Abstract
This paper summarises evidence for medicinal uses of opioids; harms related to the extra-medical use and dependence upon these drugs, and for a wide range of interventions to address the harms related to extra-medical opioid use. Finally, we use mathematical modelling to estimate harms and explore the overall health benefits of opioid agonist treatment (OAT) in a range of settings that vary in levels of opioid use and associated harms (overdose, HIV, HCV, suicide, accidental injuries) and responses. Estimates in 2017 suggest 40.5 million people were dependent upon opioids (40.5 million people, 95%UI 34.3–47.9 million) and 109,500 people died from opioid overdose (10.5,800–113,600). OAT can be highly effective in reducing illicit opioid use and improving multiple health and social outcomes, including reduced overall mortality and key causes of death including overdose, suicide, and other injuries. Modelling suggested scaling-up and retaining people in OAT, including providing OAT in prison, could avert a median of 7.7%, 14.5% and 25.9% deaths over the next 20 years (compared to scenarios without OAT) in Kentucky, Kyiv and Tehran, with more impact achieved in Tehran and Kyiv due to the added benefits on HIV mortality.. Other pharmacological and non-pharmacological treatments have varying levels of evidence for effectiveness and patient acceptability. Other effective interventions are those focused on preventing harms associated with problematic opioid use. Despite strong evidence for the effectiveness of a range of interventions to improve the health and well-being of people who are dependent on opioids, coverage is low even in high income countries. Treatment quality may be less than desirable, and considerable human, social, and economic harms arise from the criminalisation of illicit opioid use and dependence. Alternative policy frameworks are recommended that adopt a human rights and public health-based approach, do not make drug use a criminal behaviour and seek to reduce drug related harm at the population level.
Background
Opioids are among the world’s oldest known psychoactive drugs, with the use of derivatives from the opium poppy recorded for thousands of years. A wide range of opioids are used for medicinal and recreational purposes, and include natural (i.e., plant-based), semi-synthetic, and synthetic opioids (see webappendix A). Opioids are WHO-listed essential medicines for acute and cancer pain, palliative care, and treatment of opioid dependence1 (throughout this review we use the term dependence to be consistent with WHO’s International Classification of Diseases [ICD]; see Panel A for terminology). Use of opioids for extra-medical purposes is illegal in most countries, with punishments ranging from fines to incarceration. In many countries, people who are opioid dependent comprise a significant proportion of people who are incarcerated for drug-related offences or other crimes2.
Panel A: Common terminology used to describe patterns of opioid use
A range of terms are listed below that are used in the opioid literature137.
Medical opioid use:
use of opioids as prescribed for pain and in the treatment of opioid dependence.
Extra-medical opioid use:
Encompasses use of pharmaceutical opioids either without a prescription (i.e. obtained from outside the formal medical system) or not as directed by a doctor, without excluding the possibility that the user may have medically driven reasons for using the opioid.
Illicit opioid use:
use of non-pharmaceutical opioids for extra-medical purposes.
Diverted opioids:
this term can be used in varied ways, some of which are more stigmatising (e.g. use in a broad sense, including if a patient does not take medication “as directed”). We propose a more restrictive definition,137 which denotes medications diverted from licit sources to unintended individuals or the illicit marketplace: selling/trading, sharing, or giving away of prescription medications to others (either intentionally or involuntarily).
Aberrant opioid behaviours:
a range of patient practices that fall outside those usually expected in treatment of pain or opioid dependence; could have varied reasons including ambivalence about treatment, under-treatment, side-effects or emerging opioid-related problems.
Non-adherent opioid use:
use of opioids by the individual to whom they were prescribed, but are not taken in accordance with prescription directions or other conditions of treatment not met137.
Hazardous opioid use:
a pattern of opioid use that increases the risks of harmful consequences (physical, psychological) for the user or others. It has been defined under “Factors influencing health status and encounters with health services” in ICD-11.
Opioid addiction:
a term widely used by the public and health‐care professionals, and in the pain literature. It is mainly used as a synonym for opioid dependence but is not a current diagnostic term. In common usage, this term invokes a range of social constructs that are value‐laden; many people prefer not to use this term.
Opioid abuse:
a historical term used to refer to a drug use disorder (DSM-IV); it is also a more general term denoting use that is disapproved of.
Opioid use disorder:
can have varied definitions: in DSM-IV, ICD-10 and ICD-11, denotes either abuse/harmful use or dependence; in DSM-5 denotes any use disorder (mild, moderate or severe) and may be further classified as in early or sustained remission or in a controlled (e.g., prison, hospital) environment. ICD differs from DSM-5 in that DSM-5 criteria are met when any two of 11 criteria are endorsed (including criteria previously categorised as DSM-IV abuse); ICD-11 retains the concept of dependence.
Opioid dependence:
a maladaptive pattern of opioid use involving a constellation of behaviours including physiological signs of dependence, loss of control over use, craving and preoccupation with non‐therapeutic use, and continued use despite causing harm (used in ICD-10, ICD-11, DSM-IV). This term is not used in DSM-5.
Harmful opioid use:
used by WHO in its ICD classification of use disorder (similar to DSM-IV abuse).
Illicit opioid dependence:
dependence upon illicitly produced opioids, including heroin and illicitly manufactured synthetic opioids.
Pharmaceutical opioid dependence:
dependence upon pharmaceutical opioids, which may develop under medical supervision138. It may also occur via use of diverted pharmaceutical opioids.
Abstinence:
no longer using the drug that was causing problems; may still be taking agonists as prescribed.
Recovery:
definitions vary; however, can refer to a process of change through which people improve their health and wellness, live self-directed lives, and strive to reach their full potential139.
We use extra-medical use to refer to use of illicit opioids, and/or pharmaceutical opioid use either without a prescription or not as directed by a doctor, while allowing that the user may have medically driven reasons for using the opioid. Extra-medical opioid use occurs worldwide. Many people use opioids initially because they enjoy their effects (see Panel B for some perspectives from people who use drugs), without necessarily choosing opioid dependence and long-term health and social consequences. The complex intersection of illicit opioid use with increased rates of prescribing for medical purposes in many high-income countries, most notably the United States (US), has brought increasing attention to extra-medical opioid use and opioid-related harms.
Panel B: Some perspectives from people who use drugs*
What would you like people to know about people who use drugs?
He/she could be anyone: their brother, sister, fiancé, professor they like the most, singer or actor they’ve a crush on or anyone…people who use drugs are simply not bad people. (Male, 39, Nepal)
We are human we are OK people. We are parents, good and bad like everyone else. We are not demons. We hold down responsible jobs, we care, we’re intelligent, we love, we are creative, we have the same faults as others. (Male, 65, France)
All professions, all governments etc would have significant numbers of drug users among them. People who use drugs are just the same as everyone else - human beings. (Unidentified)
I am, and other people who use drugs are, capable complex multifaceted human beings, capable of being trustworthy, responsible, with ethics, in loving relationships, managing career, education, hobbies, a home, animals. I am smart and I make decisions about my drug use and my drug choices and my breaks…I am a sentient being trapped by laws that makes one drug legal and the next illegal. One route of administration [is] socially acceptable and topical and the other a pariah. (Female, 43, Australia)
There are many different reasons why people from many walks of life choose to take drugs including to self-medicate, alleviate pain, stress, boredom, to relax, have fun, socialise, escape… (Female, 53–58, Australia)
What are the current gaps in the availability, quality and suitability of drug treatment services, health services, and harm reduction services for people who use drugs?
Violation of the rights of PUD, absence of PUD in planning & designing of the program results in to low-quality services and involvement. (Male, 39 years, Nepal)
In terms of health, we just need access to the same as non-drug users but with added expertise so that specific health problems that may occur are not excluded from the treatment package and are delivered by people who know what they are doing. (Male, 65, France)
Most of the drug treatment services in North-east are in the form of Rehabilitation Facilities and all of them does not provide evidence based systematic treatment module. Again, some of the treatment centre and their service providers are not well educated about the psychotherapy and base concept of addiction as a disease. Here, patients are used to suffer and deprived from all necessary amenities inside the rehab in the name of treatment. (Male, 33, India)
Drug treatment services, health services, and harm reduction services need to value and prioritise peer & lived experience for staff, more drug positivity and total abstinence should not be seen as the only goal, some people might just want to get to a healthier place with their use. (Female, 36, Australia)
What do services need to have in place to make people who use drugs feel welcome and want to access services?
Sympathy, non-judgmental and welcoming environment (Male, 39 years, Nepal)
Active involvement of drug using community, gender responsiveness. (Unidentified) (Male, 43, South Africa)
How can people who use drugs and other stakeholders work together to improve the health of people who use drugs?
Remove the prejudice and stigma from both sides and we might actually get somewhere. (Male, 65, France)
Create an environment of trust. (Male, 39, Nepal)
Share experiences and networking of organisations (Male, 42, Burundi)
Promote, allow and listen to feedback by people who use drugs…without bias, stereotype…create and maintain avenues for feedback, suggestions, comments, compliments within services and agencies. (Female, 53–58, Australia)
What is the single best thing that could be done to improve the lives of people who use drugs?
Decriminalisation of drug use. (Male, 49 years, Nepal; Unknown; Female, 43, Australia; Male, 43, Wurundjeri country; Male, 43, South Africa; Female, 36, Australia; Male, 35, USA; Male, 53, Australia;; Male, 58, Australia; Male, 54, Australia);
Legalisation of drug use (Male, 51, UK; Male, 35, USA; Female, 41, Australia; Female, 61, Australia)
Promote human rights of people who use drugs (Male, 42, Burundi); recognise the humanity of people who use drugs (Female, 53–58, Australia; Female, 35, India)
Opioid dependence (as defined in ICD) involves a cluster of symptoms including impaired control over use, prominence of use of a substance in a person’s life and physiological symptoms including tolerance and withdrawal3,4. In North America, the term opioid use disorder (OUD) (from the American Psychiatric Association’s DSM-55) is often used in preference to opioid dependence.
This paper summarises evidence for medicinal uses of opioids, and harms related to the extra-medical use of and dependence upon these drugs. We also summarise the evidence for a wide range of interventions to address harms related to extra-medical opioid use. Finally, we use mathematical modelling to estimate harms and explore the overall health benefits of opioid agonist treatment (OAT) in a range of settings with varying levels of opioid use and associated harms (overdose, HIV, HCV, suicide, accidental injuries) and responses. The approaches used in reviewing literature for each of the sections are detailed in the appendix.
Medicinal uses of opioids
WHO lists opioids as essential medicines for acute and cancer pain, palliative care, and opioid dependence1 (see webappendix B including Panel B1 for a discussion of evidence for medicinal use of opioids). In some countries, particularly the United States (US) and Canada, there have been considerable increases in the prescribing of opioids for a wide range of chronic non-cancer pain (CNCP) conditions over the past two decades. Evidence for the long-term use of opioids for CNCP is limited,6,7 and subject to considerable controversy.
International Narcotics Control Board (INCB) data on country-level use of pharmaceutical opioids (Figure 1; appendix C details how data are collected), shows opioid analgesic use is low in Africa, Asia, Central America, the Caribbean, South America, eastern and south-eastern Europe8.
Figure 1a:
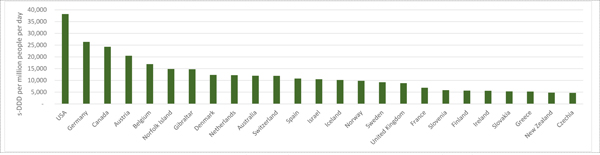
Countries with the highest no. standardised defined daily doses (s-DDDs) of opioid analgesics consumed per million people per day, 2016
In countries with the highest opioid consumption (Figure 1), most use is for CNCP. The US far exceeds other nations, consuming 68% of the world’s prescribed opioid analgesics between 2011–20138. These levels of overprescribing are in part responsible for the unprecedented increase in opioid dependence and overdose in the US.9
Epidemiology of extra-medical opioid use and dependence
There are varied trends across countries in patterns and harms of extra-medical opioid use and dependence. Illicitly produced heroin has traditionally been the dominant opioid used extra-medically. The notable exceptions have been source countries and their close neighbours such as Afghanistan and Iran, where opium has been the most common and heroin use and injecting are now increasing 10.
In many parts of South America, the Middle East and Africa opioids like tramadol are prescribed for pain. There are also reports of significant extra-medical use of tramadol, accompanied by dependence, overdose, and death11; there is some suggestion that much of this is illicitly produced tramadol12. Similarly, problems related to extra-medical use of over-the-counter codeine are reportedly common in Nigeria, Kenya, Zimbabwe, and Chad13. There is evidence that substantial amounts of opioids, including fentanyl, are illicitly manufactured in Mexico14, China and India15.
In high-income countries, increased prescribing of opioids for CNCP has produced iatrogenic dependence and subsequent increases in illicit opioid use, most prominently in the US and Canada. From the 1990s to around 201116 aggressive promotion, under-regulation, and overprescribing of pharmaceutical opioids, increased opioid dependence and overdose deathssubstantially17. In the US, interventions were introduced from 201018 to reduce the supply and extra-medical use of prescribed opioids (e.g., limits on prescribing, prescription monitoring programs, “pill mill” laws, abuse-deterrent reformulations,). These privileged reducing supply over reducing demand and increasing access to interventions for people who had developed problematic opioid use. From the late-2000s onwards16, there were increases in heroin supply and transitions to heroin use and injection 19–21. A “third wave” from around 201322 involved an influx of highly potent synthetic opioids such as illicitly manufactured fentanyl23,24 (see also Peacock et al22 in this series.) The severity of the opioid problem has reduced adult life expectancy in the US for two consecutive years, a first since 1964. In British Columbia in Canada increased use of fentanyl and carfentanyls has reduced life expectancy despite access to universal health care and considerable harm reduction and treatment services. There have been similar but far less dramatic shifts in opioid prescribing, illicit opioid use and overdose in some other countries25.
Prevalence of opioid dependence
The Global Burden of Disease (GBD) study estimated national, regional, and global prevalence of opioid dependence in 201726 (Figure 2a; see Appendix F for methods and Table F1 for regional and global opioid dependence estimates). Globally, the age-standardised rate of opioid dependence was 510 people per 100,000 population (95% uncertainty interval [UI] 430–605; 40.5 million people, 95%UI 34.3–47.9 million). The highest estimated prevalence in 2017 was in the US (age standardised rate of 1,347 per 100,000 (95%UI 1,136–1,609; 4.8 million people, 95%UI 4.1–5.6 million). High rates of opioid dependence were also estimated in the Middle East and East Asia.
Figure 1b:
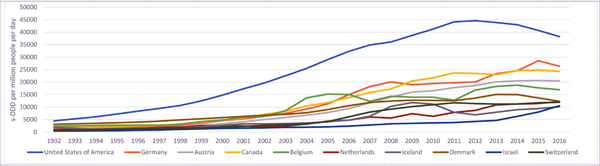
Opioid analgesic consumption in highest countries (as at 2016), no. standardised defined daily doses (s-DDDs) of opioid analgesics consumed per million people per day, 1990–2016
Source: Data provided by the International Narcotics Control Board. Used 3-year rolling averages. Opioid analgesics includes codeine, dextropropoxyphene, dihydrocodeine, fentanyl, hydrocodone, hydromorphone, morphine, ketobemidone, oxycodone, pethidine, tilidine and trimeperidine.
It is important to note that there are gaps in reporting of data by member states and there may be differences in the quality of data reported across countries. See Appendix C for details on how INCB data are collected from member states and analysed. Please note that these figures report on a subset of opioids used for analgesia and do not include the full list of narcotic drugs the full list of narcotic drugs reported in the INCB’s annual reports.
Not everyone who uses opioids extra-medically develops opioid dependence27. A 1991 US population survey suggested that around one in four people who had used opioids (largely heroin) experienced dependence at some time prior to interview28, whereas a UK study adjusting for potential biases estimated that two in three heroin users might become dependent27. A recent study of US population-based surveys over 15 years suggested that approximately 30% of people had developed heroin dependence within a year of initiating use29. A 2011 Iranian population survey found half (49%) of those who had used opioids (mainly opium) in the past year were opioid dependent30. Dependence can also be context-specific: a seminal 1974 study of US Vietnam war veterans found one in three used heroin in Vietnam, of whom 59% were dependent during that time, but 98% remitted from dependence upon returning to the US31.
Estimates of the risk of developing opioid dependence among people prescribed opioids vary widely. In studies of people prescribed opioids for any pain condition, the median risk developing dependence has been estimated at 5% (range 0–31%)32; among people with CNCP prescribed opioids long-term in primary care, estimates vary between 3–26%7. A systematic review (n=15, mostly US surveys) estimated that 15% of people with past-year extra-medical opioid use (95%CI 14–17%) might be dependent33 (see appendix E for methods).
The course of opioid dependence
Opioid dependence is best characterised as a chronic, relapsing condition with periods of active use, abstinence and relapse over years or decades, interspersed with periods of treatment and/or incarceration34. These periods place individuals at heightened risks of serious adverse consequences. There is an elevated risk of mortality from overdose,35–37 during treatment initiation or discontinuation, or when tolerance is reduced after a period of abstinence, treatment cessation, and release from incarceration.
There are few studies on the natural history of pharmaceutical opioid dependence. Some data from the US suggest elevated risk of transition from extra-medical prescription opioid use to injectable heroin19–21.
Risk factors for opioid dependence
Risk factors include genetic, early life, and environment. The social and contextual risk s for extra-medical prescription opioid and illicit opioid use38 include: drug availability, peer substance use30,39,40; social norms about substance use41; adverse childhood experiences, including social disadvantage, family history of drug use42, childhood maltreatment43, parental conflict44 and problematic parental relationships45,30,46–48. Individual risk factors include being male, externalising disorders in childhood49 and poor educational attainment50. Drug dependence is partially heritable, but probably a genetic disposition to drug use disorders in general rather than opioids in particular51.
Comorbid substance use and mental health problems
People who use extra-medical opioids typically use multiple substances and often have comorbid polysubstance use disorders52,53 and mental illnesses54. These relationships are not necessarily causal but problematic non-opioid use47,55 and depression, anxiety and post-traumatic stress disorders47,55 markedly increase the risk of opioid dependence. Similarly, use of alcohol, stimulants, benzodiazepines52 and mental health problems56 reduce positive treatment outcomes for opioid dependence and increasing overdose risk (see later section on overdose risk). Additional interventions are required when psychiatric comorbidity is present (see Hall et al57 and Farrell et al58 in this series).
Opioid overdose
Prevalence of fatal opioid overdose
Fatal opioid overdose is a major adverse outcome of prescribed and extra-medical opioid use that is increasing in the US, the United Kingdom, Canada, Australia and across Europe59. Globally, GBD 201760 estimated that there were around 109,500 opioid overdose deaths (see appendix F, Table F1), 43% of which were in the US. As shown in Figure 2b, the highest estimated fatal opioid overdose rates per 100,000 population were in the Russian Federation, Eastern European countries and the US.
Not all overdoses are fatal; many more non-fatal overdoses occur than fatal overdoses. A cohort of people prescribed opioids in Washington State, US, found a rate of 0.13 non-fatal opioid-related overdoses per 100 person-years (PY), and 0.017 fatal overdoses per 100PY (7.6:1 ratio).61 By contrast, in a cohort of PWID in Vancouver, Canada, there were 12.0 non-fatal overdoses per 100PY62 and 0.89 fatal overdoses per 100PY – a ratio of 13.5:1.62
Mechanisms of opioid overdose
At higher doses, opioids suppress respiratory rhythm generation and reduce normal physiological responsiveness of central and peripheral chemoreceptors (more detail on overdose mechanisms is presented in webappendix G). As a result, CO2 levels rise and hypoxia develops,63 breathing rates decrease and eventually stop.
People who use opioids long-term develop tolerance to euphoria faster than to respiratory depression.64 Fentanyl and its structural analogues rapidly enter the brain after intravenous injection, accelerating respiratory depression65 and inducing “respiratory paralysis.”
Risk factors for fatal opioid-related overdose
Systemic disease comorbidity may increase the risk of fatal opioid overdose e.g. via increased sensitivity to respiratory depressive effects impaired cardiac function, or impairment of opioid metabolism because of impaired liver or kidney function. Prolonged hypoxia and loss of consciousness from multiple overdoses may aggravate systemic disease66,67.
Fatal opioid-related deaths often involve alcohol, benzodiazepines, cocaine and amphetamines68. Concomitant or polydrug use greatly increases the risks of opioid overdose69–74 via synergistic respiratory depressant effects70,74; reverse tolerance to respiratory depression73,75; and 3) increase risk of relapse in abstinent heroin users76. The use of gabapentinoids prescribed to treat chronic pain, has increased amongst heroin users,74,77–81 and have been found along with opioids at post mortem74,82,83. An increased risk of opioid-related deaths has also been reported for patients prescribed opioids and gabapentin for the treatment of pain84.
Other health and social harms among people who use opioids
Non-fatal harms among people with opioid dependence
Table 1 summarises evidence for opioid dependence as a risk factor for a range of adverse outcomes (see webappendix H for methodology). Much of the evidence comes from studies of people who use heroin, most of whom inject; there are fewer studies among people who use pharmaceutical opioids extra-medically, and who use opioids without injecting.
Table 1:
Existing evidence for adverse outcomes among people who are opioid dependent
| People dependent on illicit opioids | People prescribed pharmaceutical opioids | |||||||
|---|---|---|---|---|---|---|---|---|
| Effect | Level of evidence | Sources | Effect | Level of evidence | Sources | |||
| Non-Fatal outcomes | ||||||||
| Receptive syringe sharing (past year) | ↑ | 25.5% (16.7, 34.3%) PWID | C | 85 | NA | |||
| HIV incidence | ↑ | 0.8–10.7 per 100 PY PWID | C | 107 | NA | |||
| HIV prevalence | ↑ | 17.8% (10.8, 24.8%) PWID | C | 85 | No review level quantitative evidence | 88 | ||
| HCV incidence | ↑ | 5.9–42.0 per 100 PY PWID | C | 108 | NA | |||
| HCV prevalence (HCV antibody) | ↑ | 52.3% (42.4, 62.1%) PWID | C | 85 | No review level quantitative evidence | 88 | ||
| HCV prevalence (HCV RNA) | ↑ | 39.2% (31.6, 47.0%) PWID | C | 163 | No review level quantitative evidence | 88 | ||
| Skin and soft tissue infections… | ||||||||
| …current | ↑ | 6.1–32.0% PWID | C | 86 | No review level quantitative evidence | 88 | ||
| …past 6–12 months | ↑ | 6.9–37.3% PWID | C | 86 | No review level quantitative evidence | 88 | ||
| …ever | ↑ | 6.2–68.6% PWID | C | 86 | No review level quantitative evidence | 88 | ||
| Infective endocarditis (ever) | ↑ | 0.5–11.8% PWID | C | 86 | No review level quantitative evidence | 88 | ||
| Quality of life | ↓ | No review level quantitative evidence | C | 87 | No review level quantitative evidence | 88 | ||
| Mental health (depression, anxiety) | ↑ | No review level quantitative evidence | ↑ | No review level quantitative evidence | 88 | |||
| Criminal activity | ↑ | RaRa 5.84 (1.36, 10.32) SYNTH | C | 89 | NA | |||
| Contact with criminal justice system | ↑ | RaRa 2.97 (1.43, 4.51) SYNTH | C | 89 | NA | |||
| Non-fatal overdose (ever) | ↑ | 41.5% (34.6–48.4%)PWID | C | 164 | ↑ | No review level quantitative evidence | C | 164 |
| Poor neonatal outcomes… | ||||||||
| …low birth weight | ↑ | RR 4.61 (2.78–7.65) | C | 165 | ? | RR 1.36 (0.83–2.22) METH | C | 165 |
| …neonatal abstinence syndrome | ↑ | 50–95% | C | 166 | ↑ | 47–57% | B | 167 |
| …pre-term birth | ↑ | TBC | 168 | ↑ | 7–19% | B | 167 | |
| Fatal outcomes | ||||||||
| Overdose | ↑ | SMR 58.43 (38.09–89.64) | C | 100 | ↑ | CMR 0.63 per 1000PY (0.18–2.23) | C | 101 |
| Other accidental injuries | ↑ | SMR 6.85 (4.41–10.64) | C | 100 | No data | C | 101 | |
| Suicide | ↑ | SMR 8.52 (6.00–12.10) | C | 100 | No data | C | 101 | |
| Cancer | ↑ | SMR 2.69 (1.84–3.92) | C | 100 | No data | C | 101 | |
| AIDS-related | ↑ | SMR 18.50 (8.15–41.99) | C | 100 | No data | C | 101 | |
| Viral hepatitis | ↑ | SMR 35.94 (16.06–80.42) | C | 100 | No data | C | 101 | |
| Overall mortality | ↑ | SMR 9.90 (7.52–13.05) | C | 100 | ↑ | CMR 23.84 per 1000PY (9.21–61.75) | C | 101 |
Note: For details of the search strategies used please see Appendix H and Appendix I. PWID – based on studies of people who inject drugs, not necessarily opioids specifically. SYNTH – authors did not pool estimates, but we did for this review. METH – studies examining women on methadone versus controls. RR – relative risk. RaRa – rate ratio. SMR – standardised mortality ratio. NA – reviews of pharmaceutical opioid dependent people specifically were not located. ND – reviews indicated that no estimates could be found for this outcome.
A Consistent conclusions across meta-analyses, high quality systematic reviews, or multiple randomised controlled trials
Evidence from one or two randomised controlled trials only
High quality systematic reviews of cohort, case-control or cross-sectional studies
Systematic reviews with inconsistent conclusions from authors or of cross-sectional studies; OR multiple consistent ecological studies
Cross-sectional association, case series suggesting outcome, single cohort study
HIV and HCV are a major risk for people who inject drugs (PWID).85 The prevalence of opioid injecting varies widely geographically and in the main type of opioid used e.g. in South Asian countries, non-injecting routes of administration have been the most common. Table 1 also shows that skin and soft tissue infections86, as well as infective endocarditis86, are significant risks among PWID. Other consequences associated with opioid dependence, include poorer quality of life87, mental health problems88, increased criminal activity89, and involvement with the criminal justice system89.
Prolonged heroin use is associated with damage to brain white matter90–92, changes in the connectivity between cortical and subcortical regions93 and a decrease in grey matter density94,95. Chronic extra-medical opioid use results in repeated periods of hypoxia during non-fatal overdoses. The number of non-fatal heroin overdoses predicts neurocognitive impairment96, though less than other drugs97, head injury, psychiatric comorbidity, and chronic infection (HCV and HIV)98,99.
Mortality in people with using illicit opioids and prescribed opioids for chronic pain
A systematic review of mortality among people who use opioids (see webappendix I) found 97 eligible cohorts of people using opioids extra-medically 100 and nine cohorts of people prescribed opioids for chronic non-cancer pain101. The pooled all-cause crude mortality rate (CMR) in people using extra-medical opioids was 1.7 per 100 person-years (PY; 95%CI 1.5–1.9), nearly 10 times the expected rate for people of that age (standardised mortality ratio (SMR 9.9; 95% CI 7.5–13.1)). Among people injecting extra-medical opioids, the pooled SMR was 14.1 (95% CI 10.1–19.7), highlighting a higher risk among injectors. CMRs were highest for overdose deaths (0.5 per 100PY; 95% CI 0.5–0.6), but AIDS and liver disease were major causes of mortality (AIDS CMR 0.2 per 100PY; 95% CI 0.1–0.3; liver-related CMR 0.2 per 100PY; 95% CI 0.1–0.3).
In people prescribed opioids for CNCP, the pooled all-cause mortality rate was high (2.4 per 100PY PY; 95% CI 0.9–6.2) owing to the age and poor health of these samples. Overdose comprised a small proportion of mortality (pooled overdose CMR 0.06 per 100PY; 95% CI 0.01–0.2). T
Reducing harms among people who use opioids
Impact of methadone or buprenorphine treatment on outcomes
Varying terms (and their acronyms) are used to describe long-acting opioid agonists or partial agonists for the treatment of opioid dependence, including ‘methadone maintenance treatment’ and opioid agonist treatment (OAT102. In this paper we use OAT to refer to methadone or buprenorphine specifically, though all terms have limitations103.
OAT is the most effective treatment for opioid dependence and a WHO Essential Medicine1. It reduces harms across multiple health outcomes (Table 2 (see webappendix J for methods and the searches undertaken, and modelling Panel E). Other opioids can be used in the treatment of opioid dependence; these are summarised in webappendix J in Panel J1). A range of other services are often provided in addition to OAT, depending upon the setting, and may include other medical care, mental health services, vocational and other assistance, and provision of naloxone.
Table 2:
Existing evidence for impacts* of opioid agonist treatment with methadone or buprenorphine
| Effect | Level of evidence | Sources | ||
|---|---|---|---|---|
| OAT vs. no treatment in the community | ||||
| Opioid use | ↓ | RR 0.48 (0.41, 0.55) SYNTH | A | 106 |
| Injecting frequency | ↓ | SMD −0.59 (−0.91, −0.26) SYNTH | A | 106 |
| Injecting risk (sharing needles/syringes) | ↓ | RR 0.53 (0.4, 0.7) SYNTH | A | 106 |
| HIV linkage to care and treatment | ↑ | HR 1.87 (1.50, 2.33) | C | 109 |
| HIV treatment adherence | ↑ | OR 2.14 (1.41, 3.26) | C | 109 |
| HIV treatment attrition/discontinuation | ↓ | OR 0.77 (0.63, 0.95) | C | 109 |
| HIV viral suppression | ↑ | OR 1.45 (1.21, 1.73) | C | 109 |
| HIV incidence | ↓ | RR 0.46 (0.32, 0.67) | C | 107 |
| HCV testing | ↑ | OR 1.73 (1.19, 2.51) | C | 169 |
| HCV linkage to care and treatment | ↑ | OR 1.40 (0.90, 2.17) | C | 169 |
| HCV treatment sustained virological response | × | OR 0.75 (0.45, 1.25) | C | 169 |
| HCV incidence | ↓ | RR 0.50 (0.40, 0.63) | C | 108 |
| Skin and soft tissue infections | ? | NE | D | 86 |
| Mental health problems | ↓ | SMD 0.49 (0.35, 0.63) | C | 170 |
| Quality of life (social – WHOQOL-BREF) | ↑ | SMD 0.29 (0.16, 0.42) | C | 170 |
| Criminal activity | ↓ | SMD −0.57 (−1.00, −0.13) | 110 | |
| Contact with the criminal justice system | × | RR 0.75 (0.46, 1.23) | C | 171 |
| Overdose mortality | ↓ | RaRa 0.25 (0.18, 0.36) SYNTH | C | 111 |
| Suicide mortality | ↓ | RaRa 0.48 (0.39, 0.59) | E | 112 |
| Other injury mortality | ↓ | RaRa 0.40 (0.34, 0.46) | E | 112 |
| All-cause mortality | ↓ | RaRa 0.33 (0.28, 0.39) SYNTH | C | 111 |
| OAT vs. no treatment in prison | ||||
| Unsanctioned opioid use | ↓ | NE | B | 172 |
| Injecting frequency | ↓ | NE | B | 172 |
| Injecting risk behaviour | ↓ | NE | B | 172 |
| HIV incidence | ? | NE | B | 172 |
| HCV incidence | ? | NE | B | 172 |
| Prison infractions | ↓ | NE | E | 172 |
| Criminal activity (post-release) | ? | NE | B | 172 |
| Reincarceration | ? | NE | C | 172 |
| OAT engagement (post-release) | ↑ | NE | B | 173,174 |
| HIV treatment adherence (post-release) | NR | |||
| HIV viral suppression (post-release) | NR | |||
| Overdose/suicide/injury mortality (in prison) | ↓ | aHR 0.13 (0.05, 0.35) | E | 175 |
| All-cause mortality (in prison) | ↓ | aHR 0.26 (0.13, 0.50) | E | 175 |
| All-cause mortality (4 weeks post-release) | ↓ | aHR 0.25 (0.14, 0.45) | E | 114,115 |
| Buprenorphine vs. methadone (ref) | ||||
| Retention in treatment | ↓ | RR 0.83 (0.72, 0.95) | A | 117 |
| Mortality during induction (4 weeks) | ↓ | RaRa 0.28 (0.08, 0.95) SYNTH | C | 111,118 |
| Mortality remainder in treatment | ND | RaRa 0.68 (0.44, 1.04) SYNTH | C | 111,118 |
| Mortality following cessation (4 weeks) | ND | RaRa 0.62 (0.16, 2.42) SYNTH | C | 111,118 |
| Neonatal outcomes… | ||||
| …Head circumference | ↑ | RCT WMD 0.91cm (0.14 1.66) | B | 119 |
| …Low birth weight | ↓ | RCT WMD 324g (32, 617) | B | 119 |
| …Preterm birth | ↓ | RR 0.40 (0.18, 0.91) | B | 119 |
For details of the search strategies used please see Appendix J.
Please note that the comparator groups used vary across outcomes; they can include a placebo or active control or an out-of-treatment comparator.
SYNTH – we pooled estimates for this review. NR – no quantification located. NE – no quantitative synthesis reported. ND – no difference between methadone and buprenorphine detected. RR – relative risk. RaRa – rate ratio. RCT – randomised controlled trial. WMD – weighted mean difference. SMD – standardised mean difference. HR – hazard ratio. OR – odds ratio.
Presence or absence of effect
OAT does not appear to have a significant effect upon the outcome
This outcome may be increased by OAT
This outcome is decreased by OAT
Unclear if OAT has an impact on this outcome
Level of evidence
Consistent conclusions across meta-analyses, high quality systematic reviews, or multiple RCTs
Evidence from one or two randomised controlled trials only
High quality systematic reviews of cohort, case-control or cross-sectional studies
Systematic reviews with inconsistent conclusions from authors; OR multiple consistent ecological studies
Cross-sectional association, case series suggesting outcome, single cohort study
The best evidence for OAT and other interventions is in people using illicit opioids (especially heroin) and/or PWID. Some evidence suggests that outcomes are also positive for OAT for people with any pharmaceutical opioid dependence104; evidence is less clear for opium dependence105.
Table 2 summarises the evidence for the impact of OAT on outcomes in people who are opioid dependent (. It reduces injecting risk behaviour106 and risk of HIV and HCV acquisition107,108, increases engagement in the HIV109 and HCV cascade of care, reduces criminal activity110 and reduces all-cause111 and overdose111 mortality. There is weaker evidence that it may reduce o suicide and accidental injuries112. OAT is highly cost-effective, and cost-saving when costs of crime are included113.
The protective effect of OAT on mortality is marked in people who experience incarceration, especially during the highest risk periods in the first weeks post-incarceration, and after release from prison114,115. This could reduce HIV and HCV transmission, which is also elevated at these times116. However, OAT is rarely available in these settings. Our modelling (Panel E) shows that scaling-up OAT in prisons and the community could avert up between 23.9% and 75.0% more deaths over 20 years than only scaling-up in the community, suggesting that interruptions in OAT during incarceration may limit its population benefits.
Panel E: Mathematical modelling of the impact of improving coverage and quality of OAT on multiple health outcomes across three global settings
We performed modelling to comprehensively evaluate the overall health benefits of OAT amongst PWID in 3 global settings: Kiev (Ukraine), Appalachian Kentucky (USA) and Tehran (Iran) (for details see Webappendix M). The single-sex, dynamic model, calibrated within a Bayesian framework to data from each setting, captures effects of OAT on all-cause mortality, injury, overdose and self-harm/suicide deaths, HIV and HCV transmission, HIV treatment outcomes, and incarceration (which has been linked to greater risk of HIV and HCV transmission among PWID116). The contrasting settings were chosen because of differences in HIV and HCV epidemics, overdose mortality and intervention coverage, their geographical spread and data availability (Figure E1).
Figure E1:
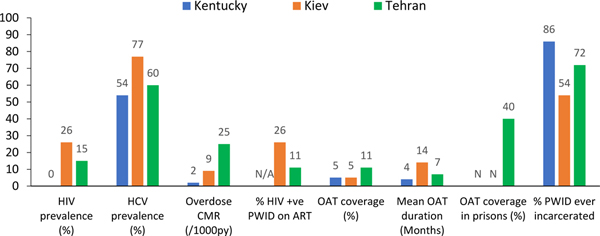
Summary of parameters across modelled settings
Model analyses estimated the lives lost due to an individual’s drug use, and the contribution that different harms (overdose, injury, suicide, HIV and HCV mortality) have to this health loss (with current OAT coverage). Analyses then evaluated how scaling-up the coverage of OAT or increasing the duration of OAT can reduce this health loss and which beneficial effects of OAT are most important for improving health in each setting. Model analyses evaluated the percentage of deaths averted and life years gained (LYG) compared to a scenario with no OAT, of: current OAT coverage (Status Quo Scenario); scaling-up OAT coverage to 40% in the community, as recommended by WHO/UNAIDS (Scale-up Scenario A); additionally, increasing the average duration of OAT to 2 years (Scale-up Scenario B); additionally, scaling-up OAT in prison (Scale-up Scenario C).
Figure E2 shows that current levels of OAT are projected to have negligible impact on mortality in Kentucky and Kiev but substantial impact in Tehran where OAT coverage is greatest and provided in prisons (Figure E2); averting 0.8% (95%CrI: 0.5–1.4), 0.3% (95%CrI: 0.1–0.6) and 6.5% (2.5 – 12.1) of deaths and gaining 119.6 (95%CrI: 61.8–206.1), 18.5 (95%CrI: 9.1–32.8) and 1062.6 (95%CrI: 385.0–2003.3) life-years per 1,000 PWID over the next 20 years in Kiev, Kentucky and Tehran, respectively.. Scaling-up OAT in the community (Scale-up Scenario A) could avert between 2.4% (Kentucky; 95%CrI: 1.2–4.5) and 12.5% (Tehran; 95%CrI: 6.0–18.0) of deaths and gain between 161.3 (Kentucky; 95%CrI: 84.6–258.9) and 1878.9 (Tehran; 95%CrI: 859.9–3262.8) life-years per 1,000 PWID over the next 20 years. This equates to between 12.7% (Tehran; 6.6–18.5) and 19.3% (Kiev; 95%CrI: 16.3–21.6) of overdose deaths averted (Figure E2 and Table E1). Model analyses suggest that extending the average duration of OAT (Scale-up Scenario B) would have additional benefits, particularly on reducing overdose deaths; with between 4.4% (Kentucky; 95%CrI: 2.4–8.5) and 16.7% (Tehran; 95%CrI: 9.1–22.4) of all deaths averted and 1.6–2.3 times more overdose deaths averted compared to scaling-up OAT without improving retention. Model projections also show that interruptions in OAT due to incarceration may limit the impact of OAT; in Tehran, for example, 25.9% (95%CrI: 16.7–33.2) of deaths and 56.2% (95%CrI: 19.4–78.6) of HIV deaths could be averted if OAT is also provided in prisons with retention upon release (Scale-up Scenario C). However, limited impact on HCV deaths is achieved due to many of the HCV deaths occurring among those with existing infections prior to OAT scale-up.
The impact of scaling-up OAT on all-cause mortality varies substantially between the three settings primarily because of differences in how the varied harms associated with drug use contribute to mortality among PWID. This impact is greatest in Kiev and Tehran where the primary cause of death associated with drug use is projected to be HIV (27.8% (95%CrI: 20.1–36.5) and 21.3% (95%CrI: 6.5–42.7) of all deaths in Kiev and Tehran, respectively). Lower impact of scaling-up OAT is achieved in Kentucky, where overdose is the primary cause of death associated with drug use and only accounts for 8.1% (95%CrI: 4.4–15.7) of all deaths. This is further demonstrated by how each effect of OAT contributed to the overall impact of scaling-up OAT in each setting. For Scale-up Scenario C, for example, the effect of OAT on HIV transmission was the most important effect in reducing mortality in Kiev and Tehran accounting for 41.3% (95%CrI: 28.6–60.1) and 69.1% (95%CrI: 31.7–92.9) of deaths averted, respectively, whilst in Kentucky, the most important effect was the effect of OAT on overdose, accounting for 55.0% (95%CrI: 32.7–75.8) of deaths averted.
Figure E1: Causes of death among PWID and ex-PWID; 2020–2040.
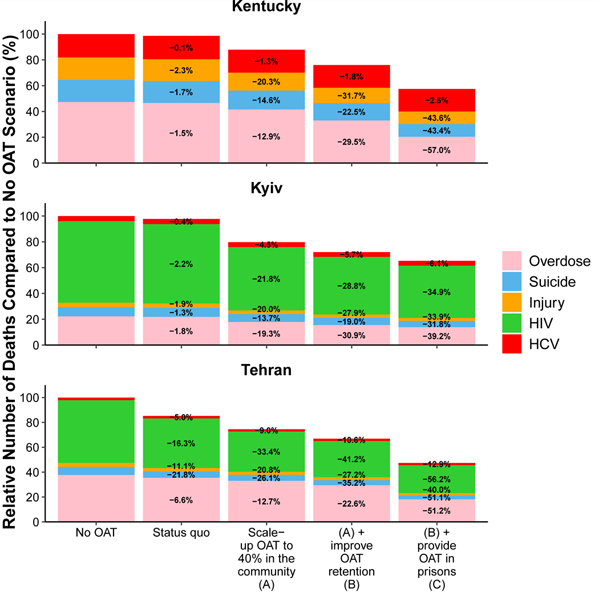
Figure shows the median percentage of deaths due to overdose, suicide, injury, HIV, HCV or other causes under the following strategies: if there were no OAT from 2020; if OAT was scaled-up to 40% coverage among PWID in the community; if OAT was scaled-up to 40% coverage among PWID in the community and the average duration of OAT is increased to 2-years; if OAT was scaled-up to 40% coverage among PWID in the community, incarcerated PWID enrol onto OAT at the same rate and the average duration of OAT is increased to 2-years. Deaths from other natural causes which account for 22%, 54% and 82% of deaths in Tehran, Kiev and Kentucky in Status Quo projections, respectively, are not shown.
Table E1:
Deaths averted with current and scaled-up OAT coverages compared to no OAT scenario. Cells show median relative reduction in preventable deaths due to different causes with 95% credibility intervals in parentheses. Negative reductions (i.e. increases) in deaths are possible for some causes due to competing risks.
| Status quo | Scale-up OAT to 40% in the community (A) | (A) + improve OAT retention (B) | (B) + provide OAT in prisons (C) | |
|---|---|---|---|---|
| Median % reduction in deaths (95%CrI) | Median % reduction in deaths (95%CrI) | Median % reduction in deaths (95%CrI) | Median % reduction in deaths (95%CrI) | |
| Kentucky | ||||
| HCV | 0.1% (0 – 0.3) | 1.3% (−0.3 – 3.1) | 1.8% (−0.7 – 4.7) | 2.6% (−1.4 – 7) |
| Overdose | 1.5% (0.2 – 2.1) | 12.9% (2.2 – 17) | 29.5% (23.1 – 38) | 57% (48.1 – 62.9) |
| Suicide | 1.7% (1.2 – 2.3) | 14.6% (12.1 – 17.7) | 22.5% (18.2 – 29) | 43.4% (35.8 – 50.8) |
| Injury | 2.3% (1.8 – 3) | 20.3% (18.1 – 22.7) | 31.7% (25.7 – 37.1) | 43.6% (39.1 – 50) |
| Other | 0% (0 – 0.2) | 0.2% (−0.4 – 2) | 0.1% (−0.7 – 2.9) | 0% (−1.3 – 3.6) |
| Total | 0.3% (0.1 – 0.6) | 2.4% (1.2 – 4.5) | 4.4% (2.4 – 8.5) | 7.7% (4.4 – 13.3) |
| Kiev | ||||
| HIV | 2.2% (1.2 – 3.7) | 21.8% (15.2 – 30.1) | 28.8% (19.6 – 39.5) | 34.9% (24.4 – 45.8) |
| HCV | 0.4% (0 – 0.9) | 4.5% (0.4 – 8.2) | 5.7% (0.3 – 10.9) | 6.1% (−1 – 12) |
| Overdose | 1.8% (1.1 – 3) | 19.3% (16.3 – 21.6) | 30.9% (22.4 – 35.4) | 39.2% (29.7 – 43.5) |
| Suicide | 1.3% (0.8 – 2) | 13.7% (11.6 – 15.3) | 19% (14.1 – 23) | 31.8% (25.4 – 40.1) |
| Injury | 1.9% (1.2 – 3) | 20% (18.3 – 21.4) | 27.9% (22.3 – 31.9) | 33.9% (27.1 – 37.9) |
| Other | −'0.1% (−0.2 – 0.1) | −'0.6% (−2.2 – 0.7) | −'0.7% (−3.2 – 0.9) | −'1% (−3.9 – 1.1) |
| Total | 0.8% (0.5 – 1.4) | 8.6% (6.8 – 11.5) | 11.7% (8.8 – 16.4) | 14.5% (11.3 – 20.4) |
| Tehran | ||||
| HIV | 16.3% (5.2 – 44.4) | 33.4% (11.2 – 63.4) | 41.2% (14.0 – 70.3) | 56.2% (19.4 – 78.6) |
| HCV | 5.0% (0.6 – 17.6) | 9.0% (1.6 – 25.7) | 10.6% (1.0 – 30.0) | 12.9% (−2.9 – 35.6) |
| Overdose | 6.6% (1.6 – 10.2) | 12.7% (6.6 – 18.5) | 22.6% (16.6 – 28.1) | 51.2% (43.3 – 56.5) |
| Suicide | 21.8% (16 – 25.6) | 26.1% (21.9 – 30.1) | 35.2% (29.5 – 39.4) | 51.1% (40.2 – 57.6) |
| Injury | 11.1% (4.6 – 14.5) | 20.8% (18.1 – 23.3) | 27.2% (23.2 – 30.3) | 40.1% (33.8 – 45.2) |
| Other | −'2.0% (−3.5 - −0.7) | −'3.8% (−6.4 - −1.8) | −'5.1% (−8.9 - −2.8) | −'8.3% (−14.5 - −4.5) |
| Total | 6.5% (2.5 – 12.1) | 12.5% (6.0 – 18.0) | 16.7% (9.1 – 22.4) | 25.9% (16.7 – 33.2) |
Methadone and buprenorphine differ on some outcomes. Retention is higher in patients on higher doses of methadone (>80mg daily) and lower for buprenorphine and lower methadone doses (<60mg daily)117. Two studies have found lower risk of mortality during induction onto methadone vs. buprenorphine, but evidence is unclear on differences at other points in or out of treatment111,118. Among opioid dependent women who are pregnant, neonatal outcomes may be superior for women maintained on buprenorphine119.
Higher doses of methadone and buprenorphine increase retention in treatment120. There is low quality evidence that supervised dosing (i.e. doses provided as directly observed doses by a pharmacist or other clinical worker) does not improve retention121. There is insufficient evidence to assess the effectiveness of urine drug screening (UDS) during OAT upon retention122. Guidance on quality OAT provision developed by WHO is based on evidence more than a decade old (Panel C summarises WHO guidance; text in bold indicates guidance based on more recent evidence).
Panel C –. Guidance for opioid agonist treatment (OAT) for the treatment of opioid dependence
The most recent international guidelines for OAT for opioid dependence were released by the World Health Organization (WHO) in 2009176, and do not reflect current practice or guidelines elsewhere, including several treatment modalities like slow-released morphine and injectable and implantable forms of OAT that are now included in standard of practice and national guidelines in many countries. The below text combines older recommendations from the WHO guidelines along with some key updates (noted in bold text) guided by more recent evidence and featured in national guidelines. It will be important for WHO to update the WHO guidelines given the developments in this field in the decade since the guidelines were published.
Due to better retention and greater cost effectiveness, higher-dose methadone might be considered the preferred OAT medication176. There are multiple reasons why buprenorphine may be preferred, including less rigid supervision and client preference, better safety profile, experience of adverse effects or medication interactions with methadone and poor response to methadone176.
Initial methadone dose depends upon level of tolerance and is 10mg to 30mg (not more than 30mg) per day. Doses should be escalated rapidly with monitoring for symptoms of opioid excess to promote lower relapse and higher retention. Higher maintenance doses (range 60–120mg per day) result in reduced extra-medical opioid use and better retention. Initial buprenorphine dose for clients will vary; for those with moderate neuroadaptation it should be 4–8mg, and maintenance doses should minimally be at least 8mg (range: 8mg to 24 mg)176. Buprenorphine doses higher than 24mg per day are associated with diversion of the medication.
Doses should be directly supervised early in treatment, especially with methadone. Supervision of buprenorphine doses should be variable, and in some cases, home inductions can occur with experienced clinicians. Take-away doses (i.e. doses provided to the patient for unsupervised dosing) should be provided especially when benefits of reduced frequency of attendance outweigh medication diversion risks, and this should be regularly reviewed176.
The WHO recommendations do not address urine drug screening during OAT. Urine drug testing requirements are variable based on settings. When deployed, however, they should be used to guide OAT dosing and concomitant psychosocial counselling – not discontinuation of OAT, which may result in relapse, overdose and death.
Clients on methadone can be successfully transferred to buprenorphine, but only when methadone doses have been lowered sufficiently, generally below 40mg.
Pharmacological treatment of opioid dependence should be widely accessible. OAT can be successfully provided in multiple settings, including specialty addiction treatment, hospitals, primary care and other office-based settings, pharmacies and in criminal justice settings.
Best practice is considered to involve a range of other interventions as needed by clients, including counselling, psychiatric treatment and social supports including assistance with housing, employment, education and legal problems. Psychosocially assisted pharmacological treatment should not be compulsory because not all patients need it138. Links to psychosocial counselling, HIV, TB and hepatitis treatment should be available176.
Synthesis of available evidence suggests that in many settings, delivery of OAT in routine clinical practice may be sub-optimal. Average doses in some countries appear to be below the minimum recommended dose, and access to unsupervised dosing is often limited, particularly for methadone.
Current OAT provision around the globe
A 2017 review123 found very low levels of OAT coverage among PWID (Figure L1). Only 86 of 179 countries with evidence of injecting drug use provided OAT. Coverage was most often low according to WHO indicators (defined as <20 OAT recipients per 100 PWID per year); only 20 countries (5% of the global PWID population) were implementing high-coverage OAT (>=40 OAT recipients per 100 PWID)123. Retention in OAT can also be poor (Figure L2 in webappendix L presents data on retention in cohorts in multiple countries). Our mathematical modelling (see Panel E) demonstrates that scaling-up OAT from low to high coverage among PWID could avert 2.4–8.1% of all deaths, 9.8–19.3% of overdose deaths and 21.8–34.9% of HIV deaths in people who injected drugs in Kentucky, Tehran and Kiev over the next 20 years if OAT achieved 40% coverage of PWID outside of prisons. Even greater impacts are projected if OAT retention was improved (to 2 years) and OAT was provided in criminal justice settings (see Panel E and below).
In a systematic review of OAT in routine clinical practice (search details in Appendix L) sub-optimal dosing of methadone and buprenorphine was common.124 There was greater access to unsupervised dosing with buprenorphine than methadone124. Limiting access to unsupervised dosing can mean daily clinic attendance which interferes with employment, education and family responsibilities, and is a barrier to treatment entry and retention. Use of urine drug screening in OAT is widespread but there is little evidence that it improves clinical outcomes 122. Requirements in some countries that OAT client details are reported to law enforcement agencies are a significant barrier to treatment 124. OAT could be improved by greater involvement of clients in service design and delivery. Panel D discusses a range of ways in which OAT access, retention and outcomes could be improved.
Panel D –. Improving opioid agonist treatment access, retention and outcomes
There are many opportunities to improve the quality of care provided in OAT. Key barriers to treatment entry include: lack of unsupervised dosing, extensive assessment processes, delays to treatment following presentation, mandatory engagement in psychosocial counselling, siloing of OAT in specialist clinics, and geographically distant clinics and pharmacies for medication delivery.
In many countries, there is a requirement for official registration of a patient due to the scheduling of opioid medications. This information can sometimes be shared with police (either by law, or in practice), and clients may be targeted by police, lose their driver’s license, experience restrictions on employment, and other adverse consequences177,178.
Opportunities to improve OAT access, retention and outcomes:
Rapid access to OAT with minimal assessment to establish opioid dependence in several settings such as emergency rooms, community-based settings, primary care and criminal justice settings
OAT prescribing in a range of settings, beyond specialised settings including primary care, and OAT dispensing in community pharmacies
Elimination of compulsory counselling requirements (e.g. in the US); instead, make provision of psychosocial services that may be accessed on a voluntary basis
Minimise intensity of care as people stabilise in treatment (e.g., transition to less supervision as patients become clinically stable)
Increased and rapid access to unsupervised dosing, particularly for people prescribed buprenorphine or stabilised on methadone
Minimise use of urine drug screening; use results of such screening to improve care (i.e. dose adjustment) rather than to terminate treatment
Low-threshold and adapted care for disengaged individuals, such as those who are homeless or with mental health issues (e.g., many required assessments can be obtained after starting OAT)
Access to alternative agonist treatments such as slow release oral morphine and injectable opioid agonist therapy for individuals who do not respond to methadone or buprenorphine
There are few data on the impacts of flexible OAT provision on the diversion of OAT medications. The safety profile of buprenorphine (especially in comparison to illicit opioids such as fentanyl and heroin) means that these harms are limited. In the UK supervised community pharmacy provision of methadone reduced methadone-related overdoses despite an almost 400% increase in patient numbers125.
Modelling impact of OAT scale-up on health outcomes
Panel E presents model projections for 3 global settings (Kiev (Ukraine), Perry County (Kentucky, USA) and Tehran (Iran) to evaluate the overall health benefits of OAT among PWID (model details are presented in webappendix M). Scaling-up OAT to UNAIDS/WHO recommended coverages of 40% among PWID in the community (current coverage in each setting is 5–11%) could prevent 2.4–8.6% of all deaths among PWID and people who previously injected drugs over 2020–2040, with overdose deaths accounting for 22.1–43.7% of all deaths averted. These mortality reductions would increase up to 1.8-fold if OAT treatment duration increases to 2 years) and up to 3.2-fold if retention was improved (to 2 years) and access provided to OAT in prisons and on release. Increasing OAT coverage, improving retention, and providing OAT in prisons could reduce 57.0% of all overdose deaths among PWID in Kentucky and 58.7% of HIV deaths among PWID and people who previously injected drugs in Tehran.
Our modelling shows that OAT also reduces other causes of mortality and the risk of HIV and HCV transmission, and rates of incarceration while improving HIV treatment outcomes. The mortality benefits vary across settings depending upon whether the major causes of death are due to HIV, as in Kiev and Tehran (where the impact of OAT is greatest), or overdoses, such as Kentucky. Our findings also highlight the importance of improving OAT retention (confirmed elsewhere118,126), and increasing the availability in prisons for maximising reductions in mortality. Importantly, our analyses focused on the impact of OAT on mortality rather than quality of life which will likely underestimate the population-level impact of OAT on HIV and HCV morbidity and other aspects of quality of life87. Nevertheless, our findings still suggest that it is imperative to expand OAT and improve the quality of OAT provided to PWID globally.
Opioid antagonist treatment
Oral naltrexone is ineffective and has little appeal for many people who are opioid dependent.127 Extended-release naltrexone (XR-NTX) reduces extra-medical opioid use more than placebo or treatment referral. The need to withdraw from opioids before initiating XR-NTX limits its use, e.g. 37% of persons in studies of people withdrawing from opioids before XR-NTX induction did not start treatment.128 Among those who initiate, XR-NTX treatment may have similar efficacy to buprenorphine in reducing opioid use short-term.128 Reviews have concluded there is very limited and “inconsistent” evidence128 on adherence and retention in XR-NTX compared to OAT.128 An economic evaluation in a clinical trial comparing XR-NTX to sublingual buprenorphine-naloxone concluded that buprenorphine was more cost-effective than XR-NTX at 24 and 36 weeks129.
As with OAT35, mortality risk increases after cessation of XR-NTX 130. This suggests that relapse to opioid use is likely after treatment cessation, and the increased risk of overdose need to be clearly communicated to people leaving treatment.
Other interventions and policies to reduce opioid-related harms
Table 3 summarises our review of reviews of the effects of various interventions on injecting risk behaviour, extra-medical opioid use, HIV and HCV incidence, quality of life, and fatal overdoses, suicides and accidental injuries (Appendix J). There are several limitations in the evidence. First, the amount and quality of evidence for many interventions is much lower than for OAT. Second, few interventions had evidence on their impacts on all the outcomes we considered and most had a limited impact on a limited number of outcomes (in contrast to the wider impacts for OAT).
Table 3:
Current evidence for effects of interventions to address key outcomes and behaviours among people who use opioids extra-medically
| Injecting risk behaviours | Extra-medical opioid use | HIV incidence | HCV incidence | Skin and soft tissue infections | Quality of life | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Effect | Size of effect | Level | Sources | Effect | Size of effect | Level | Sources | Effect | Size of effect | Level | Sources | Effect | Size of effect | Level | Sources | Effect | Size of effect | Level | Sources | Effect | Size of effect | Level | Sources |
| Provision of sterile injecting equipment | ↓ | aOR 0.52 (0.32, 0.83) |
APWID | 131 | - | - | - | - | ↓ | OR/HR/RR 0.42 (0.22, 0.81) |
CPWID | 132 | ↓? | RR 0.77 (0.38, 1.54) | C | 108 | ? | - | D | 86 | - | - | - | - |
| Condom provision | - | - | - | - | - | - | - | - | ↓ | RR 0.29, (0.20, 0.43) |
AGEN | 179 | ? | - | C | 180 | - | - | - | - | - | - | - | - |
| Naloxone | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Drug consumption rooms | ↓ | RR 0.31 (0.17, 0.55) |
CPWID | 181 | - | - | - | - | ? | - | D | 182 | ? | - | D | 182 | ↓ | OR 0.47 (0.23, 0.94) | E | 86 | - | - | - | - |
| Peer-based self-help groups | - | - | - | - | ↓? | - | BALC | 139 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Psychosocial interventions | ↓ | SMD −0.43 (−0.69, −0.18) |
A | 183 | ↓ | WMES
−0.18 (−0.30, −0.06) |
A | 184 | ? | - | D | 182 | ? | - | D | 182 | ? | - | E | 185 | - | - | - | - |
| Opioid detoxification alone | × | |||||||||||||||||||||||
| Oral opioid antagonists | × | NE | A | 186 | × | RR 1.39 (0.61, 3.17) | A | 127 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Extended-release opioid antagonists | ↓ | NE | A | 128 | ↓ | NE | A | 128,187 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Opioid agonist treatment | ↓ | RR 0.53 (0.4, 0.7) SYNTH | A | 106 | ↓ | RR 0.48 (0.41, 0.55) SYNTH | A | 106 | ↓ | RR 0.46 (0.32, 0.67) |
C | 107 | ↓ | RR 0.50 (0.40, 0.63) | C | 108 | ? | - | D | 86 | ↑ | SMD 0.29 (0.16, 0.42) | C | 170 |
| Residential rehabilitation | ↓ | NE | C | 188 | ↓ | NE | C | 188 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| HIV testing + informing of serostatus | ↓ | NE | DPWID | 135 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| HCV testing + informing of serostatus | × | aOR 0.97 (0.94, 1.00) |
CPWID | 189 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| HIV treatment | × | aOR 0.78 (0.42–1.45) |
D | 190 | - | - | - | - | ↓ | - | D | 134 | - | - | - | - | - | - | - | - | - | - | - | - |
| HCV treatment | ↓ | NE | DPWID | 133 | ↓ | NE | DPWID | 133 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| STI treatment | - | - | - | - | - | - | - | - | ↓ | AGEN | 191–193 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Suicide prevention strategies | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Opioid prescribing limits | - | - | - | - | ↓? | NE | DGEN | 194 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Abuse-deterrent opioid formulations | ↓ | NE | D | 144,195 | ? | NE | D | 144 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Prescription opioid monitoring programs | - | - | - | - | ↓? | NE | DGEN | 194,196 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Compulsory drug treatment/drug detention centres | ↑ | NE | C* | 143,197 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Criminalisation of drug use | ↑ | NE | CPWID | 147 | ↑ | NE | CPWID | 147 | ||||||||||||||||
| Overdose | Suicide | Other injuries | Overall mortality | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Effect | Size of effect | Level | Sources | Effect | Size of effect | Level | Sources | Effect | Size of effect | Level | Sources | Effect | Size of effect | Level | Sources |
| Provision of sterile injecting equipment | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Condom provision | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Naloxone | ↓ | - | D | 198 | - | - | - | - | - | - | - | - | - | - | - | - |
| Drug consumption rooms | ↓ | - | D | 199 | - | - | - | - | - | - | - | - | - | - | - | - |
| Peer-based self-help groups | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Psychosocial interventions | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Opioid detoxification alone | ||||||||||||||||
| Oral opioid antagonists | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Extended-release opioid antagonists | ↓? | - | A | 128 | - | - | - | - | - | - | - | - | ? | - | A | 128,187 |
| Opioid agonist treatment | ↓ | RaRa 0.25 (0.18, 0.36) SYNTH | C | 111 | ↓ | E | 112 | ↓ | E | 112 | ↓ | RaRa 0.33 (0.28, 0.39) SYNTH | C | 111 | ||
| Residential rehabilitation | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| HIV testing + informing of serostatus | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| HCV testing + informing of serostatus | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| HIV treatment | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| HCV treatment | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| STI treatment | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Medication for suicide prevention | - | - | - | - | ? | - | AGEN | 200 | - | - | - | - | - | - | - | - |
| Opioid prescribing limits | ↓? | - | DGEN | 194 | - | - | - | - | - | - | - | - | - | - | - | - |
| Abuse-deterrent opioid formulations | ? | - | DGEN | 144 | - | - | - | - | - | - | - | - | ? | - | D | 144 |
| Prescription opioid monitoring programmes | ? | - | DGEN | 145,196 | - | - | - | - | - | - | - | - | ? | - | D | 145 |
| Compulsory drug treatment/drug detention centres | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Criminalisation of drug use | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
Notes on codes used in this table
Presence or absence of effect
× This intervention does not appear to have a significant effect upon the outcome
↑ This outcome may be increased by the intervention
↓This outcome is decreased by the intervention
- No evidence could be located of the impact of this intervention upon the outcome
? unclear evidence on impact of this intervention on the outcome
Level of evidence
A Consistent conclusions across meta-analyses, high quality systematic reviews, or multiple randomised controlled trials
B Evidence from one or two randomised controlled trials only
C High quality systematic reviews of cohort, case-control or cross-sectional studies
D Systematic reviews with inconsistent conclusions from authors or cross-sectional; OR multiple consistent ecological studies
E Cross-sectional association, case series suggesting outcome, single cohort study
GEN Evidence drawn from people who may not specifically use any drugs (including opioids)
PWID People who inject drugs that may not include opioids
ALC Note that this evidence pertains to people attending alcoholics anonymous
NE no pooled quantitative estimate reported
UNotes on codes used in this table
Presence or absence of effect
× This intervention does not appear to have a significant effect upon the outcome
↑ This outcome may be increased by the intervention
↓This outcome is decreased by the intervention
- No evidence could be located of the impact of this intervention upon the outcome
? unclear evidence on impact of this intervention on the outcome
Level of evidence
A Consistent conclusions across meta-analyses, high quality systematic reviews, or multiple randomised controlled trials
B Evidence from one or two randomised controlled trials only
C High quality systematic reviews of cohort, case-control or cross-sectional studies
D Systematic reviews with inconsistent conclusions from authors or cross-sectional; OR multiple consistent ecological studies
E Cross-sectional association, case series suggesting outcome, single cohort study
GEN Evidence drawn from people who may not specifically use any drugs (including opioids)
PWID People who inject drugs that may not include opioids
ALC Note that this evidence pertains to people attending alcoholics anonymous
NE no pooled quantitative estimate reported
The provision of clean injecting equipment via needle and syringe programmes (NSP) reduces injecting risk in PWID131. There is stronger evidence for reduced HIV 132 than HCV incidence108. Testing for and treating HIV and HCV had positive impacts in people who use drugs133–135. Many countries are increasing access to naloxone to reverse opioid overdose. The rationale for this intervention is clear136;it is uncertain what scale of provision is required to reduce opioid-related overdose mortality in the population.
Despite their low efficacy, psychosocial interventions remain the most commonly delivered interventions. Evidence suggests benefits of contingency management, cognitive behavioural therapy and relapse prevention in substance use disorders137. Evidence for their use in opioid dependence is more mixed137, including whether they improve outcomes over OAT alone137,138. The more important factors may be quality of clinician training and supervision, the therapeutic alliance between the clinician and client and integration of clinician and peer-led psychosocial support services.137
Peer-led groups such as Narcotics Anonymous (NA) are one of the most common interventions globally. These abstinence-oriented programs involve peer support and regular attendance at group meetings. There is some evidence that of efficacy of Alcoholics Anonymous in reducing alcohol use for people who are alcohol dependent139, but less for NA.
Medically supervised opioid detoxification using tapered doses of opioids assists people to complete withdrawal140 but most people relapse to opioid use without additional interventions140 with an increased risk of overdose. Supervised withdrawal is often followed by inpatient treatment in a therapeutic community. There was insufficient evidence to assess whether these are more effective than other interventions because of very high dropout before completion 141.
Compulsory drug detention centres (CDDCs) are used in some East and Southeast Asian countries. For example, in Malaysia142, people may be forced to enter a CDDC after a positive urine drug test, police suspicion of drug use, or at family’s request; detention is for 2 years, with few effective treatments provided and 18 months’ supervision post-release. A review concluded that there was no evidence for positive outcomes and some evidence of harm143; a recent study reported a swifter relapse to opioid use among opioid users leaving CDDCs than among people leaving methadone treatment142.
“Abuse-deterrent” formulations (ADF) of pharmaceutical opioids have been introduced to reduce extra-medical opioid use (e.g. making them more difficult to inject). A systematic review144 concluded that there was promising but inconclusive” evidence that a tamper-resistant sustained-release formulation of oxycodone was “comparable or better” in reducing extra-medical use, and other opioid ADF“. The evidence was insufficient to assess its effectiveness in reducing overdose144. Cost-effectiveness modelling suggested that ADF opioids costs $231,500 to prevent one new case of extra-medical opioid use, $80,500 to prevent one abuse-year, and $1.36 billion to prevent an overdose death144.
Evidence for prescription drug monitoring programs (PDMP) and “pain clinic laws” (e.g. requiring registration, physician ownership, prescribing restrictions, and detailed record-keeping) is mixed145 and insufficient given the low-strength evidence, high risk of bias and some evidence of increases in heroin overdoses145. Unless it is obligatory for prescribers to use the system, PDMPs are unlikely to be of benefit. These interventions at best reduce new cases of opioid dependence but are an insufficient response to opioid use dependence and related harms146.
A recent systematic review of associations between indicators of criminalisation of drug use (e.g. exposure to incarceration and street-level policing) and patterns of injecting drug use, risk behaviours and HIV prevalence147. A formal synthesis was not possible but 86% of studies found that criminalisation of drug use was associated with greater injecting risk and HIV prevalence, and reduced engagement with OAT, harm reduction services, and the HIV care cascade147. Incarceration also increases risks of HCV infection116. People who use drugs see decriminalisation – as distinct from legalisation - of drug use as critical in reducing drug related harm and improving quality of life (e.g. Panel B).
The future of opioids: What are the anticipated changes, opportunities and challenges for the coming decade?
Improving access to prescribed opioids as essential medicines requires advocacy and collaboration of multiple organisations and agencies clear guidance on opioid use for pain and alternative strategies for managing non-cancer pain.
A comprehensive and evidence-based public health response is required to significantly reduce opioid dependence and related harms over the coming decades. We need drug policy to be driven by public health and prevention of health harms; which will require decriminalisation of drug use and dependence. The global research enterprise should be supported to develop and evaluate novel interventions to prevent opioid overdose, improve the quality of treatment for opioid dependence, and identify novel treatments for opioid dependence and chronic pain.
International attention should be devoted to eliminating the marketing strategies that contributed to the sharp increase in opioid prescription and harms in North America. The presence of synthetic opioids in the illicit stimulant drug supply148 poses grave public health challenges (see Farrell et al57 in this series) because many people who use stimulants are opioid-naïve and at high risk of opioid overdose. If a public health approach will be essential to address problems arising from illicitly manufactured fentanyls and other synthetic opioids We need more research on the effectiveness and cost-effectiveness of novel interventions to prevent inadvertent exposure to synthetic opioid substances such as drug checking programs 149–151. Developing the technical capacity to detect and measure these drugs will present a significant challenge in responding to public health issues raised by highly potent synthetic opioids (see Peacock et al22 in this series).
We need to increase the global coverage and quality of care in f effective interventions such as OAT and NSP. Continua of care 152–154, such as the “cascade” of care for the treatment of HIV infection155 could be used to address gaps in OUD treatment engagement156–158. The pursuit of abstinence or “recovery” should not be the immediate or ultimate goal. Overdose prevention — and prevention of drug related harm more broadly –are more appropriate public health objectives.
We need research on how to optimise treatments for opioid dependence by listening to the views of people who use drugs on what treatments are required at different stages of dependence, i.e. population-stratified medicine (see Panel B for perspectives from people who use drugs). We need to know whether novel models of treatment and care provision (e.g., in primary care, community-based and criminal justice settings) increase access and improve population-level outcomes. Particularly, we need research on how to improve retention in treatment and how to design OAT programmes (unsupervised dosing, dedicated pharmacies etc.) to best prevent overdose. This should include innovative “low threshold” OAT, including street-based outreach programs, mobile clinics, and home-based induction that shown promise159. Finally, we need to quantify the impact of interventions other than OAT on overdose and other health outcomes to design a population-based approach to treatment choices for people who are opioid dependent.
Opioid agonist and antagonist medications for the treatment of opioid dependence may not be effective for all patients160. Extended-release formulations of OAT may overcome challenges posed by the need for daily or near-daily dosing. In the United States, the first buprenorphine implant for the treatment of opioid dependence was approved by the FDA in 2016161 and depot buprenorphine in 2017. Implementation research is needed to determine their attractiveness to patients162, improve patient and population outcomes (including retention), and provide them to scale. We need to identify patient characteristics that predict successful induction and adherence to XR-NTX and reduce overdose risks during and after treatment with XR-NTX.128
Finally, the optimal policy and regulatory framework for opioids is one that minimises the health and social harms arising from extra-medical opioid use and dependence, while ensuring access to prescribed opioids as essential medications. Further work is needed to identify ideal policy and regulatory frameworks.
Opioid dependence is the third most important substance use disorder (after tobacco and alcohol) in terms of contribution to morbidity and premature mortality. The coverage of interventions to prevention opioid-related health harms is woefully inadequate in most countries. National and international drug policy, clinical guidance and research is needed to reduce the scale of opioid-related harm in the world. Drug policies need to move from a focus on criminal justice to public health. Prevention of harm should be the goal of clinical guidance and strategies – led by research into how to optimise combinations of pharmacological, psychological, and harm reduction interventions.
Supplementary Material
Figure 2:
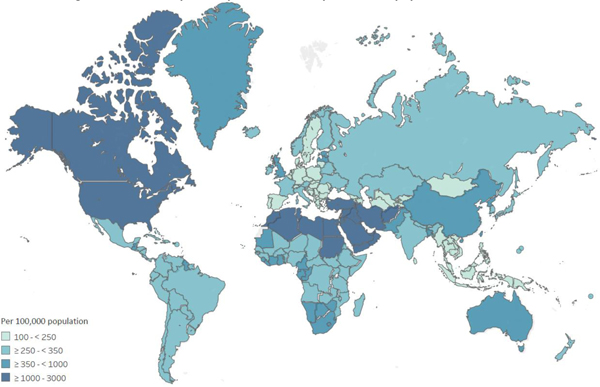
Estimated prevalence of opioid dependence and opioid overdose mortality, Global Burden of Disease study 2017
2a. Estimated age-standardised opioid dependence cases per 100,000 population
Figure 2b:
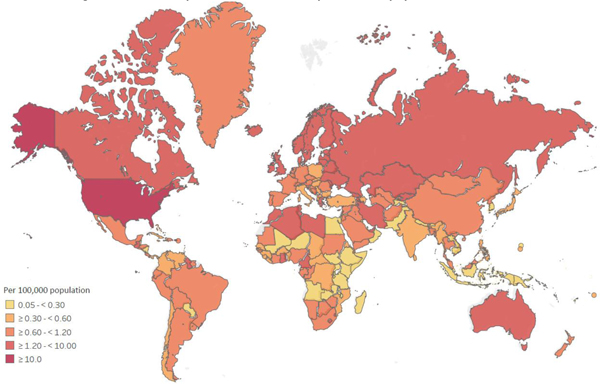
Estimated age-standardised opioid overdose deaths per 100,000 population
Source: Global Burden of Disease 201726,60. For details on the methods used please see Appendix E.
Key messages.
Opioids are essential medicines for the treatment of acute and cancer pain and opioid dependence. Access to opioids for medicinal purposes varies widely across the globe and is often inadequate.
By contrast, ready access to illicit opioids and in some countries, overprescribing of pharmaceutical opioids, mean that extra-medical opioid use and dependence are increasing globally, and causing substantial loss of health and life.
There is an increasingly diverse number and sources of opioids both naturally and synthetically produced. The proliferation of highly potent opioids in some countries is substantial increasing in overdose mortality.
Many people with problematic or dependent use of opioids will experience a long-term cycle of use, abstinence, and relapse, including periods in and out of treatment. The criminalisation of illicit opioid use in most countries means that many face arrest and incarceration, which increases health harms.
Manifold adverse health outcomes are elevated among people with problematic or dependent opioid use e.g. overdose, suicide, accidental injuries and infectious diseases, including HIV and hepatitis C virus. Mortality risk in people using illicit opioids around 10 times higher than the general population. Overdose risk is elevated after a period of abstinence from use and loss of tolerance, as may occur following incarceration, and in the context of polydrug use.
Opioid agonist treatment (OAT) is the most effective treatment for opioid dependence. It reduces illicit opioid use and reduces overall mortality and deaths from overdose, suicide, and other injuries. Modelling suggests that compared to a scenario with no OAT, scaling-up OAT to 40% coverage in the community, increasing average duration of OAT to two years and providing OAT in prisons would avert between 7.7–25.9% of all deaths, 39.2–57.0% of overdose deaths and 34.9–56.2% of HIV-related deaths over the next 20 years by also preventing incarceration, reducing the risk of HIV and HCV transmission, and improving HIV treatment outcomes.
Other pharmacological and non-pharmacological treatments are available, with varying levels of evidence for effectiveness and patient acceptability. Other effective interventions include those focused on preventing harms associated with problematic opioid use.
Despite strong evidence for the effectiveness a range of interventions to improve the health and well-being of people who are dependent on opioids, coverage is low even in high income countries and treatment quality suboptimal. Policy changes that prioritise prevention of opioid-related harms and scale-up evidence-based programs, health care services, and drug treatment services are urgently needed.
The criminalisation of illicit opioid use and dependence produces considerable avoidable human, social, and economic harms. Policy frameworks need to adopt a human rights and public health-based approach, by not making drug use a criminal offence and seeking to reduce drug related harm at the population level.
Acknowledgements
Thanks to Lucy Thi Tran (BPsySc(Hons)), NDARC, UNSW Sydney; Thomas Santo Jr (MPH), NDARC, UNSW Sydney; Harry Jin (MPH), Department of Epidemiology, Brown University School of Public Health; Behzad Hajarizadeh (MD, MPH, PhD), Kirby Institute, UNSW Sydney; and Samantha Colledge (BPsySc), NDARC, UNSW Sydney for their assistance with some of the reviews summarised here. Thanks to Christinah Mukandavire (PhD), Imperial College London; and Linda Gowing (PhD), University of Adelaide for provision of data from their reviews. Thanks to Judy Chang (M. Social Science (International Development)), INPUD; Jude Byrne (RN), AIVL. Thanks to, Ratul Dey, Emily Ebdon (BFA), Anglicare Tasmania; Binod Gurung (BA), SATHI SAMUHA, ANPUD; Joe Horvath, Shayne Kilford, Mick Mathews, P. Moore (BA), Hepatitis S.A., Drug and Alcohol Service S.A.; Martin McCusker, Lambeth Service User Council; Eric Nsengiyumva (MComm), Burundi Association for people who use drugs; Peter Parkes, CAHMA; Bishnu Fueal Sharma (Dip. Psychosocial and Counselling), Tribhuwan University; Peter Sidaway, NTAHC; Nandini Thapa, India Drug Users Forum; Connie van Staden. Thanks to those who assisted with location of data to inform the mathematical modelling summarised here: Pavlo Smyrnov (MPH), Alliance for Public Health; Maryam Alavi (PhD), Kirby Institute, UNSW Sydney; April Young, Jennifer Havens.
Funding
The Australian National Drug and Alcohol Research Centre (NDARC), UNSW Sydney, provided funding towards the costs of this review. NDARC is supported by funding from the Australian Government Department of Health under the Drug and Alcohol Program. LD and SL are supported by NHMRC Fellowships. SL and LD are supported by National Institute of Health (NIH) grants National Institute on Drug Abuse (NIDA) (R01DA1104470). LD, JS and MH acknowledge support from NHMRC (APP1150078). The Kirby Institute is funded by the Australian Government Department of Health and Ageing. The views expressed in this publication do not necessarily represent the position of the Australian Government. JS and PV acknowledge support from NIDA (R01DA033679). JS, MH and PV acknowledge support from NIHR Health Protection Research Unit (HPRU) in Evaluation of Interventions at University of Bristol. MH is an NIHR senior investigator and acknowledges NIHR School of Public Health Research. PV acknowledges support from the NIHR HPRU in Blood Borne and Sexually Transmitted Infections at University College London and National Institute for Drug Abuse (grant number R01 DA037773-01A1). BDLM is supported by NIH grants from the National Institute of General Medical Sciences (P20GM125507) and NIDA (DP2-DA040236). GH is supported by National Institute for Drug Abuse (grant number RO1DA036975). FLA is supported on research related to this grant from the National Institute on Drug Abuse (K24 DA017072, R01 DA025943, R01 DA029910, R01 DA030768, R01 DA033679, R01 DA030762, R21 DA041953).
Conflict of interest
LD has received investigator-initiated untied educational grants for studies of opioid medications in Australia from Indivior, Mundipharma and Seqirus. SL has received investigator-initiated untied educational grants from Indivior. JG is a consultant and adviser for and has received research grants from Abbvie, Cepheid, Gilead Sciences, and Merck/MSD. MH reports personal fees from Gilead, Abbvie, and MSD. JS reports non-financial support from Gilead Sciences. JB has received research support from Gilead Sciences and consulting fees from AbbVie, Gilead Sciences and Merck/MSD, in relation to hepatitis C virus treatment for people who use drugs. PV has received research support from Gilead Sciences and honoraria from AbbVie and Gilead, in relation to hepatitis C virus treatment for people who inject drugs. FLA has grants from Gilead and Merck and on advisory boards to Gilead, Merck and Abbvie. All other authors declare no competing interests.
Footnotes
These perspectives were submitted by people who use drugs in response to an email inviting input circulated in March 2019.
References
- 1.World Health Organization. WHO Model List of Essential Medicines. World Health Organization; 2017. [Google Scholar]
- 2.Fazel S, Yoon IA, Hayes AJ. Substance use disorders in prisoners: an updated systematic review and meta-regression analysis in recently incarcerated men and women. Addiction 2017; 112(10): 1725–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders. Clinical descriptions and diagnostic guidelines. Geneva: World Health Organisation; 1992. [Google Scholar]
- 4.World Health Organization. ICD-11 Beta Draft. 2016. http://apps.who.int/classifications/icd11/browse/l-m/en (accessed 4th November 2016).
- 5.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition). Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 6.Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database of Systematic Reviews, 2010; 1(CD006605). doi: 10.1002/14651858.CD006605.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou R, Turner JA, Devine EB, et al. The Effectiveness and Risks of Long-Term Opioid Therapy for Chronic Pain: A Systematic Review for a National Institutes of Health Pathways to Prevention WorkshopEffectiveness and Risks of Long-Term Opioid Therapy for Chronic Pain. Annals of internal medicine 2015; N/A(N/A): N/A-N/A. [DOI] [PubMed] [Google Scholar]
- 8.Berterame S, Erthal J, Thomas J, et al. Use of and barriers to access to opioid analgesics: a worldwide, regional, and national study. Lancet 2016; 387(10028): 1644–56. [DOI] [PubMed] [Google Scholar]
- 9.Volkow ND, Jones EB, Einstein EB, Wargo EM. Prevention and Treatment of Opioid Misuse and Addiction: A Review. JAMA psychiatry 2019; 76(2): 208–16. [DOI] [PubMed] [Google Scholar]
- 10.Cottler LB, Ajinkya S, Goldberger BA, et al. Prevalence of drug and alcohol use in urban Afghanistan: epidemiological data from the Afghanistan National Urban Drug Use Study (ANUDUS). The Lancet Global Health 2014; 2(10): e592–e600. [DOI] [PubMed] [Google Scholar]
- 11.UNODC. World Drug Report 2018. Vienna: United Nations; https://www.unodc.org/wdr2018/, 2018. [Google Scholar]
- 12.WHO Expert Committee on Drug Dependence. Critical Review Report, Tramadol. 41st ECDD Meeting. Geneva: World Health Organization, 2018. [Google Scholar]
- 13.Kiunguyu K. Codeine syrup addiction is an opioid epidemic that is sweeping the African continent. This is Africa 2018; https://thisisafrica.me/codeine-addiction-opioid-epidemic-sweeping-africa/. Accessed on February 18th 2019. [Google Scholar]
- 14.Associated Press. Mexico raids lab producing fentanyl in capital. Associated Press; 2018. [Google Scholar]
- 15.Singh D. Mumbai: Anti-Narcotics Cell seizes drug worth Rs 1000 crore. India Today; 2018. [Google Scholar]
- 16.Ciccarone D. The triple wave epidemic: Supply and demand drivers of the US opioid overdose crisis. International Journal of Drug Policy 2019. doi: 10.1016/jdrugpo.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annual review of public health 2015; 36: 559–74. [DOI] [PubMed] [Google Scholar]
- 18.Rutkow L, Chang H-Y, Daubresse M, Webster DW, Stuart EA, Alexander GC. Effect of Florida’s Prescription Drug Monitoring Program and Pill Mill Laws on Opioid Prescribing and UseFlorida Prescription Drug Monitoring Program, Pill Mill Laws, and OpioidsFlorida Prescription Drug Monitoring Program, Pill Mill Laws, and Opioids. JAMA Internal Medicine 2015; 175(10): 1642–9. [DOI] [PubMed] [Google Scholar]
- 19.Cerdá M, Santaella J, Marshall BDL, Kim JH, Martins SS. Nonmedical Prescription Opioid Use in Childhood and Early Adolescence Predicts Transitions to Heroin Use in Young Adulthood: A National Study. The Journal of Pediatrics 2015; 167(3): 605–12.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martins SS, Sarvet A, Santaella-Tenorio J, Saha T, Grant BF, Hasin DS. Changes in US Lifetime Heroin Use and Heroin Use Disorder: Prevalence From the 2001–2002 to 2012–2013 National Epidemiologic Survey on Alcohol and Related Conditions. JAMA psychiatry 2017; 74(5): 445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banerjee G, Edelman EJ, Barry DT, et al. Non-medical use of prescription opioids is associated with heroin initiation among US veterans: a prospective cohort study. Addiction 2016; 111(11): 2021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peacock A, Bruno R, Gisev N, et al. New psychoactive substances: Challenges for drug surveillance, control, and public health responses. The Lancet 2019; 394(10209): 1668–84. doi: 10.1016/S0140-6736(19)32231-7. [DOI] [PubMed] [Google Scholar]
- 23.Gladden RM, Martinez P, Seth P. Fentanyl Law Enforcement Submissions and Increases in Synthetic Opioid-Involved Overdose Deaths - 27 States, 2013–2014. MMWR Morbidity and mortality weekly report 2016; 65(33): 837–43. [DOI] [PubMed] [Google Scholar]
- 24.Ciccarone D. Fentanyl in the US heroin supply: A rapidly changing risk environment. International Journal of Drug Policy 2017; 46: 107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudd RA, Seth P, David F, Scholl L. Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010–2015. MMWR Morbidity and mortality weekly report 2016; 65(5051): 1445–52. [DOI] [PubMed] [Google Scholar]
- 26.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet 2018; 392(10159): 1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sweeting M, De Angelis D, Ades A, Hickman M. Estimating the prevalence of ex-injecting drug use in the population. Statistical methods in medical research 2009; 18(4): 381–95. [DOI] [PubMed] [Google Scholar]
- 28.Anthony JC, Warner L, Kessler R. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Experimental and Clinical Psychopharmacology 1994; 2(3): 244–68. [Google Scholar]
- 29.Rivera O, Havens JR, Parker MA, Anthony JC. Risk of heroin dependence in newly incident heroin users. JAMA Psychiatry 2018; 75(8): 863–4. doi: 10.1001/jamapsychiatry.2018.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amin-Esmaeili M, Rahimi-Movaghar A, Sharifi V, et al. Epidemiology of illicit drug use disorders in Iran: prevalence, correlates, comorbidity and service utilization results from the Iranian Mental Health Survey. Addiction 2016; 111(10): 1836–47. [DOI] [PubMed] [Google Scholar]
- 31.Robins L, Davis D, Nurco D. How Permanent Was Vietnam Drug Addiction? American Journal of Public Health 1974; 64(12 Supplement): 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minozzi S, Amato L, Davoli M. Development of dependence following treatment with opioid analgesics for pain relief: a systematic review. Addiction 2013; 108(4): 688–98. [DOI] [PubMed] [Google Scholar]
- 33.Ellerstrand J, Ferrari A, Degenhardt L, Whiteford H, Leung J. A systematic review of the global prevalence of prescription opioid non-medical use with an estimate of prescription opioid dependence NDARC Technical Report No. 337. Sydney: UNSW Sydney, 2018. [Google Scholar]
- 34.Hser YI, Haffman V, Grella CE, & Anglin MD. A 33-year follow-up of narcotics addicts. Archives of General Psychiatry 2001; 58(5): 503–8. [DOI] [PubMed] [Google Scholar]
- 35.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ 2017; 357: j1550. doi: 10.1136/bmj.j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merrall ELC, Kariminia A, Binswanger IA, et al. Meta-analysis of drug-related deaths soon after release from prison. Addiction 2010; 105(9): 1545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimber J, Larney S, Hickman M, Randall D, Degenhardt L. Mortality risk of opioid substitution therapy with methadone versus buprenorphine: a retrospective cohort study. The Lancet Psychiatry; 2(10): 901–8. [DOI] [PubMed] [Google Scholar]
- 38.Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug and Alcohol Dependence 2006; 83(Supplement 1): S4–S7. [DOI] [PubMed] [Google Scholar]
- 39.Ford JA, Rigg KK. Racial/Ethnic Differences in Factors That Place Adolescents at Risk for Prescription Opioid Misuse. Prevention Science 2015; 16(633). doi: 10.1007/s11121-014-0514-y. [DOI] [PubMed] [Google Scholar]
- 40.Fergusson DM, Boden JM, Horwood LJ. The developmental antecedents of illicit drug use: Evidence from a 25 year longitudinal study. Drug and Alcohol Dependence 2008; 96: 167–77. [DOI] [PubMed] [Google Scholar]
- 41.Lascala E, Friesthler B, Gruenwald PJ. Population ecologies of drug use, drinking and related problems In: Stockwell T, Gruenwald P, Toumbourou J, Loxley W, eds. Preventing Harmful Substance Use: The Evidence Base for Policy and Practice. Chichester: John Wiley & Sons; 2005. [Google Scholar]
- 42.Needle RH, Su S, Doherty WJ. Divorce, remarriage, and adolescent drug involvement: A longitudinal study. Journal of Marriage and the Family 1990; 52: 157–69. [Google Scholar]
- 43.Conroy E, Degenhardt L, Mattick RP, Nelson EC. Child maltreatment as a risk factor for opioid dependence: Comparison of family characteristics and type and severity of child maltreatment with a matched control group. Child Abuse & Neglect 2009; 33(6): 343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fergusson DM, Horwood LJ, Lynskey MT. Parental separation, adolescent psychopathology, and problem behaviors. Journal of the American Academy of Child and Adolescent Psychiatry 1994; 33(8): 1122–31; discussion 31–3. [DOI] [PubMed] [Google Scholar]
- 45.Cohen DA, Richardson J, LaBree L. Parenting behaviors and the onset of smoking and alcohol use: A longitudinal study. Pediatrics 1994; 94: 368–75. [PubMed] [Google Scholar]
- 46.Daniel JZ, Hickman M, Macleod J, et al. Is socioeconomic status in early life associated with drug use? A systematic review of the evidence. Drug and alcohol review 2009; 28(2): 142–53. [DOI] [PubMed] [Google Scholar]
- 47.Saha TD, Kerridge BT, Goldstein RB, et al. Nonmedical Prescription Opioid Use and DSM-5 Nonmedical Prescription Opioid Use Disorder in the United States. J Clin Psychiatry 2016; 77(6): 772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Macleod J, Hickman M, Jones HE, et al. Early life influences on the risk of injecting drug use: case control study based on the Edinburgh Addiction Cohort. Addiction 2013; 108(4): 743–50. [DOI] [PubMed] [Google Scholar]
- 49.Lynskey MT, Fergusson DM. Childhood conduct problems and attention deficit behaviors and adolescent alcohol, tobacco and illicit drug use. Journal of Abnormal Child Psychology 1995; 23: 281–302. [DOI] [PubMed] [Google Scholar]
- 50.Townsend L, Flisher A, King G. A Systematic Review of the Relationship between High School Dropout and Substance Use. Clinical Child and Family Psychology Review 2007; 10(4): 295–317. [DOI] [PubMed] [Google Scholar]
- 51.Berrettini W. A brief review of the genetics and pharmacogenetics of opioid use disorders. Dialogues in clinical neuroscience 2017; 19(3): 229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Witkiewitz K, Vowles KE. Alcohol and Opioid Use, Co-Use, and Chronic Pain in the Context of the Opioid Epidemic: A Critical Review. Alcoholism, clinical and experimental research 2018; 42(3): 478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rajabi A, Dehghani M, Shojaei A, Farjam M, Motevalian SA. Association between tobacco smoking and opioid use: A meta-analysis. Addictive Behaviors 2019; 92: 225–35. [DOI] [PubMed] [Google Scholar]
- 54.Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet 2010; 376(9738): 367–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katz C, El-Gabalawy R, Keyes KM, Martins SS, Sareen J. Risk factors for incident nonmedical prescription opioid use and abuse and dependence: Results from a longitudinal nationally representative sample. Drug and Alcohol Dependence 2013; 132(1): 107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Langdon KJ, Dove K, Ramsey S. Comorbidity of opioid-related and anxiety-related symptoms and disorders. Current Opinion in Psychology 2019; 30: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hall W, et al. Public health implications of legalising the production and sale of cannabis for medical and recreational use. The Lancet in preparation. [DOI] [PubMed] [Google Scholar]
- 58.Farrell M, et al. Global patterns of stimulant use: Assessment of harms and responses. The Lancet in preparation. [Google Scholar]
- 59.EMCDDA. European Drug Report 2018. Lisbon. http://www.emcdda.europa.eu/edr2018_en. Accessed on May 1st 2019, 2018.
- 60.GBD 2017 causes of death collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England) 2018; 392(10159): 1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med 2010; 152(2): 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caudarella A, Dong H, Milloy MJ, Kerr T, Wood E, Hayashi K. Non-fatal overdose as a risk factor for subsequent fatal overdose among people who inject drugs. Drug and Alcohol Dependence 2016; 162: 51–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pattinson KTS. Opioids and the control of respiration. British Journal of Anaesthesia 2008; 100(6): 747–58. [DOI] [PubMed] [Google Scholar]
- 64.White JM, Irvine RJ. Mechanisms of fatal opioid overdose. Addiction 1999; 94(7): 961–72. [PubMed] [Google Scholar]
- 65.Suzuki J, El-Haddad S. A review: Fentanyl and non-pharmaceutical fentanyls. Drug and Alcohol Dependence 2017; 171: 107–16. [DOI] [PubMed] [Google Scholar]
- 66.Seltenhammer MH, Marchart K, Paula P, et al. Micromorphological changes in cardiac tissue of drug-related deaths with emphasis on chronic illicit opioid abuse. Addiction 2013; 108(7): 1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alinejad S, Ghaemi K, Abdollahi M, Mehrpour O. Nephrotoxicity of methadone: a systematic review. Springerplus 2016; 5(1): 2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jones JD, Mogali S, Comer SD. Polydrug abuse: A review of opioid and benzodiazepine combination use. Drug and Alcohol Dependence 2012; 125(1): 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Darke S, Hall W. Levels and correlates of polydrug use among heroin users and regular amphetamine users. Drug and Alcohol Dependence 1995; 39(3): 231–5. [DOI] [PubMed] [Google Scholar]
- 70.Darke S, Hall W. Heroin overdose: Research and evidence-based intervention. Journal of Urban Health 2003; 80(2): 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hickman M, Carrivick S, Paterson S, et al. London audit of drug-related overdose deaths: characteristics and typology, and implications for prevention and monitoring. Addiction 2007; 102(2): 317–23. [DOI] [PubMed] [Google Scholar]
- 72.Brecht M-L, Huang D, Evans E, Hser Y-I. Polydrug use and implications for longitudinal research: Ten-year trajectories for heroin, cocaine, and methamphetamine users. Drug and Alcohol Dependence 2008; 96(3): 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hill R, Lyndon A, Withey S, et al. Ethanol Reversal of Tolerance to the Respiratory Depressant Effects of Morphine. Neuropsychopharmacology 2016; 41(3): 762–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lyndon A, Audrey S, Wells C, et al. Risk to heroin users of polydrug use of pregabalin or gabapentin. Addiction 2017; 112(9): 1580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hill R, Dewey WL, Kelly E, Henderson G. Oxycodone-induced tolerance to respiratory depression: reversal by ethanol, pregabalin and protein kinase C inhibition. Br J Pharmacol 2018; 175(12): 2492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stenbacka M, Beck O, Leifman A, Romelsjo A, Helander A. Problem drinking in relation to treatment outcome among opiate addicts in methadone maintenance treatment. Drug and alcohol review 2007; 26(1): 55–63. [DOI] [PubMed] [Google Scholar]
- 77.Grosshans M, Lemenager T, Vollmert C, et al. Pregabalin abuse among opiate addicted patients. European Journal of Clinical Pharmacology 2013; 69(12): 2021–5. [DOI] [PubMed] [Google Scholar]
- 78.McNamara S, Stokes S, Kilduff R, Shine A. Pregabalin abuse amongst opioid substitution treatment patients. Irish Medical Journal. [PubMed] [Google Scholar]
- 79.Bastiaens L, Galus J, Mazur C. Abuse of Gabapentin is Associated with Opioid Addiction. Psychiatric Quarterly 2016; 87(4): 763–7. [DOI] [PubMed] [Google Scholar]
- 80.Evoy KE, Morrison MD, Saklad SR. Abuse and Misuse of Pregabalin and Gabapentin. Drugs 2017; 77(4): 403–26. [DOI] [PubMed] [Google Scholar]
- 81.Tomko JR, Prasad KM, Kubas S, Simpson T. The association of gabapentin use and dose with substance use disorders prior to inpatient mental health treatment: A cross-sectional study. The Primary Care Companion for CNS Disorders 2018; 20(4). doi: 10.4088/pcc.18m02291. [DOI] [PubMed] [Google Scholar]
- 82.Häkkinen M, Vuori E, Kalso E, Gergov M, Ojanperä I. Profiles of pregabalin and gabapentin abuse by postmortem toxicology. Forensic Science International 2014; 241: 1–6. [DOI] [PubMed] [Google Scholar]
- 83.Slavova S, Miller A, Bunn TL, et al. Prevalence of gabapentin in drug overdose postmortem toxicology testing results. Drug and Alcohol Dependence 2018; 186: 80–5. [DOI] [PubMed] [Google Scholar]
- 84.Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W. Gabapentin, opioids, and the risk of opioid-related death: A population-based nested case–control study. PLoS Medicine 2017; 14(10): e1002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. The Lancet Global Health 2017; 5(12): e1192–e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Larney S, Peacock A, Mathers BM, Hickman M, Degenhardt L. A systematic review of injecting-related injury and disease among people who inject drugs. Drug Alcohol Depend 2017; 171: 39–49. [DOI] [PubMed] [Google Scholar]
- 87.De Maeyer J, Vanderplasschen W, Broekaert E. Quality of life among opiate-dependent individuals: A review of the literature. International Journal of Drug Policy 2010; 21(5): 364–80. [DOI] [PubMed] [Google Scholar]
- 88.Lake S, Kennedy MC. Health outcomes associated with illicit prescription opioid injection: A systematic review. Journal of addictive diseases 2016; 35(2): 73–91. [DOI] [PubMed] [Google Scholar]
- 89.Hayhurst KP, Pierce M, Hickman M, et al. Pathways through opiate use and offending: A systematic review. International Journal of Drug Policy 2017; 39: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li W, Li Q, Zhu J, et al. White matter impairment in chronic heroin dependence: A quantitative DTI study. Brain Research 2013; 1531: 58–64. [DOI] [PubMed] [Google Scholar]
- 91.Qiu Y, Jiang G, Su H, et al. Progressive White Matter Microstructure Damage in Male Chronic Heroin Dependent Individuals: A DTI and TBSS Study. PLoS ONE 2013; 8(5): e63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wollman SC, Alhassoon OM, Stern MJ, et al. White matter abnormalities in long-term heroin users: a preliminary neuroimaging meta-analysis. The American Journal of Drug and Alcohol Abuse 2015; 41(2): 133–8. [DOI] [PubMed] [Google Scholar]
- 93.Fareed A, Kim J, Ketchen B, et al. Effect of heroin use on changes of brain functions as measured by functional magnetic resonance imaging, a systematic review. Journal of addictive diseases 2017; 36(2): 105–16. [DOI] [PubMed] [Google Scholar]
- 94.Yuan Y, Zhu Z, Shi J, et al. Gray matter density negatively correlates with duration of heroin use in young lifetime heroin-dependent individuals. Brain and Cognition 2009; 71(3): 223–8. [DOI] [PubMed] [Google Scholar]
- 95.Qiu Y-w, Jiang G-h, Su H-h, et al. The impulsivity behavior is correlated with prefrontal cortex gray matter volume reduction in heroin-dependent individuals. Neuroscience Letters 2013; 538: 43–8. [DOI] [PubMed] [Google Scholar]
- 96.Darke S, Sims J, McDonald S, Wickes W. Cognitive impairment among methadone maintenance patients. Addiction 2000; 95(5): 687–95. [DOI] [PubMed] [Google Scholar]
- 97.Ersche KD, Sahakian BJ. The neuropsychology of amphetamine and opiate dependence: implications for treatment. Neuropsychology review 2007; 17(3): 317–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Loeber S, Nakovics H, Kniest A, Kiefer F, Mann K, Croissant B. Factors affecting cognitive function of opiate-dependent patients. Drug and Alcohol Dependence 2012; 120(1): 81–7. [DOI] [PubMed] [Google Scholar]
- 99.Darke S, Hall W. Heroin overdose: Research and evidence-based intervention. Journal of Urban Health : Bulletin of the New York Academy of Medicine 2003; 80(2): 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Larney S, Tran LT, Leung J, Santo T Jr, Santomauro D, Hickman M, Peacock A, Stockings E, Degenhardt L. All-cause and cause-specific mortality among people using extramedical opioids: A systematic review and meta-analysis. JAMA Psychiatry 2019. doi: 10.1001/jamapsychiatry.2019.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Larney S, et al. Mortality among people using pharmaceutical opioids: Systematic review and meta-analysis of cohort studies. in preparation. [Google Scholar]
- 102.Wakeman SE. Medications For Addiction Treatment: Changing Language to Improve Care. Journal of addiction medicine 2017; 11(1): 1–2. [DOI] [PubMed] [Google Scholar]
- 103.Samet JH, Fiellin DA. Opioid substitution therapy-time to replace the term. Lancet (London, England) 2015; 385(9977): 1508–9. [DOI] [PubMed] [Google Scholar]
- 104.Nielsen S, Larance B, Degenhardt L, Gowing L, Kehler C, Lintzeris N. Opioid agonist treatment for pharmaceutical opioid dependent people. Cochrane Database Syst Rev 2016; (5): CD011117. [DOI] [PubMed] [Google Scholar]
- 105.Rahimi-Movaghar A, Amin-Esmaeili M, Hefazi M, Yousefi-Nooraie R. Pharmacological therapies for maintenance treatments of opium dependence. Cochrane Database of Systematic Reviews 2013; 1(CD007775). doi: 10.1002/14651858.CD007775.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gowing L, Farrell MF, Bornemann R, Sullivan LE, Ali R. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev 2011; 8(CD004145). doi: 10.1002/14651858.CD004145.pub4. [DOI] [PubMed] [Google Scholar]
- 107.MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: Systematic review and meta-analysis. BMJ 2012; 345: e5945. doi: 10.1136/bmj.e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database of Systematic Reviews 2017; 9(CD012021). doi: 10.1002/14651858.CD012021.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Low AJ, Mburu G, Welton NJ, et al. Impact of Opioid Substitution Therapy on Antiretroviral Therapy Outcomes: A Systematic Review and Meta-Analysis. Clinical Infectious Diseases 2016; 63(8): 1094–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maglione MA, Raaen L, Chen C, et al. Effects of medication assisted treatment (MAT) for opioid use disorder on functional outcomes: A systematic review. J Subst Abuse Treat 2018; 89: 28–51. [DOI] [PubMed] [Google Scholar]
- 111.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ 2017; 357: j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Degenhardt L, Randall D, Hall W, Law M, Butler T, Burns L. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: risk factors and lives saved. Drug Alcohol Depend 2009; 105(1–2): 9–15. [DOI] [PubMed] [Google Scholar]
- 113.Chetty M, Kenworthy JJ, Langham S, Walker A, Dunlop WC. A systematic review of health economic models of opioid agonist therapies in maintenance treatment of non-prescription opioid dependence. Addict Sci Clin Pract 2017; 12(1): 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Degenhardt L, Larney S, Kimber J, et al. The impact of opioid substitution therapy on mortality post-release from prison: retrospective data linkage study. Addiction 2014; 109(8): 1306–17. [DOI] [PubMed] [Google Scholar]
- 115.Marsden J, Stillwell G, Jones H, et al. Does exposure to opioid substitution treatment in prison reduce the risk of death after release? A national prospective observational study in England. Addiction 2017; 112: 1408–18. doi: 10.1111/add.13779. [DOI] [PubMed] [Google Scholar]
- 116.Stone J, Fraser H, Lim AG, et al. Incarceration history and risk of HIV and hepatitis C virus acquisition among people who inject drugs: a systematic review and meta-analysis. Lancet Infect Dis 2018; 18(12): 1397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews 2014; 2(CD002207). doi: 10.1002/14651858.CD002207.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hickman M, Steer C, Tilling K, Lim AG, Marsden J, Millar T, Strang J, Telfer M, Vickerman P, Macleod J. The impact of buprenorphine and methadone on mortality: a primary care cohort study in the United Kingdom. Addiction 2018; 113(8): 1461–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zedler BK, Mann AL, Kim MM, et al. Buprenorphine compared with methadone to treat pregnant women with opioid use disorder: a systematic review and meta-analysis of safety in the mother, fetus and child. Addiction 2016; 111(12): 2115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Faggiano F, Vigna-Taglianti F, Versino E, Lemma P. Methadone maintenance at different dosages for opioid dependence. Cochrane Database of Systematic Reviews 2003; 3(CD002208). doi: 10.1002/14651858.CD002208. [DOI] [PubMed] [Google Scholar]
- 121.Saulle R, Vecchi S, Gowing L. Supervised dosing with a long-acting opioid medication in the management of opioid dependence. Cochrane Database of Systematic Reviews 2017. doi: 10.1002/14651858.CD011983.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McEachern J, Adye-White L, Priest KC, Moss E, Gorfinkel L, Wood E, Cullen W, Klimas J. Lacking evidence for the association between frequent urine drug screening and health outcomes of persons on opioid agonist therapy. International Journal of Drug Policy 2019; 64: 30–33. doi: 10.1016/j.drugpo.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Larney S, Peacock A, Leung J, et al. Global, regional, and country-level coverage of interventions to prevent and manage HIV and hepatitis C among people who inject drugs: a systematic review. The Lancet Global Health 2017; 5(12): e1208–20. doi: 10.1016/S2214-109X(17)30373-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jin H, Marshall B, Degenhardt L, et al. Opioid agonist therapy in clinical practice globally: A systematic review of criteria for treatment entry, methadone and buprenorphine doses, access to unsupervised doses, and urine drug screening practices. Drug and Alcohol Dependence in preparation. [Google Scholar]
- 125.Strang J, Hall W, Hickman M, Bird SM. Impact of supervision of methadone consumption on deaths related to methadone overdose (1993–2008): analyses using OD4 index in England and Scotland. BMJ (Clinical research ed) 2010; 341: c4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cornish R, Macleod J, Strang J, Vickerman P, Hickman M. Risk of death during and after opiate substitution treatment in primary care: prospective observational study in UK General Practice Research Database. BMJ 2010; 341: c5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database of Systematic Reviews 2011; 4(CD001333). doi: 10.1002/14651858.CD001333.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jarvis BP, Holtyn AF, Subramaniam S, et al. Extended-release injectable naltrexone for opioid use disorder: A systematic review. Addiction 2018; 113: 1188–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Murphy SM, McCollister KE, Leff JA, et al. Cost-Effectiveness of Buprenorphine-Naloxone Versus Extended-Release Naltrexone to Prevent Opioid Relapse. Ann Intern Med 2019; 170(2): 90–8. doi: 10.7326/M18-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Saucier R, Wolfe D, Dasgupta N. Review of Case Narratives from Fatal Overdoses Associated with Injectable Naltrexone for Opioid Dependence. Drug Safety 2018; 41: 981–8. doi: 10.1007/s40264-018-0653-3. [DOI] [PubMed] [Google Scholar]
- 131.Turner KM, Hutchinson S, Vickerman P, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction 2011; 106(11): 1978–88. [DOI] [PubMed] [Google Scholar]
- 132.Aspinall EJ, Nambiar D, Goldberg DJ, et al. Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: a systematic review and meta-analysis. International Journal of Epidemiology 2014; 43(1): 235–48. [DOI] [PubMed] [Google Scholar]
- 133.Caven M, Malaguti A, Robinson E, Fletcher E, Dillon J. Impact of Hepatitis C treatment on behavioural change in relation to drug use in people who inject drugs: a systematic review. The International Journal of Drug Policy 2019; 72: 169–76. doi: 10.1016/j.drugpo.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 134.Reddon H, Marshall BDL, Milloy MJ. Elimination of HIV transmission through novel and established prevention strategies among people who inject drugs. The Lancet HIV 2019; 6(2): e128–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schlumberger M, Desenclos J, Papaevangelou G, Richardson S, Ancelle-Park R. Knowledge of HIV serostatus and preventive behaviour among European injecting drug users: Second study. European journal of epidemiology 1999; 15(3): 207–15. [DOI] [PubMed] [Google Scholar]
- 136.Strang J, Bird SM, Dietze P, Gerra G, McLellan AT. Take-home emergency naloxone to prevent deaths from heroin overdose. BMJ (Clinical research ed) 2014; 349: g6580. [DOI] [PubMed] [Google Scholar]
- 137.Day E, Mitcheson L. Psychosocial interventions in opiate substitution treatment services: does the evidence provide a case for optimism or nihilism? 2017; 112(8): 1329–36. [DOI] [PubMed] [Google Scholar]
- 138.Amato L, Minozzi S, Davoli M, Vecchi S. Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database of Systematic Reviews 2011; 10(CD004147). doi: 10.1002/14651858.CD004147.pub4. [DOI] [PubMed] [Google Scholar]
- 139.Humphreys K, Blodgett JC, Wagner TH. Estimating the efficacy of Alcoholics Anonymous without self-selection bias: an instrumental variables re-analysis of randomized clinical trials. Alcoholism, clinical and experimental research 2014; 38(11): 2688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Amato L, Davoli M, Minozzi S, Ferroni E, Ali R, Ferri M. Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database of Systematic Reviews, 2013; 2(CD003409). doi: 10.1002/14651858.CD003409.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Smith LA, Gates S, Foxcroft D. Therapeutic communities for substance related disorder. Cochrane Database of Systematic Reviews 2006; 1(CD005338). doi: 10.1002/14651858.CD005338.pub2. [DOI] [PubMed] [Google Scholar]
- 142.Wegman MP, Altice FL, Kaur S, et al. Relapse to opioid use in opioid-dependent individuals released from compulsory drug detention centres compared with those from voluntary methadone treatment centres in Malaysia: a two-arm, prospective observational study. The Lancet Global Health 2017; 5(2): e198–e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Werb D, Kamarulzaman A, Meacham MC, et al. The effectiveness of compulsory drug treatment: A systematic review. The International journal on drug policy 2016; 28: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.New England Comparative Effectiveness Public Advisory Council. Abuse-Deterrent Formulations of Opioids: Effectiveness and Economic Impact. New England: https://icer-review.org/material/adf-final-report/: Institute for Clinical and Economic Review, 2017. [Google Scholar]
- 145.Fink DS, Schleimer JP, Sarvet A, et al. Association Between Prescription Drug Monitoring Programs and Nonfatal and Fatal Drug Overdoses: A Systematic Review. Ann Intern Med 2018; 168(11): 783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen Q, Larochelle MR, Weaver DT, et al. Prevention of Prescription Opioid Misuse and Projected Overdose Deaths in the United States. JAMA network open 2019; 2(2): e187621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.DeBeck K, Cheng T, Montaner JS, et al. HIV and the criminalisation of drug use among people who inject drugs: a systematic review. The Lancet HIV 2017; 4(8): e357–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.US Drug Enforcement Administration. Cocaine laced with fentanyl leads to multiple deaths, overdoses [press release]. https://www.dea.gov/press-releases/2018/09/14/cocaine-laced-fentanyl-leads-multiple-deaths-overdoses. Accessed on May 9th 2019; 2018.
- 149.Tupper KW, McCrae K, Garber I, Lysyshyn M, Wood E. Initial results of a drug checking pilot program to detect fentanyl adulteration in a Canadian setting. Drug Alcohol Depend 2018; 190: 242–5. [DOI] [PubMed] [Google Scholar]
- 150.Krieger MS, Goedel WC, Buxton JA, et al. Use of rapid fentanyl test strips among young adults who use drugs. The International Journal on Drug Policy 2018; 61: 52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McGowan CR, Harris M, Platt L, Hope V, Rhodes T. Fentanyl self-testing outside supervised injection settings to prevent opioid overdose: Do we know enough to promote it? The International Journal on Drug Policy 2018; 58: 31–6. [DOI] [PubMed] [Google Scholar]
- 152.Simmons R, Ireland G, Irving W, et al. Establishing the cascade of care for hepatitis C in England-benchmarking to monitor impact of direct acting antivirals. Journal of Viral Hepatitis 2018; 25(5): 482–90. [DOI] [PubMed] [Google Scholar]
- 153.Stokes A, Berry KM, McHiza Z, et al. Prevalence and unmet need for diabetes care across the care continuum in a national sample of South African adults: Evidence from the SANHANES-1, 2011–2012. PLoS ONE 2017; 12(10): e0184264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shih YC, Ganz PA, Aberle D, et al. Delivering high-quality and affordable care throughout the cancer care continuum. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 2013; 31(32): 4151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 2011; 52(6): 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Socías ME, Volkow N, Wood E. Adopting the ‘cascade of care’ framework: an opportunity to close the implementation gap in addiction care? Addiction 2016; 111(12): 2079–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Williams A, Nunes E, Olfson M. To battle the opioid overdose epidemic, deploy the ‘cascade of care’ model. Health Affairs Blog 2017. [Google Scholar]
- 158.Williams AR, Nunes EV, Bisaga A, et al. Developing an opioid use disorder treatment cascade: A review of quality measures. Journal of Substance Abuse Treatment 2018; 91: 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kourounis G, Richards BDW, Kyprianou E, Symeonidou E, Malliori M-M, Samartzis L. Opioid substitution therapy: Lowering the treatment thresholds. Drug and Alcohol Dependence 2016; 161: 1–8. [DOI] [PubMed] [Google Scholar]
- 160.Volkow ND. Medications for opioid use disorder: bridging the gap in care. The Lancet 2018; 391(10118): 285–7. [DOI] [PubMed] [Google Scholar]
- 161.Voelker R. Implant for opioid dependence. JAMA 2016; 316(1): 24. doi: 10.1001/jama.2016.8067. [DOI] [Google Scholar]
- 162.Neale J, Tompkins CNE, McDonald R, Strang J. Implants and depot injections for treating opioid dependence: Qualitative study of people who use or have used heroin. Drug Alcohol Depend 2018; 189: 1–7. [DOI] [PubMed] [Google Scholar]
- 163.Grebely J, Larney S, Peacock A, et al. Global, regional, and country-level estimates of hepatitis C infection among people who have recently injected drugs. Addiction 2018; 114: 150–66. doi: 10.1111/add.14393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Martins SS, Sampson L, Cerda M, Galea S. Worldwide Prevalence and Trends in Unintentional Drug Overdose: A Systematic Review of the Literature. Am J Public Health 2015; 105(11): e29–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hulse GK, Milne E, English DR, Holman CD. The relationship between maternal use of heroin and methadone and infant birth weight. Addiction 1997; 92(11): 1571–9. [PubMed] [Google Scholar]
- 166.Keegan J, Parva M, Finnegan M, Gerson A, Belden M. Addiction in pregnancy. Journal of addictive diseases 2010; 29(2): 175–91. [DOI] [PubMed] [Google Scholar]
- 167.Jones HE, Kaltenbach K, Heil SH, et al. Neonatal Abstinence Syndrome after Methadone or Buprenorphine Exposure. New England Journal of Medicine 2010; 363(24): 2320–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yazdy MM, Desai RJ, Brogly SB. Prescription Opioids in Pregnancy and Birth Outcomes: A Review of the Literature. Journal of Pediatric Genetics 2015; 4(2): 56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Grebely J, et al. The impact of opioid agonist treatment on engagement with the hepatitis C treatment cascade: A systematic review and meta-analysis. Journal of Hepatology in preparation. [Google Scholar]
- 170.Feelemyer JP, Jarlais DCD, Arasteh K, Phillips BW, Hagan H. Changes in quality of life (WHOQOL-BREF) and addiction severity index (ASI) among participants in opioid substitution treatment (OST) in low and middle income countries: an international systematic review. Drug & Alcohol Dependence 2014; 134: 251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Perry AE, Neilson M, Martyn-St James M, et al. Pharmacological interventions for drug-using offenders. Cochrane Database of Systematic Reviews 2015; 6(CD010862). doi: 10.1002/14651858.CD010862.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hedrich D, Alves P, Farrell M, Stover H, Moller L, Mayet S. The effectiveness of opioid maintenance treatment in prison settings: a systematic review. Addiction 2012; 107(3): 501–17. [DOI] [PubMed] [Google Scholar]
- 173.Kinlock TW, Gordon MS, Schwartz RP, O’Grady K, Fitzgerald TT, Wilson M. A randomized clinical trial of methadone maintenance for prisoners: Results at one-month post-release. Drug and Alcohol Dependence 2007; 91: 220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rich JD, McKenzie M, Larney S, et al. A randomised trial of methadone continuation versus forced withdrawal upon incarceration. The Lancet 2015; 386: 350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Larney S, Gisev N, Farrell M, et al. Opioid substitution therapy as a strategy to reduce deaths in prison: retrospective cohort study. BMJ Open 2014; 4(e004666). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.World Health Organization. Guidelines for the Psychosocially Assisted Pharmacological Treatment of Opioid Dependence. Geneva: WHO Press, World Health Organization, 2009. [PubMed] [Google Scholar]
- 177.Bojko MJ, Mazhnaya A, Makarenko I, et al. “Bureaucracy & Beliefs”: Assessing the barriers to accessing opioid substitution therapy by people who inject drugs in Ukraine. Drug-Educ Prev Polic 2015; 22(3): 255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bojko MJ, Dvoriak S, Altice FL. At the crossroads: HIV prevention and treatment for people who inject drugs in Ukraine. Addiction 2013; 108(10): 1697–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Giannou FK, Tsiara CG, Nikolopoulos GK, et al. Condom effectiveness in reducing heterosexual HIV transmission: a systematic review and meta-analysis of studies on HIV serodiscordant couples. Expert Review of Pharmacoeconomics & Outcomes Research 2016; 16(4): 489–99. [DOI] [PubMed] [Google Scholar]
- 180.Platt L, Minozzi S, Reed J, et al. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta-analysis. Addiction 2018; 113(3): 545–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Milloy MJ, Wood E. Emerging role of supervised injecting facilities in human immunodeficiency virus prevention. Addiction 2009; 104(4): 620–1. [DOI] [PubMed] [Google Scholar]
- 182.MacArthur GJ, van Velzen E, Palmateer N, et al. Interventions to prevent HIV and hepatitis C in people who inject drugs: A review of reviews to assess evidence of effectiveness. International Journal of Drug Policy 2014; 25(1): 34–52. [DOI] [PubMed] [Google Scholar]
- 183.Gilchrist G, Swan D, Widyaratna K, et al. A Systematic Review and Meta-analysis of Psychosocial Interventions to Reduce Drug and Sexual Blood Borne Virus Risk Behaviours Among People Who Inject Drugs. AIDS & Behavior 2017; 21(7): 1791–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Copenhaver MM, Johnson BT, Lee IC, Harman JJ, Carey MP. Behavioral HIV risk reduction among people who inject drugs: Meta-analytic evidence of efficacy. Journal of Substance Abuse Treatment 2006; 31(2): 163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ivan M, Rodgers C, Maher L, van Beek I. Reducing injecting-related injury and diseases in people who inject drugs: Results from a clinician-led brief intervention. Aust Fam Physician 2016; 45(3): 129–33. [PubMed] [Google Scholar]
- 186.Jahagirdar D, Wright M-D. Naltrexone for Opioid Use Disorders: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines. Ottowa: CADTH, 2017. [PubMed] [Google Scholar]
- 187.Larney S, Gowing L, Mattick RP, Farrell M, Hall W, Degenhardt L. A systematic review and meta-analysis of naltrexone implants for the treatment of opioid dependence. Drug and alcohol review 2014; 33(2): 115–28. [DOI] [PubMed] [Google Scholar]
- 188.Malivert M, Fatseas M, Denis C, Langlois E, Auriacombe M. Effectiveness of therapeutic communities: A systematic review. European Addiction Research 2012; 18(1): 1–11. [DOI] [PubMed] [Google Scholar]
- 189.Spelman T, Morris MD, Zang G, et al. A longitudinal study of hepatitis C virus testing and infection status notification on behaviour change in people who inject drugs. J Epidemiol Community Health 2015; 69(8): 745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kuyper L, Milloy MJ, Marshall BD, et al. Does initiation of HIV antiretroviral therapy influence patterns of syringe lending among injection drug users? Addict Behav 2011; 36(5): 560–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Manhart L, Holmes K. Randomized controlled trials of individual-level, population-level, and multilevel interventions for preventing sexually transmitted infections: what has worked? The Journal of Infectious Diseases 2005; 191(S1): 7–24. [DOI] [PubMed] [Google Scholar]
- 192.Shahmanesh M, Patel V, Mabey D, Cowan F. Effectiveness of interventions for the prevention of HIV and other sexually transmitted infections in female sex workers in resource poor setting: a systematic review. Tropical Medicine & International Health 2008; 13(5): 659–79. [DOI] [PubMed] [Google Scholar]
- 193.Elwy A, Hart G, Hawkes S, Petticrew M. Effectiveness of interventions to prevent sexually transmitted infections and human immunodeficiency virus in heterosexual men: a systematic review. Archives of Internal Medicine 2002; 162(16): 1818. [DOI] [PubMed] [Google Scholar]
- 194.Kertesz SG, Gordon AJ. A crisis of opioids and the limits of prescription control: United States. Addiction 2019; 114(1): 169–80. [DOI] [PubMed] [Google Scholar]
- 195.Rauck RL. Mitigation of IV Abuse Through the Use of Abuse-Deterrent Opioid Formulations: An Overview of Current Technologies. Pain Practice; 19(4): 443–54. doi: 10.1111/papr.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dowell D, Zhang K, Noonan RK, Hockenberry JM. Mandatory Provider Review And Pain Clinic Laws Reduce The Amounts Of Opioids Prescribed And Overdose Death Rates. Health Affairs 2016; 35(10): 1876–83. doi: 10.1377/hlthaff.2016.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.World Health Organization. Assessment of compulsory treatment of people who use drugs in Cambodia, China, Malaysia and Viet Nam: An application of selected human rights principles. Manila: World Health Organization, 2009. [Google Scholar]
- 198.Mueller SR, Walley AY, Calcaterra SL, Glanz JM, Binswanger IA. A review of opioid overdose prevention and naloxone prescribing: Implications for translating community programming into clinical practice. Substance abuse 2015; 36(2): 240–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Potier C, Laprevote V, Dubois-Arber F, Cottencin O, Rolland B. Supervised injection services: What has been demonstrated? A systematic literature review. Drug and Alcohol Dependence 2014; 145: 48–68. [DOI] [PubMed] [Google Scholar]
- 200.Hawton K, Witt KG, Taylor Salisbury TL, et al. Pharmacological interventions for self‐harm in adults. Cochrane Database of Systematic Reviews 2015; 7(CD011777). doi: 10.1002/14651858.CD011777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


