Abstract
Activins selectively stimulate follicle-stimulating hormone (FSH) secretion by pituitary gonadotrope cells. More recently, other members of the TGFbeta superfamily, the bone morphogenetic proteins (BMPs), were reported to regulate FSH synthesis. Activins and BMPs independently and synergistically stimulate transcription of the FSHbeta subunit (Fshb) gene in immortalized gonadotrope-like cells. Both ligands can signal via the activin receptor type IIA (ACVR2A) to regulate FSH synthesis in vitro. In vivo, global Acvr2a knockout mice exhibit a 60% reduction in circulating FSH relative to wild-type animals, suggesting that activins, BMPs, or related ligands might signal through additional type II receptors to regulate FSH in vivo. Although the leading candidates are ACVR2B and the BMP type II receptor (BMPR2), only the latter mediates activin or BMP2 induction of Fshb transcription in vitro. Here, we generated mice carrying a loss of function mutation in Bmpr2 specifically in gonadotropes. Puberty onset, estrous cyclicity, and reproductive organ weights were similar between control and conditional knockout females. Serum FSH and luteinizing hormone (LH) and pituitary expression of Fshb and the LHbeta subunit (Lhb) were similarly unaffected by the gene deletion in both sexes. These results suggest that BMPR2 might not play a necessary role in FSH synthesis or secretion in vivo or that another type II receptor, such as ACVR2A, can fully compensate for its absence. These data also further contribute to the emerging concept that BMPs may not be physiological regulators of FSH in vivo.
Keywords: anterior pituitary, FSH, bone morphogenetic protein, activin receptor-like kinase, Cre-lox
Ablation of BMPR2 in gonadotropes does not affect gonadotropin levels, suggesting that TGFbeta ligands other than BMPs and activins may be the primary regulators of FSH synthesis in mice.
Introduction
TGFbeta superfamily ligands play important roles in reproduction and development [1, 2]. In the mid-1980s, the activins were characterized by their capacity to selectively stimulate FSH secretion from gonadotrope cells of the anterior pituitary gland [3]. More recently, other ligands in the family, the bone morphogenetic proteins (BMPs), were similarly implicated in FSH regulation [4–7]. BMP2, for example, independently and synergistically with activin A stimulates FSHbeta subunit (Fshb) transcription in LbetaT2 cells, an immortalized gonadotrope-like cell line [4, 8].
TGFbeta ligands signal through type I and type II serine/threonine receptor complexes. Although BMPs and activins share the ability to bind to three different type II receptors [activin receptors type II A and B (ACVR2A, ACVR2B) [9–12] and the bone morphogenetic type II receptor (BMPR2) [8, 13, 14]], they signal intracellularly via distinct type I receptors. BMPs use the BMP type IA (BMPR1A or ALK3) and IB receptors (BMPR1B or ALK6), as well as the activin receptor type IA (ACVR1A or ALK2). In contrast, activins signal via ACVR1B (ALK4) and ACVR1C (ALK7) [12, 15–17].
Contrary to studies in cell lines, BMP2 fails to regulate Fshb expression in primary pituitary cultures and gonadotrope-specific depletion of the type I receptor ALK3 (alone or with ALK2) in mice does not impair FSH synthesis [18]. These data suggest that BMPs may be dispensable for Fshb expression in vivo [19]. Regardless of the relevant ligand(s), however, the relative contributions of the type II receptors ACVR2A, ACVR2B, and BMPR2 to TGFbeta superfamily regulation of FSH synthesis in vivo is presently unknown.
Acvr2a knockout animals are FSH-deficient, showing a ~ 60% reduction in serum FSH levels [20]. It is possible that the residual FSH in these mice may be mediated by activin or BMP signaling through ACVR2B or BMPR2 [21, 22]. However, knockdown of BMPR2, but not ACVR2B, impairs activin and BMP2 induction of Fshb transcription in LbetaT2 cells [23]. BMPR2’s role in FSH synthesis in vivo has not previously been determined because Bmpr2 knockout mice die during embryonic development [24]. Therefore, we used a Cre-lox approach to ablate BMPR2 specifically in murine gonadotrope cells and assessed the effects on FSH production in vivo and in vitro.
Materials and methods
Reagents
RQ1 RNase-free DNase (M6101), random hexamer primers (C1181), Moloney murine leukemia virus (MMLV)-reverse transcriptase (M1701), and RNasin (N2511) were from Promega (Madison, WI, USA). Medium 199 (M199; 31100-035), HBSS without calcium/magnesium (14170-112), and TRIzol Reagent were from Invitrogen (Burlington, ON, Canada). EvaGreen ×2 quantitative polymerase chain reaction (qPCR) MasterMix-S was from Applied Biological Materials, Inc. (Richmond, BC, Canada). Oligonucleotides were purchased from Integrated DNA Technologies, Inc. (Coralville, IA, USA). SB431542 (S4317), pancreatin (P3292), and collagenase (Type I-C0130) were from Sigma (St. Louis, MO, USA). Gentamycin (450-135-XL), ×100 antibiotic–antimycotic (450-115-EL), and deoxynucleotide triphosphates (800-401-TL) were from Wisent (St-Bruno, QC, Canada).
Mice
We crossed Bmpr2fl/fl mice [25] (obtained from https://www.mmrrc.org/) and GnrhrGRIC mice [26] to generate conditional knockouts (Bmpr2fl/fll;GnrhrGRIC/+, hereafter cKO) and littermate controls (Bmpr2fl/fl). The floxed Bmpr2 allele contains loxP sites flanking exons 4 and 5 [25]. Recombination deletes the receptor’s transmembrane domain and, through a frame-shift, the entirety of the kinase domain. In the GRIC strain, Cre is produced from a bicistronic messenger RNA (mRNA) under the control of the endogenous gonadotropin-releasing hormone receptor (Gnrhr) locus. Mice were kept in a light (lights on 07:00 to 19:00 h) and temperature controlled room, and were fed ad libitum. In order to genetically label gonadotrope cells, GnrhrGRIC/GRIC mice were crossed with B6.129(Cg)-Gt (ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J (mT/mG mice) [27] reporter mice acquired from Jackson Laboratories (Bar Harbor, Maine, strain number 007676). To generate Bmpr2fl/fl;GnrhrGRIC/+;mT/mG/+ mice (cKO;mT/mG mice), we crossed Bmpr2fl/fl;GnrhrGRIC/+ females with Bmpr2fl/fl;mT/mG/mT/mG males. All animal experiments were performed in accordance with federal guidelines and were approved by Goodman Cancer Research Centre Facility Animal Care Committee at McGill University (Animal Use Protocol No. 5204). At 9–10 weeks old, animals were weighed and sacrificed by CO2 asphyxiation. Blood, pituitary glands, and reproductive organs were harvested. Testes, ovaries, and uteri were weighed on a precision balance.
DNA extraction and genotyping
Genomic DNA was extracted from toe or tail biopsies using 0.5 ml of lysis buffer (100 mmol/L Tris–HCl (pH 8.5), 5 mmol/L ethylenediaminetetraacetic acid (pH 8.0), 200 mmol/L NaCl, 0.2% (v/v) sodium dodecyl sulphate, and 100 μg/ml proteinase K). Samples were incubated at 55°C overnight in a water bath. After centrifugation at 12 000 rpm for 10 min, the supernatant was collected and mixed by inversion with 0.5 ml isopropanol. Precipitated DNA was collected with a micropipette tip and dissolved in 40 μl of 10 mmol/L Tris, 1 mmol/L EDTA (pH 8.0). Following the same procedure, DNA was extracted from several tissues of Bmpr2fl/fl;GnrhrGRIC/+ mice to examine tissue-specific recombination. Wild-type, floxed, and recombined Bmpr2 alleles, as well as the GRIC and mT/mG alleles, were detected by PCR using the primers indicated in Table 1.
Table 1.
Genotyping and RT-qPCR primers
| Genotyping |
| Cre |
| Fwd: GGACATGTTCAGGGATCGCCAGGC |
| Rev: GCATAACCAGTGAAACAGCATTGCTG |
| Bmpr2 |
| Fwd: GGCAGACTCTGACTTTGACGCTAG |
| Rev: TTATTGTAAGTACACTGTTGCTGTC |
| Fw Rec: CACACCAGCCTTATACTCTAGATAC |
| ROSA26Sortm4(ACTB-tdTomato,-EGFP) |
| Fwd-WT: AGGGAGCTGCAGTGGAGTAG |
| Fwd-mT/mG: TAGAGCTTGCGGAACCCTTC |
| Rev: CTTTAAGCCTGCCCAGAAGA |
| RT-qPCR |
| Fshb |
| Fwd: GTGCGGGCTACTGCTACACT |
| Rev: CAGGCAATCTTACGGTCTCG |
| Lhb |
| Fwd: ACTGTGCCGGCCTGTCAACG |
| Rev: AGCAGCCGGCAGTACTCGGA |
| Bmpr2 |
| Fwd: GAATGTTGACAGGAGACCGGA |
| Rev: TTATCCAGGTCAAGGGAGGGC |
| Rpl19 |
| Fwd: CGGGAATCCAAGAAGATTGA |
| Rev: TTCAGCTTGTGGATGTGCTC |
| Actb |
| Fwd: TGGCGCTTTTGACTCAGGAT |
| Rev: GGGATGTTTGCTCCAACCAA |
Puberty onset and estrous cycle determination
After weaning, at postnatal day 21, female mice were examined daily for vaginal opening. Estrous cyclicity was then assessed for 21 consecutive days starting at 6 weeks of age. Vaginal cells obtained every morning (09:00–10:00 h) using a cotton swab dampened with sterile PBS were smeared on glass slides and stained with 0.1% (w/v) methyl blue. The cytology of the cells was examined under a microscope and stages were assigned following published guidelines [28].
Hormone assays
Blood was collected by cardiac puncture at 7:00 h on the morning of estrus in 9-week-old females as well as from 10-week-old males. After 1 h at room temperature, coagulated samples were centrifuged at 3000 g for 15 min. Serum was collected and stored at −20°C. Luteinizing hormone (LH) and FSH levels were measured in singlet by multiplex enzyme-linked immunosorbent assay (ELISA) at the Ligand Assay and Analysis Core of the Center for Research in Reproduction at the University of Virginia (Charlottesville, VA, USA). The reportable ranges for LH and FSH were 0.24–30.0 ng/ml (CV%: 1.2–4.2) and 2.40–300.0 ng/ml (CV%: 0.5–5.5), respectively.
Pituitary RNA extraction and qPCR
For female mice, pituitaries were collected at 7:00 h on the morning of estrus and immediately frozen on dry ice. Pituitaries were homogenized in TRIzol and RNA was extracted following the manufacturer’s protocol. Total RNA from pituitaries (1 μg) was reverse transcribed using (MMLV) reverse transcriptase. Expression of genes encoding the beta-subunits of FSH and LH (Fshb and Lhb) was analyzed in duplicate qPCR reactions using EvaGreen Master mix on a Corbett Rotorgene 6000 instrument (Corbett Life Science, Mortlake, NSW, Australia). Gene expression was determined relative to that of the reference gene Rpl19 or Beta-actin (Actb) using the 2-ΔΔCt method [29]. Primer sets are listed in Table 1.
Primary pituitary cultures
Primary cultures were performed as previously described [30]. Briefly, pituitaries were collected from 10-week-old cKO or control male and female mice in M199 medium supplemented with 10% (v/v) fetal bovine serum (FBS). Pituitaries were washed three times in Hank’s Balanced Salt Solution (HBSS) with 150 μmol/L of CaCl2, cut several times with a scalpel, and digested in collagenase (1.5 mg/ml) (Sigma #C-0130; diluted in HBSS with 30 mg/ml bovine serum albumin (BSA), pH 7.4, 40 μl/pituitary) at 37°C for 2 h. The suspension was then washed with 5 ml calcium-free HBSS, centrifuged for 5 min at 1000 g, and resuspended in pancreatin solution (Sigma P3292; 4.5 mg/ml in calcium-free HBSS; 40 μl/pituitary). Pancreatin digestion was performed in a 37°C water bath with manual agitation for 7 min. Finally, the cell suspension was washed three times in 5 ml M199 medium containing 10% FBS (v/v) and cells were seeded at density of 3 × 105/well in 48-well plates. After 24 h, activin A or SB431542 treatment was performed for 6 h in M199 medium containing 2% (v/v) FBS.
Fluorescence-activated cell sorting of genetically labeled gonadotropes
cKO;mT/mG (Bmpr2fl/fl;GnrhrGRIC/+;mT/mG/+) and control (GnrhrGRIC/+;mT/mG/+) mice were sacrificed at 8 weeks of age. Pituitaries from males and females were collected, pooled, and enzymatically dispersed using a protocol adapted from [30]. Briefly, pituitaries were washed three times in HBSS with 150 μmol/L of CaCl2, cut with a scalpel, and digested in collagenase (1.5 mg/ml) (Sigma #C-0130; diluted in HBSS with 30 mg/ml BSA, pH 7.4, 40 μl per pituitary) for 1.5 h 37°C. The suspension was then washed with 5 ml calcium-free HBSS, centrifuged for 5 min at 1000 g, and resuspended in pancreatin solution (Sigma P3292; 4.5 mg/ml in calcium-free HBSS; 40 μl per pituitary). Pancreatin digestion was performed in a 37°C water bath with manual agitation for 6–8 min. Cells were washed and resuspended in M199 medium containing 2% (v/v) FBS and then passed through a 70 μm nozzle at 70 psi into a Becton Dickinson FACSAria III Sorter in the IRCM Flow Cytometry Core Facility. Sorting was performed using FACSDiva software (v. 8.0.1). The pituitary cells were then run and gated to sort GFP+ and Tomato+ cells.
Statistics
Data from control and cKO mice and/or cell cultures were compared with t-tests or analyses of variance, as indicated, using GraphPad 7.0 (La Jolla, USA). Post hoc pairwise comparisons were made with Holm–Sidak’s multiple-comparisons test. Significance was assessed relative to P < 0.05.
Results
Bmpr2 is recombined specifically in gonadotropes and male germ cells of cKO mice
Adult female and male cKO animals were sacrificed and different tissues were collected for comparison of DNA recombination of the floxed Bmpr2 alleles. Cre is expressed in pituitaries and male germ cells of GRIC mice [31], the Cre-driver line used here [26]. Recombination was detected in the pituitary glands of both sexes and also in the epididymides and testes of males, but was not observed in other tissues, including the brain, ovaries, and uterus (Figure 1A). Next, we purified gonadotropes (GFP+ cells) from control and cKO mice. Recombination of the Bmpr2 allele was only observed in gonadotropes (GFP+ cells) of cKO (Figure 1B). RNA analysis of the same cells demonstrated a significant reduction of Bmpr2 transcript levels in the GFP+ population (~99%) from cKO relative to control mice (Figure 1C). Bmpr2 expression did not differ between the tomato-positive (non-gonadotrope) cells from the two genotypes.
Figure 1.

Validation of gonadotrope-specific Bmpr2 knockout mice. A) PCR analysis of genomic DNA from the indicated tissues in male and female cKO mice. The presence of a band indicates recombination of the floxed Bmpr2 allele. B) PCR analysis of genomic DNA from gonadotrope (GFP) and non-gonadotrope (Tomato, TOM) cells from pituitaries of adult male control and cKO mice. The primer set detects the recombined Bmpr2 allele. C) cDNA was prepared from total RNA purified from GFP and Tomato cells isolated from control and cKO mice. Bmpr2 expression was assessed by qPCR and measured relative to Actb expression. Note, the Bmpr2 primers overlapped the deleted exons of the recombined Bmpr2 allele.
Bmpr2 cKO females exhibit normal puberty onset and estrous cyclicity
We evaluated puberty onset and estrous cyclicity in cKO and control female littermates (n = 11/genotype). The timing of vaginal opening was similar in both genotypes (Figure 2A). Furthermore, cKO females showed normal estrous cycles between 6 to 9 weeks of age (Figure 2B).
Figure 2.

Puberty onset and estrous cyclicity are normal in cKO female mice. A) Day of vaginal opening, used as a marker of puberty onset, in cKO and control mice. Each point represents an individual mouse. The horizontal line represents the group mean. B) Proportion of time spent in each estrous cycle stage over 3 weeks in control and cKO mice. P: proestrus, E: estrus and, ME/D: metestrus/diestrus. n = 11 females per genotype.
Bmpr2 cKO have reproductive organ weights similar to those of control mice
Females (9- to 10-week-old) and males were sacrificed and their reproductive organs weighed. There were no significant differences in the weights of ovaries (Figure 3A) or uteri (Figure 3B) between cKO and control mice. Similarly, 10-week-old cKO males had comparable testis weights to their control littermates (Figure 3C).
Figure 3.

Normal reproductive organ weights in cKO and control mice. A) Ovarian and B) uterine weights (normalized to body weight) in 9-week-old control and cKO female mice. C) Testicular weights (normalized to body weight) in 10-week-old control and cKO males. Body weights did not differ between genotypes (not shown). n = 12 animals per sex and per genotype
Bmpr2 cKO mice exhibit normal serum gonadotropin levels and pituitary gonadotropin beta subunit mRNA expression
Serum FSH and LH levels were determined in 9–10-week-old cKO and control mice of both sexes. Female mice were sacrificed at 07:00 h on the morning of estrus, toward the end of the secondary FSH surge [32]. Gonadotropin levels in female (Figure 4A and B) and male (Figure 4D and E) cKOs were similar to those of control littermates. Pituitary Fshb and Lhb mRNA expression did not differ significantly between genotypes (Figure 4C and F).
Figure 4.
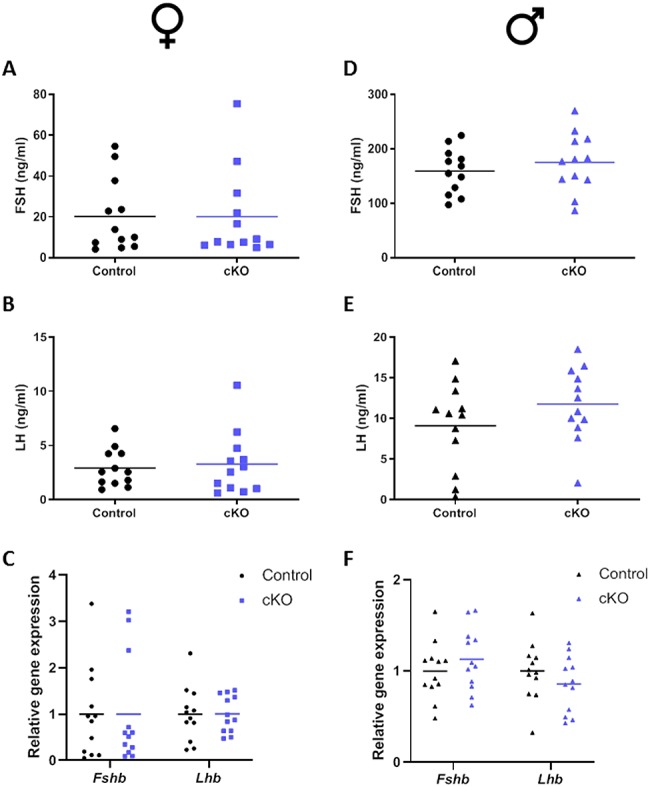
Normal serum FSH and LH levels in cKO mice. Serum and pituitaries were collected from control and cKO mice at 9–10 weeks of age. Left: Female mice; right: Male mice. Serum FSH (A and D) and LH (B and E) levels were measured by Multiplex ELISA. C and F) Pituitary Fshb and Lhb mRNA levels were assessed by quantitative RT-PCR relative to Rpl19 expression. N = 12 per sex per genotype.
Basal and activin A-stimulated Fshb expression are impaired in cultured pituitaries from cKO mice
Finally, we cultured and treated pituitaries from control and cKO mice (male and female pituitaries were used in combination). Both basal and activin A-stimulated Fshb mRNA expression were reduced in cultures from cKO mice relative to controls (Figure 5A). A type I receptor inhibitor (SB431542) fully suppressed Fshb mRNA levels in cultures from both genotypes, confirming earlier reports that Fshb expression in these cultures depends on one or more endogenous TGFbeta ligands that signal via ALK4, 5, or 7 [23]. The decrease in basal Fshb in cKO cultures further suggests that these ligands act, at least in part, via BMPR2. Lhb expression was unaffected by the gene deletion, activin A, or SB43152 in these cultures, demonstrating the specificity of the effects on Fshb (Figure 5B).
Figure 5.

Fshb expression is impaired in cultured pituitaries from cKO mice. Primary pituitary cultures were prepared from Bmpr2 cKO and control mice. Cells were treated 24 h later with 25 ng/ml activin A or 1 μmol/L SB431542 in 2% serum containing medium. Six hours later, RNA was collected and Fshb (A) and Lhb (B) mRNA levels were measured by quantitative RT-qPCR relative to Rpl19 expression. Each point represents an independent culture experiment.
Discussion
Although Acvr2a knockout mice are FSH-deficient, they produce the hormone at about 40% of the level seen in wild-type mice [20]. The residual FSH could reflect the actions of GnRH or signaling by activins (or related TGFbeta ligands) via alternate type II receptors. Gonadotrope-specific deletion of mediators of activin signaling to the Fshb promoter (SMAD3, SMAD4, and FOXL2) produces far greater reductions in FSH than global deletion of Acvr2a [33–36]. Thus, compensation via alternate receptors, in particular BMPR2 or ACVR2B, seems the most likely explanation. Acvr2b mice die shortly after birth [37], precluding their use to assess the receptor’s role in FSH synthesis in vivo. A floxed allele for Acvr2b has been developed [38], which should enable deletion of the receptor specifically in gonadotropes. This said, according to in vitro data, activins and BMP2 seem more likely to signal via BMPR2 than ACVR2B to regulate Fshb transcription [4, 8, 39]. Nonetheless, as we report here, gonadotrope-specific deletion of Bmpr2, at least on its own, has no measurable effect on FSH (or LH) synthesis or fertility in mice in vivo.
There are several potential explanations for the negative data presented here. First, it is possible that we did not effectively delete BMPR2 function in gonadotropes. This seems unlikely to us, however, the GRIC (Cre-driver) allele has proven highly effective in recombining a wide variety of floxed alleles in gonadotropes in the past [18, 33–35, 40–44]. Moreover, we show that the allele is recombined selectively in pituitaries (and specifically gonadotropes) of the mice analyzed here (Figure 1A and B). This is associated with a profound decrease in Bmpr2 mRNA levels in the gonadotropes of these mice (Figure 1C). Finally, we observed significant impairments in Fshb expression in cultured pituitaries from cKO mice, both basally and in response to exogenous activin A (Figure 5A). These data show that the gene knockout had a functional outcome in vitro. Thus, the available data indicate that Bmpr2 expression and protein function were efficiently reduced in these mice.
A second, perhaps more likely, explanation of the present data set is that BMPR2 is either unimportant for FSH synthesis in vivo or that another receptor (most likely ACVR2A) can compensate fully for its absence. Activins bind with greater affinity to ACVR2A than to BMPR2 [8, 14]. Moreover, though recent data argue against a role for BMP signaling in FSH synthesis in vivo [18, 19], if these ligands do play a role, it is now well-established that they can use ACVR2A in the absence of BMPR2 [45, 46]. Therefore, it may only be possible to delineate BMPR2’s role in gonadotrope function and FSH synthesis in the combined absence of ACVR2A. The mice used here could be crossed to the global Acvr2a knockout strain, though a more elegant approach will be to use Cre-lox to selectively ablate both genes in gonadotropes [47]. Here, the prediction is that FSH will be more greatly reduced in Bmpr2/Acvr2a double knockout mice relative to mice lacking Acvr2a alone. Of course, we cannot yet rule out a primary or compensatory role for ACVR2B.
A third possibility for the apparently negative data presented here is that we may not have examined animals or their pituitaries under conditions in which BMPR2 plays a functional role. For example, mice in these experiments were housed under highly controlled laboratory conditions. Moreover, males and females were housed separately. Perhaps under conditions that challenge the reproductive axis (e.g., following gonadectomy or in response to a prospective mate), effects of the gene deletion may have been manifested. We also only examined young adult mice and therefore cannot rule out effects during development or reproductive aging. The apparently normal gonads and reproductive tracts of the animals, however, argue against effects on gonadotropins during early postnatal development. It should also be noted that while the gene deletion did not affect FSH production in vivo, it did impair Fshb mRNA expression in cultured pituitaries from the same animals. Indeed, these in vitro results closely mirrored those observed previously in the gonadotrope-like cell line LbetaT2 [8]. These results suggest that the TGFbeta family ligands and receptors mediating FSH synthesis in vivo and in vitro may differ. Indeed, we have seen a similar pattern of results (i.e., impaired FSH production in vitro but not in vivo) in a type I receptor conditional knockout model that we will report elsewhere (Ongaro et al., unpublished).
In conclusion, according to the data presented here, BMPR2 does not play a fundamental role in the physiological regulation of FSH synthesis in vivo in mice. As a result, activins or related TGFbeta ligands likely signal preferentially via ACVR2A (and/or ACVR2B) in gonadotropes. Roles for BMPR2 and the related ACVR2B may only be manifested (or observable) when ACVR2A function is compromised. It is worth noting that loss of BMPR2 impairs the actions of BMP2 and BMP4 while promoting the actions of BMP6 and BMP7 in pulmonary artery smooth muscle cells [45]. This stems from the fact that the latter ligands signal more efficiently via ACVR2A than do the former. In the absence of BMPR2, ACVR2A functionality is unmasked. Therefore, with these observations in mind, it is formally possible that the residual FSH production in Acvr2a-deficient mice might reflect gain of function of ligands that preferential signal via BMPR2 or ACVR2B. Conditional deletion of Acvr2a and Acvr2b will enable the testing of these and related hypotheses about the nature and flexibility of TGFbeta superfamily signaling in gonadotropes in vivo and in vitro.
Acknowledgements
The authors thank Julie Lord in the IRCM Flow Cytometry Core Facility for technical assistance with cell sorting.
Conflict of interest
The authors have no conflicts of interest to disclose.
References
- 1. Matzuk MM, Kumar TR, Vassalli A, Bickenbach JR, Roop DR, Jaenisch R, Bradley A. Functional analysis of activins during mammalian development. Nature 1995; 374:354–356. [DOI] [PubMed] [Google Scholar]
- 2. Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massague JTGF. Beta signals through a heteromeric protein kinase receptor complex. Cell 1992; 71:1003–1014. [DOI] [PubMed] [Google Scholar]
- 3. Ling N, Ying SY, Ueno N, Shimasaki S, Esch F, Hotta M, Guillemin R. Pituitary FSH is released by a heterodimer of the beta-subunits from the two forms of inhibin. Nature 1986; 321:779–782. [DOI] [PubMed] [Google Scholar]
- 4. Lee KB, Khivansara V, Santos MM, Lamba P, Yuen T, Sealfon SC, Bernard DJ. Bone morphogenetic protein 2 and activin a synergistically stimulate follicle-stimulating hormone beta subunit transcription. J Mol Endocrinol 2007; 38:315–330. [DOI] [PubMed] [Google Scholar]
- 5. Takeda M, Otsuka F, Takahashi H, Inagaki K, Miyoshi T, Tsukamoto N, Makino H, Lawson MA. Interaction between gonadotropin-releasing hormone and bone morphogenetic protein-6 and -7 signaling in LbetaT2 gonadotrope cells. Mol Cell Endocrinol 2012; 348:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang HJ, Wu JC, Su P, Zhirnov O, Miller WL. A novel role for bone morphogenetic proteins in the synthesis of follicle-stimulating hormone. Endocrinology 2001; 142:2275–2283. [DOI] [PubMed] [Google Scholar]
- 7. Nicol L, Faure MO, McNeilly JR, Fontaine J, Taragnat C, McNeilly AS. Bone morphogenetic protein-4 interacts with activin and GnRH to modulate gonadotrophin secretion in LbetaT2 gonadotrophs. J Endocrinol 2008; 196:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rejon CA, Hancock MA, Li YN, Thompson TB, Hebert TE, Bernard DJ. Activins bind and signal via bone morphogenetic protein receptor type II (BMPR2) in immortalized gonadotrope-like cells. Cell Signal 2013; 25:2717–2726. [DOI] [PubMed] [Google Scholar]
- 9. Olsen OE, Wader KF, Hella H, Mylin AK, Turesson I, Nesthus I, Waage A, Sundan A, Holien T. Activin a inhibits BMP-signaling by binding ACVR2A and ACVR2B. Cell Commun Signal 2015; 13:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Willis SA, Zimmerman CM, Li LI, Mathews LS. Formation and activation by phosphorylation of activin receptor complexes. Mol Endocrinol 1996; 10:367–379. [DOI] [PubMed] [Google Scholar]
- 11. Yamashita H, ten P, Huylebroeck D, Sampath TK, Andries M, Smith JC, Heldin CH, Miyazono K. Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects. J Cell Biol 1995; 130:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Attisano L, Carcamo J, Ventura F, Weis FM, Massague J, Wrana JL. Identification of human activin and TGF beta type I receptors that form heteromeric kinase complexes with type II receptors. Cell 1993; 75:671–680. [DOI] [PubMed] [Google Scholar]
- 13. Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, Dijke P, Heldin CH, Miyazono K. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci U S A 1995; 92:7632–7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aykul S, Martinez-Hackert E. Transforming growth factor-beta family ligands can function as antagonists by competing for type II receptor binding. J Biol Chem 2016; 291:10792–10804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bernard DJ, Lee KB, Santos MM. Activin B can signal through both ALK4 and ALK7 in gonadotrope cells. Reprod Biol Endocrinol 2006; 4:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsuchida K, Nakatani M, Yamakawa N, Hashimoto O, Hasegawa Y, Sugino H. Activin isoforms signal through type I receptor serine/threonine kinase ALK7. Mol Cell Endocrinol 2004; 220:59–65. [DOI] [PubMed] [Google Scholar]
- 17. ten Dijke P, Yamashita H, Ichijo H, Franzen P, Laiho M, Miyazono K, Heldin CH. Characterization of type I receptors for transforming growth factor-beta and activin. Science 1994; 264:101–104. [DOI] [PubMed] [Google Scholar]
- 18. Zhou X, Wang Y, Ongaro L, Boehm U, Kaartinen V, Mishina Y, Bernard DJ. Normal gonadotropin production and fertility in gonadotrope-specific Bmpr1a knockout mice. J Endocrinol 2016; 229:331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ongaro L, Schang G, Ho CC, Zhou X, Bernard DJ. TGF-beta superfamily regulation of follicle-stimulating hormone synthesis by Gonadotrope cells: Is there a role for bone morphogenetic proteins? Endocrinology 2019; 160:675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matzuk MM, Kumar TR, Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature 1995; 374:356–360. [DOI] [PubMed] [Google Scholar]
- 21. Attisano L, Wrana JL, Cheifetz S, Massague J. Novel activin receptors: Distinct genes and alternative mRNA splicing generate a repertoire of serine/threonine kinase receptors. Cell 1992; 68:97–108. [DOI] [PubMed] [Google Scholar]
- 22. Bagarova J, Vonner AJ, Armstrong KA, Borgermann J, Lai CS, Deng DY, Beppu H, Alfano I, Filippakopoulos P, Morrell NW, Bullock AN, Knaus P et al. Constitutively active ALK2 receptor mutants require type II receptor cooperation. Mol Cell Biol 2013; 33:2413–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rejon CA, Ho CC, Wang Y, Zhou X, Bernard DJ, Hebert TE. Cycloheximide inhibits follicle-stimulating hormone beta subunit transcription by blocking de novo synthesis of the labile activin type II receptor in gonadotrope cells. Cell Signal 2013; 25:1403–1412. [DOI] [PubMed] [Google Scholar]
- 24. Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol 2000; 221:249–258. [DOI] [PubMed] [Google Scholar]
- 25. Beppu H, Lei H, Bloch KD, Li E. Generation of a floxed allele of the mouse BMP type II receptor gene. Genesis 2005; 41:133–137. [DOI] [PubMed] [Google Scholar]
- 26. Wen S, Schwarz JR, Niculescu D, Dinu C, Bauer CK, Hirdes W, Boehm U. Functional characterization of genetically labeled gonadotropes. Endocrinology 2008; 149:2701–2711. [DOI] [PubMed] [Google Scholar]
- 27. Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis 2007; 45:593–605. [DOI] [PubMed] [Google Scholar]
- 28. Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci 2009; Appendix 4:Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 2001; 25:402–408. [DOI] [PubMed] [Google Scholar]
- 30. Fortin J, Kumar V, Zhou X, Wang Y, Auwerx J, Schoonjans K, Boehm U, Boerboom D, Bernard DJ. NR5A2 regulates Lhb and Fshb transcription in gonadotrope-like cells in vitro, but is dispensable for gonadotropin synthesis and fertility in vivo. PLoS One 2013; 8:e59058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wen S, Ai W, Alim Z, Boehm U. Embryonic gonadotropin-releasing hormone signaling is necessary for maturation of the male reproductive axis. Proc Natl Acad Sci U S A 2010; 107:16372–16377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kovacic N, Parlow AF. Alterations in serum FSH-LH ratios in relation to the estrous cycle, pseudopregnancy, and gonadectomy in the mouse. Endocrinology 1972; 91:910–915. [DOI] [PubMed] [Google Scholar]
- 33. Fortin J, Boehm U, Deng CX, Treier M, Bernard DJ. Follicle-stimulating hormone synthesis and fertility depend on SMAD4 and FOXL2. Faseb j 2014; 28:3396–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tran S, Zhou X, Lafleur C, Calderon MJ, Ellsworth BS, Kimmins S, Boehm U, Treier M, Boerboom D, Bernard DJ. Impaired fertility and FSH synthesis in gonadotrope-specific Foxl2 knockout mice. Mol Endocrinol 2013; 27:407–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li Y, Schang G, Boehm U, Deng CX, Graff J, Bernard DJ. SMAD3 regulates follicle-stimulating hormone synthesis by pituitary Gonadotrope cells in vivo. J Biol Chem 2017; 292:2301–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li Y, Schang G, Wang Y, Zhou X, Levasseur A, Boyer A, Deng CX, Treier M, Boehm U, Boerboom D, Bernard DJ. Conditional deletion of FOXL2 and SMAD4 in Gonadotropes of adult mice causes isolated FSH deficiency. Endocrinology 2018; 159:2641–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oh SP, Li E. The signaling pathway mediated by the type IIB activin receptor controls axial patterning and lateral asymmetry in the mouse. Genes Dev 1997; 11:1812–1826. [DOI] [PubMed] [Google Scholar]
- 38. Lee SJ, Huynh TV, Lee YS, Sebald SM, Wilcox-Adelman SA, Iwamori N, Lepper C, Matzuk MM, Fan CM. Role of satellite cells versus myofibers in muscle hypertrophy induced by inhibition of the myostatin/activin signaling pathway. Proc Natl Acad Sci U S A 2012; 109:E2353–E2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ho CC, Bernard DJ. Bone morphogenetic protein 2 signals via BMPR1A to regulate murine follicle-stimulating hormone beta subunit transcription. Biol Reprod 2009; 81:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wen S, Gotze IN, Mai O, Schauer C, Leinders-Zufall T, Boehm U. Genetic identification of GnRH receptor neurons: A new model for studying neural circuits underlying reproductive physiology in the mouse brain. Endocrinology 2011; 152:1515–1526. [DOI] [PubMed] [Google Scholar]
- 41. Brown JL, Xie J, Brieno-Enriquez MA, Sones J, Angulo CN, Boehm U, Miller A, Toufaily C, Wang Y, Bernard DJ, Roberson MS. Sex- and age-specific impact of ERK loss within the pituitary Gonadotrope in mice. Endocrinology 2018; 159:1264–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boerboom D, Kumar V, Boyer A, Wang Y, Lambrot R, Zhou X, Rico C, Boehm U, Paquet M, Celeste C, Kimmins S, Bernard DJ. Beta-catenin stabilization in gonadotropes impairs FSH synthesis in male mice in vivo. Endocrinology 2015; 156:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jonak CR, Lainez NM, Boehm U, Coss D. GnRH receptor expression and reproductive function depend on JUN in the GnRH receptor-expressing cells. Endocrinology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Allen SJ, Garcia-Galiano D, Borges BC, Burger LL, Boehm U, Elias CF. Leptin receptor null mice with reexpression of LepR in GnRHR expressing cells display elevated FSH levels but remain in a prepubertal state. Am J Physiol Regul Integr Comp Physiol 2016; 310:R1258–R1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yu PB, Beppu H, Kawai N, Li E, Bloch KD. Bone morphogenetic protein (BMP) type II receptor deletion reveals BMP ligand-specific gain of signaling in pulmonary artery smooth muscle cells. J Biol Chem 2005; 280:24443–24450. [DOI] [PubMed] [Google Scholar]
- 46. Mayeur C, Leyton PA, Kolodziej SA, Yu B, Bloch KD. BMP type II receptors have redundant roles in the regulation of hepatic hepcidin gene expression and iron metabolism. Blood 2014; 124:2116–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goh BC, Singhal V, Herrera AJ, Tomlinson RE, Kim S, Faugere MC, Germain-Lee EL, Clemens TL, Lee SJ, DiGirolamo DJ. Activin receptor type 2A (ACVR2A) functions directly in osteoblasts as a negative regulator of bone mass. J Biol Chem 2017; 292:13809–13822. [DOI] [PMC free article] [PubMed] [Google Scholar]


