Abstract
Background & Aims
There is currently no data on physician preferences regarding future therapies for non-alcoholic steatohepatitis (NASH); this study explores these preferences and characteristics that are relevant to physician decision-making when choosing a potential therapy for a patient with NASH. The results were compared with those from a similar patient preference survey which was conducted in parallel.
Method
Initial exploratory 30-minute telephone interviews were conducted to inform the design of a 15-minute quantitative online specialist physicians survey, containing direct questions and a preference survey. This was based on a best-worst scaling (BWS) experiment to assess the relative importance of different treatment characteristics (attributes), followed by several paired comparison questions to understand the preference for 5 hypothetical product profiles.
Results
The answers come from 121 physicians from Canada (n = 31), Germany (n = 30), the UK (n = 30) and the USA (n = 30). The primary driving element in NASH treatment decision-making was efficacy (49.23%), defined as “[hypothetical product] impact on liver status” and “[slowing of] progression to cirrhosis”. Physicians reported the common use of non-invasive NASH diagnostic tests and 81% reported performing liver biopsy. In 57% of cases, physicians reported that “concerns related to the available diagnostic methods” limit the number of patients with biopsy-confirmed NASH.
Conclusions
This first physician preference study reveals that efficacy will be the main driver for physicians in selecting future NASH drugs. The findings also confirm the widespread use of non-invasive diagnostic tests and the reluctance to perform confirmatory liver biopsy despite guideline recommendations, mainly due to limited therapeutic options and patient refusal.
Lay summary
This study explores physician preferences in relation to future therapies for non-alcoholic steatohepatitis (NASH) and characteristics that are relevant to physician decision-making when choosing a potential therapy for a patient with NASH. The results of a short online survey completed by 121 specialist physicians determined that the primary factor that influences treatment decision-making is efficacy, and that a wide range of non-invasive techniques are used to diagnose NASH, while confirmatory liver biopsy is not performed by all physicians despite guideline recommendations.
Keywords: Non-invasive diagnostics, liver biopsy, non-alcoholic fatty liver disease, NAFLD, NASH, Best-worst scaling, liver disease, type 2 diabetes mellitus
Abbreviations: ACBC, adaptive choice-based conjoint; BWS, best-worst scaling; GPs, general practitioners; HB, hierarchical Bayesian; Hb1Ac, glycated haemoglobin; HTA, health technology assessment; NASH, non-alcoholic steatohepatitis; PCPs, primary care physicians; SC, steering committee
Graphical abstract
Highlights
-
•
This is the first study on physician and patient preferences for future NASH therapies.
-
•
Important differences between physician and patient preferences identified.
-
•
Efficacy & safety were most important for physicians and efficacy & impact on symptoms were most important for patients.
-
•
NASH is diagnosed using a range of non-invasive techniques, but not always confirmed by liver biopsy.
Introduction
Decision-making in healthcare frequently incorporates physician, regulatory and payer perspectives during drug development, health technology assessment and regulatory processes.1 NASH is a condition with no approved therapies and several drugs under development. It is therefore important to understand the preferences of patients and of the physicians treating them, when it comes to optimising treatment selection. This physician research was conducted as part of a wider preference study, which also collected information on patient preferences2 in NASH. The patient study revealed that there is limited dialogue between patients with NASH and their treating physicians, which results in limited patient knowledge about NASH, disease progression and management strategies. The current manuscript reports the physician qualitative and quantitative research. The aim of this physician study was to (i) understand physician preferences for attributes of new therapeutic options in development for NASH, (ii) to identify physicians' approach to diagnosis and management of patients with NASH, (iii) to understand if patient views and preferences are aligned with the physicians' priorities, treatment objectives and preferences and (iv) assess physicians' perspective with regards to potential future therapies in NASH.
Materials and methods
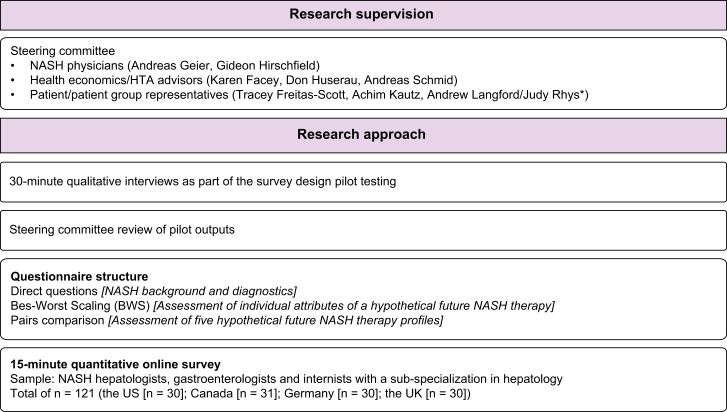
The study was conducted under the guidance of a steering committee (SC) of clinical, health technology assessment (HTA) body advisers and patient representatives (Fig. 1).
Fig. 1.
Overview of study methodology.
∗Judy Rhys replaced Andrew Langford for the final steering committee meeting.
Settings and participants
The study was conducted online among specialist physicians in Canada, Germany, the UK and the USA during December 2017. On advice from the SC, specialist practitioners were targeted for 3 reasons: i) NASH was considered a new condition, which, all healthcare professionals were still learning how to manage effectively, ii) given the first point, the diagnosis and management of these patient at the time of research was mainly with specialists (these specialists would also be the first healthcare professionals to be initiating potential new therapies in the real world), and iii) the requirement of responders to be managing a number of patients with NASH. A screener was administered prior to the main questionnaire in order to select the eligible physicians. Physicians had to be experienced, have a specialisation in hepatology, currently be treating patients with NASH and have been managing at least 40 patients in the past 3 months.
Study questionnaires
An initial set of exploratory telephone interviews with physicians (n = 18) were conducted in Canada, Germany and the UK. The initial interviews provided insights on physician perspective, unmet need and hypothetical drug profiles. These findings supported the development of the final main questionnaire for the quantitative phase of the study. As per the SC recommendations, the 15-minute online questionnaire was designed in 2 parts. Part one contained direct questions to determine physicians' knowledge, perceptions of NASH, diagnosis pathways, and reasons for their selection. Physicians responded on a multiple choice or Likert scale ranging from 1 (low) to 5 (high) or 1 (extremely low) to 7 (extremely high) depending on the type of question.
The second part was designed to capture physician preferences regarding different hypothetical NASH product profiles, using best-worst scaling (BWS) of those profiles. The study documents were consistent across the research countries and were translated into local languages (Fig. 1). The online survey used for this research is included in the supplementary materials.
BWS methodology
BWS is an approach to obtain preference scores based upon extending the method of paired comparison to multiple-choice tasks where physicians are asked to indicate which feature is their most and least preferred option.3,4
The results provide information on how much unique value each item has, which can then be transformed into utility scores. It was used to test the relative importance of 11 product characteristics (attributes) (Table S1). Using a balanced incomplete block design, a BWS experiment was developed with 11 choice task questions, displaying 3 attributes at a time. In each question, the physicians were asked to select which is the most important and which is the least important product characteristic. Hierarchical Bayesian (HB) estimation was used to estimate utility scores and 95% CIs for each attribute, which were ranked in order of importance. We introduced the country covariates to improve the model fit of the estimation and to improve the country comparison. Afterwards, fixed hypothetical product profiles were tested with paired comparison questions. Each fixed hypothetical product profile was characterised by a set of 11 attributes (same as the ones tested in BWS) each of which represented a level (feature). The attributes and hypothetical product profiles tested within this research are consistent with prior patient research,2 which used an adaptive choice-based conjoint approach (ACBC) (Table 1).
Table 1.
Comparison of importance of attributes when selecting a NASH therapy between physicians and patients.2
| Comparison of importance of attributes between patients and physicians | |||
|---|---|---|---|
| Importance of attributes for patients (% of overall profile importance)2 |
Importance of attributes for physicians (% of overall profile importance) |
||
| Methodology: ACBC | Total (n = 164) | Methodology: BWS | Total (n = 121) |
| Impact on liver status (based on test results) | 28.15 | Impact on liver status | 24.87 |
| Impact on symptoms possibly linked to my liver disease | 17.78 | Progression to cirrhosis | 24.36 |
| Impact on blood sugar (diabetes) & cholesterol (LDL-C) | 14.64 | Impact on blood sugar | 16.11 |
| Impact on weight | 12.32 | Impact on cholesterol | 9.64 |
| Impact on progression to serious damage to my liver (cirrhosis) | 11.92 | Impact on frequency of visits to the doctor | 5.15 |
| Side effects: Itching | 5.18 | Impact on fatigue and stomach pain | 4.75 |
| Side effects: Diarrhoea | 4.66 | Impact on weight | 4.13 |
| Side effects: Nausea | 3.16 | Possibility to cause itching | 3.99 |
| Frequency of visits to my doctor | 1.17 | Possibility to cause diarrhoea | 2.69 |
| Side effects: Headache | 1.03 | Possibility to cause nausea | 2.18 |
| Possibility to cause headache | 2.13 | ||
NOTE: “Impact on symptoms possibly linked to my liver disease” in patient research was equivalent to “impact of fatigue and stomach pain” in physician research (language adjusted for patient understanding). NASH, non-alcoholic steatohepatitis.
Statistical analysis
Data from the direct questions collected in the first part of the survey are reported as a percentage of responses for each category. Country-specific statistical significance testing was not undertaken. Due to the relatively small sample size in each country, statistical analysis within each country was not performed, because it could introduce the probability of false positive and false negative results during hypothesis testing. Nonetheless the overall sample size allowed analysis to be performed on the pooled sample. Analysis of physician responses to the BWS exercise was conducted using Sawtooth Software - Lighthouse Studio 9.5.3. All data was analysed and reported per country and pooled across the 4 countries.
Ethics approval
Physician survey research did not require ethical approval as per BHBIA and EphMRA guidelines, however informed consent was collected from research participants prior to the study initiation. All procedures performed in this study involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable national ethical standards. All participants were recruited and compensated in accordance with the local market research norms and regulations.
Results
A total of 121 physicians took the online questionnaire in Canada (n = 31), Germany (n = 30), the UK (n = 30) and the USA (n = 30). The sample included internists with a sub-specialisation in hepatology (n = 6), gastroenterologists (n = 85) and hepatologists (n = 30) (Table S2). The majority (75%) were hospital based, with the remainder being office-based; the UK was the only country where all physicians were hospital based (Table 2).
Table 2.
Physician sample composition by country.
| Physician place of practice | Canada | Germany | UK | USA |
|---|---|---|---|---|
| Total N | 31 | 30 | 30 | 30 |
| Hospital / centre/private clinic-based specialised in liver conditions, including NASH | 17 | 13 | 19 | 11 |
| Hospital based (not specialised in liver conditions) | 7 | 10 | 11 | 3 |
| Office-based | 7 | 7 | 0 | 16 |
Data presented as a total number of respondents who took part in the research.
Direct questions: Diagnosis practice, and perceptions of NASH
In the first part of the quantitative online questionnaire, physicians answered multiple-choice questions relating to diagnosis practice and perceptions of NASH.
Self-reported physician knowledge of NASH as a disease and its diagnosis
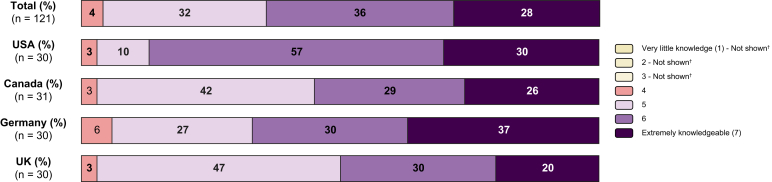
Among the total physician cohort, the majority considered themselves extremely (28%) and very knowledgeable (36%) about NASH as a disease (Fig. 2). Differences were observed across the countries in the study: in the USA, physicians rated their knowledge of NASH as “very high” in 57% of cases and “extremely high” in 30% of cases, compared to 30% and 20%, respectively, in the UK.
Fig. 2.
Physicians' self-reported level of knowledge of NASH (expressed as % of respondents in each category).
[Question: On a scale from 1 to 7, where 1=very little knowledge, and 7=extremely knowledgeable, how would you evaluate your current knowledge of NASH?] [NOTE: Scores 2-6 were not defined on the respondent screen]. NASH, non-alcoholic steatohepatitis.
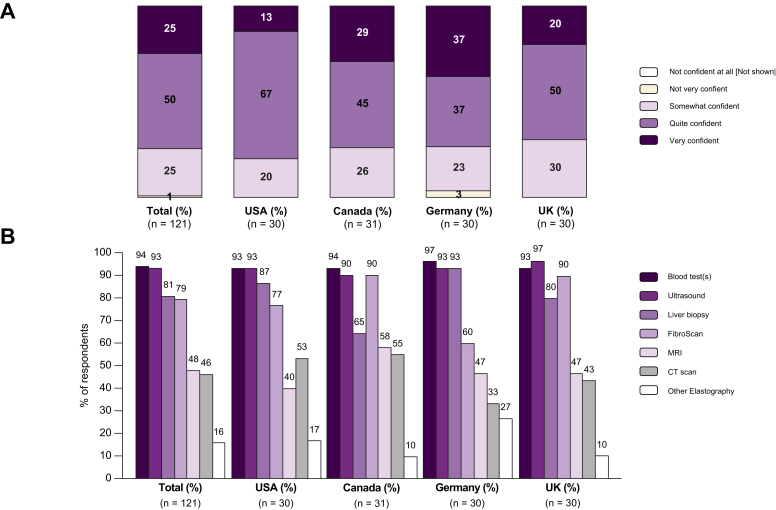
Overall, 25% of physicians reported being “very confident” in their ability to correctly and accurately diagnose NASH, however individual results varied across the 4 countries (13% in the USA, 20% in the UK, 29% in Canada and 37% in Germany), a further 25% of the physicians reported being “somewhat confident” overall in their ability to correctly and accurately diagnose NASH, again with some country variation (20% in the USA, 23% in Germany, 26% in Canada and 30% in the UK) (Fig. 3A).
Fig. 3.
Physicians' level of confidence and the tests usually used to diagnose NASH.
(A) Physicians' level of confidence when it comes to correctly diagnosing NASH (expressed as % of respondents in each category) [Question: How would you consider your level of confidence when it comes to correctly and accurately diagnosing NASH (meaning a confirmation diagnosis, not just a suspicion)?]. (B) Reported tests usually performed to diagnose NASH (expressed as a % of respondents selecting each option) [Question: What, if any, tests do you usually perform to diagnose NASH in your patients? (Please select ALL that apply and add others, we are specifically interested in understanding what tests are used in addition to or as an alternative to liver biopsy.)]; NOTE: The numbers are not mutually exclusive and expressed as %. NASH, non-alcoholic steatohepatitis.
Physician practice in diagnosing NASH
Pooled data (Fig. 3B) show the tests and procedures usually used by physicians to diagnosis NASH; multiple selections were permitted. Many non-invasive procedures are used: blood tests (94% of physicians), ultrasound (93%), and transient elastography (FibroScan) (79%). CT and MRI were reportedly used by 46% and 48% of physicians respectively. Other elastography techniques were reportedly used less frequently (in 16% of cases). Confirmatory liver biopsy was reported to be used by 81% of physicians.
The use of FibroScan, a non-invasive diagnostic tool used to estimate the degree of liver stiffness5 varied significantly between countries, with 90% of physicians in the UK and Canada indicating that it was usually performed in patients with NASH, whilst usage was only reported by 60% of German physicians.
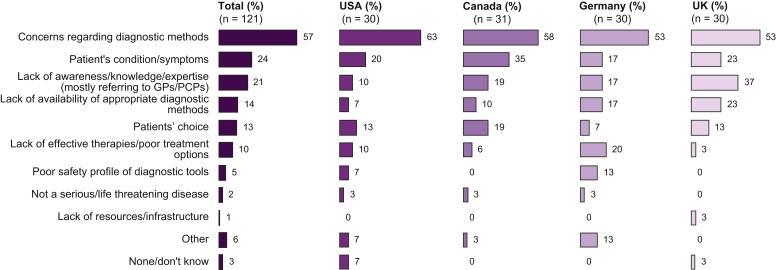
Physicians reported various reasons for the relatively low rate of patients who have confirmed NASH (Fig. 4). The most common reason across all countries was “Concerns regarding diagnostic methods” (57%). Other reasons were “Patient’s condition/symptoms” (24%) and “Lack of awareness / knowledge / expertise mostly referring to GPs/PCPs” (21%) but also “Patients choice” (13%).
Fig. 4.
Reasons for low level of confirmed NASH among patients suspected of having NASH (expressed as a % of respondents selecting the option).
[Question: In previous exploratory research, doctors told us only a small percentage of suspected patients get a confirmed NASH diagnosis (either through a biopsy or another test).If you agree, what do you think are the main reasons for this low level of confirmatory diagnosis?]. NASH, non-alcoholic steatohepatitis.
Physician preference for therapy profile and value attributes
BWS: Importance of efficacy as a factor for product choice among physicians
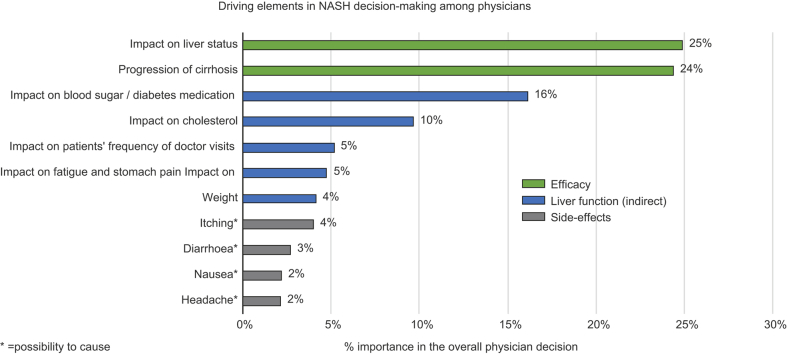
The BWS analysis revealed that the predominant feature driving decision-making when choosing a future NASH therapy was efficacy, expressed as impact on liver status and progression to cirrhosis (Table 3). Pooled data showed that these 2 features of highest importance to physician decision-making accounted for 24.9% and 24.3% of importance, respectively. The combined importance of these factors stood at 49.2%, making efficacy the single most important factor. We saw consistency of these results across markets and physician type.
Table 3.
Best-worst scaling - Mean (%) of the importance of each attribute reflecting physician preference on hypothetical products.
| Best-worst scaling scores – Mean % of attribute importance? (%) | Total (n = 121) | Canada (n = 31) | Germany (n = 30) | UK (n = 30) | US (n = 30) | Hepatologists (n = 30) | Gastroenterologists (n = 85) |
|---|---|---|---|---|---|---|---|
| Impact on liver status (ranging from liver status is stabilised, e.g. no regression of fibrosis and no worsening of inflammation [no worsening of NASH] to liver status is better, e.g. regression of fibrosis from F3 to F2 or F1 and reduction of inflammation [resolution of NASH]) | 24.87 | 26.99 | 23.00 | 24.29 | 25.13 | 24.32 | 25.17 |
| Progression of cirrhosis (ranging from slows down progression to cirrhosis to no progression to cirrhosis) | 24.36 | 26.61 | 21.93 | 24.51 | 24.33 | 23.42 | 24.72 |
| Impact on weight (ranging from no impact to weight loss by at least 5%) | 4.13 | 3.27 | 7.81 | 2.22 | 3.25 | 4.70 | 4.11 |
| Impact on fatigue and stomach pain (ranging from no impact to reduction of both) | 4.75 | 2.95 | 5.95 | 5.50 | 4.65 | 4.40 | 5.13 |
| Impact on blood sugar / diabetes medication (ranging from lowering blood sugar (HbA1c) to making current diabetes medication less effective | 16.11 | 16.12 | 13.85 | 19.03 | 15.46 | 12.48 | 17.24 |
| Impact on cholesterol (ranging from increasing the level of LDL-cholesterol in the blood to having no interaction with cholesterol-lowering medication) | 9.64 | 9.83 | 9.32 | 9.58 | 9.82 | 8.74 | 9.85 |
| Impact on frequency of patients' visits to their doctor for their liver condition (ranging from more visits to same number of visits) | 5.15 | 6.22 | 3.94 | 4.53 | 5.89 | 6.56 | 4.51 |
| Possibility to cause diarrhoea (ranging from mild to not at all) | 2.69 | 2.45 | 3.33 | 2.72 | 2.30 | 3.40 | 2.24 |
| Possibility to cause nausea (ranging from occasional to not at all) | 2.18 | 1.82 | 2.57 | 1.25 | 3.08 | 3.40 | 1.73 |
| Possibility to cause headache (ranging from occasional to not at all) | 2.13 | 1.26 | 2.69 | 2.16 | 2.43 | 3.02 | 1.83 |
| Possibility to cause itching (ranging from moderate to not at all | 3.99 | 2.49 | 5.60 | 4.21 | 3.68 | 5.55 | 3.46 |
Data presented as a total as well as, per country and by specialty. NOTE: the numbers in the table indicate the importance of each attribute (Best-worst scaling scores – Mean percentages (%)), higher numbers indicate higher importance of an attribute to the physician. Data not shown for internal medicine (n = 6) because sample size was too low. Hb1Ac, glycated haemoglobin; NASH, non-alcoholic steatohepatitis.
BWS: Impact of measures not directly related to liver function on prescribing behaviours
Factors other than liver-related efficacy represented approximately 50% of the importance attributed to the desired product profile by physicians. The additional attributes, seen as important for treatment choice were “impact on blood sugar” (16.1%), then “impact on cholesterol” (9.6%), followed by “impact on frequency of patients' visits to their doctor for their liver condition” (5.1%). Of lesser importance to physicians were “impact on fatigue and stomach pain” (4.8%) and “impact on weight” (4.1%). These results serve as an indicator of the importance hierarchy for physicians when choosing a therapy for their NASH patients.
BWS: Impact of side effects on prescribing behaviours
Physicians did not attribute the same level of importance to the potential side effects considered in the hypothetical product profiles, as they did to the other attributes tested. The most concerning side effect for physicians was “possibility to cause itching”, which accounted for 4% of the importance, followed by “possibility to cause diarrhoea”, “nausea” and “headache” which accounted for 2.7%, 2.2% and 2.1%, respectively.
Paired comparison of hypothetical product profiles
The physicians made trade-offs between 5 profiles (A-E), which had subtle variations in terms of efficacy, side effects and adverse events. The profiles were paired head-to-head in 4 scenarios designed to test the trade-offs between efficacy, side effects, adverse events and weight loss (Table 4). “Profile A” (which showed regression in fibrosis from F3 to F2 or F1, slowing progression to cirrhosis as well as a favourable side-effect profile, which did not cause diarrhoea, nausea, headache or itching) was preferred by 80% or more physicians in 3 out of 4 scenarios. In the scenario where “Profile A” was tested against “Profile C”, the latter was preferred by 69% of the physicians due to the additional benefits of “Profile C” in terms of lowering glycated haemoglobin (HbA1c) and LDL-cholesterol in the blood.
Table 4.
Head-to-head profile comparisons and physician preferences based on the profiles presented.
| Hypothetical product profile comparison | Scenario | Profiles tested | % Preference | |
|---|---|---|---|---|
| Profile Avs. Profile E | Scenario 1: Comparing improvement on fibrosis status vs. Weight loss | Profile A Liver status is better, e.g. regression of fibrosis from F3 to F2 or F1 and reduction of inflammation (resolution of NASH) Slows down progression to cirrhosis Weight loss by less than 5% of current weight Reduction of both fatigue and stomach pain No interaction with diabetes medication No interaction with cholesterol or cholesterol-lowering medication Same number of visits to doctor(s) for patient's liver condition Does not cause diarrhoea Does not cause nausea Does not cause headache Does not cause itching |
Profile E Liver status is stabilised, e.g. no regression of fibrosis and no worsening of inflammation (no worsening of NASH) Slows down progression to cirrhosis Weight loss by more than 5% of current weight No impact on fatigue or on stomach pain No interaction with diabetes medication No interaction with cholesterol or cholesterol-lowering medication Same number of visits to doctor(s) for patient's liver condition Does not cause diarrhoea Does not cause nausea Does not cause headache Does not cause itching |
Profile A: 80% Profile E: 20% |
| Profile Avs. Profile D | Scenario 2: Side effects vs. efficacy | Profile A Liver status is better, e.g. regression of fibrosis from F3 to F2 or F1 and reduction of inflammation (resolution of NASH) Slows down progression to cirrhosis Weight loss by less than 5% of current weight Reduction of both fatigue and stomach pain No interaction with diabetes medication No interaction with cholesterol or cholesterol-lowering medication Same number of visits to doctor(s) for patient's liver condition Does not cause diarrhoea Does not cause nausea Does not cause headache Does not cause itching |
Profile D Liver status is better, e.g. regression of fibrosis from F3 to F2 or F1 and reduction of inflammation (resolution of NASH) Slows down progression to cirrhosis Weight loss by less than 5% of current weight No impact on fatigue or on stomach pain No interaction with diabetes medication No interaction with cholesterol or cholesterol-lowering medication Same number of visits to doctor(s) for patient's liver condition Causes mild diarrhoea (less than 1 day out of 10) Causes occasional nausea (once a week or less) Causes occasional headache (once a week or less) Does not cause itching |
Profile A: 88% Profile D: 12% |
| Profile A vs.Profile C | Scenario 3: scenario – effect on blood sugar & LDL-cholesterol | Profile A Liver status is better, e.g. regression of fibrosis from F3 to F2 or F1 and reduction of inflammation (resolution of NASH) Slows down progression to cirrhosis Weight loss by less than 5% of current weight Reduction of both fatigue and stomach pain No interaction with diabetes medication No interaction with cholesterol or cholesterol-lowering medication Same number of visits to doctor(s) for patient's liver condition Does not cause diarrhoea Does not cause nausea Does not cause headache Does not cause itching |
Profile C Liver status is better, e.g. regression of fibrosis from F3 to F2 or F1 and reduction of inflammation (resolution of NASH) Slows down progression to cirrhosis Weight loss by less than 5% of current weight Reduction of both fatigue and stomach pain Lowers blood sugar (HbA1c) Lowers the level of LDL-cholesterol in the blood More visits to doctor(s) required for patient's liver condition Does not cause diarrhoea Does not cause nausea Does not cause headache Does not cause itching |
Profile A: 31% Profile C: 69% |
| Profile Avs. Profile B | Scenario 4: scenario – effect of doctor visits and itching | Profile A Liver status is better, e.g. regression of fibrosis from F3 to F2 or F1 and reduction of inflammation (resolution of NASH) Slows down progression to cirrhosis Weight loss by less than 5% of current weight Reduction of both fatigue and stomach pain No interaction with diabetes medication No interaction with cholesterol or cholesterol-lowering medication Same number of visits to doctor(s) for patient's liver condition Does not cause diarrhoea Does not cause nausea Does not cause headache Does not cause itching |
Profile B Liver status is better, e.g. regression of fibrosis from F3 to F2 or F1 and reduction of inflammation (resolution of NASH) Slows down progression to cirrhosis Weight loss by less than 5% of current weight Reduction of both fatigue and stomach pain No interaction with diabetes medication Increases the level of LDL-cholesterol in the blood More visits to doctor(s) required for patient's liver condition Does not cause diarrhoea Does not cause nausea Does not cause headache Causes mild to moderate itching |
Profile A: 89% Profile B:11% |
NOTE: Bold text indicates superiority to a comparator profile; bold and italic indicates inferiority to a comparator profile [this is for illustrative purposes only and was not used in physician research]. Hb1Ac, glycated haemoglobin; NASH, non-alcoholic steatohepatitis.
Comparison of patient and physician preferences
The results from this study can be compared to the patient preference study conducted in parallel with NASH patients2 (Table 1). The “impact on liver status” was considered the highest priority for both patients and physicians. By contrast, “progression to cirrhosis” which was the second highest priority for physicians, was rated only fifth highest by patients, who instead prioritised “impact on symptoms possibly linked to my liver disease”, “impact on blood sugar (diabetes) & cholesterol (LDL-C)” and “impact on weight” as being of greater importance to them.
The comparison of symptoms indicates that “impact on symptoms possibly linked to my liver disease” and “impact on weight” were ranked higher by patients (2nd and 4th most important factors, respectively). The physicians ranked the same factors lower (6th and 7th, respectively).
The ranking of side effects showed “itching” to be the most important side effect, followed by “diarrhoea”, “nausea”, and “headache” in that order for both physicians (8th, 9th, 10th and 11th, respectively) and patients (6th, 7th, 8th and 10th, respectively). The importance of “frequency of visits to the doctor” provides a notable discrepancy, for patients it was the 9th most important factor, while for the physicians it was the 5th most important factor.
Discussion
This is the first multi-country preference study conducted among physicians managing patients with NASH. The study unveils current diagnosis and management approaches in real-world practice and elicits physician preferences on hypothetical NASH therapy choice through a well-accepted methodology. The BWS methodology is commonly used to investigate preferences in healthcare,4 due to its ability to provide robust results whilst minimizing the number of questions that need be asked in the research. The key finding from the direct questions present the current approach for diagnosing and managing NASH in clinical practice.
According to American Association for the Study of Liver Diseases guidance6 and the European Association for the Study of the Liver guidelines,7 liver biopsy is required to diagnose, stage and grade NASH. In clinical practice, liver biopsy is required to establish a diagnosis, and at present it is performed in many patients in the clinical trial setting. Performing liver biopsy was reported by 81% of physicians in the pooled cohort and country differences were observed with 65% of them reporting liver biopsy in Canada compared to 93% in Germany. One relevant reason is that physicians do not consider liver biopsy in all cases if the disease management strategy will be no different due to a current lack of available treatment options for NASH. According to the physicians surveyed, concerns or unwillingness of patients to undergo liver biopsy were also important reasons not to perform it. Additionally, patients were said to express a preference for non-invasive tests to support their NASH diagnosis. Over the long-term, there are benefits associated with having a confirmatory liver biopsy, because it removes any uncertainty around the diagnosis. It remains to be seen if patient education on long-term benefits of liver biopsies will make biopsies more desired by patients.
Physicians indicated that non-invasive approaches were more commonly used as a diagnostic tool in clinical practice. For instance, the use of FibroScan was more often reported by physicians in Canada and the UK, which could be explained by the fact that it is reimbursed as standard in both countries, making it accessible to physicians treating NASH. FibroScan is useful to rule out patients with advanced disease, even in the absence of cirrhosis.8 However, the diagnostic reliability is lower in some patients, such as those with a BMI >30,5 which could result in inaccuracies when it comes to diagnosis of NASH. Data show that that in both Canada and the UK, physicians are more likely to use FibroScan than liver biopsy. Limitations in reimbursement may also guide diagnostic decisions as missing reimbursement of FibroScan could explain the lower use of this technique observed in Germany.9,10
In our view, there should be interactions and collaboration among specialists in both secondary and tertiary care with primary care physicians (PCPs), for the management of patients with NASH. Assuming these healthcare professionals have both the time and relevant knowledge and information to communicate to their patients, the patients would likely have more informed choices about their NASH tests and confirmatory diagnoses, as well as their ongoing disease management. “Lack of awareness / knowledge / expertise of PCPs” was cited by physicians in this study as a secondary reason for patients not receiving confirmatory diagnosis in the UK, Canada and Germany (Fig. 4). Therefore, PCP education could also be an important factor to improve referral and management of patients suffering from NASH in the future. Continual professional development courses for physicians, as well as other accessible sources of education, e.g. online resources, could be leveraged to help improve the number of patients being referred to specialists or receiving a confirmatory diagnosis. The fact that the confirmatory diagnosis can be made with an invasive procedure and the lack of available pharmacological treatment options may be important factors influencing diagnostic decisions. These aspects could change once targeted therapies for NASH have been approved.
The BWS exercise illustrated that both short-term (direct impact on liver status) and longer term (avoiding progression to cirrhosis) efficacy outcomes were the main factors influencing the selection of preferred hypothetical therapy profiles. Efficacy as the key value driver was consistently shown across countries and specialties. Interestingly, adverse events were judged to be of less importance compared to efficacy, indicating that physicians treating NASH are willing to accept the side-effect profile discussed above when an effective treatment becomes available.
Considering the patient preference data obtained within the previously conducted study in patients,2 both physicians and patients agree that the most important attribute when selecting a new NASH therapy is “impact on liver status”. There may be a disconnect however between the messages conveyed by physicians and the information retained by the patients; notably, physicians ranked “progression to cirrhosis” as second highest whereas patients ranked symptoms and effect on their weight higher than “progression to cirrhosis”. This difference also reflects that the proximal aspects of disease such as symptoms which might affect their health-related quality of life are more important to patients than distal effects such as future progression to a more severe stage. Another possible explanation could be around the low level of awareness that patients have about the natural progression of the disease. Understanding these different perspectives together with better patient education around their liver disease will be an important part of the patient-physician dialogue and requirement for effective disease management.
The hypothetical product profile trade-off exercise showed that given 2 options with the same efficacy on the liver (e.g. Profiles A and C), physicians selected the profile with additional clinical benefits such as lowering blood sugar and LDL-cholesterol (Profile C), with the side-effect profile seen as a tertiary consideration. Weight loss did not seem to be a defining factor in product choice for the physicians in contrast to the research in patients.2 These findings enable the creation of a design framework for an “optimal” NASH therapy from a physician perspective. This profile would have strong efficacy on the liver parameters, a positive effect on patients' blood sugar and LDL-cholesterol and an acceptable safety profile (severe or serious side effects were not tested in this research). Physicians also pointed out that without a treatment, there is no strong driver for a confirmatory liver biopsy diagnosis. Therefore, the emergence of such new treatments, could result in a higher number of patients having their NASH and fibrosis stage diagnosis confirmed by liver biopsy.
More broadly, patient preference studies and methods have been reviewed by healthcare bodies in Europe and the US, either as a pilot studies for specific methodologies11 or as a review of techniques for future reference.12 There have also been calls for improved alignment of stakeholders on patient needs early on in the drug development process.13 Physicians are typically a major stakeholder that interacts directly with patients, which is why it is important for their preferences to be known and aligned with the other stakeholders. This paper takes a step further to align NASH stakeholders on their preferences and can be used in the future to guide research and develop awareness programmes for the benefit of patients.
Study limitations
Our study has some limitations: the study was conducted among specialists treating NASH and gastroenterologists constituted the majority of the sample, therefore the results reflect their perceptions of NASH. Conversely internal medicine specialists were underrepresented, and the results cannot therefore be seen as representative of the entire medical community treating patients with NASH; PCPs/GPs were not included in this study. Similarly, most of physicians were hospital based which does not reflect the reality of primary care settings in many countries. The sample size per country was such that comparison of factors within or between countries should be viewed with caution. For the same reason, no analysis was conducted on physician preferences based on their practice setting. The paired comparison of product profiles was conducted for fixed pairs of profiles, therefore not all relevant scenarios may have been tested. Furthermore, only a limited number of product profile attributes could be tested in this survey, with the perceived relevance based on the previous qualitative research interviews. However, product profile attributes not tested may also have held importance for some physicians. Because of differences in reimbursement of diagnostic tests within individual healthcare systems, deriving generalised conclusions risks conflating the factor of physician will with physician ability to perform a specific diagnostic test; future research should take this limitation into account.
Conclusions
In conclusion, this physician preference study illustrates the general approach that specialist physicians take to diagnose NASH in clinical practice, with the extensive use of several non-invasive tests including blood tests and ultrasound. Confirmatory biopsy is infrequently performed in patients even when progressive NASH is suspected. Several factors were identified that may contribute to this decision, such as current lack of pharmacological options for NASH and patient unwillingness to undergo a confirmatory diagnosis procedure. Physician preferences for NASH treatment profiles elicit the efficacy parameter as the main driver of value. This is reflected in the impact on liver status, which is an endpoint in current development programmes, and the longer term efficacy, which is expressed as stopping progression to end-stage liver disease (cirrhosis). Additional secondary motivators seem to be related to the potential of positive effect on comorbidities in NASH including cholesterol and fasting blood glucose levels. As this is the first physician preference study in NASH, it provides an important insight into factors considered by healthcare professionals when diagnosing and managing patients with NASH and their expectations on future therapeutic profiles. These differ in some aspects from the patient-reported preferences,2 as patients value the impact of medication on symptoms more than progression to cirrhosis. Both studies provide results which are highly relevant to further optimise diagnostic and treatment pathways in NASH. These findings from both patients and physicians show the importance of collecting both perspectives and considering them in drug development programmes.
The results also highlight the importance of a patient-physician dialogue and the need to strengthen this dialogue for a mutual approach to the management of NASH. We expect that this study will support and initiate future research to expand these findings among the broader healthcare community.
Financial support
The research was funded by Novartis Pharma AG, Basel, Switzerland which is developing therapies for the treatment of NASH.
Authors’ contributions
NC and MMB drove the study, shaped the design of the study and this publication. All authors took part in overseeing this study, contributing their ideas on an ongoing basis, and reviewed this paper.
Conflict of interest
Nigel Cook and Maria-Magdalena Balp are employees of Novartis Pharma AG. Andreas Geier sits on steering committees for: Gilead, Intercept, Novartis; Advisor: AbbVie, Alexion, BMS, Gilead, Intercept, Ipsen, Novartis, Pfizer, Sequana; Speaker for: AbbVie, Alexion, BMS, CSL Behring, Falk, Gilead, Intercept, Novartis, Sequana; Provides Research Support for: Intercept (NAFLD CSG), Novartis, Kibion, Exalenz (LITMUS). Gideon Hirschfield has provided consultancy services for Gilead, Intercept, Novartis and Cymabay. Achim Kautz does not have any conflict of interest to declare. Jörn M. Schattenberg reports consultancies with AbbVie, Intercept Pharmaceuticals, Galmed, Genfit, Gilead Sciences, IQVIA, Novartis, Pfizer, Roche; research funding from Gilead Sciences, Yakult Europe B.V.; travel support: Janssen; lectures for Falk Foundation, Merck, Norgine. Andreas Schmid sits on a steering committee for Novartis and delivered a presentation for Lilly.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgments
The authors would like to acknowledge and thank Andrew Longford, Judy Rhys, Karen Facey, Tracy Freitas-Scott and Don Huserau for their valuable inputs and insights as part of the steering committee for this project; and Alexandra Chirilov, Olivia Weiss and Veruska Carboni for their support and guidance in conducting this research.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100081.
Supplementary data
References
- 1.Van Overbeeke E., Whichello C., Janssens R., Veldwijk J., Cleemput I., Simoens S. Factors and situations influencing the value of patient preference studies along the medical product lifecycle: a literature review. Drug Discov Today. 2019;24(1):57–68. doi: 10.1016/j.drudis.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Cook N., Balp M.M., Geier A., Schmid A., Hirschfield G., Kautz A. The patient perspectives on future therapeutic options in NASH and patient needs. Front Med (Lausanne) 2019;6:61. doi: 10.3389/fmed.2019.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louviere J.J., Flynn N., Marley A.A.J. Cambridge University Press; 2015. Best-Worst Scaling; Theory, Methods and Applications. [Google Scholar]
- 4.Cheung K.L., Wijnen B., Hollin I.L., Janssen E.M., Bridges J.F., Evers S.M. Using best-worst scaling to investigate preferences in health care. Pharmacoeconomics. 2016;34(12):1195–1209. doi: 10.1007/s40273-016-0429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kemp W., Roberts S. FibroScan® and transient elastography. APF. 2013;42(7):468–471. [PubMed] [Google Scholar]
- 6.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO) EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Labenz C., Huber Y., Kalliga E., Nagel M., Ruckes C., Straub B.K. Predictors of advanced fibrosis in non-cirrhotic non-alcoholic fatty liver disease in Germany. Aliment Pharmacol Ther. 2018;48(10):1109–1116. doi: 10.1111/apt.14976. [DOI] [PubMed] [Google Scholar]
- 9.G-DRG Fallpauschalen-Katalog. https://www.g-drg.de/content/download/8237/61224/version/2/file/Fallpauschalenkatalog+2019_180928.pdf Available at:
- 10.EBM Online-Version des EBM. https://www.kbv.de/html/online-ebm.php Available at:
- 11.IQWiG Online report. https://www.iqwig.de/download/GA10-03_Executive-summary-of-working-paper-1.1_Conjoint-Analysis.pdf Available at:
- 12.Medical Device Innovation Consortium FDA website online report. Appendix A: Catalog of Methods for Assessing Patient Preferences for Benefits and Harms of Medical Technologies. https://www.fda.gov/media/95960/download Available at:
- 13.Cook N., Cave J., Holtorf A.P. Patient preference studies during early drug development: aligning stakeholders to ensure development plans meet patient needs. Front Med (Lausanne) 2019;6:82. doi: 10.3389/fmed.2019.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.