. In addition, although not differentially regulated, our granulosa cell transcriptome analysis showed that granulosa cells also express other steroidogenic enzymes already at the start of the follicular phase. These include steroidogenic acute regulatory protein (STAR), regulating cholesterol transfer within the mitochondria [37], cytochrome P450 family 11 subfamily A member 1 (CYP11A1), and hydroxysteroid 17-β dehydrogenase 1 (HSD17B1), involved in the conversion of cholesterol into androgens [38] (data not shown). Similarly, in a study by Garmey et al. [39], granulosa cells of cyclic gilts had a low transcript abundance of CYP11A and STAR mRNA and protein from approximately day 3 or 5, respectively, of the follicular phase. Apparently, porcine follicular development differs from that of rats and mice, as granulosa cells in the latter species only start to express CYP11A and STAR in pre-ovulatory follicles, so at a much later stage of follicular development [38, 40]. As CYP11A1, STAR, and HSD17B1 were expressed in granulosa cells, they might contribute to follicular androgen production in sows, already at the start of the follicular phase. However, these steroidogenic enzyme transcripts were not differentially regulated in granulosa cells of sows with high vs. low percentage high-quality COCs. Therefore, it cannot explain the higher progesterone, 19-norandrostenedione, and α-testosterone concentrations in FF of sows with high COC quality. Possibly, these steroidogenic enzymes might be differentially regulated at the protein level or have a different enzymatic activity. Thus, although granulosa cells contribute to follicular androgen production in sows, they are at least at this stage of follicular development not responsible for differentially regulating androgen concentrations in FF. This confirms the existence of the classical two-cell, two-gonadotropin concept for estrogen biosynthesis in the porcine ovary, in which theca cells play the major role in androgen production and its regulation [41].
Besides genes encoding enzymes participating in steroid metabolism, sows with a high vs. low percentage high-quality COCs had a differential abundance of transcripts, which are involved in regulation of enzymes involved in ovarian steroidogenesis. Examples of these differentially expressed transcripts are POR and VEGFA, which had a higher abundance in sows with a high percentage high-quality COCS. POR supplies electrons to all CYP enzymes for catalytic activity and is essential for steroid biosynthesis [42]. VEGFA is a growth factor which regulates angiogenesis in the ovary [43] and was shown to increase CYP19A1 granulosa cell transcript abundance after addition to granulosa cell culture medium [44]. Therefore, higher POR and VEGFA might be involved in increasing ovarian steroid biosynthesis at the start of the follicular phase in sows with a high percentage high-quality COCs. Next to genes involved in ovarian steroidogenesis, we also found differential abundance of transcripts regulating ovarian follicular atresia, e.g., INHBB and GADD45A. GADD45A abundance was lower in sows with a high percentage high-quality COCs. GADD45A is involved in the DNA damage response and has a higher abundance in porcine atretic follicles [45]. GADD45A might provide a valuable marker for early atretic follicles, as it was already expressed in the antral follicles of our study which have been classified as healthy, as they did not (yet) express cleaved-caspase 3. Granulosa cell INHBB transcript abundance was higher in sows with a high percentage high-quality COCs. INHBB encodes for the B subunit of activin and inhibin complexes. Follicular atresia has been previously associated with less INHBB transcript abundance in porcine granulosa cells [45, 46].
Other genes that had a higher transcript abundance in granulosa cells of sows with a high percentage high-quality COCs encode for proteins that are protective against oxidative stress e.g., MT1A, GPX3, PRDX3, PRDX6, and SOD2. These proteins might play a role in protecting the ovarian tissues from oxidative stress [47–50]. High levels of reactive oxygen species are produced during steroid biosynthesis, which results in a higher abundance of superoxide dismutase, catalase, and glutathione peroxidase in steroid secreting areas of the ovary [51]. In our study, follicles of sows with a high percentage high-quality COCs produce more steroids, as seen by the higher concentrations of FF steroids and abundance of transcripts involved in steroidogenesis, which might explain the increased abundance of transcripts involved in the oxidative stress response.
Follicle size and health status and COC quality can be affected by environmental factors, such as the nutritional status. In the current study, ovaries were obtained within 2 h after weaning. Therefore, we analyzed the lactational metabolic state of the sows as well, as this might underlie differences in follicular development [52]. Sows with a higher percentage high-quality COCs at the day of weaning lost a lower percentage of their body weight during lactation and had a higher plasma IGF1 concentration at weaning as compared to sows with a low percentage high-quality COCs. In addition, granulosa cells of sows with a high percentage high-quality COCs express more IGF1. Peripheral IGF1 indirectly influences follicular development by binding to insulin and IGF1 receptors in the hypothalamus and pituitary and therefore stimulates the production of the gonadotropins FSH and LH [53]. In addition, both peripheral IGF1 and locally produced IGF1 are essential for follicular development, as IGF1 can activate IGF1 signaling in granulosa cells and synergize with FSH to stimulate follicular growth and steroidogenesis [54]. This effect is mediated by activation of phosphatidylinositol-dependent kinase/AKT, as shown for bovine granulosa cells in vitro [55]. In human cumulus cells, effects of FSH on cumulus cell transcript abundance of genes involved in cholesterol and steroid metabolism are minimal when IGF1R is blocked [56]. This provides further evidence for the essential role of IGF1 in regulation of steroid production. Next to this, both FSH and IGF1 have been identified as anti-apoptotic factors [57]. Therefore, the relatively low IGF1 concentration in plasma of sows with a low percentage of high-quality COCs might be insufficient to support follicular development and steroidogenesis resulting in an increased risk of follicular atresia. Indeed, the metabolic state of the sows during lactation seems to influence follicular development and FF content.
In conclusion, we found that a positive relation between follicle size and follicular fluid β-estradiol concentration already exists at the start of the follicular phase, as sows with larger follicles had a higher FF β-estradiol concentration. Follicles of sows with a high percentage high-quality COCs contained more β-estradiol, progesterone, 19-noradrenostenedione, and α-testosterone and less cortisol, which could therefore serve as potential markers to identify early atretic follicles with decreased follicular developmental competence.
By comparing transcriptomes of healthy follicles of sows with a high vs. low percentage high-quality COCs, we identified several genes involved in granulosa cell steroidogenesis (e.g., POR and VEGFA) and genes involved in regulating granulosa cell apoptosis (e.g., GADD45A and INHBB), which could serve as markers for follicle quality.
Conflict of interest
The authors declare that there are no conflicts of interest that could be perceived as precluding the impartiality of the results reported.
Funding
The reported research was funded by the Wageningen Institute for Animal Sciences (WIAS) and NWO for providing the NWO-WIAS Graduate Programme 2015 grant.
Acknowledgements
We acknowledge Evert van Schothorst and Klaas Frankena for their help with the microarray data analysis.
Supplementary Material
References
- 1. McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev 2000; 21:200–214. [DOI] [PubMed] [Google Scholar]
- 2. Rienzi L, Vajta G, Ubaldi F. Predictive value of oocyte morphology in human IVF: a systematic review of the literature. Hum Reprod Update 2010; 17:34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Broekmans F, Kwee J, Hendriks D, Mol B, Lambalk C. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 2006; 12:685–718. [DOI] [PubMed] [Google Scholar]
- 4. Algriany O, Bevers M, Schoevers E, Colenbrander B, Dieleman S. Follicle size-dependent effects of sow follicular fluid on in vitro cumulus expansion, nuclear maturation and blastocyst formation of sow cumulus oocytes complexes. Theriogenology 2004; 62:1483–1497. [DOI] [PubMed] [Google Scholar]
- 5. Lequarre A-S, Vigneron C, Ribaucour F, Holm P, Donnay I, Dalbies-Tran R, Callesen H, Mermillod P. Influence of antral follicle size on oocyte characteristics and embryo development in the bovine. Theriogenology 2005; 63:841–859. [DOI] [PubMed] [Google Scholar]
- 6. Pavlok A, Lucas-Hahn A, Niemann H. Fertilization and developmental competence of bovine oocytes derived from different categories of antral follicles. Mol Reprod Dev 1992; 31:63–67. [DOI] [PubMed] [Google Scholar]
- 7. Seneda MM, Esper CR, Garcia JM, De Oliveira JA, Vantini R. Relationship between follicle size and ultrasound-guided transvaginal oocyte recovery. Anim Reprod Sci 2001; 67:37–43. [DOI] [PubMed] [Google Scholar]
- 8. Yuan Y, Van Soom A, Leroy J, Dewulf J, Van Zeveren A, De Kruif A, Peelman L. Apoptosis in cumulus cells, but not in oocytes, may influence bovine embryonic developmental competence. Theriogenology 2005; 63:2147–2163. [DOI] [PubMed] [Google Scholar]
- 9. Nagano M, Katagiri S, Takahashi Y. Relationship between bovine oocyte morphology and in vitro developmental potential. Zygote 2006; 14:53–61. [DOI] [PubMed] [Google Scholar]
- 10. Mikkelsen AL, Lindenberg S. Morphology of in-vitro matured oocytes: impact on fertility potential and embryo quality. Hum Reprod 2001; 16:1714–1718. [DOI] [PubMed] [Google Scholar]
- 11. Wang Q, Sun Q-Y. Evaluation of oocyte quality: morphological, cellular and molecular predictors. Reprod Fertil Dev 2006; 19:1–12. [DOI] [PubMed] [Google Scholar]
- 12. Yuan B, Liang S, Jin Y-X, Kwon J-W, Zhang J-B, Kim N-H. Progesterone influences cytoplasmic maturation in porcine oocytes developing in vitro. PeerJ 2016a; 4:e2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matsuo M, Sumitomo K, Ogino C, Gunji Y, Nishimura R, Hishinuma M. Three-step in vitro maturation culture of bovine oocytes imitating temporal changes of estradiol-17β and progesterone concentrations in preovulatory follicular fluid. Arch Anim Breed 2017; 60:385–390. [Google Scholar]
- 14. Grupen CG, Mcilfatrick SM, Ashman RJ, Boquest AC, Armstrong DT, Nottle MB. Relationship between donor animal age, follicular fluid steroid content and oocyte developmental competence in the pig. Reprod Fertil Dev 2003; 15:81–87. [DOI] [PubMed] [Google Scholar]
- 15. Fortune J. Ovarian follicular growth and development in mammals. Biol Reprod 1994; 50:225–232. [DOI] [PubMed] [Google Scholar]
- 16. Soede NM, Langendijk P, Kemp B. Reproductive cycles in pigs. Anim Reprod Sci 2011; 124:251–258. [DOI] [PubMed] [Google Scholar]
- 17. Quesnel H, Pasquier A, Mounier A, Prunier A. Influence of feed restriction during lactation on gonadotropic hormones and ovarian development in primiparous sows. J Anim Sci 1998; 76:856–863. [DOI] [PubMed] [Google Scholar]
- 18. Yang H, Foxcroft G, Pettigrew J, Johnston L, Shurson G, Costa A, Zak L. Impact of dietary lysine intake during lactation on follicular development and oocyte maturation after weaning in primiparous sows. J Anim Sci 2000; 78:993–1000. [DOI] [PubMed] [Google Scholar]
- 19. Costermans NGJ, Teerds KJ, Keijer J, Knol E, Koopmanschap R, Kemp B, Soede N. Follicular development of sows at weaning in relation to estimated breeding value for within-litter variation in piglet birth weight. Animal 2019a; 13:554–563. [DOI] [PubMed] [Google Scholar]
- 20. Alvarez GM, Dalvit GC, Achi MV, Migeuz MS, Cetica PD. Immature oocyte quality and maturational competence of porcine cumulus-oocyte complexes subpopulations. Biocell 2009; 33:167–177. [PubMed] [Google Scholar]
- 21. Blokland MH, Van Tricht EF, Van Ginkel LA, Sterk SS. Applicability of an innovative steroid-profiling method to determine synthetic growth promoter abuse in cattle. J Steroid Biochem Mol Biol 2017; 174:265–275. [DOI] [PubMed] [Google Scholar]
- 22. Da Silva CLA, Laurenssen B, Knol E, Kemp B, Soede N. Relationships between ovulation rate and embryonic and placental chracteritsics in multiparous sows at 35 days of pregnancy. Relationship between ovulation rate and embryonic characteristics in gilts at 35 d of pregnancy. J Anim Sci 2017; 95:3160–3172. [DOI] [PubMed] [Google Scholar]
- 23. Costermans NGJ, Keijer J, van Schothorst EM, Kemp B, Keshtkar S, Bunschoten A, Soede NM, Teerds KJ. In ovaries with high or low variation in follicle size, granulosa cells of antral follicles exhibit distinct size-related processes. Mol Hum Reprod 2019; 25:614–624. [DOI] [PubMed] [Google Scholar]
- 24. Graddy L, Kowalski A, Simmen F, Davis S, Baumgartner W, Simmen R. Multiple isoforms of porcine aromatase are encoded by three distinct genes. J Steroid Biochem Mol Biol 2000; 73:49–57. [DOI] [PubMed] [Google Scholar]
- 25. Grant SA, Hunter M, Foxcroft G. Morphological and biochemical characteristics during ovarian follicular development in the pig. J Reprod Fertil 1989; 86:171–183. [DOI] [PubMed] [Google Scholar]
- 26. Ainsworth L, Tsang B, Downey B, Marcus G, Armstrong D. Interrelationships between follicular fluid steroid levels, gonadotropic stimuli, and oocyte maturation during preovulatory development of porcine follicles. Biol Reprod 1980; 23:621–627. [DOI] [PubMed] [Google Scholar]
- 27. Liu J, Koeningsfeld AT, Cantley TC, Boyd CK, Kobayashi Y, Lucy MC. Growth and the initiation of steroidogenesis in porcine follicles are associated with unique patterns of gene expression for individual components of the ovarian insulin-like growth factor system. Biol Reprod 2000; 63:942–952. [DOI] [PubMed] [Google Scholar]
- 28. Bertoldo M, Holyoake P, Evans G, Grupen C. Follicular progesterone levels decrease during the period of seasonal infertility in sows. Reprod Domest Anim 2011; 46:489–494. [DOI] [PubMed] [Google Scholar]
- 29. Conley A, Howard H, Slanger W, Ford J. Steroidogenesis in the preovulatory porcine follicle. Biol Reprod 1994; 51:655–661. [DOI] [PubMed] [Google Scholar]
- 30. Mahajan DK. Pig model to study dynamics of steroids during ovarian follicular growth and maturation In: Conn PM. (eds.), Sourcebook of Models for Biomedical Research. Humana Press Inc: Totowa, New Jersey, 2008: 425–436.
- 31. Tilly J, Kowalski K, Schomberg D, Hsueh A. Apoptosis in atretic ovarian follicles is associated with selective decreases in messenger ribonucleic acid transcripts for gonadotropin receptors and cytochrome P450 aromatase. Endocrinology 1992; 131:1670–1676. [DOI] [PubMed] [Google Scholar]
- 32. Pan Z, Zhang J, Lin F, Ma X, Wang X, Liu H. Expression profiles of key candidate genes involved in steroidogenesis during follicular atresia in the pig ovary. Mol Biol Rep 2012; 39:10823–10832. [DOI] [PubMed] [Google Scholar]
- 33. Westhof G, Westhof KF, Braendle WL, Dizerega GS. Differential steroid secretion and gonadotropin response by individual tertiary porcine follicles in vitro. Possible physiologic role of atretic follicles. Biol Reprod 1991; 44:461–468. [DOI] [PubMed] [Google Scholar]
- 34. Guthrie H, Cooper B. Follicular atresia, follicular fluid hormones, and circulating hormones during the midluteal phase of the estrous cycle in pigs. Biol Reprod 1996; 55:543–547. [DOI] [PubMed] [Google Scholar]
- 35. Grupen CG, Armstrong DT. Relationship between cumulus cell apoptosis, progesterone production and porcine oocyte developmental competence: temporal effects of follicular fluid during IVM. Reprod Fertil Dev 2010; 22:1100–1109. [DOI] [PubMed] [Google Scholar]
- 36. Yuan H-J, Han X, He N, Wang G-L, Gong S, Lin J, Gao M, Tan J-H. Glucocorticoids impair oocyte developmental potential by triggering apoptosis of ovarian cells via activating the Fas system. Sci Rep 2016; 6:24036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 1994; 269:28314–28322. [PubMed] [Google Scholar]
- 38. Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev 2004; 25:947–970. [DOI] [PubMed] [Google Scholar]
- 39. Garmey JC, Guthrie H, Garrett WM, Stoler MH, Veldhuis JD. Localization and expression of low-density lipoprotein receptor, steroidogenic acute regulatory protein, cytochrome P450 side-chain cleavage and P450 17-α-hydroxylase/C17–20 lyase in developing swine follicles: in situ molecular hybridization and immunocytochemical studies. Mol Cell Endocrinol 2000; 170:57–65. [DOI] [PubMed] [Google Scholar]
- 40. Ronen-Fuhrmann T, Timberg R, King SR, Hales KH, Hales DB, Stocco DM, Orly J. Spatio-temporal expression patterns of steroidogenic acute regulatory protein (StAR) during follicular development in the rat ovary. Endocrinology 1998; 139:303–315. [DOI] [PubMed] [Google Scholar]
- 41. Magoffin DA. Ovarian theca cell. Int J Biochem Cell Biol 2005; 37:1344–1349. [DOI] [PubMed] [Google Scholar]
- 42. Miller WL, Agrawal V, Sandee D, Tee MK, Huang N Choi JH, Morrissey K, Giacomini KM. Consequences of POR mutations and polymorphisms. Mol Cell Endocrinol 2011; 336:174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McFee RM, Cupp AS. Vascular contributions to early ovarian development: potential roles of VEGFA isoforms. Reprod Fertil Dev 2013; 25:333–342. [DOI] [PubMed] [Google Scholar]
- 44. Mishra SR, Bharati J, Rajesh G, Chauhan VS, Sharma GT, Bag S, et al. Fibroblast growth factor 2 (FGF2) and vascular endothelial growth factor A (VEGFA) synergistically promote steroidogenesis and survival of cultured buffalo granulosa cells. Anim Reprod Sci 2017; 179:88–97. [DOI] [PubMed] [Google Scholar]
- 45. Terenina E, Fabre S, Bonnet A, Monniaux D, Robert-Granié C, Sancristobal M, Sarry J, Vignoles F, Gondret F, Monget P. Differentially expressed genes and gene networks involved in pig ovarian follicular atresia. Physiol Genomics 2016; 49:67–80. [DOI] [PubMed] [Google Scholar]
- 46. Tu F., Pan Z., Yao Y., Liu H., Liu S., Xie Z., & Li Q.. miR-34a targets the inhibin beta B gene, promoting granulosa cell apoptosis in the porcine ovary. Genet Mol Res 2014; 13(2):2504–2512. [DOI] [PubMed] [Google Scholar]
- 47. Espey L, Ujioka T, Okamaru H, Richards J. Metallothionein-1 messenger RNA transcription in steroid-secreting cells of the rat ovary during the periovulatory period. Biol Reprod 2003; 68:1895–1902. [DOI] [PubMed] [Google Scholar]
- 48. Alscher DM, Braun N, Biegger D, Stuelten C, Gawrosnki K, Mürdter TE, Kuhlmann U, Fritz P. Induction of metallothionein in proximal tubular cells by zinc and its potential as an endogenous antioxidant. Kidney Blood Press Res 2005; 28:127–133. [DOI] [PubMed] [Google Scholar]
- 49. Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz RM. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology 1998; 139:4008–4011. [DOI] [PubMed] [Google Scholar]
- 50. Espinosa-Diez C, Miguel V, Mennerich D, Kietzmann T, Sánchez-Pérez P, Cadenas S, Lamas S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol 2015; 6:183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hanukoglu I. Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metab Rev 2006; 38:171–196. [DOI] [PubMed] [Google Scholar]
- 52. Hazeleger W, Soede NM, Kemp B. The effect of feeding strategy during the pre-follicular phase on subsequent follicular development in the pig. Domest Anim Endocrinol 2005; 29:362–370. [DOI] [PubMed] [Google Scholar]
- 53. Wolfe A, Divall S, Wu S. The regulation of reproductive neuroendocrine function by insulin and insulin-like growth factor-1 (IGF-1). Front Neuroendocrinol 2014; 35:558–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xia P, Tekpetey FR, Armstrong DT. Effect of IGF-I on pig oocyte maturation, fertilization, and early embryonic development in vitro, and on granulosa and cumulus cell biosynthetic activity. Mol Reprod Dev 1994; 38:373–379. [DOI] [PubMed] [Google Scholar]
- 55. Mani A. M., Fenwick M. A., Cheng Z., Sharma M. K., Singh D., Wathes D. C. IGF1 induces up-regulation of steroidogenic and apoptotic regulatory genes via activation of phosphatidylinositol-dependent kinase/AKT in bovine granulosa cells. Reproduction 2010; 139(1):139–151. [DOI] [PubMed] [Google Scholar]
- 56. Stocco C., Baumgarten S. C., Armouti M., Fierro M. A., Winston N. J., Scoccia B., Zamah A. M. Genome-wide interactions between FSH and insulin-like growth factors in the regulation of human granulosa cell differentiation. Hum Reprod 2017; 32(4):905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Guthrie H, Garrett W, Cooper B. Follicle-stimulating hormone and insulin-like growth factor-I attenuate apoptosis in cultured porcine granulosa cells. Biol Reprod 1998; 58:390–396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


